
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 04 September 2023
Sec. Cancer Epidemiology and Prevention
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1224753
Background: Epidemiological evidence suggests an association between lifestyle habits (smoking, alcohol consumption, tea, coffee intake, etc.) and gastric cancer (GC). However, the causal relationship remains uncertain. Therefore, the purpose of this study was to ascertain whether there is a causal connection between them.
Methods: Two-sample Mendelian randomization (MR) analysis was performed using the publicly available Genome Wide Association Study summary datasets using six methods: inverse variance weighting (IVW), weighted median, MR using a Robust Adjusted Profile Score (MR.Raps), MR using a Robust Adjusted Profile Score (MR-PRESSO), Radial regression of MR, and Causal Analysis Using Summary Effect Estimates (CAUSE). A sensitivity analysis was conducted to assess the robustness of the results.
Results: In an East Asian population, we found that increased tea intake reduced the risk of GC [odds ratio (OR)= 0.90, 95% confidence interval (CI)= 0.82-0.99, P = 0.037] while there was a positive association between smoking and GC (OR = 1.58, 95% CI = 1.04-2.39, P = 0.032). No causal relationship between alcohol and coffee intake and GC. Sensitivity analyses demonstrated the robustness of these causal associations.
Conclusions: Our study suggests that tea intake may reduce the risk of GC, for which smoking is a potential risk factor. Nevertheless, a larger and more diverse sample size is needed for further validation.
According to the International Agency for Research on Cancer’s (IARC) 2020 findings, gastric cancer (GC), a highly aggressive and lethal malignancy, is the fourth most prevalent cause of cancer mortality globally and the fifth most common cancer overall (1–3). According to statistics (3), there were 479,000 new cases of gastric cancer and 374,000 deaths in China in 2020, accounting for 44.0% and 48.6% of new gastric cancer cases and deaths worldwide, respectively. The prevalence of cancer screening and improvements in food hygiene have led to a gradual decline in the incidence and mortality of GC over the past half-century. However, in recent years, the incidence of GC has rebounded in some countries due to the disruption of the gastric microbiological environment as a result of the inappropriate use of antibiotics and acid inhibitors (4, 5). Due to the lack of clinical signs and symptoms of early GC, most patients are in the middle or late stages at the time of diagnosis. The high cost of clinical treatment, poor prognosis, and short survival period add to the disease burden of GC. Therefore, the study of its risk factors is crucial for the prevention and treatment of gastric cancer.
Currently, various factors have been reported that may correlate with the development of GC. In addition to inflammation (6), infection (7), environmental, immune, and genetic factors (8), lifestyle factors may also influence lifetime cancer risk (9). In observational studies, smoking (10), alcohol consumption (11), coffee (12), and tea intake (13, 14) have been identified as factors associated with GC. Nevertheless, as a result of inconsistent findings and potential biases (15, 16), including residual confounding, reverse causality, and misclassification, it remains unclear whether these associations between lifestyle habits are causal.
Mendel’s second law states that genotypes are fixed before illness onset and alleles are randomly assigned to progeny gametes at the time of gamete production. In addition, as a result of genetic variation being used as an exposure tool (e.g., smoking, alcohol consumption, coffee, and tea intake), Mendelian randomization (MR) studies can improve causal inference by reducing residual confounding and reverse causality (17–19). Here, we conducted an MR study to explore genetically predicted associations between smoking, alcohol, coffee, and tea intake with gastric cancer-related phenotypes.
We downloaded publicly available abstract-level genome-wide association study (GWAS) datasets from the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol (https://gwas.mrcieu.ac.uk/). We conducted two-sample bivariate MR analyses to assess the causal effects of lifestyle factors (smoking, alcohol, coffee, and tea intake) and GC.
We selected relevant phenotypes (smoking, alcohol, coffee, and tea intake) from European and East Asian populations as exposures to analyze their correlation with GC in East Asian populations, and thus distinguish whether this correlation is biased by population stratification. The genome-wide Association Study (GWAS) pooled statistics for GC included 34,652 subjects of East Asian descent (6,563 GC cases and 195,745 controls) (20). Table S1 shows detailed information on the data used. There were no raw data requirements for this MR study since we used publicly available or published data. Ethical approval for all GWAS studies was obtained from the appropriate institutional review board. Therefore, ethics committee approval is no longer required for this study.
When evaluating the causal connection between an exposure and an outcome in MR, genetic variant(s) are employed as IVs. The fundamental conditions for a genetic variant to satisfy an IV are summarized as follows: (1) the variation and exposure are connected; (2) the variation has no connection to any confounding factor in the exposure-outcome relationship; (3) with the possible exception of its relationship to the exposure, the variation has no effect on the result.
At the outset of the research design, single nucleotide polymorphisms (SNPs) (P < 5 × 10-5) that are strongly related with exposure were selected as IVs. We only used independent SNPs with r2 < 0.01 within a distance of 5,000 kb in order to remove the chain disequilibrium (LD) bias (21). Therefore, the default parameters of the TwoSampleMR package in R (physical distance threshold, 5,000 kb, r2 < 0.01) were set for aggregating the data and clipping possible LD genetic variation. Furthermore, we used the PhenoSCanner database, which includes a wealth of information on SNPs that may be associated with disease and phenotype (22), to exclude SNPs that were associated between lifestyle factors and GC. Finally, our analysis for palindromic SNPs was excluded those whose minor allele frequency (MAF) was less than 0.3 (23). In order to prevent weak instrumental variable bias, we calculated the F-statistics for each IV-SNP according to this formula: F= (β/SE)2 (24). IVs that had an F-statistic of less than 10 were regarded as weak instruments and were rejected.
Inverse variance weighted (IVW) analysis was employed as the primary approach after choosing the suitable SNPs for exposures, and we augmented this with the use of numerous MR techniques based on various IVs assumptions, including weighted median (WM) (25), MR using a Robust Adjusted Profile Score (MR.Raps) (26), MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) (27), Radial regression of MR (Radial MR) (28) and causal analysis using summary effects estimation (CAUSE) (29) as sensitivity analysis to validate the robustness of the main IVW estimates. IVW can force the intercept term in weighted linear regression to zero to determine the causal effect value, and all three assumptions must be met in order for the causal effect estimation to be unbiased. IVW can be used to integrate multiple SNPs to obtain consistent estimates of causal effect values (30). For WM, at least 50% of SNPs are effective as IVs, which can generate strong causal effect values (31). The MR-PRESSO method can be used to identify anomalous data points and generate revised estimates after deletion (32). MR-RAPS can make robust inferences when containing weak IVs (33). Radial MR identifies outliers and repeats the MR analysis after eliminating heterogeneous SNPs (28).
To improve the accuracy of causal effects, we additionally calculated Cochran’s Q statistic to quantify heterogeneity across causal estimates of all SNPs in the IVW technique. In order to evaluate the horizontal pleiotropy impact of IVs, MR-Egger regression was performed. MR-Egger can use the intercept term to evaluate pleiotropy. When the intercept term is equal to zero, the results of MR-Egger are consistent with IVW, proving the absence of horizontal pleiotropy (34). For selected IVs, we used the MR-PRESSO global test to examine whether there were any outliers with variant-specific causal estimates that were significantly different from the rest. Using the leave-one-plot function from the TwoSampleMR package in R, we carried out a leave-one-out analysis to further confirm the stability of the analytic results (24).
MR analysis stastistics were performed using the following R version 4.2.0 packages: “TwoSampleMR” (24, 35), “MR-PRESSO” (36), and “CAUSE” (37).
To prepare our analyses for investigating the effect of lifestyle habits on GC risk, we performed chain imbalance clustering, and palindromic SNPs. We additionally exclude SNPs related to confounding factors by searching for pleiotropic associations between the SNPs used and other traits in PhenoScanner (www.phenoscanner.medschl.cam.ac.uk), and did not find any such associations. Thereafter, 135, 66, 50, and 56 SNPs from East Asian populations and 86, 179, 119, and 151 SNPs from European populations were included as IVs for smoking, alcohol intake, coffee intake, and tea intake, respectively. The F-statistic indicated no bias due to weak instrumentation (F > 10, Table S2-9).
As shown in Figure 1, the IVW analysis showed no causal relationship between smoking [Odds ratio (OR) = 2.07, 95% confidence interval (CI) = 0.71-6.02, P = 0.180], alcohol consumption (OR = 1.05, 95% CI = 0.94-1.18, P = 0.359), coffee intake (OR = 1.27, 95% CI = 0.86-1.87, P = 0.227) and tea intake (OR = 0.86, 95% CI = 0.67-1.11, P = 0.251) and GC. There was no evidence of substantial heterogeneity (PQ > 0.05 in Cochran’s Q test and Pintercept> 0.05 in MR-Egger) or horizontal pleiotropy (Pglobal > 0.05 in MR-PRESSO global test) in these causal estimates when sensitivity analyses were performed (Table S10). The results of the analysis of scatterplots, forest plots, funnel plots are shown in Supplementary Figures S1–S3. Meanwhile, this causal effect cannot be attributed to any single IV, as indicated by the leave-one-out analysis (Supplementary Figure S4).
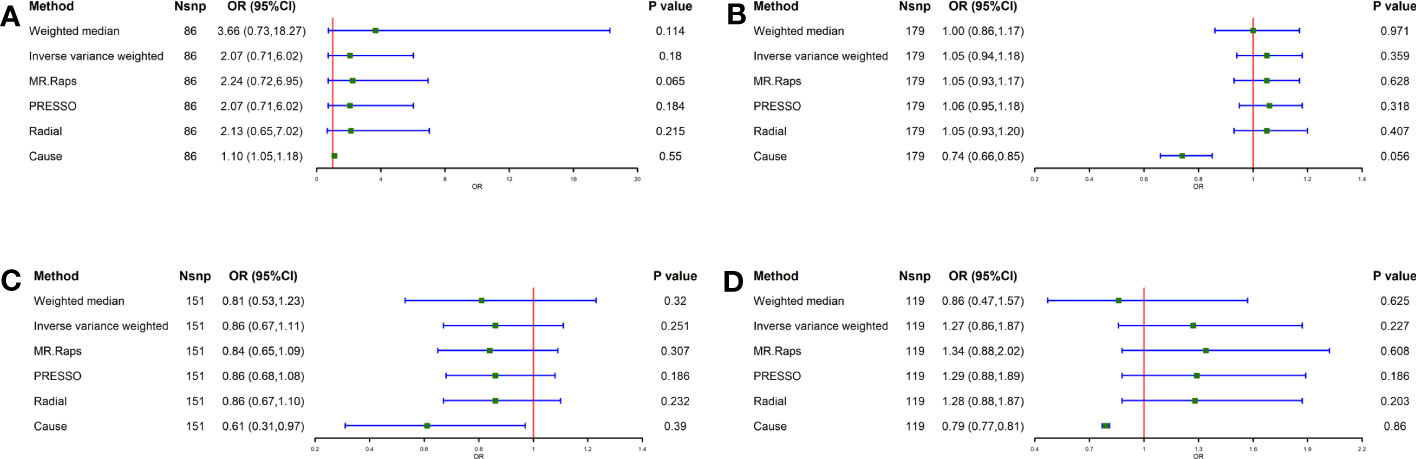
Figure 1 Causal effect of Lifestyle Factors in European on GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Tea intake and GC; (D) Coffee intake and GC; The green point means the effect (OR). GC, Gastric cancer.
To determine the correlation between lifestyle habits and GC, the IVW method showed a possible positive association between smoking and GC (OR = 1.58, 95% CI = 1.04-2.39, P = 0.032) and a negative association between tea intake and GC (OR = 0.90, 95% CI = 0.82-0.99, P = 0.037) (Figure 2). In addition, for alcohol consumption and coffee intake, no causal relationship was found between either and GC (alcohol consumption: P = 0.562, coffee intake: P = 0.623) (Figure 2). Furthermore, the MR-Egger and MR-PRESSO methodologies, as well as Cochran’s Q-statistic, show that there is no heterogeneity or pleiotropy in these studies (Table S11). The results of the scatterplot, forest plot, and funnel plot analyses are shown in Supplementary Figures S5–7, respectively. All figures are consistent with the results of the above analyses. No one SNP was found to have a significant impact on the overall outcome of GC for these causal associations, according to the leave-one-out sensitivity analysis (Supplementary Figure S8).
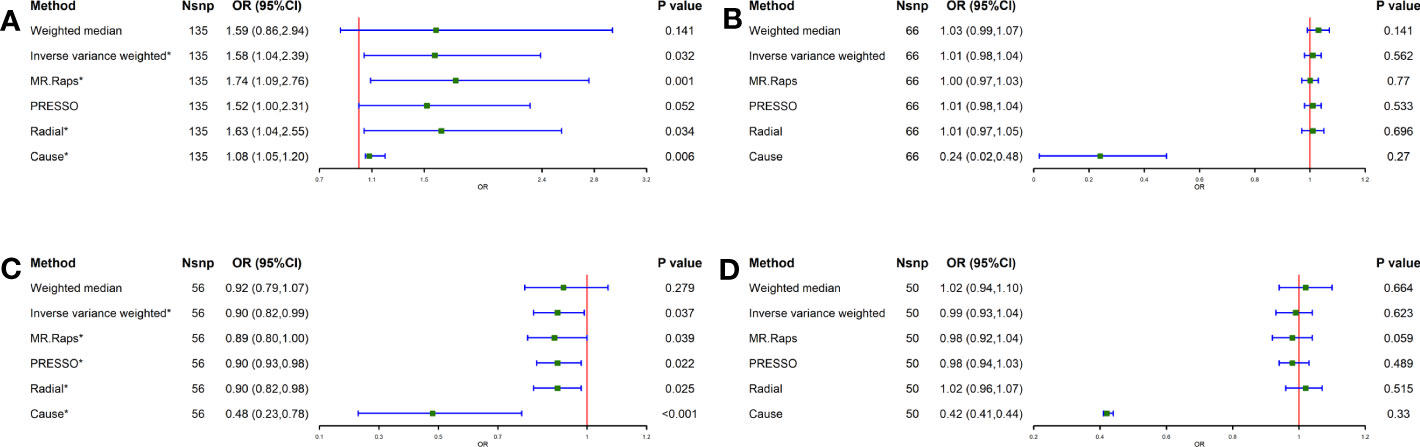
Figure 2 Causal effect of Lifestyle Factors in Eastern Asia on GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Tea intake and GC; (D) Coffee intake and GC; The green point means the effect (OR). GC, Gastric cancer.
Our study examined the causal relationship between lifestyle factors (smoking, alcohol, coffee, and tea intake) and GC lesions using a two-sample MR approach. We come to the conclusion that in an East Asian community, tea consumption is adversely linked with GC whereas smoking is favorably associated with increasing incidence of GC. We also found no causal relationship between alcohol and coffee intake with GC. Also, certain lifestyle choices (smoking, alcohol, tea, and coffee intake) as exposure in the European population and outcome analysis in the East Asian population did not reveal a causal relationship. Our findings were valid and stable in IVW analyses before and after excluding outlier SNPs and were also stable in sensitivity analyses.
Among the various habits that play a role in GC development, the impact of smoking has been considered. Numerous studies have shown that smokers are more likely than non-smokers to get stomach cancer. The study showed that never smoking or quitting for more than 10 years ago were associated with a reduced risk of GC at a mean follow-up of 11.4 years (OR = 0.64, 95% CI = 0.54-0.75) (38). Moy et al. found an estimated risk of 80% for GC in a group of smokers (39). Smoking was named by the IARC as a risk factor for the development of GC in 2002, and it was also noted that there is enough evidence to imply a causal link between smoking and GC (40). Tobacco is a biologically plausible tumour-promoting behavior and cigarette smoke has been reported to contain more than 7,000 toxic chemicals, including carcinogens such as nitrosamines, polycyclic aromatic hydrocarbons, acrylamides, volatile organic compounds and cadmium, which can increase the risk of cancer (10, 41). There is a possibility that these carcinogens and toxins may directly damage DNA. Since DNA controls the growth and function of cells, damages to DNA can alter normal cell growth patterns and further cause abnormal gastric epithelial cells to become cancerous (42, 43). Secondly, the bacterium Helicobacter pylori (H. pylori) is a significant risk factor for GC and its complete eradication has been shown to slow the occurrence of GC (44), while Pan et al. also suggest that smoking is not only a known risk factor for the development of GC, but is also linked to the failure of H. pylori eradication (38). What’s more, tobacco smoke reduces gastric defence mechanisms (45) and levels in mucus (46) gastric secretions by decreasing vitamin C concentrations and also increases bile reflux (47). Stomach cancer precursor lesions may form and grow as a result of increased bile acid concentrations in stomach contents and compromised gastric defense systems (48).
Our findings regarding the negative association between tea intake and GC are consistent with most, but not all, previous studies. The inverse association between tea consumption and GC can be explained by a number of biological processes. In previous studies, polyphenols in tea have been hypothesized to prevent cancer by regulating DNA methylation, histone modifications, and epigenetic abnormalities in microRNA (49). Studies has also revealed that the major polyphenols in tea (theaflavins and catechins) have inhibitory effects on cancer cell proliferation, tumor growth, angiogenesis, metastasis and inflammation while also inducing apoptosis to prevent tumourigenesis (50, 51). Additionally, several polyphenols in tea, such as theobromine and theaflavin, have been shown to have antioxidant properties in laboratory studies (52).
Our results for alcohol intake and coffee intake did not indicate a causal relationship with GC, possibly due to the following reasons: GC in cardia and non-cardia sites may have different etiologies. As for the anatomic site of gastric cancer, previous studies have evaluated the association between alcohol intake and site-specific risk, but the results have been inconsistent (53, 54). Furthermore, in a prospective study of Japanese men and women, light to heavy alcohol consumption was found to be associated with an increased risk of gastric cancer incidence in a dose-response manner in men, whereas a null association was seen in women (55). Meanwhile, regarding the subsites of GC, a significant positive correlation between coffee consumption and gastric cardia cancer and zero correlation with gastric non-cardia cancer was observed in the NIH-AARP study (56). It is worth noting that in this study, we did not categorize gastric cancer and the gender of the population, etc., which may have made our findings show that there is no causal relationship between alcohol consumption and GC.
The greatest strength of this study is the two-sample MR study design. As a result, MR analyses is an excellent method for understanding whether exposure factors contribute to a disease’s development. Moreover, we investigated the possibility of pleiotropy by using the most recent methodological developments. Based on the results of different sensitivity analyses, little evidence of pleiotropy can be found. As a result, our causal estimates are reliable and robust.
It is important to note, however, that our MR analyses do have some limitations. Firstly, due to the limited information available in the IEU GWAS database, it was not possible to assess the effect of type of tea. Secondly, the identification and evaluation of abnormal genetic variants involves multiple methods, however mediators or pleiotropy cannot be completely excluded as possible influences. Thirdly, the intensity of IV depends on the sample size of the GWAS, and a larger GWAS is needed to identify more genetic variation in MR. Finally, our results are instructive for disease prevention, but further validation and analysis are still needed due to the limitations of the selected GAWS data.
In summary, the results of the MR analysis suggest a causal relationship between smoking and tea intake with GC in the East Asian population. This result has some implications for the prevention and diagnosis of GC. However, due to the limitations of the study, this hypothesis needs to be supported by more high-quality cohort studies or randomized controlled trials with a larger and more diverse samples population.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WH designed this study protocol and supervised the study. YG and ZW drafted the manuscript. KL performed the data analysis. YQ polished the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2022JQ-898) and Chinese Medicine Research Project of Xi’an Health Commission (Program No. SZJ202101).
We appreciate the Key Research and Development (R&D) Projects of Shaanxi Province for funding our project. We thank all individuals providing and conducting data collection for this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1224753/full#supplementary-material
Supplementary Figure 1 | Scatterplot of the causal association between Lifestyle Factors in European and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 2 | Forest plot of the causal association between Lifestyle Factors in European and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 3 | Funnel plot of the causal association between Lifestyle Factors in European and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 4 | Leave-one-out test plot of the causal association between Lifestyle Factors in European and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 5 | Scatterplot of the causal association between Lifestyle Factors in Eastern Asia and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 6 | Forest plot of the causal association between Lifestyle Factors in Eastern Asia and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 7 | Funnel plot of the causal association between Lifestyle Factors in Eastern Asia and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
Supplementary Figure 8 | Leave-one-out test plot of the causal association between Lifestyle Factors in Eastern Asia and GC. (A) Smoking and GC; (B) Alcohol intake and GC; (C) Coffee intake and GC; (D) Tea intake and GC. GC, Gastric cancer.
1. Pan C, Deng D, Wei T, Wu Z, Zhang B, Yuan Q, et al. Metabolomics study identified bile acids as potential biomarkers for gastric cancer: A case control study. Front endocrinol (2022) 13:1039786. doi: 10.3389/fendo.2022.1039786
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London England) (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep (2017) 19(8):36. doi: 10.1007/s11894-017-0575-8
5. Park JY, Herrero R. Recent progress in gastric cancer prevention. Best Pract Res Clin Gastroenterol (2021) 50-51:101733. doi: 10.1016/j.bpg.2021.101733
6. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad gastroenterol (2019) 14(1):26–38. doi: 10.5114/pg.2018.80001
7. González CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter (2013) 18 Suppl 1:34–8. doi: 10.1111/hel.12082
8. Yu S, Chen Z, Cheng J, Shi X, Liu J, Zhong P, et al. Case-control study on CYP4B1 gene polymorphism and susceptibility to gastric cancer in the chinese Han population. BMC Med Genomics (2022) 15(1):223. doi: 10.1186/s12920-022-01367-w
9. Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/american institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr (2020) 150(4):663–71. doi: 10.1093/jn/nxz268
10. Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health (2020) 42:e2020004. doi: 10.4178/epih.e2020004
11. Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer (Oxford England: 1990) (2015) 51(18):2820–32. doi: 10.1016/j.ejca.2015.09.010
12. Parra-Lara LG, Mendoza-Urbano DM, Bravo JC, Salamanca CH, Zambrano ÁR. Coffee consumption and its inverse relationship with gastric cancer: an ecological study. Nutrients (2020) 12(10):3028. doi: 10.3390/nu12103028
13. Karagulle M, Fidan E, Kavgaci H, Ozdemir F. The effects of environmental and dietary factors on the development of gastric cancer. J BUON (2014) 19(4):1076–82.
14. Martimianaki G, Alicandro G, Pelucchi C, Bonzi R, Rota M, Hu J, et al. Tea consumption and gastric cancer: a pooled analysis from the Stomach cancer Pooling (StoP) Project consortium. Br J cancer (2022) 127(4):726–34. doi: 10.1038/s41416-022-01856-w
15. Sheerah H, Keyang L, Eshak ES, Cui R, Shirai K, Muraki I, et al. Association of tea consumption and the risk of gastric cancer in Japanese adults: the Japan Collaborative Cohort Study. BMJ Open (2020) 10(10):e038243. doi: 10.1136/bmjopen-2020-038243
16. Alicandro G, Tavani A, La Vecchia C. Coffee and cancer risk: a summary overview. Eur J Cancer Prev (2017) 26(5):424–32. doi: 10.1097/CEJ.0000000000000341
17. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
18. Fu L, Wang Y, Hu YQ. Causal effects of B vitamins and homocysteine on obesity and musculoskeletal diseases: A Mendelian randomization study. Front Nutr (2022) 9:1048122. doi: 10.3389/fnut.2022.1048122
19. Birney E. Mendelian randomization. Cold Spring Harbor Perspect Med (2022) 12(4):a041302. doi: 10.1101/cshperspect.a041302
20. Ishigaki K, Akiyama M, Kanai M, Takahashi A, Kawakami E, Sugishita H, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet (2020) 52(7):669–79. doi: 10.1038/s41588-020-0640-3
21. Chen S, Luo X, Zhao J, Liang Z, Gu J. Exploring the causality between ankylosing spondylitis and atrial fibrillation: A two-sample Mendelian randomization study. Front Genet (2022) 13:951893. doi: 10.3389/fgene.2022.951893
22. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinf (Oxford England) (2016) 32(20):3207–9. doi: 10.1093/bioinformatics/btw373
23. Wang W, Zhang L, Cao W, Xia K, Huo J, Huang T, et al. Systematic screening of associations between medication use and risk of neurodegenerative diseases using a mendelian randomization approach. Biomedicines (2023) 11(7):1930. doi: 10.3390/biomedicines11071930
24. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet (2017) 13(11):e1007081. doi: 10.1371/journal.pgen.1007081
25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
26. Freuer D, Meisinger C, Linseisen J. Causal relationship between dietary macronutrient composition and anthropometric measures: A bidirectional two-sample Mendelian randomization analysis. Clin Nutr (Edinburgh Scotland) (2021) 40(6):4120–31. doi: 10.1016/j.clnu.2021.01.047
27. Wang Q, Wang R, Chen C, Feng Y, Ye Z, Zhan M, et al. Educational attainment and endometrial cancer: A Mendelian randomization study. Front Genet (2022) 13:993731. doi: 10.3389/fgene.2022.993731
28. Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol (2018) 47(4):1264–78. doi: 10.1093/ije/dyy101
29. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
30. Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet (2016) 48(5):481–7. doi: 10.1038/ng.3538
31. Xu Q, Chen C, You R, Ni L, Chen S, Peng B. Causal association between major depressive disorder and coronary heart disease: a two-sample bidirectional mendelian randomization study. BMC Med Genomics (2023) 16(1):183. doi: 10.1186/s12920-023-01625-5
32. Wang Q, Liu F, Tuo Y, Ma L, Feng X. Associations between obesity, smoking behaviors, reproductive traits and spontaneous abortion: a univariable and multivariable Mendelian randomization study. Front endocrinol (2023) 14:1193995. doi: 10.3389/fendo.2023.1193995
33. Zhao Q, Chen Y, Wang J, Small DS. Powerful three-sample genome-wide design and robust statistical inference in summary-data Mendelian randomization. Int J Epidemiol (2019) 48(5):1478–92. doi: 10.1093/ije/dyz142
34. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
35. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife (2018) 7:e34408. doi: 10.7554/eLife.34408
36. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
37. Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet (2020) 52(7):740–7. doi: 10.1038/s41588-020-0631-4
38. Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut (2016) 65(1):9–18. doi: 10.1136/gutjnl-2015-309197
39. Moy KA, Fan Y, Wang R, Gao YT, Yu MC, Yuan JM. Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer epidemiol Biomarkers prevention: Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2010) 19(9):2287–97. doi: 10.1158/1055-9965.EPI-10-0362
40. Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clinics North America (2002) 11(2):235–56. doi: 10.1016/S1055-3207(02)00002-9
41. Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol (2009) 10(11):1033–4. doi: 10.1016/S1470-2045(09)70326-2
42. Dyke GW, Craven JL, Hall R, Garner RC. Smoking-related DNA adducts in human gastric cancers. Int J cancer (1992) 52(6):847–50. doi: 10.1002/ijc.2910520602
43. Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene (2002) 21(48):7435–51. doi: 10.1038/sj.onc.1205803
44. Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. Jama (2004) 291(2):187–94. doi: 10.1001/jama.291.2.187
45. Jarosz M, Dzieniszewski J, Dabrowska-Ufniarz E, Wartanowicz M, Ziemlanski S. Tobacco smoking and vitamin C concentration in gastric juice in healthy subjects and patients with Helicobacter pylori infection. Eur J Cancer Prev (2000) 9(6):423–8. doi: 10.1097/00008469-200012000-00008
46. Ma JJ, Hou DQ, Zhang QB, Korsten MA. Reversal of the gastric effects of nicotine by nitric oxide donor treatment. Digestion (2001) 63(2):102–7. doi: 10.1159/000051877
47. Müller-Lissner SA. Bile reflux is increased in cigarette smokers. Gastroenterology (1986) 90(5 Pt 1):1205–9. doi: 10.1016/0016-5085(86)90386-0
48. Nakamura M, Haruma K, Kamada T, Mihara M, Yoshihara M, Sumioka M, et al. Cigarette smoking promotes atrophic gastritis in Helicobacter pylori-positive subjects. Digest Dis Sci (2002) 47(3):675–81. doi: 10.1023/A:1017901110580
49. Bag A, Bag N. Tea polyphenols and prevention of epigenetic aberrations in cancer. J Natural sci biol Med (2018) 9(1):2–5. doi: 10.4103/jnsbm.JNSBM_46_17
50. Sasazuki S, Inoue M, Miura T, Iwasaki M, Tsugane S. Plasma tea polyphenols and gastric cancer risk: a case-control study nested in a large population-based prospective study in Japan. Cancer epidemiol Biomarkers prevention: Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2008) 17(2):343–51. doi: 10.1158/1055-9965.EPI-07-0428
51. Lambert JD. Does tea prevent cancer? Evidence from laboratory and human intervention studies. Am J Clin Nutr (2013) 98(6 Suppl):1667s–75s. doi: 10.3945/ajcn.113.059352
52. Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database: J Biol Database curation (2010) 2010:bap024. doi: 10.1093/database/bap024
53. Wang PL, Xiao FT, Gong BC, Liu FN. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget (2017) 8(58):99013–23. doi: 10.18632/oncotarget.20918
54. Rota M, Pelucchi C, Bertuccio P, Matsuo K, Zhang ZF, Ito H, et al. Alcohol consumption and gastric cancer risk-A pooled analysis within the StoP project consortium. Int J cancer (2017) 141(10):1950–62. doi: 10.1002/ijc.30891
55. Li Y, Eshak ES, Shirai K, Liu K, Dong JY, Iso H, et al. Alcohol consumption and risk of gastric cancer: the Japan collaborative cohort study. J Epidemiol (2021) 31(1):30–6. doi: 10.2188/jea.JE20190304
Keywords: gastric cancer, Mendelian randomization, smoking, alcohol consumption, tea intake, coffee intake
Citation: Tan Y, Wei Z, Liu K, Qin Y and Hui W (2023) Lifestyle habits and gastric cancer in an East Asian population: a Mendelian randomization study. Front. Oncol. 13:1224753. doi: 10.3389/fonc.2023.1224753
Received: 25 June 2023; Accepted: 14 August 2023;
Published: 04 September 2023.
Edited by:
Wei Zhang, Northwestern University, United StatesReviewed by:
Xiangxiang Jiang, University of South Carolina, United StatesCopyright © 2023 Tan, Wei, Liu, Qin and Hui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenqi Hui, aHVpd3FoczIzQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.