- 1Department of Orthopedics, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan, China
- 2Modern Educational Technology Center, Henan University of Economics and Law, Zhengzhou, Henan, China
- 3Department of Medical Oncology, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan, China
Background: Effective adjuvant therapy for osteosarcoma is necessary for improved outcomes. Previous studies demonstrated that apatinib plus doxorubicin-based chemotherapy may improve the efficacy of neoadjuvant therapy. This study aimed to clarify the effectiveness and safety of apatinib plus doxorubicin and cisplatin (AP) as neoadjuvant therapy for osteosarcoma.
Methods: The clinical data of osteosarcoma patients who underwent neoadjuvant therapy and surgery between August 2016 and April 2022 were retrospectively collected and analyzed. Patients were divided into two groups: the apatinib plus AP (apatinib + AP) group and the methotrexate, doxorubicin, and cisplatin (MAP) group.
Results: This study included 42 patients with nonmetastatic osteosarcoma (19 and 23 patients in the apatinib + AP and MAP groups, respectively). The 1- and 2-year disease-free survival rates in the apatinib + AP group were higher than those in the MAP group, but the difference was not significant (P=0.165 and 0.283, respectively). Some adverse events were significantly more common in the apatinib + AP group than in the MAP group, including oral mucositis (grades 3 and 4) (52.6% vs. 17.4%, respectively, P=0.023), limb edema (47.4% vs. 17.4%, respectively, P=0.049), hand-foot syndrome (31.6% vs. 0%, respectively, P=0.005), proteinuria (26.3% vs. 0%, respectively, P=0.014), hypertension (21.1% vs. 0%, respectively, P=0.035), and hypothyroidism (21.1% vs. 0%, respectively, P=0.035). No drug-related deaths occurred. There was no statistically significant difference in the incidence of postoperative complications between the groups (P>0.05).
Conclusion: The present study suggests that apatinib + AP may be a promising candidate for neoadjuvant therapy for osteosarcoma, warranting further validation in prospective randomized controlled clinical trials with long-term follow-up.
1 Introduction
Osteosarcoma is one of the most common osteogenic malignancies. There are 2,000–3,000 newly diagnosed cases annually in China (1, 2). The standard treatment for non-metastatic osteosarcoma is preoperative chemotherapy (neoadjuvant chemotherapy), surgery, and postoperative adjuvant chemotherapy (2–4). The purpose of neoadjuvant chemotherapy is to 1) eliminate small metastases that may exist; 2) shrink the tumor, increase the rate of limb salvage, and reduce the recurrence rate of osteosarcoma; and 3) determine the effect of chemotherapy to facilitate the formulation of plans for postoperative chemotherapy, thus improving the curative effect (5, 6). The drug regimen used as neoadjuvant chemotherapy has been studied repeatedly and in detail. The currently recognized regimen is a combination of methotrexate, adriamycin (doxorubicin), and cisplatin (MAP), or doxorubicin and cisplatin (AP) (6, 7). Approximately 50% of patients initially diagnosed with non-metastatic osteosarcoma develop metastasis and ultimately do not survive, even after receiving neoadjuvant chemotherapy (6, 8, 9). Therefore, new therapeutic drugs and methods are urgently required.
Apatinib was marketed in 2014 in China as the first domestically produced multi-target tyrosine kinase inhibitor (TKI) (10). Targets that are inhibited by apatinib include VEGFR1, VEGFR2, c-RET, c-KIT, and c-SRC (11, 12). Several studies have demonstrated that apatinib can inhibit the proliferation, invasion, and migration of osteosarcoma cells in vitro (13–15) and is effective in treating patients with advanced osteosarcoma (16).
Our previous study has demonstrated that apatinib ameliorates doxorubicin-induced migration and cancer stemness of osteosarcoma cells (17). This suggests that apatinib combined with neoadjuvant chemotherapy may achieve better outcomes in non-metastatic osteosarcoma than the current standard regimen. However, no reports have confirmed this. Some of our patients with osteosarcoma were treated with apatinib plus neoadjuvant chemotherapy in clinical practice. In the present study, we retrospectively collected and analyzed the clinical data of these patients and summarized the effectiveness and safety of this treatment regimen.
2 Materials and methods
2.1 Patient
All osteosarcoma patients included in this retrospective study were treated between August 2016 and April 2022. This study was approved by the Institutional Review Board of Henan Cancer Hospital and was conducted in accordance with the guidelines and principles of the Declaration of Helsinki. All patients included in the present study met the following criteria: 1) pathologically confirmed osteosarcoma; 2) no evidence of distant metastasis; 3) received apatinib + AP or MAP neoadjuvant and postoperative adjuvant therapy; 4) underwent limb salvage surgery or amputation.
2.2 Treatment protocol
Based on the different drugs received, patients were divided into two groups: the apatinib + AP group and the MAP group. In the MAP group, patients received preoperative chemotherapy comprising two 5-week cycles of doxorubicin 37.5 mg/m2/day for 2 days, cisplatin 60 mg/m2/day for 2 days, and methotrexate 12 g/m2 (18). Surgery was scheduled after two cycles of preoperative chemotherapy. The patients received another four cycles of treatment postoperatively, similar to preoperative chemotherapy.
Some patients chose to receive apatinib plus AP treatment (apatinib + AP group). In this group, all patients received preoperative therapy consisting of cisplatin 60 mg/m2 and doxorubicin 37.5 mg/m2 per day on days 1−2. Each patient received six cycles of chemotherapy, which were repeated every 3 weeks. Surgery was scheduled after two cycles of chemotherapy. Postoperatively, the patients received another four cycles of treatment, similar to preoperative chemotherapy. Patients in parallel received 500 mg (those with body surface area [BSA] ≥1.5 m2) or 250 mg (those with BSA <1.5 m2) apatinib per day, starting on day 3. Apatinib was interrupted during chemotherapy and interrupted for 2 weeks postoperatively and then continued until 1 year postoperatively.
Adverse events (AEs) were determined according to the Common Terminology Criteria for Adverse Events version 4.0. For patients who could not tolerate AEs, the dose of apatinib was reduced to 250 mg/day or 125 mg/day.
Limb salvage surgery or amputation was performed after two cycles of preoperative therapy. All surgeries were aimed at achieving complete resection of the primary lesion. Apatinib was interrupted for 2 weeks postoperatively and then continued until 1 year postoperatively.
2.3 Evaluation
The Response Evaluation Criteria in Solid Tumors (version 1.1) was used to evaluate the effectiveness of neoadjuvant treatment. Tumor responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease. The disease control rate (DCR) was defined as the sum of the rates of CR, PR and SD. The differences in alkaline phosphatase (ALP) serum level changes post-neoadjuvant therapy, tumor cell necrosis rate, and 1- and 2-year disease-free survival (DFS) rates between the two groups were evaluated. Tumor responses were evaluated according to the response evaluation criteria in solid tumors (version 1.1). DFS was defined as the time from the surgery to the first occurrence of signs of recurrence or metastasis. The between-group rates of drug-related AEs and surgery-related complications were compared. Surgery-related complications were graded using the Clavien–Dindo grading system.
2.4 Statistical analysis
Quantitative variables are presented as numerical values (percentages), medians (ranges), or medians (interquartile range). Categorical data were compared using Fisher’s exact test, and continuous variables were compared using Student’s t-test. Progression-free survival was estimated using the Kaplan–Meier method. The univariate Cox proportional hazards model was used to analyze the relationship between clinicopathological parameters and DFS. Statistical analyses were performed using SPSS (version 21.0; IBM, Armonk, NY, USA). All statistical analyses were two-sided, and a P-value <0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
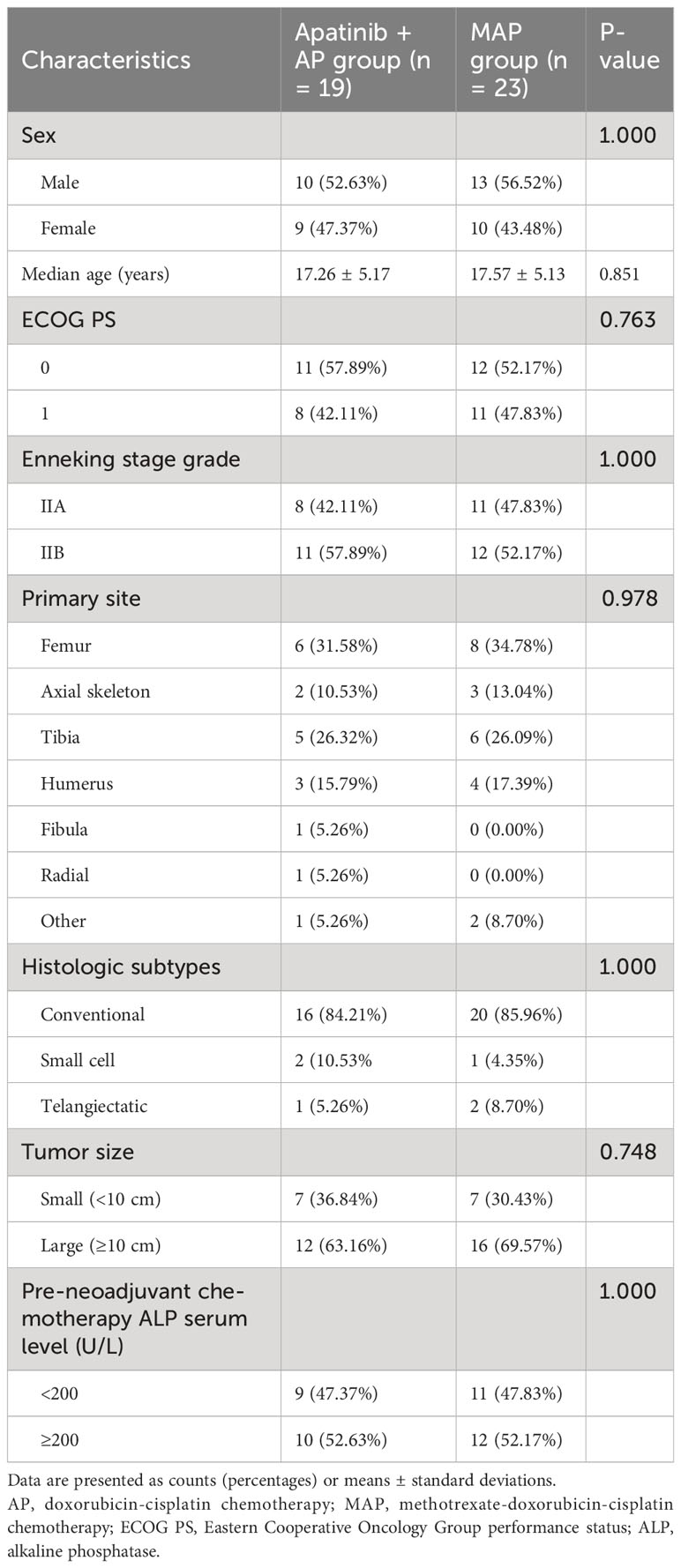
A total of 42 osteosarcoma patients met the eligibility criteria for this study, with 19 and 23 patients included in the apatinib + AP and MAP groups, respectively. The median follow-up period was 28 (9–50) and 22 (9–42) months for the apatinib + AP and MAP groups, respectively.
The baseline characteristics of the patients are presented in Table 1. All patients in both groups were younger than 30 years of age. The median ages of the patients at diagnosis were 18.0 (13.0–21.0) and 18.0 (14.5–20.5) years in the apatinib + AP and MAP groups, respectively. All patients in both groups were Enneking Stage II at the time of treatment initiation. The primary lesions were most commonly located in the long bones of the extremities. The diameters of the primary lesions in most patients in the two groups were >10 cm. The pre-neoadjuvant chemotherapy ALP serum level was >200 U/L in more than half of the patients in both groups. There were no significant differences between the groups in baseline characteristics (P>0.05, Table 1).
Four patients in the apatinib + AP group experienced recurrence or metastasis during the maintenance treatment with apatinib, leading to the discontinuation of apatinib treatment. The remaining 15 patients successfully completed a 1-year maintenance treatment.
3.2 Clinical effectiveness
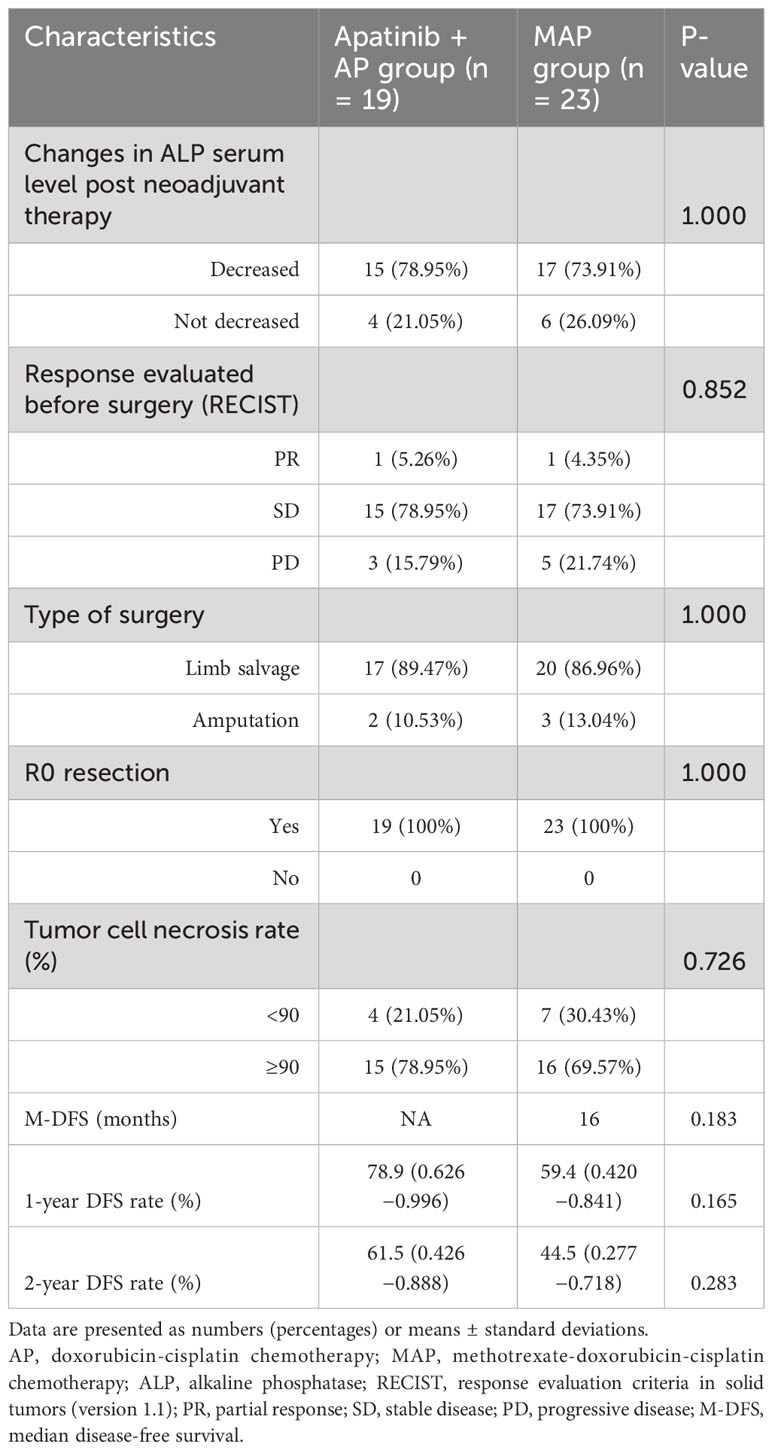
Preoperative evaluation of the efficacy of neoadjuvant therapy revealed that 78.95% (15/19) and 73.91% (17/23) of patients in the apatinib + AP and MAP groups, respectively, experienced a reduction in ALP serum levels after neoadjuvant therapy (Table 2).
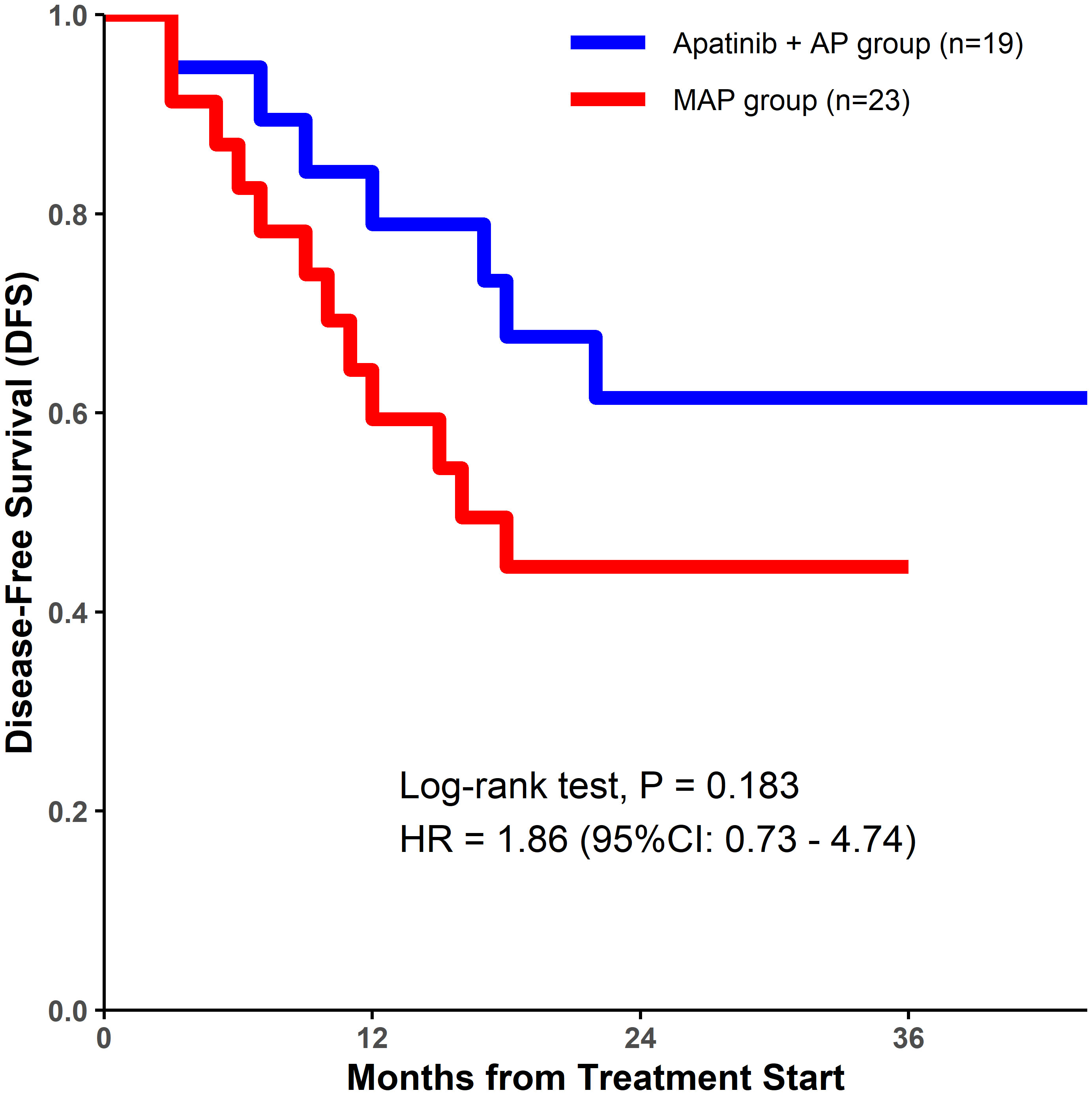
Although a decrease in ALP level was observed in a higher percentage of patients in the apatinib + AP group than in the MAP group, there were no significant differences between the two groups with respect to the DCR (84.21% vs. 78.26%, P=0.852; Table 2), tumor cell necrosis rate ≥90% (78.95% vs. 69.57%, P=0.726; Table 2), 1-year DFS rate (78.9% vs. 59.4%, P=0.165; Table 2, Figure 1), and the 2-year DFS rate (61.5% vs. 44.5%, P=0.283; Table 2, Figure 1).
3.3 Toxicity and complications
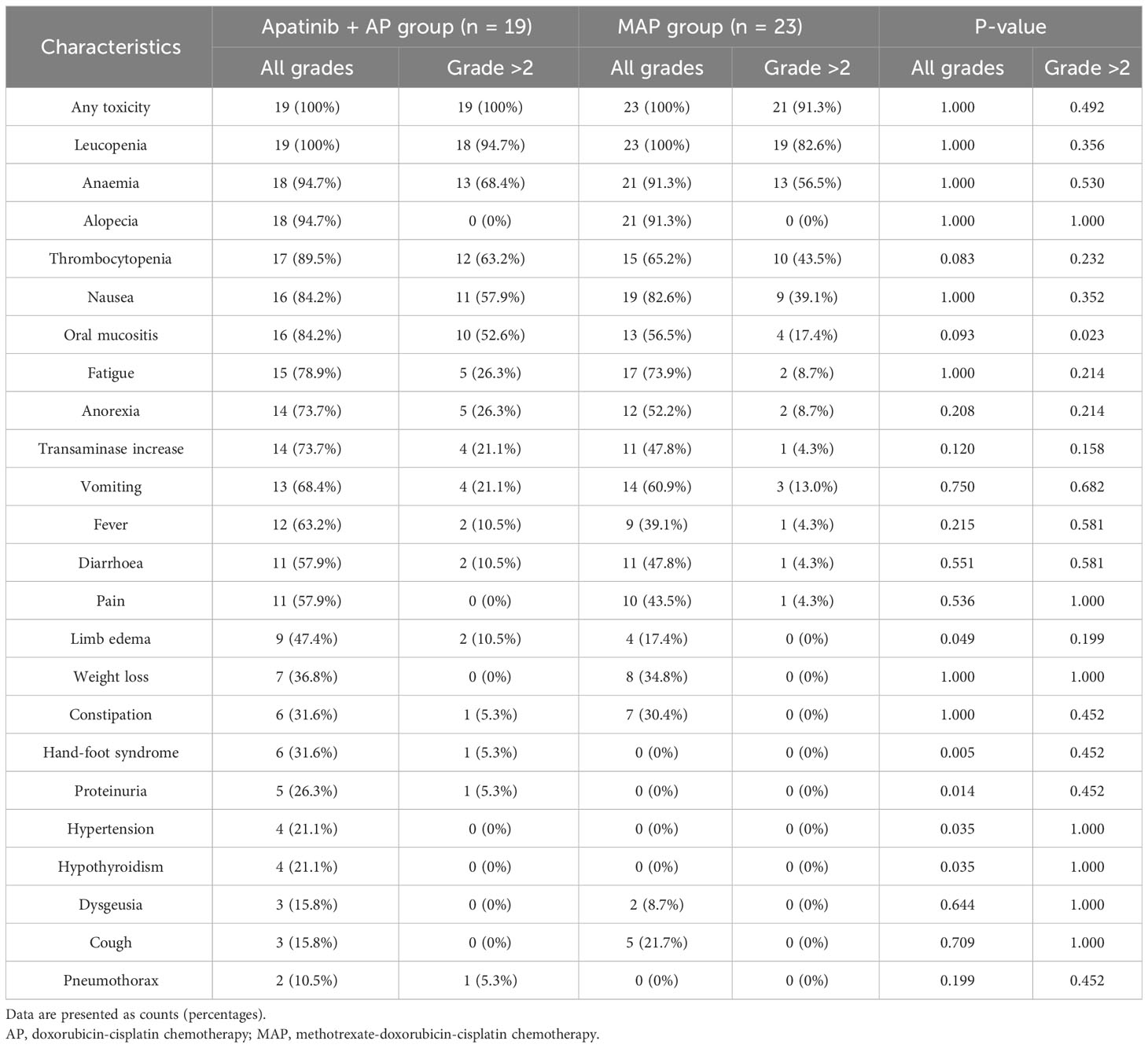
Patients in the apatinib + AP group experienced more AEs than those in the MAP group. Some AEs were significantly more common in the apatinib + AP group than in the MAP group (P<0.05), and these included oral mucositis (grades 3 and 4) (52.6% vs. 17.4%, respectively, P=0.023), limb edema (47.4% vs. 17.4%, respectively, P=0.049), hand-foot syndrome (31.6% vs. 0%, respectively, P=0.005), proteinuria (26.3% vs. 0%, respectively, P=0.014), hypertension (21.1% vs. 0%, respectively, P=0.035), and hypothyroidism (21.1% vs. 0%, respectively, P=0.035; Table 3).
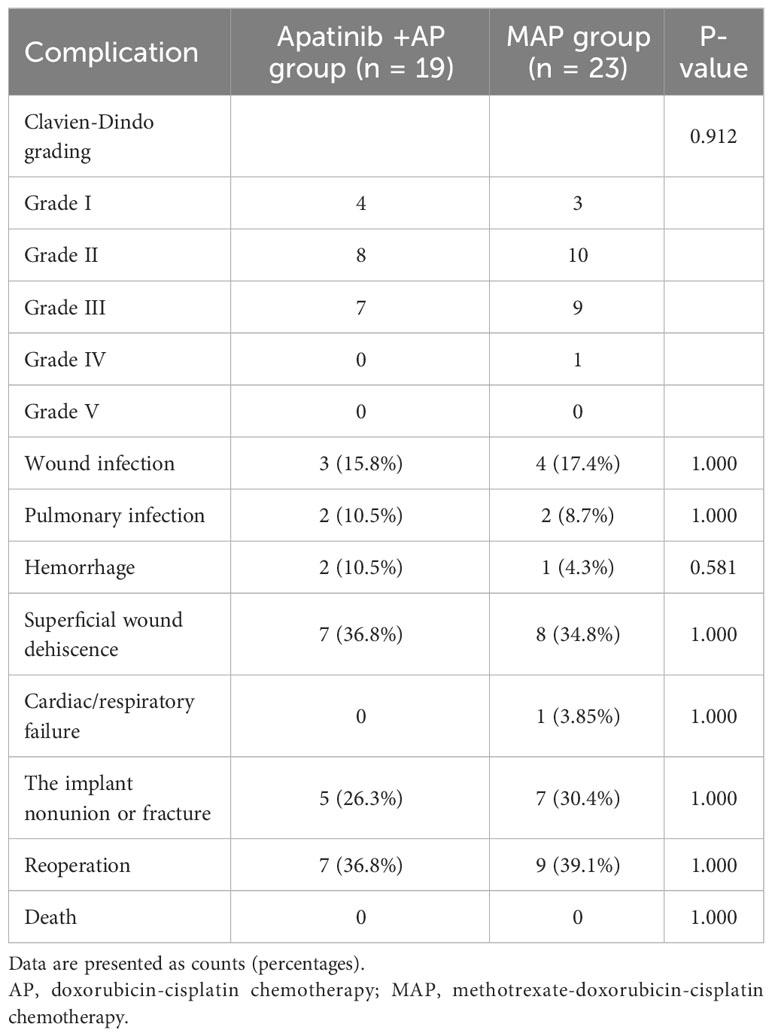
Postoperative complications in each group are shown in Table 4. A grade IV complication (cardiac failure) occurred in one patient in the MAP group (Table 4). There was no statistically significant difference in the incidence of postoperative complications between the two groups (P>0.05, Table 4). No drug- and surgery-related deaths occurred.
3.4 Univariate Cox regression analysis
Univariate Cox regression analysis was performed to determine the relationship between DFS and the clinical characteristics of the patients in this study. In the apatinib + AP group, patients with decreased ALP serum levels after neoadjuvant therapy, ≥90% tumor cell necrosis rate, and disease control after neoadjuvant therapy had significantly longer DFS (P<0.05, Figure 2). In the MAP group, patients with a primary tumor located in the axial skeleton, ≥90% tumor cell necrosis rate, and disease control after neoadjuvant therapy had significantly longer DFS (P<0.05, Figure 3).
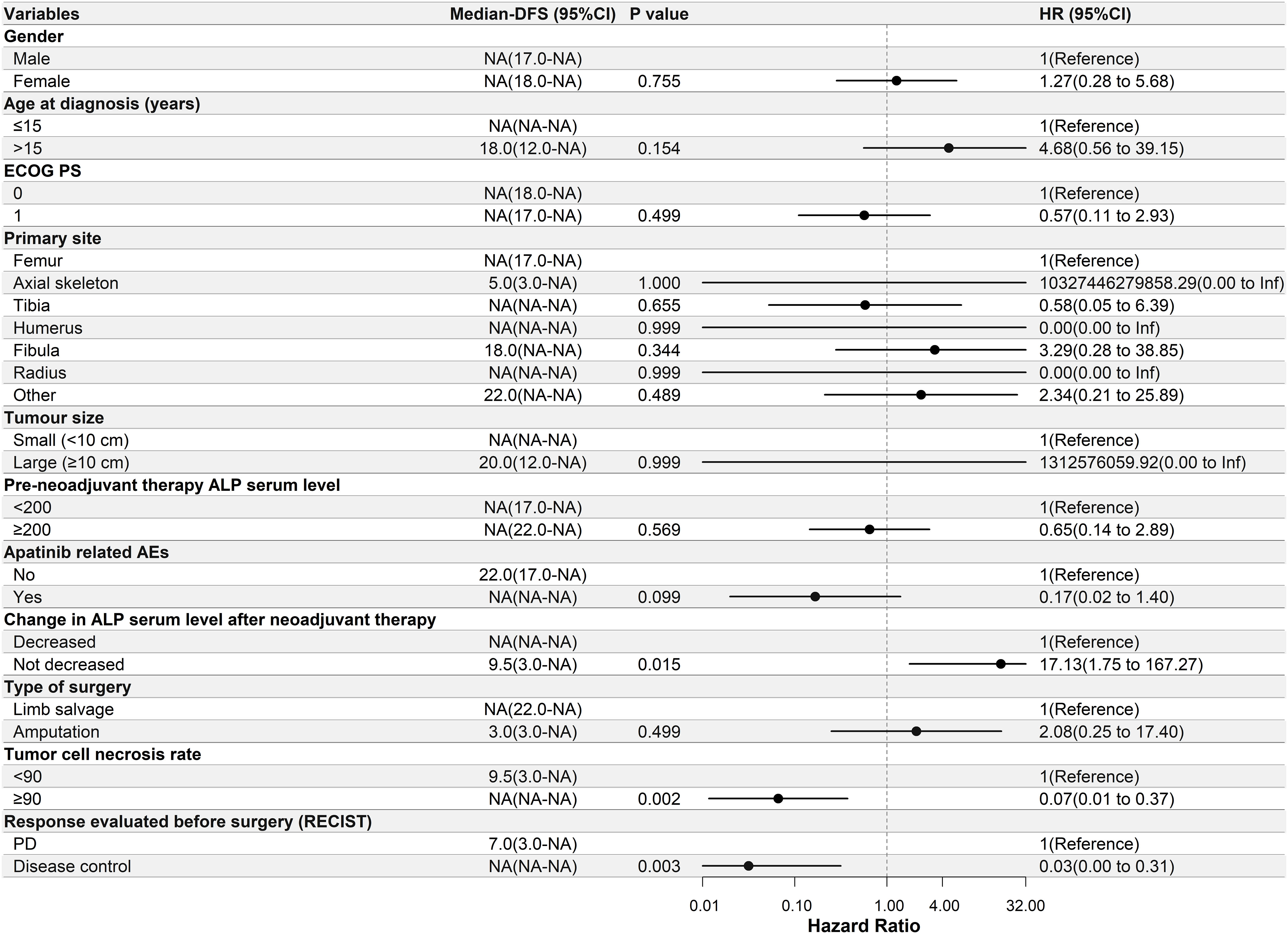
Figure 2 Univariate Cox regression analysis of the relationship between clinicopathological parameters and disease-free survival (DFS) in the apatinib + AP group. Patients with decreased alkaline phosphatase serum levels after neoadjuvant therapy, ≥90% tumor cell necrosis rate, and disease control had significantly longer DFS. ECOG PS, Eastern Cooperative Oncology Group performance status; ALP, alkaline phosphatase; AEs, adverse events; RECIST, Response Evaluation Criteria in Solid Tumors; PD, progressive disease; NA, Not Applicable.
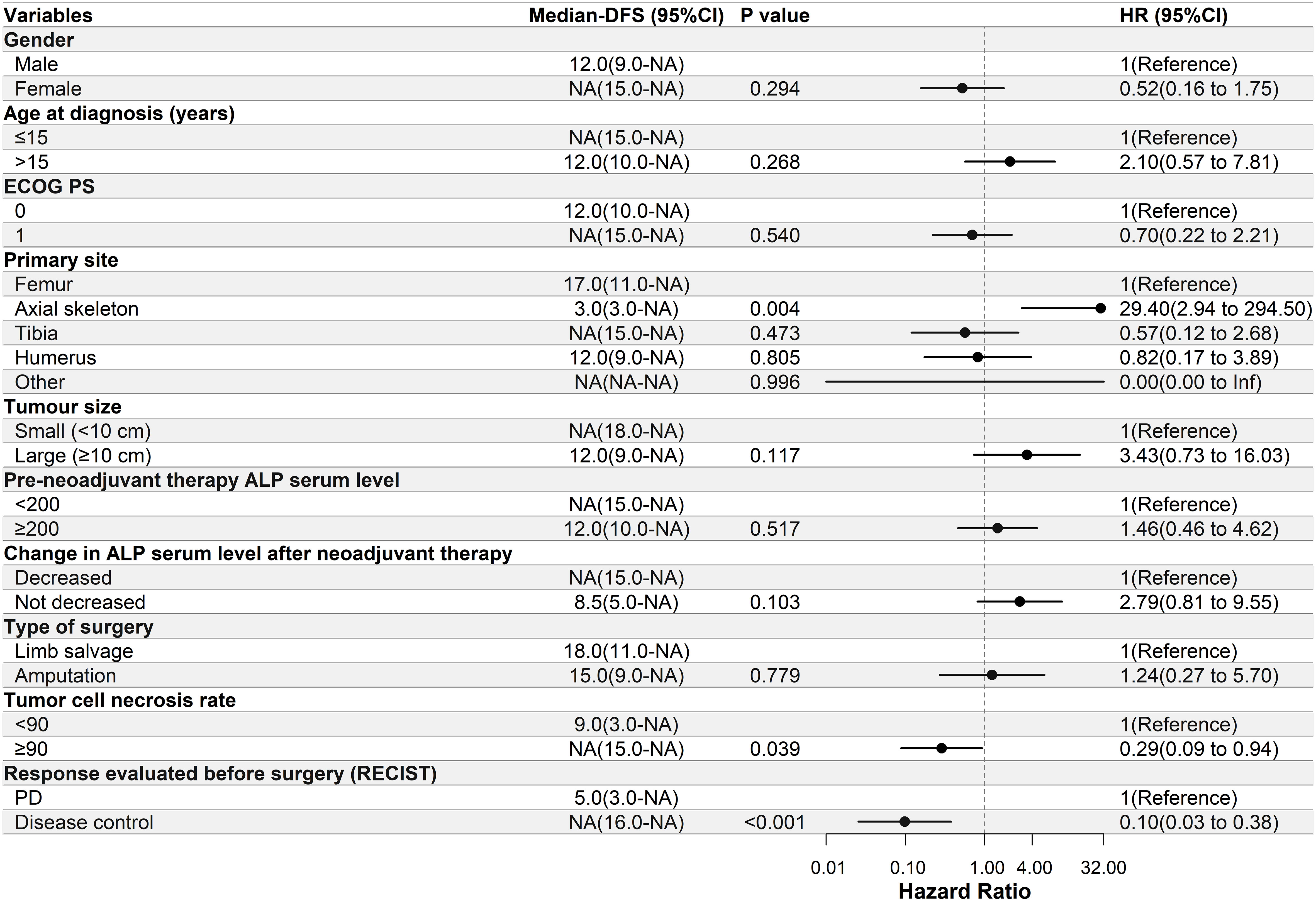
Figure 3 Univariate Cox regression analysis of the relationship between clinicopathological parameters and disease-free survival (DFS) in the methotrexate-doxorubicin-cisplatin group. Patients with a primary site located in the axial skeleton, ≥90% tumor cell necrosis rate, and disease control had significantly longer DFS. NA, Not Applicable. ECOG PS, Eastern Cooperative Oncology Group performance status; ALP, alkaline phosphatase; RECIST, Response Evaluation Criteria in Solid Tumors; PD, progressive disease; NA, Not Applicable.
4 Discussion
This study is the first to report the safety and effectiveness of apatinib plus AP for the neoadjuvant treatment of patients with osteosarcoma. Based on the different treatments received, patients were divided into the apatinib + AP and MAP groups. AEs were more prevalent in patients treated with apatinib plus AP than in those treated with MAP. The 1- and 2-year DFS rates in the apatinib +AP group were higher than those in the MAP group, but the difference was not significant.
Perioperative chemotherapy has repeatedly been shown to be indispensable as a standard of care for non-metastatic osteosarcoma (3, 7). However, chemotherapy drugs used remain controversial (8). The history of neoadjuvant chemotherapy in osteosarcoma is a balance between efficacy and toxicity, and researchers have tried to improve efficacy by increasing the dose or number of different drugs used as much as possible while maintaining a tolerable level of toxicity (3, 19). Currently, AP and MAP chemotherapies are generally recognized as efficacious, but they have a high toxicity rate, and their cure rate should be further improved (8). Improvements in neoadjuvant therapy for osteosarcoma will continue as new drugs are being developed (20–22).
Since it was launched, apatinib, a multi-target TKI, has been shown to have good efficacy in the treatment of advanced osteosarcoma that has failed multi-line therapy (23). Our previous study demonstrated that apatinib can reverse the resistance of osteosarcoma cells to doxorubicin (17). Different studies have also demonstrated inhibition of the targets of apatinib to be beneficial for the treatment of osteosarcoma (24). At present, multiple clinical trials of TKI combined with chemotherapy in the treatment of sarcoma have achieved benign results (25, 26). In the present study, the tumor necrosis rate and 1- and 2-year DFS rates in the apatinib +AP group were higher than those in the MAP group. This suggests that the addition of apatinib to neoadjuvant therapy for osteosarcoma could achieve better outcomes. This confirms the results of our previous study and similar studies (27, 28). However, the improvement in efficacy in the present study was not significant. We believe that the reason for this might be the limited number of patients. Therefore, it is necessary to further assess the efficacy of apatinib as neoadjuvant treatment of osteosarcoma in a prospective randomized controlled clinical trial with a large sample size.
Several previous studies have demonstrated that apatinib is highly toxic. This has led to a reduction in the dose used in clinical practice for the treatment of various malignancies from 750 mg to 500 mg (29, 30). Studies on various tumor types have demonstrated that apatinib combined with chemotherapy can be severely toxic (30, 31). This is an important reason apatinib has not been tested as neoadjuvant therapy for osteosarcoma, despite the evidence for efficacy in advanced sarcomas. In the present study, to reduce the toxicity of apatinib when combined with chemotherapy, the AP regimen was used for the combined group, which was also recommended as first-line treatment by the National Comprehensive Cancer Network guidelines (32). Nevertheless, the results of this study show that AEs were more prevalent in the apatinib + AP group than in the MAP group. However, these significantly increased adjuvant treatment-related AEs did not hinder the output of surgical treatment, let alone lead to adjuvant treatment-related death. This suggests that apatinib combined with AP in the neoadjuvant setting results in an acceptable level of toxicity. Here, we emphasize three points. First, in this study, the initial dose of apatinib was individualized according to the patient’s BSA, and the dose of apatinib was dynamically adjusted according to the occurrence of AEs. This is an important point for clinical decision-making and study design. Second, patients older than 30 years of age were excluded from receiving the combined regimen, which was fully considered from the outset of the study. Older patients have been excluded from several studies of neoadjuvant treatment in osteosarcoma (33). Younger patients appear to be more tolerant of the AEs caused by apatinib combined with chemotherapy. In addition, it is worth noting that in this study, seven patients younger than 10 years of age did not experience AEs when receiving apatinib combined with neoadjuvant chemotherapy. This suggests that apatinib can be safely added to neoadjuvant therapy in children with osteosarcoma. Finally, the maintenance treatment with apatinib after the MAP regimen is also a worthwhile treatment option. This approach not only avoids the toxicity of chemotherapy plus apatinib but also preserves the benefits of these two treatment options. The maintenance with apatinib after MAP may even be better than the apatinib + AP regimen.
Researchers have attempted to find the best evaluation system for neoadjuvant therapy for osteosarcoma (34–36). We found that decreased ALP serum levels after neoadjuvant therapy, ≥90% tumor cell necrosis rate, and disease control were significantly associated with longer DFS in the present study. This is similar to the results of other studies (12). However, it is unclear which method is the most suitable for efficacy evaluation and prediction of survival rates in patients treated with apatinib combined with AP neoadjuvant therapy. Answering this question requires further prospective studies and long-term follow-ups.
This study had some limitations, including its retrospective nature, small sample size, and short follow-up period. All these factors make it difficult to analyze the differences in outcomes and complications. Prospective registered clinical trials are required to continue investigating the effectiveness of the apatinib + AP regimen. Moreover, further research on the efficacy of apatinib in postoperative maintenance therapy for osteosarcoma is warranted.
5 Conclusions
The results of this study suggest that apatinib + AP may be a promising candidate for neoadjuvant therapy for osteosarcoma, warranting further validation in prospective randomized controlled clinical trials with long-term follow-up.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Henan Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Miaopu Funds from Henan Cancer Hospital.
Acknowledgments
We are grateful to all the Chinese patients and their family members for their cooperation in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer (2009) 125(1):229–34. doi: 10.1002/ijc.24320
2. Moukengue B, Lallier M, Marchandet L, Baud'huin M, Verrecchia F, Ory B, et al. Origin and therapies of osteosarcoma. Cancers (Basel) (2022) 14(14):3265. doi: 10.3390/cancers14143503
3. Benjamin RS. Adjuvant and neoadjuvant chemotherapy for osteosarcoma: A historical perspective. Adv Exp Med Biol (2020) 1257:1–10. doi: 10.1007/978-3-030-43032-0_1
4. Jiang ZY, Liu JB, Wang XF, Ma YS, Fu D. Current status and prospects of clinical treatment of osteosarcoma. Technol Cancer Res t (2022) 21(null):e001361. doi: 10.1177/15330338221124696
5. Papakonstantinou E, Stamatopoulos A, Athanasiadis DI, Kenanidis E, Potoupnis M, Haidich AB, Tsiridis E. Limb-salvage surgery offers better five-year survival rate than amputation in patients with limb osteosarcoma treated with neoadjuvant chemotherapy. A systematic review and meta-analysis. J Bone Oncol (2020) 25:100319. doi: 10.1016/j.jbo.2020.100319
6. Argenziano M, Tortora C, Pota E, Di Paola A, Di Martino M, Di Leva C, et al. Osteosarcoma in children: not only chemotherapy. Pharm (Basel) (2021) 14(9):923. doi: 10.3390/ph14090923
7. Belayneh R, Fourman MS, Bhogal S, Weiss KR. Update on osteosarcoma. Curr Oncol Rep (2021) 23(6):71. doi: 10.1007/s11912-021-01053-7
8. Smrke A, Anderson PM, Gulia A, Gennatas S, Huang PH, Jones RL. Future directions in the treatment of osteosarcoma. Cells (2021) 10(1):172. doi: 10.3390/cells10010172
9. Cole S, Gianferante DM, Zhu B, Mirabello L. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer-am Cancer Soc (2022) 128(11):2107–18. doi: 10.1002/cncr.34163
10. Scott LJ. Apatinib: A review in advanced gastric cancer and other advanced cancers. Drugs (2018) 78(7):747–58. doi: 10.1007/s40265-018-0903-9
11. Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci (2011) 102(7):1374–80. doi: 10.1111/j.1349-7006.2011.01939.x
12. Tian Z, Niu X, Yao W. Efficacy and response biomarkers of apatinib in the treatment of Malignancies in China: A review. Front Oncol (2021) 11:749083. doi: 10.3389/fonc.2021.749083
13. Zheng B, Ren T, Huang Y, Guo W. Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem Biophys Res Commun (2018) 495(2):1695–701. doi: 10.1016/j.bbrc.2017.12.032
14. Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis (2017) 8(8):e3015. doi: 10.1038/cddis.2017.422
15. Han G, Guo Q, Ma N, Bi W, Xu M, Jia J. Apatinib inhibits cell proliferation and migration of osteosarcoma via activating LINC00261/miR-620/PTEN axis. Cell Cycle (2021) 20(18):1785–98. doi: 10.1080/15384101.2021.1949132
16. Yao H, Chen X, Tan X. Efficacy and safety of apatinib in the treatment of osteosarcoma: a single-arm meta-analysis among Ch inese patients. BMC Cancer (2021) 21(1):449. doi: 10.1186/s12885-021-08154-3
17. Tian ZC, Wang JQ, Ge H. Apatinib ameliorates doxorubicin-induced migration and cancer stemness of osteosarcoma cells by inhibiting Sox2 via STAT3 signalling. J Orthop Translat (2020) 22:132–41. doi: 10.1016/j.jot.2019.07.003
18. Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol (2015) 33(20):2279–87. doi: 10.1200/JCO.2014.60.0734
19. Múdry P, Kýr M, Rohleder O, Mahdal M, Staniczková Zambo I, Ježová M, et al. Štěrba: Improved osteosarcoma survival with addition of mifamurtide to conventional chemotherapy – Observational prospective single institution analysis. J Bone Oncol (2021) 28:100361. doi: 10.1016/j.jbo.2021.100362
20. Ratti C, Botti L, Cancila V, Galvan S, Torselli I, Garofalo C, et al. Trabectedin overrides osteosarcoma differentiative block and reprograms the tumor immune environment enabling effective combination with immune checkpoint inhibitors. Clin Cancer Res (2017) 23(17):5149–61. doi: 10.1158/1078-0432.CCR-16-3186
21. Liverani C, Mercatali L, Spadazzi C, La Manna F, De Vita A, Riva N, et al. CSF-1 blockade impairs breast cancer osteoclastogenic potential in co-culture systems. Bone (2014) 66(null):214–22. doi: 10.1016/j.bone.2014.06.017
22. Recine F, Bongiovanni A, Riva N, Fausti V, De Vita A, Mercatali L, et al. Update on the role of trabectedin in the treatment of intractable soft tissue sarcomas. Onco Targets Ther (2017) 10(null):1155–64. doi: 10.2147/OTT.S127955
23. Yang QK, Chen T, Wang SQ, Zhang XJ, Yao ZX. Apatinib as targeted therapy for advanced bone and soft tissue sarcoma: a dilemma of reversing multidrug resistance while suffering drug resistance itself. Angiogenesis (2020) 23(3):279–98. doi: 10.1007/s10456-020-09716-y
24. Tian Z, Niu X, Yao W. Receptor tyrosine kinases in osteosarcoma treatment: which is the key target? Front Oncol (2020) 10:1642. doi: 10.3389/fonc.2020.01642
25. Somaiah N, Van Tine BA, Wahlquist AE, Milhem MM, Hill EG, Garrett-Mayer E, et al. A randomized, open-label, phase 2, multicenter trial of gemcitabine with pazopanib or gemcitabine with docetaxel in patients with advanced soft-tissue sarcoma. Cancer (2021) 127(6):894–904. doi: 10.1002/cncr.33216
26. Schmoll HJ, Lindner LH, Reichardt P, Heissner K, Kopp HG, Kessler T, et al. Efficacy of pazopanib with or without gemcitabine in patients with anthracycline- and/or ifosfamide-refractory soft tissue sarcoma: final results of the PAPAGEMO phase 2 randomized clinical trial. JAMA Oncol (2021) 7(2):255–62. doi: 10.1001/jamaoncol.2020.6564
27. Gong T, Huang Q, Tang F, Wang Y, Li Z, Luo Y, et al. Activity and safety of apatinib monotherapy or apatinib combined with chemotherapy for patients with metastatic or unresectable osteosarcoma over the age of 40 years: A retrospective analysis. Front Oncol (2022) 12:1031787. doi: 10.3389/fonc.2022.1031787
28. Xie L, Xu J, Sun X, Li X, Liu K, Liang X, et al. Apatinib plus ifosfamide and etoposide for relapsed or refractory osteosarcoma: A retrospective study in two centres. Oncol Lett (2021) 22(1):552. doi: 10.3892/ol.2021.12813
29. Tian Z, Wang X, Liu Z, Wang J, Yao W, Zhao Y, et al. Safety and efficacy of combination therapy with apatinib and doxorubicin in metastatic soft tissue sarcomas: an observational study from multiple institutions. Cancer Manag Res (2019) 11:5293–300. doi: 10.2147/CMAR.S207150
30. Shao F, Zhang H, Yang X, Luo X, Liu J. Adverse events and management of apatinib in patients with advanced or metastatic cancers: A review. Neoplasma (2020) 67(4):715–23. doi: 10.4149/neo_2020_190801N701
31. Zheng Y, Yang X, Yan C, Feng R, Sah BK, Yang Z, et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: A single-arm, open-label, phase II trial. Eur J Cancer (2020) 130:12–9. doi: 10.1016/j.ejca.2020.02.013
32. Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, et al. NCCN guidelines insights: bone cancer, version 2.2017. J Natl Compr Canc Netw (2017) 15(2):155–67. doi: 10.6004/jnccn.2017.0017
33. Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer (2019) 109:36–50. doi: 10.1016/j.ejca.2018.11.027
34. Guenther LM, Rowe RG, Acharya PT, Swenson DW, Meyer SC, Clinton CM, et al. Response Evaluation Criteria in Solid Tumors (RECIST) following neoadjuvant chemotherapy in osteosarcoma. Pediatr Blood Cancer (2018) 65(4):e26896. doi: 10.1002/pbc.26896
35. Lin P, Yang P-F, Chen S, Shao Y-Y, Xu L, Wu Y, et al. A Delta-radiomics model for preoperative evaluation of Neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imaging (2020) 20(1):7. doi: 10.1186/s40644-019-0283-8
Keywords: apatinib, doxorubicin, cisplatin, osteosarcoma, neoadjuvant therapy, adverse events
Citation: Wang J, Zhang F, Dong S, Yang Y, Gao F, Liu G, Zhang P, Wang X, Du X and Tian Z (2023) Apatinib plus chemotherapy for non-metastatic osteosarcoma: a retrospective cohort study. Front. Oncol. 13:1227461. doi: 10.3389/fonc.2023.1227461
Received: 23 May 2023; Accepted: 30 October 2023;
Published: 13 November 2023.
Edited by:
Hong Duan, Sichuan University, ChinaReviewed by:
Yong Zhou, Sichuan University, ChinaAlessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Copyright © 2023 Wang, Zhang, Dong, Yang, Gao, Liu, Zhang, Wang, Du and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhichao Tian, dGlhbnpoaWNoYW95eUAxNjMuY29t
 Jiaqiang Wang
Jiaqiang Wang Fan Zhang1
Fan Zhang1 Zhichao Tian
Zhichao Tian