- 1Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3GCP center, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objectives: To analyze clinicopathological risk factors and regular pattern of regional lymph node metastasis (LNM) in Chinese patients with T1 breast cancer and the effect on overall survival (OS) and disease-free survival (DFS).
Materials and methods: Between 1999 and 2020, breast cancer patients meeting inclusion criteria of unilateral, no distant metastatic site, and T1 invasive ductal carcinoma were reviewed. Clinical pathology characteristics were retrieved from medical records. Survival analysis was performed using Kaplan−Meier methods and an adjusted Cox proportional hazards model.
Results: We enrolled 11,407 eligible patients as a discovery cohort to explore risk factors for LNM and 3484 patients with stage T1N0 as a survival analysis cohort to identify the effect of those risk factors on OS and DFS. Compared with patients with N- status, patients with N+ status had a younger age, larger tumor size, higher Ki67 level, higher grade, higher HR+ and HER2+ percentages, and higher luminal B and HER2-positive subtype percentages. Logistic regression indicated that age was a protective factor and tumor size/higher grade/HR+ and HER2+ risk factors for LNM. Compared with limited LNM (N1) patients, extensive LNM (N2/3) patients had larger tumor sizes, higher Ki67 levels, higher grades, higher HR- and HER2+ percentages, and lower luminal A subtype percentages. Logistic regression indicated that HR+ was a protective factor and tumor size/higher grade/HER2+ risk factors for extensive LNM. Kaplan−Meier analysis indicated that grade was a predictor of both OS and DFS; HR was a predictor of OS but not DFS. Multivariate survival analysis using the Cox regression model demonstrated age and Ki67 level to be predictors of OS and grade and HER2 status of DFS in stage T1N0 patients.
Conclusion: In T1 breast cancer patients, there were several differences between N- and N+ patients, limited LNM and extensive LNM patients. Besides, HR+ plays a dual role in regional LNM. In patients without LNM, age and Ki67 level are predictors of OS, and grade and HER2 are predictors of DFS.
1 Introduction
Breast cancer is the malignant tumor with the highest incidence in the world and has one of the highest cancer mortality rates, accounting for 24.5% of all new malignant tumors in women (1). With the popularization of breast cancer screening, an increasing number of breast cancer cases are detected at an early stage (2). It is generally considered that increasing tumor size correlates with a high risk of lymph node involvement (3). However, some patients with small-sized breast cancer also have lymph node metastasis (LNM), even extensive LNM, when diagnosed (4). In general, patients with 4 or more metastatic lymph nodes (N2-3) are considered to have “extensive LNM” (5). It has been reported that approximately 27.8% of T1 breast cancer patients have LNM and approximately 0.7% extensive LNM at the time of diagnosis (4). Several studies have indicated that small tumors might be a surrogate for biologically aggressive disease, especially in extensive node-positive disease (4, 5). Therefore, it is of great significance to explore factors associated with LNM, the difference between limited LNM and extensive LNM, and effects on the survival of patients with small tumors.
Previous research has established that many factors are associated with LNM, including age at diagnosis, tumor size, hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, and Ki67 level (3, 6–8). Several studies have proven that race is also an important factor influencing LNM and survival (9–11). Nevertheless, the different risk factors between limited LNM and extensive LNM remain unclear. To date, there are only a few reports evaluating factors of LNM in the Chinese population, and those reports had limited sample sizes (12, 13).
In this study, we reviewed clinicopathological characteristics of T1 breast cancer patients in a Chinese population. We compared clinicopathological factors of LNM, including the difference between N- and N+, limited N+(N1) and extensive N+(N2/3). We also analyzed the effect on survival to gain a better understanding of LNM prediction and prognosis for patients with small tumors.
2 Materials and methods
2.1 Patients and materials
Breast cancer patients meeting the inclusion criteria of female, unilateral, no distant metastatic site, and T1 invasive ductal carcinoma in the Cancer Hospital of Chinese Academy of Medical Sciences were reviewed from January 1999 to September 2020. Patients with distant metastasis at diagnosis or primary malignant tumors of other organs in the past were excluded. Medical records were collected, including age and clinical pathology features, such as tumor size, grade, Ki67 level, HR status, HER2 status and lymph node involvement. In addition, we enrolled eligible patients from 2009 to 2017 as a survival analysis cohort to identify the effect of risk factors on the overall survival (OS) and disease-free survival (DFS) of T1 breast cancer patients. Survival time was calculated from the date of diagnosis to the occurrence of the event or the censoring date. The study was approved and a waiver of the informed consent of study participants was granted by the Ethics Committee of the Cancer Hospital of Chinese Academy of Medical Sciences.
2.2 Statistical analysis
Data were analyzed using SPSS software (version 23.0, SPSS Inc., Chicago, IL). Differences were evaluated using the χ2 test, the Fisher test, or the independent samples t test according to their characteristics. Multiple factor analysis was performed using logistic regression. To evaluate risk factors for LNM, we compared the characteristics of lymph node-negative (N-) patients with those of lymph node-positive (N+) patients. Then, we analyzed differences between N1 patients and N2/3 patients to validate the determination of limited LNM and extensive LNM. Survival analysis was carried out using Kaplan−Meier methods and an adjusted Cox proportional hazards model; p values less than 0.05 were considered statistically significant.
3 Results
3.1 Descriptive statistics
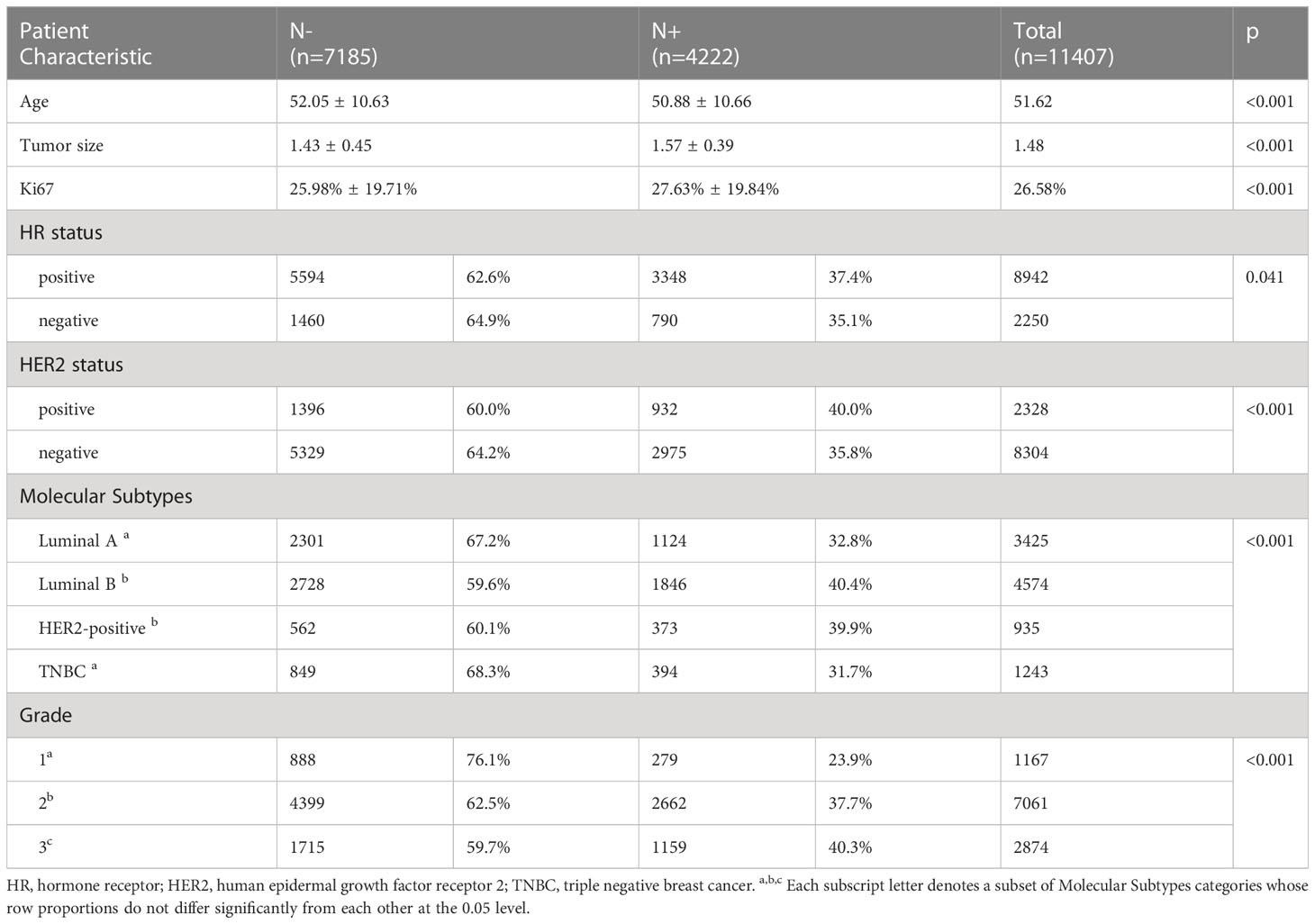
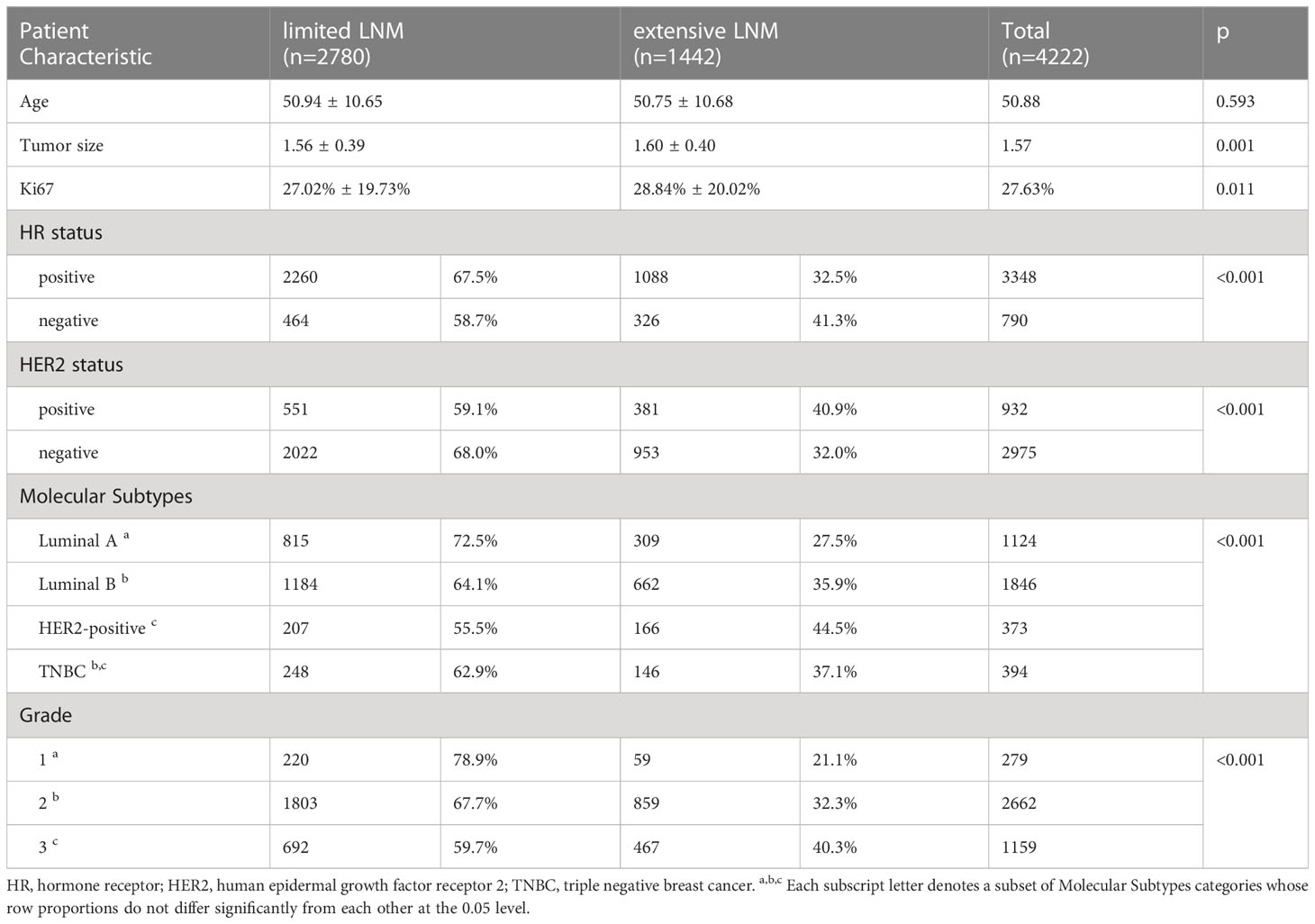
We enrolled 11,407 eligible breast cancer patients with stage T1 as a discovery cohort (Table 1): 7185 N- patients and 4222 N+ patients. Among the patients with N+ status, 2780 patients had N1 stage and 1442 patients N2/3.
3.2 Determination of LNM
Compared with N- patients, N+ patients were younger (50.88 ± 10.66 vs. 52.05 ± 10.63, p<0.001) (Table 1); the tumor size was larger in the N+ group (1.57 ± 0.39 vs. 1.43 ± 0.45, p<0.001), and Ki67 levels were higher (27.63% ± 19.84% vs. 25.98% ± 19.71%, p<0.001). We also compared differences in HR and HER2 status, molecular subtype and grade. The percentage of HR+ in the N+ group was higher than that in the HR- group (37.4% vs. 35.1%, p=0.041), and that of HER2+ in the N+ group was higher than that of HER2- (40.0% vs. 35.8%, p<0.001). In the N+ group, the percentage of luminal B and HER2-positive subtypes was higher (p<0.001) than that of luminal A and triple-negative breast cancer (TNBC) subtypes. There was also a greater percentage of higher grade in the N+ group (Grade 3/2/1: 40.3% vs. 37.7% vs. 23.9%, respectively, p<0.001). It is possible to hypothesize that these conditions are more likely to occur in patients with lymph node involvement, including younger age, larger tumor size, higher Ki67 level, HR+, HER2+, luminal B and HER2-positive subtypes, and higher grade.
Based on the difference between N- and N+ patients, logistic regression was conducted to identify risk factors for LNM, indicating that age, tumor size, grade, HR status, and HER2 status were significant indicators of lymph node involvement (Table 2). In contrast, age was a protective factor (OR, 0.987, 95%CI, 0.983 to 0.991, p<0.001). Tumor size was a risk factor (OR, 2.084, 95%CI, 1.862 to 2.332, p<0.001). Grade 2 (OR, 1.718, 95% CI, 1.451 to 2.035, p<0.001) and grade 3 (OR, 1.788, 95%CI, 1.452 to 2.201, p<0.001) were risk factors, as were HR positivity (OR, 1.249, 95%CI, 1.095 to 1.424, p=0.001) and HER2 positivity (OR, 1.168, 95%CI, 1.045 to 1.306, p=0.006).
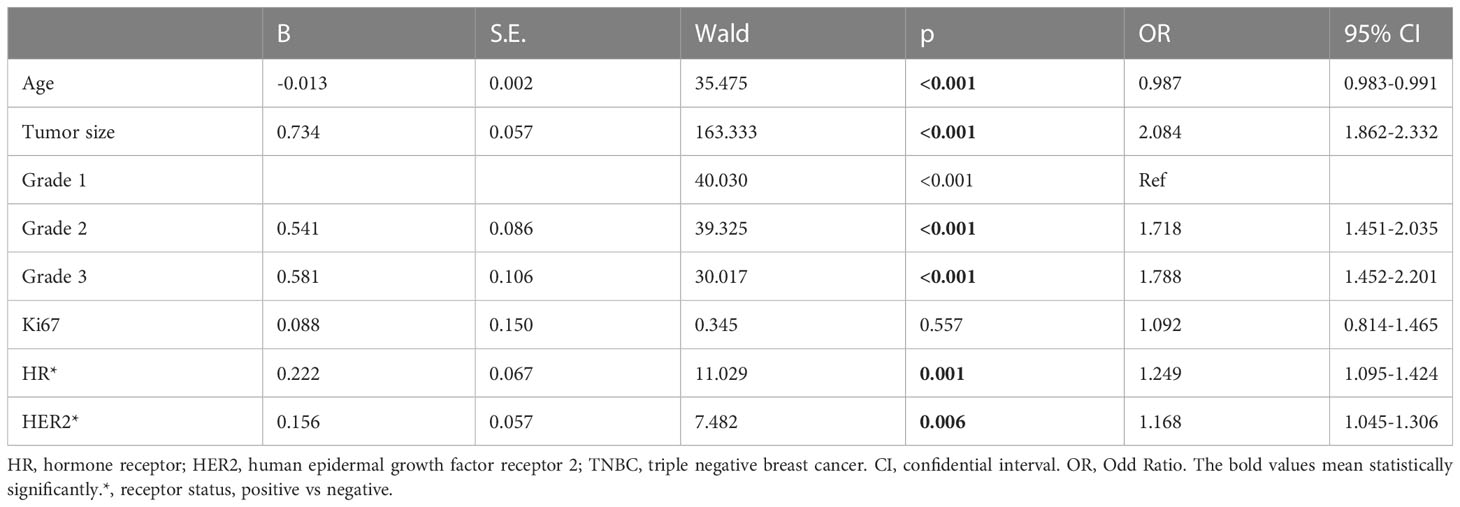
Table 2 Logistic Regression on high risk factors of lymph node metastasis comparing to non-lymph node involvement in the discovery cohort with stage T1 patients.
3.3 Determination of extensive LNM compared with limited LNM
Compared with patients with limited LNM (1-3 lymph nodes involved, N1), those with extensive LNM (more than or equal to 4 lymph nodes involved, N2/3) had similar ages at diagnosis (50.75 ± 10.68 vs. 50.94 ± 10.65, p=0.593) (Table 3). Tumor size was larger in the extensive LNM group (1.60 ± 0.40 vs. 1.56 ± 0.39, p=0.001), and the Ki67 level was higher (28.84% ± 20.02% vs. 27.02% ± 19.73%, p=0.011). Then, we compared differences in HR/HER2 status, molecular subtypes and grade. In the extensive LNM group, the percentage of HR- was higher than that of HR+ (41.3% vs. 32.5%, p<0.001); in the extensive LNM group, the percentage of HER2+ was higher than that of HER2- (40.9% vs. 32.0%, p<0.001). The percentage of HER2-positive subtypes was also higher than that of luminal B and luminal A subtypes in the extensive LNM group (44.5% vs. 35.9% vs. 27.5%, p<0.001). The percentage of TNBC subtypes was higher than that of luminal A subtypes but was not different from that of luminal B and HER2-positive subtypes. There was also a higher percentage of higher grade in the extensive LNM group (grade 3/2/1: 40.3% vs. 32.3% vs. 21.1%, p<0.001). These results further support the hypothesis that these factors, including larger tumor size, higher Ki67 level, HR-, HER2+, non-luminal A subtypes and higher grade, increase risk of extensive LNM.
Based on the difference between limited LNM and extensive LNM patients, logistic regression was conducted to identify risk factors for extensive LNM. Logistic regression indicated that tumor size, grade, HR status, and HER2 status were significant indicators of extensive LNM (Table 4). Tumor size was a risk factor (OR, 1.240, 95%CI, 1.019 to 1.510, p=0.032). Grade 2 (OR, 1.731, 95%CI, 1.216 to 2.463, p=0.002) and grade 3 (OR, 2.225, 95%CI, 1.494 to 3.312, p<0.001) were also risk factors compared to grade 1. HR positivity was a protective factor (OR, 0.016, 95%CI, 0.620 to 0.952, p=0.016) and HER2 positivity a risk factor for extensive LNM (OR, 1.212, 95%CI, 1.012 to 1.451, p=0.036).
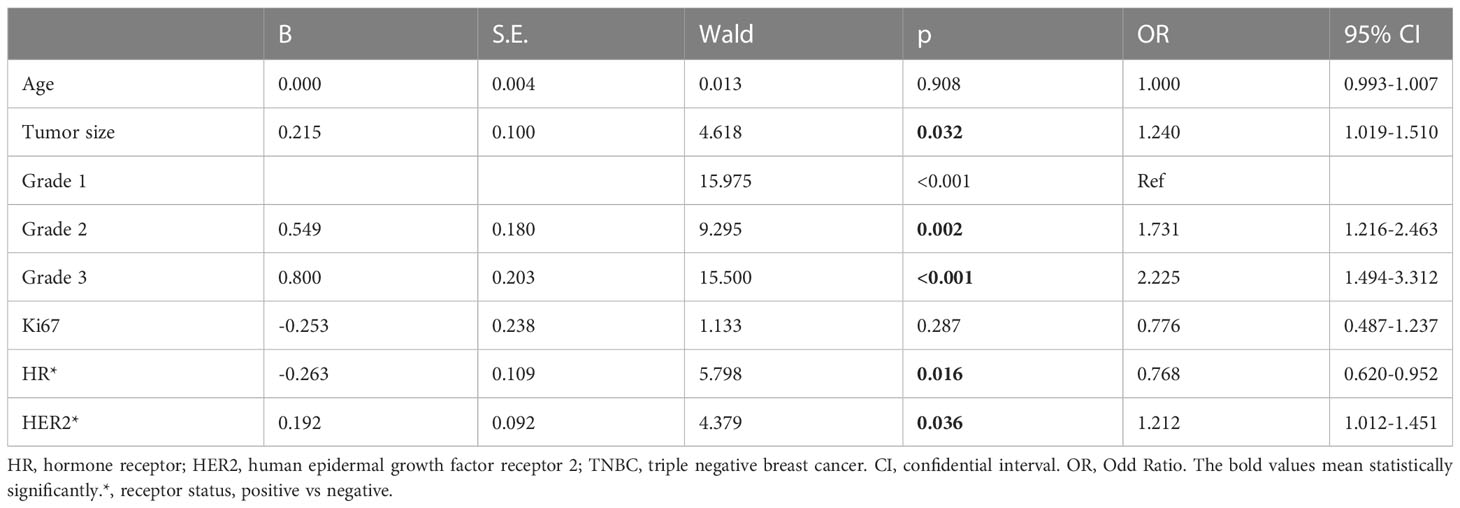
Table 4 Logistic Regression on high risk factors of extensive lymph node metastasis comparing with limited lymph node metastasis in the discovery cohort with stage T1N+ patients.
3.4 Effects of different clinical factors on survival
There were 3484 patients in the survival analysis cohort (Table S1). Age at diagnosis was 52.23 ± 10.46 years. The mean tumor size was 1.45 ± 0.46 cm, and the Ki67 level was 27.35% ± 20.50%. The percentage of HR+ patients was 76.1%, and that of HER2+ patients was 21.1%. There were approximately 31.5% luminal A, 40.1% luminal B, 8% HER2-positive and 12.1% TNBC subgroup patients. Percentages of grade 1, grade 2 and grade 3 were 12.1%, 60.6%, and 25.3%, respectively.
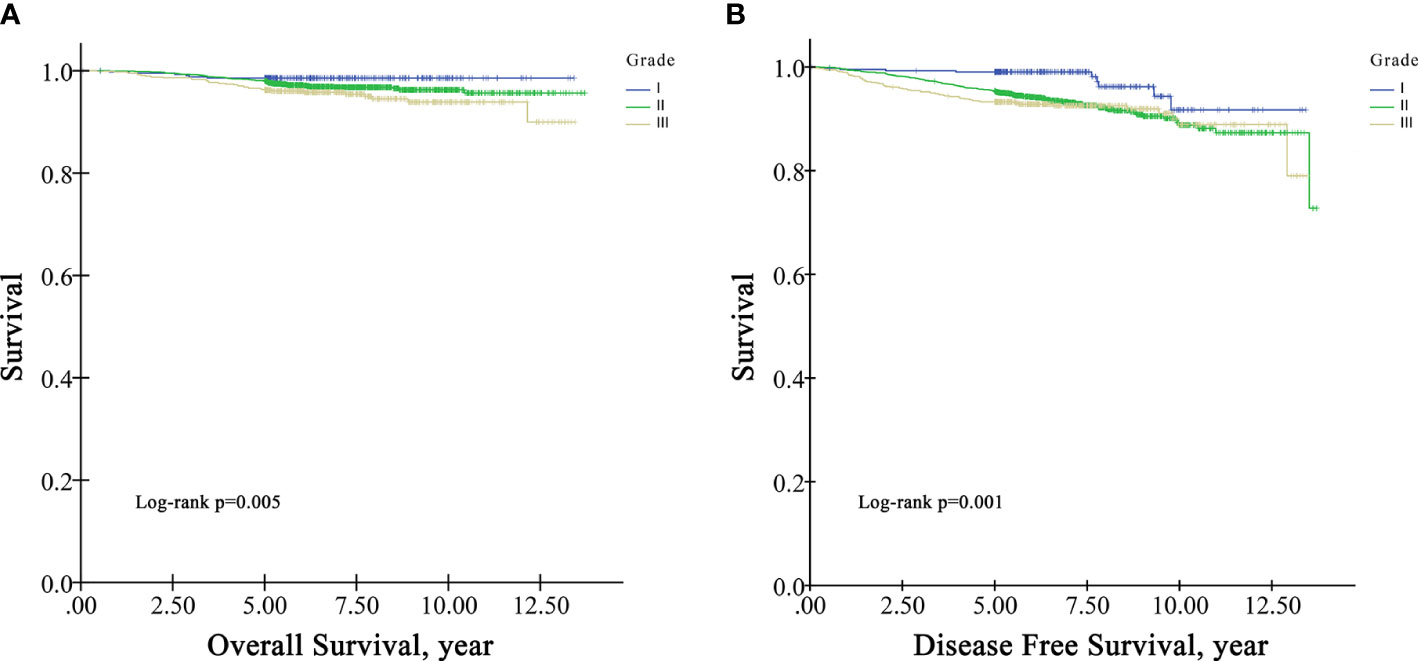
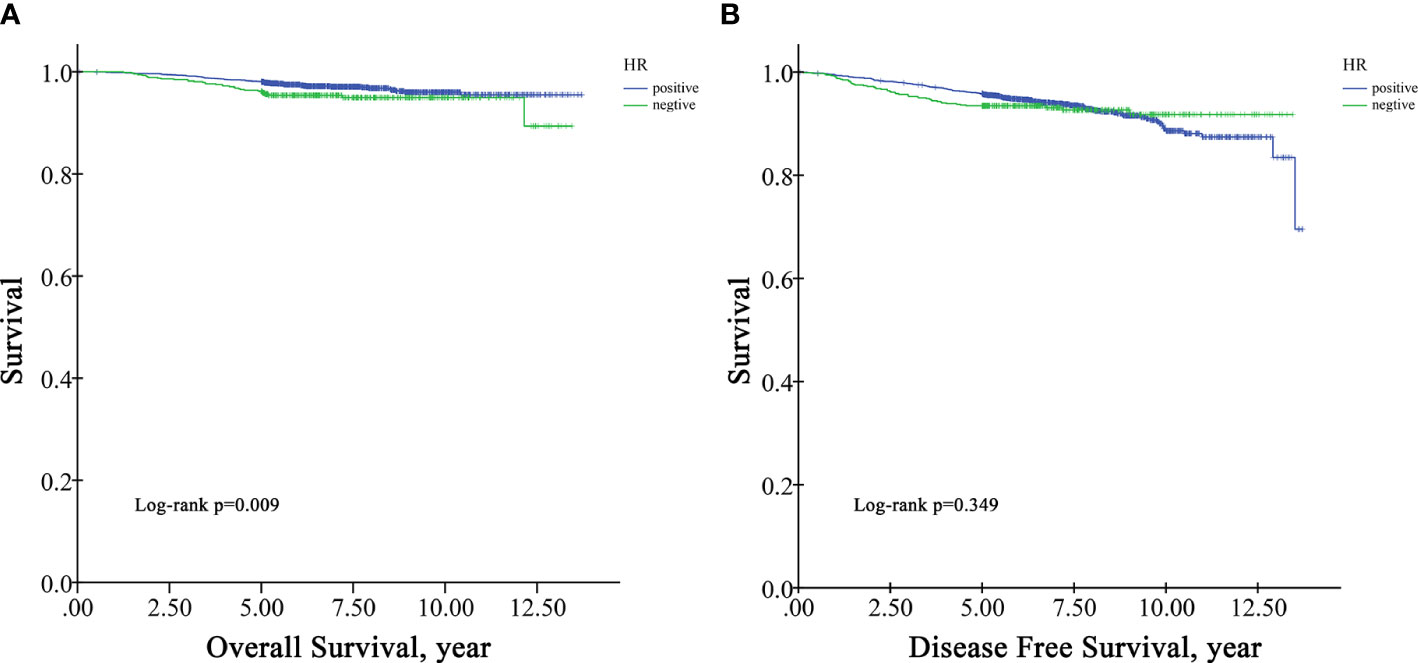
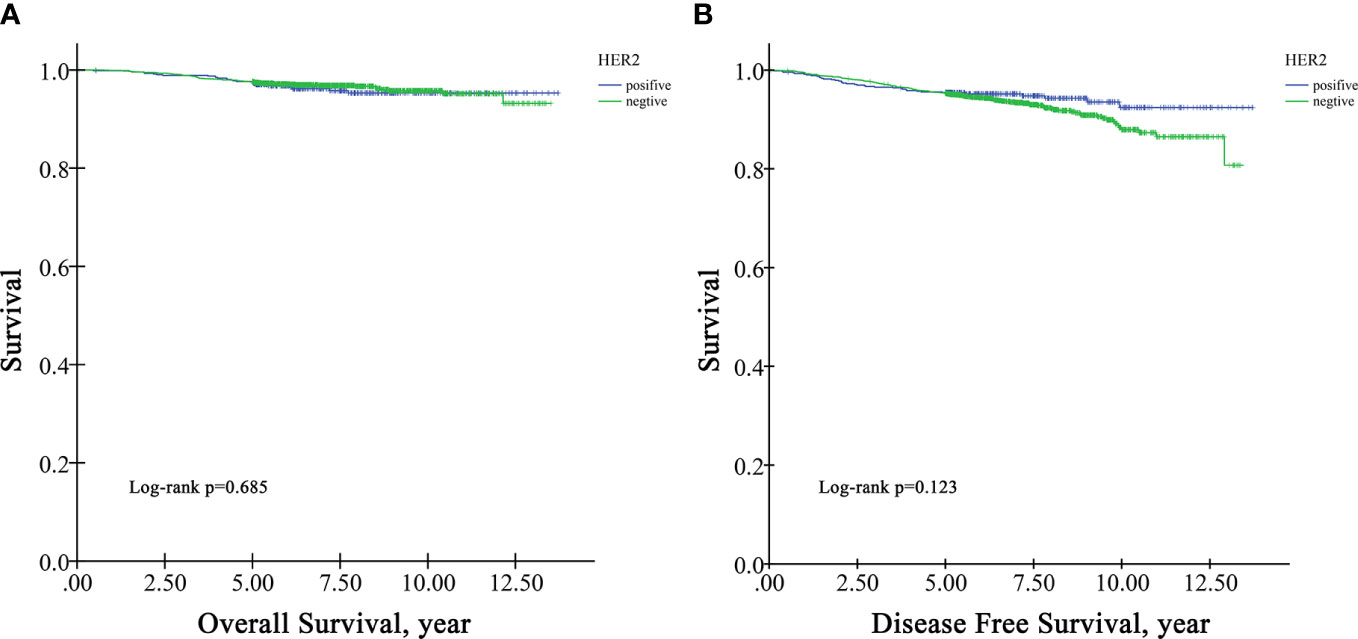
In the survival analysis cohort, the mean follow-up time after diagnosis was 6.60 years, and the median follow-up time was 5.71 years. There were 107 deaths and 216 patients with disease progression in this cohort. The OS rate at five years was 97.5%, and the DFS rate at five years was 95.13%. According to Kaplan−Meier survival analysis, higher grade patients had shorter OS (log-rank p=0.005) and DFS (log-rank p=0.001) than lower grade patients (Figure 1). Moreover, patients with negative HR had shorter OS than those with positive HR (log-rank p=0.009) (Figure 2A), but DFS was not significantly different between these two groups (log-rank p=0.349) (Figure 2B), and there was no significant difference in OS and DFS between the HER2-positive group and the HER2-negative group (Figure 3).
According to multivariate survival analysis using the Cox regression model, several factors were independent predictors of OS and DFS in the validation cohort (Table 5). Age at diagnosis (HR=1.022, 95%CI=1.001-1.043, p=0.045) and Ki67 level (HR=6.367, 95%CI=1.863-21.761, p=0.003) were predictors of OS and higher grade (Grade II, HR=3.715, 95%CI=1.500-9.199, p=0.005. grade III, HR=4.321, 95%CI=1.612-11.581, p=0.004) and HER2 status (HR=0.607, 95%CI=0.402-0.917, p=0.018) were predictors of DFS in stage T1N0 patients.
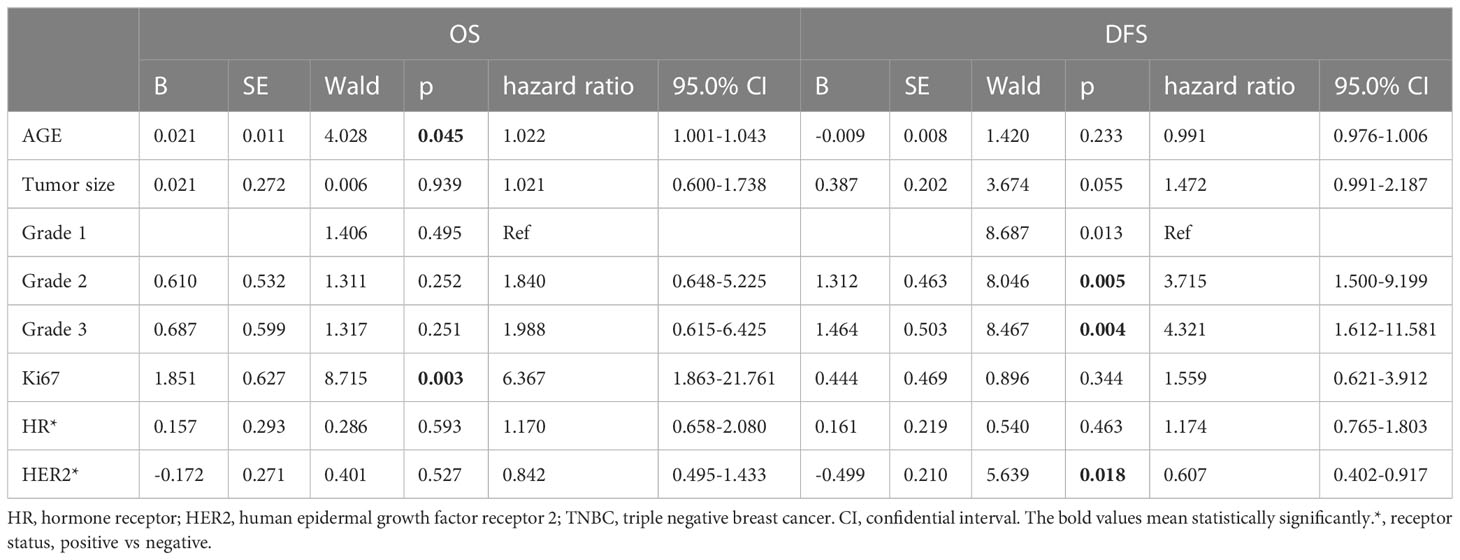
Table 5 The effects of different clinical factors on Overall Survival and Disease Free Survival in the survival analysis cohort with stage T1N0 patients.
4 Discussion
Small-sized breast cancer with LNM is a special kind of aggressive breast cancer, especially with extensive LNM. In this study, we sought to determine risk factors and the regular pattern of LNM in small-sized tumors (defined as T1 tumors), between no metastasis (N-) to metastasis (N+), and between limited metastasis (N1) to extensive metastasis (N2/N3). We also sought to determine whether these risk factors affect the OS and DFS of patients with small tumors without LNM.
According to our analysis, younger age at diagnosis is a risk factor for lymph node involvement. However, age was not found to be a risk factor for extensive LNM compared to limited LNM. There were similarities between the findings of this study and those by other researchers (7, 14–16). Younger age has also been reported as a risk factor for distant metastasis (17). Our study found age to be an independent risk predictor of OS in patients with small tumors without LNM. These results are in line with those of previous studies (5, 18, 19).
In our study, tumor size was associated with LNM, including cases without LNM to tumors with extensive LNM. Some studies have postulated a convergence between tumor size and LNM (15, 20). T1N0 breast cancer has good prognosis, and our study found that tumor size was not a predictor of OS or DFS in these patients. Nonetheless, for tumors with LNM, small tumor size is associated with survival (5).
Our study results also showed Ki67 level to be a risk factor for LNM. The importance of Ki67 levels in predicting LNM in patients with small tumors has been noted (8, 21, 22). One unanticipated finding was that the Ki67 level was an independent predictor of OS in patients with small tumors without LNM. Previous studies have indicated that the level of Ki67 is an independent prognostic factor of breast cancer-specific survival (BCSS) and DFS in breast cancer patients (23, 24), and our study confirmed the value of the level of Ki67 in prediction of survival in small-sized tumor patients.
Importantly, high histological grade was associated with LNM in our study. Similar to the role of tumor size, higher grade increased LNM risk from limited metastasis to extensive involvement. In addition, grade was a predictor of OS and DFS in patients with small tumors without LNM. In accordance with the present results, previous studies have demonstrated that high grade indicates a high labeling index, rapid replication, and a greater early relapse rate than low grade (22, 25). Therefore, high grade can be used for predicting LNM and survival in patients with small tumors.
The most striking and unanticipated finding was that HR+ plays a dual role in regional LNM: although HR+ status was a risk factor for LNM, in N+ patients, HR+ status was a protective factor against extensive LNM. Consistent with the literature, there are some studies indicating that HR+ status is a protective factor against LNM (7, 22), but there are also several studies showing that HR+ status is a risk factor, as we observed in our study (6, 26, 27). To our knowledge, this is the first study to identify this dual role in regional LNM. It is possible to hypothesize that HR+ status is likely to increase but also restrict risk of LNM. One possible explanation is that estrogen is important for tumor growth, and required for intratumoral lymphangiogenesis which could increase the risk of LNM, but in the lymph node microenvironment, ER is dysfunctional and the sensitivity is altered, which could also influence LNM (28). We also found that HR+ status was a protective predictor of OS in univariate survival analysis but failed to find a tendency in multivariate survival analysis. In accordance with the present results, previous studies have indicated that HR positivity is a protective factor for DFS and OS in small-tumor patients (18, 29). Further work including subgroup analysis is needed to confirm these findings.
In our study, we found HER2+ status to be a risk factor for LNM with small tumors. These results mirror those of previous studies (8, 26). We also found HER2+ status to be an independent protective factor of DFS in multivariate survival analysis. HER2+ status is reportedly associated with poor prognosis (30, 31), especially in patients with stage T3/T4 tumors (31). For small-sized tumors, Gonzalez-Angulo et al. (2009) concluded that HER2 positivity is an independent risk factor for DFS in T1a and T1b breast cancer without node positivity (29). This discrepancy might be attributed to racial differences. Indeed, several studies have indicated that ethnicity is associated with survival of breast cancer patients (11, 18). We also observed higher DFS in the HER2+ group in another hospital in China (32). Another possible alternative explanation of our findings is that anti-HER2 therapy increases the DFS of patients with small tumors. In general, patients with HER2 positivity and stage T1b/T1c should undergo anti-HER2 therapy. Several studies have indicated that HER2+ patients have increased DFS after anti-HER2 therapy (33), but few prospective studies have compared the prognosis of HER2- patients and HER2+ patients treated with anti-HER2 therapy. Further research is required to evaluate the impact of HER2+ status.
There are several limitations in our study. First, information for real-world treatment was not reviewed when we conducted survival analysis. Although we only included patients with small-sized tumors without LNM, not all of the patients underwent standard treatment, which may lead to bias in survival analysis. Second, the sample size used for survival analysis was small, possibly influencing its significance, especially for the HER2+ group. Larger samples are needed for replication.
5 Conclusion
Our study describes the first and largest cohort of small-sized breast cancer patients in a single center in China. We reveal several factors associated with LNM for T1 tumors, including age, tumor size, Ki67 level, grade, and HR and HER2 status. We found that HR+ status plays a dual role in regional LNM. We also evaluated the effect of the above risk factors on the survival of T1 tumor patients. Univariate analysis indicated grade and HR status to be predictors of OS, with grade also being a predictor of DFS. Multiple regression analysis showed that age and Ki67 level are predictors of OS and that grade and HER2 are predictors of DFS in patients with small-sized tumors without LNM. The findings of our study have a number of important implications for prediction of LNM and survival in patients with small tumors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved and a waiver of the informed consent of study participants was granted by the Ethics Committee of the Cancer Hospital of Chinese Academy of Medical Sciences.
Author contributions
GL and YW contributed to the conception, supervision, and funding of the study. GL, CG and ZX collected the medical data. QD and HC conducted the data analyses. GL, CG and ZX wrote the original draft. XW and YT were responsible for the critical revision of the manuscript or important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This research was funded in part by the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2020B15 to GL and LC2022A02 to Y.W.) and the CAMS Innovation Fund for Medical Sciences (2021-I2M-1-014 to Y.W.).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1217869/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Marcadis AR, Morris LGT, Marti JL. Relative survival with early-stage breast cancer in screened and unscreened populations. Mayo Clinic Proc (2022) 97(12):2316–23. doi: 10.1016/j.mayocp.2022.08.006
3. Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann surgery. (1999) 230(5):692–6. doi: 10.1097/00000658-199911000-00012
4. Wo JY, Chen K, Neville BA, Lin NU, Punglia RS. Effect of very small tumor size on cancer-specific mortality in node-positive breast cancer. J Clin Oncol (2011) 29(19):2619–27. doi: 10.1200/JCO.2010.29.5907
5. Zheng YZ, Wang XM, Fan L, Shao ZM. Breast cancer-specific mortality in small-sized tumor with stage IV breast cancer: A population-based study. oncologist. (2021) 26(2):e241–50. doi: 10.1002/onco.13567
6. Gann PH, Colilla SA, Gapstur SM, Winchester DJ, Winchester DP. Factors associated with axillary lymph node metastasis from breast carcinoma: descriptive and predictive analyses. Cancer (1999) 86(8):1511–9. doi: 10.1002/(SICI)1097-0142(19991015)86:8<1511::AID-CNCR18>3.0.CO;2-D
7. Brenin DR, Manasseh DM, El-Tamer M, Troxel A, Schnabel F, Ditkoff BA, et al. Factors correlating with lymph node metastases in patients with T1 breast cancer. Ann Surg Oncol (2001) 8(5):432–7. doi: 10.1007/s10434-001-0432-7
8. Lale A, Yur M, Özgül H, Alkurt EG, Yıldırım N, Aygen E, et al. Predictors of non-sentinel lymph node metastasis in clinical early stage (cT1-2N0) breast cancer patients with 1-2 metastatic sentinel lymph nodes. Asian J surgery. (2020) 43(4):538–49. doi: 10.1016/j.asjsur.2019.07.019
9. Zhao YX, Liu YR, Xie S, Jiang YZ, Shao ZM. A nomogram predicting lymph node metastasis in T1 breast cancer based on the surveillance, epidemiology, and end results program. J Cancer. (2019) 10(11):2443–9. doi: 10.7150/jca.30386
10. Yi M, Meric-Bernstam F, Ross MI, Akins JS, Hwang RF, Lucci A, et al. How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer (2008) 113(1):30–7. doi: 10.1002/cncr.23514
11. Zhang C, Zhang C, Wang Q, Li Z, Lin J, Wang H. Differences in stage of cancer at diagnosis, treatment, and survival by race and ethnicity among leading cancer types. JAMA network Open (2020) 3(4):e202950. doi: 10.1001/jamanetworkopen.2020.2950
12. Wang X, Wang X, Zhang Y, Zhang D, Song Z, Meng Q, et al. Development of the prediction model based on clinical-imaging omics: molecular typing and sentinel lymph node metastasis of breast cancer. Ann Trans Med (2022) 10(13):749. doi: 10.1186/s12967-022-03274-1
13. Fu F, Zhang Y, Sun J, Zhang C, Zhang D, Xie L, et al. Predictors of sentinel lymph node metastasis in Chinese women with clinical T1-T2 N0 breast cancer and a normal axillary ultrasound. Acta radiologica (Stockholm Sweden 1987). (2022) 63(11):1463–8. doi: 10.1177/02841851211054191
14. Min Y, Wei X, Chen H, Xiang K, Yin G, Feng Y. Identifying clinicopathological risk factors of the regional lymph node metastasis in patients with T(1-2) mucinous breast cancer: A population-based study. J Oncol (2021) 2021:3866907. doi: 10.1155/2021/3866907
15. Capdet J, Martel P, Charitansky H, Lim YK, Ferron G, Battle L, et al. Factors predicting the sentinel node metastases in T1 breast cancer tumor: an analysis of 1416 cases. Eur J Surg Oncol (2009) 35(12):1245–9. doi: 10.1016/j.ejso.2009.06.002
16. Rivadeneira DE, Simmons RM, Christos PJ, Hanna K, Daly JM, Osborne MP. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surgeons. (2000) 191(1):1–6. doi: 10.1016/S1072-7515(00)00310-0
17. Min Y, Liu X, Hu D, Chen H, Chen J, Xiang K, et al. Risk factors, prognostic factors, and nomogram for distant metastasis in breast cancer patients without lymph node metastasis. Front endocrinol (2021) 12:771226. doi: 10.3389/fendo.2021.771226
18. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. Jama (2015) 313(2):165–73. doi: 10.1001/jama.2014.17322
19. Ryu JM, Lee HJ, Yoon TI, Lee ES, Lee SJ, Jung JH, et al. Breast cancer-specific mortality in small-sized tumor with node-positive breast cancer: a nation-wide study in Korean breast cancer society. Breast Cancer Res Treat (2016) 159(3):489–98. doi: 10.1007/s10549-016-3943-4
20. Benson JR, Weaver DL, Mittra I, Hayashi M. The TNM staging system and breast cancer. Lancet Oncol (2003) 4(1):56–60. doi: 10.1016/S1470-2045(03)00961-6
21. Singh Y, Nambu H, Yoshizawa K, Hatano T, Hioki K, Tsubura A. Factors related to axillary lymph node metastasis in T1 breast carcinoma. Oncol Rep (1998) 5(2):459–62. doi: 10.3892/or.5.2.459
22. Bader AA, Tio J, Petru E, Bühner M, Pfahlberg A, Volkholz H, et al. T1 breast cancer: identification of patients at low risk of axillary lymph node metastases. Breast Cancer Res Treat (2002) 76(1):11–7. doi: 10.1023/A:1020231300974
23. Pathmanathan N, Balleine RL, Jayasinghe UW, Bilinski KL, Provan PJ, Byth K, et al. The prognostic value of Ki67 in systemically untreated patients with node-negative breast cancer. J Clin pathol (2014) 67(3):222–8. doi: 10.1136/jclinpath-2013-201793
24. Shin GW, Zhang Y, Kim MJ, Su MY, Kim EK, Moon HJ, et al. Role of dynamic contrast-enhanced MRI in evaluating the association between contralateral parenchymal enhancement and survival outcome in ER-positive, HER2-negative, node-negative invasive breast cancer. J magnetic resonance Imaging JMRI. (2018) 48(6):1678–89. doi: 10.1002/jmri.26176
25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology (1991) 19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x
26. Holm-Rasmussen EV, Jensen MB, Balslev E, KrOman N, Tvedskov TF. Reduced risk of axillary lymphatic spread in triple-negative breast cancer. Breast Cancer Res Treat (2015) 149(1):229–36. doi: 10.1007/s10549-014-3225-y
27. Tvedskov TF, Jensen MB, Balslev E, Ejlertsen B, KrOman N. Stage migration after introduction of sentinel lymph node dissection in breast cancer treatment in Denmark: a nationwide study. Eur J Cancer (Oxford Engl 1990). (2011) 47(6):872–8. doi: 10.1016/j.ejca.2010.11.022
28. Harrell JC, Dye WW, Allred DC, Jedlicka P, Spoelstra NS, Sartorius CA, et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res (2006) 66(18):9308–15. doi: 10.1158/0008-5472.CAN-06-1769
29. Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol (2009) 27(34):5700–6. doi: 10.1200/JCO.2009.23.2025
30. Gwark SC, Lee HS, Lee Y, Lee SB, Sohn G, Kim J, et al. Clinical implication of HER2 status in hormone receptor-positive mucinous breast cancer. Ann Surg Oncol (2019) 26(7):2166–74. doi: 10.1245/s10434-019-07332-9
31. Ménard S, Balsari A, Tagliabue E, Camerini T, Casalini P, Bufalino R, et al. Biology, prognosis and response to therapy of breast carcinomas according to HER2 score. Ann Oncol (2008) 19(10):1706–12. doi: 10.1093/annonc/mdn369
32. Zhao X, Yang X, Fu L, Yu K. Associations of estrogen receptor, progesterone receptor, human epidemic growth factor receptor-2 and ki-67 with ultrasound signs and prognosis of breast cancer patients. Cancer Manage Res (2021) 13:4579–86. doi: 10.2147/CMAR.S276422
Keywords: breast cancer, small-sized tumor with extensive metastasis, hormone receptor, clinical pathology, lymph node metastasis
Citation: Liu G, Xing Z, Guo C, Dai Q, Cheng H, Wang X, Tang Y and Wang Y (2023) Identifying clinicopathological risk factors for regional lymph node metastasis in Chinese patients with T1 breast cancer: a population-based study. Front. Oncol. 13:1217869. doi: 10.3389/fonc.2023.1217869
Received: 06 May 2023; Accepted: 21 July 2023;
Published: 04 August 2023.
Edited by:
Nosheen Masood, Fatima Jinnah Women University, PakistanReviewed by:
Shuheng Bai, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaAnam Nayab, University of Science and Technology of China, China
Copyright © 2023 Liu, Xing, Guo, Dai, Cheng, Wang, Tang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yipeng Wang, eWlkb2N0b3I5OUAxMjYuY29t; Yu Tang, dGFuZ3l1QGNpY2Ftcy5hYy5jbg==; Xiang Wang, eGlhbmd3QHZpcC5zaW5hLmNvbQ==
†These authors share first authorship
 Gang Liu
Gang Liu Zeyu Xing
Zeyu Xing Changyuan Guo2†
Changyuan Guo2† Xiang Wang
Xiang Wang Yipeng Wang
Yipeng Wang