
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 10 October 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1229936
Primary hepatic squamous cell carcinoma (SCC) is extremely rare, and only a few dozen cases have been reported to date. It can barely be diagnosed before histopathological examination, which necessitates the exclusion of metastatic tumors. In this case, we present a 60-year-old female patient with no comorbidity. As laboratory tests and imaging examinations were not diagnostic, ultrasonography (US)-guided liver biopsy was performed and eventually revealed a definitive pathological diagnosis of hepatic SCC. After excluding metastasis, the diagnosis of primary hepatic SCC was established, and then chemotherapy and immunotherapy were performed. Additionally, a comprehensive literature search was conducted on primary hepatic SCC using PubMed, Google Scholar, and Web of Science, and a total of 53 articles were retrieved with a time range from 1972 to 2022. A critical analysis was then performed to evaluate previous literature focusing on the clinical characteristics, imaging features, treatments, and prognosis.
Primary hepatic squamous cell carcinoma (SCC) is extremely rare with only a few dozen cases being reported till now (1). Unlike hepatocellular carcinoma, the most common malignancy in the liver, primary hepatic SCC lacks distinctive imaging characteristics and can only be definitively diagnosed through histopathological examination, necessitating the exclusion of metastatic tumors (2). The pathogenesis of primary hepatic SCC remains unclear, which may relate to liver cysts and hepatolithiasis. However, some patients have no obvious causes (3). Currently, there is no standardized treatment approach in place for primary hepatic SCC. Surgical resection, interventional therapy, chemotherapy, and more recently immunotherapy have been employed based on individual patient characteristics; however, the majority of patients exhibit a poor prognosis with short survival (4–6). In this study, we present a case of primary hepatic SCC in an elderly patient without comorbidities. Additionally, we conducted a comprehensive analysis of relevant literature, focusing on the clinical characteristics, imaging findings, treatments, and prognosis.
In August 2020, a 60-year-old female patient was admitted to our hospital due to upper abdominal discomfort and fullness, without abdominal pain, fever, jaundice, or weight loss. Physical examination revealed a soft abdomen without tenderness, rebound tenderness, or positive Murphy’s sign. Laboratory examinations upon admission revealed leukocytosis and anemia (white blood cell (WBC) 19.79 × 109/L, neutrophil 79.7%, lymphocyte 12.4%, and hemoglobin 102 g/L). Liver function tests indicated elevated alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT) (ALP 170 U/L and GGT 136 U/L), and other indicators (alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and direct bilirubin (DBIL)) were within normal range. There was no history of hepatitis B virus or hepatitis C virus infection reported by the patient. The tumor marker of SCC exhibited significant elevation, and carbohydrate antigen 19-9 (CA19-9) showed a slight increase, while alpha-fetoprotein (AFP), CA72-4, and carcinoembryonic antigen (CEA) levels were normal. C-reactive protein (CRP) level was significantly elevated, while procalcitonin (PCT) level remained normal.
Conventional US revealed a heterogeneous hypoechoic mass in the right posterior lobe of the liver, exhibiting an ill-defined boundary and irregular shape, which involved the right branch of the portal vein. Contrast-enhanced US (CEUS) demonstrated earlier enhancement, displaying an irregular hyperenhancement pattern with multiple non-enhancement areas within during the arterial phase, then washout early in the portal venous phase, and eventually marked washout in the late phase (Figures 1A–E). The abdominal computed tomography (CT) revealed a 6.3 × 6.8 cm patchy heterogeneous low-density mass with an ill-defined boundary in the right lobe of the liver, accompanied by dilation of some intrahepatic biliary ducts adjacent to the lesion in the right lobe. Contrast-enhanced CT (CECT) demonstrated mild-to- moderate heterogeneous enhancement during both arterial and portal venous phases within the mass, while the margin enhanced with a central necrotic area was devoid of enhancement. The degree of enhancement was more pronounced in the delayed phase. The mass involving segments V, VII, and VIII, the right branch of the portal vein was not clearly visualized, possibly due to tumor invasion (Figures 1F–I). Multiple lymph nodes were identified in the hilar region, peripancreatic region, and portacaval space, some of which demonstrated metastatic disease (Figures 1F–I). Positron emission tomography/CT (PET/CT) revealed no discernible lesions in the lung, gastrointestinal tract, or other organs, thus indicating a primary malignant liver tumor with lymph node metastasis. Based on the aforementioned examination, primary malignant lesions originating from intrahepatic biliary ducts were considered a possibility. In order to determine the pathology of the lesion, a US-guided liver biopsy was performed and ultimately revealed a pathological diagnosis of SCC. Immunohistochemistry analysis demonstrated CK7 (individual cells +), CK19 (partial +), Hep (−), Ki-67 proliferation index approximately 40%, CD34 (+), P63 (+), CK5/6 (+), GPC-3 (−), CK (+), and arginase-1 (−). Genetic testing revealed a PD-L1 tumor proportion score (TPS) of 1%, genomic profiling status 1 (GPS 1), tumor mutational burden (TMB) with 24.93 mutations per megabase (Mb), and microsatellite instability-high status (MSI-H). Finally, it was determined that the lesion originated from intrahepatic biliary ducts and was diagnosed as primary hepatic SCC. Due to the involvement of multiple hepatic segments, the right branch of the portal vein, and extensive lymph node metastasis, after a multidisciplinary team discussion, surgical intervention (major liver resection) was not considered the primary treatment option for this patient. She underwent treatment with gemcitabine–cisplatin (GP) chemotherapy regimen (gemcitabine 1.4 g and cisplatin 40 mg on day 1 and day 8, q21day), which is commonly used for the management of SCC in both lung and head and neck regions. Following the completion of two cycles of chemotherapy, a CECT scan was performed, revealing an increase in the size of the mass to 11.9 × 9.4 cm. According to the result of genetic testing, the treatment regimen was modified to PD-L1 combined chemotherapy (pembrolizumab 100 mg on day 1 and albumin-bound paclitaxel 400 mg on day 2, q21day). Following completion of the treatment, the patient was discharged automatically. The clinical course is shown in Figure 2. In July 2021, she returned to our hospital for a follow-up ultrasound examination, which revealed a reduction in tumor size to approximately 4.9 × 3.9 cm. However, unfortunately, she died of obstructive jaundice and extrahepatic metastasis 4 months later with an overall survival time of 15 months.
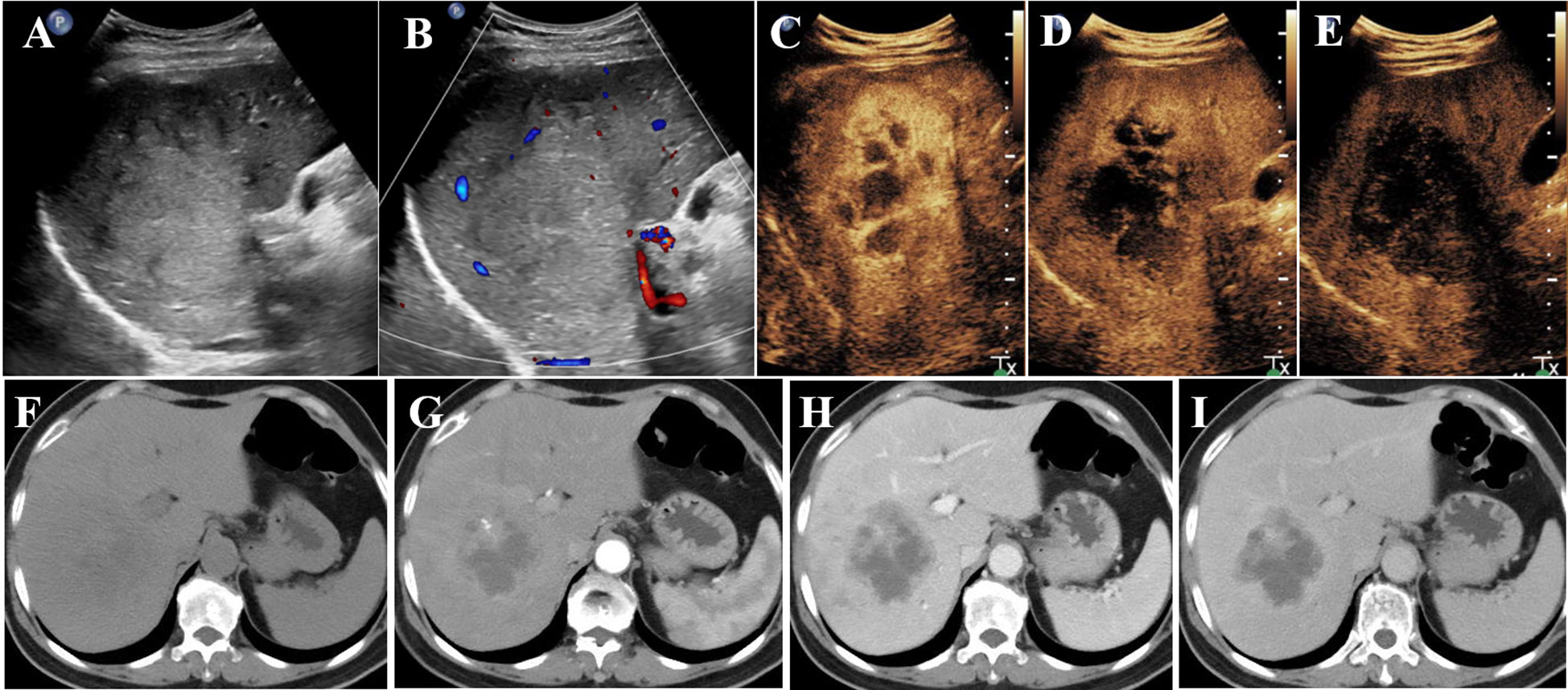
Figure 1 Conventional US revealed a mass (A, B) in the right lobe of the liver; CEUS showed irregular hyperenhancement with non-enhancement areas in the arterial phase (C), then washed out early in the portal venous phase (D), and finally marked washed out in the late phase (E). CT revealed a heterogeneous low-density mass in the right lobe of the liver (F); CECT showed mild-to- moderate heterogeneous enhancement in the arterial phase (G) and portal venous phase (H) in the mass. The enhancement degree was more obvious in the delayed stage (I). US, ultrasonography; CEUS, contrast-enhanced ultrasonography; CECT, contrast-enhanced computed tomography.
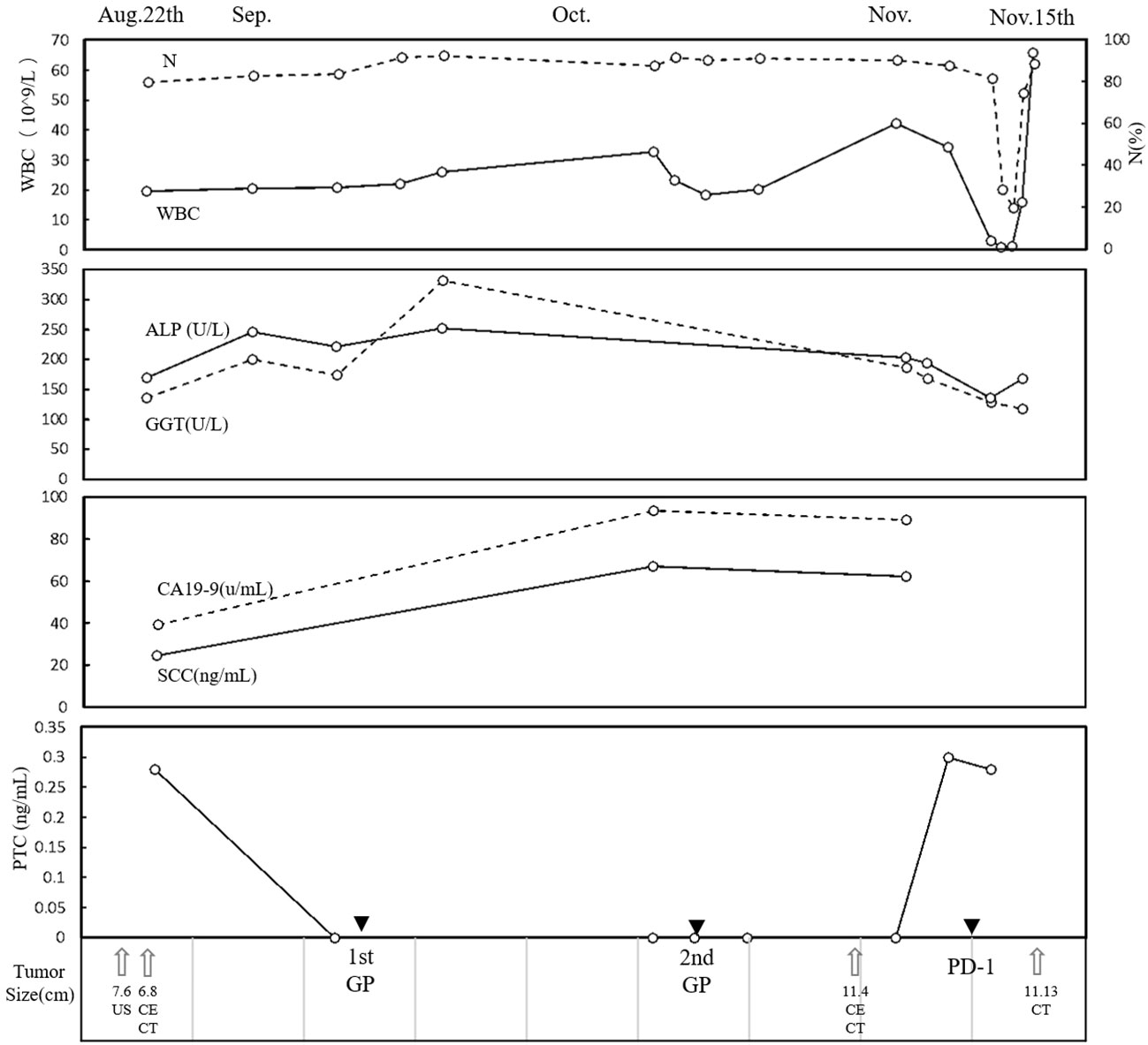
Figure 2 Clinical course. The time course of WBC, N, ALP, GGT, CA19-9, SCC, and PCT. Therapies and tumor size are indicated at the bottom. WBC, white blood cell; N, neutrophil; ALP, alkaline phosphatase; GGT, gamma glutamyl transpeptidase; CA19-9, carbohydrate antigen 19-9; SCC, serum squamous cell carcinoma antigen; PCT, procalcitonin.
The literature search strategy was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for a systematic review. The search was performed in PubMed, Google Scholar, and Web of Science with a time range from 1972 to 2022. The research strategy employed various combinations of the following terms: primary hepatic squamous cell carcinoma, ((primary squamous cell carcinoma)) AND (liver), primary hepatic SCC, (primary SCC) AND (liver). Only English-language articles with full-text availability were included in this review analysis. Two authors (L.Z. and Y.Z.) screened all manuscripts according to PRISMA guidelines, excluding duplicate records and non-relevant reports. L.Z. and Y.Z evaluated all included literature independently. Data extraction and summarization were carried out by L.Z. and Y.Z., resolving any disagreements through discussion. A total of 53 articles were included.
Primary hepatic SCC is an exceedingly rare malignancy, with only a few dozen cases documented in the English literature worldwide. A comprehensive search was conducted on PubMed, Google Scholar, and Web of Science, yielding a total of 53 articles published between 1972 and 2022 (1, 3–54). This article aims to provide a concise overview of the clinical characteristics, imaging findings, diagnosis and treatments associated with primary hepatic SCC.
In the majority of primary hepatic SCC cases, there are no identifiable risk factors for liver tumors, such as hepatitis B infection, hepatitis C infection, or cirrhosis. However, it may be associated with hepatic cysts or hepatolithiasis, but in some cases, no comorbidity was observed. The etiology of primary hepatic SCC remains elusive. It is postulated that the presence of hepatic cysts and hepatolithiasis may be associated with primary hepatic SCC, as chronic inflammation caused by infection of hepatic cysts and irritation of bile ducts by stones could potentially induce squamous metaplasia and subsequent transformation (41, 50). Ciliated hepatic foregut cysts (CHFCs) are rare cystic lesions found in the liver, which have the potential to undergo malignant transformation, leading to the development of primary SCC (55). The clinical manifestations of hepatic primary SCC lack specificity, and there are no specific indicators in laboratory examinations and imaging findings, leading to the patients being typically diagnosed at an advanced stage. Abdominal pain is the most common symptom, while less than half of the patients experience weight loss. Jaundice may be associated with liver damage or cholestasis. Laboratory tests lack specificity, and a history of hepatitis is usually absent in these patients; however, some individuals exhibit abnormalities in liver function. The levels of AFP were within normal range in all patients, while elevated levels of CEA, CA125, and CA19-9 were relatively common. However, these markers lacked specificity. Leukocytosis was observed in approximately 30% of patients, with most of them not reporting fever. This may be attributed to tumor-related leukocytosis (TRL) (48). The occurrence of TRL, a paraneoplastic syndrome, has been reported in 10%–20% of cancer patients and is associated with a poor prognosis. It has been observed that TRL does not respond well to radiotherapy or chemotherapy, leading to poor survival outcomes (56, 57). The general characteristics and treatments are summarized in Table 1.
The imaging findings of primary hepatic SCC lack a typical enhancement pattern, with most CT scans showing low-density heterogeneous masses, some of which may be accompanied by hepatic cysts or intrahepatic biliary duct stones. After contrast enhancement, a significant number of cases exhibit rim enhancement or delayed enhancement, resembling the patterns observed in intrahepatic cholangiocarcinoma (ICC). Differentiation from liver metastasis and liver abscess is necessary, changes in tumor markers may provide insights into the differential diagnosis. We have summarized the CECT imaging findings reported in the literature (Table 2). Contrast-enhanced magnetic resonance imaging (CEMRI) and CEUS were performed in a limited number of cases, yielding no typical findings. In CEMRI, the lesion may exhibit peripheral enhancement or demonstrate a high and irregular enhancement pattern (34, 46, 47). CEUS reveals heterogeneous enhancement during the arterial phase followed by washout in the late phase, indicative of malignancy (35, 44). In the present study, the patient had no history of liver disease; CECT revealed mild-to- moderate enhancement in the arterial and portal venous phases within the mass, with a more pronounced degree of enhancement observed in the delayed stage. This finding suggests an origin from the intrahepatic bile duct, consistent with previous literature reports. The CEUS demonstrated irregular hyperenhancement during the arterial phase, followed by early washout in the portal venous phase within the mass and ultimately significant washout in the late phase, resembling characteristics typically seen in ICC. Thus, the inconsistency in enhancement patterns observed between CEUS and CECT implies that, apart from ICC and vascular liver tumors, primary hepatic SCC should also be taken into consideration.
The diagnosis of primary hepatic SCC necessitates a comprehensive systemic examination to exclude the presence of metastatic lesions, and the final diagnosis still requires pathological analysis and immunohistochemistry. Given the rarity of primary hepatic SCC, there is currently no standardized treatment method. Surgical intervention, interventional therapy, chemotherapy, and immunotherapy can be considered based on the patient’s overall condition and tumor characteristics (1, 4, 41, 50). We conducted an analysis of the treatment regimen and overall survival time among the patients. The patients were categorized into four groups based on different treatment modalities. Those who underwent surgery or interventional therapy (such as transarterial chemoembolization (TACE) and ablation) were assigned to either the surgery group or the intervention group. The non-operative group encompassed radiotherapy, chemotherapy, immunotherapy, or combination therapy. It should be noted that some patients’ treatments were not mentioned (NM). As shown in Figure 3, patients who underwent surgery exhibited better overall survival, potentially attributed to the predominance of early-stage tumors amenable to radical treatment. Conversely, patients receiving non-operative management exhibited shorter survival times due to their inability to undergo radical surgery. Additionally, primary hepatic SCC cases may exhibit resistance toward radiotherapy and chemotherapy, further contributing to a poor prognosis. In recent years, the application of immunotherapy in cancer has provided new hope for primary hepatic SCC. In our case, the patient was given an initial treatment with a GP chemotherapy regimen administered, which is commonly employed for head and neck SCC as well as lung SCC. However, the patient demonstrated a poor response to chemotherapy, resulting in tumor progression following two cycles of treatment. The patient exhibited a significantly elevated level of WBC, which may be associated with chemotherapy resistance. As genetic testing showed PD-L1 TPS 1%, TMB 24.93 mutations/Mb, GPS 1, and MSI-H, pembrolizumab, a PD-1 inhibitor, was administered due to its approval for the treatment of various cancer types when TMB is ≥10 mutations/Mb or in cases of MSI-H. Additionally, the values of TPS and GPS further support this therapeutic approach (58). A significant reduction in the size of the lesion after 1 year demonstrated the efficacy of the treatment. The combination of surgery and PD-1 inhibitors has consistently shown improved outcomes when available (3, 4). Therefore, we hypothesize that leukocytosis in patients with primary hepatic SCC may indicate resistance to chemoradiotherapy, prompting genetic testing to determine eligibility for immunotherapy.

Figure 3 Overall survival time of primary hepatic SCC with different treatments. NM, not mentioned; SCC, squamous cell carcinoma.
In conclusion, we present a case report of primary hepatic SCC, summarizing the clinical characteristics, imaging features, treatments, and prognosis from relevant literature. Treatment options should be tailored based on the patient’s overall condition and tumor characteristics. Additionally, genetic testing may aid in determining the suitability of immunotherapy as a radical treatment alternative when conventional approaches are not feasible. This is particularly important for patients with leukocytosis, which suggests potential resistance to radiotherapy and chemotherapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Third Central Hospital of Tianjin Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization, XJ; data curation, LZ and YZ; writing—original draft preparation, LZ; writing—review and editing, YZ, JD, ZQ, and HZ; supervision, XJ. All authors contributed to the article and approved the submitted version.
This work was funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-074C).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Xiao J, Ma L, Li J, Yin B, Liang J, Wang J. Primary squamous cell carcinoma of the liver is rare but hostile: Case series and comprehensive review of the literature. Cancer Manag Res (2021) 13:829–37. doi: 10.2147/CMAR.S290523
2. Giorgio A, De Luca M, Gatti P, Matteucci P, Giorgio V. CEUS LI-RADS categories to distinguish hepatocellular carcinoma and non-hepatocellular carcinoma Malignancies. Radiology (2020) 296(2):E121–2. doi: 10.1148/radiol.2020200623
3. Kang LM, Yu DP, Zheng Y, Zhou Y-H. Primary squamous cell carcinoma of the liver: A case report. World J Clin cases (2022) 10(19):6744–9. doi: 10.12998/wjcc.v10.i19.6744
4. Wang K, De L, JN S, Wang CN, Gao G, Hu YK, et al. Primary hepatic squamous cell carcinoma with high microsatellite instability shows good response to programmed cell death 1 inhibitor as adjuvant therapy. Hepatology (2021) 74(3):1695–7. doi: 10.1002/hep.31737
5. Atiq M, Ammar AS, Ali RM, Haider S, Ahmed I, Dar FS, et al. Primary squamous cell carcinoma of liver. First case report from Pakistan and South Asia. Int J Surg Case Rep (2022) 99:107655. doi: 10.1016/j.ijscr.2022.107655
6. Naik S, Waris W, Carmosino L, Mehrishi A, Saif MW. Primary squamous cell carcinoma of the liver. J Gastrointestin Liver Dis (2009) 18(4):487–9.
7. Greenwood N, Orr WM. Primary squamous-cell carcinoma arising in a solitary non-parasitic cyst of the liver. J Pathol (1972) 107(2):145–8. doi: 10.1002/path.1711070211
8. Bloustein PA, Silverberg SG. Squamous cell carcinoma originating in an hepatic cyst. Case report with a review of the hepatic cyst-carcinoma association. Cancer (1976) 38(5):2002–5. doi: 10.1002/1097-0142(197611)38:53.0.co;2-9
9. Song E, Kew MC, Grieve T, Isaacson C, Myburgh JA. Primary squamous cell carcinoma of the liver occurring in association with hepatolithiasis. Cancer (1984) 53(3):542–6. doi: 10.1002/1097-0142(19840201)53:3<542::AID-CNCR2820530328>3.0.CO;2-3
10. Banbury J, Conlon KC, Ghossein R, Brennan MF. Primary squamous cell carcinoma within a solitary nonparasitic hepatic cyst. J Surg Oncol (1994) 57(3):210–2. doi: 10.1002/jso.2930570316
11. Gresham GA, Rue LW 3rd. Squamous cell carcinoma of the liver. Hum Pathol (1985) 16(4):413–6. doi: 10.1016/S0046-8177(85)80234-3
12. Arase Y, Endo Y, Hara M, Kumada H, Ikeda K, Yoshiba A. Hepatic squamous cell carcinoma with hypercalcemia in liver cirrhosis. Acta Pathol Jpn (1988) 38(5):643–50. doi: 10.1111/j.1440-1827.1988.tb02336.x
13. Clements D, Newman P, Etherington R, Lawrie BW, Rhodes J. Squamous carcinoma in the liver. Gut (1990) 31(11):1333–4. doi: 10.1136/gut.31.11.1333
14. Roediger WE, Dymock RB. Primary squamous carcinoma of the liver: clinical and histopathological features. Aust N Z J Surg (1991) 61(9):720–2. doi: 10.1111/j.1445-2197.1991.tb00331.x
15. Nieweg O, Slooff MJ, Grond J. A case of primary squamous cell carcinoma of the liver arising in a solitary cyst. HPB Surg (1992) 5(3):203–8. doi: 10.1155/1992/32474
16. Lombardo FP, Hertford DE, Tan LK, Kazam E, Ramirez de Arellaro E. Epidermoid cyst of the liver complicated by microscopic squamous cell carcinoma: CT, ultrasound, and pathology. J Comput Assist Tomogr (1995) 19(1):131–4. doi: 10.1097/00004728-199501000-00025
17. Weimann A, Klempnauer J, Gebel M, Maschek H, Bartels M, Ringe B. Squamous cell carcinoma of the liver originating from a solitary non-parasitic cyst case report and review of the literature. HPB Surg (1996) 10(1):45–9. doi: 10.1155/1996/97680
18. Shinagawa T, Tadokoro M, Takagi M, Yasuda R, Adachi Y, Komoriyama H, et al. Primary squamous cell carcinoma of the liver: a case report. Acta Cytol (1996) 40(2):339–45. doi: 10.1159/000333765
19. Chou YY, Lee WJ, Su CT, Hsu HC. Case report: primary cystic keratinizing squamous cell carcinoma of the liver in a patient with treated nasopharyngeal carcinoma. J Gastroenterol Hepatol (1997) 12(3):229–32. doi: 10.1111/j.1440-1746.1997.tb00413.x
20. Monteagudo M, Vidal G, Moreno M, Bella R, Díaz MJ, Colomer O, et al. Squamous cell carcinoma and infection in a solitary hepatic cyst. Eur J Gastroenterol Hepatol (1998) 10(12):1051–3. doi: 10.1097/00042737-199812000-00012
21. Vick DJ, Goodman ZD, Ishak KG. Squamous cell carcinoma arising in a ciliated hepatic foregut cyst. Arch Pathol Lab Med (1999) 123(11):1115–7. doi: 10.5858/1999-123-1115-SCCAIA
22. Asanuma N, Hagiwara K, Matsumoto I, Matsuda M, Nakamura F, Kouhara H, et al. PTHrP-producing tumor: squamous cell carcinoma of the liver accompanied by humoral hypercalcemia of Malignancy, increased IL-6 and leukocytosis. Intern Med (2002) 41(5):371–6. doi: 10.2169/internalmedicine.41.371
23. de Lajarte-Thirouard AS, Rioux-Leclercq N, Boudjema K, Gandon Y, Ramée M-P, Turlin B, et al. Squamous cell carcinoma arising in a hepatic forgut cyst. Pathol Res Pract (2002) 198(10):697–700. doi: 10.1078/0344-0338-00323
24. Furlanetto A, Dei Tos AP. Squamous cell carcinoma arising in a ciliated hepatic foregut cyst. Virchows Arch (2002) 441(3):296–8. doi: 10.1007/s00428-002-0668-z
25. Saito T, Harada K, Tsuneyama K, Hirano M, Amaya S, Sasaki M, et al. Primary squamous cell carcinoma of the liver producing parathyroid hormone-related protein. J Gastroenterol (2002) 37(2):138–42. doi: 10.1007/s005350200010
26. Kaji R, Sasaki N, Tateishi I, Nagata E, Okabe Y, Yoshida T, et al. A case report of primary hepatic squamous cell carcinoma that remarkably responded to low dose arterial injection of anti-cancer drugs. Kurume Med J (2003) 50(1-2):71–5. doi: 10.2739/kurumemedj.50.71
27. Boscolo G, Jirillo A, Da Pian P. Complete remission of poorly differentiated squamous liver carcinoma after systemic chemotherapy and surgery. A case report. Tumori (2005) 91(1):71–2. doi: 10.1177/030089160509100113
28. Hsieh CB, Chen CJ, Yu JC, Chang T-M, Gao H-W, Liu Y-C. Primary squamous cell carcinoma of the liver arising from a complex liver cyst: report of a case. Surg Today (2005) 35(4):328–31. doi: 10.1007/s00595-004-2941-z
29. Odemiş B, Köksal AS, Yüksel O, Kacar S, Turhan N. Squamous cell cancer of the liver arising from an epidermoid cyst: case report and review of the literature. Dig Dis Sci (2006) 51(7):1278–84. doi: 10.1007/s10620-006-8049-0
30. Yuki N, Hijikata Y, Kato M, Kawahara K, Wakasa K. Squamous cell carcinoma as a rare entity of primary liver tumor with grave prognosis. Hepatol Res (2006) 36(4):322–7. doi: 10.1016/j.hepres.2006.08.004
31. Lee H-L, Liu Y-Y, Yeh C-N, Chiang K-C, Chen T-C, Jan Y-Y. Primary squamous cell carcinoma of the liver: a successful surgically treated case. World J Gastroenterol (2006) 12(33):5419–21. doi: 10.3748/wjg.v12.i33.5419
32. Abbas R, Willis J, Kinsella T, Siegel C, Sanabria J. Primary squamous cell carcinoma of the main hepatic bile duct. Can J Surg (2008) 51(4):E85–6.
33. Zhang X, Wang Z, Dong Y. Squamous cell carcinoma arising in a ciliated hepatic foregut cyst: case report and literature review. Pathol Res Pract (2009) 205(7):498–501. doi: 10.1016/j.prp.2008.12.003
34. Spaggiari M, Di Benedetto F, Ballarin R, Losi L, Cautero N, De Ruvo N, et al. Primary squamous cell carcinoma of the liver associated with Caroli’s disease: a case report. Onkologie (2011) 34(4):193–5. doi: 10.1159/000326999
35. Iimuro Y, Asano Y, Suzumura K, Yada A, Hirano T, Iijima H, et al. Primary squamous cell carcinoma of the liver: an uncommon finding in contrast-enhanced ultrasonography imaging. Case Rep Gastroenterol (2011) 5(3):628–35. doi: 10.1159/000334425
36. Zhu KL, Li DY, Jiang CB. Primary squamous cell carcinoma of the liver associated with hepatolithiasis: a case report. World J Gastroenterol (2012) 18(40):5830–2. doi: 10.3748/wjg.v18.i40.5830
37. Zhao R, Zhu K, Wang R, Gao J, Cui K, Yu F, et al. Primary squamous cell carcinoma of the liver: A case report and review of the literature. Oncol Lett (2012) 4(6):1163–6. doi: 10.3892/ol.2012.894
38. Choi MK, Kim GH, Song GA, Nam HS, Yi YS, Ahn KH, et al. Primary squamous cell carcinoma of the liver initially presenting with pseudoachalasia. Gut Liver (2012) 6(2):275–9. doi: 10.5009/gnl.2012.6.2.275
39. Wilson JM, Groeschl R, George B, Turaga KK, Patel PJ, Saeian K, et al. Ciliated hepatic cyst leading to squamous cell carcinoma of the liver - A case report and review of the literature. Int J Surg Case Rep (2013) 4(11):972–5. doi: 10.1016/j.ijscr.2013.07.030
40. Morito K, Kai K, Miyoshi A, Kubo H, Ide T, Azama S, et al. Primary squamous cell carcinoma of the liver concomitant with primary colon cancer: report of a case. Clin J Gastroenterol (2013) 6(2):134–8. doi: 10.1007/s12328-012-0341-2
41. Zhang XF, Du ZQ, Liu XM, Lv Y. Primary squamous cell carcinoma of liver: Case series and review of literatures. Med (United States) (2015) 94(28):1–8. doi: 10.1097/MD.0000000000000868
42. Yoo TK, Kim BI, Han EN, Kim DH, Yoo JH, Lee SJ, et al. Primary squamous cell carcinoma of the liver: a case report. Clin Mol Hepatol (2016) 22(1):177–82. doi: 10.3350/cmh.2016.22.1.177
43. Mao JX, Teng F, Yuan H, Liu C, Fu H, Sun KY, et al. Primary hepatic squamous cell carcinoma with abdominal incision metastasis after hepatectomy. Hepatobiliary Pancreat Dis Int (2019) 18(2):194–8. doi: 10.1016/j.hbpd.2018.12.004
44. Bondini S, Leoni S, Bolondi L. Squamous cell carcinoma of the liver: metastasis or primary neoplasm? J Clin Ultrasound (2005) 33(9):477–8. doi: 10.1002/jcu.20173
45. Avezbadalov A, Aksenov S, Kaplan B, Jung G. Asymptomatic primary squamous cell carcinoma of the liver. J Community Support Oncol (2014) 12(2):75–6. doi: 10.12788/jcso.0019
46. Lubana SS, Singh N, Seligman B, Tuli SS, Heimann DM. First reported case of primary intrahepatic cholangiocarcinoma with pure squamous cell histology: A case report. Am J Case Rep (2015) 16:438–44. doi: 10.12659/AJCR.894609
47. Yamada K, Shinoura S, Kikuchi K. Hepatic squamous cell carcinoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Case Rep Gastrointest Med (2021) 2021:9939898. doi: 10.1155/2021/9939898
48. Wang Y, Pan Y, Zhou Z. An unusual cause of fever in a man with a liver mass. Gastroenterology (2020) 159(3):842–4. doi: 10.1053/j.gastro.2020.03.080
49. Sun Y, Jin G. Primary squamous cell carcinoma of the liver: A case report. J Int Med Res (2021) 49(6):3000605211021275. doi: 10.1177/03000605211021275
50. Fakhreddine O, Fadlallah Y, Turfa J, Hassan MA, Chamseddine N, Assi HI. Primary squamous cell carcinoma of the liver: Case report and review of literature. Case Rep Oncol (2022) 15(2):480–5. doi: 10.1159/000523857
51. Tuminello F, Castiglione D, Broggi G, Vecchio GM, Basile A, Puleo S, et al. Primary squamous cell carcinoma of the liver: an unexpected pathological finding. Egypt Liver J (2020) 10(17):45–9. doi: 10.1186/s43066-020-00032-0
52. Li W, Guo H, Zhou H, Meng Z. Primary hepatic squamous carcinoma concurrent with gastric signet ring cell carcinoma demonstrated by 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Korean J Intern Med (2021) 36(5):1253–5. doi: 10.3904/kjim.2020.436
53. Okuda Y, Abe T, Ikeda M, Kurihara K, Shimizu A, Oshita A, et al. Curative surgery for primary squamous cell carcinoma of the liver: A rare case study. Clin J Gastroenterol (2023) 16(2):263–9. doi: 10.1007/s12328-022-01740-3
54. Lee HL, Fu CK, Chien LY, Chen L-M. Primary squamous cell carcinoma of the liver with good response to carboplatin and 5-flurouracil: A case report. Medicina (Kaunas) (2022) 58(12):1864. doi: 10.3390/medicina58121864
55. Hughes DL, Tsakok M, Patel N, Rendek A, Bungay H, Silva MA. Ciliated hepatic foregut cysts: Not as rare as previously believed. Int J Surg Pathol (2023) 31(3):260–7. doi: 10.1177/10668969221095263
56. Roh JL, Lee H, Choi SH, Nam SY, Kim SY. Tumor-related leukocytosis predictive of recurrence and survival in patients with oral cavity squamous cell carcinoma. Oral Dis (2019) 25(6):1511–8. doi: 10.1111/odi.13138
57. Wilcox RA. Cancer-associated myeloproliferation: Old association, new therapeutic target. Mayo Clin Proc (2010) 85(7):656–63. doi: 10.4065/mcp.2010.0077
Keywords: primary hepatic squamous cell carcinoma, clinical course, clinical characteristics, imaging findings, treatments and prognosis
Citation: Zhao L, Zhou Y, Ding J, Qin Z, Zhou H and Jing X (2023) Primary hepatic squamous cell carcinoma: case report and systematic review of the literature. Front. Oncol. 13:1229936. doi: 10.3389/fonc.2023.1229936
Received: 14 June 2023; Accepted: 18 September 2023;
Published: 10 October 2023.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Nádia Ghinelli Amôr, Campinas State University, BrazilCopyright © 2023 Zhao, Zhou, Ding, Qin, Zhou and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Jing, ZHIuamluZ3hpYW5nQHZpcC4xNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.