- 1Medical Research Center, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Medical Laboratory Technology, The University of Haripur, Haripur, Khyber Pakhtunkhwa, Pakistan
- 3Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN, United States
- 4Department of Biomedical Informatics, Vanderbilt University School of Medicine, Nashville, TN, United States
- 5Department of Mathematics and Statistics, The University of Haripur, Haripur, Khyber Pakhtunkhwa, Pakistan
- 6Department of Pathology, Shaukat Khanum Memorial Cancer Hospital and Research Center (SKMCH&RC), Lahore, Pakistan
- 7Zhengzhou Key Laboratory of Children’s Infection and Immunity, Children’s Hospital Affiliated to Zhengzhou University, Zhengzhou, China
- 8Henan Key Laboratory of Precision Diagnosis of Respiratory Infectious Diseases, Zhengzhou Key Laboratory of Precision Diagnosis of Respiratory Infectious Diseases, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Background: Breast Cancer (BC) stands out as the widely prevalent malignancy among all the types of cancer affecting women worldwide. There is significant evidence that the pathogenicity of BC may be altered by Human Papillomavirus (HPV) infection; however, conclusive data are not yet available.
Methods: By searching five databases, including EMBASE, IBECS, PubMed, Scopus, Science Direct, Google Scholar, and Web of Science, a thorough systematic analysis was conducted on the prevalence of HPV in BC patients from 1990 to June 30, 2022. After applying extensive eligibility criteria, we selected 74 publications for further analysis based on the prevalence of HPV infections in breast tissues. All of the data were analyzed using a random-effects meta-analysis, Cochran Q test and I2 statistic were used to calculate the heterogeneity of the prevalence among these studies using subgroup analysis. Variations in the HPV prevalence estimates in different subgroups were evaluated by subgroup meta-analysis.
Results: In total, 3156 studies were initially screened, resulting in 93 full-text studies reviewed, with 74 meeting inclusion criteria. Among a total of 7156 BC biopsies, the pool prevalence of HPV was 25.6% (95% CI= 0.24-0.33, τ2 = 0.0369 with significant heterogeneity between estimates (I2 = 97% and p< 0.01). Consequently, 45 studies with available controls were further studied, and the prevalence of HPV in case-control studies was 26.2% with overall odds 5.55 (95% CI= 3.67-8.41, I2 = 38%, τ2 = 1.4878, p< 0.01). Further subgroup analysis of HPV revealed HPV-16 had a maximum prevalence of 9.6% (95% CI= 3.06-11.86, I2 = 0%, τ2 = 0.6111, p< 0.01). Among different geographical regions, Europe reported the maximum prevalence of HPV, i.e., 39.2% (95% CI=1.29-7.91, I2 = 18%, τ2 = 1.2911, p< 0.01). Overall distribution showed HPV-18 was a frequent HPV subtype reported in Australia.
Conclusion: Current study provides a global estimate of HPV prevalence in BC patients and demonstrates a significant association between this virus and BC etiology. Nevertheless, we recommend further investigation into the underlying mechanism is essential to validate this hypothesis.
1 Introduction
Breast Cancer (BC) is the most frequently detected in women, accounting for the sixth highest cancer mortality rate and the most frequent cancer in females worldwide (1, 2). Besides, this malignancy has a substantially higher fatality rate than lung and colorectal cancers (2, 3). BC is attributed to the lives of one woman every minute and over 1,400 women every day. Recent estimates show BC affected 2.3 million women worldwide in 2020, resulting in about 0.68 million fatalities (4). Alarmingly, if the problem is not addressed, new cases might reach 2.7 million by 2030, with a death toll of 0.87 million (5). In the recent two decades, the global BC incidence has grown alarmingly, attributed to an upsurge in identified and/or undeclared risk factors. However, some risk factors may be important oncogenic infectious agents (6–8). BC is caused by several etiological variables, such as prolonged or excessive estrogen exposure brought on by early menarche (under 12 years), late menopause (> fifty-five years), increased exposure to radiation, nulliparity, alcohol addiction, and fat-rich diet (9). Amongst different factors, BC has been strongly linked to viral infection; these factors ranked between 20 and 50% of BC cases (10–12). Studies have suggested that oncoviruses, notably the Human Papillomavirus (HPV), may be risk factors for breast carcinogenesis (13). Additionally, it has been suggested that the development of BC may be influenced by the Epstein-Barr virus (EBV), the Mouse Mammary Tumor Virus (MMTV), and the Bovine Leukemia Virus (BLV) (14).
HPV is a non-enveloped double-stranded DNA virus whose genome is divided into three regions: the long control region (LCR), the early region (which encodes E1, E2, and E4-E7), and the late region (encodes L1 and L2) (15–17). E6 and E7 oncoproteins largely promote host cellular proliferation (18). The carcinogenic risk of HPVs is used to categorize subtypes into either Low-Risk (LR) or High-Risk (HR) subtypes (19). Approximately 15 HPV subtypes have been identified as HR-HPVs (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82) (20, 21), with their relative carcinogenic risk assessed from a variety of epidemiological studies (21). LR-HPVs typically result in anogenital and cutaneous warts, whereas HR-HPVs are responsible for approximately 4.5% of all diagnosed human malignancies, including head and neck cancers (such as oral, tonsil, and throat cancers) as well as anogenital cancers (such as cervical, anal, vulvar, vaginal, and penile cancers) (14, 22–26).
The transmission of HPV can happen in either sexual or nonsexual interactions. Direct skin-to-skin contact during sexual activity with a person infected with the virus is the most prevalent way for genital HPV to spread (27). It is suggested that HPV may enter the body through various routes, including cuts, scrapes, and mucosal membranes, and can cause infection in the stratum basale’s—the lowest layer of the stratified epithelium (27). Multiple routes exist for transferring HPV from infected sites to breast tissues, as depicted in Figure 1. First, HPV virions can spread to the breasts from other body parts (most commonly the head, neck, or cervical region) via the circulatory or lymphatic systems (28, 29). Second, the transmission involves the transfer of HPV to the breast through genital-breast sexual activity-related nipple or micro-lesions in the breast skin (27, 30).
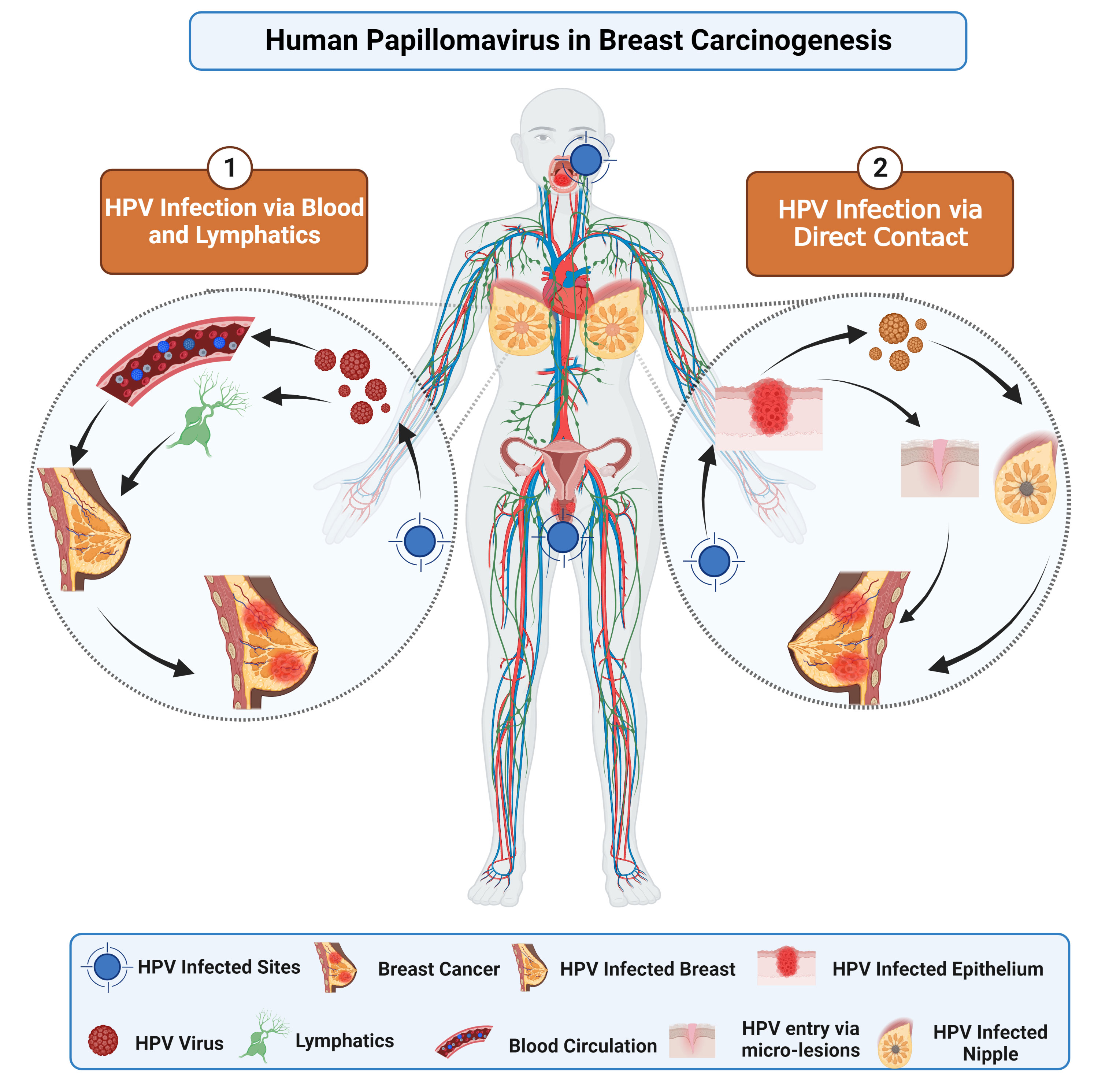
Figure 1 This image depicts the potential pathways implicated in BC pathogenesis. The oncogenic proteins of HPV, E6 and E7, interfere with different pathways by inactivating tumor suppressor genes. Consequently, overexpression of E6 and E7 may contribute to BC progression.
In BC patients, the rate of HPV infection is anywhere between 0% to 86.2% (31); however, certain researchers have not found any association with HPV in breast carcinogenesis (32–34). The epidemiology of HPV types varies among different populations. Other investigations have discovered that the pathogenicity of HPV is determined by its genotypes (35). HPV16, HPV18, and HPV33 were frequently reported and responsible for 70% of BC cases globally (32, 36). There is currently a debate surrounding studies investigating HPV prevalence in BC. The first association between HPV and BC was first reported by, Lonardo and colleagues in 1992 (37). In the last several decades, multiple studies have highlighted the presence of HPV DNA in BC cells (2, 31, 38); certain studies have reported the absence of HPV DNA in BC tissues (39–42).
Though the involvement of HPV in breast carcinogenesis is still debatable, understanding it is essential in devising effective new preventative and treatment measures for women with BC. Consequently, the present systematic review and meta-analysis aim to address this information gap by providing an up-to-date estimate of the overall prevalence of HPV and its subtypes in BC patients worldwide.
2 Methodology
2.1 Search strategy
We systematically analyzed publications on HPV prevalence in BC patients published between 1990 and June 30, 2022. We used five databases: EMBASE, IBECS, PubMed, Scopus, Science Direct, Google Scholar, and Web of Science. Different keywords or MeSH terms were used to find relevant data, with the articles limited written in English (Supplementary File).
2.2 Eligibility criteria
By screening the title, reading the abstract, and reviewing the publications, the inclusion criteria guaranteed that the study was relevant. All cross-sectional, case-based, and prevalence-based research was also included. The following studies, on the other hand, were excluded: (i) studies published in a language other than English, (ii) studies composed of letters, commentaries, reviews, series, editorial, and commission, (iii) duplicate studies, and (iv) non-relevant studies that did not report any correlation between HPV and BC.
2.3 Data extraction
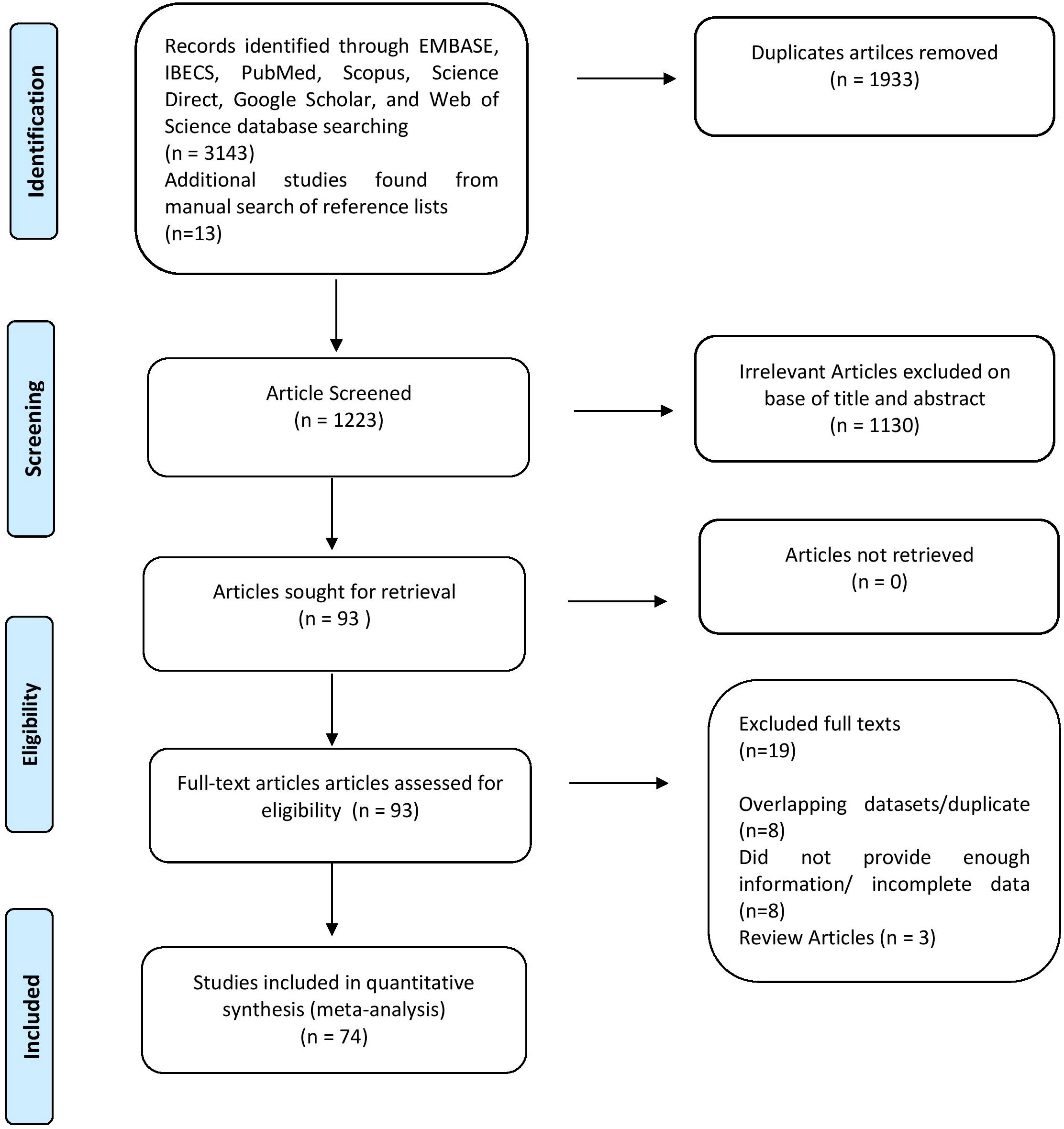
Following selecting relevant publications, three reviewers (U.A.A., A.A.K., and N.A.) independently reviewed the titles and abstracts of articles to be read in full text. Within three decades, 3156 studies were researched using electronic databases. After a thorough evaluation, 74 pertinent papers were included. Information about the author(s), year of publication, area, sample size, prevalence of HPV in BC, HPV subtypes, coinfection frequency, and detection technique was acquired for each. Results were classified following the Preferred Reporting Items for Systematic and Meta-analyses (PRISMA) guideline, as shown in Figure 2.
2.4 Quality assessment of studies
Additionally, using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, three reviewers (U.A.A, A.A.K., and N.A) independently assessed the methodological quality of each included study (43). Disagreements about the quality assessment standards were settled by discussion and consensus with other study authors.
2.5 Statistical analysis
After extracting the data using Microsoft Excel, we conducted a statistical analysis using R version (4.2.3) for meta-analysis using different packages. HPV infection prevalence was determined using the binomial distribution, and the standard error for each study was computed. We use random-effects models to adjust for the high heterogeneity of the studies. Otherwise, it generates study impediments, mostly used to distinguish between unique research variants. We assessed the heterogeneity among the studies using the Cochran Q test and I2 statistic, and we presented the results graphically using forest plots (showing the effect size [ES] with a 95% confidence interval [95% CI]). Therefore, the I2 statistic, which ranges from 0 to 100%, reveals that heterogeneity, rather than chance, is the primary difference across research in systematic reviews and meta-analyses. I2 values of 25%, 50%, and 70% indicate low, medium, and high trial heterogeneity. However, subgroup meta-analysis were used to identify the potential sources of statistical heterogeneity.
3 Results
In this systematic review and meta-analysis, we found 3156 studies after a baseline online search between 1992 and June 30, 2022, whereas 06 were discovered after manually searching reference lists, as shown in Supplementary Table 1. Following the exclusion of 1933 duplicate research, a total of 1223 papers were evaluated. In addition, 1130 of the articles were eliminated for being irrelevant. The remaining 93 pieces of research were extensively evaluated, and 19 full-text publications were removed owing to disparities in the nature and outcomes of investigations. In the end, only 74 studies met the inclusion criteria, and data were retrieved for the meta-analysis. A detailed PRISMA diagram depicting the publication trajectory of this meta-analysis is given in Figure 2.
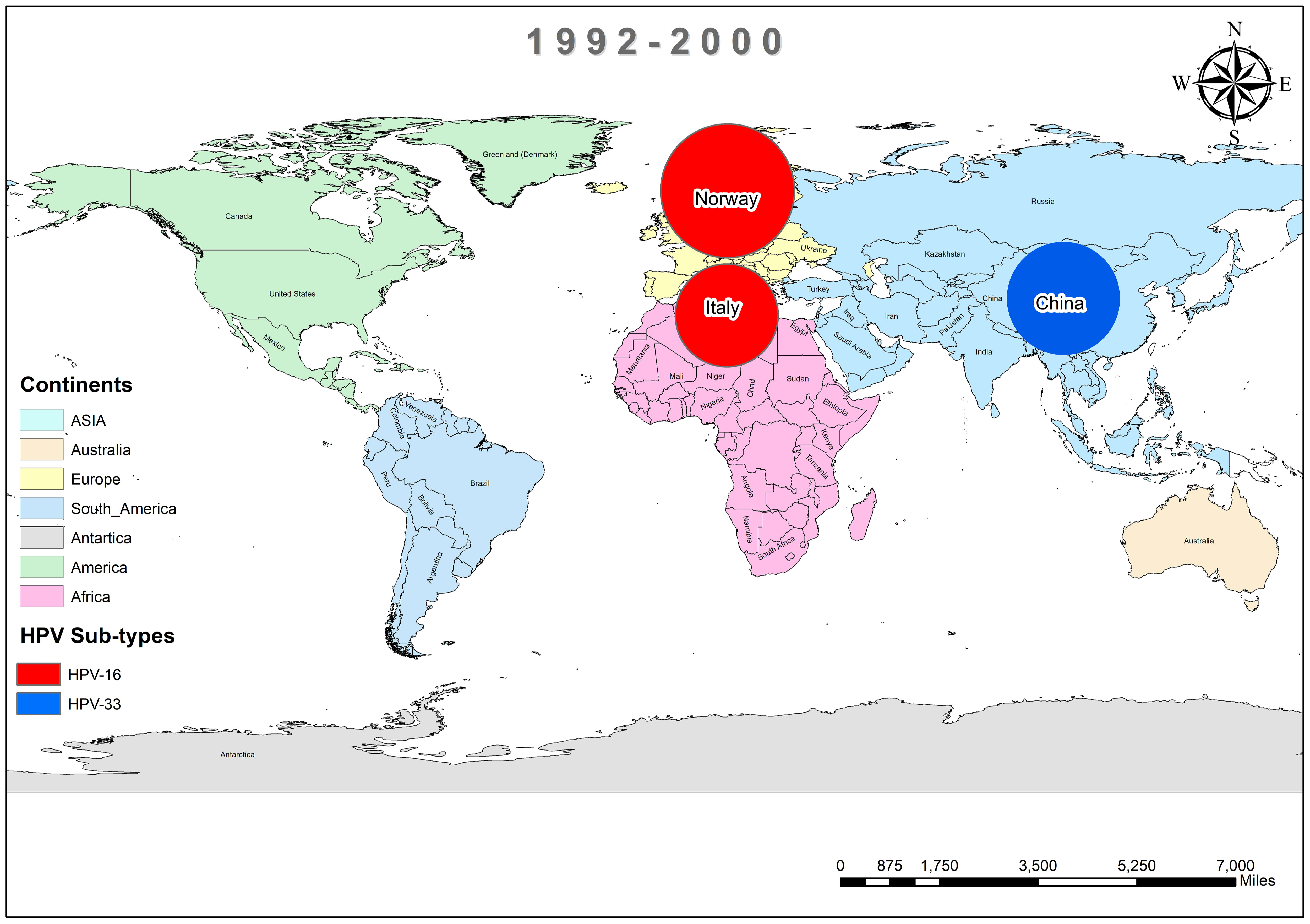
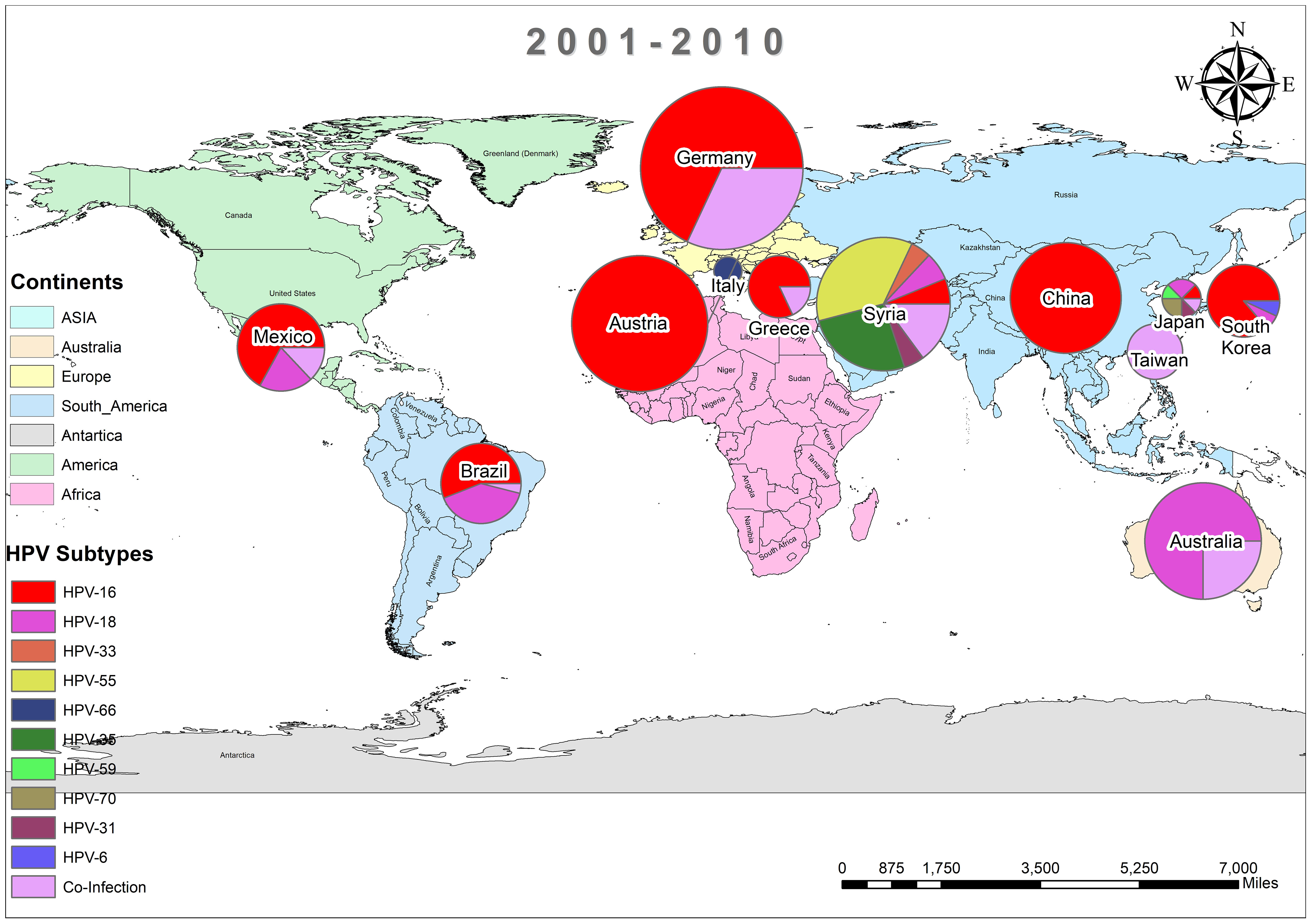
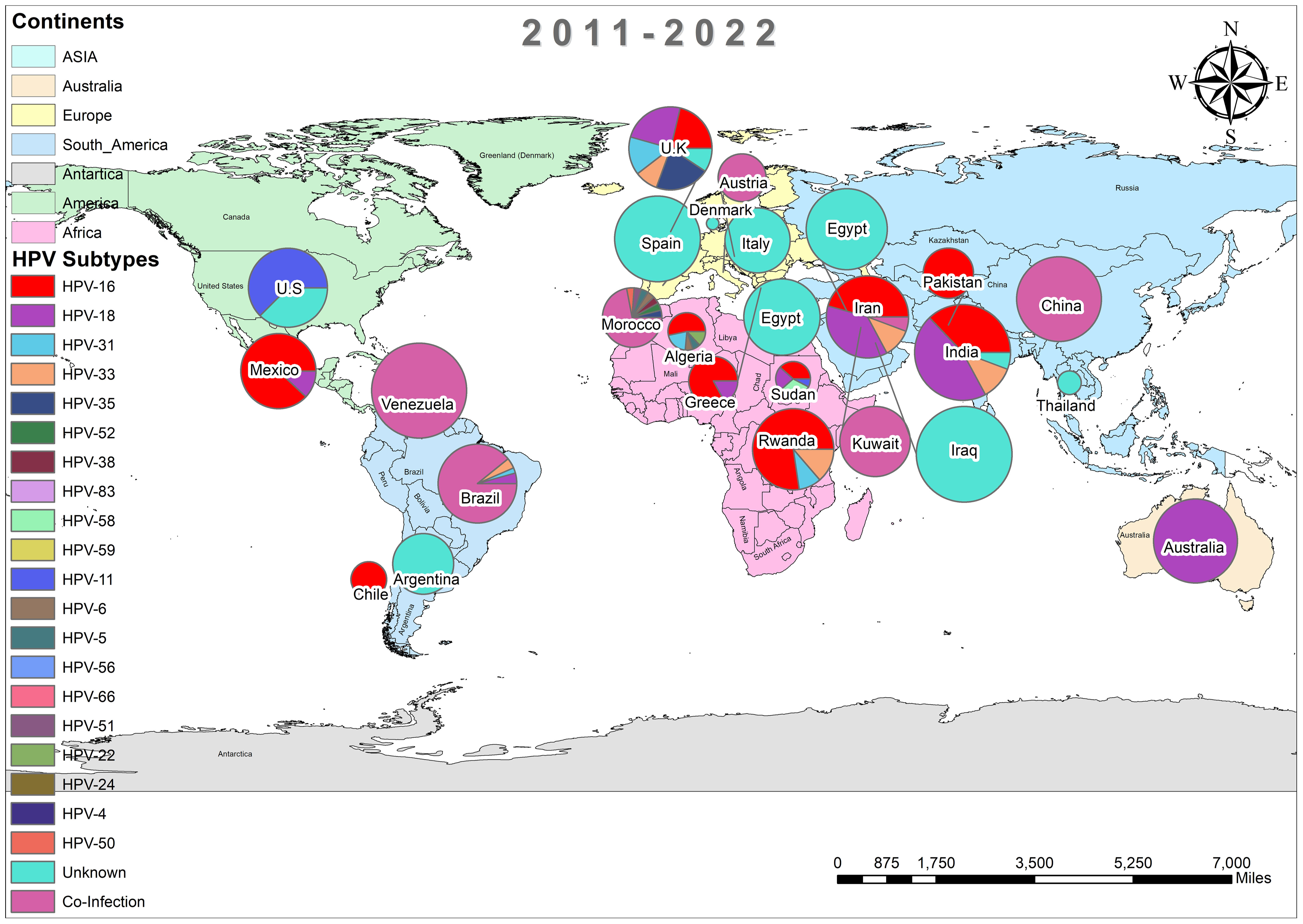
In addition, the overall prevalence of HPV and the subtypes in individuals diagnosed with BC was calculated for each decade between 1990 and 2022 across all 74 studies. From 1992 to 2000, only three studies reported HPV in breast tissues, primarily subtype HPV-16 and 33, as depicted in Figure 3 (37, 44, 45). In contrast, a wider range of HPV subtypes was reported in sixteen studies undertaken between 2001-2010, as illustrated in Figure 4 (1, 39, 46–59). During this time frame, HPV was reported in BC patients in Germany (86%) (48), followed by Austria (64%) (47), Syria (61%) (53), China (60%) (59) and similarly other reported in Figure 4. Amongst, the maximum diversity of HPV subtypes was reported in Syria by Akil et al. (12), with the majority of HPV-31 (n=58), followed by HPV-35 (n=39), HPV-18 (n=11), HPV-16 (n=9), and HPV-33 (n=8). Furthermore, between 2011-2022, fifty-six studies reported the occurrence of HPV in BC patients around the globe, as shown in Figure 5 (10, 30, 31, 60–112). In this decade, the maximum frequency of HPV was reported in Qatar (65%) (110), followed by India (65%) (97), Venezuela (64%) (95), Spain (52%) (92), China (51%) (79), Australia (50%) (61, 64), and remaining are depicted in Figure 5.
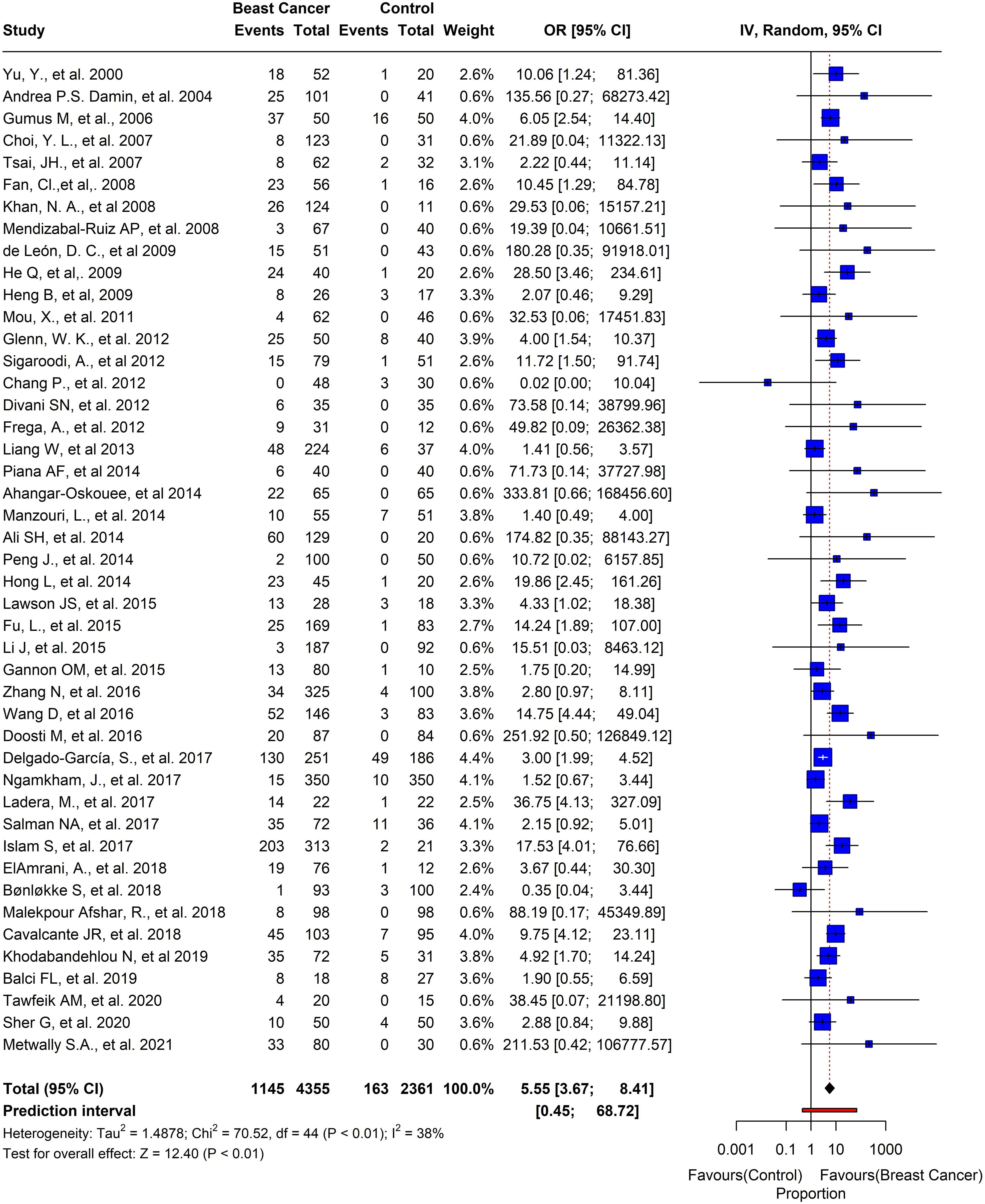
Out of the 74 studies we analyzed, the prevalence of HPV in BC was noteworthy. The pooled prevalence showed that 1839 out of 7156 participants tested positive for HPV, with an overall prevalence of 25.6% (95% CI= 0.24-0.33, I2 = 97%, τ2 = 0.0369, p = 0) (Figure 6). Moreover, we selected 45 studies that had control samples for comparison. The pooled prevalence of HPV in BC samples and control samples was 26.2%, and overall odds of 5.55 (95% CI= 3.67-8.41, I2 = 38%, τ2 = 1.4878, p< 0.01) (Figure 7).
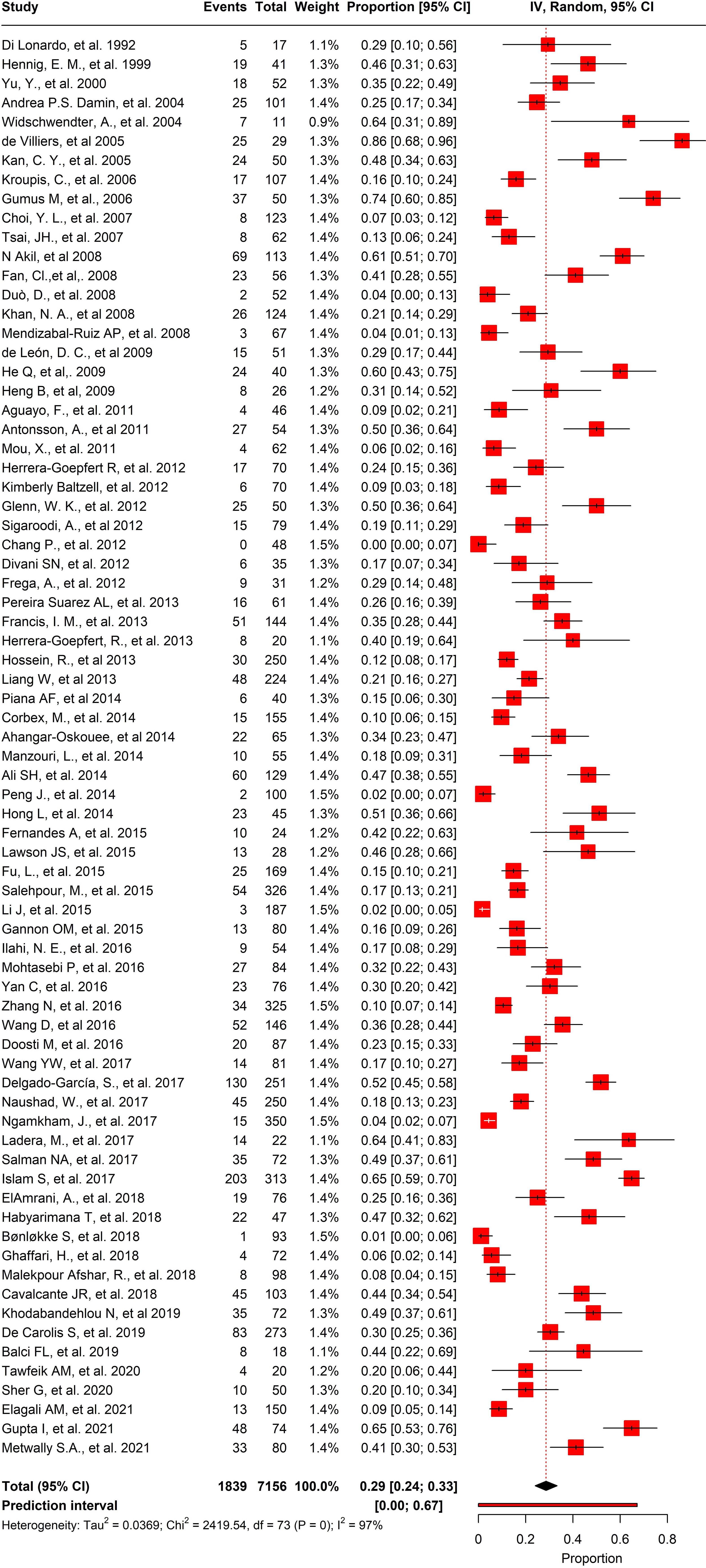
Figure 6 Overall pool prevalence of HPV reported in BC tissues in both case-control and cases-only tissues around the globe from 1992-to-2022.
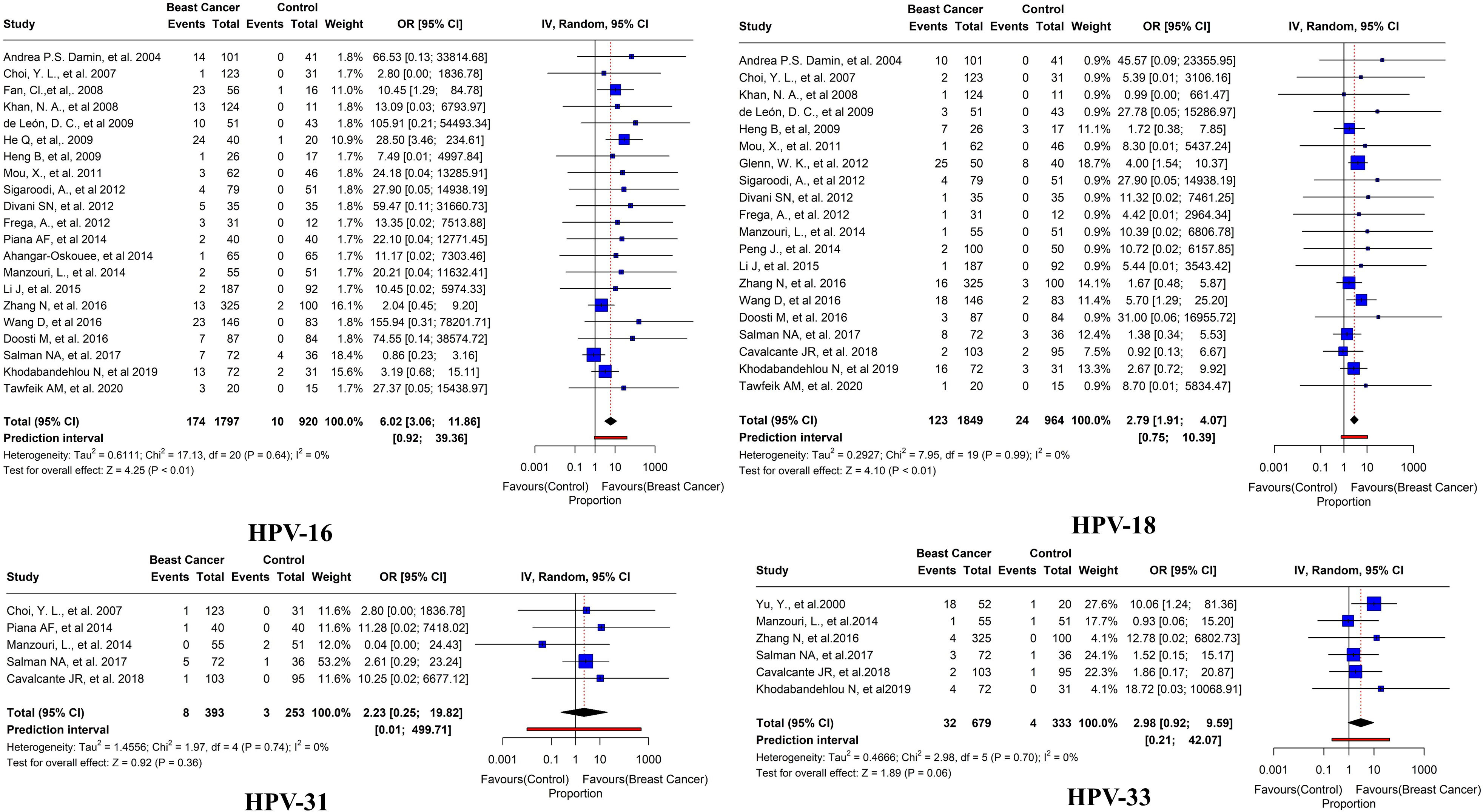
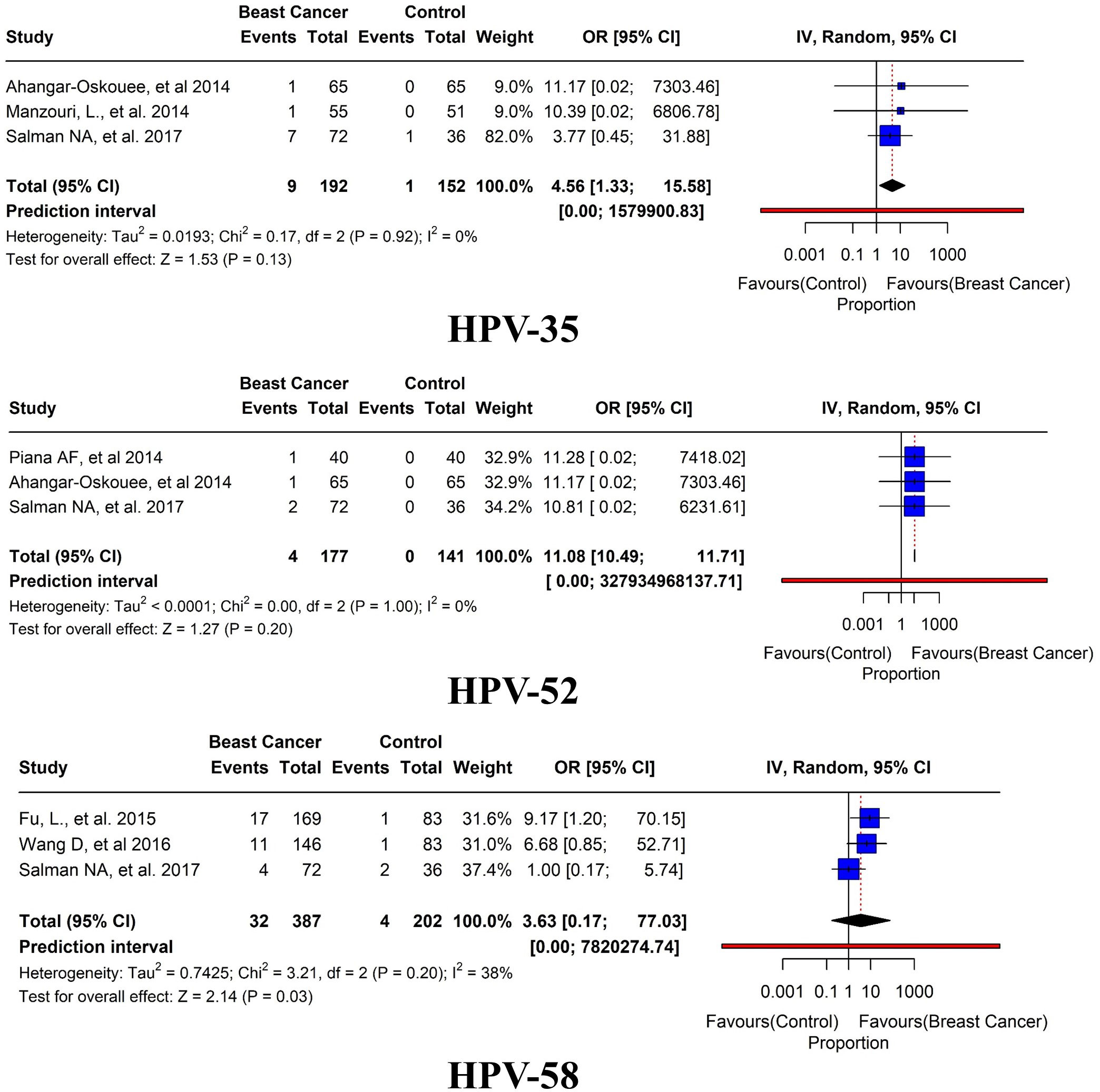
Of these 45 studies, the further prevalence of HPV subtypes was also studied. HPV-16 was reported in BC tissues in 21 studies with an overall prevalence of 9.6% (95% CI= 3.06-11.86, I2 = 0%, τ2 = 0.6111, p< 0.01). HPV-18 was reported in BC tissues in 20 studies with a prevalence of 6.6% (95% CI=1.91-4.07, I2 = 0%, τ2 = 0.2927, p< 0.01). HPV-31 has been reported in 5 studies with an overall prevalence of 2% (95% CI= 0.25-19.82, I2 = 0%, τ2 = 1.4556, p = 0.36). HPV-33 was reported in 6 studies with an overall prevalence of 4.7% (95% CI= 0.92-9.59, I2 = 0%, τ2 = 0.4666, p = 0.06) (Figure 8). HPV 35 was reported in only three studies with an overall prevalence of 4.6% (95% CI=1.33-15.58, I2 = 0%, τ2 = 0.0193, p = 0.13). HPV 52 was reported in only 3 studies with an overall prevalence of 2.3% (95% CI=10.49-11.71, I2 = 0%, τ2<0.0001, p = 0.20). In addition, HPV 58 was reported in only three studies with an overall prevalence of 8.2% (95% CI=0.17-77.03, I2 = 38%, τ2 = 0.7425, p = 0.03) (Figure 9).
We conducted a sub-group analysis to examine the regional variations of the prevalence of HPV in BC patients. Surprisingly, Asia had the highest number of studies (n=25) with a pooled prevalence of 22.7% (95% CI=3.54-11.51, I2 = 45%, τ2 = 1.5917, p< 0.01). In Europe, nine studies reported positive HPV cases with an overall prevalence of 39.1% (95% CI=1.29-7.91, I2 = 18%, τ2 = 1.2911, p< 0.01). In America, six studies reported positive HPV cases with an overall prevalence of 30.3% (95% CI=2.10-501.13, I2 = 44%, τ2 = 1.2743, p< 0.01). In Australia, two studies reported positive HPV cases with an overall prevalence of 29.2% (95% CI=0.05-216.65, I2 = 0%, τ2 = 0.0662, p< 0.01). Furthermore, in Africa, three studies reported positive HPV cases with an overall prevalence of 31.8% (95% CI=0.09-1175.06, I2 = 0%, τ2 = 1.5228, p = 0.05) (Figure 10).
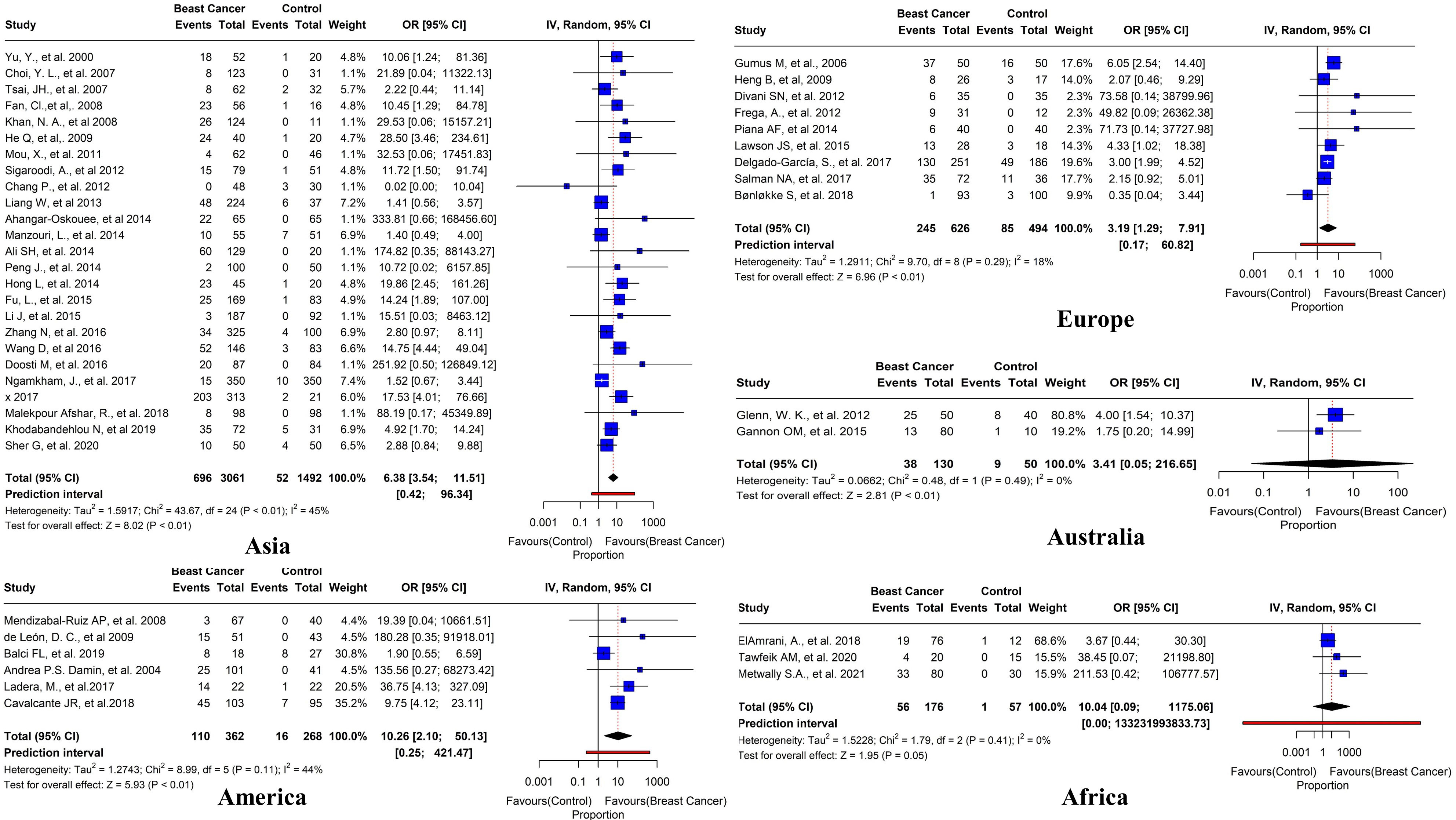
Figure 10 Prevalence of HR HPV subtypes in breast tissues in case-control studies reported in different regions worldwide (Asia, America, Europe, Australia, and Africa).
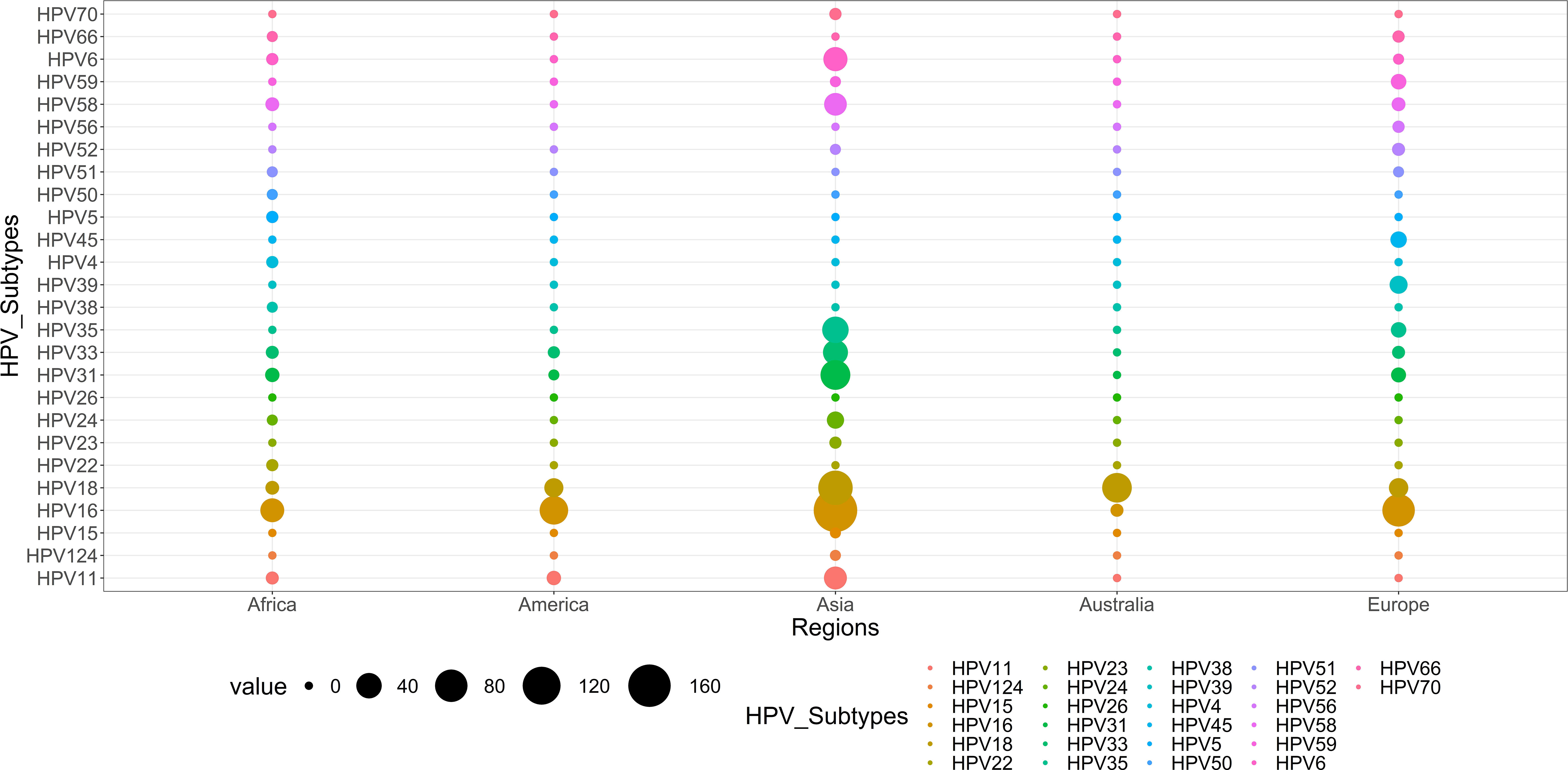
Figure 11 illustrates the global diversity of HPV subtypes (n=26) across five continents: Asia, Africa, America, Europe, and Australia. Whereas the distinct color represents each continent, the relative frequency of each HPV subtype is shown by the thickness of the chords, as shown in Figure 12. HPV-16 is the dominant subtype in all regions, comprising 342 out of 689 cases. Asia and Europe exhibit the highest HPV-16 cases, with 170 and 80 cases, respectively. Moreover, HPV-18 ranks as the second most common subtype, with 193 cases. The distribution of the other subtypes displays considerable variation across the regions. For example, Australia has an exceptionally high percentage of HPV-18 cases (95.4%); HPV-33 is common in Asia (38) and Africa (3) but scarce in other regions (2 or less). Likewise, HPV-35 is prevalent in Europe (7) and Asia (44) but absent in other regions. Conversely, HPV-31 is largely restricted to Africa (5) and Asia (60), while HPV-45 is mainly limited to Europe (9). The subtypes HPV-23, HPV-4, HPV-26, HPV-15, and HPV-124 in Africa; HPV-70 and HPV-50 in Asia; and HPV-24 and HPV-38 in Europe are extremely rare and only detected in one region.

Figure 12 The diversity and distribution of HPV types across five continents. The chords represent the relative frequency of each HPV type, while the colors denote the different continents: Asia, Africa, America, Europe, and Australia.
4 Discussion
The causal role of HPV in breast cancer has been a subject of debate for a long time, but recent studies have provided convincing evidence of a positive association between HPV infection and breast cancer development. However, there are still many unresolved questions and challenges in this area of research. Most studies detected HPV using conventional or nested PCR using commercial primers for the L1 gene (capsid protein). After positive results and using primers for E6 and E7 genes or sequencing, they reported probable causes of false positive and false negative results and limitation factors related to the diagnostic techniques used in those studies, like DNA/RNA quality and viral load (113). Approximately 13-15 of the 200 HPV subtypes are HR and associated with various cancers (19). Our comprehensive meta-analysis results revealed that the pooled prevalence of HPV infection among females with BC was 25.6% (95% CI= 0.24-0.33, I2 = 97%, τ2 = 0.0364, p=0). This finding underscores the urgency of identifying novel risk factors for BC development.
We stratified the control breast tissues and control group to reveal a remarkable finding: a significant difference between breast and control tissues. The pooled prevalence of HPV in BC tissues was 26.2%, with overall odds of 5.55 (95% CI= 3.67-8.41, I2 = 38%, τ2 = 1.4878, p< 0.01). The I2 value across subgroups was below 50%, indicating acceptable heterogeneity and emphasizing the importance of control selection. Multiple meta-analyses have reported similar results, showing a significant association of HPV with BC. For instance, a meta-analysis of 37 case-control studies containing 3,607 BC cases and 1,728 controls reported overall odds of 6.22 (95% CI = 4.25 to 9.12, p = 0.0002) (114). Another meta-analysis of nine case-control studies reported odds of 5.9 (95% CI = 3.26–10.67) (38). Bae and colleagues performed a meta-analysis of 22 studies and reported odds of 4.02 (95% CI: 2.42–6.68) (2). Similarly, a meta-analysis of ten case–control studies containing 447 BC cases and 275 controls showed an increased breast carcinoma risk with HPV positivity (OR = 3.63, 95% CI = 1.42–9.27) (115). However, the association between HPVs and BC is supported by the consistency of different detection methods of HPVs, which indicate a significantly higher prevalence of HPVs in breast cancer than in control tissues (116).
This review and meta-analysis encompass 3156 articles published over the last three decades. We assessed 74 publications to determine the prevalence of HPV in BC tissues after a rigorous evaluation of 1223 studies, of which 1130 were deemed inadequate. Our findings have been supported by Simoes et al. (38) meta-analyses conducted in Europe, North America, and Australia. According to the studies, HPV infection was more common in Iranian BC patients than in European women but less common in North American and Australian women. Worldwide HPV infection in BC reported up to 86%, indicating disparities between countries may be influenced by demographic factors and geographical differences (2, 38, 117). The conclusion drawn from the previously published figures—approximately one in four women diagnosed with BC have HPV infection (115).
There was a substantial variation in the prevalence of HPV strains observed across various populations (118). HR HPV infections, including HPV 16, 18, and 33, reported as the common causes of genital atypical lesions and cancer, have been identified in BC (56, 119). Intriguingly, HPV 11, 16, 18, and 33 were the most common types in European women with BC, whereas HPV 52, 59, and 83, either HR or LR subtypes, were more prevalent in Asian women (50, 119, 120). In this research, we analyzed HR subtypes of HPV, namely HPV 16, 18, 31, 33, 35, 52, and 58. Our study revealed that all these HR HPVs were associated with an elevated risk of developing BC (p<0.05). Our study corroborates the existing literature and suggests that HPV infection may have a causal or contributory effect on BC initiation and progression. The prevalence of HR HPVs in BC tissue is six times higher than in normal and benign breast tissue controls (121). Moreover, HPV-16 was reported in BC tissues in 21 studies with an overall prevalence of 9.7% (95% CI= 3.15-11.73, I2 = 0%, τ2 = 0.5766, p< 0.01). The second most common type found in BC tissues, as reported in 21 studies, was HPV-18 with a prevalence of 6.6% (95% CI=1.95-4.04, I2 = 0%, τ2 = 0.2734, p< 0.01).
In this study, we aimed to investigate the prevalence and distribution of different HPV subtypes in BC tissues from various geographical regions. We found that HPV-16 was the most frequent subtype in Asia, America, Europe, and Africa, while HPV-18 was more prevalent in Australia. A total of 45 studies were examined, with the majority (n=25) published in Asia, indicating a prevalence of (22.7%) overall. We also observed regional variations in the prevalence of HPV among BC patients, with Europe having the highest rate (39.1%), followed by Africa (31.8%), Australia (30.5%), and America (30.3%). Our results suggest that the prevalence of HPV in BC patients varies by location, which may have implications for developing regionally specific methods for preventing and treating BC caused by HPV.
The regional variation of HPV infection and its association with breast cancer may be attributed to multiple factors, such as sexual behavior, hygiene, screening, vaccination, and socioeconomic status. For instance, Asia has a high prevalence of HPV in breast cancer (22.7%) due to its large population of women with multiple sexual partners, low condom use, and limited access to cervical cancer screening and HPV vaccination (122–127). Conversely, Europe has a lower prevalence of HPV in breast cancer (13.4%) due to its more developed health systems, higher awareness of HPV prevention, and higher coverage of HPV vaccination (128–130). Moreover, the oncogenic potential and distribution of HPV subtypes vary across regions (131). For example, HPV 16 and 18, the most common and carcinogenic subtypes of HPV, are more prevalent in breast cancer tissues than other subtypes but are not evenly distributed worldwide (119, 131, 132). Therefore, the regional variation of HPV subtypes may influence the risk of developing breast cancer. Furthermore, the availability and quality of data, the methods of detection, and the selection of samples may affect the estimation of HPV prevalence in breast cancer by country and region (119, 133). However, these results may also be influenced by the heterogeneity of the studies, the sensitivity and specificity of the assays, and the representativeness of the samples (134, 135).
The link between HPV and BC has not been conclusively established, which raises questions about the potential underlying mechanism. The simple detection of HPV is insufficient evidence to prove the virus’s role as a causal agent in the development of BC pathophysiology, and this fact has to be addressed. On the other hand, it is predicted that HPV infection will commence the progression of BC, ultimately resulting in accumulative changes over time similar to the process of cervical carcinogenesis (136). Many hypotheses were offered. Elevated levels of inflammatory cytokines (IL-1, IL-6, IL-17, TGF-, TNF-, and NF-kB) and tumor growth were associated with HPV (137). On the other hand, an essential functional protein, the E6 protein, interacts with p53 and collaborates with BCL2 antagonist/killer (BAK 34) to induce chromosomal instability and apoptotic resistance (138). On the other hand, E7 proteins interact with retinoblastoma (RB) proteins, which triggers the release of E2F (transcription factor), which enhances cellular proliferation. The overexpression of S-phase genes like cyclin A and E is facilitated by E7, which concurrently suppresses cyclin-dependent kinase inhibitors (WAF1/p21) and kinesin-like protein (KIP1/p27) (18, 139). Breast and ovarian cancer susceptibility gene-1 (BRCA1) and BRCA2 are critical biological components linked to HPV proteins. The tumor suppressor activities of these genes are widely recognized for their ability to repair DNA damage and prevent tumor formation, while these proteins activate c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) to induce apoptosis (140–142). The interaction of E7 and E6 with BRCA1 suppresses several BRCA1-mediated activities (143). In addition, in HPV-induced carcinogenesis, the overexpression of E6 and E7 oncogenes has significant biological importance and role in cancer progression (118, 144, 145). In addition, Michael B. Burns’s study team showed that mutations and deletions in the DNA cytosine deaminase APOBEC3B (A3B), which inhibits retroviral replication, might increase BC risk (146). The team of Vieira and Ohba found out in 2014 that HPV could potentially modify the expression of APOBEC3B (A3B) (147, 148). Hence, it is feasible to hypothesize that HPV may contribute to early BC development by influencing APOBEC3B (A3B) (114, 146).
This systematic review aimed to determine the global prevalence of HPV in BC tissues. In addition, we conducted a meta-analysis of case-control studies, which provided more reliable and informative results than studies that included only positive cases. This study provides a more comprehensive picture of the prevalence patterns of HPV infection by incorporating primary studies from different geographical regions around the globe. It also identifies the most vulnerable communities for targeted interventions.
4.1 Limitations
One of the limitations of our study was the inability to determine the association between HPV and BC mortality, as none of the primary studies in our systematic review/meta-analysis addressed this issue. Moreover, we could not examine the effect of coinfections on BC aggressiveness due to insufficient data. To establish the causal role of HPV in BC, it is essential to detect both integrated and free virus DNA, which will improve the methodological quality of HPV detection.
5 Conclusion
To estimate the global prevalence of HPV in BC tissues, we performed a meta-analysis of data from multiple studies conducted within the last 30 years. Our findings indicate that HPV infection is a risk factor for developing BC. In addition, we discovered that the prevalence of HPV infection in BC patients is substantially higher than in the general population. Incorporating HPV testing of breast ductal lavage, nasopharyngeal discharge, and breast milk into the cervical screening program is essential for determining breast cancer risk and facilitating early detection. In addition, HPV vaccination may reduce the incidence of breast cancer. However, no research has investigated the link between HPV vaccination and cancer occurrence. Given the heterogeneity and high likelihood of HPV infection in BC lesions, additional rigorous studies employing standardized testing methods are necessary to clarify the role of HPV in BC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
UA, NA, and AK were involved in the conceptualization, formal analysis, data collection, visualization, and writing the first draft of the manuscript. SA, SKa, UA, SKh, and XG did the data validation, statistical analysis, data interpretation, and figure editing. UA, XG, ZY, and JL were involved in conceptualizing, designing, and supervising the study and did the final revision of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved the final version of the manuscript.
Funding
The Science and Technology Developmental Plan of Henan Province (Grant No.232102520025), The National Foreign Expert Program of China (Grant No. QN2022026001L), and Zhengzhou University start-up grant for high talent full time researchers
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1219161/full#supplementary-material
References
1. Kroupis C, Markou A, Vourlidis N, Dionyssiou-Asteriou A, Lianidou ES. Presence of high-risk human papillomavirus sequences in breast cancer tissues and association with histopathological characteristics. Clin Biochem (2006) 39:727–31. doi: 10.1016/j.clinbiochem.2006.03.005
2. Bae J-M, Kim EH. Human papillomavirus infection and risk of breast cancer: a meta-analysis of case-control studies. Infect Agents cancer. (2016) 11:1–8. doi: 10.1186/s13027-016-0058-9
3. Yu Y, Xiao C, Tan L, Wang Q, Li X, Feng Y. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J cancer. (2014) 110:724–32. doi: 10.1038/bjc.2013.768
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
5. World Health Organization (WHO). Breast cancer (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
6. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: Cancer J Clin (2005) 55:74–108. doi: 10.3322/canjclin.55.2.74
7. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J cancer. (2006) 118:3030–44. doi: 10.1002/ijc.21731
8. Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe (2014) 15:295–305. doi: 10.1016/j.chom.2014.02.003
9. Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res (2004) 6:1–6. doi: 10.1186/bcr921
10. Li J, Ding J, Zhai K. Detection of human papillomavirus DNA in patients with breast tumor in China. PLoS One (2015) 10:e0136050. doi: 10.1371/journal.pone.0136050
11. de Lima E G, do Amaral C MM, Peixe F CQ, Gurgel A PAD, da Costa Silva Neto J C, de Freitas A. Putative mechanisms of viral transmission and molecular dysregulation of mammary epithelial cells by human papillomavirus: implications for breast cancer. Curr Mol Med (2016) 16:650–9. doi: 10.2174/1566524016666160805120410
12. Alibek K, Kakpenova A, Mussabekova A, Sypabekova M, Karatayeva N. Role of viruses in the development of breast cancer. Infect Agents cancer. (2013) 8:1–6. doi: 10.1186/1750-9378-8-32
13. Park IH, Ko K, Joo J, Park B, Jung S-Y, Lee S, et al. High volumetric breast density predicts risk for breast cancer in postmenopausal, but not premenopausal, Korean women. Ann Surg Oncol (2014) 21:4124–32. doi: 10.1245/s10434-014-3832-1
14. Lawson JS, Salmons B, Glenn WK. Oncogenic viruses and breast cancer: mouse mammary tumor virus (MMTV), bovine leukemia virus (BLV), human papilloma virus (HPV), and epstein–barr virus (EBV). Front Oncol (2018) 8:1. doi: 10.3389/fonc.2018.00001
15. Burk RD, Chen Z, Van Doorslaer K. Human papillomaviruses: genetic basis of carcinogenicity. Public Health Genomics (2009) 12:281–90. doi: 10.1159/000214919
16. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev (2003) 16:1–17. doi: 10.1128/CMR.16.1.1-17.2003
17. Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene (2003) 22:5201–7. doi: 10.1038/sj.onc.1206554
18. Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev cancer. (2002) 2:342–50. doi: 10.1038/nrc798
19. Alhamlan FS, Alfageeh MB, Al Mushait MA, Al-Badawi IA, Al-Ahdal MN. Human papillomavirus-associated cancers. Microbial Pathogenesis: Infection and Immunity. (2021) 1–4. doi: 10.1007/978-3-030-67452-6_1
20. Muñoz N, Bosch FX, De Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New Engl J Med (2003) 348:518–27. doi: 10.1056/NEJMoa021641
21. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet (2007) 370:890–907. doi: 10.1016/S0140-6736(07)61416-0
22. De Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J cancer. (2017) 141:664–70. doi: 10.1002/ijc.30716
23. Asiaf A, Ahmad ST, Mohammad SO, Zargar MA. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev (2014) 23:206–24. doi: 10.1097/CEJ.0b013e328364f273
24. Buchanan TR, Graybill WS, Pierce JY. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum Vaccines Immunotherapeutics (2016) 12:1352–6. doi: 10.1080/21645515.2016.1147634
25. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine (2012) 30:F12–23. doi: 10.1016/j.vaccine.2012.07.055
26. De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol (2012) 13:607–15. doi: 10.1016/S1470-2045(12)70137-7
27. Stevens-Simon C, Nelligan D, Breese P, Jenny C, Douglas JM Jr. The prevalence of genital human papillomavirus infections in abused and nonabused preadolescent girls. Pediatrics (2000) 106:645–9. doi: 10.1542/peds.106.4.645
28. Lawson JS, Glenn WK, Salyakina D, Clay R, Delprado W, Cheerala B, et al. Human papilloma virus identification in breast cancer patients with previous cervical neoplasia. Front Oncol (2016) 5:298. doi: 10.3389/fonc.2015.00298
29. Bodaghi S, Wood LV, Roby G, Ryder C, Steinberg SM, Zheng Z-M. Could human papillomaviruses be spread through blood? J Clin Microbiol (2005) 43:5428–34. doi: 10.1128/JCM.43.11.5428-5434.2005
30. Chang P, Wang T, Yao Q, Lv Y, Zhang J, Guo W, et al. Absence of human papillomavirus in patients with breast cancer in north-west China. Med Oncol (2012) 29:521–5. doi: 10.1007/s12032-011-9945-5
31. Mou X, Chen L, Liu F, Shen Y, Wang H, Li Y, et al. Low prevalence of human papillomavirus (HPV) in Chinese patients with breast cancer. J Int Med Res (2011) 39:1636–44. doi: 10.1177/147323001103900506
32. Hsu C-R, Lu T-M, Chin LW, Yang C-C. Possible DNA viral factors of human breast cancer. Cancers (2010) 2:498–512. doi: 10.3390/cancers2020498
33. Moradi A, Mobasheri E, Tabarraei A, Bakhshandeh Nosrat S, Azarhosh R, Alizadeh S, et al. Molecular epidemiology of Human Papillomaviruses in breast cancer, Golestan province of Iran. Med Lab J (2009) 3:0–0.
34. Tahmasebi Fard Z, Abdirad A, Saatian M, Arefian L. Association between human Papillomavirus (HPV) and breast cancer in Iranian patients. Med Sci J Islamic Azad Univesity-Tehran Med Branch (2013) 23:120–6.
35. Sudarshan SR, Schlegel R, Liu X. Two conserved amino acids differentiate the biology of high-risk and low-risk HPV E5 proteins. J Med Virol (2022) 94(9):4565–75. doi: 10.1002/jmv.27829
36. Ashrafi GH, Haghshenas MR, Marchetti B, O'Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J cancer. (2005) 113:276–83. doi: 10.1002/ijc.20558
37. Di Lonardo A, Venuti A, Marcante ML. Human papillomavirus in breast cancer. Breast Cancer Res Treat (1992) 21:95–100. doi: 10.1007/bf01836955
38. Simões PW, Medeiros LR, Pires PDS, Edelweiss MI, Rosa DD, Silva FR, et al. Prevalence of human papillomavirus in breast cancer: a systematic review. Int J Gynecologic Cancer (2012) 22:343–7. doi: 10.1097/IGC.0b013e31823c712e
39. Heng B, Glenn WK, Ye Y, Tran B, Delprado W, Lutze-Mann L, et al. Human papilloma virus is associated with breast cancer. Br J Cancer (2009) 20101:1345–50. doi: 10.1038/sj.bjc.6605282
40. de Cremoux P, Thioux M, Lebigot I, Sigal-Zafrani B, Salmon R, Sastre-Garau X. No evidence of human papillomavirus DNA sequences in invasive breast carcinoma. Breast Cancer Res Treat (2008) 109:55–8. doi: 10.1007/s10549-007-9626-4
41. Lindel K, Forster A, Altermatt HJ, Greiner R, Gruber G. Breast cancer and human papillomavirus (HPV) infection: no evidence of a viral etiology in a group of Swiss women. breast (2007) 16:172–7. doi: 10.1016/j.breast.2006.09.001
42. Hedau S, Kumar U, Hussain S, Shukla S, Pande S, Jain N, et al. Breast cancer and human papillomavirus infection: no evidence of HPV etiology of breast cancer in Indian women. BMC cancer. (2011) 11:1–10. doi: 10.1186/1471-2407-11-27
43. National Heart Labi. Study Quality Assessment Tools- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (2021). Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
44. Hennig EM, Suo Z, Thoresen S, Holm R, Kvinnsland S, Nesland JM. Human papillomavirus 16 in breast cancer of women treated for high grade cervical intraepithelial neoplasia (CIN III). Breast Cancer Res Treat (1999) 53:121–35. doi: 10.1023/a:1006162609420
45. Yu Y, Morimoto T, Sasa M, Okazaki K, Harada Y, Fujiwara T, et al. Human papillomavirus type 33 DNA in breast cancer in Chinese. Breast Cancer (Tokyo Japan) (2000) 7:33–6. doi: 10.1007/bf02967185
46. Damin AP, Karam R, Zettler CG, Caleffi M, Alexandre CO. Evidence for an association of human papillomavirus and breast carcinomas. Breast Cancer Res Treat (2004) 84:131–7. doi: 10.1023/B:BREA.0000018411.89667.0d
47. Widschwendter A, Brunhuber T, Wiedemair A, Mueller-Holzner E, Marth C. Detection of human papillomavirus DNA in breast cancer of patients with cervical cancer history. J Clin Virol (2004) 31(4):292–7. doi: 10.1016/j.jcv.2004.06.009
48. de Villiers EM, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res BCR. (2005) 7:R1–11. doi: 10.1186/bcr940
49. Kan CY, Iacopetta BJ, Lawson JS, Whitaker NJ. Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J cancer. (2005) 93(8):946–8. doi: 10.1038/sj.bjc.6602778
50. Gumus M, Yumuk PF, Salepci T, Aliustaoglu M, Dane F, Ekenel M, et al. HPV DNA frequency and subset analysis in human breast cancer patients' normal and tumoral tissue samples. J Exp Clin Cancer Res (2006) 25(4):515–21.
51. Choi YL, Cho EY, Kim JH, Nam SJ, Oh YL, Song SY, et al. Detection of human papillomavirus DNA by DNA chip in breast carcinomas of Korean women. Tumour Biol (2007) 28:327–32. doi: 10.1159/000124238
52. Tsai JH, Hsu CS, Tsai CH, Su JM, Liu YT, Cheng MH, et al. Relationship between viral factors, axillary lymph node status and survival in breast cancer. J Cancer Res Clin Oncol (2007) 133:13–21. doi: 10.1007/s00432-006-0141-5
53. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer (2008) 599:404–7. doi: 10.1038/sj.bjc.6604503
54. C-l F, J-h Z, C-y Hu. Expression of human papillomavirus in mammary carcinoma and its possible mechanism in carcinogenesis. Virologica Sin (2008) 23:226–31. doi: 10.1007/s12250-008-2914-2
55. Duò D, Ghimenti C, Migliora P, Pavanelli MC, Mastracci L, Angeli G. Identification and characterization of human papillomavirus DNA sequences in Italian breast cancer patients by PCR and line probe assay reverse hybridization. Mol Med Rep (2008) 1:673–7. doi: 10.3892/mmr_00000011
56. Khan N, Castillo A, Koriyama C, Kijima Y, Umekita Y, Ohi Y, et al. Human papillomavirus detected in female breast carcinomas in Japan. Br J cancer. (2008) 99:408–14. doi: 10.1038/sj.bjc.6604502
57. Mendizabal-Ruiz AP, Morales JA, Ramírez-Jirano LJ, Padilla-Rosas M, Morán-Moguel MC, Montoya-Fuentes H. Low frequency of human papillomavirus DNA in breast cancer tissue. Breast Cancer Res Treat (2009) 114:189–94. doi: 10.1007/s10549-008-9989-1
58. de León DC, Montiel DP, Nemcova J, Mykyskova I, Turcios E, Villavicencio V, et al. Human papillomavirus (HPV) in breast tumors: prevalence in a group of Mexican patients. BMC Cancer (2009) 229:26. doi: 10.1186/1471-2407-9-26
59. He Q, Zhang SQ, Chu YL, Jia XL, Wang XL. The correlations between HPV16 infection and expressions of c-erbB-2 and bcl-2 in breast carcinoma. Mol Biol Rep (2009) 36:807–12. doi: 10.1007/s11033-008-9249-9
60. Aguayo F, Khan N, Koriyama C, González C, Ampuero S, Padilla O, et al. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect Agents Cancer (2011) 236:7. doi: 10.1186/1750-9378-6-7
61. Antonsson A, Spurr TP, Chen AC, Francis GD, McMillan NA, Saunders NA, et al. High prevalence of human papillomaviruses in fresh frozen breast cancer samples. J Med Virol (2011) 83:2157–63. doi: 10.1002/jmv.22223
62. Herrera-Goepfert R, Khan NA, Koriyama C, Akiba S, Pérez-Sánchez VM. High-risk human papillomavirus in mammary gland carcinomas and non-neoplastic tissues of Mexican women: no evidence supporting a cause and effect relationship. Breast (2011) 20(2):184–9. doi: 10.1016/j.breast.2010.11.006
63. Baltzell K, Buehring GC, Krishnamurthy S, Kuerer H, Shen HM, Sison JD. Limited evidence of human papillomavirus in [corrected] breast tissue using molecular in situ methods. Cancer (2012) 1118:1212–20. doi: 10.1002/cncr.26389
64. Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PloS One (2012) 7:e48788. doi: 10.1371/journal.pone.0048788
65. Sigaroodi A, Nadji SA, Naghshvar F, Nategh R, Emami H, Velayati AA. Human papillomavirus is associated with breast cancer in the north part of Iran. TheScientificWorldJournal (2012) 2012:837191. doi: 10.1100/2012/837191
66. Divani SN, Giovani AM. Detection of human papillomavirus DNA in fine needle aspirates of women with breast cancer. Arch Oncol (2012) 20:12–4. doi: 10.2298/AOO1202012D
67. Frega A, Lorenzon L, Bononi M, De Cesare A, Ciardi A, Lombardi D, et al. Evaluation of E6 and E7 mRNA expression in HPV DNA positive breast cancer. Eur J Gynaecol Oncol (2012) 33:164–7.
68. Pereira Suarez AL, Lorenzetti MA, Gonzalez Lucano R, Cohen M, Gass H, Martinez Vazquez P, et al. Presence of human papilloma virus in a series of breast carcinoma from Argentina. PloS One (2013) 8:e61613. doi: 10.1371/journal.pone.0061613
69. Francis IM, Al-Ayadhy B, Al-Awadhi S, Kapila K, Al-Mulla F. Prevalence and correlation of human papilloma virus and its types with prognostic markers in patients with invasive ductal carcinoma of the breast in kuwait. Sultan Qaboos Univ Med J (2013), 13(4):527–33. doi: 10.12816/0003311
70. Herrera-Goepfert R, Vela-Chávez T, Carrillo-García A, Lizano-Soberón M, Amador-Molina A, Oñate-Ocaña LF, et al. High-risk human papillomavirus (HPV) DNA sequences in metaplastic breast carcinomas of Mexican women. BMC Cancer (2013) 13(1). doi: 10.1186/1471-2407-13-445
71. Hossein R, Behzad S, Tahar M, Azadeh NA. Prevalence of human papillomavirus genotypes associated with cervical and breast cancers in iran. Monoclonal antibodies immunodiagnosis immunotherapy (2013) 32(6):399–403. doi: 10.1089/mab.2013.0047
72. Liang W, Wang J, Wang C, Lv Y, Gao H, Zhang K, et al. Detection of high-risk human papillomaviruses in fresh breast cancer samples using the hybrid capture 2 assay. J Med Virol (2013) 85:2087–92. doi: 10.1002/jmv.23703
73. Piana AF, Sotgiu G, Muroni MR, Cossu-Rocca P, Castiglia P, De Miglio MR. HPV infection and triple-negative breast cancers: an Italian case-control study. Virol J (2014) 2111:190. doi: 10.1186/s12985-014-0190-3
74. Corbex M, Bouzbid S, Traverse-Glehen A, Aouras H, McKay-Chopin S, Carreira C, et al. Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from algeria compared with other types of breast cancer tumors. PLoS One (2014) 9:e114559. doi: 10.1371/journal.pone.0114559
75. Ahangar-Oskouee M, Shahmahmoodi S, Jalilvand S, Mahmoodi M, Ziaee AA, Esmaeili H-A, et al. No detection of'high-risk'human papillomaviruses in a group of Iranian women with breast cancer. Asian Pacific J Cancer Prev (2014) 15:4061–5. doi: 10.7314/APJCP.2014.15.9.4061
76. Manzouri L, Salehi R, Shariatpanahi S, Rezaie P. Prevalence of human papilloma virus among women with breast cancer since 2005-2009 in Isfahan. Adv BioMed Res (2014) 3:75. doi: 10.4103/2277-9175.125873
77. Ali SH, Al-Alwan NA, Al-Alwany SH. Detection and genotyping of human papillomavirus in breast cancer tissues from Iraqi patients. East Mediterr Health J (2014) 1820:372–7. doi: 10.26719/2014.20.6.372
78. Peng J, Wang T, Zhu H, Guo J, Li K, Yao Q, et al. Multiplex PCR/mass spectrometry screening of biological carcinogenic agents in human mammary tumors. J Clin Virol (2014) 61:255–9. doi: 10.1016/j.jcv.2014.07.010
79. Hong L, Tang S. Does HPV 16/18 infection affect p53 expression in invasive ductal carcinoma? An experimental study. Pak J Med Sci (2014) 30(4):789–92. doi: 10.12669/pjms.304.4534
80. Gannon OM, Antonsson A, Milevskiy M, Brown MA, Saunders NA, Bennett IC. No association between HPV positive breast cancer and expression of human papilloma viral transcripts. Sci Rep (2015) 145:18081. doi: 10.1038/srep18081
81. Fernandes A, Bianchi G, Feltri AP, Pérez M, Correnti M. Presence of human papillomavirus in breast cancer and its association with prognostic factors. Ecancermedicalscience (2015) 9:548. doi: 10.3332/ecancer.2015.548
82. Lawson JS, Glenn WK, Salyakina D, Clay R, Delprado W, Cheerala B, et al. Human papilloma virus identification in breast cancer patients with previous cervical neoplasia. Front Oncol (2015) 5:298. doi: 10.3389/fonc.2015.00298
83. Fu L, Wang D, Shah W, Wang Y, Zhang G, He J. Association of human papillomavirus type 58 with breast cancer in Shaanxi province of China. J Med Virol (2015) 87:1034–40. doi: 10.1002/jmv.24142
84. Salehpour M, Tayyebi Meibodi N, Teimourpour R, Ghorani-Azam A, Sepahi S, Rostami S, et al. Frequency of human papillomavirus genotypes 6, 11, 16, 18 and 31 in paraffin-embedded tissue samples of invasive breast carcinoma, North- East of Iran. Iranian J Pathol (2015) 10:192–8.
85. Ilahi NE, Anwar S, Noreen M, Hashmi SN, Murad S. Detection of human papillomavirus-16 DNA in archived clinical samples of breast and lung cancer patients from North Pakistan. J Cancer Res Clin Oncol (2016) 142:2497–502. doi: 10.1007/s00432-016-2251-z
86. Mohtasebi P, Rassi H, Maleki F, Hajimohammadi S, Bagheri Z, Fakhar Miandoab M, et al. Detection of human papillomavirus genotypes and major BRCA mutations in familial breast cancer. Monoclonal antibodies immunodiagnosis immunotherapy (2016) 35:135–40. doi: 10.1089/mab.2015.0081
87. Yan C, Teng ZP, Chen YX, Shen DH, Li JT, Zeng Y. Viral etiology relationship between human papillomavirus and human breast cancer and target of gene therapy. BioMed Environ Sci (2016) 29(5):331–9. doi: 10.3967/bes2016.043
88. Zhang N, Ma ZP, Wang J, Bai HL, Li YX, Sun Q, et al. Human papillomavirus infection correlates with inflammatory Stat3 signaling activity and IL-17 expression in patients with breast cancer. Am J Transl Res (2016) 8:3214–26.
89. Wang D, Fu L, Shah W, Zhang J, Yan Y, Ge X, et al. Presence of high risk HPV DNA but indolent transcription of E6/E7 oncogenes in invasive ductal carcinoma of breast. Pathol Res Pract (2016) 212:1151–6. doi: 10.1016/j.prp.2016.09.009
90. Doosti M, Bakhshesh M, Zahir ST, Shayestehpour M, Karimi-Zarchi M. Lack of evidence for a relationship between high risk human papillomaviruses and breast cancer in iranian patients. Asian Pac J Cancer Prev (2016) 17:4357–61.
91. Wang YW, Zhang K, Zhao S, Lv Y, Zhu J, Liu H, et al. HPV Status and Its Correlation with BCL2, p21, p53, Rb, and Survivin Expression in Breast Cancer in a Chinese Population. BioMed Res Int (2017) 2017:6315392. doi: 10.1155/2017/6315392
92. Delgado-García S, Martínez-Escoriza JC, Alba A, Martín-Bayón TA, Ballester-Galiana H, Peiró G, et al. Presence of human papillomavirus DNA in breast cancer: a Spanish case-control study. BMC Cancer (2017) 817:320. doi: 10.1186/s12885-017-3308-3
93. Naushad W, Surriya O, Sadia H. Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: A possible etiological role of viruses in breast cancer. Infection Genet Evol (2017) 54:230–7. doi: 10.1016/j.meegid.2017.07.010
94. Ngamkham J, Karalak A, Chaiwerawattana A, Sornprom A, Thanasutthichai S, Sukarayodhin S, et al. Prevalence of human papillomavirus infection in breast cancer cells from Thai women. Asian Pac J Cancer Prev (2017) 2718:1839–45. doi: 10.22034/apjcp.2017.18.7.1839
95. Ladera M, Fernandes A, López M, Pesci-Feltri A, Ávila M, Correnti M. Presence of human papillomavirus and Epstein-Barr virus in breast cancer biopsies as potential risk factors. Gaceta Mexicana Oncologia (2017) 16:103–8. doi: 10.24875/j.gamo.17000018
96. Salman NA, Davies G, Majidy F, Shakir F, Akinrinade H, Perumal D, et al. Association of High Risk Human Papillomavirus and Breast cancer: A UK based Study. Sci Rep (2017) 277:43591. doi: 10.1038/srep43591
97. Islam S, Dasgupta H, Roychowdhury A, Bhattacharya R, Mukherjee N, Roy A, et al. Study of association and molecular analysis of human papillomavirus in breast cancer of Indian patients: Clinical and prognostic implication. PloS One (2017) 12:e0172760. doi: 10.1371/journal.pone.0172760
98. ElAmrani A, Gheit T, Benhessou M, McKay-Chopin S, Attaleb M, Sahraoui S, et al. Prevalence of mucosal and cutaneous human papillomavirus in Moroccan breast cancer. Papillomavirus Res (Amsterdam Netherlands) (2018) 5:150–5. doi: 10.1016/j.pvr.2018.04.003
99. Habyarimana T, Attaleb M, Mazarati JB, Bakri Y, El Mzibri M. Detection of human papillomavirus DNA in tumors from Rwandese breast cancer patients. Breast Cancer (Tokyo Japan) (2018) 25:127–33. doi: 10.1007/s12282-018-0831-2
100. Bønløkke S, Blaakær J, Steiniche T, Høgdall E, Jensen SG, Hammer A, et al. Evidence of no association between human papillomavirus and breast cancer. Front Oncol (2018) 8:209. doi: 10.3389/fonc.2018.00209
101. Ghaffari H, Nafissi N, Hashemi-Bahremani M, Alebouyeh MR, Tavakoli A, Javanmard D, et al. Molecular prevalence of human papillomavirus infection among Iranian women with breast cancer. Breast disease (2018) 37:207–13. doi: 10.3233/bd-180333
102. Malekpour Afshar R, Balar N, Mollaei HR, Arabzadeh SA, Iranpour M. Low prevalence of human papilloma virus in patients with breast cancer, Kerman; Iran. Asian Pac J Cancer Prev (2018) 2919:3039–44. doi: 10.31557/apjcp.2018.19.11.3039
103. Cavalcante JR, Pinheiro LGP, Almeida PRC, Ferreira MVP, Cruz GA, Campelo TA, et al. Association of breast cancer with human papillomavirus (HPV) infection in Northeast Brazil: molecular evidence. Clinics (Sao Paulo) (2018) 1873:e465. doi: 10.6061/clinics/2018/e465
104. Khodabandehlou N, Mostafaei S, Etemadi A, Ghasemi A, Payandeh M, Hadifar S, et al. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC Cancer (2019) 1419:61. doi: 10.1186/s12885-019-5286-0
105. De Carolis S, Storci G, Ceccarelli C, Savini C, Gallucci L, Sansone P, et al. HPV DNA associates with breast cancer malignancy and it is transferred to breast cancer stromal cells by extracellular vesicles. Front Oncol (2019) 9:860. doi: 10.3389/fonc.2019.00860
106. Balci FL, Uras C, Feldman SM. Is human papillomavirus associated with breast cancer or papilloma presenting with pathologic nipple discharge? Cancer Treat Res Commun (2019) 19:100122. doi: 10.1016/j.ctarc.2019.100122
107. Tawfeik AM, Mora A, Osman A, Moneer MM, El-Sheikh N, Elrefaei M. Frequency of CD4+ regulatory T cells, CD8+ T cells, and human papilloma virus infection in Egyptian Women with breast cancer. Int J Immunopathology Pharmacol (2020) 34:2058738420966822. doi: 10.1177/2058738420966822
108. Sher G, Salman NA, Kulinski M, Fadel RA, Gupta VK, Anand A, et al. Prevalence and type distribution of high-risk human Papillomavirus (HPV) in breast cancer: a qatar based study. Cancers (2020) 12:1528. doi: 10.3390/cancers12061528
109. Elagali AM, Suliman AA, Altayeb M, Dannoun AI, Parine NR, Sakr HI, et al. Human papillomavirus, gene mutation and estrogen and progesterone receptors in breast cancer: A cross-sectional study. Pan Afr Med J (2021) 38:1–16. doi: 10.11604/pamj.2021.38.43.22013
110. Gupta I, Jabeen A, Al-Sarraf R, Farghaly H, Vranic S, Sultan AA, et al. The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Hum Vaccin Immunother (2021) 317:982–9. doi: 10.1080/21645515.2020.1802977
111. Metwally SA, Abo-Shadi MA, Abdel Fattah NF, Barakat AB, Rabee OA, Osman AM, et al. Presence of HPV, EBV and HMTV viruses among Egyptian breast cancer women: molecular detection and clinical relevance. Infect Drug Resist (2021) 14:2327–39. doi: 10.2147/idr.S313219
112. Glenn WK, Whitaker NJ, Lawson JS. High risk human papillomavirus and Epstein Barr virus in human breast milk. BMC Res notes. (2012) 15:477. doi: 10.1186/1756-0500-5-477
113. Joshi D, Buehring GC. Are viruses associated with human breast cancer? Scrutinizing the molecular evidence-response to James F. Holland and Beatriz GT Pogo. Breast Cancer Res Treat (2012) 136:306–7. doi: 10.1007/s10549-011-1921-4
114. Ren C, Zeng K, Wu C, Mu L, Huang J, Wang M. Human papillomavirus infection increases the risk of breast carcinoma: a large-scale systemic review and meta-analysis of case-control studies. Glan Surg (2019) 8:486. doi: 10.21037/gs.2019.09.04
115. Li N, Bi X, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and sporadic breast carcinoma risk: a meta-analysis. Breast Cancer Res Treat (2011) 126:515–20. doi: 10.1007/s10549-010-1128-0
116. Lawson JS, Glenn WK. Catching viral breast cancer. Infect Agents cancer. (2021) 16:1–11. doi: 10.1186/s13027-021-00366-3
117. de Villiers E-M, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res (2004) 7:1–11. doi: 10.1186/bcr940
118. Islam MS, Chakraborty B, Panda CK. Human papilloma virus (HPV) profiles in breast cancer: future management. Ann Trans Med (2020) 8:650. doi: 10.21037/atm-19-2756
119. Wang T, Chang P, Wang L, Yao Q, Guo W, Chen J, et al. The role of human papillomavirus infection in breast cancer. Med Oncol (2012) 29:48–55. doi: 10.1007/s12032-010-9812-9
120. Joshi D, Buehring GC. Are viruses associated with human breast cancer? Scrutinizing the molecular evidence. Breast Cancer Res Treat (2012) 135:1–15. doi: 10.1007/s10549-011-1921-4
121. Schlichting JA, Soliman AS, Schairer C, Harford JB, Hablas A, Ramadan M, et al. Breast cancer by age at diagnosis in the Gharbiah, Egypt, population-based registry compared to the United States surveillance, epidemiology, and end results program, 2004–2008. BioMed Res Int (2015) 2015). doi: 10.1155/2015/381574
122. Domingo EJ, Noviani R, Noor MRM, Ngelangel CA, Limpaphayom KK, Van Thuan T, et al. Epidemiology and prevention of cervical cancer in Indonesia, Malaysia, the Philippines, Thailand and Vietnam. Vaccine (2008) 26:M71–9. doi: 10.1016/j.vaccine.2008.05.039
123. Awan UA, Khattak AA. Has Pakistan failed to roll back HPV? Lancet Oncol (2022) 23:e204. doi: 10.1016/S1470-2045(22)00141-3
124. Awan UA, Guo X, Khattak AA, Hassan U, Bashir S. HPV vaccination and cervical cancer screening in Afghanistan threatened. Lancet Infect Diseases (2023) 23:141–2. doi: 10.1016/S1473-3099(22)00868-4
125. Gerend MA, Magloire ZF. Awareness, knowledge, and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health (2008) 42:237–42. doi: 10.1016/j.jadohealth.2007.08.022
126. Foss AM, Hossain M, Vickerman PT, Watts CH. A systematic review of published evidence on intervention impact on condom use in sub-Saharan Africa and Asia. Sexually transmitted infections (2007) 83:510–6. doi: 10.1136/sti.2007.027144
127. Schellekens MC, Dijkman A, Aziz MF, Siregar B, Cornain S, Kolkman-Uljee S, et al. Prevalence of single and multiple HPV types in cervical carcinomas in Jakarta, Indonesia. Gynecologic Oncol (2004) 93:49–53. doi: 10.1016/j.ygyno.2003.12.015
128. Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med (2021) 144:106399. doi: 10.1016/j.ypmed.2020.106399
129. Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. HPV-vaccination and cancer cervical screening in 53 WHO European Countries: an update on prevention programs according to income level. Cancer Med (2019) 8:2524–34. doi: 10.1002/cam4.2048
130. Nguyen-Huu N-H, Thilly N, Derrough T, Sdona E, Claudot F, Pulcini C, et al. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine (2020) 38:1315–31. doi: 10.1016/j.vaccine.2019.11.081
131. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J cancer (2003) 88:63–73. doi: 10.1038/sj.bjc.6600688
132. Pirog EC, Lloveras B, Molijn A, Tous S, Guimerà N, Alejo M, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Modern Pathol (2014) 27:1559–67. doi: 10.1038/modpathol.2014.55
133. Benevolo M, Vocaturo A, Caraceni D, French D, Rosini S, Zappacosta R, et al. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol (2011) 49:2643–50. doi: 10.1128/JCM.02570-10
134. Ogilvie G, Patrick D, Schulzer M, Sellors J, Petric M, Chambers K, et al. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sexually transmitted infections (2005) 81:207–12. doi: 10.1136/sti.2004.011858
135. Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Muzio LL, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007). Ann Oncol (2008) 19:1681–90. doi: 10.1093/annonc/mdn372
136. Malhone C, Longatto-Filho A, Filassi JR. Is human papilloma virus associated with breast cancer? A review of the molecular evidence. Acta Cytologica (2018) 62:166–77. doi: 10.1159/000487700
137. Khodabandehlou N, Mostafaei S, Etemadi A, Ghasemi A, Payandeh M, Hadifar S, et al. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC cancer. (2019) 19:1–11. doi: 10.1186/s12885-019-5286-0
138. Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev (2000) 14:3065–73. doi: 10.1101/gad.182100
139. Yim E-K, Park J-S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat (2005) 37:319–24. doi: 10.4143/crt.2005.37.6.319
140. Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer (2007) 7:937–48. doi: 10.1038/nrc2054
141. Rosen E, Fan S, Isaacs C. BRCA1 in hormonal carcinogenesis: basic and clinical research. Endocrine-Related Cancer (2005) 12:533–48. doi: 10.1677/erc.1.00972
142. Santivasi W, Wang H, Wang T, Yang Q, Mo X, Brogi E, et al. Association between cytosolic expression of BRCA1 and metastatic risk in breast cancer. Br J Cancer (2015) 113:453–9. doi: 10.1038/bjc.2015.208
143. Zhang Y, Fan S, Meng Q, Ma Y, Katiyar P, Schlegel R, et al. BRCA1 interaction with human papillomavirus oncoproteins. J Biol Chem (2005) 280:33165–77. doi: 10.1074/jbc.M505124200
144. Motoyama S, Ladines-Llave CA, Luis Villanueva S, Maruo T. The role of human papilloma virus in the molecular biology of cervical carcinogenesis. Kobe J Med Sci (2004) 50:9–19.
145. Mammas IN, Sourvinos G, Giannoudis A, Spandidos DA. Human papilloma virus (HPV) and host cellular interactions. Pathol Oncol Res (2008) 14:345–54. doi: 10.1007/s12253-008-9056-6
146. Burns MB, Lackey L, Carpenter MA, Rathore A, Land AM, Leonard B, et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature (2013) 494:366–70. doi: 10.1038/nature11881
147. Ohba K, Ichiyama K, Yajima M, Gemma N, Nikaido M, Wu Q, et al. In vivo and in vitro studies suggest a possible involvement of HPV infection in the early stage of breast carcinogenesis via APOBEC3B induction. PLoS One (2014) 9:e97787. doi: 10.1371/journal.pone.0097787
Keywords: human papillomavirus, breast cancer, viral oncology, HPV subtypes, breast carcinogenesis, meta-analysis, polymerase chain reaction (PCR), case-control studies
Citation: Awan UA, Khattak AA, Ahmed N, Guo X, Akhtar S, Kamran S, Yongjing Z, Liu J and Khan S (2023) An updated systemic review and meta-analysis on human papillomavirus in breast carcinogenesis. Front. Oncol. 13:1219161. doi: 10.3389/fonc.2023.1219161
Received: 08 May 2023; Accepted: 17 July 2023;
Published: 11 August 2023.
Edited by:
Ala-Eddin Al Moustafa, Qatar University, QatarReviewed by:
James Sutherland Lawson, University of New South Wales, AustraliaJ. Guilherme Gonçalves - Nobre, University of Lisbon, Portugal
Copyright © 2023 Awan, Khattak, Ahmed, Guo, Akhtar, Kamran, Yongjing, Liu and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Usman Ayub Awan, dXNtYW4uYXl1YjExMUBnbWFpbC5jb20=; Zhao Yongjing, emhhb3lvbmdqaW5nMjQzMkAxNjMuY29t; Jianbo Liu, amJsaXV6ekB6enUuZWR1LmNu; Xingyi Guo, eGluZ3lpLmd1b0B2dW1jLm9yZw==; Suliman Khan, c3VsaW1hbi5raGFuMThAbWFpbHMudWNhcy5hYy5jbg==; c3VsaW1hbi5raGFuMThAZ21haWwuY29t
 Usman Ayub Awan
Usman Ayub Awan Aamer Ali Khattak
Aamer Ali Khattak Noman Ahmed
Noman Ahmed Xingyi Guo
Xingyi Guo Sohail Akhtar
Sohail Akhtar Shehrish Kamran6
Shehrish Kamran6 Suliman Khan
Suliman Khan