- 1Department of Thoracic and Cardiovascular Surgery, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Department of Bioinformatics and Computational Biology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 3Department of Translational Molecular Pathology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 4Department of Thoracic/Head and Neck Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 5Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 6Department of Pathology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
Background: Malignant pleural mesothelioma (MPM) is associated with poor prognosis despite advances in multimodal therapeutic strategies. While patients with resectable disease may benefit from added survival with oncologic resection, patient selection for mesothelioma operations often relies on both objective and subjective evaluation metrics. We sought to evaluate factors associated with improved overall survival (OS) in patients with mesothelioma who underwent macroscopic complete resection (MCR).
Methods: Patients with MPM who received neoadjuvant therapy and underwent MCR were identified in a prospectively maintained departmental database. Clinicopathologic, blood-based, and radiographic variables were collected and included in a Cox regression analysis (CRA). Response to neoadjuvant therapy was characterized by a change in tumor thickness from pretherapy to preoperative scans using the modified RECIST criteria.
Results: In this study, 99 patients met the inclusion criteria. The median age of the included patients was 64.7 years, who were predominantly men, had smoking and asbestos exposure, and who received neoadjuvant therapy. The median change in tumor thickness following neoadjuvant therapy was –16.5% (interquartile range of -49.7% to +14.2%). CRA demonstrated reduced OS associated with non-epithelioid histology [hazard ratio (HR): 3.06, 95% confidence interval (CI): 1.62–5.78, p < 0.001] and a response to neoadjuvant therapy inferior to the median (HR: 2.70, CI: 1.55–4.72, p < 0.001). Patients who responded poorly (below median) to neoadjuvant therapy had lower median survival (15.8 months compared to 38.2 months, p < 0.001).
Conclusion: Poor response to neoadjuvant therapy in patients with MPM is associated with poor outcomes even following maximum surgical cytoreduction and should warrant a patient-centered discussion regarding goals of care and may therefore help guide further therapeutic decisions.
1 Introduction
The prognosis associated with the diagnosis of malignant pleural mesothelioma (MPM) surrounds patient and clinicopathologic factors; however, it remains poor despite robust efforts to modulate oncologic outcomes with novel neoadjuvant therapies (1, 2). With the increased use of multimodal therapy (3), there remains an equipoise in selecting patients who may benefit from resection. While the inception of enhanced recovery pathways reduced surgical morbidity, the convalescence associated with macroscopic complete resection (MCR) is protracted and often associated with decreased quality of life (4) and potential detrimental effects for the receipt of other established or experimental therapies, regardless of whether pleurectomy and decortication or extrapleural pneumonectomy was performed.
The current clinical staging metrics have been insufficient in providing clinicians with objective tools to inform patient-centered prognosis discussions (5). Due to a deficiency of meaningful prognostic measures, multiple groups have suggested scoring systems (6–8) or factors (9) that can help prognostication of this patient population. While these may inform prognosis in patients with MPM often prior to receipt of any therapy, patient and disease characteristics that might affect overall survival in patients undergoing resection for MPM remain underutilized.
Surgical decisions are currently based on patient and disease factors, patient’s wishes, and surgeon’s experience, using insight on the aggressivity and biologic behavior of this disease. Thus, we sought to augment the surgical decision-making process by investigating a series of environmental, clinicopathologic, treatment response, blood-based, and radiographic factors that might reflect disease and host physiology. We aimed to integrate blood-based and radiographic changes that mirror the dynamic pathological milieu impacted by neoadjuvant therapy into our analyses to enhance the surgical decision process for resectable MPM.
2 Materials and methods
2.1 Study design, population, and treatment
A prospectively maintained single-institution departmental database was retrospectively queried for patients with MPM, evaluated by a multidisciplinary team, who underwent MCR following neoadjuvant therapy between April 2005 and September 2019 at the University of Texas MD Anderson Cancer Center (MDACC). This study was undertaken following approval by MDACC’s Institutional Review Board with a waiver of individual informed consent (PA 2019-1197).
Patient and disease-specific variables obtained included smoking status, asbestos exposure, baseline pulmonary function, and performance status, as well as clinical and pathological stage (adjusted to the eighth edition American Joint Commission on Cancer staging) and histopathology. A subgroup of patients with epithelioid histology was defined a priori. Peripheral blood samples were collected prior to the start of neoadjuvant systemic therapy and again following the completion of therapy but prior to surgery. These samples were the source of peripheral blood-based data that included absolute lymphocyte, absolute neutrophil, and platelet counts. These were then used to calculate neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). Radiographic data were obtained from computed tomography (CT) scans completed prior to the initiation of neoadjuvant systemic therapy, during neoadjuvant systemic therapy, and included the last scan prior to the resection. Tumor thickness was measured according to the revised modified RECIST (mRECIST) (10, 11) criteria. All scans included met the minimum quality standard as suggested by the criteria. The radiographic response to neoadjuvant systemic therapy was categorized by the change in tumor thickness from prior to systemic therapy to the most recent preoperative scan as a percentage change. Tumor response was also stratified using the mRECIST criteria, which defined partial response (PR) as a decrease in tumor thickness of at least 30%, stable disease (SD) as a decrease of <30% or an increase of <20% in tumor thickness, and progressive disease (PD) as an increase in tumor thickness of at least 20%. Overall survival was defined from the initiation of neoadjuvant systemic therapy until date of death or censored at the last follow-up. Stratification of the cohort by 20 months’ survival was performed, which characterized a clinically meaningful threshold, considering the historical median overall survival of patients with resectable MPM, in addition to surgical convalescence (4), and as a comparison to 18 months’ survival achieved in the immunotherapy cohort of Checkmate 743 trial.
2.2 Statistical methods
A paired T-test was used to compare the clinical to pathological tumor and nodal statuses in order to assess concordance. A Cox proportional hazards regression model and multivariable linear regression model were used to assess and identify variables that may inform overall survival. Covariates were included in the models if they satisfied Wald’s backward elimination using a probability value threshold of 0.200. The Kaplan–Meier method and a log-rank test were used to further determine the survival probability of patients with MPM stratified by radiographic response to neoadjuvant systemic therapy. A subgroup analysis defined a priori was performed and included only patients with epithelioid disease. Collinearity was assessed using variance inflation factors. Analyses were performed using R Studio (version 1.4.1717, PBC, Boston, MA, USA) and GraphPad Prism (version 9.3.1, GraphPad Software, San Diego, CA, USA).
3 Results
3.1 Environmental, clinicopathologic, circulomic, and radiomic characteristics
There were 99 patients who met the inclusion criteria during the study period. The median age of the patients in this cohort was 64.7 years [interquartile range (IQR): 59.3–68.1]; the majority of the patients were men (n = 74, 74.7%), had smoking exposure (ever smoker, n = 53, 53.5%), and had asbestos exposure (n = 74, 74.7%). Patients were most commonly diagnosed with clinical stage I disease (n = 52, 52.5%) and clinical stage III disease (n = 27, 27.3%). However, upstaging following resection was significantly common (p < 0.001) in 58 patients (58.6%), leading to pathological stage III (n = 55, 55.6%) and IV (n = 23, 23.2%) predominance. This was secondary to a significant rate of upstaging in both tumor (p < 0.001) and nodal statuses (p < 0.001). The cohort included patients who predominantly received a platinum-based doublet neoadjuvant therapy (n = 86, 86.9%), which included combination therapy with immunotherapy in four patients (4.0%). Targeted therapy was used in 17 patients (17.2%). The median length of neoadjuvant therapy was 1.97 months (IQR: 1.07–2.17). The median length of time from the cessation of neoadjuvant therapy to resection was 1.25 months (IQR: 0.81–1.97). The majority of resected tumors in this cohort were found to be composed of epithelioid histology (n = 75, 75.8%). These characteristics are summarized in Table 1. The peripheral blood marker characteristics are summarized in Table 1.
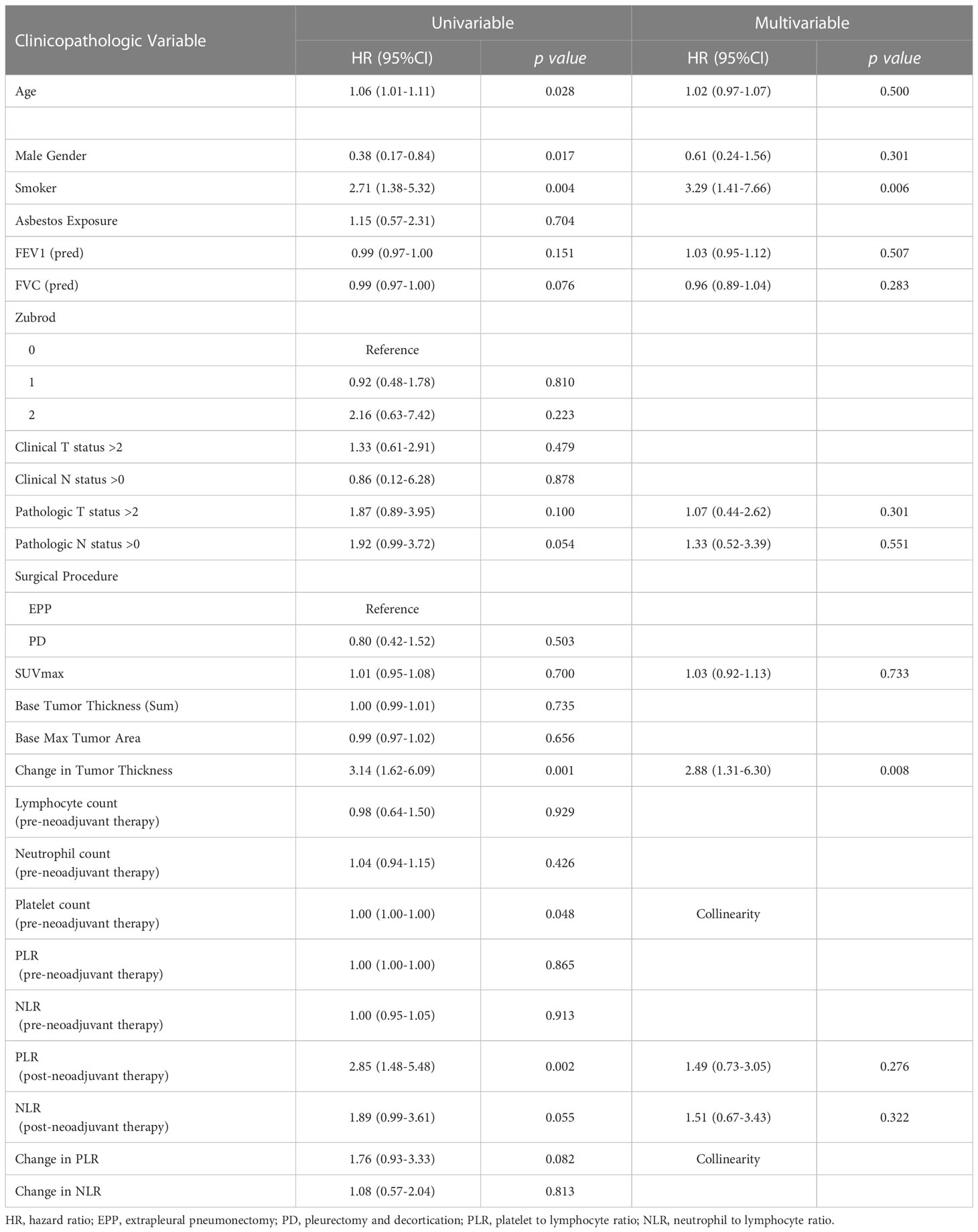
Table 1 Multivariable analysis of patients with epithelioid type malignant pleural mesothelioma reflecting characteristics significantly affecting overall survival.
The median pre-neoadjuvant therapy tumor thickness was 59.9 mm (IQR: 43.6–82.2), and the median thickness observed post-neoadjuvant therapy was 53.4 mm (IQR: 23.5–72.3). The median change in tumor thickness from the pre-neoadjuvant therapy CT scan to the most recent preoperative CT scan was -16.5% (IQR: -49.7 to 14.2). PR was observed in 41 patients (41.4%) while 39 patients (39.4%) had SD and 19 patients (19.2%) had PD (Figures 1A, B).
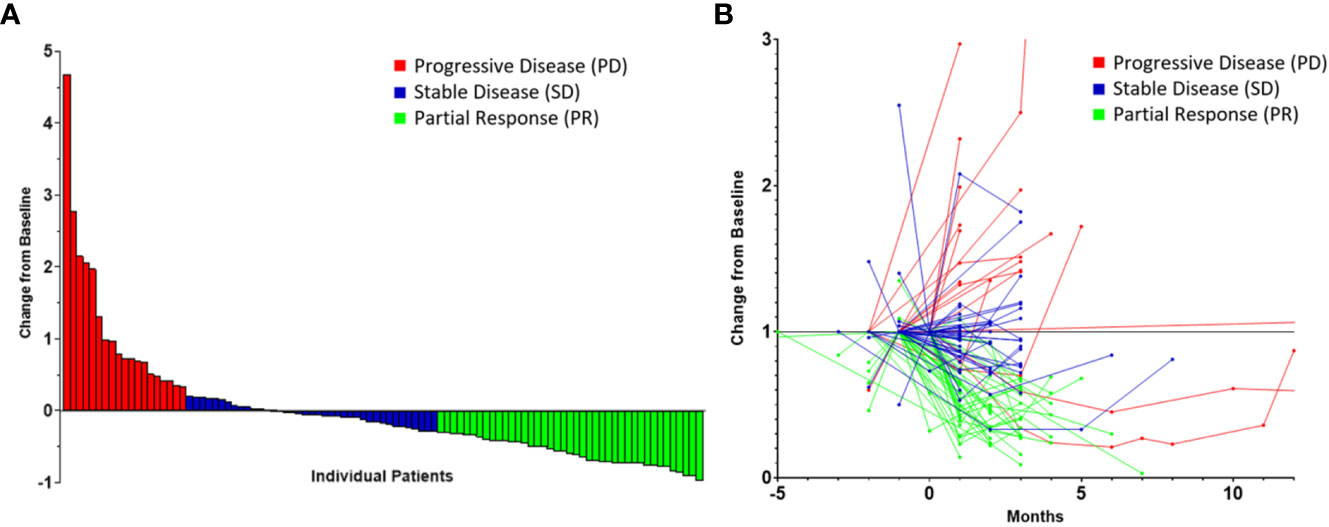
Figure 1 Radiomic data highlighting tumor response to neoadjuvant therapy in patients with malignant pleural mesothelioma stratified by response according to the modified RECIST criteria represented as a waterfall plot (A) and as a spaghetti plot (B). Time of 0 month represents the initiation of neoadjuvant therapy.
3.2 Survival analysis
Following univariate analyses, age, gender, smoking status, histology, pathologic nodal status greater than 0, and change in tumor thickness from neoadjuvant therapy met the criteria for inclusion in the multivariable Cox regression analysis. The regression analysis showed that patients with non-epithelioid MPM has a significant risk of mortality [hazard ratio (HR): 3.06, 95% confidence interval (CI): 1.62–5.78, p = 0.001]. Patients with pathologically proven nodal disease also were found to be at a survival disadvantage (HR: 1.92, CI: 1.06–3.48, p = 0.031). A change in tumor thickness that represented a response to neoadjuvant therapy inferior to the median response (tumors that decreased in size by 16.5% or less) was also associated with a greater risk of mortality (HR: 2.70, CI: 1.55–4.72, p < 0.001). The results of this analysis are summarized in Table 2 and graphically represented in Figure 2A. Furthermore, Kaplan–Meier analysis showed that patients with a response inferior to a 16.5% decrease in thickness or disease progression on neoadjuvant therapy had a median survival of 15.77 months (CI: 11.83–23.78), while those with a response better than a 16.5% decrease in tumor thickness achieved a median survival of 38.17 months (CI: 25.59–54.66, p < 0.001, Figure 3A).
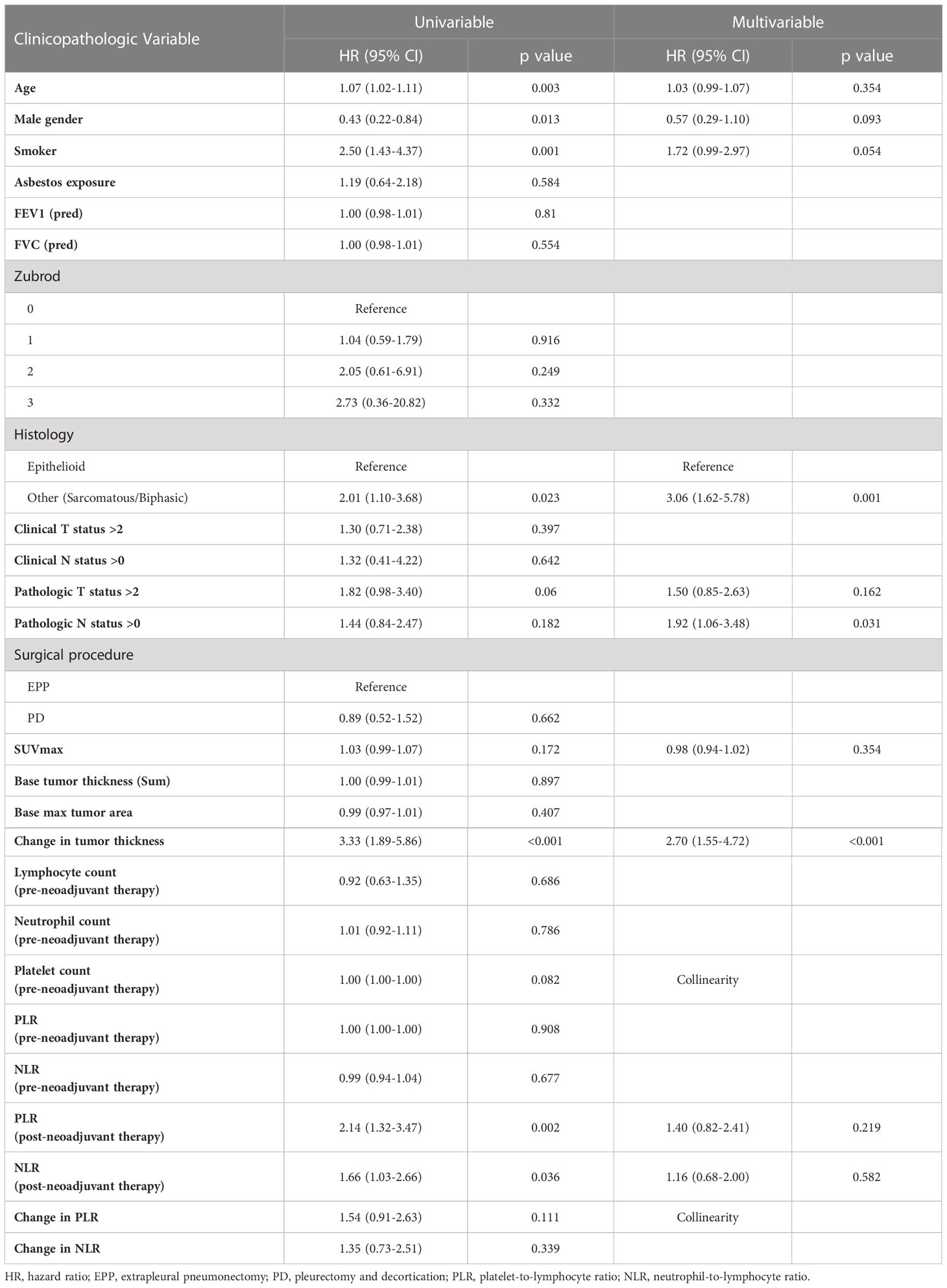
Table 2 Multivariable analysis of patients with malignant pleural mesothelioma reflecting characteristics significantly affecting overall survival.
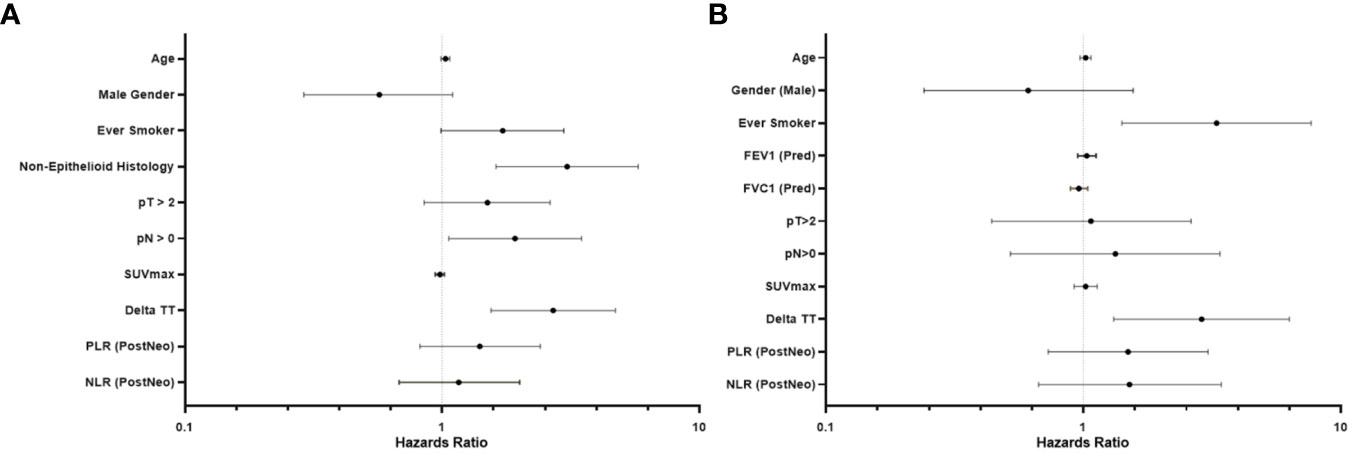
Figure 2 Forest plot representing the results of a multivariable analysis investigating survival beyond 20 months in patients with malignant pleural mesothelioma (A) and subgroup (epithelioid histology only) multivariable analysis (B).
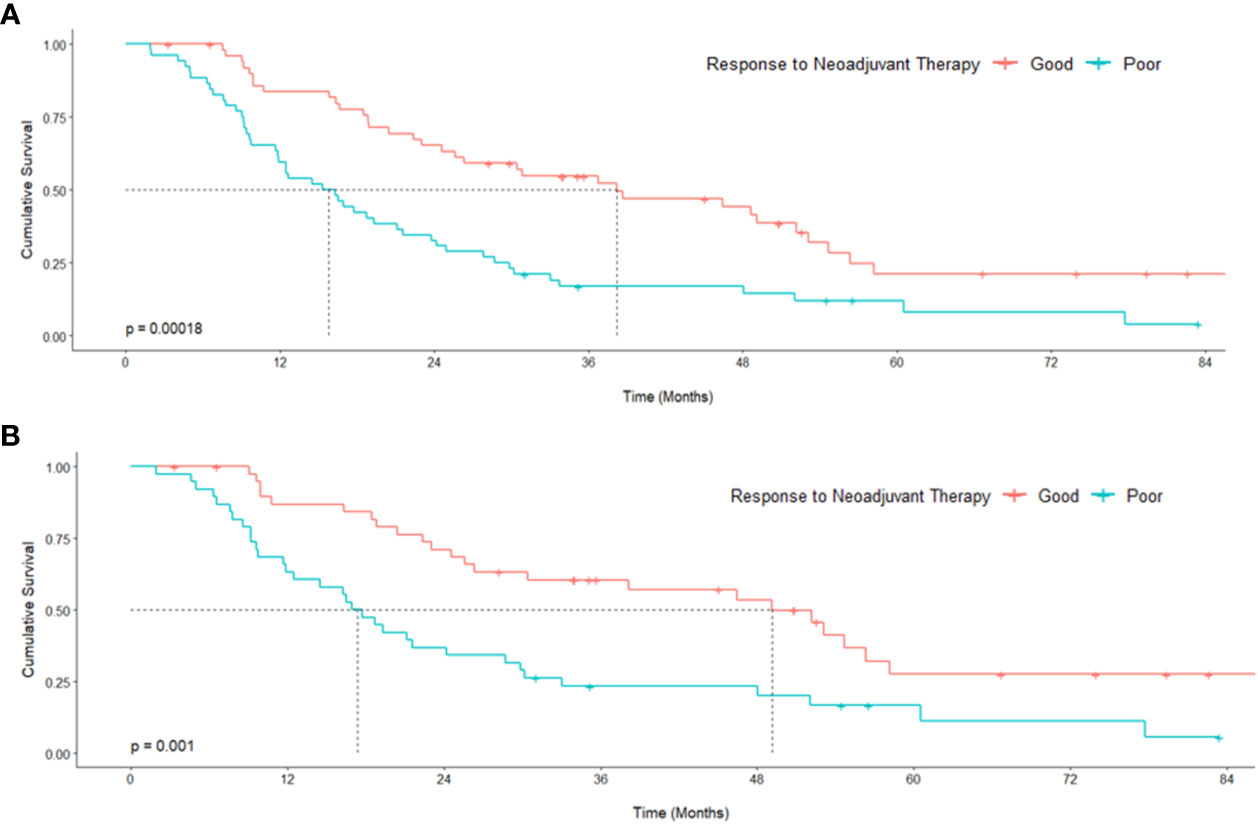
Figure 3 Kaplan–Meier curves representing survival analysis of a cohort of patients with malignant pleural mesothelioma (A) and subgroup [epithelioid histology only (B)] stratified around the median tumor response to neoadjuvant therapy. p values originate from a log-rank test comparing median survival.
Univariate analyses of the subgroup of patients with epithelioid histology showed that age, gender, smoking status, pulmonary function test results, maximum standardized uptake value (SUVmax), change in tumor thickness, post-neoadjuvant therapy NLR and PLR met the inclusion criteria in our multivariable Cox regression analysis. The regression analysis revealed that smoking status had a significant effect on mortality (HR: 3.29, CI: 1.41–7.66, p = 0.006) as did change in tumor thickness from neoadjuvant therapy (HR: 2.88, CI: 1.31–6.30, p = 0.008, Table 3; Figure 2B). In this subgroup, Kaplan–Meier analysis showed that patients with a poor response to neoadjuvant therapy achieved a median survival of 17.33 months (CI: 11.86–29.83), while those with a good response attained a median survival of 49.11 months (CI: 26.31–Not Reached, p = 0.001, Figure 3B).
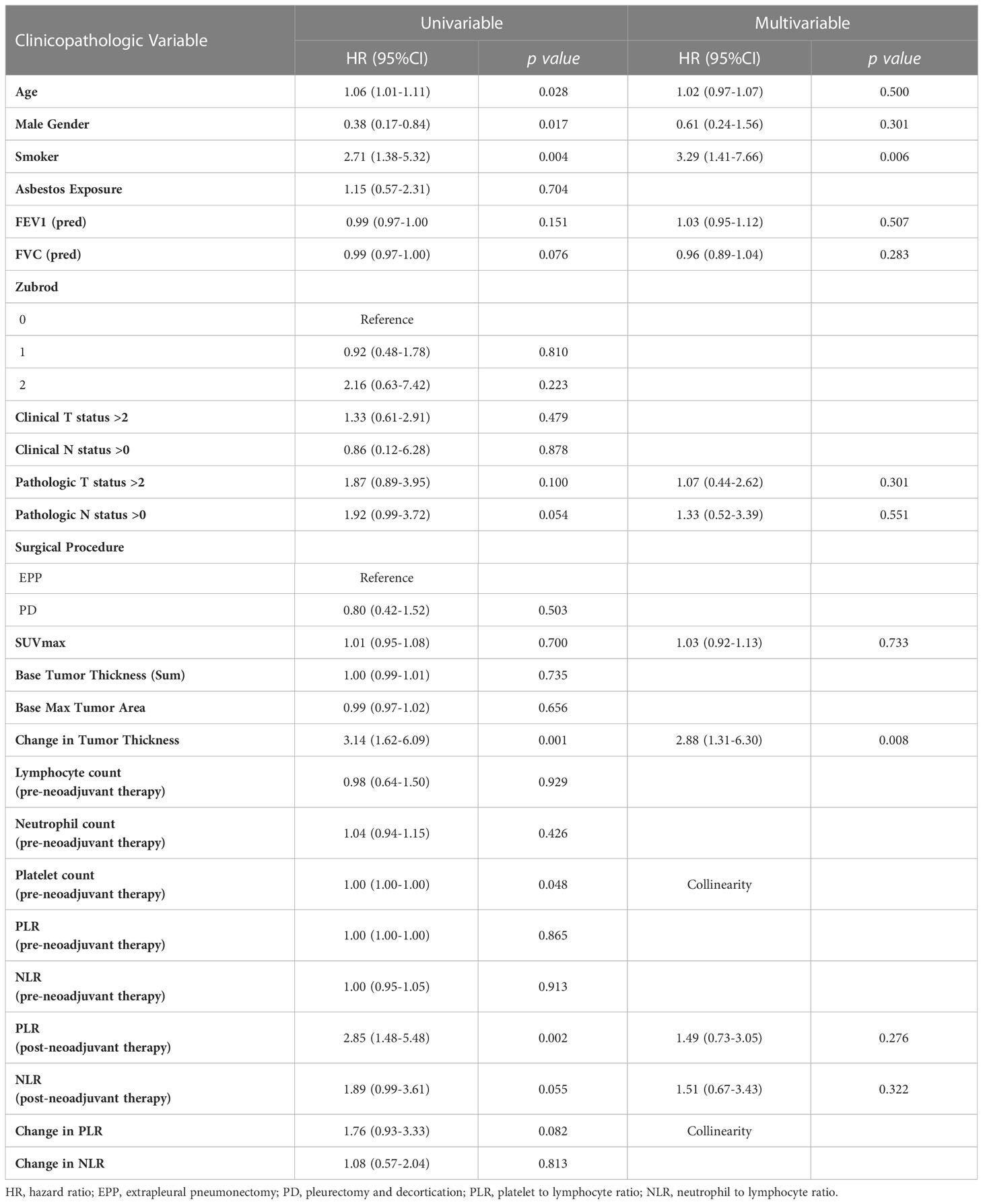
Table 3 Multivariable analysis of patients with epithelioid type malignant pleural mesothelioma reflecting characteristics significantly affecting overall survival.
4 Discussion
We report the survival probability of patients with MPM deemed to have undergone the “best” mesothelioma operation as judged by an operating surgeon, namely, maximum complete reduction, following neoadjuvant therapy. We found that patients with non-epithelioid MPM, nodal involvement, and less than 16.5% radiographic response to neoadjuvant therapy were at greater risk of early mortality following resection; the mortality of this group was similar to mesothelioma outcomes achieved without surgery. The median length of neoadjuvant chemotherapy in this group was 2 months, which permitted an investigation of pretherapy to presurgery tumor change, accounting for surgical decision-making. In the subgroup analysis of patients with epithelioid MPM, we found that smoking status and the lack of response to neoadjuvant therapy were associated with poor survival probability. In both our main analysis and our subgroup analysis, we observed that patients who underwent MCR following at least 16.5% radiographic response to neoadjuvant therapy obtained a survival benefit twice as long compared to those who did not respond to neoadjuvant therapy. This is a finding of significant clinical importance in such a rapidly lethal disease as MPM. Additionally, it provides a more powerful prediction of outcomes than nodal status and may more accurately represent cancer behavior and biology.
While patients with MPM can obtain meaningful outcomes from multimodal therapy, patient selection for resection continues to be intricately related to a compilation of factors. As we have observed, disease-specific factors should inform surgical decisions that mirror patient’s wishes, considering the significant convalescence associated with resection. Specifically in patients with epithelioid MPM, who are often referred for surgical consideration, who may have a median survival of 15.8 months following poor response to neoadjuvant therapy. Considering the significant survival benefit achieved with nivolumab plus ipilimumab (1) (median survival of 18.1 months) in patients with unresectable MPM, further investigations must consider the use of immunotherapy in patients who may have responded poorly to neoadjuvant chemotherapy and who may not benefit from surgical resection.
The current landscape in trimodality therapy, which includes resection, is rapidly evolving (12). Developments of neoadjuvant and maintenance protocols, with combination therapy or immunotherapy alone, are currently enrolling (13) or have recently completed accrual (14). Furthermore, neoadjuvant radiotherapy has also undergone investigation (15) with an ongoing trial exploring the benefits associated with oligofractionated radiotherapy followed by resection (15). Importantly, as meaningful survival benefits are obtained with systemic therapy in unresectable disease (16), the value of surgical resection is also being evaluated in clinical trials such as MARS2 comparing chemotherapy with neoadjuvant chemotherapy plus surgery (17). Advancements are also reported with novel systemic therapy such as T-cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) blockade (18).
This study is associated with limitations, which relate to the heterogeneity in surveillance imaging in this cohort; however, we were able to obtain tumor measurements from all patients included prior to the initiation of neoadjuvant therapy and prior to the resection. Similarly, we noted that using clinical stage was not reliable, considering the significant upstaging following resection, and thus, we decided to use pathological tumor and nodal statuses in order for our multivariate models to be adequately adjusted. Additionally, as represented in Figure 1B, there was a small group of patients who initially appeared to have a great response to systemic therapy who ultimately had progressive disease. This group may benefit from tailored therapy. Furthermore, the overall survival was calculated from the time of neoadjuvant therapy initiation rather than the date of diagnosis in order to alleviate additional heterogeneity in this cohort; however, this may not reflect true overall survival. Interobserver variability in measuring radiographic tumor change was minimized by having a single researcher performing the assessments, blinded to survival data at the time of the measurement. In conclusion, cytoreductive operations should be considered with increased carefulness in patients who do not respond to neoadjuvant therapy, as defined by radiographic metrics, which can serve as a possible surrogate for cancer biology. In the context of suboptimal response to neoadjuvant therapy, the patient’s wishes and quality of life must be considered prior to the pursuit of operative management. The involvement of patients in clinical trials or prospective treatment protocols must continue to be encouraged in order to further enhance therapeutic strategies in MPM.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by MDACC’s Institutional Review Board with a waiver of individual informed consent (PA 2019-1197). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ND: conceptualization, investigation, visualization, methodology, writing–original draft, writing–review and editing. NZ and DM: visualization, methodology, writing–original draft, writing–review and editing. HF, JO, KT, EP, JZ, PL, MBA, and MA: investigation, writing–review and editing. WH, RM, RR, DR, JR, SS, and AV: patient accrual, writing–review and editing. AW: pathology review, writing–review and editing. AT: resources, funding acquisition, writing–review and editing. CH and BS: conceptualization, investigation, visualization, resources, supervision, funding acquisition, methodology, writing–original draft, project administration, writing–review and editing. All authors have contributed to the final article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the generous philanthropic contributions to The University of Texas MD Anderson Lung Cancer Moon Shots Program, the Gil and Dody Weaver Foundation, the Bill and Katie Weaver Charitable Trust, the MD Anderson Cancer Center Support Grant P30 CA016672, and generous funding from the Mason Family Research Fund.
Acknowledgments
The study was also supported by the Translational Molecular Pathology-Immunoprofiling (TMP-IL) Moon Shots Platform at the Department of Translational Molecular Pathology, the University of Texas MD Anderson Cancer Center.
Conflict of interest
Author JZ reports grants from Merck, grants and personal fees from Johnson and Johnson and Novartis, personal fees from Bristol Myers Squibb, AstraZeneca, GenePlus, Innovent, and Hengrui outside the submitted work. Author PL reports support from AstraZeneca, as well as consulting fees from Varian, Viewray, Genentech, John & Johnson, payments for lectures from Varian, Viewray, and AstraZeneca, support for attending meetings from the Radiosurgery Society, and is an adviser for Genentech, Viewray, AstraZeneca. Author PL is on the board of directors of the Radiosurgery Society. Author CH declares research funding to institution from Sanofi, Dragonfly, BTG, Iovance, Obsidian, and Avenge; scientific advisory board member of Briacell with stock options; personal fees from Nanobiotix and speaker fees/honorarium from SWOG and SITC outside the scope of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet (British edition) (2021) 397(10272):375–86. doi: 10.1016/S0140-6736(20)32714-8
2. Peters S, Scherpereel A, Cornelissen R, Oulkhouir Y, Greillier L, Kaplan MA, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol (2022). doi: 10.1016/j.annonc.2022.01.074
3. Tsao AS, Lindwasser OW, Adjei AA, Adusumilli PS, Beyers ML, Blumenthal GM, et al. Current and future management of malignant mesothelioma: A consensus report from the national cancer institute thoracic malignancy steering committee, international association for the study of lung cancer, and mesothelioma applied research foundation. J Thorac Oncol (2018) 13(11):1655–67. doi: 10.1016/j.jtho.2018.08.2036
4. Bueno R, Opitz I. Surgery in malignant pleural mesothelioma. J Thorac Oncol (2018) 13(11):1638–54. doi: 10.1016/j.jtho.2018.08.001
5. Ambrogi V, Mineo TC. Clinical and biologic prognostic factors in malignant pleural mesothelioma. Thorac Cancer (2012) 3(4):289–302. doi: 10.1111/j.1759-7714.2012.00127.x
6. Yeap BY, De Rienzo A, Gill RR, Oster ME, Dao MN, Dao NT, et al. Mesothelioma risk score: A new prognostic pretreatment, clinical-molecular algorithm for malignant pleural mesothelioma. J Thorac Oncol (2021). doi: 10.1016/j.jtho.2021.06.014
7. Meniawy TM, Creaney J, Lake RA, Nowak AK. Existing models, but not neutrophil-to-lymphocyte ratio, are prognostic in malignant mesothelioma. Br J Cancer (2013) 109(7):1813–20. doi: 10.1038/bjc.2013.504
8. Chollet B, Guinde J, Laroumagne S, Dutau H, Astoul P. Does the LENT score risk-stratify patients with malignant pleural mesothelioma? An observational study. Thorac Cancer (2021) 12(11):1752–6. doi: 10.1111/1759-7714.13987
9. Guzmán-Casta J, Carrasco-CaraChards S, Guzmán-Huesca J, Sánchez‐Ríos CP, Riera‐Sala R, Martínez‐Herrera JF, et al. Prognostic factors for progression-free and overall survival in malignant pleural mesothelioma. Thorac Cancer (2021) 12(7):1014–22. doi: 10.1111/1759-7714.13814
10. Tsao AS, Gladish GW, Gill RR. Revised modified RECIST criteria in malignant pleural mesothelioma (Version 1.1): A step forward in a long race. J Thorac Oncol (2018) 13(7):871–3.
11. Tsao AS, Garland L, Redman M, Kernstine K, Gandara D, Marom EM. A practical guide of the southwest oncology group to measure malignant pleural mesothelioma tumors by RECIST and modified RECIST criteria. J Thorac Oncol (2011) 6(3):598–601. doi: 10.1097/JTO.0b013e318208c83d
12. Govindan R, Aggarwal C, Antonia SJ, Davies M, Dubinett SM, Ferris A, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of lung cancer and mesothelioma. J Immunother Cancer (2022) 10(5):e003956. doi: 10.1136/jitc-2021-003956
13. Forde PM, Scherpereel A, Tsao AS. Use of immune checkpoint inhibitors in mesothelioma. Curr Treat options Oncol (2019) 20(2):1–10. doi: 10.1007/s11864-019-0613-x
14. Burt B, Ramineni M, Jang H, Ramos D, Splawn T, Espinoza M. A randomized trial of neoadjuvant PD-L1 versus PD-L1 Plus CTLA-4. Blockade in patients with malignant pleural mesothelioma. Am Assoc Thorac Surg 101st Annu Meeting (2021) 2021.
15. Cho BCJ, Donahoe L, Bradbury PA, Leighl N, Keshavjee S, Hope A, et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): final results from a single-centre, phase 2 trial. Lancet Oncol (2021) 22(2):190–7. doi: 10.1016/S1470-2045(20)30606-9
16. Kitajima K, Maruyama M, Yokoyama H, Minami T, Yokoi T, Nakamura A, et al. Response to immune checkpoint inhibitor therapy in patients with unresectable recurrent malignant pleural mesothelioma shown by FDG-PET and CT. Cancers (2021) 13(5):1098. doi: 10.3390/cancers13051098
17. Lim E, Darlison L, Edwards J, Elliott D, Fennell DA, Popat S, et al. Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open (2020) 10(9):e038892–e. doi: 10.1136/bmjopen-2020-038892
Keywords: malignant pleural mesothelioma, neoadjuvant therapy, radiographic response, cytoreductive resection, patient-centered care
Citation: Deboever N, Zhou N, McGrail DJ, Tomczak K, Oliva JL, Feldman HA, Parra E, Zhang J, Lee PP, Antonoff MB, Hofstetter WL, Mehran RJ, Rajaram R, Rice DC, Roth JA, Swisher SS, Vaporciyan AA, Altan M, Weissferdt A, Tsao AS, Haymaker CL and Sepesi B (2023) Radiographic response to neoadjuvant therapy in pleural mesothelioma should serve as a guide for patient selection for cytoreductive operations. Front. Oncol. 13:1216999. doi: 10.3389/fonc.2023.1216999
Received: 10 May 2023; Accepted: 10 July 2023;
Published: 11 August 2023.
Edited by:
Han-Yu Deng, Sichuan University, ChinaReviewed by:
Olivia Lauk, University Hospital Zuerich, SwitzerlandFrancesco Fiore, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 Deboever, Zhou, McGrail, Tomczak, Oliva, Feldman, Parra, Zhang, Lee, Antonoff, Hofstetter, Mehran, Rajaram, Rice, Roth, Swisher, Vaporciyan, Altan, Weissferdt, Tsao, Haymaker and Sepesi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cara L. Haymaker, Y2hheW1ha2VyQG1kYW5kZXJzb24ub3Jn
 Nathaniel Deboever
Nathaniel Deboever Nicolas Zhou
Nicolas Zhou Daniel J. McGrail2
Daniel J. McGrail2 Katarzyna Tomczak
Katarzyna Tomczak Jacqueline L. Oliva
Jacqueline L. Oliva Edwin Parra
Edwin Parra Jianjun Zhang
Jianjun Zhang Ravi Rajaram
Ravi Rajaram Jack A. Roth
Jack A. Roth Stephen S. Swisher
Stephen S. Swisher Mehmet Altan
Mehmet Altan Annikka Weissferdt
Annikka Weissferdt Anne S. Tsao
Anne S. Tsao Cara L. Haymaker
Cara L. Haymaker Boris Sepesi
Boris Sepesi