
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 September 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1226366
This article is part of the Research Topic Clinical and Translational Research in Prostate Cancer - Volume II View all 6 articles
 Dae Hyuk Chung1
Dae Hyuk Chung1 Jang Hee Han1,2
Jang Hee Han1,2 Seung-Hwan Jeong1,2
Seung-Hwan Jeong1,2 Hyeong Dong Yuk1,2
Hyeong Dong Yuk1,2 Chang Wook Jeong1,2
Chang Wook Jeong1,2 Ja Hyeon Ku1,2
Ja Hyeon Ku1,2 Cheol Kwak1,2*
Cheol Kwak1,2*Objective: Lymphatic invasion in prostate cancer is associated with poor prognosis. However, there is no consensus regarding the clinical and prognostic value of lymphatic invasion. This study aimed to investigate the prognostic value of lymphatic invasion in biochemical recurrence (BCR) and compare the recurrence rates between patients with lymphatic invasion and lymph node metastasis.
Methods: We retrospectively analyzed 2,207 patients who underwent radical prostatectomy (RP) without pelvic lymph node dissection (PLND) and 742 patients who underwent RP with PLND for clinically localized or locally advanced prostate cancer, between 1993 and 2020, at Seoul National University Hospital. Kaplan–Meier analysis was performed to estimate BCR-free survival (BCRFS) using the log-rank test. The Cox proportional hazards model was used to identify the significant factors for BCR. Propensity score matching was performed with a 1:2 ratio to match age, initial PSA level, pathological T stage, and Gleason score to exclude confounding effects.
Results: Of the 2,207 patients who underwent RP without PLND, lymphatic invasion (L1Nx) was observed in 79 (3.5%) individuals. Among the 742 patients who underwent RP with PLND, lymph node metastases were found in 105 patients (14.2%). In patients with lymph node metastasis, lymphatic invasion was observed in 50 patients (47.6%), whereas lymphatic invasion was observed in 53 patients (8.3%) among those without lymph node metastasis. In patients who underwent RP without PLND, Kaplan–Meier analysis showed significantly poorer BCR-free survival in the L1Nx group than in the L0Nx group (p < 0.001). In patients who underwent RP with PLND, the L1N0, L0N1, and L1N1 groups showed significantly worse prognoses than the L0N0 group (p < 0.001). However, there was no significant difference in BCRFS between the L1N0 and lymph node metastasis groups, including the L0N1 and L1N1 groups. After propensity score matching at a 1:2 ratio, the L1Nx group showed significantly poorer outcomes in terms of BCRFS than the L0Nx group (p = 0.05). In addition, the L1N0 group showed a significantly worse prognosis than the L0N0 group after propensity score matching.
Conclusion: Lymphatic invasion in radical prostatectomy specimens is an independent prognostic factor, which can complement lymph node status for predicting biochemical recurrence. Considering lymphatic invasion as an adverse pathological finding, similar to lymph node metastasis, adjuvant therapy could be considered in patients with lymphatic invasion.
Lymph nodes near the primary cancer are considered the first site of metastasis (1, 2), and the existence of lymph node metastasis is known to be a crucial prognostic factor in many cancers (3–5). Patients with prostate cancer with lymph node metastasis have a poor prognosis (6, 7). Several retrospective studies have demonstrated that pathological N1 stage (N1) after radical prostatectomy (RP) with pelvic lymph node dissection (PLND) is a risk factor for recurrence (8, 9). To reduce relapse in patients with N1 disease after RP with PLND, current guidelines suggest androgen deprivation therapy with or without radiation therapy after surgery (10, 11).
Similar to that in other cancers, lymphatic invasion in prostate cancer is associated with lymph node metastasis (12). Several retrospective studies have revealed that lymphatic invasion (L1) in radical prostatectomy specimens is a risk factor for biochemical recurrence (BCR) (13, 14). Therefore, the International Collaboration on Cancer Reporting (ICCR) recommends providing information regarding the presence of lymphovascular invasion in radical prostatectomy specimens (15).
Although L1 is associated with poor prognosis in prostate cancer, there is no definite consensus on the clinical and prognostic value of lymphatic invasion. The aim of our study was to evaluate the prognostic value of L1 and determine whether L1 can be a complement or substitute for lymph node status in predicting recurrence.
This study was approved by the Institutional Review Board (IRB No. H-305-041-1430) of Seoul National University Hospital. The requirement for informed consent was waived due to the retrospective nature of the study. All the research and related protocols used in this study were performed in accordance with the principles of the Declaration of Helsinki.
We conducted a retrospective study of 2,207 patients who underwent RP without PLND and 742 patients who underwent RP with PLND for clinically localized or locally advanced prostate cancer between 1993 and 2020 at the Seoul National University Hospital. The clinical data of these patients were acquired retrospectively by reviewing their electronic medical records. All surgeries were performed by several surgeons at a single center using one of the following techniques: open, laparoscopic, or robot-assisted laparoscopy. PLND was performed according to risk stratification or clinical stage, and all PLND was performed in standard extent.
All tumor-containing blocks were analyzed by experienced genitourinary pathologists according to standard procedures, including complete embedding of the entire prostate. The histopathological findings from the resected specimens included Gleason score, pathological T stage, pathological N stage, lymphatic invasion, venous invasion, and perineural invasion. Unlike other institutes that only report lymphovascular invasion from specimens, we separately analyzed lymphatic and venous invasions.
We retrospectively collected clinical data from patients, including age, Eastern Cooperative Oncology Group (ECOG) performance status grade, and initial serum prostate-specific antigen (PSA) level, which was defined as the PSA value just before prostate biopsy. Postoperative serum PSA levels were measured every 3 months, 2 years after surgery, and every 6 months thereafter. BCR was defined as a detectable or rising PSA ≥0.2 ng/ml, followed by a confirmatory PSA of ≥0.2 ng/ml. All patients with PSA persistence after radical prostatectomy were excluded from the study. Adjuvant radiation therapy or adjuvant androgen deprivation therapy (ADT) was administered to patients with adverse pathological factors, including positive surgical margins and N1 stage.
All continuous variables were described as mean ± standard deviation, whereas categorical variables were described as frequency (percentage). Categorical variables were analyzed using chi-square tests and numerical variables were compared using a two-tailed Student’s t-test. Kaplan–Meier analysis was performed to estimate BCR-free survival using the log-rank test for significance assessment.
To verify the independence of lymphatic invasion as a factor for BCR, propensity score matching was performed with a 1:2 ratio to match age, initial PSA level, pathological T stage, and Gleason score to exclude confounding effects. The Cox proportional hazards model was used to identify significant factors for BCR. All statistical analyses were performed using XLSTAT (version 2021.5-Life Sciences). Statistical significance was set at p < 0.05.
In total, 2,207 patients underwent RP without PLND, and lymphatic invasion (L1Nx) was observed in 79 patients (3.5%). Table 1 compares the clinicopathological features between with lymphatic invasion (L1Nx) and without lymphatic invasion (L0Nx) in patients who underwent RP without PLND. The initial serum PSA level was significantly higher in the L1Nx group than in the L0Nx group (13.89 versus 9.28, p < 0.0001). Furthermore, the pathological T stage and Gleason score tended to be higher in L1Nx patients, indicating adverse pathological features (p < 0.0001). Venous invasion, perineural invasion, and positive surgical margin were also higher in the L1Nx group than in the L0Nx group (p < 0.0001). Adjuvant radiation therapy was administered to eight patients (0.4%) in the L0Nx group, but none in the L1Nx group. Adjuvant ADT was given to 41 patients (1.9%) in the L0Nx group but four patients (5.1%) in the L1Nx group. However, there was no significant difference in the proportion of adjuvant therapy between L0Nx and L1Nx group.
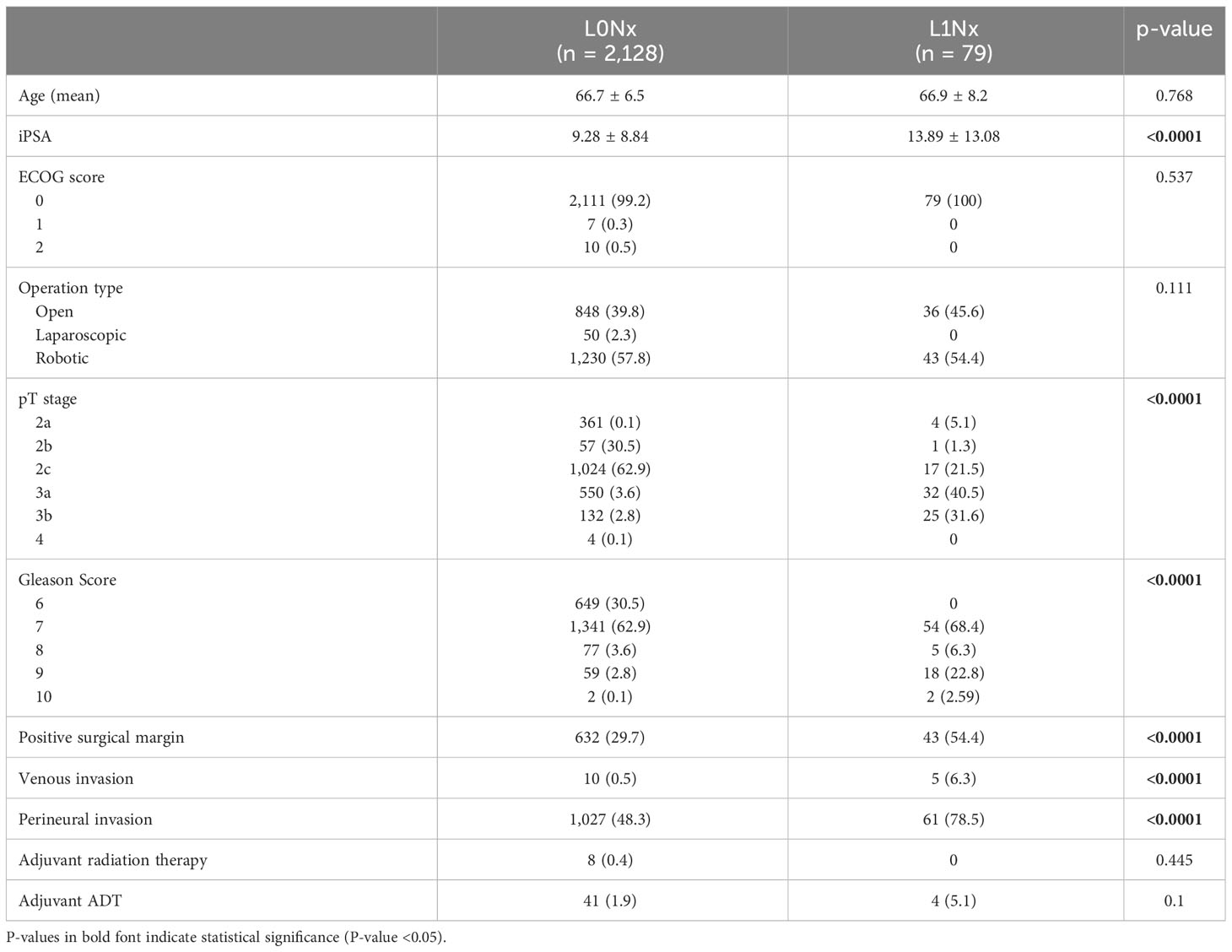
Table 1 Clinicopathological data between with lymphatic invasion (L1Nx) and without lymphatic invasion (L0Nx) in patients who underwent radical prostatectomy (RP) without pelvic lymph node dissection (PLND).
Among the 742 patients who underwent RP with PLND, lymph node metastases were found in 105 patients (14.2%). In patients with lymph node metastasis, lymphatic invasion was observed in 50 patients (47.6%), whereas lymphatic invasion was observed in 53 patients (8.3%) among those without lymph node metastasis. Table 2 shows a comparison of clinicopathological features in pN0 patients between with lymphatic invasion (L1N0) and without lymphatic invasion (L0N0). The mean age was significantly higher in the L1N0 group than that in the L0N0 group (66.3 versus 68.3 years, p = 0.038). As shown in Table 2, the initial serum PSA level, pathological T stage, Gleason score, venous invasion, perineural invasion, and positive surgical margin in the L1N0 group were also significantly higher in the L1N0 group compared with L0N0 group (all p < 0.001). Adjuvant radiation therapy was given to nine patients (1.5%) in the L0N0 group but none in the L1N0 group. Adjuvant ADT was administered to 16 patients (2.7%) in the L0N0 group but three patients (5.7%) in the L1N0 group. However, there was also no significant difference in the proportion of adjuvant therapy between L0N0 and L1N0 group. Table 3 shows a comparison of clinicopathological features among the L1N0, L0N1, and L1N1 groups. There were significant differences only in the initial serum PSA level (p = 0.002) and the number of patients who received adjuvant ADT (p = 0.012).
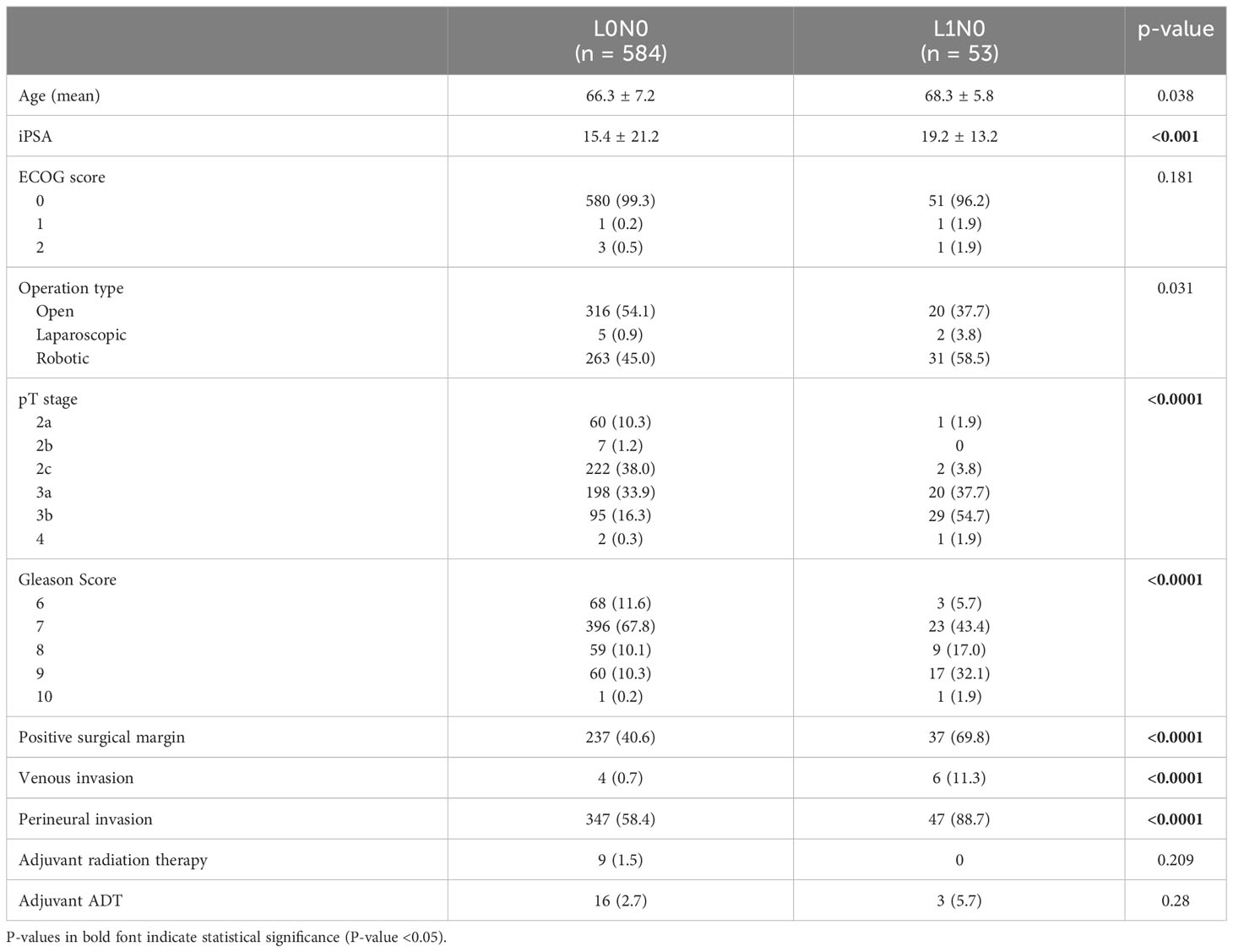
Table 2 Clinicopathological data between with lymphatic invasion (L1N0) and without lymphatic invasion (L0N0) in pathological node-negative patients who underwent RP with PLND.
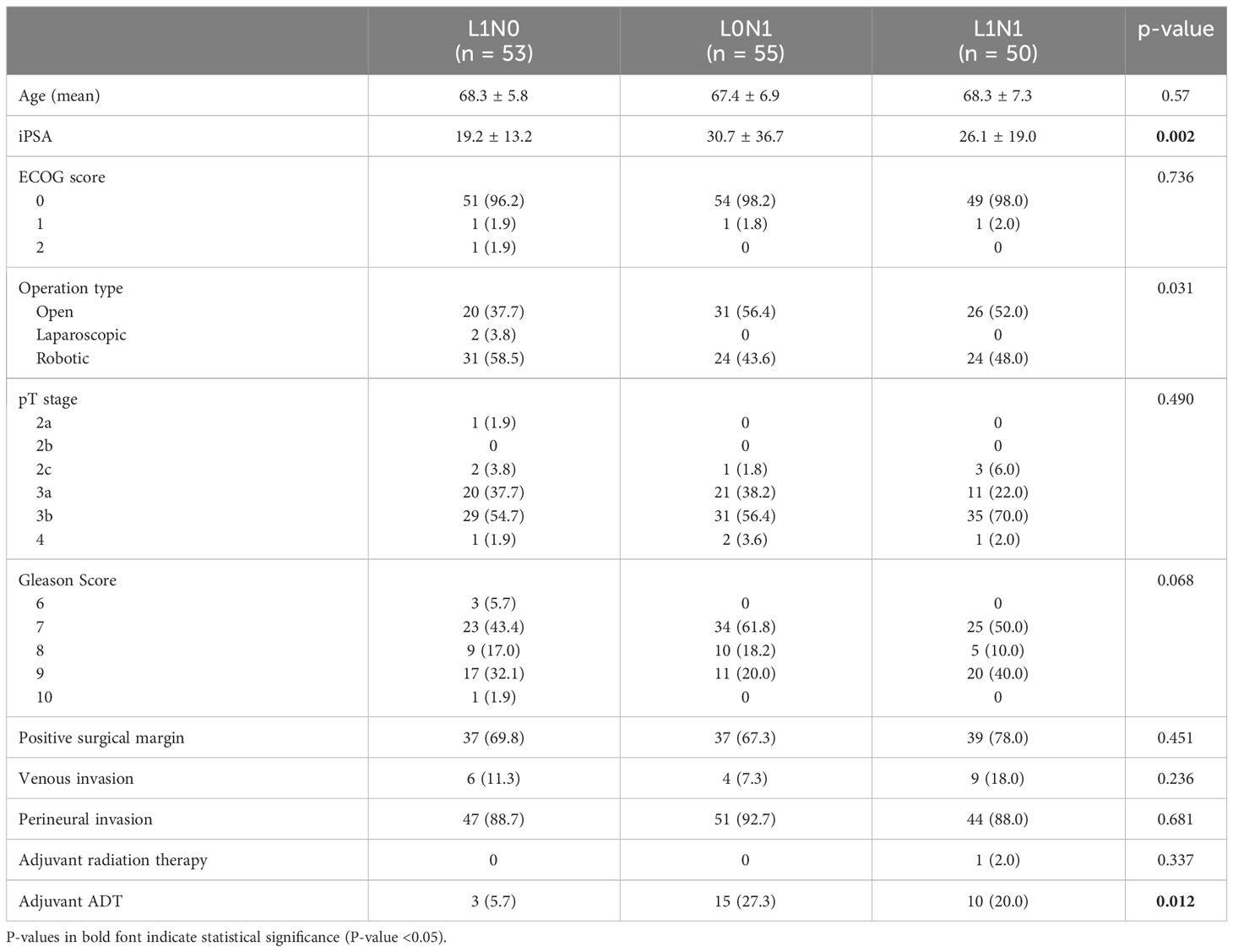
Table 3 Clinicopathological data among the L1N0, L0N1, and L1N1 group in patients who underwent RP with PLND.
Figure 1 shows a comparison of biochemical recurrence-free survival (BCRFS) according to the presence or absence of lymphatic invasion after RP without PLND. The L1Nx group showed significantly worse BCRFS than the L0Nx group (p < 0.001).
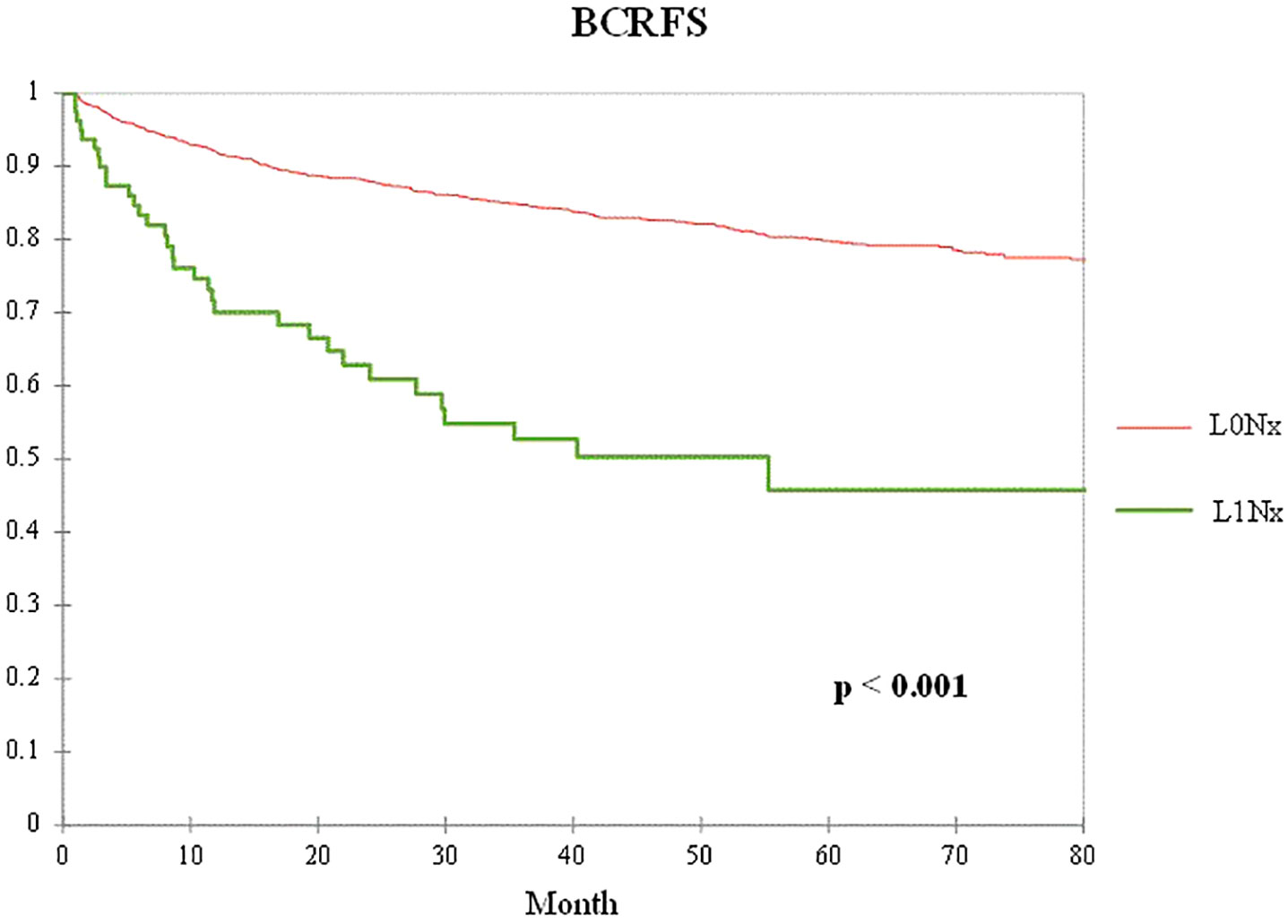
Figure 1 Kaplan–Meier curve of the biochemical recurrence-free survival (BCRFS) between the L0Nx group (red line) and the L1Nx group (green line) in patients who underwent radical prostatectomy (RP) without pelvic lymph node dissection (PLND).
Figure 2 shows BCFRS according to the combination of pathological lymph node metastasis and lymphatic invasion status in patients who underwent RP with PLND. The L1N0, L0N1, and L1N1 groups had significantly worse prognoses than the L0N0 group (p < 0.001). However, there was no significant difference in BCRFS between the L1N0 and lymph node metastasis groups, including the L0N1 and L1N1 groups.
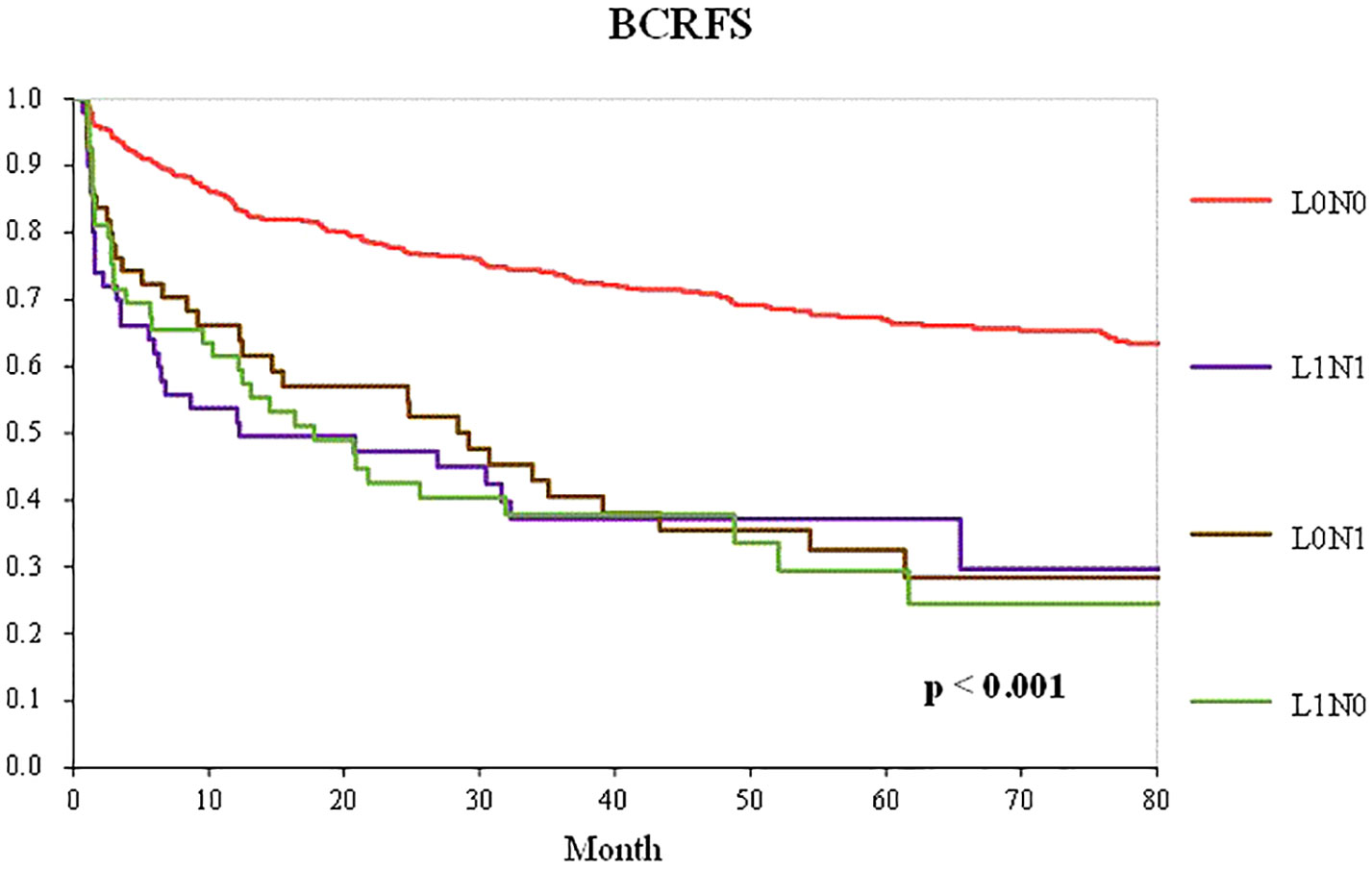
Figure 2 Kaplan–Meier curve of the BCRFS according to the combination of pathological lymph node metastasis and lymphatic invasion status in patients who underwent RP with PLND (L0N0 group—red line; LINO group—green line; LONI group—brown line; LIN1 group—purple line).
According to the multivariate Cox regression analysis of BCRFS between the L0N0 group and the L1N0 group, lymphatic invasion was identified as a significant predictor of BCR (hazard ratio [HR], 1.64; 95% confidence interval [CI], 1.21–2.02). Moreover, other covariates, including a high Gleason score, pathological T stage, and positive surgical margin also showed a detrimental prognostic impact on BCR (Table 4).
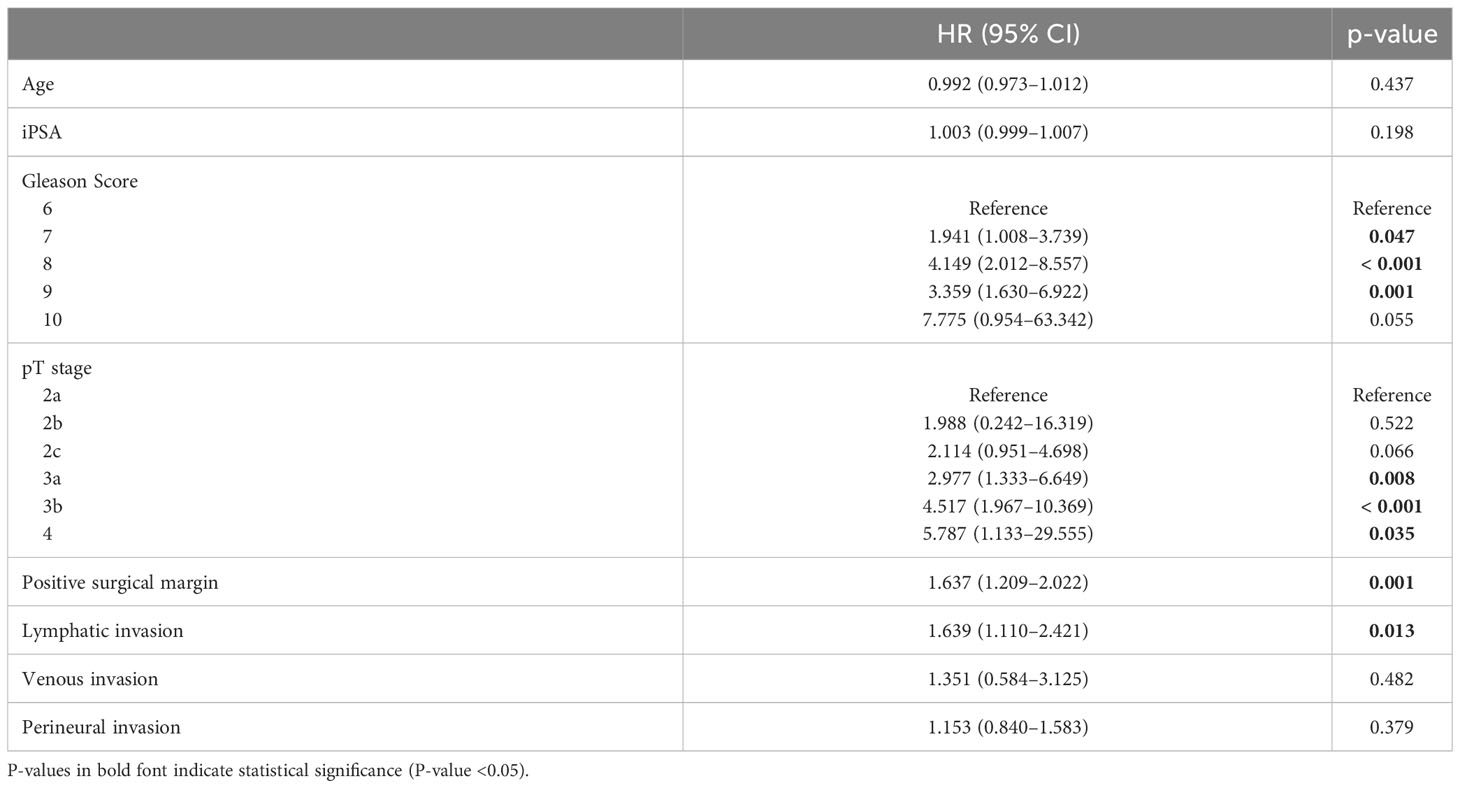
Table 4 Multivariable Cox regression analysis predicting BCR between L0N0 group and L1N0 group in patients who underwent RP with PLND.
To control for the influence of age, initial serum PSA level, pathological T stage, and Gleason score in patients who underwent RP without PLND, 79 patients in the L1Nx group were matched in a 1:2 ratio with 2,131 patients in the L0Nx group. After propensity score matching, the clinicopathological characteristics of the two groups were well-balanced, with no significant differences among all variables, except perineural invasion (Table 5). Figure 3 shows the Kaplan–Meier curve of the BCFRS between the L0Nx and L1Nx groups after propensity score matching at a 1:2 ratio. Even after propensity score matching, the L1Nx group showed significantly poorer BCRFS than the L0Nx group (p = 0.05).
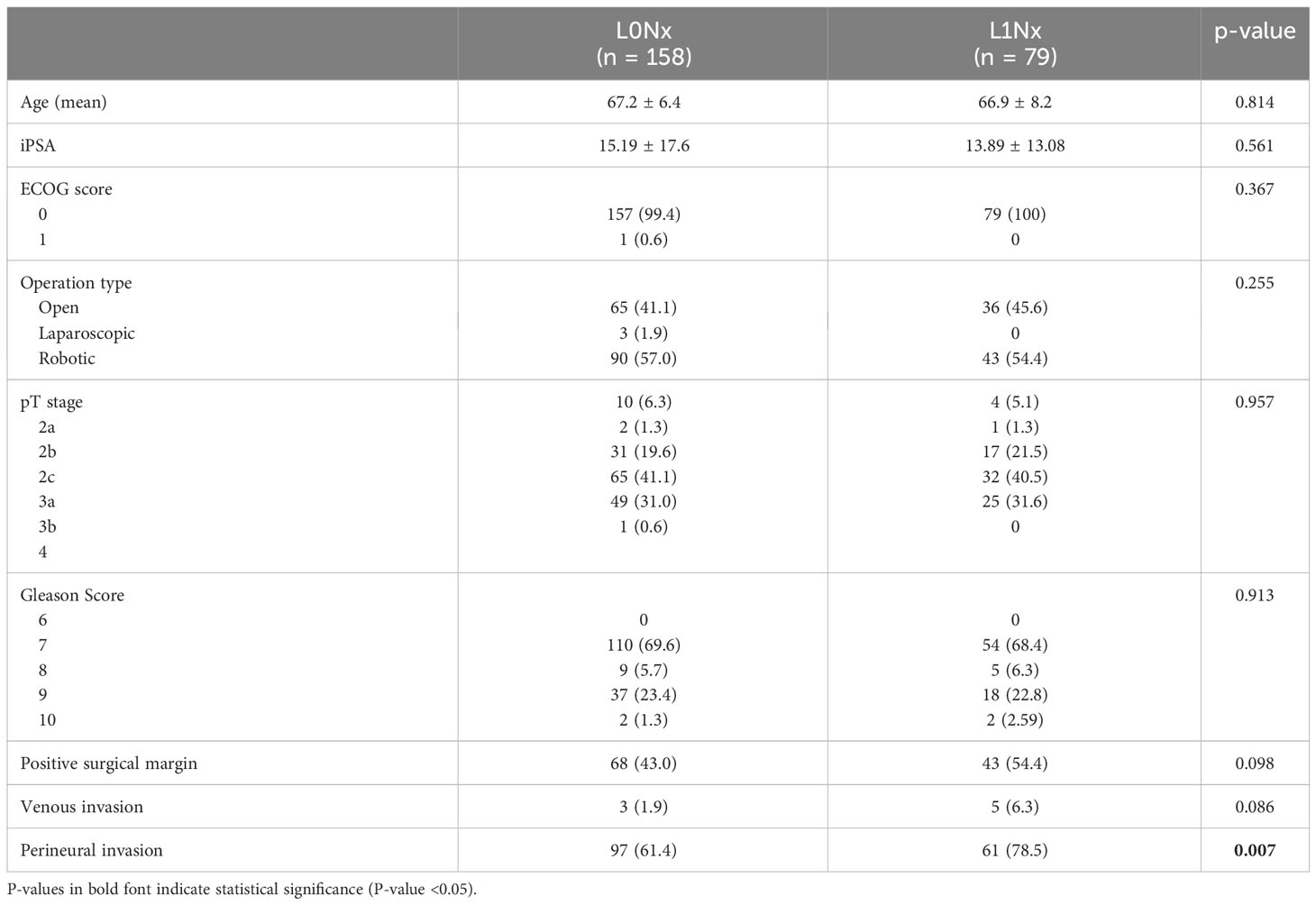
Table 5 Comparison of baseline variables for the between the L0Nx group and the L1Nx group after 1:2 propensity score matching in patients who underwent RP without PLND.
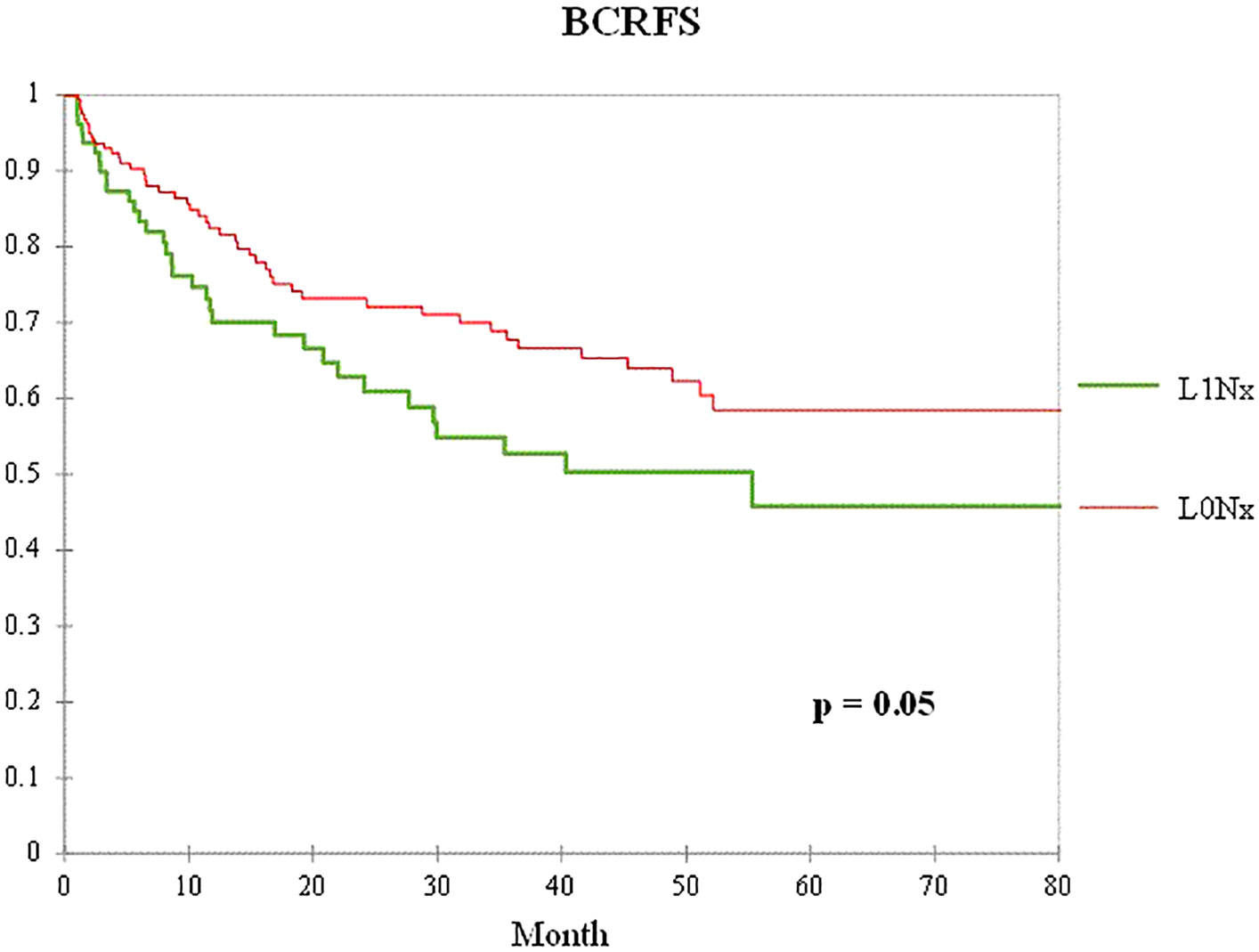
Figure 3 Kaplan–Meier curve of the BCRFS between the L0Nx group (red line) and the L1Nx group (green line) after 1:2 propensity score matching in patients who underwent RP without PLND.
Among the patients who underwent radical prostatectomy with pelvic lymph node dissection, propensity score matching was performed in a 1:2 ratio between 53 patients in the L1N0 group and 584 patients in the L0N0 group. The baseline variables were also well-balanced, with no significant differences among the other variables except for venous invasion (Table 6). Figure 4 shows the Kaplan–Meier curve of the BCFRS after propensity score matching between the L0N0 and L1N0 groups. The L1N0 group showed a significantly worse prognosis than the L0N0 group after propensity score matching (p = 0.009).

Table 6 Comparison of baseline variables for between the L0N0 group and the L1N0 group after 1:2 propensity score matching in patients who underwent RP with PLND.
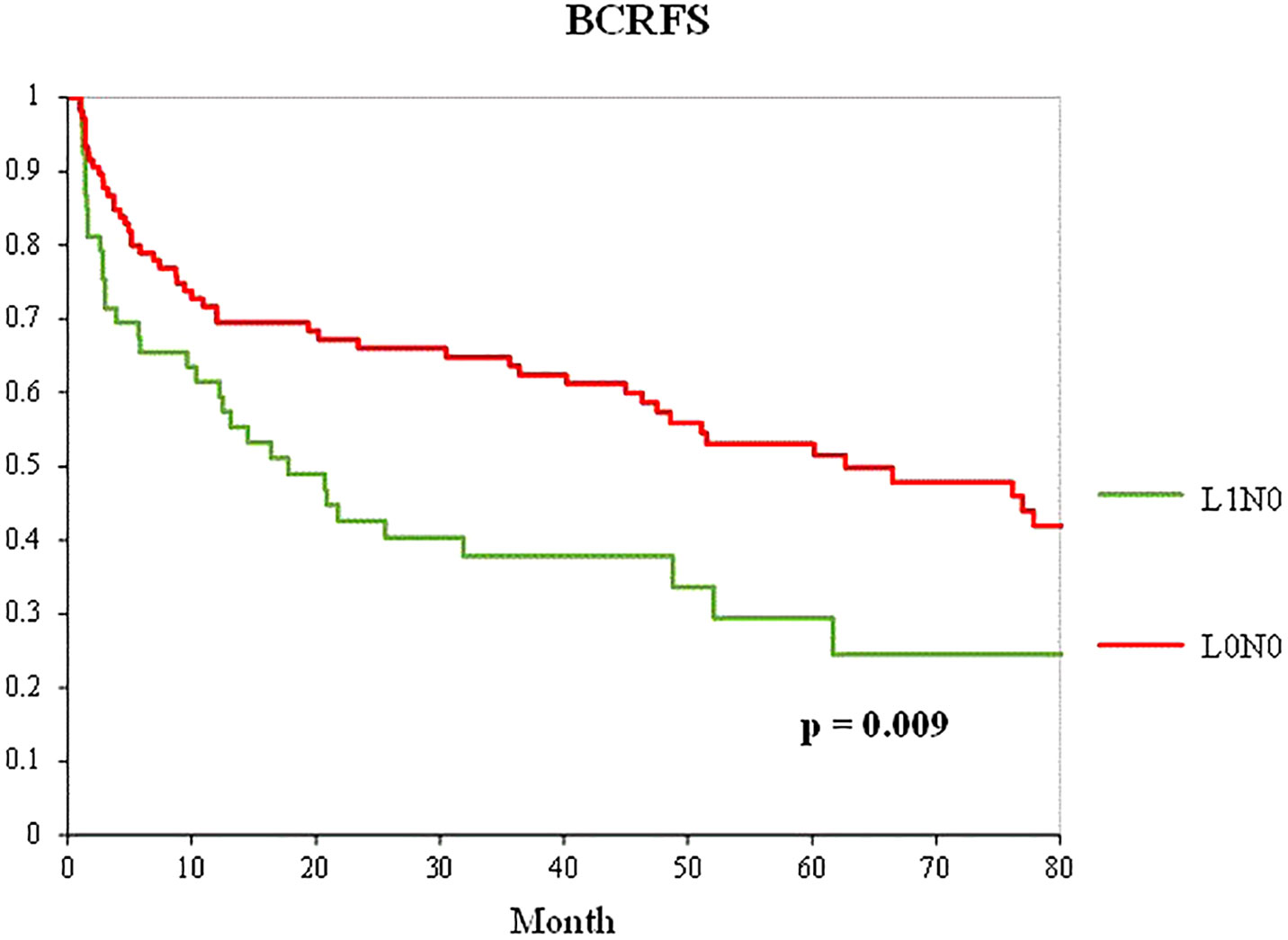
Figure 4 Kaplan–Meier curve of the BCRFS between the L0N0 group (red line) and the L1N0 (green line) group after 1:2 propensity score matching in patients who underwent RP with PLND.
In the present study, we analyzed the risk of BCR in the presence of lymphatic invasion among patients who underwent RP without PLND. We identified significant differences in BCRFS between the L0Nx and L1Nx groups before and after propensity score matching. Second, we evaluated the effects of lymphatic invasion on BCR with respect to lymph node metastasis in patients who underwent RP with PLND. The lymphatic invasion without lymph node metastasis (L1N0) group showed similar BCRFS to the pN1 group in the Kaplan–Meier curve. Furthermore, after propensity score matching between the L0N0 group and the L1N0 group, lymphatic invasion was the only significant predictor of BCR. BCRFS also showed a significant difference between the two groups after propensity score matching.
Most previous studies have demonstrated the prognostic impact of lymphovascular invasion in prostate cancer, which integrates both lymphatic and venous invasion. In the present study, we separated lymphatic and venous invasions from lymphovascular invasion. A few recent studies have analyzed the prognostic impact of lymphatic invasion alone. Wilczak et al. retrospectively examined 14,528 patients who underwent radical prostatectomy and studied the prognostic value of lymphatic invasion (16). It was concluded that lymphatic invasion provides prognostic information comparable to that of lymph node status. However, there was a huge gap in the number of patients between the two groups, and no balance of covariates was achieved between them. Thus, we performed propensity score matching to overcome the limitations of this retrospective observational study. Yamashita et al. prospectively analyzed 183 consecutive patients with high-risk prostate cancer who underwent robot-assisted radical prostatectomy with extended lymph node dissection and showed that lymphatic invasion was an independent significant predictor of BCR (17). Despite the advantages of this prospective study, the sample size was small, and the follow-up period after surgery was relatively short.
Although propensity score matching performed well, we obtained one question about our results. Perineural (Table 5), venous (Table 6), and lymphatic invasion also showed significant differences. Several studies have demonstrated that perineural invasion in prostate cancer is an independent prognostic factor (18, 19); however, to the best of our knowledge, no studies have investigated the prognostic significance of venous invasion alone. There may be a correlation between perineural, venous, and lymphatic invasion; hence, further studies are required to validate this association.
This study has some limitations. First, we retrospectively analyzed the patients. Second, several surgeons performed the radical prostatectomies. Third, clinical data were obtained from a single center. Fourth, we did not incorporate pathological variants such as the cribriform pattern and intraductal carcinoma of the prostate in our study. Fifth, the number of patients received adjuvant treatment is too few to be used as accurate factor. To overcome these limitations, a randomized prospective trial including pathological variants is required to verify the results of the present study.
Lymphatic invasion in radical prostatectomy specimens is an independent prognostic factor, which can complement lymph node status for predicting biochemical recurrence. Considering lymphatic invasion as an adverse pathologic finding, similar to lymph node metastasis, adjuvant therapy could be considered in patients with lymphatic invasion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Institutional Review Board (IRB No. H-2305-041-1430) of Seoul National University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective nature of the study.
Conceptualization: DC. Data collection: DC, S-HJ, JH, and HY. Data analysis DC, S-HJ, and CK. Data visualization: DC and S-HJ. Data interpretation: DH, S-HJ, JH, HY, CJ, JK, and CK. Manuscript writing: DC. Supervision: DC, S-HJ, and CK. All authors contributed to the article and approved the submitted version.
This study was supported by Seoul National University Hospital.
We would like to thank Editage (www.editage.co.kr) for editing and reviewing this manuscript for English language.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Van Trappen PO, Pepper MS. Lymphatic dissemination of tumour cells and the formation of micrometastases. Lancet Oncol (2002) 3:1. doi: 10.1016/s1470-2045(01)00621-0
2. Sleeman JP. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res (2002) 157:55–81. doi: 10.1007/978-3-642-57151-0_6
3. de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest ,PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med (2009) 361:7. doi: 10.1056/NEJMoa0904832
4. Gunji Y, Suzuki T, Hori S, Hayashi H, Matsubara H, Shimada H, et al. Prognostic significance of the number of metastatic lymph nodes in early gastric cancer. Dig Surg (2003) 20:2. doi: 10.1159/000069392
5. Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol (2006) 24:22. doi: 10.1200/JCO.2006.06.8866
6. Cheng L, Zincke H, Blute ML, Bergstralh EJ, Scherer B, Bostwick DG. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer (2001) 91:1. doi: 10.1002/1097-0142(20010101)91:1<66::aid-cncr9>3.0.co;2-p
7. Bernstein AN, Shoag JE, Golan R, Halpern JA, Schaeffer EM, Hsu WC, et al. Contemporary incidence and outcomes of prostate cancer lymph node metastases. J Urol. (2018) 199:6. doi: 10.1016/j.juro.2017.12.048
8. Touijer KA, Mazzola CR, Sjoberg DD, Scardino PT, Eastham JA. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur Urol. (2014) 65:1. doi: 10.1016/j.eururo.2013.03.053
9. Marra G, Valerio M, Heidegger I, Tsaur I, Mathieu R, Ceci F, et al. Management of patients with node-positive prostate cancer at radical prostatectomy and pelvic lymph node dissection: A systematic review. Eur Urol Oncol (2020) 3:5. doi: 10.1016/j.euo.2020.08.005
10. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol (2006) 7:6. doi: 10.1016/S1470-2045(06)70700-8
11. Gupta M, Patel HD, Schwen ZR, Tran PT, Partin AW. Adjuvant radiation with androgen-deprivation therapy for men with lymph node metastases after radical prostatectomy: identifying men who benefit. BJU Int (2019) 123:2. doi: 10.1111/bju.14241
12. Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol (2006) 19:3. doi: 10.1038/modpathol.3800546
13. Yee DS, Shariat SF, Lowrance WT, Maschino AC, Savage CJ, Cronin AM, et al. Prognostic significance of lymphovascular invasion in radical prostatectomy specimens. BJU Int (2011) 108:4. doi: 10.1111/j.1464-410X.2010.09848.x
14. Cheng L, Jones TD, Lin H, Eble JN, Zeng G, Carr MD, et al. Lymphovascular invasion is an independent prognostic factor in prostatic adenocarcinoma. J Urol. (2005) 174(6):2181–5. doi: 10.1097/01.ju.0000181215.41607.c3
15. Kench JG, Judge M, Delahunt B, Humphrey PA, Kristiansen G, Oxley J, et al. Dataset for the reporting of prostate carcinoma in radical prostatectomy specimens: Updated recommendations from the International Collaboration on Cancer Reporting. Virchows Ar.ch. (2019) 475:3. doi: 10.1007/s00428-019-02574-0
16. Wilczak W, Wittmer C, Clauditz T, Minner S, Steurer S, Büscheck F, et al. Marked prognostic impact of minimal lymphatic tumor spread in prostate cancer. Eur Urol. (2018) 74(3):376–86. doi: 10.1016/j.eururo.2018.05.034
17. Yamashita S, Muraoka S, Wakamiya T, Kikkawa K, Kohjimoto Y, Hara I. Prognostic impact of lymphatic invasion in patients with high-risk prostate cancer after robot-assisted radical prostatectomy and extended lymph node dissection: A single-institution prospective cohort study. Cancers (Basel). (2022) 14:14. doi: 10.3390/cancers14143466
18. Zhang LJ, Wu B, Zha ZL, Qu W, Zhao H, Yuan J, et al. Perineural invasion as an independent predictor of biochemical recurrence in prostate cancer following radical prostatectomy or radiotherapy: A systematic review and meta-analysis. BMC Urol. (2018) 18:1. doi: 10.1186/s12894-018-0319-6
Keywords: prostate cancer, lymphatic invasion, lymph node metastasis, biochemical recurrence, radical prostatectomy
Citation: Chung DH, Han JH, Jeong S-H, Yuk HD, Jeong CW, Ku JH and Kwak C (2023) Role of lymphatic invasion in predicting biochemical recurrence after radical prostatectomy. Front. Oncol. 13:1226366. doi: 10.3389/fonc.2023.1226366
Received: 25 May 2023; Accepted: 21 August 2023;
Published: 11 September 2023.
Edited by:
Sifeng Qu, Shandong University, ChinaReviewed by:
Yoichiro Okubo, Kanagawa Cancer Center, JapanCopyright © 2023 Chung, Han, Jeong, Yuk, Jeong, Ku and Kwak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheol Kwak, bWRyYWZhZWxAc251LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.