- Department of Obstetrics and Gynecology, Breast Center, Gynecologic Oncology Center and CCC Munich, University Hospital, LMU Munich, Munich, Germany
Introduction: Safety and tolerability of COVID-19 vaccines were demonstrated by several clinical trials which led to the first FDA/EMA approvals in 2021. Because of mass immunizations, most social restrictions were waived with effects on quality of life. Therefore, our a-priori hypothesis was that COVID-19 vaccination impacted the health-related quality of life (HR-QoL) in patients with breast and gynecological cancer.
Methods: From March 15th until August 11th, 2022, fully vaccinated patients with breast and gynecological cancer treated in the oncological outpatient clinics of the Department of Obstetrics and Gynecology, LMU University Hospital, Munich, Germany filled out a vaccine related QoL survey. Patients were asked about demographics (age, comorbidities), clinical parameters related to previous COVID-19 infections, and HR-QoL related parameters (living situation, responsibilities in everyday life). Subsequently, a questionnaire with 12 items was designed using a 5-point Likert scale (0 – strongly disagree/4 – strongly agree), covering the aspects health and therapy, social environment, participation in everyday life and overall assessment.
Results: By August 11th, 2022, 108 out of 114 (94.7%) patients had received at least three doses of COVID-19 vaccine and six patients at least two doses. More than half of the surveyed patients were >55y (52.6%; mean: 55.1y, range 29-86y). Patients with breast cancer (n= 83) had early (59.0%) or metastatic cancer (41.0%); gynecological cancers (n=31) also included metastatic (54.8%) and non-metastatic cancer (45.2%). 83.3% of the patients stated that COVID-19 vaccination had a positive impact on their HR-QoL. Furthermore, 29 patients (25.4%) had undergone a COVID-19 infection. These patients reported self-limiting symptoms for a median duration of 5.9 days and no hospital admissions were registered.
Conclusions: Our study demonstrates that vaccination against COVID-19 was positively associated with HR-QoL in patients with breast and gynecological cancer. Furthermore, vaccinated patients who underwent COVID-19 disease experienced only self-limiting symptoms.
Introduction
The COVID-19 pandemic showed a meaningful impact on oncology care: Delays and interruptions of both diagnosis and therapy were reported (1, 2). In addition, after starting systemic treatment for cancer, many patients experience side-effects that lead to immunosuppression (3), which may favor a severe course of COVID-19 infection. Lastly, multiple psychological impacts on the quality of life have been demonstrated (4).
Safety and tolerability of Conmirnaty (BioNTech/Pfizer), Vaxzevria (Astra Zeneca), and COVID-19 vaccine Moderna was demonstrated by several clinical trials which resulted in the first FDA/EMEA approvals in 2021 (5). However, data on safety of vaccines and courses of COVID-19 infections in cancer patients remain limited (6).
Still, in order to demonstrate that the vaccination is worthwhile, its benefit should not only be limited to its safety and tolerability but also be associated with patients’ satisfaction. Especially during a pandemic, factors such as social environment or regular participation in everyday life have a crucial influence on quality of life (QoL) (7). These aspects have been noticeably influenced by the COVID-19 pandemic since its beginnings in 2020 (8).
In particular, oncology patients restricted themselves to a greater extent than the rest of the population. Consequently, QoL in this group might have been affected even more than the general population, before and after vaccination. Importantly, studies on safety and tolerability of the COVID-19 vaccination did not include QoL data. However, this aspect is clinically especially meaningful when counselling oncologic patients regarding the clinical benefit of COVID-19 vaccination. Several side effects of systemic therapy such as neutropenia (increased possibility of infections), anemia, thrombocytopenia (increased possibility of bleeding), thromboembolic events, cardiac toxicity, fatigue, and chemotherapy-induced nausea/vomiting (CINV) could negatively influence health-related quality of life (HR-QoL) and these conditions could be additionally aggravated by COVID-19 (9–12).
The aim of this study is to evaluate the impact of COVID-19 vaccination on HR-QoL in patients with breast or gynecological cancer. We also aim to assess the clinical manifestation of COVID-19 disease in these vaccinated patients. The objectives of our study are to assess different quality of life aspects and investigate their impact on oncologic patients receiving COVID vaccine.
Methods
Study population and data collection
The study received ethics approval from the Ludwig-Maximilians-University (LMU) Munich Ethics Committee in February 2022 (number of ethical approval: 21-1237). Signed informed consent was obtained from all participants. From March 15th until August 11th, 2022, vaccinated patients with breast or gynecological cancer receiving oncological treatment in the oncological outpatient clinics of the LMU Department of Obstetrics and Gynecology were asked to fill out the quality of life (QoL) survey (consecutive sampling). The survey (Supplementary Figure 1) contained questions regarding demographics (age, comorbidities, current disease and treatment, COVID vaccination) and lifestyle parameters (working and living situation, responsibilities in everyday life, e.g., caring for children, grandchildren, or pets), as well as duration and symptoms in the case of a COVID-19 infection.
The above-mentioned questions were followed by a self-designed questionnaire with 12 items using a 5-point Likert scale (0 –not at all/4 –very much), covering the aspects health and therapy, social environment, participation in daily life, and overall assessment (Supplementary Figure 2). The collected data were analyzed anonymously.
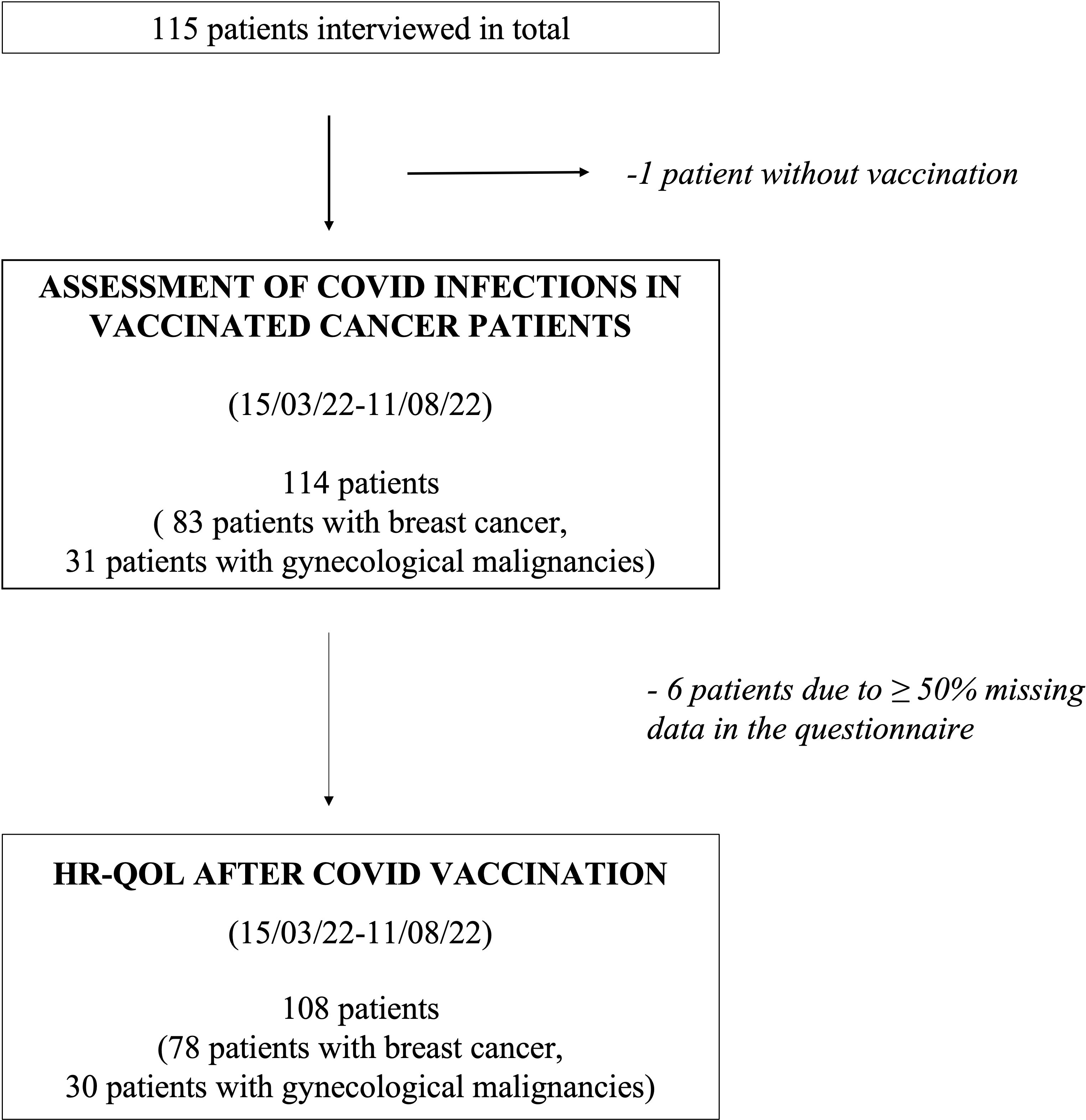
Figure 1 describes the process of data collection: In total, 115 patients received the survey, of whom one was not vaccinated against COVID-19 and thus excluded from the study. All other patients had at least received two doses of vaccine. The remaining 114 patients were analyzed regarding their indicated demographic and clinical parameters as well as symptoms and their duration during COVID-19 infection, if applicable. Six patients had ≥50% of the requested data in the subsequent questionnaire missing and were thus not included in the evaluation of the vaccine-related HR-QoL questionnaire (Figure 1).
Study definitions
Patients with breast and gynecological cancer receiving oncological treatment in the oncological outpatient clinics of the LMU Department of Obstetrics and Gynecology were included in the study. Types of anti-cancer therapy included neoadjuvant, adjuvant, and maintenance therapy as well as therapy for locally recurrent or metastatic disease. Therapy regimen consisted of chemotherapy +/- targeted therapy (e.g., taxane- or carboplatin-based, gemcitabine), targeted therapies (HER2-targeted therapies, bevacizumab, and PARP-inhibitors), immunotherapy +/- chemotherapy (PD-1 and PD-L1 inhibitors) as well as endocrine-based targeted therapy (CDK4/6-inhibitors or PIK3CA-inhibitors in combination with aromatase-inhibitors or Fulvestrant) and endocrine-based therapy.
Statistical analysis
Patient characteristics and endpoints were summarized. The scores according to the Likert scale of the QoL-questionnaire were added to a total score with only one item being reverse-scored (item 1.0 on negative influence of the vaccine on HR-QoL). Furthermore, basic questionnaire parameters such as floor and ceiling effects were assessed. 15% missing data were set as the acceptable threshold for individual items. Internal consistency was evaluated by calculating Cronbach’s alpha, which was considered as indicating good internal consistency with a value more than 0.7.
Statistical analysis was performed using R Studio, Version 1.4.1103. Fisher’s exact test was used to test for differences between specific groups. All statistical tests were performed two-sided. P-values of <0.05 were considered statistically significant. Power calculation and sample size justification were performed a priori using the program “G*Power”. For the calculations performed using Fisher’s exact test, a total sample size of 69 patients was required to achieve an actual power of 0.8 with a significance level of 0,05 (alpha error), reporting a medium effect size of 0.4 for calculations with up to three degrees of freedom.
Questionnaire construction
The vaccine related QoL- questionnaire consists of 12 items covering five superordinate topics:
item 1.1 assesses patients’ HR-QoL with regard to side effects of the COVID-19 vaccine.
item 2.1 – 2.3 (subscale 2) cover the aspect “Health and therapy” since previously conducted studies showed that “concerns of contracting COVID-19 infection were correlated with lower scores of global QoL and the emotional functioning scale” (13).
item 3.1 – 3.3 (subscale 3) focus on the impact of the vaccination on social environment (mainly family and friends). Due to ongoing restrictions regarding social life during COVID-19 many patients lacked the support of family or friends (8), which is especially important during a cancer diagnosis and outcome of therapy (14, 15). Travelling and work precaution for caregivers raised concern among cancer patients (16), which might lead to inner-familiar conflicts (item 3.1).
item 4.1 – 4.4 (subscale 4) assess the impact of the vaccine on participation in everyday life in various aspects (socially, work-related, leisure activities, sports), which have a measurable impact on cancer patients’ life (17–20).
item 5.1 examines the overall perception of the patients regarding a positive impact of COVID-19 vaccination on their HR-QoL.
We did not carry out a pilot study before using the questionnaire as the topic of COVID-19 was very current at that time and we wanted to collect results as up to date as possible. To still prove the scientific quality of the questionnaire used we assessed missing data rates, floor/ceiling effects as well as Cronbachs alpha prove internal consistency.
The missing data rate in the vaccine related QoL questionnaire was extremely low (1.6%), exclusive item 4.1, which could only be completed by those patients currently working (not those currently on sick leave/no longer working/already retired; item completed by 20 patients). Missing data were distributed across 9 of the 11 items with no item having more than 5 missing responses.
Total scores (n=108) ranged from 2 – 44 (possible range: 0 – 44), with mean 20.9, SD 10.0, and median 20. No participants had minimum scores. 2 participants (1.9%) had maximum scores (threshold for ceiling effects set at 15%).
Cronbachs alpha for the whole vaccine related QoL-questionnaire was 0.89. Furthermore, it was assessed for subscale 2, 3 and 4 (as “subscale” 1 and 5 only consisted of one item, respectively). Cronbach’s α for “health and therapy” was 0.89 (n =108); for “social environment” 0.77 (n = 108) and for “participation in everyday life” 0.9 (n = 107).
Results
Demographic characteristics and clinical presentation
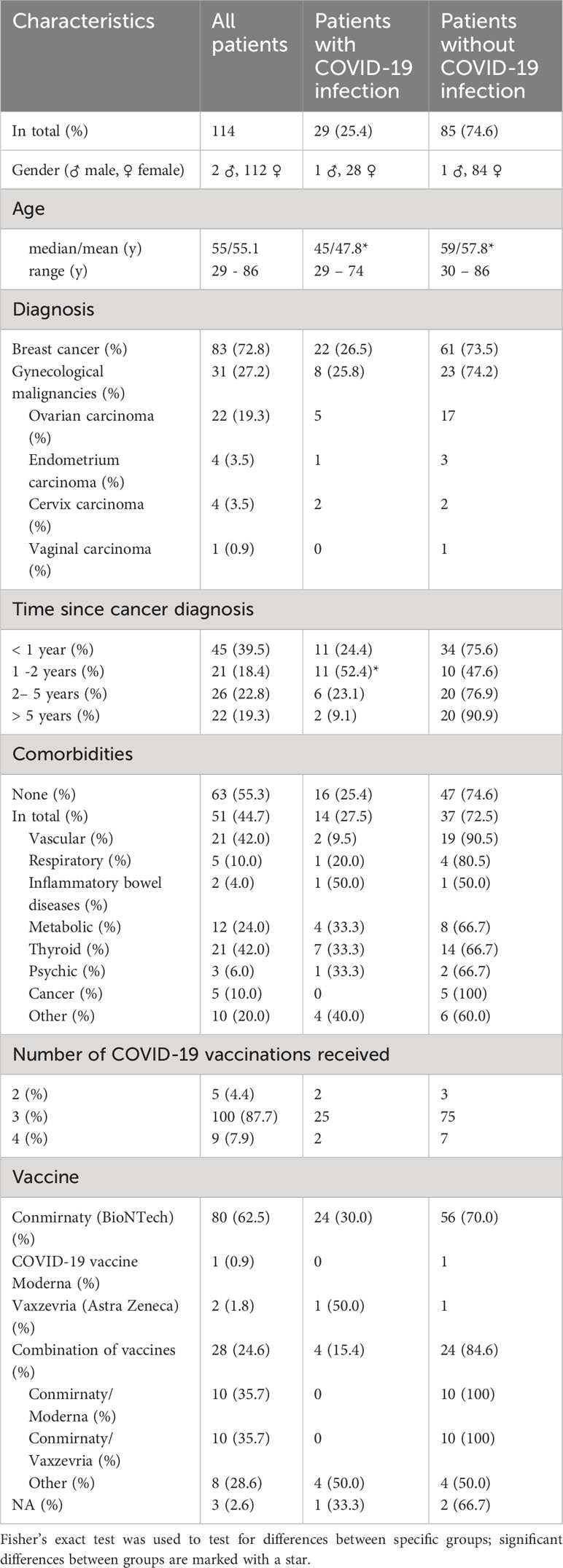
A total of 114 patients (83 patients with breast cancer and 31 with gynaecological malignancies) were interviewed, of whom 25.4% previously had a COVID-19 infection. Clinical characteristics of patients were stratified by COVID-19 infection (Table 1). Mean age in breast cancer and gynecological cancer patients was 55.1 years (Median 55y, range 29 – 86y). Patients who experienced a COVID-19 infection after vaccination were significantly younger than those with no COVID-19 (47.8y vs. 57.8y, p <0.01).
72.8% of the surveyed patients were diagnosed with breast cancer and 27.2% with gynecological cancers such as ovarian carcinoma, endometrium carcinoma, cervix carcinoma and vaginal carcinoma (Table 1). 44.7% of the patients indicated at least one comorbidity, of these mostly thyroid (42.0%) or vascular pathologies (42.0%). No significant differences could be observed between cancer type or co-morbidities and COVID-19 infection.
94.7% of the patients had received at least three COVID-19 vaccinations. 62.5% received the vaccine Conmirnaty (by BioNTech/Pfizer) and 24.6% of the patients a combination of vaccines (35.7% of those were combinations of Conmirnaty/Vaxzevria by Astra Zeneca and 35.7% Conmirnaty/COVID-19 vaccine Moderna).
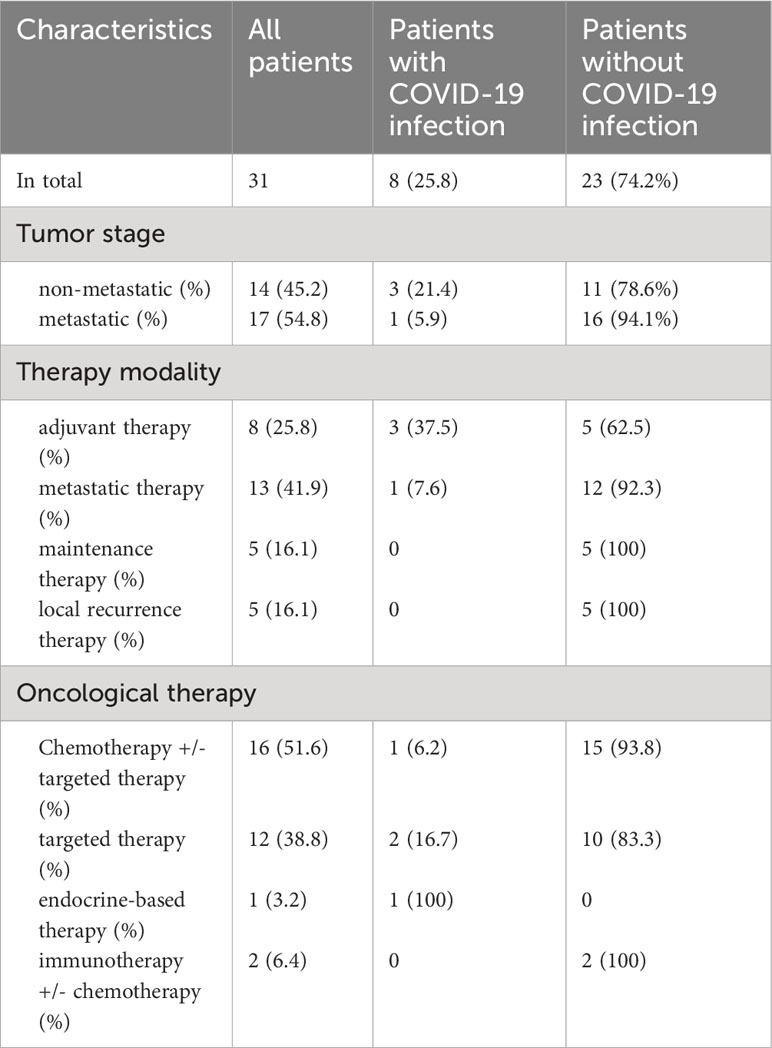
59.0% of the 83 breast cancer patients had early breast cancer and 41.0% metastatic disease; most of them were in the metastatic (41.0%) or neoadjuvant (32.5%) therapy setting. The majority received chemotherapy +/- targeted therapy (51.8%) (Table 2). In contrast, patients with gynecological cancer mostly had metastatic disease (54.8%). Gynaecological cancer patients were in the metastatic (41.9%) or adjuvant (25.8%) oncological therapy setting and also mostly received chemotherapy +/- targeted therapy (51.6%) (Table 3).
No significant differences could be observed regarding a potential influence of both, patient and therapy characteristics, on whether the patients got infected with COVID-19 or not (Tables 2, 3).
COVID-19 infections – clinical presentation
Figure 2 displays the symptoms of the vaccinated cancer-patients during COVID-19 infections (n=29). The most frequent symptoms during infection were cold symptoms such as coughing (51.7%), and rhinitis (51.7%) followed by flu symptoms fever (37.9%) and headache (34.5%). Median duration of symptoms was 5.9 days (median: 6d, range: 0-18d).
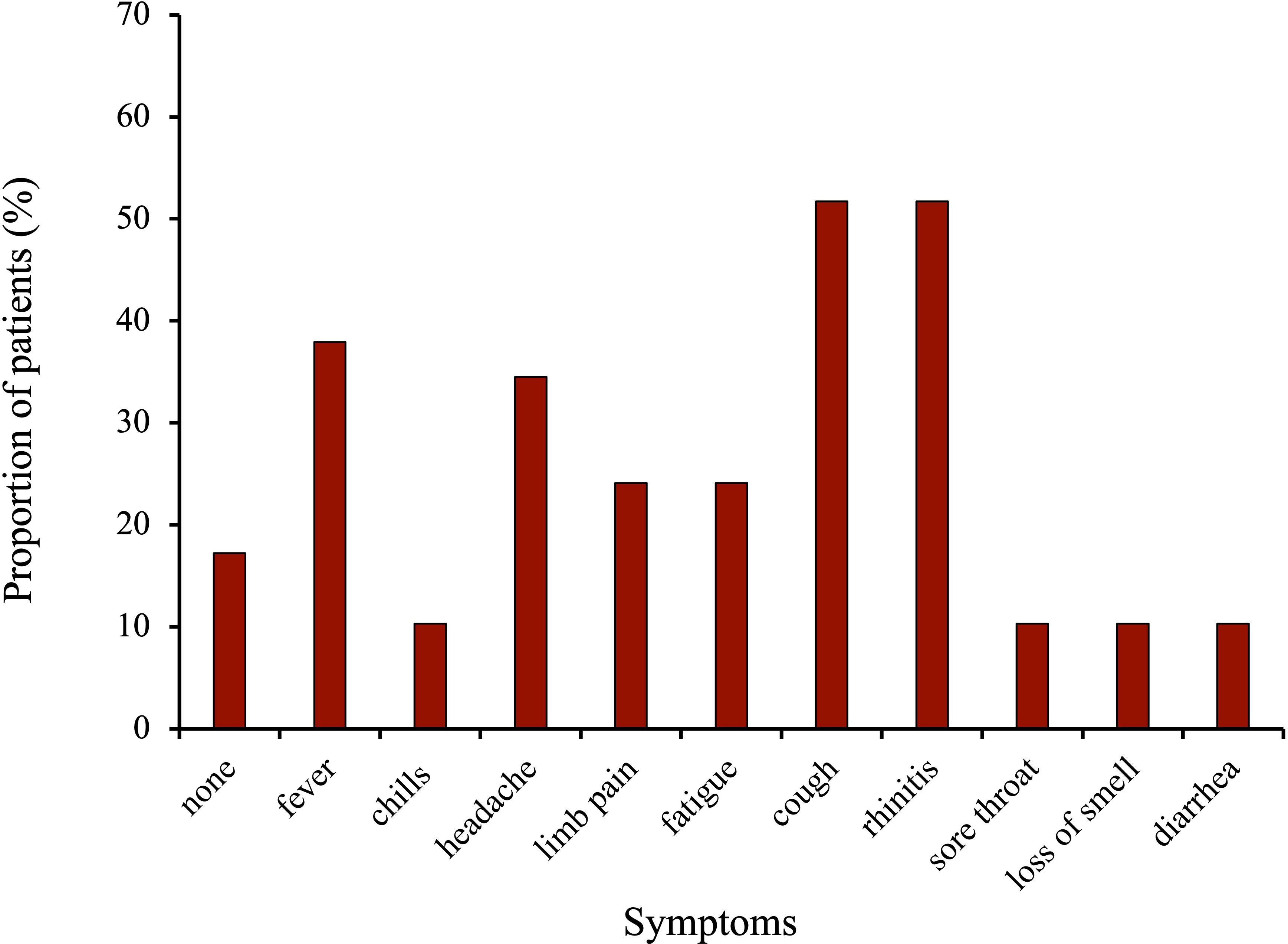
Figure 2 Symptoms during COVID-19 infection in the vaccinated breast and gynecological cancer patients.
Supplementary Figure 3 presents the COVID-19 infections based on the date of the first positive PCR test. The majority of the patients (82.8%) got infected with COVID-19 in 2022, more specifically, in March 2022 (31.0%).
Lifestyle impact on acquiring of COVID-19 infections
Table 4 summarizes patient lifestyle parameters and COVID-19 infections. Patients who were employed got significantly more often infected (p < 0.05). If counted together, those who were still active at work (employed and self-employed) caught more infections than those unemployed, retired, housewives, or students (p < 0.01). The infection rate was significantly lower in the retired patients (p < 0.01).
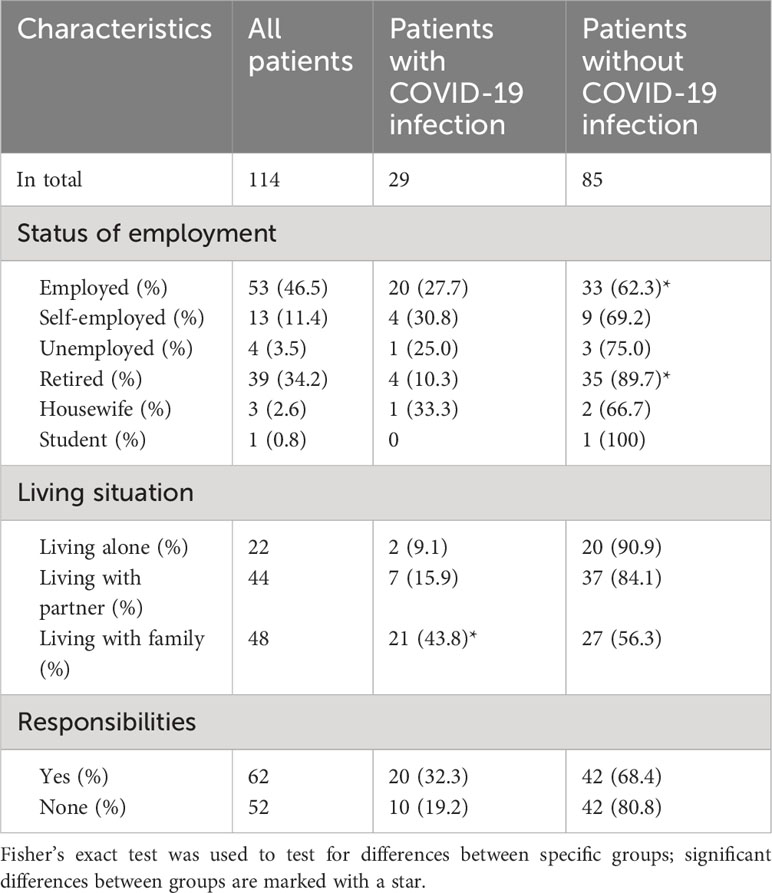
Table 4 Lifestyle parameters that may potentially influence the probability of COVID-19 infection in the surveyed patients.
Moreover, patients living with their families reported more frequently (significant, p < 0.01) an infection with COVID-19 than those living alone or with their partner. Patients with responsibilities in their everyday life (n=62, of those 71.0% involved in childcare, 16.1% taking care of their parents, and 37.1% of their pets (multiple entries possible)) showed a higher proportion of COVID-19 infections than those without (32.3% vs. 19.2%, not significant, p=0.1).
Vaccine related QoL in patients with breast and gynecological cancer
Table 5 summarizes the mean total scores of the vaccine related QoL questionnaire of different groups of patients. Here, higher scores indicate a higher impact of COVID-19 vaccination on the patients’ HR-QoL. Breast cancer patients showed a tendency towards a higher mean total score (22.5 vs. 17.1, not significant, p=0.07). Patients with comorbidities had significantly lower mean total scores than those without comorbidities (19.2 vs. 22.6, p <0.05) and patients with metastatic disease reached significantly lower mean total scores than patients with non-metastatic cancers (20.3 vs. 21.6, p<0.05). However, this effect was only present in the breast cancer cohort; the gynaecological cancer patients with metastatic disease and those with comorbidities reached higher scores compared to those with non-metastatic disease and no comorbidities (Tab. 5). No significant differences could be shown regarding the total scores of patients with different oncological therapies, although patients receiving endocrine-based therapy showed higher total scores (not significant, p>0.05, Table 5). Patients receiving oral therapies also tended to show higher scores than those with i.v. therapies (not significant, p >0.05). Age of the patients, their status of employment, living situation and their responsibilities in everyday life did not appear to significantly influence the impact of the vaccine on their HR-QoL.
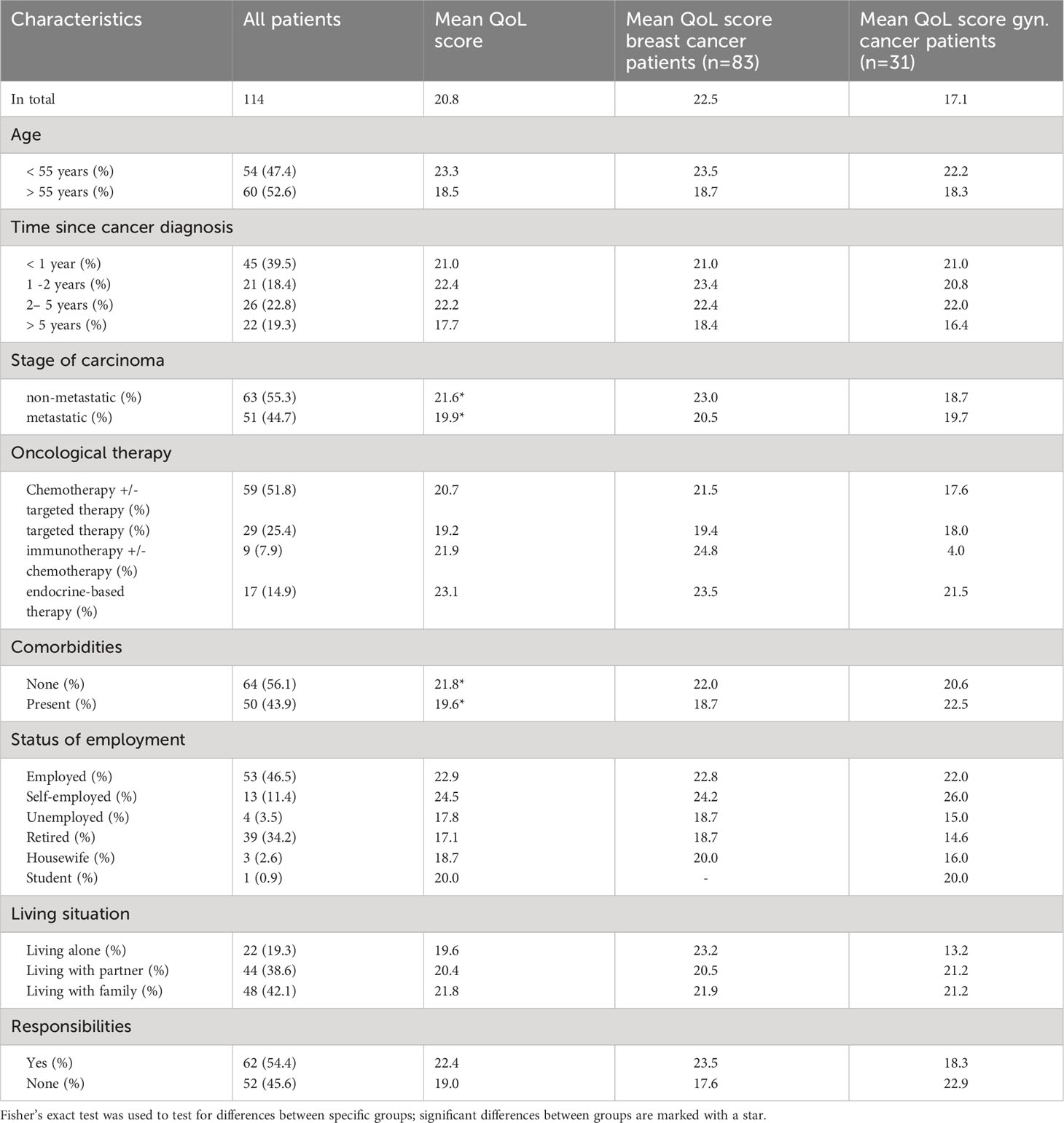
Table 5 Parameters that may influence the vaccine related HR-QoL in the surveyed patients and their corresponding mean total scores in the questionnaire.
Figure 3A presents the mean scores of the individual items of the vaccine related QoL questionnaire and Figure 3B displays the distribution of the scores for the individual items. The patients strongly denied the statement, that the COVID-19 vaccination had negative influence on their QoL (mean item score of item 1.1 = 3.6, Figure 3A). Reasons for stating “a bit” of negative influence on QoL (Figure 3B) were flu-like symptoms and local reactions at the vaccination site.
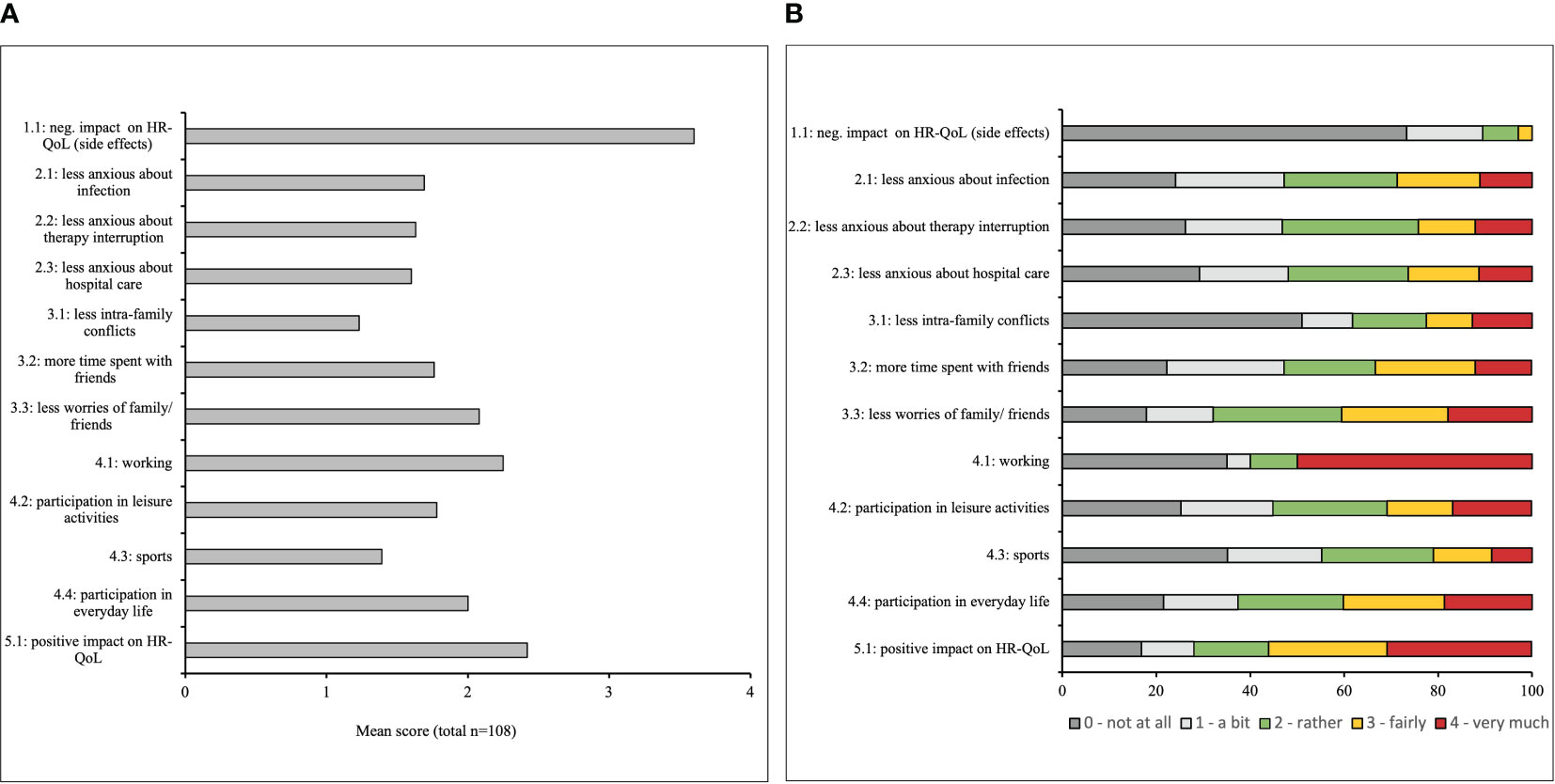
Figure 3 (A) Mean item score (possible range: 1 – 4) in the COVID-19 vaccine-related Health-related quality of life (HR-QoL) questionnaire. Higher scores indicate a higher impact of the vaccine on the HR-QoL in the surveyed breast and gynecological cancer patients. (B) Distribution of response options for all items in the COVID 19 vaccine-related HR-QoL questionnaire. The patients were asked to what extent the COVID-19 vaccine impacted their HR-QoL regarding different aspects, given the answer options “very much”, “fairly”, “rather”, “a bit” and “not at all”.
Regarding the items assessing “health and therapy” (items 2.1 – 2.3), more than a quarter of the patients agreed fairly or very much to the statement that due to vaccination they would worry less on developing COVID-19, interruption of treatment/therapy due to the COVID-19 pandemic, and seeking medical assistance for health problems (31%, 26%, 28%, respectively, Figure 3B).
Item 3.1 on less conflicts within the family due to complete vaccine protection in the third subscale ’social environment“, received the lowest degree of agreement with a mean score of 1.2 (Figure 3A). 36% of the patients agreed fairly or very much on meeting more with friends since complete vaccine protection (Figure 3B).
Additionally, 30.8% of the surveyed patients indicated fairly or very much to further engage in leisure activities (item 4.2), 21.0% fairly or very much to do more sports (item 4.3) and 40.2% fairly or very much to participate more in everyday-life (item 4.4) due to vaccine protection (Figure 3B).
Overall, 60% of the patients stated fairly or very much that the COVID-19 vaccination had a positive impact on their QoL (item 5, mean 2.5), whereas only 16.8% indicated the vaccination did “not at all” have that positive impact (Figure 3B).
Discussion
The present study first evaluated to which extend COVID-19 vaccination might be associated with HR-QoL in patients with breast and gynecological cancer. Our data showed a noticeable although not statistically significant improvement of the patients’ quality of life due to their COVID-19 vaccination: 78.5% participated more in their everyday life (38.3% a bit or rather more; 40.2% fairly or very much more), 77.8% of the patients spent more time with their friends (44.4% a bit or rather more; 33.4% fairly or very much more), and 64.8% of the patients spent more time practicing sports after receiving complete vaccine protection (43.8% a bit or rather more; 21.0% fairly or very much more). Furthermore, 83.3% of the surveyed patients reported a positive effect on their quality of life after receiving the vaccine. Second, we evaluated COVID-19 infections in fully vaccinated patients with breast and gynecological cancer. The symptoms of the surveyed patients were self-limiting, and no hospital admissions were reported.
Our questionnaire showed good psychometric results regarding acceptance and internal consistency. Our findings from the questionnaire might reassure undecided patients regarding COVID-19 vaccination: A previous study conducted one year ago at our gynecological outpatient clinic demonstrated a rate of only 61.1% of cancer patients willing to receive the vaccine (21). This study also showed no vaccine-related serious events and self-limiting vaccine-related adverse events of mostly short duration. This is in line with our data from item 1 of the questionnaire (Figures 3A, B), with 77% of the surveyed patients stating the vaccination did not at all negatively influence their QoL.
Approximately 25% of the patients in the present survey worried less about interruption of their oncological therapy and seeking medical help in the case of health-related problems. The reluctancy observed in the beginning of the pandemic (2) may have led to less diagnostic procedures and thus less treatment of non-COVID-19-related diseases (22).
A previously conducted study reported a positive influence on patients’ global HR-QoL, physical, social, emotional, cognitive and role functioning if they had been working before diagnosis (17). 50% of our currently working patients agreed very strong that returning physically to work after vaccination positively impacted their HR-QoL. Besides working, according to the ESMO-ESO international consensus guidelines for advanced breast cancer as well as other studies physical exercise, sports, or yoga are suggested to further improve QoL, fitness and fatigue (18, 23). 64.8% of the surveyed patients reported to do more sports due to COVID-19 vaccination, which reveals another positive impact of the vaccine on their HR-QoL.
A study by Vuagnat et al. showed a higher impact of the presence of comorbidities on the course of the COVID-19 disease than the oncological therapy which patients were undergoing (24). Breast cancer patients with comorbidities significantly showed lower total scores than those without comorbidities in the vaccine-related QoL-questionnaire, indicating less of a positive influence of the vaccination on their QoL. This might be due to the fact, that these patients have a higher risk of having severe courses of COVID-19 (especially those with vascular, metabolic or respiratory comorbidities) (25, 26), and thus limit themselves, their social contacts and participation in everyday life more strictly – vaccinated or not. The same could apply for patients with metastatic disease, since according to the ACHOCC-19 Study metastatic disease is associated with a higher mortality due to COVID-19 (27). Compared to patients with non-metastatic cancer, patients with metastatic disease showed significantly lower total scores in our study implying less benefit of the vaccine for their HR-QoL. The fact, that the effect was not significant among gynecological cancer patients might be related to the time of diagnose and stage of disease in gynecological malignancies, which are more often diagnosed at an advanced stage. Therefore, patients with non-metastatic cancer are more likely to be impaired by their disease compared to (early) breast cancer patients with non-metastatic cancer.
Our results show a COVID-19 infection rate of 25.4% in patients having received at least two doses of vaccine (booster rate 95.6%) All of these patients reported self-limiting symptoms and no case of hospitalization was noted. Compared to the general population in Germany with an infection rate of 38.78% (proportion of fully vaccinated persons: 76.3%, 62.0% having received a booster vaccine), the infection rate in the surveyed cohort of vaccinated patients with breast and gynecological cancer is considerably lower than in the general population (28).
The majority of the surveyed patients developed cold- or flu-like symptoms (Figure 2). A study by Rüthrich et al. which enrolled 435 cancer patients including 59% with solid tumors (18.5% with breast and gynecological cancer) and 54% with active malignancy observed a rate of 27.5% patients being admitted to ICU (29). Most common symptoms were fever (34%), coughing (24.5%) and excessive tiredness (18.9%). This is in line with our results which showed fever in 37.9%, coughing in 51.7%, and fatigue in 24.1% of the patients. Interestingly, we had a much a higher amount (51.7% vs 24.5%) of patients with coughing but no cases of admittance to ICU in the vaccinated cohort. The above-mentioned study was conducted in 2020, meaning the patients were not vaccinated and probably infected with the alpha variant, whereas 93.1% of the patients surveyed here got infected from October 2021 onwards (Supplementary Figure 3), when the predominant virus variant in Germany was Omikron, which is associated with a milder course of the disease than the variants before (30).
Interestingly, patients who received a combination of vaccines (Conmirnaty/Moderna, Conmirnaty/Vaxzevria) showed fewer infection rates than those who received only Conmirnaty. Due to the small cohort size, this difference is not significant. Two independent reviews reported good immunogenicity and a stronger T cellular immunity in patients with a combination of ChAdOx1 and Conmirnaty, which may be a reason for our clinical observation (31, 32).
A review performed by Lasagna et al. in 2020 explored the question, whether the gut microbiota and estrogen levels in breast cancer patients could influence the course of their COVID-19 infections (33). A possible conclusion of the data discussed in the paper was that breast cancer patients with hormone receptor positive breast cancer may be better protected against COVID-19 infections due to higher estrogen levels, which showed a negative correlation with severity of COVID-19 infection in a multi-center study performed in China in 2020 (34). By implication, this raises the question whether endocrine-based therapy increases the risk for more severe COVID-19 infections. Our results from Table 2 showed that 4/16 (25.0%) of the breast cancer patients treated with endocrine-based therapy had a COVID-19 infection. The mean duration of symptoms was 6.4 days (n=5) vs. 5.6 days in those patients not receiving endocrine based therapies (n=18), which would thus support this hypothesis (not significant due to the small number of patients).
Limitations of our study mostly consist of the small cohort included in the study. Second, the study had a monocentric design, and all patients were recruited from the two LMU gynecological outpatient clinics in Munich. In addition, we did not have a control group as almost all patients in the oncological outpatient clinics had received at least two vaccinations at the time of the survey. Further studies are thus required to validate our findings.
Conclusion
Overall, the conducted study pointed out that the COVID-19 vaccine had a positive impact on patients’ HR-QoL. Moreover, in an ambulatory setting, fully vaccinated patients with breast and gynecological cancer showed predominantly mild courses of COVID-19 infection without hospital admissions. These results should be considered when consulting cancer patients regarding COVID-19 vaccination.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study received ethics approval from the Ludwig-Maximilians-University (LMU) Munich Ethics Committee in February 2022 (number of ethical approval: 21-1237). Signed informed consent was obtained from all participants.
Author contributions
MF, RW, NH, and AC-R contributed to conception and design of the study. MF, AK, AS, EW and AC-R contributed to acquisition of data. MF performed the statistical analysis, FB, FT, SM and AC-R were engaged in analysis and interpretation of data. MF, RW, NH and AC-R drafted the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
RW received honoraria from Agendia, Amgen, Aristo, Astra Zeneca, Boehringer Ingelheim, Carl Zeiss, Celgene, Daiichi-Sankyo, Eisai, Exact Sciences, Genomic Health, Gilead, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Mylan, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Sanofi Genzyme, Seattle Genetics/Seagen, Tesaro Bio, Teva, Veracyte, Viatris, FOMF, Aurikamed, Clinsol. FT reports grants and personal fees from AstraZeneca, Clovis, Eisai, Medac, MSD, PharmaMar, Roche, and Tesaro/GSK. SM reports research funding, advisory board, honorary or travel expenses: AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, Medac, MSD, Novartis, Nykode, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, Tesaro NH reports honoraria for lectures and/or consulting from AstraZeneca, Daiichi-Sankyo, Gilead, Lilly, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, Sanofi, Sandoz, Seagen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1217805/full#supplementary-material
References
1. Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat (2020) 184(1):249–54. doi: 10.1007/s10549-020-05828-7
2. de Joode K, Dumoulin DW, Engelen V, Bloemendal HJ, Verhaij M, van Laarhoven HWM, et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients’ perspective. Eur J Cancer (2020) 136:132–9. doi: 10.1016/j.ejca.2020.06.019
3. Rolston KV. Infections in cancer patients with solid tumors: A review. Infect Dis Ther (2017) 6(1):69–83. doi: 10.1007/s40121-017-0146-1
4. Ciążyńska M, Pabianek M, Szczepaniak K, Ułańska M, Skibińska M, Owczarek W, et al. Quality of life of cancer patients during coronavirus disease (COVID-19) pandemic. Psychooncology (2020) 29(9):1377–9. doi: 10.1002/pon.5434
5. EMA EMA. COVID-19 vaccines: authorised(2021). Available at: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section.
6. Aboueshia M, Hussein MH, Attia AS, Swinford A, Miller P, Omar M, et al. Cancer and COVID-19: analysis of patient outcomes. Future Oncol (2021) 17(26):3499–510. doi: 10.2217/fon-2021-0121
7. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv (2017) 11(2):211–32. doi: 10.1007/s11764-016-0580-1
8. Bargon CA, Batenburg MCT, van Stam LE, Mink van der Molen DR, van Dam IE, van der Leij F, et al. Impact of the COVID-19 pandemic on patient-reported outcomes of breast cancer patients and survivors. JNCI Cancer Spectr (2021) 5(1):pkaa104. doi: 10.1093/jncics/pkaa104
9. Hamer J, McDonald R, Zhang L, Verma S, Leahey A, Ecclestone C, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer (2017) 25(2):409–19. doi: 10.1007/s00520-016-3417-6
10. Aapro M, Lyman GH, Bokemayer C, Rapoport BL, Mathieson N, Koptelova N, et al. Supportive care in patients with cancer during the COVID-19 pandemic. ESMO Open (2021) 6(1):100038. doi: 10.1016/j.esmoop.2020.100038
11. Johnston SR. The role of chemotherapy and targeted agents in patients with metastatic breast cancer. Eur J Cancer (2011) 47 Suppl 3:S38–47. doi: 10.1016/S0959-8049(11)70145-9
12. Westin SN, Labrie M, Litton JK, Blucher A, Fang Y, Vellano CP, et al. Phase ib dose expansion and translational analyses of olaparib in combination with capivasertib in recurrent endometrial, triple-negative breast, and ovarian cancer. Clin Cancer Res (2021) 27(23):6354–65. doi: 10.1158/1078-0432.CCR-21-1656
13. Jeppesen SS, Bentsen KK, Jørgensen TL, Holm HS, Holst-Christensen L, Tarpgaard LS, et al. Quality of life in patients with cancer during the COVID-19 pandemic - a Danish cross-sectional study (COPICADS). Acta Oncol (2021) 60(1):4–12. doi: 10.1080/0284186X.2020.1830169
14. Nausheen B, Gidron Y, Peveler R, Moss-Morris R. Social support and cancer progression: a systematic review. J Psychosom Res (2009) 67(5):403–15. doi: 10.1016/j.jpsychores.2008.12.012
15. Usta YY. Importance of social support in cancer patients. Asian Pac J Cancer Prev (2012) 13(8):3569–72. doi: 10.7314/APJCP.2012.13.8.3569
16. Moraliyage H, De Silva D, Ranasinghe W, Adikari A, Alahakoon D, Prasad R, et al. Cancer in lockdown: impact of the COVID-19 pandemic on patients with cancer. Oncologist (2021) 26(2):e342–4. doi: 10.1002/onco.13604
17. Schmidt ME, Scherer S, Wiskemann J, Steindorf K. Return to work after breast cancer: The role of treatment-related side effects and potential impact on quality of life. Eur J Cancer Care (Engl) (2019) 28(4):e13051. doi: 10.1111/ecc.13051
18. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res (2018) 20(1):124. doi: 10.1186/s13058-018-1051-6
19. Leclerc AF, Foidart-Dessalle M, Tomasella M, Coucke P, Devos M, Bruyère O, et al. Multidisciplinary rehabilitation program after breast cancer: benefits on physical function, anthropometry and quality of life. Eur J Phys Rehabil Med (2017) 53(5):633–42. doi: 10.23736/S1973-9087.17.04551-8
20. Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC. Breast cancer and social environment: getting by with a little help from our friends. Breast Cancer Res (2016) 18(1):54. doi: 10.1186/s13058-016-0700-x
21. Forster M, Wuerstlein R, Koenig A, Amann N, Beyer S, Kaltofen T, et al. COVID-19 vaccination in patients with breast cancer and gynecological Malignancies: A German perspective. Breast (2021) 60:214–22. doi: 10.1016/j.breast.2021.10.012
22. Figueroa JD, Gray E, Pashayan N, Deandrea S, Karch A, Vale DB, et al. The impact of the Covid-19 pandemic on breast cancer early detection and screening. Prev Med (2021) 151:106585. doi: 10.1016/j.ypmed.2021.106585
23. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol (2020) 31(12):1623–49. doi: 10.1016/j.annonc.2020.09.010
24. Vuagnat P, Frelaut M, Ramtohul T, Basse C, Diakite S, Noret A, et al. COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res (2020) 22(1):55. doi: 10.1186/s13058-020-01293-8
25. Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health (2020) 13(12):1833–9. doi: 10.1016/j.jiph.2020.07.014
26. Müller-Wieland D, Marx N, Dreher M, Schnell O. COVID-19 and cardiovascular comorbidities. Exp Clin Endocrinol Diabetes (2022) 130(3):178–89. doi: 10.1055/a-1269-1405
27. Ospina AV, Bruges R, Mantilla W, Triana I, Ramos P, Aruachan S, et al. Impact of COVID-19 infection on patients with cancer: experience in a latin american country: the ACHOCC-19 study. Oncologist (2021) 26(10):e1761–73. doi: 10.1002/onco.13861
28. R.K.I. COVID-19: Number of cases in Germany (2022). Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html/.
29. Rüthrich MM, Giessen-Jung C, Borgmann S, Classen AY, Dolff S, Grüner B, et al. COVID-19 in cancer patients: clinical characteristics and outcome-an analysis of the LEOSS registry. Ann Hematol (2021) 100(2):383–93. doi: 10.1007/s00277-020-04328-4
30. Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci (2021) 25(24):8012–8. doi: 10.26355/eurrev_202112_27652
31. Chiu NC, Chi H, Tu YK, Huang YN, Tai YL, Weng SL, et al. To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination. Expert Rev Vaccines (2021) 20(10):1211–20. doi: 10.1080/14760584.2021.1971522
32. Fiolet T, Kherabi T, MacDonald C-J, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect (2022) 28(2):202–21. doi: 10.1016/j.cmi.2021.10.005
33. Lasagna A, Zuccaro V, Ferraris E, Corbella M, Bruno R, Pedrazzoli P. COVID-19 and breast cancer: may the microbiome be the issue? Future Oncol (2021) 17(2):123–6. doi: 10.2217/fon-2020-0764
34. Ding T, Zhang J, Wang T, Cui P, Chen Z, Jiang J, et al. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome coronavirus 2 infection: A cross-sectional multicenter study in Wuhan, China. Clin Infect Dis (2021) 72(9):e240–8. doi: 10.1093/cid/ciaa1022
Keywords: SARS-CoV-2 pandemic, COVID-19 vaccination, breast cancer, gynecological cancer, health-related quality of life
Citation: Forster M, Wuerstlein R, Koenig A, Stefan A, Wiegershausen E, Batz F, Trillsch F, Mahner S, Harbeck N and Chelariu-Raicu A (2023) Health-related quality of life and patient-centred outcomes with COVID-19 vaccination in patients with breast cancer and gynaecological malignancies. Front. Oncol. 13:1217805. doi: 10.3389/fonc.2023.1217805
Received: 05 May 2023; Accepted: 28 September 2023;
Published: 12 October 2023.
Edited by:
Areerat Suputtitada, Chulalongkorn University, ThailandReviewed by:
Amina Aquil, Hassan Premier University, MoroccoShuzhao Chen, First Affiliated Hospital of Shantou University Medical College, China
Copyright © 2023 Forster, Wuerstlein, Koenig, Stefan, Wiegershausen, Batz, Trillsch, Mahner, Harbeck and Chelariu-Raicu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anca Chelariu-Raicu, QW5jYS5DaGVsYXJpdS1SYWljdUBtZWQudW5pLW11ZW5jaGVuLmRl
 Marie Forster
Marie Forster Rachel Wuerstlein
Rachel Wuerstlein Alexander Koenig
Alexander Koenig Alexandra Stefan
Alexandra Stefan Falk Batz
Falk Batz Fabian Trillsch
Fabian Trillsch Nadia Harbeck
Nadia Harbeck