
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 29 June 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1229853
Introduction: Treatment of children with medulloblastoma (MB) includes surgery, radiation therapy (RT) and chemotherapy (CT). Several treatment protocols and clinical trials have been developed over the time to maximize survival and minimize side effects.
Methods: We performed a systematic literature search in May 2023 using PubMed. We selected all clinical trials articles and multicenter studies focusing on MB. We excluded studies focusing exclusively on infants, adults, supratentorial PNETs or refractory/relapsed tumors, studies involving different tumors or different types of PNETs without differentiating survival, studies including <10 cases of MB, solely retrospective studies and those without reference to outcome and/or side effects after a defined treatment.
Results: 1. The main poor-prognosis factors are: metastatic disease, anaplasia, MYC amplification, age younger than 36 months and some molecular subgroups. The postoperative residual tumor size is controversial.
2. MB is a collection of diseases.
3. MB is a curable disease at diagnosis, but survival is scarce upon relapse.
4. Children should be treated by experienced neurosurgeons and in advanced centers.
5. RT is an essential treatment for MB. It should be administered craniospinal, early and without interruptions.
6. Craniospinal RT dose could be lowered in some low-risk patients, but these reductions should be done with caution to avoid relapses.
7. Irradiation of the tumor area instead of the entire posterior fossa is safe enough.
8. Hyperfractionated RT is not superior to conventional RT
9. Both photon and proton RT are effective.
10. CT increases survival, especially in high-risk patients.
11. There are multiple drugs effective in MB. The combination of different drugs is appropriate management.
12. CT should be administered after RT.
13. The specific benefit of concomitant CT to RT is unknown.
14. Intensified CT with stem cell rescue has no benefit compared to standard CT regimens.
15. The efficacy of intraventricular/intrathecal CT is controversial.
16. We should start to think about incorporating targeted therapies in front-line treatment.
17. Survivors of MB still have significant side effects.
Conclusion: Survival rates of MB improved greatly from 1940-1970, but since then the improvement has been smaller. We should consider introducing targeted therapy as front-line therapy.
Medulloblastoma (MB) was first described in 1925 and is to date the most common malignant brain tumor of childhood (1). It is an embryonal tumor that arises in the cerebellum and is characterized by the presence of small blue round cells. It has a peak age of diagnosis around 6-8 years of age (2). Almost 25-35% of MB occur in children under 3 years old and about 50% are diagnosed before the age of 5 years old (3, 4). Since 2011, it has been classified into four molecular subgroups: WNT-MB, SHH-MB, Group 3 MB and Group 4 MB (2, 5). Progressively, different subtypes are being redefined based on further genomic findings (6). There is increasing awareness of the importance of these molecular subgroups in terms of prognosis and specific therapeutic management (7, 8).
Treatment of MB generally includes surgery, chemotherapy (CT) and radiation therapy (RT) (2). Treatment of MB has progressed greatly over the past 70 years. The earliest treatment protocols date back to the 1960s (9) (Tables 1–4). Different treatment protocols have been designed internationally to find the best management of MB (Figure 1) (Tables 1–4).
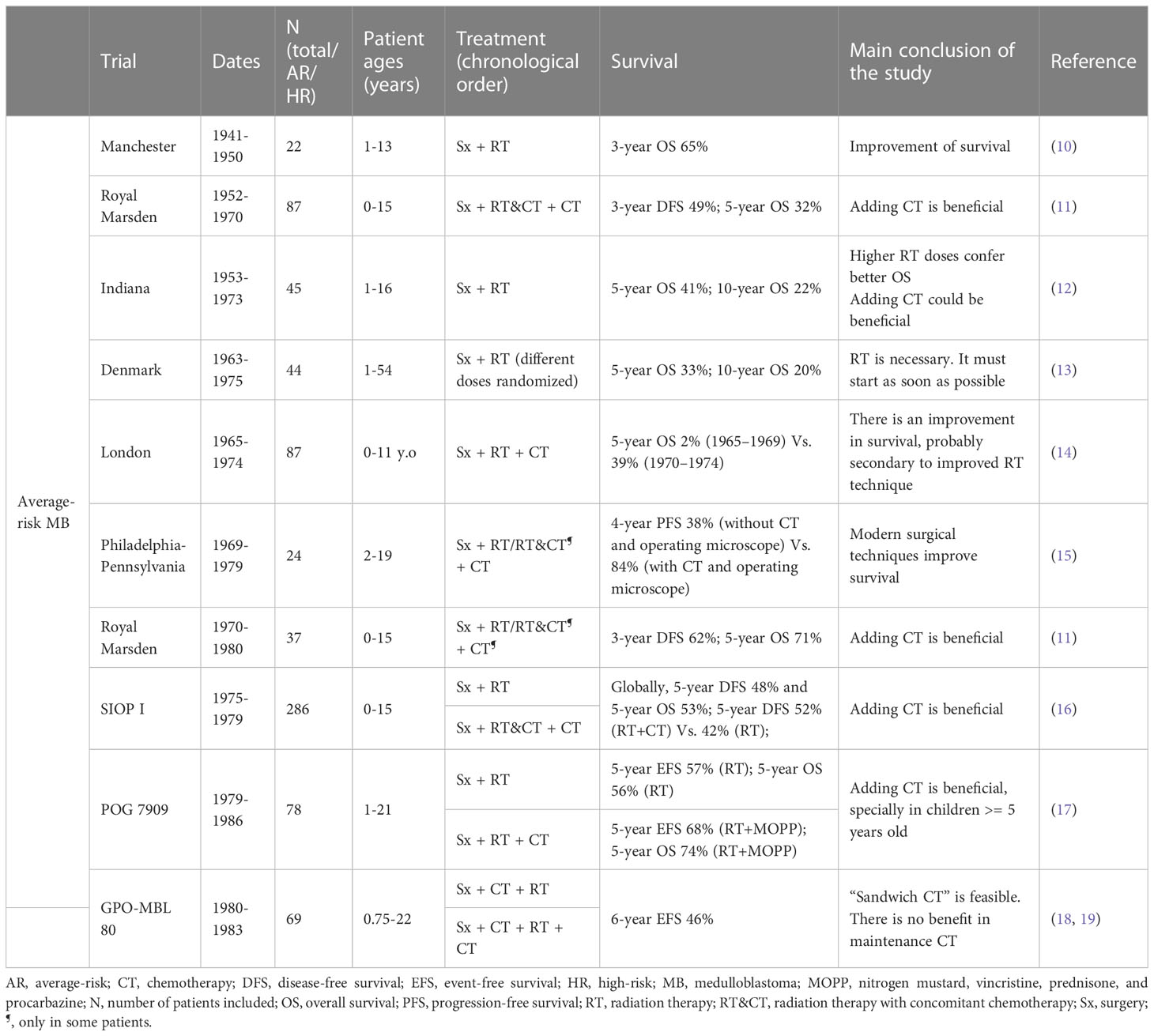
Table 1 Protocols of treatment developed over the time for patients with MB without classification by risk.
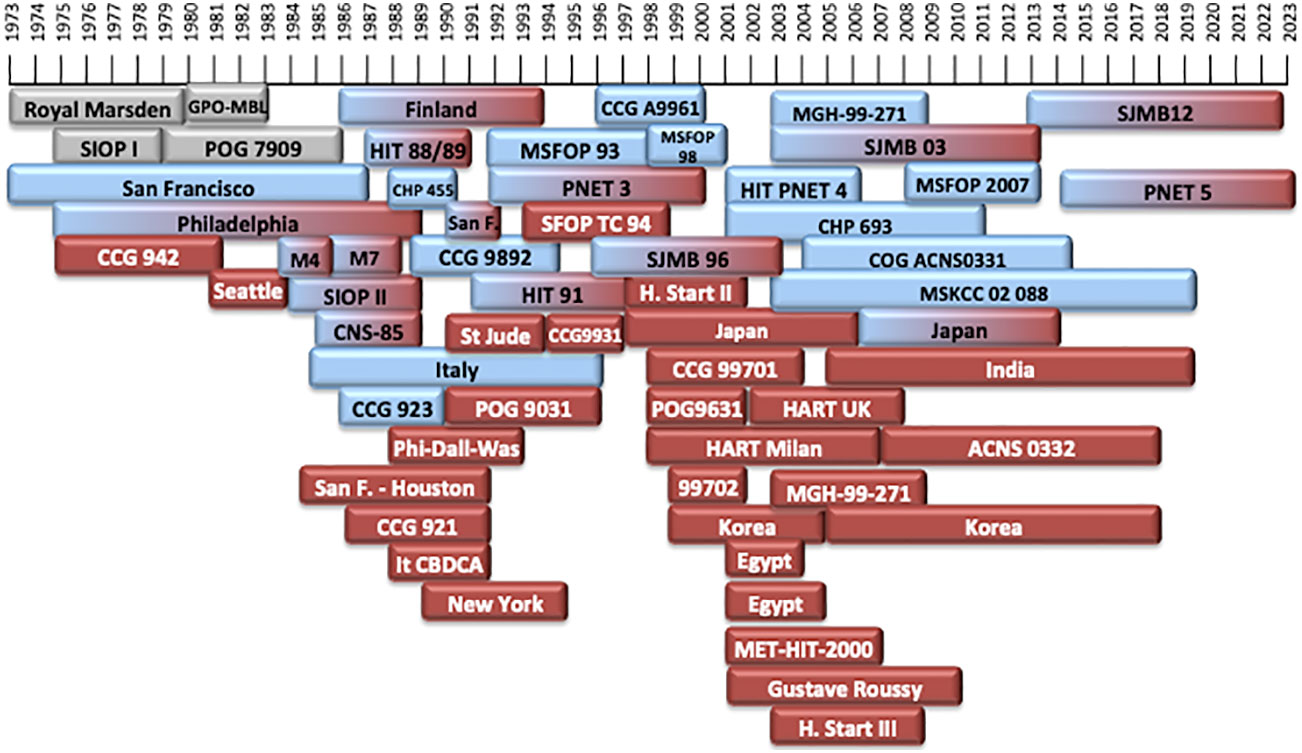
Figure 1 Timeline of the different treatment protocols developed over the last 50 years. In grey: treatment protocols of non-defined risk MB (NR-MB). In blue: treatment protocols for patients with average-risk MB (AR-MB). In red: treatment protocols for patients with high-risk MB (HR-MB). Protocols with gradient colors included patients both with AR-MB or HR-MB.
Around 1960, the standard of care of MB consisted on surgery and post-operative craniospinal irradiation (CSI), with a 5-year overall survival (OS) being around 30-50% (16, 20–22). In 1975, the first multi-center randomized trial of the International Society of Pediatric Oncology (SIOP I (16)) was designed. Since then, survival rates are better (Figure 2).
The aim of this paper is to review, synthesize and analyze the design and outcome of the different treatment protocols used in 0-18 year-old children affected with MB. Therefore, we would critically understand the rationale for the current management of children with MB. In this regard, we conducted a systematic review, analyzing the clinical trials and multicenter studies performed since the 1940s, their design, hypothesis and conclusions. Since treatment is considerably different in children under 3 years of age, for the purpose of this review we will exclude trials based exclusively in infants and children younger than 3 years of age.
We performed a comprehensive search through the indexed database PubMed Central in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (68) (Figure 3). The research string included the word “medulloblastoma”. Afterwards, we selected the following filters: “clinical trial”, “randomized controlled trial” and “multicenter study”, so that all clinical trials and prospective studies were included. We initially conducted the search on January 31st, 2022 and we updated it on May 11th, 2023.
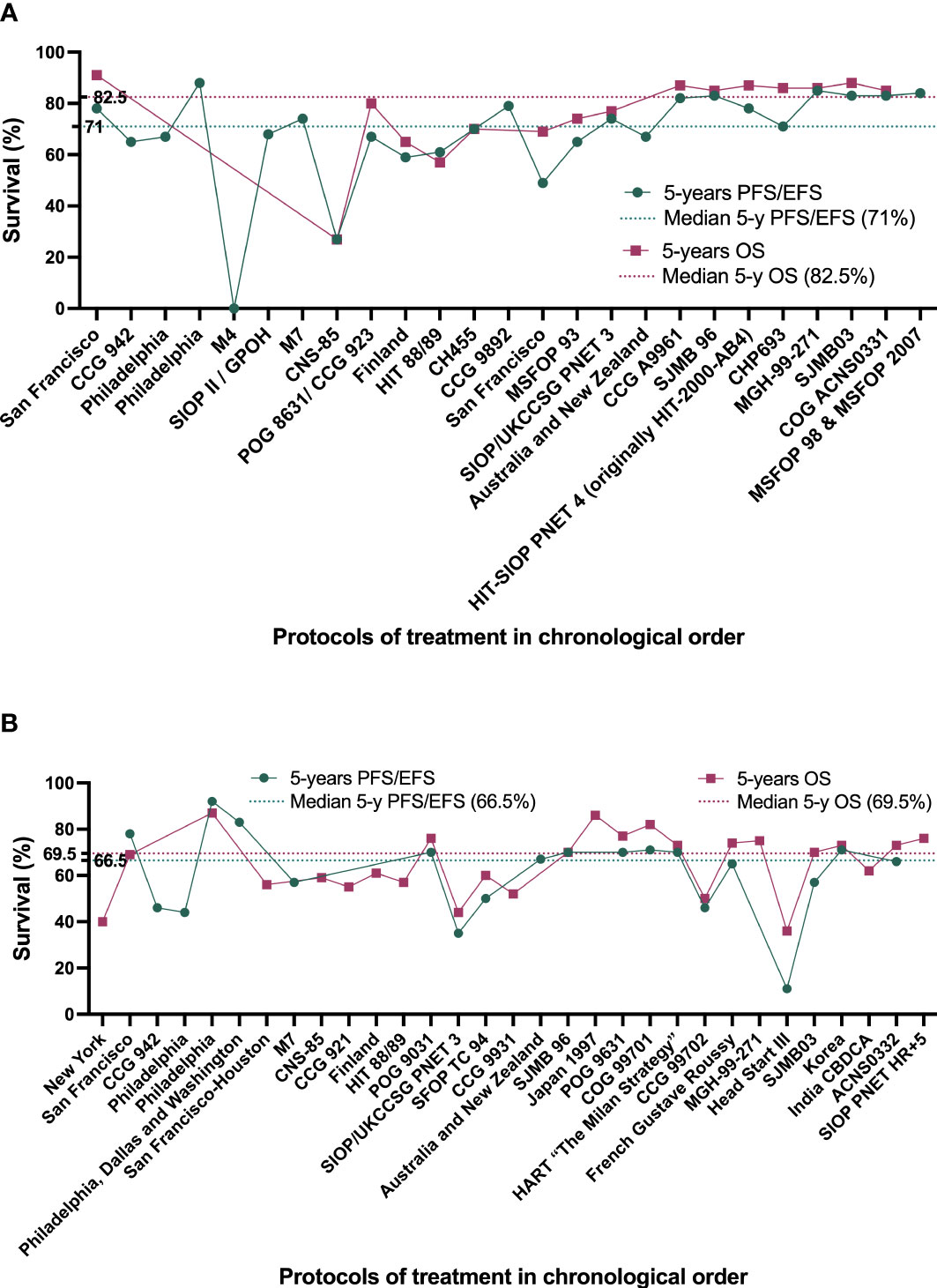
Figure 3 Best survival rates achieved by the different treatment protocols developed since 1963 by chronological order. (A) treatment protocols for patients with average-risk MB (AR-MB). (B) treatment protocols for patients with high-risk MB (HR-MB).
Two investigators (MPOM and LIML) read the titles and abstracts of all the papers that met the criteria. We selected articles written in any language focusing on outcomes and/or side effects after a defined treatment, randomized or not. There were no restrictions regarding the date of publication, language or country of origin of the articles. We subsequently excluded studies focusing exclusively on supratentorial PNETs, studies on refractory/relapsed tumors, studies focused exclusively on patients younger than 3 years of age, studies focused exclusively on patients older than 18, studies involving different tumors without differentiating survival according to different histologies, studies including fewer than 10 cases of MB, trials on different types of PNETs, studies based solely on retrospective data and those without reference to outcome and/or side effects after a defined treatment.
The selected papers were deeply reviewed by MPOM. Their bibliographies were also manually screened for other relevant studies to ensure that the search was complete. We extracted the following information from each clinical trial: trial name, dates the trial was open, number of patients included, patient risk classification, doses of RT administered (craniospinal, boost, fractions, boost area and duration), type of CT administered (before RT, after RT and/or concomitant to RT, doses and timing of each drug), and main conclusions of the trial. When available, the rationale of each treatment protocol was also analyzed.
The total selection process is summarized in Figure 2. A total of 442 papers were included for initial review. Title and abstract screening resulted in exclusion of 215 studies. Therefore, 227 articles concerning the outcomes and conclusions of the different treatment protocols over time were selected for full-text review. Subsequently, 113 articles were excluded for meeting one or more exclusion criteria. One article was not available in full-text version. A supplementary search of the reference list generated 41 additional papers. Finally, a total of 155 papers were included to develop this systematic review. Based on the information gathered, we answer some of the key questions regarding MB and its management over time.
One of the milestones in the management of MB has been the classification of patients into prognostic groups, in order to obtain the best survival rates without unnecessary long-term toxicity. Currently, the five main poor-prognostic factors are: age less than 36 months, metastatic disease (according to Chang Stage), postoperative residual tumor volume ≥1.5 cm2, anaplastic histology and MYC amplification (1). The San Francisco (9) treatment protocol (1966-1987), the European SIOP I (16) trial (1975-1979), and the American CCG 942 (21) trial (1975-1981) were the first trials that defined some of these poor prognosis factors (9, 16, 21) (Tables 1, 3, 4).
The first studies that adjusted treatment based on the risk were performed at the Children’s Hospital of Philadelphia (22) (1983-1991), the Children’s National Medical Center in Washington (24) (1988-1993) and the Children’s Medical Center in Dallas (24) (1988-1993). In these studies, children were classified into “standard-risk” or “poor-risk” groups (22, 24). Children older than 5 years of age in the standard-risk group were treated with RT alone, whereas children younger than 5 years of age in the standard-risk and children older than 18 months in the poor-risk group were treated with RT plus CT. There was a major difference in outcomes between children treated with RT alone and those treated with RT+CT: 5-year disease-free survival (DFS) was 52% and 88%, respectively (22) (Tables 3, 4). This is even more remarkable considering that 82% of patients of the latter group met the poor-risk criteria (22). Since then, patients have been classified into average/standard risk (AR-MB) and high-risk (HR-MB) groups. Lately, given the excellent prognosis of certain molecular subtypes, some protocols have created a new low-risk group (LR-MB) (5, 85).
Disease extent was shown to be important both in the Philadelphia-Washington-Dallas (22, 24) treatment plan (5-year progression-free survival (PFS) of 90% for patients with localized disease Vs. 67% in metastatic disease (24)) and in the CCG 921 (32) randomized phase III study (5-year PFS for M0, M1 and M2 was 70%, 57% and 40%, respectively (32)). This has subsequently been confirmed in posterior trials, such as the French Society of Pediatric Oncology (SFOP) study of 1993 (45) which confirmed that M1 tumors should be considered high-risk (Tables 3, 4).
Another prognostic factor is the postoperative bulky residual tumor size (≥1.5 cm2) in M0 tumors: 5-year PFS were 78% and 54% depending on whether the residual tumor was less than 1.5 cm2 or not in the CCG 921 (32) study. Similarly, the 5-year PFS of patients who achieved a complete response with CT before RT was 57% in the HIT 88/89 trial, compared with 20% in patients that did not achieve it (34). In contrast, the presence of residual disease in patients M0 was not associated with an inferior outcome in the POG 9031 (40) trial. Although this has traditionally been stated, there are some recent reports evidencing that the residual tumor size may be of less importance than initially thought (8, 86), especially after taking into account molecular subgroup classification (87) (Tables 3, 4).
The presence of anaplasia is also considered a high-risk feature, even in patients who otherwise fulfill standard risk criteria (8, 53, 77).
Children younger than 36 months old have worse prognosis than older children (3). The biology of MB in infants is different than that of older children and they present more frequently with metastatic disease. Moreover, they mostly receive radiation-sparing treatment protocols based on CT and high-dose CT due to the high susceptibility of RT-induced neurocognitive deficits in these young children (3, 4). For all this, age under than 36 months old is considered to be one of the main poor prognostic factors. Many reports outline the behavioral and prognostic differences between infants, children and adults (88).
Prolonged duration of symptoms before diagnosis does not seem to be a poor prognostic factor. On the contrary, it has been associated with lower metastatic stages, some specific molecular subgroups and better survival rates (89, 90).
An extraordinary milestone in the history of MB has been the definition of its molecular subtypes (5). This recent molecular classification enables us a deeper understanding of tumor biology and pathophysiology. What was originally thought to be a single disease is now up to 12 different diseases (6). This classification is going to become increasingly important over time. This has recently been demonstrated by the results of the SJMB03 (8) (2003-2013) and the ACNS0332 (7) (2007-2018) trials. These trials document that survival rates differ dramatically between molecular groups. In this regard, the ACNS0332 (7) trial found 5-year event-free survival (EFS) rates of 92.9% for WNT, 49.6% for SHH, 64.2% for group 3 and 65.4% for group 4 (p=0.06). Moreover, it has been observed that prognosis can be excellent in patients with MB of a specific molecular subgroup, despite presenting high-risk features. In this respect, the SJMB03 trial found a 5-year PFS in high-risk WNT-MB of 100%, compared to only 67% in standard-risk group 3-MB (8). The high-risk group of patients treated with the SJMB03 trial show dramatic differences in survival, with 5-year PFS rates of 100% for WNT and <30% for SHH (8) (Tables 3, 4).
The excellent survival rates observed in the WNT molecular subgroup has led to the definition of a new low-risk treatment group. This low-risk group includes patients with molecular WNT-MB (defined by the presence of nuclear beta-catenin positivity by immunohistochemistry and the CTNNB1 mutation, and, in some protocols, associated with the presence of monosomy 6) without any high-risk features (i.e. age >= 16 years old, large-cell-anaplastic histology, metastatic disease, MYC amplification and/or residual tumor volume >1,5 cm2). There are currently 3 ongoing trials based on a de-escalated treatment for this good-prognosis WNT subgroup: SJMB12 stratum W1 (NCT01878617, from 2013 to present), PNET5 MB-LR (NCT02066220, from 2014 to present) and COG ACNS1422 (NCT02724579, from 2017 to present) trials (Table 2). Molecular subgrouping is the future and should be taken into account when defining treatment intensity.
Survival rates have improved greatly over time. Overall, in children older than 3 years of age, 5-year OS is around 70-85% in AR-MB and 60-65% in HR-MB (1, 2, 91) (Figure 4). Approximately two-thirds of patients with MB fall into the standard-risk group.
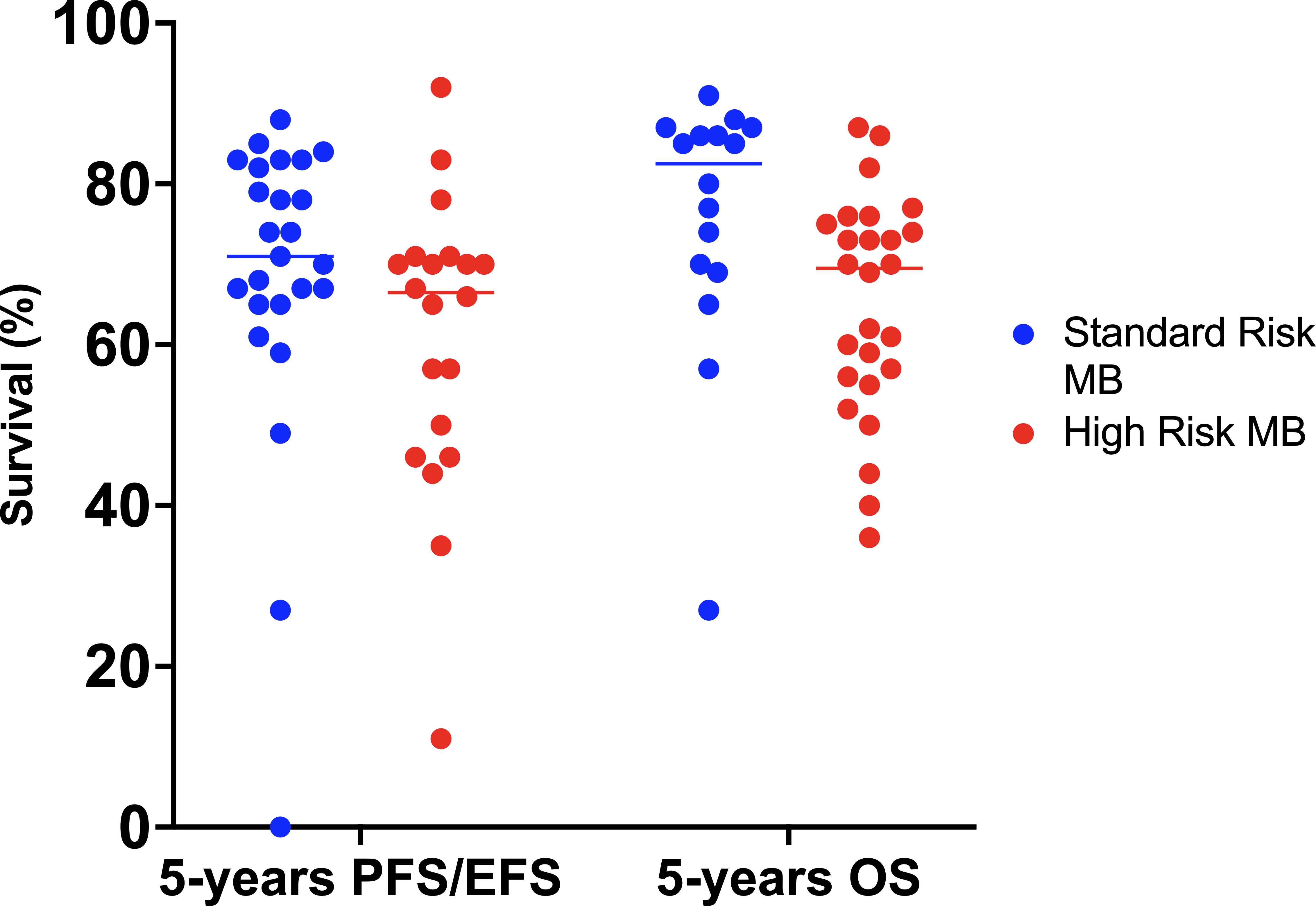
Figure 4 Best survival rates achieved in the different treatment protocols since 1970. EFS, event-free survival; MB, medulloblastoma; OS, overall survival; PFS, progression-free survival.
Considering molecular subtype, patients with AR-MB have a 5-year PFS of 100% for WNT-MB, 77.5% for SHH-MB, 66.7% for group 3 MB and 87.3% for group 4 MB. For patients with HR-MB, the 5-year PFS rates are 100% for WNT-MB, 25% for SHH-MB, 40.6% for group 3 MB and 68.1% for group 4 MB (8).
Recurrences occur in about 30% of children and can occur >5 years after diagnosis. There is usually only one chance to cure children with MB; survival is poor in case of relapse (<10% al 5 years, even for initial standard-risk patients) (2, 7, 91).
The importance of good surgery was soon demonstrated. The University Hospitals of Philadelphia and Pennsylvania incorporated in December 1974 the use of computer tomographic scan for the planning of the tumor surgical approach and the use of binocular operating microscope. These two techniques dramatically improved patients survival (4-year PFS of 84% with these techniques Vs. 38% without) (15). The use of novel technologies, such as navigated intraoperative ultrasonography, should be implemented, as they improve the complete response rate and lower the morbidity (92).
Similarly, the SIOP I (16) trial (1975-1979) found statistically significant differences in survival depending on where the children were treated (5-year OS in major centers 57% Vs. 42% in minor centers) (16).
The introduction of post-operative craniospinal irradiation (CSI) in the management of MB started to give the patient some chance of cure (20). The Head Start III trial (2003-2009) obtained very poor survival rates in children between 6-10 years old (5-year EFS 11%, 5-year OS 36%) (63). This was probably secondary to the absence of RT (it was only indicated in patients ≥6 years old with residual disease) or its very late administration (after 5 cycles of CT and one cycle of high-dose CT with stem cell rescue). Therefore, it can be stated that RT in MB is an essential part of the treatment. It is included in the treatment plan of every child older than 3 years of age affected by MB. The RT technique, dosing schedule, boost extension and even patient positioning (93) have changed over the time. The RT technique, dosing schedule, boost extension and even patient positioning (93) have changed over the time (Figure 5).
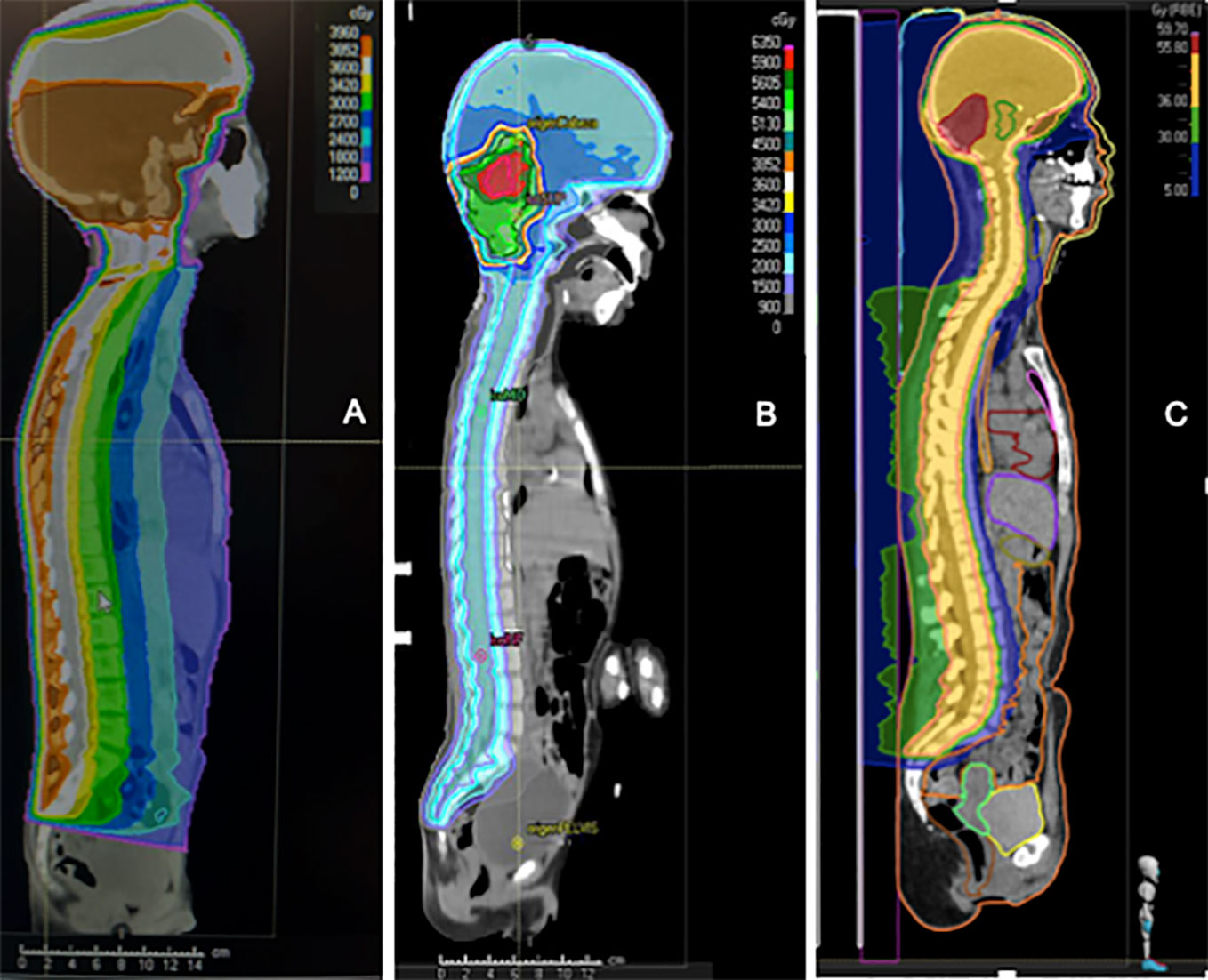
Figure 5 Comparison of the dosimetry of three patients with medulloblastoma who received craniospinal irradiation with a local boost. (A) old-fashioned 3D Conformal Radiation Therapy (3D-CRT) with a unique posterior photon beam. (B) modern Intensity-modulated radiation therapy (IMRT) photon technique; (C) modern proton technique.
Craniospinal RT is delivered since the 1940s, in accordance with post-mortem findings that revealed tumor spread when the entire brain and cord were not treated (10). RT should include some high-risk areas for recurrence, such as cribriform plaques or temporal lobes (91).
RT in MB should be administered early and without interruptions. The timing of RT should not be delayed by any cause, not even by the administration of adjuvant CT because otherwise survival decreases. This was demonstrated in the HIT-SIOP PNET 4 and HIT 91 trials (42, 43, 81) (Tables 3, 4). The current recommendation is to start RT no later than 40 days after surgery (ideally within 28 days of surgery) (91). Similarly, the SIOP/UKCCSG PNET3 study also demonstrated that prolonging the duration of RT more than 50 days had a negative impact on outcome and should be avoided (94) (Tables 3, 4).
RT should be administered in experienced centers and with strict quality control, in order to avoid relapses secondary to radiotherapeutic errors, as experienced in the past (95). One study found that 23% of patients received less than the 95% of the prescribed dose (96). Targeting deviations occur in 49-71% of patients, so it is essential to use quality control techniques (97, 98). The quality of RT technique is strongly correlated with outcome (97).
Early protocols showed improved survival rates with increasing the RT dose to the posterior fossa and spinal axis (12, 20). Subsequently, and because of the deleterious effects of RT on the developing brain, various groups have attempted to reduce the RT dose to reduce long-term sequelae. Nowadays, craniospinal RT doses depend on prognostic groups. Doses of 18 Gy, 23.4 Gy and 36 Gy, at 1.8 Gy/fraction, are generally considered adequate for low-risk, standard-risk and high-risk patients, respectively, with boost in the primary tumor up to 54-55.8 Gy (1, 2).
Initially, the craniospinal RT dose was 35-36 Gy. Since then, various clinical trials have been developed reducing the dose of CSI (Figure 6). One of the first studies reducing craniospinal RT dose was performed in San Francisco (9) (1966-1987) and found no increased tumor recurrence rate despite reducing CSI from 30-40 Gy to 25 Gy combined with CT both in standard and high-risk patients (9). Although not reaching statistical significance between both groups as a whole, DFS rates in the high-risk group did differ (5-year DFS of 78% for standard CSI dose Vs. 39% in reduced-CSI dose and CT) (9) (Tables 3, 4).
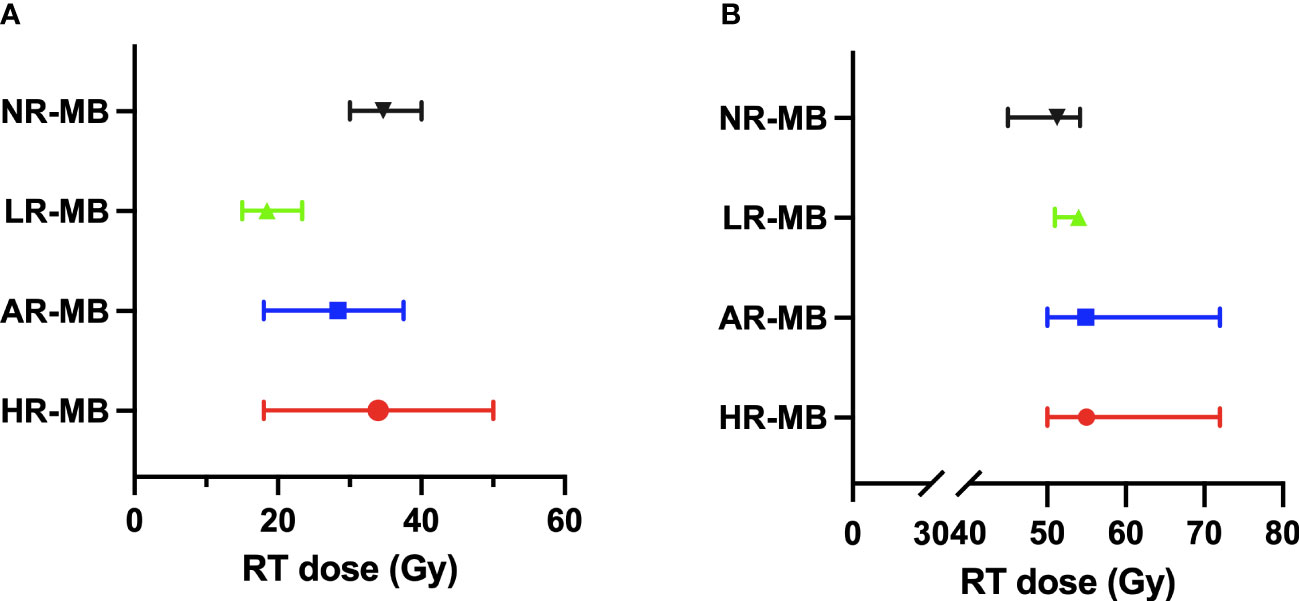
Figure 6 Radiation Therapy (RT) doses administered in the different treatment protocols classified by risk. The median dose is shown with the range between the maximum and minimum dose. (A) Craniospinal RT dose. (B) Boost RT dose. AR-MB, average-risk medulloblastoma; Gy, gray; HR-MB, high-risk medulloblastoma; LR-MB, low-risk medulloblastoma; NR-MB, non-defined risk medulloblastoma.
In patients with AR-MB, there are several treatment protocols that randomized different doses of RT (Figure 6). The POG 8631/CCG 923 (70, 71) trial (1986-1990) attempted to reduce the craniospinal RT dose from 36 to 23.4 Gy (without any CT treatment), but it was prematurely closed due to a significant increase in the risk of early relapse in the reduced-dose RT group detected in the interim analysis in 1990 (73). Posterior analysis revealed that over time these differences narrowed, reaching no statistical difference in 8-year EFS (67% for 36 Gy and 52% for 23.4 Gy) (71). The SIOP II (26) trial (1984-1989) also randomized the craniospinal RT dose (35 Vs. 25 Gy) and demonstrated a non-statistically significant better outcome in those with standard craniospinal RT dose (5-year EFS was 67.6% Vs. 55.3%) and a particularly poor outcome in patients who received both pre-irradiation CT and low craniospinal RT dose (5-year EFS 41.7%) (26). Subsequently, the CCG 9892 (73) pilot study (1989-1994) found similar survival rates when a reduced dose of craniospinal RT (23.4 Gy) was combined with concurrent VCR and followed by maintenance CT based on the combination of CDDP, VCR and CCNU (73). This was later confirmed in the phase III CCG A9961 (77, 78) study (1996-2000). In Europe, the MSFOP 93 (74) study (1991-1998) reduced the craniospinal RT dose to 25 Gy, preceded by CT, with worse results (5-year PFS of 64.8%) (74). Concurrently, the multicenter SJMB 96 (49, 79) trial (1996-2003) combined reduced-dose craniospinal RT with dose-intensive CT, achieving a 5-year EFS rate of 83% (49, 79) (Tables 3, 4).
Subsequent attempts to further reduce the CSI dose to 18 Gy have shown an increased risk of relapse, according to the results observed in the CHP455 (72) pilot trial (1988-1990), in the single-arm pilot study CHP693 (82) (2001-2010), and in the COG ACNS0331 (83) randomized phase III trial (2004-2012) (Table 4). Interestingly, children in the CHP455 pilot trial who did not relapse in the first two years, did not relapse in the following ten years and show less neurocognitive impairment.
These different trials demonstrate that it is feasible to use slightly reduced doses of craniospinal RT (23.4 Gy) in standard-risk patients if CT is added after RT, with or without concomitant CT to RT. This approach has become the standard of care for patients with standard-risk MB (1, 2, 99).
In patients with HR-MB, craniospinal RT doses are higher (Figure 6). The most frequent CSI dose is 36 Gy (range 35–39 Gy). In the San Francisco-Houston (27) phase II study (1984-1992), the reduction of CSI dose to 24 Gy resulted in decreased survival (27). Conversely, the Japanese Pediatric Brain Tumor Consortium (51, 66) (1997-2006 and 2006-2014) despite administering a reduced neuraxis dose of 18 Gy, obtained optimal survival rates [5-year PFS of 82.1% (51)] (Table 4).
In the Philadelphia-Washington-Dallas (Packer et al., 1991, 1994) treatment plan (1983-1989 in Philadelphia and 1988-1993 in Washington-Dallas), RT doses differed according to patient age (full-dose was 36 Gy CSI with a local boost up to 54-55.8 Gy and reduced-dose was 23.4 Gy and 50.4-55.8 Gy of boost), with no differences observed in outcome (Packer et al., 1994) (Table 4).
Currently, the standard of care for high-risk patients remains 36 Gy of craniospinal RT dose plus a boost to the primary tumor and the bulky metastases (2).
In patients fulfilling low-risk features, the RT dose has been further reduced (to 15 Gy craniospinal with a 51 Gy boost to the TB in the SJMB12 trial stratum W1; and to 18 Gy craniospinal with a 54 Gy boost to the TB in the ACNS1422 and PNET5 MB-LR (Table 2). The results of these trials are still pending.
Attempts to further reduce the craniospinal RT dose should be undertaken with caution, to avoid repeating the negative experiences of the past (25, 70–72, 83).
RT administered exclusively to the tumor bed (TB) plus a safety margin rather than to the entire posterior fossa (PF) has been administered in several studies (Figure 7) and is today the standard of care (83). The goal of this reduction is to decrease toxicity without compromising outcome. This safe reduction had been debated since the late 1990s (1, 2, 91, 100, 101). The non-randomized trials SJMB 96 (49, 79) and MSFOP 98 (80) had had positive results (5-year cumulative incidence of PF failure of 4.9% (79) and 0% (80), respectively). Finally, its safety was demonstrated in the randomized phase III COG ACNS0331 (83) trial: the 5-year EFS for involved field RT and PF irradiation was 82.5% and 80.5%, respectively (83). However, according to the results of the French M4 (25) protocol (1984-1985), RT should always include the supratentorial area because otherwise the outcome is very poor (6-year DFS of 18% (25)) with a high incidence of relapse within the supratentorial area (Tables 3, 4). Nevertheless, RT administered exclusively to the tumor site is not sufficient, and CSI should always be performed.
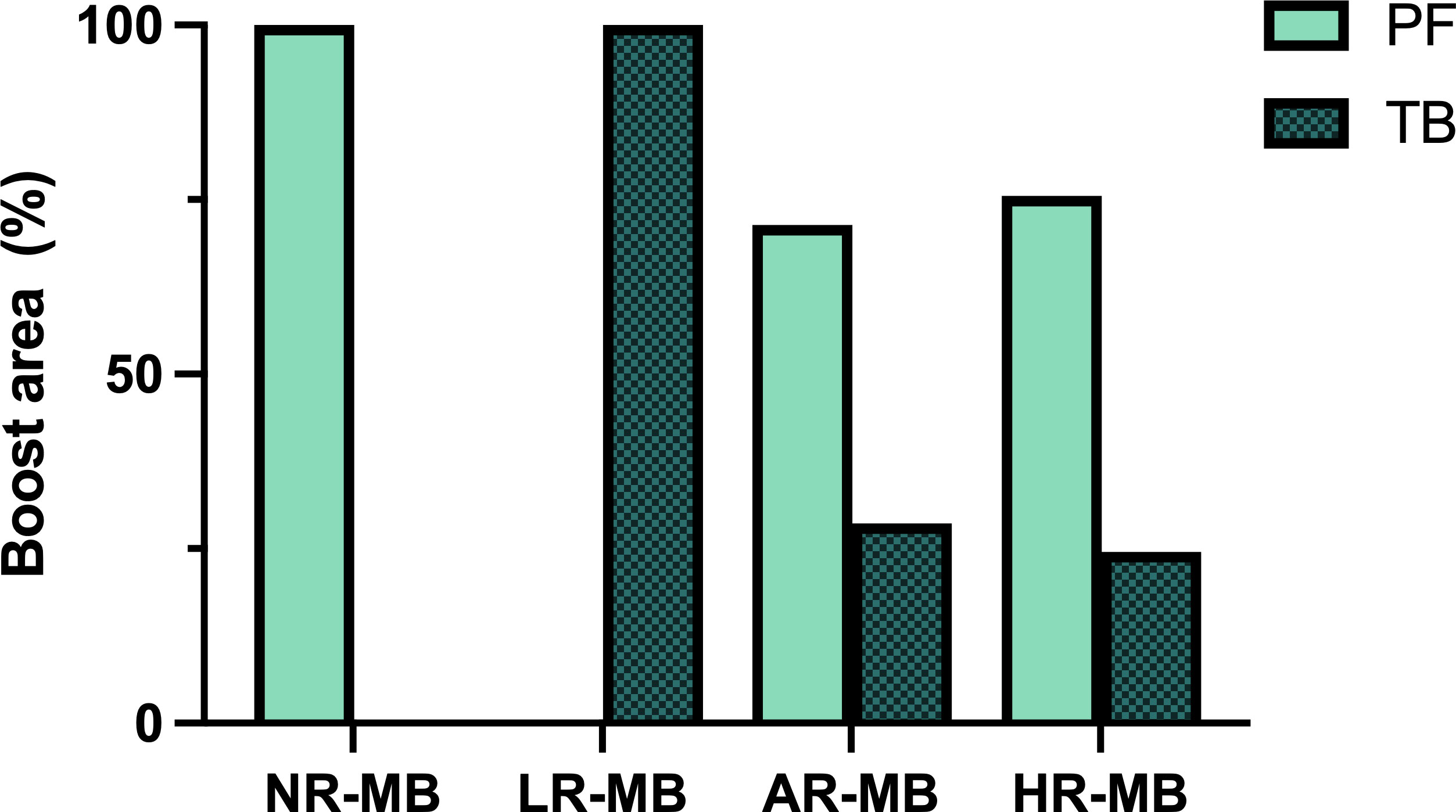
Figure 7 Boost area administered in the different treatment protocols. AR-MB, average-risk medulloblastoma; HR-MB, high-risk medulloblastoma; LR-MB, low-risk medulloblastoma; NR-MB, non-defined risk medulloblastoma; PF, posterior fossa; TB, tumor bed.
Subsequently, with the improvement in RT techniques, some groups began to evaluate the effect of hyperfractionated RT (HFRT). Some of the first studies include the New York pilot study (36) (1989-1995) and the San Francisco phase II study (38) (1990-1992). The New York trial included patients with high-risk features and consisted of HFRT followed by CT. They found excellent long-term PFS in the subgroup of patients with high T stages but M0 (6.5-year PFS of 95%) (36). The San Francisco trial included patients with AR-MB and HR-MB and consisted of HFRT that was only followed by maintenance CT in the poor-risk group. They observed lower PFS rates in the AR-MB group and no superior outcomes in the HR-MB group compared to other studies (38). Based on the results of the two aforementioned pilot trials (36, 38), the CCG 9931 (46) phase II pilot study (1994-1997) for patients with HR-MB combined 5 cycles of pre-RT CT with HFRT over 2 months and achieved a 5-year EFS of 43% (46) (Tables 3, 4).
In AR-MB, the French started using HFRT in 1998 in the non-randomized MSFOP 98 (80) treatment protocol (1998-2001) and observed very good 6-year EFS rates (75%) despite avoiding CT (80). Additionally, the randomized multicenter HIT-SIOP PNET 4 (81) trial (2001-2006) compared the traditional RT schedule (CSI 23.4 Gy and up to 54 Gy in posterior fossa) with the hyperfractionated schedule (1 Gy twice a day over 48 days) plus concurrent weekly VCR and maintenance CT in both treatment arms. In this study, HFRT was not superior to conventional RT [5-year EFS was 78% for HFRT and 77% for conventional RT (81)] (Table 3). Therefore, conventional RT remained the standard of care.
Further on, the efficacy and feasibility of Hyperfractionated Accelerated RT (HART) for metastatic MB was tested. It was first used by the Italians (1998-2007 (54), who combined it with pre-RT CT and maintenance CT with good tolerance and promising results [5-year EFS of 70% (54)]. Subsequently, the British (61) (2002-2008) used the same strategy of RT followed by maintenance CT but were unable to reproduce the Italian excellent results [3-year EFS of 59% (61, 102)] (Table 4).
Overall, neither HFRT nor HART have shown to be more beneficial than the standard administration scheme, so they are not currently recommended (81).
Technological advances have led to the development of proton RT, which reduces the damage to surrounding healthy tissue. Disease control and outcomes are similar with proton and photon RT in AR-MB (103). Proton RT has been used in MB in the non-randomized phase II trial MGH-99-271 (62) (2003-2009), with similar survival rates (5-year EFS of 85% patients with AR-MB and of 70% for patients with high-to-intermediate risk MB) and lower toxicity rates for the entire cohort. They report less grade 3-4 hearing loss (16% at 5 years Vs. 24% in the CCG A9961), superior intellectual outcome and no proton-induced cardiac, pulmonary or gastrointestinal toxicity (62). However, these results are from patients with different ages, RT doses, boost volumes and follow-up time, so the results should be interpreted with caution. Furthermore, the comparison is against outdated photon RT techniques. The comparison between proton RT and advanced photon techniques (such as Intensity Modulated Radiation Therapy [IMRT] or Volumetric Modulated Arc Therapy [VMAT]) has not been explored. In any case, proton RT is effective but not without side effects; neuroendocrine deficits were still reported in 55% of patients at 5-year follow-up (62). Both photon and proton RT are effective in the treatment of MB.
Precisely, this is the first question that international groups tried to answer. The Royal Marsden Hospital (11) developed a pilot study with 37 patients to test the feasibility of administering concurrent and adjuvant CT. This pilot study achieved a very significant improvement in 5-year OS (71%) (11) compared to historical control (32%) and was the basis for the SIOP I trial (Table 1).
In 1975, two parallel prospective randomized trials were developed with the aim of confirming that CT improved survival: the European SIOP I (16) trial (1975-1979), and the American CCG 942 (21) trial (1975-1981). In both studies, there were two randomized arms: surgery and RT Vs. surgery, RT with concurrent VCR and followed by 8 cycles of adjuvant CT every 6 weeks based on CCNU and VCR and, in the American, associated with prednisone (Tables 3, 4) (16, 21). The SIOP I trial was prematurely closed in 1979 because there was a significant difference in the event-free survival (EFS) of both arms (53% for the arm with CT and 48% for the arm without CT, p=0.005). Subsequent follow-up revealed relapses in the CT group, narrowing the difference in EFS between both arms. Nevertheless, CT was proven to be beneficial in some specific subgroups (16). The CCG 942 trial concluded that CT was only beneficial for metastatic MB (21). Similarly, the randomized POG 7909 (17) trial (1979-1986) also found a benefit towards CT, in this case based on the MOPP regimen (nitrogen mustard, VCR, prednisone and procarbazine) (17). The Philadelphia-Washington-Dallas (22, 24) treatment plan (1983-1989 in Philadelphia and 1988-1993 in Washington-Dallas) combined CT and RT for children classified into the “poor-risk” group (22). They received RT with concomitant VCR and 8 cycles of adjuvant CT every 6 weeks based on CCNU, CDDP and VCR. In this study, patients with metastatic MB achieved a 5-year PFS of 67% (24) (Tables 1, 3, 4).
Based on the multiple studies outlined, we can conclude that both CT and RT are effective in MB and that the combination of both has a synergistic effect. CT has been shown to be beneficial in the treatment of MB, especially in the high-risk subgroup (16, 21). The number of cycles administered varies in the different treatment protocols (Figure 8).
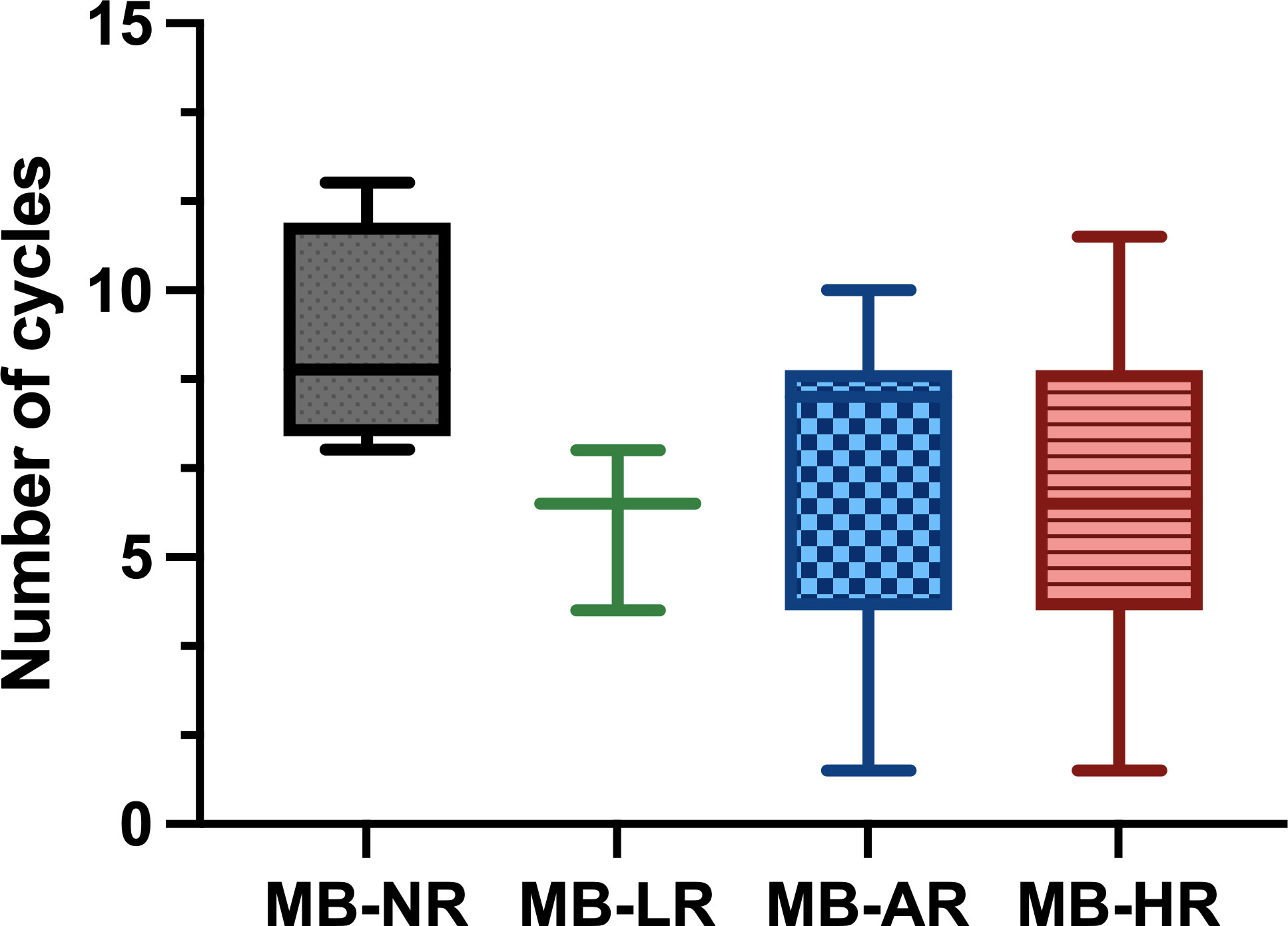
Figure 8 Number of chemotherapy (CT) cycles administered in the different treatment protocols classified by risk. The median dose is shown with the range between the maximum and minimum dose. Gy, gray; MB-AR, average-risk medulloblastoma; MB-HR, high-risk medulloblastoma; MB-LR, low-risk medulloblastoma; MB-NR, non-defined risk medulloblastoma.
Suitable CT regimens are based on the combination of multiple drugs, the most commonly used being vincristine (VCR), cisplatin (CDDP) and cyclophosphamide (CP) (Figure 9). Although each treatment protocol has its peculiarities, administration of 6 to 9 cycles of a combination of CDDP, CCNU, VCR or CP every 4-8 weeks after completion of RT is a frequent approach (SIOP I (16), CCG 942 (21), Philadelphia-Dallas-Washington (24), San Francisco ‘90 (38), CCG 9892 (73), New York ‘89 (36), HIT 91 (42, 43), CCG A9961 (77, 78), HIT SIOP PNET4 (81), MET-HIT-2000-AB4 (59), COG 99701 (53), COG ACNS0332 (7), COG ACNS0331 (83), SJMB12, PNET5 and COG ANCS1422) (Tables 1–4).
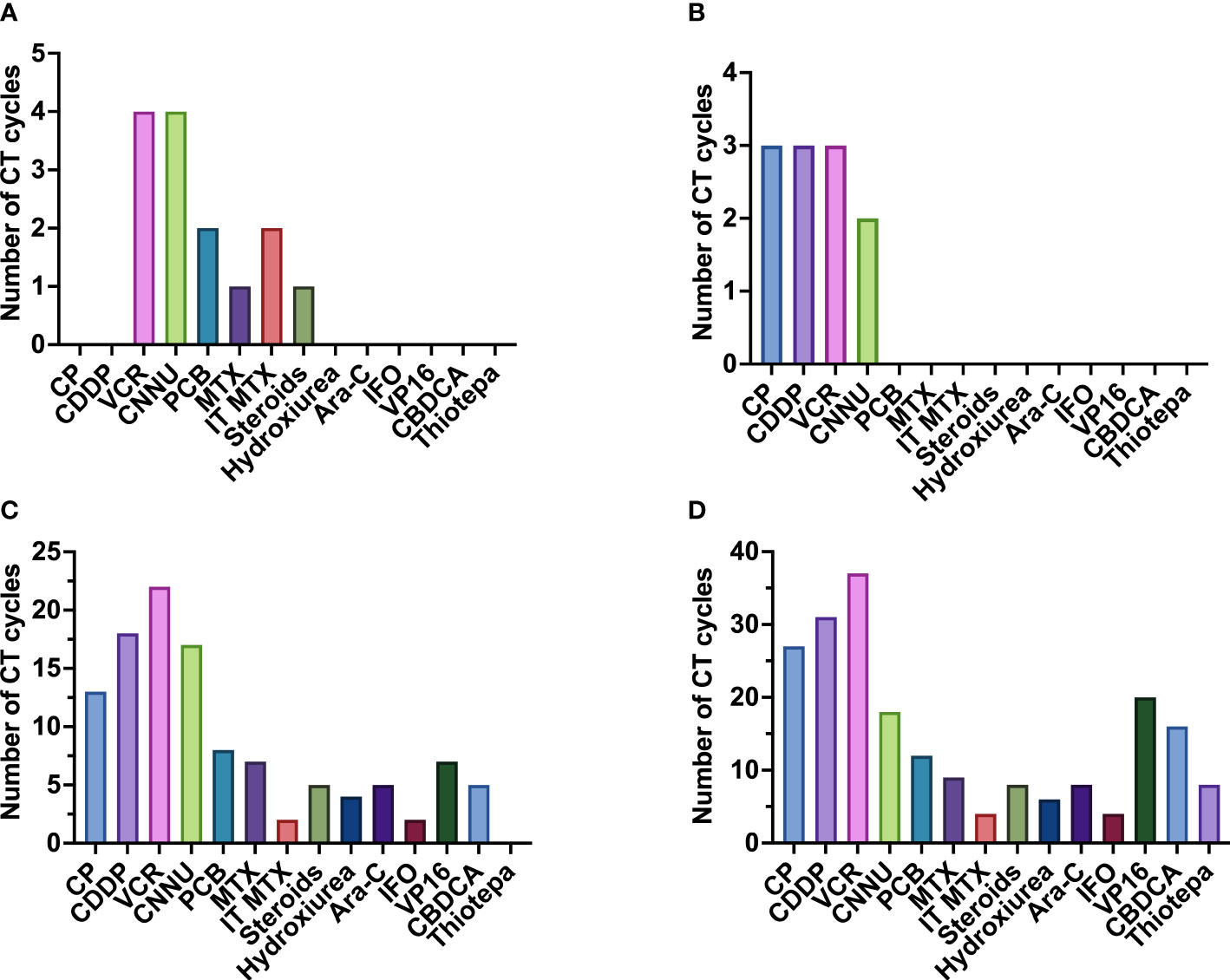
Figure 9 Frequency of administration of the different chemotherapeutic drugs administered, divided by risk. (A) chemotherapeutic drugs used in non-defined risk medulloblastoma (NR-MB); (B) chemotherapeutic drugs used in low-risk medulloblastoma (LR-MB); (C) chemotherapeutic drugs used in average-risk medulloblastoma (AR-MB); (D) chemotherapeutic drugs used in high-risk medulloblastoma (HR-MB). Gy, gray; Ara-C, cytarabine; CBDCA, carboplatin; CCNU, lomustine; CDDP, cisplatin; CP, cyclophosphamide; IFO, ifosfamide; IT-MTX, intrathecal methotrexate; MTX, methotrexate; PCB, procarbazine; VCR, vincristine; VP-16, etoposide.
Carboplatin (CBDCA) has been used instead of CDDP in an attempt to diminish CDDP-derived ototoxicity and nephrotoxicity. The Italian MB group tested it a long time ago (1988-1992) with good results in some patients (1 complete response, 7 partial responses, 4 stable disease and 1 progressive disease after 2 cycles) (35). Afterwards, two other studies tested the use of CBDCA: the investigational protocol in St. Jude (39) (which administered CBDCA prior to RT in patients with HR-MB) and the American study (76) (which administered CBDCA after RT in patients with AR-MB). Both studies obtained disappointing results. The American Study failed to achieve better results than with CDDP (76). In the St Jude protocol, they observed a slightly increased risk of progressive disease, although results should be interpreted with caution due to the reduced number of patients included (39).
Some CT regimens have been tested in the subgroup of high-risk patients with the aim of increasing survival by rising intensity. One of these regimens includes the intensive “8-in-1” regimen, that includes CP, CDDP, VCR, CCNU, procarbazine, steroids, hydroxyurea and cytarabine. This regimen has been part of different studies (SIOP II (26), M7 (28, 29), CCG 921 (32), SFOP (45), among others). The CCG 921 compared the “8-in-1” regimen (administered before and after RT) with the VCP regimen after RT (based on VCR, CCNU and prednisone). The VCP regimen was superior to the “8-in-1” scheme [5-year PFS was 63% and 45%, respectively (32)] (Table 4).
However, the studies have mostly been single-arm clinical trials, so it is not possible to fully compare different CT schemes. When randomized studies comparing two different CT schemes have been performed, the results have either shown non-statistical difference between the two approaches (e.g., CCG A9961 (78), COG 99701 (53)) or the differences have been mainly due to different timing in RT administration (e.g. HIT 91 (42, 43), CCG 921 (32)) (Tables 3, 4).
Over time, there have been several attempts to assess whether pre-irradiation CT (“sandwich CT”) improved survival (Figure 10). The first studies to test this were the GPO-MBL 80 (18, 19) study (1980-1983) and the randomized multicenter SIOP II (26) trial (1984-1989). In the German study, all patients received pre-RT CT based on procarbazine, VCR and methotrexate (MTX), but were randomized to receive or not CT after RT. In the SIOP II, the standard-risk group were randomized to receive RT alone versus sandwich CT based on the same drugs used in the German study, while children in the high-risk group were randomized to receive or not pre-irradiation CT (sandwich CT), but all received RT and CT after RT, based on CCNU and VCR. Both studies showed no-statistically significant difference in outcomes (in GPO-MBL 80, 6-year EFS was 46% (18, 19); in SIOP II patients with AR-MB had 6-year EFS was 64.7% (RT alone) and 58.9% (sandwich CT); patients with HR-MB had 5-year EFS was 52.8% (no sandwich CT) Vs. 56.3% (sandwich CT) (26) (Tables 1, 3, 4). Simultaneously, the French M7 Cooperative Study (28) (1985-1988) combined pre-irradiation CT based on the “8-in-1” regimen and two cycles of high-dose MTX (in the average-risk group the MTX was given before RT, while in the high-risk group MTX was administered concomitantly to RT) followed by RT and, in the high-risk group only, followed by 4 more cycles of “8-in-1” CT. The M7 trial obtained 5-year DFS of 74% for patients with AR-MB and 57% for high-risk patients (28). At the same time, the POG 8695 (31) pilot study (1986-1990) found discouraging results when CT was administered before RT. There was a 43% of objective response rate and 23% of patients had progressive disease during CT, leaving a 2-year PFS of only 40% (31). Subsequently, the randomized POG 9031 (40) trial (1990-1996) compared outcomes of children treated with 3 cycles of CDDP and VP16 either before or after RT, with non-statistically significant difference between the treating arms (5-year EFS for pre-RT CT was 66% Vs. 70% for RT first) (40). Despite this, objective response rates were 66% for pre-RT CT and 86% for RT first (p=0.01) (40) (Tables 3, 4).
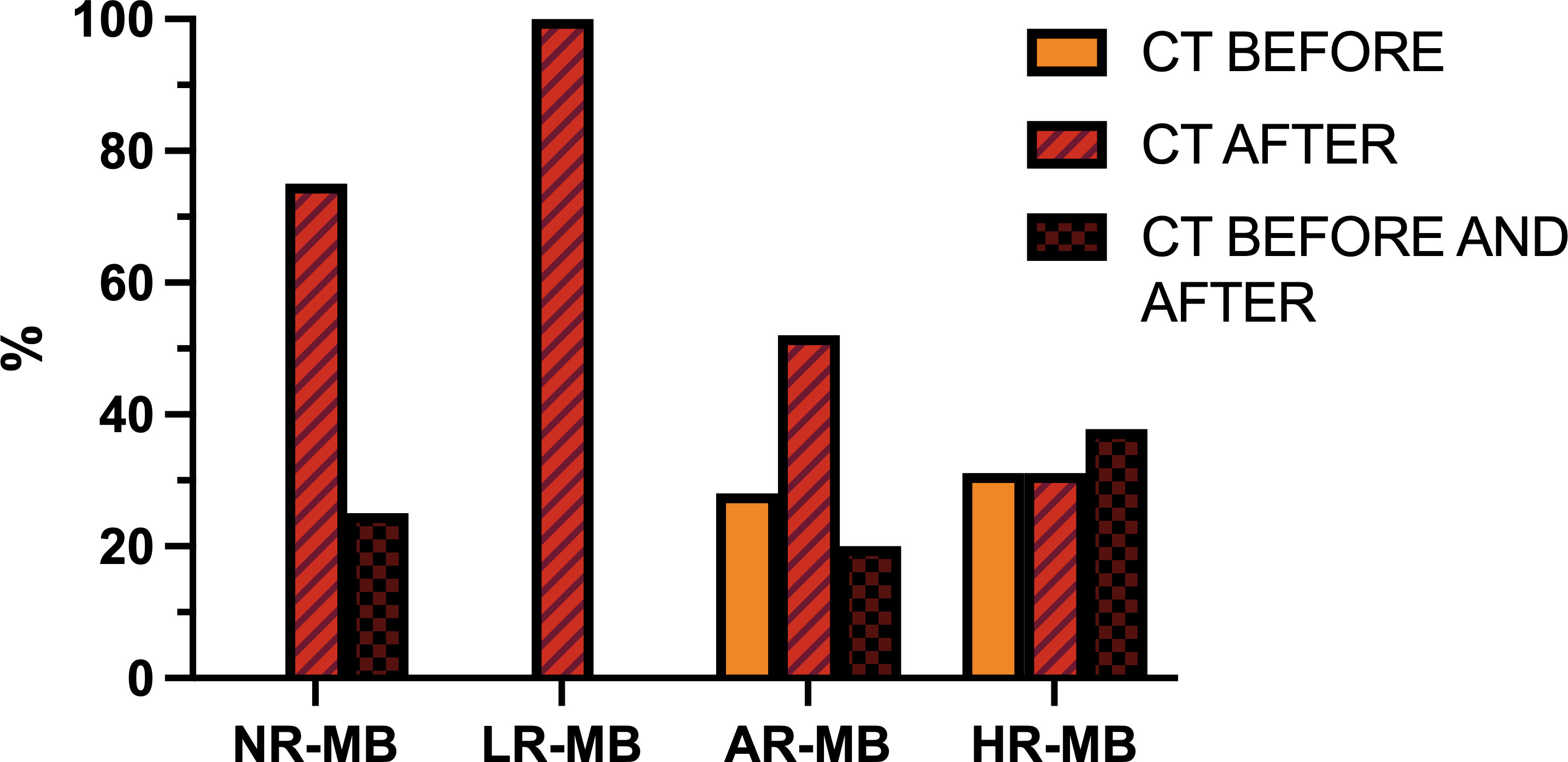
Figure 10 Timing of chemotherapy (CT) with respect to radiation therapy administration (before, after or both), divided by risk. AR-MB, average-risk medulloblastoma; HR-MB, high-risk medulloblastoma; LR-MB, low-risk medulloblastoma; NR-MB, non-defined risk medulloblastoma.
The GPOH phase II pilot study HIT 88/89 (34) (1987-1991) and the MSFOP93 (74) study (1991-1998) examined the efficacy of pre-irradiation CT in children with AR-MB, achieving a 5-year PFS of 64.8% (34, 74). The results of the HIT 88/89 study led to the development of the randomized HIT 91 (42, 43) trial (1991-1997), which compared RT followed by maintenance CT Vs. the sandwich treatment scheme used in the earlier HIT 88/89 pilot study, with VCR administration concurrent with RT in all cases. In addition, patients with inadequate response after sandwich CT and RT also received maintenance CT. This trial confirmed that pre-irradiation CT and subsequent delay in RT administration had a negative impact on outcome in M0 patients (10-year EFS with sandwich CT Vs. post-irradiation CT was 53% and 83%, respectively) and in M1 patients (the 10-year EFS with sandwich CT Vs. maintenance CT was 36% and 71%, respectively), but, in contrast, the outcome was similar in M2/3 patients regardless of the treatment received (10-year EFS with sandwich Vs. maintenance CT was 40% and 32%, respectively) (42, 43) (Tables 3, 4).
Simultaneously, the randomized SIOP/UKCCSG PNET3 (75) trial (1992-2000) compared, in non-metastatic patients, RT alone Vs. sandwich CT prior to RT with more intense CT regimen than the earlier SIOP trial, based on VCR, etoposide (VP16), CBDCA and CP. All M2/M3 patients received both RT and CT. This trial obtained opposite results to the previous ones in non-metastatic patients: pre-irradiation CT significantly improved EFS compared to RT alone (5-year EFS was 74.2%, while 5-year EFS for RT alone was 59.8%), without a non-statistically significant improvement in OS (5-year OS was 76.7% for pre-irradiation CT+RT and 64.9% for RT alone) (75). For M2/M3 patients, outcome rates were similar (5-year EFS of 34.7% (44)). The differences observed in non-metastatic patients could be explained by the absence of CT at any time in patients treated with early RT, which is known to be beneficial. Nevertheless, the EFS rates of the HIT 91 trial remain better than those achieved with the SIOP/UKCCSG PNET3 treatment plan. Concurrently, the French SFOP (45) study for metastatic MB (1993–1999) obtained a 5-year EFS of 49.8% alternating 8-in-1 cycles with CBDCA and VP16 before and after RT (45) (Tables 3, 4).
These different trials have failed to confirm the benefit of pre-RT CT. On the contrary, there is concern regarding the risk of delaying RT secondary to the administration of CT. In this regard, the different trials show a 9.6-33% progression rate during CT treatment, prior to the initiation of RT (31, 37, 40, 42, 45, 46). At St. Jude Hospital they analyzed failure patterns in infants and children with MB treated with pre-RT CT from 1984 to 1993 and observed that the risk of neuroaxial progression increased with CT duration (104). Currently, it is recommended to administer CT 4 weeks after ending RT and, ideally, during RT.
CT plays an important role in enhancing the effect of RT. Various treatment protocols administer CT concomitant to RT, but the frequency varies depending on the risk classification (Figure 11). The most widely used drug is weekly VCR (SIOP I (16), CCG 942 (21), Philadelphia-Washington-Dallas (22, 24), CHP455 (72), CCG 9892 (73), HIT 91 (42, 43), PNET4 (81), CHP693 (82), COG ACNS0331 (83), among others). In an attempt to intensify treatment, daily CBDCA was used as a radiosensitizer together with weekly VCR during RT in the phase I/II study COG 99701 (53) (1998-2004) with promising results (5-year PFS of 59-71% (53)). This effect was subsequently tested in the trial ACNS0332 (7) (2007-2018), which randomized whether or not to add daily CBDCA to weekly VCR during RT. Overall, the trial showed no benefit (5-year EFS 66.4% with Vs. 59.2% without CBDCA, p=0.11) (7). The trial results showed exclusively a benefit in molecular group 3 (5-year EFS 73.2% with CBDCA Vs. 53.7% without CBDCA, p=0.047) (7). In the remaining molecular subgroups, it was even found to be detrimental in some molecular subgroups (e.g., Wnt and SHH) (7) (Table 2). Once the PNET5 study is concluded, it may finally be elucidated whether daily administration of CBDCA during RT is advisable or not. In any case, it is clear that the toxicity of weekly VCR during RT is lower than the one observed with daily CBDCA.
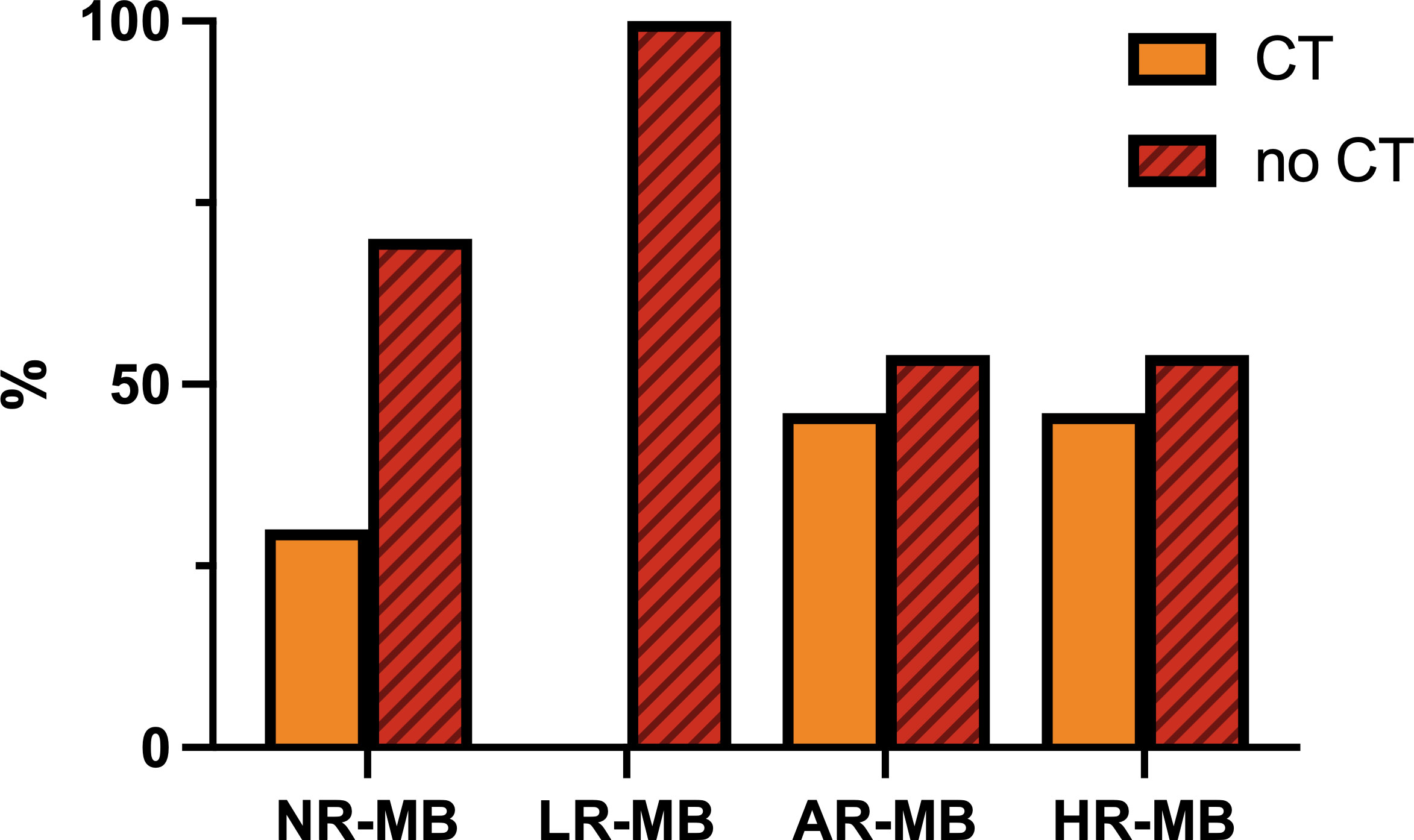
Figure 11 Frequency of administration of chemotherapy (CT) concomitant to radiation therapy, divided by risk. AR-MB, average-risk medulloblastoma; LR-MB, low-risk medulloblastoma; NR-MB, non-defined risk medulloblastoma; HR-MB, high-risk medulloblastoma.
Other treatment schemes using other drugs include: the POG 9631 (52) trial (1998-2002), which administered a 3-week course of daily VP16 during RT with good survival rates and good tolerability once the daily dose was reduced to 35 mg/m2/day instead of the original 50 mg/m2/day (52); the MGH-99-271 (62) trial, in which patients received VCR, CBDCA, VCR+CBDCA, oral VP16 or no CT; and finally, the Japanese Pediatric Brain Tumor Consortium (1997-2006 and 2006-2014) (51), who used intrathecal MTX and intravenous VCR, CDDP, VP16 and CP with a promising 5-year PFS of 82.1% despite reducing CSI to 18 Gy (51) (Tables 3, 4).
Except for the PNET5 study, there have been no studies focused on the randomization of CT during RT. There are no studies that exclusively evaluate the benefit of the addition of VCR during RT, so the effectiveness of its administration cannot be assured. Randomized studies conducted in the past (SIOP I (16), CCG 942 (21), Philadelphia-Washington-Dallas (22, 24)) randomize the administration of CT or no CT (concomitant VCR and maintenance CT), and all of them show a benefit of CT administration. However, the proportion of benefit due to concomitant VCR administration is unclear. The CCG 921 (32) trial randomized two regimens of CT: regimen A, which included VCR during RT followed by maintenance CT based on VCP regimen, and regimen B, which included two cycles of “8in1” CT before RT and eight cycles of “8in1” CT after RT, without any CT during RT. In this trial there was a benefit toward regimen A. Although this benefit could be justified in part by the increased dose intensity of VCR, it is more likely due to the earlier administration of RT. Similarly, the HIT 91 (42, 43) trial also showed a benefit of the arm including VCR during RT. However, it is likely that this benefit was secondary to the delay and higher rate of interruptions in RT administration in the other arm (Tables 3, 4).
Weekly VCR primarily, but also daily CBDCA (alone or in combination of both), are frequently administered as radiosensitizers concomitant to RT. The specific benefit of their administration has not been explored in depth.
The use of intensified CT with stem cell rescue has also been tested with the aim of increasing survival. The multicenter SJMB 96 (49, 79) trial (1996-2003) combined a risk-adapted CSI dose with 4 cycles of dose-intensive CT based on VCR, CDDP and CP followed by stem cell rescue with good results (5-year EFS of 83% for localized AR-MB, 66% for M patients and 70% for patients with HR-MB) despite reducing total VCR and CDDP doses and treatment time compared to other established regimens (49, 79)). The SJMB03 (8) trial used the same CT scheme as its predecessor and obtained similar results (5-year PFS of 83.2% for patients with AR-MB and 58.7% for the HR-MB ones) (8) (Tables 3, 4).
It was also tested in the Gustave Roussy (60) (2001-2010) and PNET HR+5 (67) (2009-2012) trials for high-risk patients. Both protocols used 2 cycles of CBDCA+VP16, followed by 2 cycles of high-dose thiotepa followed by stem cell rescue, RT and maintenance CT, with similar outcomes (5-year EFS of 65% for the French and 5-year PFS of 76% for the PNET HR+5) (60, 67) (Table 4).
It was also tested by the Koreans (56) (1999-2005), who used 1-2 cycles of high-dose CT with a combination of several drugs (CP + melphalan, CBDCA + thiotepa + VP16, or busulfan + melphalan) followed by stem cell rescue in high-risk patients aged 1-16 years old with similar outcomes (3-year EFS of 83% in older than 3 years old, and 3-year EFS of 62.5% in younger than 3) (Table 4).
In contrast, the SFOP (45) and the CCG 99702 (55) trials for patients with HR-MB obtained poorer survival rates (5-year EFS 49.8% and 46%, respectively), the latter due to a high-incidence of sinusoidal obstructive syndrome (45, 55). The Head Start II (50) (1997-2003) and III (63) (2003-2009) trials, included children younger than 10 years old and used myeloablative CT as consolidation of a previous induction CT. In the Head Start II (50), it failed to maintain a good survival rate despite excellent response rates observed with the induction CT (response rate to induction CT 91%; 3-year EFS of 49%) (50). The Head Start III trial (2003-2009) obtained average survival rates as a whole (5-year EFS of 46%), but very poor survival rates in 6-10 year old children (63) (Table 4).
Finally, the Japanese Pediatric Brain Tumor Consortium (51, 66) (1997-2006 and 2006-2014) obtained very good survival rates (3-year PFS 93.9% for patients with AR-MB and 5-year PFS of 82.1% for HR-MB) despite reducing neuraxis irradiation dose to 18 Gy by combining it with concomitant intrathecal MTX, intravenous CP, CDDP, VP16 and VCR and one cycle of thiotepa and melphalan followed by stem cell rescue (51, 66) (Tables 3, 4). However, this trial had a small sample size, so the results should be interpreted carefully (99).
Despite good results in the past, schemes based on intensified CT or high-dose CT followed by autologous stem cell rescue are not widely used as they have not shown any real benefit compared to standard CT (49, 105). However, given the excellent survival rates reported by the Japanese (51, 66), this could be a matter of debate.
Various regimens (GPO-MBL 80 (18, 19), Italy 1985 (69), CNS-85 (30), Japan 1997 (51), Japan 2006 (66)) include intrathecal/intraventricular administration of MTX (Tables 1, 3, 4). Its administration is even more frequent in radiation-sparing treatment protocols for infants with MB (3, 4). The Italian study is the only one to randomize its administration, and concludes that it is not advisable because it does not improve survival and worsens the cognitive outcome of these patients (69). On the contrary, children treated with CT and intraventricular MTX according to the (MET-)HIT-2000-BIS4 study showed similar IQ scores compared to children with ependymoma treated with focal RT (106). In the Japanese Pediatric Brain Tumor Consortium studies, intrathecal MTX administration was safe but, despite having excellent survival rates, it is not possible to determine the contribution of intrathecal MTX to these results. They believe that its administration allows to safely decrease the CSI dose to 18 Gy (51, 66).
The randomized trial ACNS0332 (7), based on experience with neuroblastoma, also tested the survival-enhancing potential of isotretinoin as a proapoptotic agent. This randomization was closed in 2005 due to the absence of a significant effect (5-year EFS of 68.6% with isotretinoin Vs. 67.8% without it) (7).
Other novel therapies, including targeted treatments such as vismodegib, are currently being investigated in the SJMB12 trial (NCT01878617, from 2013 to present). The efficacy of this approach remains unknown to date, but it is essential to move forward and include these new targeted therapies.
Long-term survivors of MB suffer neurological deficits, hearing loss, endocrine deficits, growth restriction, cognitive impairment, increased risk of stroke, social deficits and impaired quality of life, among others (107, 108).
CSI is known to be a major cause of cognitive impairment. This deterioration is inversely related to the age of the patient, having a great impact in patients younger than 7-10 years old (69, 106, 109). It is also directly related to RT dose and is progressive over several years after treatment (69, 109, 110). However, reducing the RT dose does not provide the decrease in side effects that might be expected. Analysis of the intellectual outcome of children treated with the CCG A9961 trial found that the intellectual decline observed in children receiving CSI with 23.4 Gy was similar to that of previous studies administering 36 Gy (111). Conversely, the SJMB 96 trial did show that patients receiving 23.4 Gy had less cognitive impairment than patients receiving 36-39.6 Gy, and it was especially important in children younger than 7 years old (112). Patients treated with HFRT had better verbal activity in children younger than 8, but, on the contrary, had worse growth, and had no other significant differences observed compared to patients treated with conventional RT (113).
Intrathecal MTX was shown to have a detrimental effect on cognitive function by the Italian group, again especially in children younger than 10 years old, without an improvement in survival (69), but this is opposite to the results shown in the (MET-)HIT-2000-BIS4 trial (106).
Patients included in the SIOP/UKCCSG PNET3 study who received CT prior RT were found to have worse health status, poorer quality of life and more behavioral and emotional problems compared to patients who received RT alone (114). However, this study showed that patients with CT+RT had a significantly better EFS than those treated exclusively with RT (75), so these side effects may not be so bad after all.
Similarly, some of the CT drugs used in the treatment of MB have well-known intrinsic side effects and dose reductions are often needed due to toxicity, especially in older patients. Dose reductions of VCR or CDDP do not seem to affect survival (115, 116), so it might be advisable to use lower doses in future trials. Regarding CCDP-induced hearing loss, amifostine has been applied with positive results, especially in patients with AR-MB (117).
But not all the side effects are treatment-related. Tumor location is also important. Tumors arising in the vermis induce emotional disturbances whereas hemispheric tumors cause cognitive deterioration (69). Moreover, different molecular subgroups have different toxicities (118, 119). Other risk factors for further neurocognitive impairment include post-operative mutism and higher intelligence at diagnosis (110).
The improvement in treatment-related toxicity has been small. Despite reducing RT dose, despite avoiding some drugs, despite applying prophylactic treatments and despite technological advances, treatment-related toxicity remains significant and most long-term survivors experience side effects. Cognitive and behavioral therapies have been applied with positive results, especially in young children, and should be applied more widely (120, 121). Other interventions have been proven ineffective (122).
The treatment of MB has improved significantly over time. The improvement was big from 1950s to 1970s with the introduction of RT and CT. Since then, the improvement has been smaller. The results of the different studies and trials performed, even if they have yielded negative results, have been the basis for reaching the survival rates we are achieving today. Among the various studies performed, some are worth highlighting. Firstly, those performed at the Royal Marsden Hospital (11), which demonstrated the benefit of adding CT to the treatment of MB. In addition, the San Francisco (9) treatment protocol (1966–1987) obtained impressive survival rates (5-year OS 91%) (9). Similarly, the Japanese have obtained excellent survival rates that deserve attention (51, 66). Some other studies (25, 38, 42, 70) have shown that reduction of treatment intensity should be done with cautioun to avoid poor outcomes. Furthermore, the COG ACNS0331 trial has shown that it is feasible to administer RT boost exclusively to the tumor bed instead of the whole posterior fossa (83). Finally, the SJMB03 trial has proven the prognostic implications of molecular subgrouping in MB (8).
Children should be treated in experimented centers, where they should receive the best treatment known to date and with the most modern technological means. Adequate management of MB in children >3 years old consists of:
1. First, surgery, with maximum safe resection but without significant secondary neurological deficits;
2. Secondly, RT. It should be administered early (ideally within the first 28 days after surgery), craniospinal, with boost to the tumor bed, over 6 weeks, without interruption, and probably with concomitant CT. Proton or photon therapies are acceptable, provided that modern photon techniques such as IMRT are used.
3. Thirdly, CT. A good option is to administer 6-9 cycles of a combination of CDDP, CCNU, VCR and CP every 4-8 weeks.
4. Fourthly, we should consider introducing targeted therapy, such as SHH pathway inhibitors, as front-line therapy.
5. Finally, we should also implement the use of therapies known to reduce side effects, such as cognitive and behavioral therapies and amifostine.
It is crucial to continue international collaboration so that studies rapidly include a sufficient number of patients to be able to draw conclusions quickly. The development of clinical trials involves major effort. Parents of children participate generously in studies to advance knowledge. However, some studies are communicating their results more than 10 years after their closure so it is impossible to benefit from their results. It is critical to communicate the results as soon as possible and to avoid repeating what has already been demonstrated.
The next step now is to further emphasize the importance of risk classification according to molecular features. We should keep trying to adjust treatment to reduce treatment-related side effects, but without decreasing survival. However, we must be careful because the worst side effect is disease progression and death.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
MO-M, BL-I, and ÁT provided concept, design, and manuscript preparation. MO-M and LM-L performed the search and read the titles and abstracts of all the papers that met the criteria. MO-M deeply reviewed the selected papers and wrote the first draft. All authors contributed to the article and approved the submitted version.
This research is supported by the Jérôme Lejeune Foundation, the Álvaro Entrecanales Foundation and the HM Hospitals Research Foundation (FiHM). The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, nor in the writing of the manuscript or the decision to publish the results.
We acknowledge Dr. Rosa Alonso and Dr. Felipe Calvo for the images provided. We acknowledge the Jérôme Lejeune Foundation, the Álvaro Entrecanales Foundation and the HM Hospitals Research Foundation (FiHM) for the economical support for the development of this paper in the context of the research thesis of MPOM. We acknowledge Ana Jiménez Rivero for her comments and corrections to the article. We aknowledge the patients and their families for participating in these studies, and specially to Ari, without whom this paper would never have existed.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ramaswamy V, Taylor MD. Medulloblastoma: from myth to molecular. J Clin Oncol (2017) 35(21):2355–63. doi: 10.1200/JCO.2017.72.7842
2. Northcott PA, Robinson GW, Kratz CP, Mabbott DJ, Pomeroy SL, Clifford SC, et al. Medulloblastoma. Nat Rev Dis Prim (2019) 5(1):11. doi: 10.1038/s41572-019-0063-6
3. Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med (2005) 352(10):978–86. doi: 10.1056/NEJMoa042176
4. von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol (2011) 13(6):669–79. doi: 10.1093/neuonc/nor025
5. Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol (2011) 29(11):1408–14. doi: 10.1200/JCO.2009.27.4324
6. Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell (2017) 31(6):737–54.e6. doi: 10.1016/j.ccell.2017.05.005
7. Leary SES, Packer RJ, Li Y, Billups CA, Smith KS, Jaju A, et al. Efficacy of carboplatin and isotretinoin in children with high-risk medulloblastoma. JAMA Oncol (2021) 7(9):1313. doi: 10.1001/jamaoncol.2021.2224
8. Gajjar A, Robinson GW, Smith KS, Lin T, Merchant TE, Chintagumpala M, et al. Outcomes by clinical and molecular features in children with medulloblastoma treated with risk-adapted therapy: results of an international phase III trial (SJMB03). J Clin Oncol (2021) 39(7):822–35. doi: 10.1200/JCO.20.01372
9. Halberd FE, Wara WM, Fippin LF, Edwards MSB, LEVIN VA, Davis RL, et al. Low-dose craniospinal radiation therapy for medulloblastoma. Int J Radiat Oncol (1991) 20(4):651–4. doi: 10.1016/0360-3016(91)90004-N
10. PATERSON E, FARR RF. Cerebellar medulloblastoma: treatment by irradiation of the whole central nervous system. Acta Radiol (1953) 39(4):323–36. doi: 10.3109/00016925309136718
11. Bloom HJG. Intracranial tumors: response and resistance to therapeutic endeavors, 1970–1980. Int J Radiat Oncol (1982) 8(7):1083–113. doi: 10.1016/0360-3016(82)90056-6
12. Mealey J, Hall PV. Medulloblastoma in children. J Neurosurg (1977) 46(1):56–64. doi: 10.3171/jns.1977.46.1.0056
13. Nüchel B, Andersen AP. Medulloblastoma: treatment results. Acta Radiol Oncol Radiat Phys Biol (1978) 17(4):305–11. doi: 10.3109/02841867809127933
14. McIntosh N. Medulloblastoma–a changing prognosis? Arch Dis Child (1979) 54(3):200–3. doi: 10.1136/adc.54.3.200
15. Norris DG, Bruce DA, Bruce DA, Bruce DA, Byrd RL, Schut L, et al. Improved relapse-free survival in medulloblastoma utilizing modern techniques. Neurosurgery (1981) 9(6):661–4. doi: 10.1227/00006123-198112000-00008
16. Tait DM, Thornton-Jones H, Bloom HJG, Lemerle J, Morris-Jones P. Adjuvant chemotherapy for medulloblastoma: the first multi-centre control trial of the international society of paediatric oncology (SIOP I). Eur J Cancer Clin Oncol (1990) 26(4):463–9. doi: 10.1016/0277-5379(90)90017-N
17. Krischer JP, Ragab AH, Kun L, Kim TH, Laurent JP, Boyett JM, et al. Nitrogen mustard, vincristine, procarbazine, and prednisone as adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg (1991) 74(6):905–9. doi: 10.3171/jns.1991.74.6.0905
18. Neidhardt M, Habermalz HJ, Henze G, Langermann HJ. Medulloblastoma study of the German society for pediatric oncology, a first report. Klin Padiatr (1982) 194(4):257–61. doi: 10.1055/s-2008-1033814
19. Neidhardt M, Bailey CC, Gnekow A, Kleihues P, Michaelis J, Wellek S. [Medulloblastoma therapy studies MBL 80 and MED 84 of the society of pediatric oncology and the société internationale d’Oncologie pédiatrique (SIOP)]. Klin Padiatr (1987) 199(3):188–92. doi: 10.1055/s-2008-1026787
20. Harisiadis L, Chang CH. Medulloblastoma in children: a correlation between staging and results of treatment. Int J Radiat Oncol (1977) 2(9–10):833–41. doi: 10.1016/0360-3016(77)90181-X
21. Evans AE, Jenkin RD, Sposto R, Ortega JA, Wilson CB, Wara W, et al. The treatment of medulloblastoma. results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg (1990) 72(4):572–82. doi: 10.3171/jns.1990.72.4.0572
22. Packer RJ, Sutton LN, Goldwein JW, Perilongo G, Bunin G, Ryan J, et al. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg (1991) 74(3):433–40. doi: 10.3171/jns.1991.74.3.0433
23. Pendergrass TW, Milstein JM, Geyer JR, Mulne AF, Kosnik EJ, Morris JD, et al. Eight drugs in one day chemotherapy for brain tumors: experience in 107 children and rationale for preradiation chemotherapy. J Clin Oncol (1987) 5(8):1221–31. doi: 10.1200/JCO.1987.5.8.1221
24. Packer RJ, Sutton LN, Elterman R, Lange B, Goldwein J, Nicholson HS, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg (1994) 81(5):690–8. doi: 10.3171/jns.1994.81.5.0690
25. Bouffet E, Bernard JL, Frappaz D, Gentet JC, Roche H, Tron P, et al. M4 protocol for cerebellar medulloblastoma: supratentorial radiotherapy may not be avoided. Int J Radiat Oncol Biol Phys (1992) 24(1):79–85. doi: 10.1016/0360-3016(92)91025-I
26. Bailey CC, Gnekow A, Wellek S, Jones M, Round C, Brown J, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. international society of paediatric oncology (SIOP) and the (German) society of paediatric oncology (GPO): SIOP II. Med Pediatr Oncol (1995) 25(3):166–78. doi: 10.1002/mpo.2950250303
27. Prados MD, Wara W, Edwards MSB, Ater J, Rabbit J, Lamborn K, et al. Treatment of high-risk medulloblastoma and other primitive neuroectodermal tumors with reduced dose craniospinal radiation therapy and multi-agent nitrosourea-based chemotherapy. Pediatr Neurosurg (1996) 25(4):174–81. doi: 10.1159/000121120
28. Gentet JC, Bouffet E, Doz F, Tron P, Roche H, Thyss A, et al. Preirradiation chemotherapy including “eight drugs in 1 day” regimen and high-dose methotrexate in childhood medulloblastoma: results of the M7 French cooperative study. J Neurosurg (1995) 82(4):608–14. doi: 10.3171/jns.1995.82.4.0608
29. Bouffet E, Gentet JC, Doz F, Tron P, Roche H, Plantaz D, et al. Metastatic medulloblastoma: the experience of the French cooperative M7 group. Eur J Cancer (1994) 30(10):1478–83. doi: 10.1016/0959-8049(94)00256-5
30. Pezzotta S, Cordero di Montezemolo L, Knerich R, Arrigoni M, Barbara A, Besenzon L, et al. CNS-85 trial: a cooperative pediatric CNS tumor study–results of treatment of medulloblastoma patients. Childs Nerv Syst (1996) 12(2):87–96. doi: 10.1007/BF00819502
31. Mosijczuk AD, Nigro MA, Thomas PR, Burger PC, Krischer JP, Morantz RA, et al. Preradiation chemotherapy in advanced medulloblastoma. A Pediatr Oncol Group pilot study. Cancer (1993) 72(9):2755–62. doi: 10.1002/1097-0142(19931101)72:9%3C2755::AID-CNCR2820720937%3E3.0.CO;2-V
32. Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the children’s cancer group 921 randomized phase III study. J Clin Oncol (1999) 17(3):832–45. doi: 10.1200/JCO.1999.17.3.832
33. Ilveskoski I, Saarinen UM, Perkkiö M, Salmi TT, Lanning M, Mäkipernaa A, et al. Chemotherapy with the “8 in 1” protocol for malignant brain tumors in children: a population-based study in Finland. Pediatr Hematol Oncol (1996) 13(1):69–80. doi: 10.3109/08880019609033373
34. Kühl J, Müller HL, Berthold F, Kortmann RD, Deinlein F, Maass E, et al. Preradiation chemotherapy of children and young adults with malignant brain tumors: results of the German pilot trial HIT’88/’89. Klin Padiatr (1998) 210(4):227–33. doi: 10.1055/s-2008-1043883
35. Mastrangelo R, Lasorella A, Riccardi R, Colosimo C, Iavarone A, Tornesello A, et al. Carboplatin in childhood Medulloblastoma/PNET: feasibility of an in vivo sensitivity test in an “up-front” study. Med Pediatr Oncol (1995) 24(3):188–96. doi: 10.1002/mpo.2950240309
36. Allen JC, Donahue B, DaRosso R, Nirenberg A. Hyperfractionated craniospinal radiotherapy and adjuvant chemotherapy for children with newly diagnosed medulloblastoma and other primitive neuroectodermal tumors. Int J Radiat Oncol (1996) 36(5):1155–61. doi: 10.1016/S0360-3016(96)00450-6
37. Tornesello A, Mastrangelo S, Piciacchia D, Bembo V, Colosimo C, Di Rocco C, et al. Progressive disease in children with medulloblastoma/PNET during preradiation chemotherapy. J Neurooncol (1999) 45(2):135–40. doi: 10.1023/A:1006133404936
38. Prados MD, Edwards MSB, Chang SM, Russo C, Davis R, Rabbitt J, et al. Hyperfractionated craniospinal radiation therapy for primitive neuroectodermal tumors: results of a phase II study. Int J Radiat Oncol (1999) 43(2):279–85. doi: 10.1016/S0360-3016(98)00413-1
39. Heideman RL, Kovnar EH, Kellie SJ, Douglass EC, Gajjar AJ, Walter AW, et al. Preirradiation chemotherapy with carboplatin and etoposide in newly diagnosed embryonal pediatric CNS tumors. J Clin Oncol (1995) 13(9):2247–54. doi: 10.1200/JCO.1995.13.9.2247
40. Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol (2013) 31(23):2936–41. doi: 10.1200/JCO.2012.43.9984
41. Kortmann R-D. The chemotherapy before or after radiation therapy does not influence survival of children with high-risk medulloblastomas: results of the multicenter and randomized study of the Pediatric Oncology Group (POG 9031). Random Control Trial Strahlenther Onkol (2014) 190(1):106–8. doi: 10.1007/s00066-013-0491-2
42. Kortmann R-D, Kühl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the german prospective randomized trial hit ’91. Int J Radiat Oncol (2000) 46(2):269–79. doi: 10.1016/S0360-3016(99)00369-7
43. Hoff Kv, Hinkes B, NU G, Deinlein F, Mittler U, Urban C, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT‘91. Eur J Cancer (2009) 45(7):1209–17. doi: 10.1016/j.ejca.2009.01.015
44. Taylor RE, Bailey CC, Robinson KJ, Weston CL, Walker DA, Ellison D, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer (2005) 41(5):727–34. doi: 10.1016/j.ejca.2004.12.017
45. Verlooy J, Mosseri V, Bracard S, Tubiana AL, Kalifa C, Pichon F, et al. Treatment of high risk medulloblastomas in children above the age of 3 years: a SFOP study. Eur J Cancer (2006) 42(17):3004–14. doi: 10.1016/j.ejca.2006.02.026
46. Allen J, Donahue B, Mehta M, Miller DC, Rorke LB, Jakacki R, et al. A phase II study of preradiotherapy chemotherapy followed by hyperfractionated radiotherapy for newly diagnosed high-risk medulloblastoma/primitive neuroectodermal tumor: a report from the children’s oncology group (CCG 9931). Int J Radiat Oncol Biol Phys (2009) 74(4):1006–11. doi: 10.1016/j.ijrobp.2008.09.019
47. Kellie SJ, Wong CKF, Pozza LD, Waters KD, Lockwood L, Mauger DC, et al. Activity of postoperative carboplatin, etoposide, and high-dose methotrexate in pediatric CNS embryonal tumors: results of a phase II study in newly diagnosed children. Med Pediatr Oncol (2002) 39(3):168–74. doi: 10.1002/mpo.10137
48. Stewart CF, Iacono LC, Chintagumpala M, Kellie SJ, Ashley D, Zamboni WC, et al. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J Clin Oncol (2004) 22(16):3357–65. doi: 10.1200/JCO.2004.10.103
49. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol (2006) 7(10):813–20. doi: 10.1016/S1470-2045(06)70867-1
50. Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH, et al. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol (2004) 22(24):4881–7. doi: 10.1200/JCO.2004.12.126
51. Yamasaki K, Okada K, Soejima T, Sakamoto H, Hara J. Strategy to minimize radiation burden in infants and high-risk medulloblastoma using intrathecal methotrexate and high-dose chemotherapy: a prospective registry study in Japan. Pediatr Blood Cancer (2020) 67(1):e28012. doi: 10.1002/pbc.28012
52. Esbenshade AJ, Kocak M, Hershon L, Rousseau P, Decarie J-C, Shaw S, et al. A phase II feasibility study of oral etoposide given concurrently with radiotherapy followed by dose intensive adjuvant chemotherapy for children with newly diagnosed high-risk medulloblastoma (protocol POG 9631): a report from the children’s oncology gro. Pediatr Blood Cancer (2017) 64(6):e26373. doi: 10.1002/pbc.26373
53. Jakacki RI, Burger PC, Zhou T, Holmes EJ, Kocak M, Onar A, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a children’s oncology group phase I/II study. J Clin Oncol (2012) 30(21):2648–53. doi: 10.1200/JCO.2011.40.2792
54. Gandola L, Massimino M, Cefalo G, Solero C, Spreafico F, Pecori E, et al. Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol (2009) 27(4):566–71. doi: 10.1200/JCO.2008.18.4176
55. Nazemi KJ, Shen V, Finlay JL, Boyett J, Kocak M, Lafond D, et al. High incidence of veno-occlusive disease with myeloablative chemotherapy following craniospinal irradiation in children with newly diagnosed high-risk CNS embryonal tumors: a report from the children’s oncology group (CCG-99702). Pediatr Blood Cancer (2016) 63(9):1563–70. doi: 10.1002/pbc.26074
56. Sung KW, Yoo KH, Cho EJ, Koo HH, Lim DH, Shin HJ, et al. High-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk or relapsed medulloblastoma or supratentorial primitive neuroectodermal tumor. Pediatr Blood Cancer (2007) 48(4):408–15. doi: 10.1002/pbc.21064
57. Abd El-Aal HH, Mokhtar MM, Habib EE, El-Kashef AT, Fahmy ES. Medulloblastoma: conventional radiation therapy in comparison to chemo radiation therapy in the post-operative treatment of high-risk patients. J Egypt Natl Canc Inst (2005) 17(4):301–7.
58. Salama MM, Ghorab EM, Al-Abyad AG, Al-Bahy KM. Concomitant weekly vincristine and radiation followed by adjuvant vincristine and carboplatin in the treatment of high risk medulloblastoma: ain shams university hospital and sohag cancer center study. J Egypt Natl Canc Inst (2006) 18(2):167–74.
59. von Bueren AO, Kortmann R-D, von Hoff K, Friedrich C, Mynarek M, Müller K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol (2016) 34(34):4151–60. doi: 10.1200/JCO.2016.67.2428
60. Dufour C, Kieffer V, Varlet P, Raquin MA, Dhermain F, Puget S, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuro-ectodermic tumors. Pediatr Blood Cancer (2014) 61(8):1398–402. doi: 10.1002/pbc.25009
61. Taylor RE, Howman AJ, Wheatley K, Brogden EE, Large B, Gibson MJ, et al. Hyperfractionated accelerated radiotherapy (HART) with maintenance chemotherapy for metastatic (M1–3) medulloblastoma – a safety/feasibility study. Radiother Oncol (2014) 111(1):41–6. doi: 10.1016/j.radonc.2014.01.022
62. Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol (2016) 17(3):287–98. doi: 10.1016/S1470-2045(15)00167-9
63. Dhall G, O’Neil SH, Ji L, Haley K, Whitaker AM, Nelson MD, et al. Excellent outcome of young children with nodular desmoplastic medulloblastoma treated on “Head start” III: a multi-institutional, prospective clinical trial. Neuro Oncol (2020) 22(12):1862–72. doi: 10.1093/neuonc/noaa102
64. Lee JW, Lim DH, Sung KW, Cho HW, Ju HY, Hyun JK, et al. Promising survival rate but high incidence of treatment-related mortality after reduced-dose craniospinal radiotherapy and tandem high-dose chemotherapy in patients with high-risk medulloblastoma. Cancer Med (2020) 9(16):5807–18. doi: 10.1002/cam4.3199
65. Gupta T, Sinha S, Chinnaswamy G, Vora T, Prasad M, Bhat V, et al. Safety and efficacy of concurrent carboplatin during full-dose craniospinal irradiation for high-risk/metastatic medulloblastoma in a resource-limited setting. Pediatr Blood Cancer (2021) 68(5):1–9. doi: 10.1002/pbc.28925
66. Okada K, Soejima T, Sakamoto H, Hirato J, Hara J. Phase II study of reduced-dose craniospinal irradiation and combination chemotherapy for children with newly diagnosed medulloblastoma: a report from the Japanese pediatric brain tumor consortium. Pediatr Blood Cancer (2020) 67(11):1–6. doi: 10.1002/pbc.28572
67. Dufour C, Foulon S, Geoffray A, Masliah-Planchon J, Figarella-Branger D, Bernier-Chastagner V, et al. Prognostic relevance of clinical and molecular risk factors in children with high-risk medulloblastoma treated in the phase II trial PNET HR+5. Neuro Oncol (2021) 23(7):1163–72. doi: 10.1093/neuonc/noaa301
68. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
69. Riva D, Giorgi C, Nichelli F, Bulgheroni S, Massimino M, Cefalo G, et al. Intrathecal methotrexate affects cognitive function in children with medulloblastoma. Neurology (2002) 59(1):48–53. doi: 10.1212/WNL.59.1.48
70. Deutsch M, Thomas PRM, Krischer J, Boyett JM, Albright L, Aronin P, et al. Results of a prospective randomized trial comparing standard dose neuraxis irradiation (3,600 cGy/20) with reduced neuraxis irradiation (2,340 cGy/13) in patients with low-stage medulloblastoma. Pediatr Neurosurg (1996) 24(4):167–77. doi: 10.1159/000121042
71. Thomas PR, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol (2000) 18(16):3004–11. doi: 10.1200/JCO.2000.18.16.3004
72. Goldwein JW, Radcliffe J, Packer RJ, Sutton LN, Lange B, Rorke LB, et al. Results of a pilot study of low-dose craniospinal radiation therapy plus chemotherapy for children younger than 5 years with primitive neuroectodermal tumors. Cancer (1993) 71(8):2647–52. doi: 10.1002/1097-0142(19930415)71:8<2647::AID-CNCR2820710833>3.0.CO;2-S
73. Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a children’s cancer group study. J Clin Oncol (1999) 17(7):2127–7. doi: 10.1200/JCO.1999.17.7.2127
74. Oyharcabal-Bourden V, Kalifa C, Gentet JC, Frappaz D, Edan C, Chastagner P, et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French society of pediatric oncology study. J Clin Oncol (2005) 23(21):4726–34. doi: 10.1200/JCO.2005.00.760
75. Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the international society of paediatric Oncology/United kingdom children’s cancer study group PNET-3 study. J Clin Oncol (2003) 21(8):1581–91. doi: 10.1200/JCO.2003.05.116
76. Bergman I, Jakacki RI, Heller G, Finlay J. Treatment of standard risk medulloblastoma with craniospinal irradiation, carboplatin, and vincristine. Med Pediatr Oncol (1997) 29(6):563–7. doi: 10.1002/(SICI)1096-911X(199712)29:6<563::AID-MPO8>3.0.CO;2-I
77. Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol (2006) 24(25):4202–8. doi: 10.1200/JCO.2006.06.4980
78. Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of children’s oncology group trial A9961. Neuro Oncol (2013) 15(1):97–103. doi: 10.1093/neuonc/nos267
79. Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 gy) followed by conformal posterior fossa (36 gy) and primary site irradiation (55.8 gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol (2008) 70(3):782–7. doi: 10.1016/j.ijrobp.2007.07.2342
80. Carrie C, Grill J, Figarella-Branger D, Bernier V, Padovani L, Habrand JL, et al. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: long-term results of MSFOP 98. J Clin Oncol (2009) 27(11):1879–83. doi: 10.1200/JCO.2008.18.6437
81. Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol (2012) 30(26):3187–93. doi: 10.1200/JCO.2011.39.8719
82. Minturn JE, Mochizuki AY, Partap S, Belasco JB, Lange BJ, Li Y, et al. A pilot study of low-dose craniospinal irradiation in patients with newly diagnosed average-risk medulloblastoma. Front Oncol (2021) 11:744739/full(September). doi: 10.3389/fonc.2021.744739/full
83. Michalski JM, Janss AJ, Vezina LG, Smith KS, Billups CA, Burger PC, et al. Children’s oncology group phase III trial of reduced-dose and reduced-volume radiotherapy with chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol (2021) 39(24):2685–97. doi: 10.1200/JCO.20.02730
84. Carrie C, Kieffer V, Figarella-Branger D, Masliah-Planchon J, Bolle S, Bernier V, et al. Exclusive hyperfractionated radiation therapy and reduced boost volume for standard-risk medulloblastoma: pooled analysis of the 2 French multicentric studies MSFOP98 and MSFOP 2007 and correlation with molecular subgroups. Int J Radiat Oncol Biol Phys (2020) 108(5):1204–17. doi: 10.1016/j.ijrobp.2020.07.2324
85. Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, et al. β-catenin status predicts a favorable outcome in childhood medulloblastoma: the united kingdom children’s cancer study group brain tumour committee. J Clin Oncol (2005) 23(31):7951–7. doi: 10.1200/JCO.2005.01.5479
86. Gajjar A, Sanford RA, Bhargava R, Heideman R, Walter A, Li Y, et al. Medulloblastoma with brain stem involvement: the impact of gross total resection on outcome. Pediatr Neurosurg (1996) 25(4):182–7. doi: 10.1159/000121121
87. Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol (2016) 17(4):484–95. doi: 10.1016/S1470-2045(15)00581-1
88. Dasgupta A, Gupta T, Sridhar E, Shirsat N, Krishnatry R, Goda JS, et al. Pediatric patients with SHH medulloblastoma fail differently as compared with adults: possible implications for treatment modifications. J Pediatr Hematol Oncol (2019) 41(8):e499–505. doi: 10.1097/MPH.0000000000001484
89. Gerber NU, von Hoff K, von Bueren AO, Treulieb W, Deinlein F, Benesch M, et al. A long duration of the prediagnostic symptomatic interval is not associated with an unfavourable prognosis in childhood medulloblastoma. Eur J Cancer (2012) 48(13):2028–36. doi: 10.1016/j.ejca.2011.11.012
90. Ramaswamy V, Remke M, Shih D, Wang X, Northcott PA, Faria CC, et al. Duration of the pre-diagnostic interval in medulloblastoma is subgroup dependent. Pediatr Blood Cancer (2014) 61(7):1190–4. doi: 10.1002/pbc.25002
91. Padovani L, Horan G, Ajithkumar T. Radiotherapy advances in paediatric medulloblastoma treatment. Clin Oncol (2019) 31(3):171–81. doi: 10.1016/j.clon.2019.01.001
92. El Beltagy MA, Atteya MME. The benefits of navigated intraoperative ultrasonography during resection of fourth ventricular tumors in children. Child’s Nerv Syst (2013) 29(7):1079–88. doi: 10.1007/s00381-013-2103-y
93. Huang F, Parker W, Freeman CR. Feasibility and early outcomes of supine-position craniospinal irradiation. Pediatr Blood Cancer (2009) 54(2)322–5. doi: 10.1002/pbc.22215
94. Taylor RE, Bailey CC, Robinson KJ, Weston CL, Ellison D, Ironside J, et al. Impact of radiotherapy parameters on outcome in the international society of paediatric Oncology/United kingdom children’s cancer study group PNET-3 study of preradiotherapy chemotherapy for M0-M1 medulloblastoma. Int J Radiat Oncol (2004) 58(4):1184–93. doi: 10.1016/j.ijrobp.2003.08.010
95. Carrie C, Alapetite C, Mere P, Aimard L, Pons A, Kolodie H, et al. Quality control of radiotherapeutic treatment of medulloblastoma in a multicentric study: the contribution of radiotherapy technique to tumour relapse. Radiother Oncol (1992) 24(2):77–81. doi: 10.1016/0167-8140(92)90282-Y
96. Benk V, Bouhnik H, Raquin MA, Kalifa C, Habrand JL. Quality control of low dose craniospinal irradiation for low risk medulloblastoma. Br J Radiol (1995) 68(813):1009–13. doi: 10.1259/0007-1285-68-813-1009
97. Carrie C, Hoffstetter S, Gomez F, Moncho V, Doz F, Alapetite C, et al. Impact of targeting deviations on outcome in medulloblastoma: study of the French society of pediatric oncology (SFOP). Int J Radiat Oncol Biol Phys (1999) 45(2):435–9. doi: 10.1016/S0360-3016(99)00200-X
98. Dietzsch S, Braesigk A, Seidel C, Remmele J, Kitzing R, Schlender T, et al. Pretreatment central quality control for craniospinal irradiation in non-metastatic medulloblastoma. Strahlentherapie und Onkol (2021) 197(8):674–82. doi: 10.1007/s00066-020-01707-8
99. Gajjar A. Medulloblastoma: improving cure rates in tandem with reduction in short-term toxicities and long-term treatment-related morbidities. Pediatr Blood Cancer (2020) 67:1–2. doi: 10.1002/pbc.28645
100. Fukunaga-Johnson N, Lee JH, Sandler HM, Robertson P, McNeil E, Goldwein JW. Patterns of failure following treatment for medulloblastoma: is it necessary to treat the entire posterior fossa? Int J Radiat Oncol Biol Phys (1998) 42(1):143–6. doi: 10.1016/S0360-3016(98)00178-3
101. Tian S, Sudmeier LJ, Zhang C, Madden NA, Buchwald ZS, Shu H-KG, et al. Reduced-volume tumor-bed boost is not associated with inferior local control and survival outcomes in high-risk medulloblastoma. Pediatr Blood Cancer (2020) 67(1):e28027. doi: 10.1002/pbc.28027
102. Vivekanandan S, Breene R, Ramanujachar R, Traunecker H, Pizer B, Gaze MN, et al. The UK experience of a treatment strategy for pediatric metastatic medulloblastoma comprising intensive induction chemotherapy, hyperfractionated accelerated radiotherapy and response directed high dose myeloablative chemotherapy or maintenance chemothera. Pediatr Blood Cancer (2015) 62(12):2132–9. doi: 10.1002/pbc.25663
103. Eaton BR, Esiashvili N, Kim S, Weyman EA, Thornton LT, Mazewski C, et al. Clinical outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: a comparison of disease control and overall survival. Int J Radiat Oncol (2016) 94(1):133–8. doi: 10.1016/j.ijrobp.2015.09.014
104. Hartsell WF, Gajjar A, Heideman RL, Langston JA, Sanford RA, Walter A, et al. Patterns of failure in children with medulloblastoma: effects of preirradiation chemotherapy. Int J Radiat Oncol (1997) 39(1):15–24. doi: 10.1016/S0360-3016(97)00136-3
105. Ramaswamy V, Remke M, Adamski J, Bartels U, Tabori U, Wang X, et al. Medulloblastoma subgroup-specific outcomes in irradiated children: who are the true high-risk patients? Neuro Oncol (2016) 18(2):291–7. doi: 10.1093/neuonc/nou357
106. Ottensmeier H, Schlegel PG, Eyrich M, Wolff JE, Juhnke B-O, von Hoff K, et al. Treatment of children under 4 years of age with medulloblastoma and ependymoma in the HIT2000/HIT-REZ 2005 trials: neuropsychological outcome 5 years after treatment. PloS One (2020) 15(1):e0227693. doi: 10.1371/journal.pone.0227693
107. King AA, Seidel K, Di C, Leisenring WM, Perkins SM, Krull KR, et al. Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: a report from the childhood cancer survivor study. Neuro Oncol (2016) 19(5):now242. doi: 10.1093/neuonc/now242
108. Holland AA, Colaluca B, Bailey L, Stavinoha PL. Impact of attention on social functioning in pediatric medulloblastoma survivors. Pediatr Hematol Oncol (2018) 35(1):76–89. doi: 10.1080/08880018.2018.1440333
109. Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a pediatric oncology group study. J Clin Oncol (1998) 16(5):1723–8. doi: 10.1200/JCO.1998.16.5.1723
110. Ris MD, Walsh K, Wallace D, Armstrong FD, Holmes E, Gajjar A, et al. Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr Blood Cancer (2013) 60(8):1350–7. doi: 10.1002/pbc.24496
111. Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J Clin Oncol (2001) 19(15):3470–6. doi: 10.1200/JCO.2001.19.15.3470
112. Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol (2005) 23(24):5511–9. doi: 10.1200/JCO.2005.00.703
113. Kennedy C, Bull K, Chevignard M, Culliford D, Dörr HG, Doz F, et al. Quality of Survival and Growth in Children and Young Adults in the PNET4 European Controlled Trial of Hyperfractionated Versus Conventional Radiation Therapy for Standard-Risk Medulloblastoma. Int J Radiat Oncol [Internet] (2014) 88(2):292–300. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0360301613031866.
114. Bull KS, Spoudeas HA, Yadegarfar G, Kennedy CR. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: a follow-up study in PNET 3 trial survivors–on behalf of the CCLG (formerly UKCCSG). J Clin Oncol (2007) 25(27):4239–45. doi: 10.1200/JCO.2006.08.7684
115. von Bueren AO, von Hoff K, Benesch M, Rutkowski S. Dose reductions of vincristine in children with medulloblastoma treated in the maintenance arm of the prospective multicenter trial HIT’91. Klin Padiatr (2009) 221(6):396–7. doi: 10.1055/s-0029-1238278
116. Rao AAN, Wallace DJ, Billups C, Boyett JM, Gajjar A, Packer RJ. Cumulative cisplatin dose is not associated with event-free or overall survival in children with newly diagnosed average-risk medulloblastoma treated with cisplatin based adjuvant chemotherapy: report from the children’s oncology group. Pediatr Blood Cancer (2014) 61(1):102–6. doi: 10.1002/pbc.24670
117. Gurney JG, Bass JK, Onar-Thomas A, Huang J, Chintagumpala M, Bouffet E, et al. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro Oncol (2014) 16(6):848–55. doi: 10.1093/neuonc/not241
118. Moxon-Emre I, Taylor MD, Bouffet E, Hardy K, Campen CJ, Malkin D, et al. Intellectual outcome in molecular subgroups of medulloblastoma. J Clin Oncol (2016) 34(34):4161–70. doi: 10.1200/JCO.2016.66.9077
119. Jabarkheel R, Amayiri N, Yecies D, Huang Y, Toescu S, Nobre L, et al. Molecular correlates of cerebellar mutism syndrome in medulloblastoma. Neuro Oncol (2020) 22(2):290–7. doi: 10.1093/neuonc/noz158
120. Poggi G, Liscio M, Pastore V, Adduci A, Galbiati S, Spreafico F, et al. Psychological intervention in young brain tumor survivors: the efficacy of the cognitive behavioural approach. Disabil Rehabil (2009) 31(13):1066–73. doi: 10.1080/09638280802509546
121. KASATKIN V, DEVIATERIKOVA A, SHURUPOVA M, KARELIN A. The feasibility and efficacy of short-term visual-motor training in pediatric posterior fossa tumor survivors. Eur J Phys Rehabil Med (2022) 58(1):51–9. doi: 10.23736/S1973-9087.21.06854-4
122. Palmer SL, Leigh L, Ellison SC, Onar-Thomas A, Wu S, Qaddoumi I, et al. Feasibility and efficacy of a computer-based intervention aimed at preventing reading decoding deficits among children undergoing active treatment for medulloblastoma: results of a randomized trial. J Pediatr Psychol (2014) 39(4):450–8. doi: 10.1093/jpepsy/jst095
Keywords: Medulloblastoma, children, survival, surgery, radiotherapy, chemotherapy, cancer treatment protocol, side effects
Citation: Osuna-Marco MP, Martín-López LI, Tejera ÁM and López-Ibor B (2023) Questions and answers in the management of children with medulloblastoma over the time. How did we get here? A systematic review. Front. Oncol. 13:1229853. doi: 10.3389/fonc.2023.1229853
Received: 30 May 2023; Accepted: 13 June 2023;
Published: 29 June 2023.
Edited by:
Kevin X. Liu, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Mohamed Saad Zaghloul, Cairo University, EgyptCopyright © 2023 Osuna-Marco, Martín-López, Tejera and López-Ibor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta P. Osuna-Marco, bWFydGFvc3VuYW1hcmNvQGdtYWlsLmNvbQ==
†ORCID: Marta P Osuna-Marco, orcid.org/0000-0002-2169-9266Laura I Martín-López, orcid.org/0000-0001-7591-5473Águeda M Tejera, orcid.org/0000-0002-6703-9466Blanca López-Ibor, orcid.org/0000-0002-6419-6773
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.