- The First Clinical College, Changsha Medical University, Changsha, China
Purpose: Our aim was to conduct a meta-analysis and systematic review in order to compare the diagnostic efficacy of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI in patients with biochemically recurrent after radical prostatectomy and biochemically recurrent prostate cancers (BCR) after hybrid RT and RP.
Methods: Up until February 2023, we searched PubMed, Embase, and Web of Science for pertinent papers. Studies examining the utility of 68Ga-PSMA-11 PET/CT or PET/MRI as a screening tool for biochemically recurrent prostate cancer were included. To measure heterogeneity, we employed the I2 statistic. In cases of substantial heterogeneity (I2 > 50%), we used the random effect model to produce a forest plot. In other cases, we utilized the fixed model. Furthermore, we assessed the quality of the studies included using the Quality Assessment of Diagnostic Performance Studies (QUADAS-2) method.
Results: In total, 37 studies involving 8409 patients were examined. For 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI, the combined total detection rate was 0.70 (95% CI: 0.65-0.75) and 0.71 (95% CI:0.67-0.75), respectively. 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI did not substantially differ in terms of the overall detection rate for BCR (P = 0.58). The detection rate was unaffected by the PSA values (all P > 0.05).
Conclusion: The diagnostic efficacy of 68Ga-PSMA-11 PET/CT appears to be equivalent to that of 68Ga-PSMA-11 PET/MRI in detecting biochemically recurrent prostate cancer. Nonetheless, it should be noted that not all studies have used pathological biopsies as the gold standard. Therefore, additional larger prospective studies are needed to address this issue.
Systematic review registration: identifier CRD42023410039.
1 Introduction
One of the most prevalent diseases in the world, prostate cancer (PCa) has an annual incidence increase of 3% from 2014 to 2019 (1). Radiation therapy and radical surgery are the two most frequently used treatments for prostate cancer. A rise in prostate-specific antigen (PSA) levels following treatment, however, is a sign that over 30% of people may still experience disease recurrence (2, 3). In clinical practice, biochemical recurrence (BCR) of PCa is fairly typical. BCR is defined as an absolute rise in PSA level of 2 ng/ml over the lowest post-treatment PSA level following radiation therapy (RT) or a serum PSA level exceeding a threshold of 0.2 ng/ml twice after radical prostatectomy (RP) (4).
Imaging techniques are advised for individuals with biochemical recurrence who have serum prostate-specific antigen levels greater than 10 ng/mL or PSA doubling times shorter than 6 months (5). However, the ability of these traditional imaging techniques to diagnose aggressive lesions, bone involvement, and nodal metastases is restricted. It is essential to discover more sophisticated imaging techniques to detect the metastasis of the early BCR in order to increase diagnostic precision and select an appropriate treatment strategy.
EANM/SNMMI guidelines recently provided updated guidance and standards for the indication, acquisition, and interpretation of PSMA PET/CT for prostate cancer imaging (Fendler et al.). Currently, several guidelines highlight the superior accuracy of PSMA-ligand PET for staging primary disease (EAU, ESMO, NCCN) or consider additional value (ASCO) in this setting. PSMA-ligand PET/CT evaluation of BCR/BCP is recommended in documents produced by the EAU, ASCO, and NCCN (Fendler et al.). Moreover, evidence is growing in terms of PSMA-guided treatments, particularly metastases-directed therapy (Ceci et al., Rovera et al., Fendler et al., Phillips et al.) (6–9).
A type II membrane glycoprotein with 750 amino acids, prostate-specific membrane antigen(PSMA), is highly produced in prostate cancer cells (10). As a result, PSMA is thought to be a good candidate for PCa PET scanning. There are several radiopharmaceuticals that target PSMA, including 68Ga-PSMA-11, 18F-DCFPyL, and 18F-PSMA-1007 (11, 12).
Gallium-68 (68Ga)-labeled prostate-specific membrane antigen (PSMA-11), a new PET radiopharmaceutical, has recently gained attention as a promising imaging tool for the identification of recurrent prostate cancer. In patients with rising PSA levels, 68Ga-PSMA-11 PET has demonstrated great sensitivity and specificity for the detection of recurrent prostate cancer. However, uncertainty persists over the ideal imaging mode for 68Ga-PSMA-11 PET.
Despite systematic reviews or meta-analyses have evaluated the diagnostic efficacy of 68Ga-PSMA-11PET/CT and PET/MRI in earlier study, the amount of included article is insufficient (10, 13). This meta-analysis will enable more detailed and objective comparison of the diagnostic performance of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11PET/MRI in detecting biochemical recurrent prostate. Our aim was to conduct a meta-analysis and systematic review in order to compare the diagnostic efficacy of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI in patients with biochemically recurrent prostate cancer in patient-based analysis.
2 Manuscript formatting
2.1 Materials and methods
This article was written according to Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy (PRISMA-DTA) guidelines. Moreover, our registration number is CRD42023410039.
2.1.1 Search strategy
The search strategy described below was used to perform a thorough search of the PubMed, Embase, and Web of Science databases until February 2023. (1) PET MRI OR PET MR OR positron emission tomography/magnetic resonance imaging OR PET CT OR positron emission tomography OR positron emission tomography/computed tomography; (2) regeneration OR recurrent OR relapse OR recrudescence; (3) prostate cancers OR prostate neoplasm OR prostate tumor OR prostatic tumor. For the reference list, we also go through the search and consider articles that may meet the inclusion criteria.
2.1.2 Inclusion and exclusion criteria
Only study that fulfilled all of the following requirements were included: (1) articles evaluating the diagnostic performance of 68Ga-PSMA-11 PET/CT or 68Ga-PSMA-11 PET/MRI for biochemically recurrent prostate cancer in patient-based analysis; (2) number of patients ≥ 10; (3) retrospective or prospective studies; (4) English articles. The exclusion criteria were: (1) Irrelevant topic; (2) duplicated articles; (3) case reports, abstract, letters, review, or meta-analysis; (4) The full-text versions of the selected articles were screen to see if they fulfilled the inclusion criteria after the titles and abstracts of the articles were assessed in accordance with the inclusion and exclusion criteria. Disagreements among the researchers were resolved by consensus.
2.2 Quality assessment and data extraction
Two researchers separately evaluated the included studies’ quality using the Quality Assessment of Diagnostic Performance Studies (QUADAS-2) method. The applicability and bias risk of each study was assessed. Regarding bias risk and applicability, each study was given a rating of high, low, or unclear. A third reviewer was involved to resolve any possible conflicts. For the study, RevMan (version 5.4) was employed.
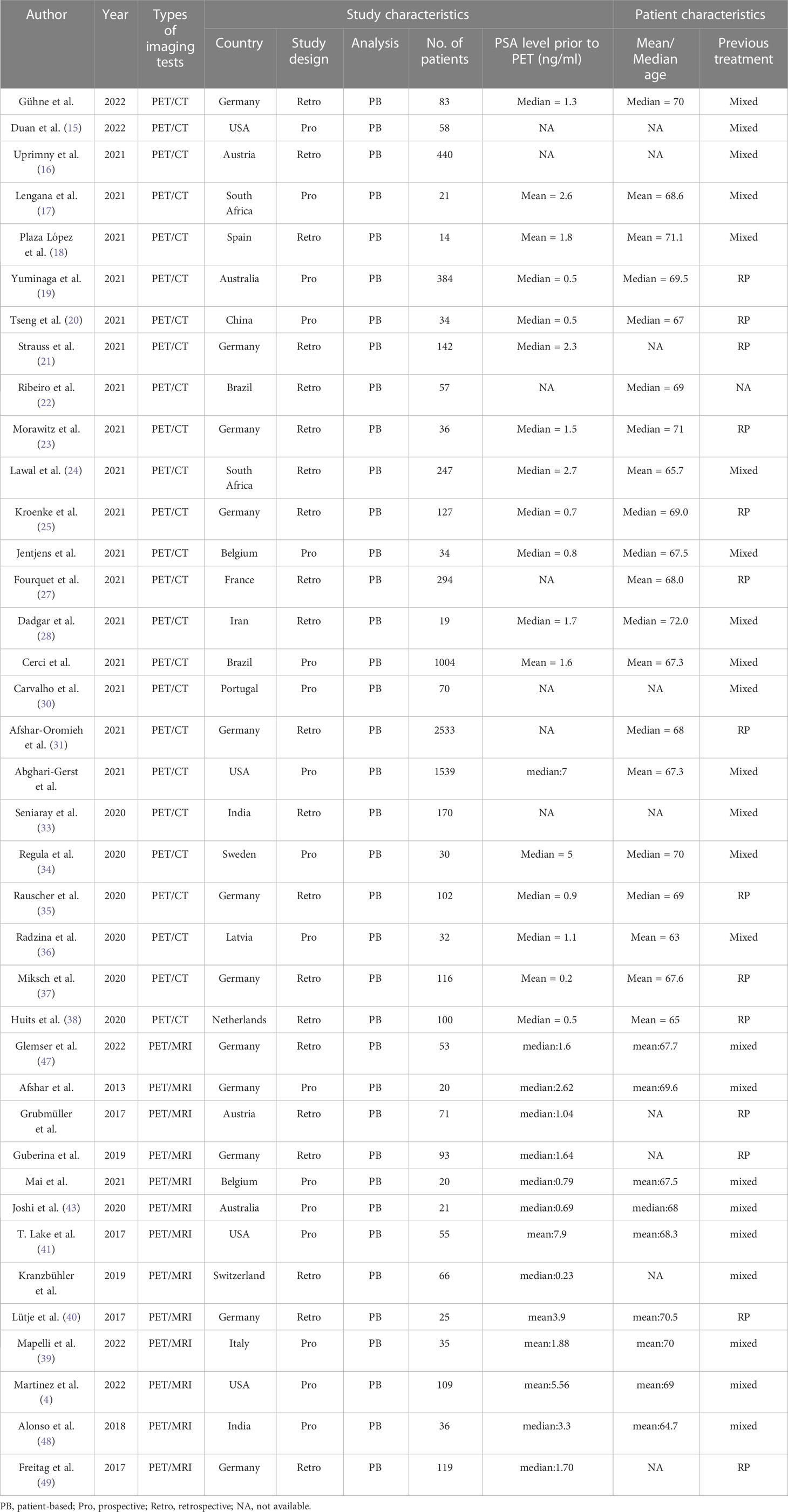
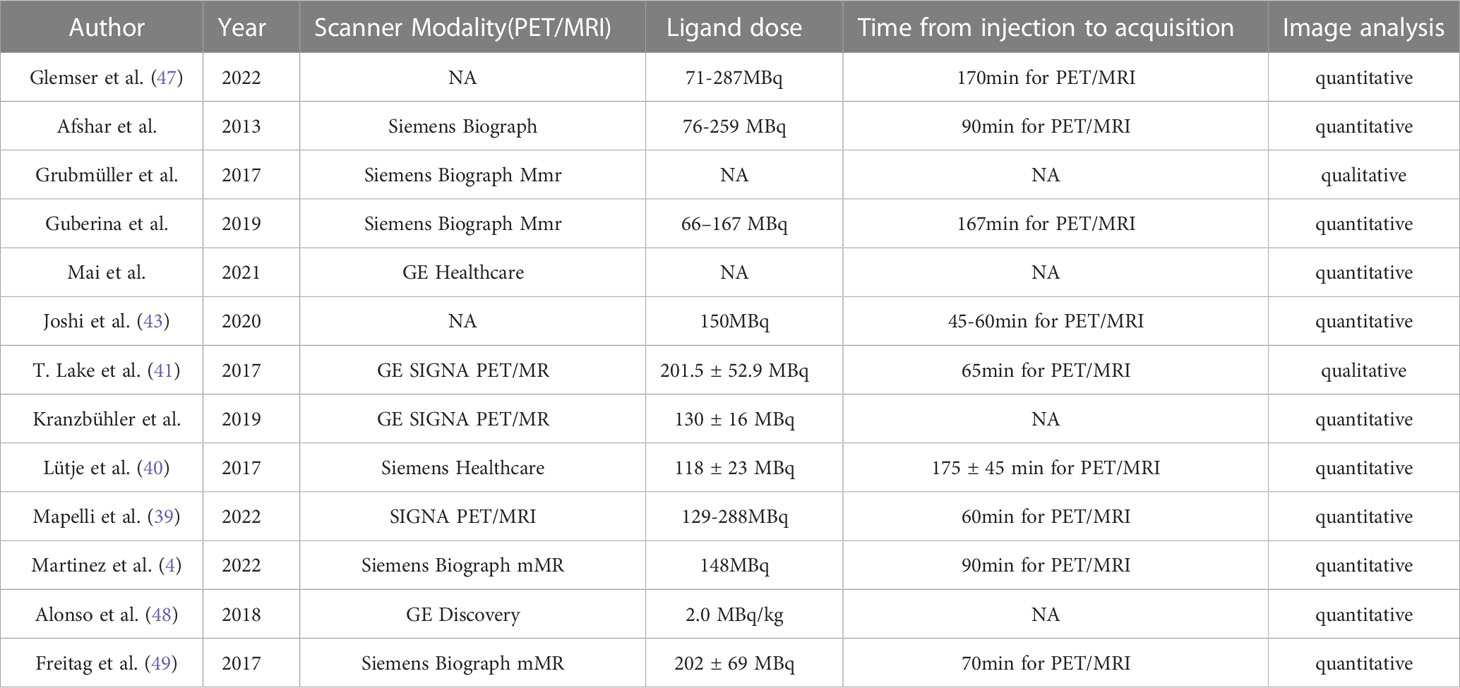
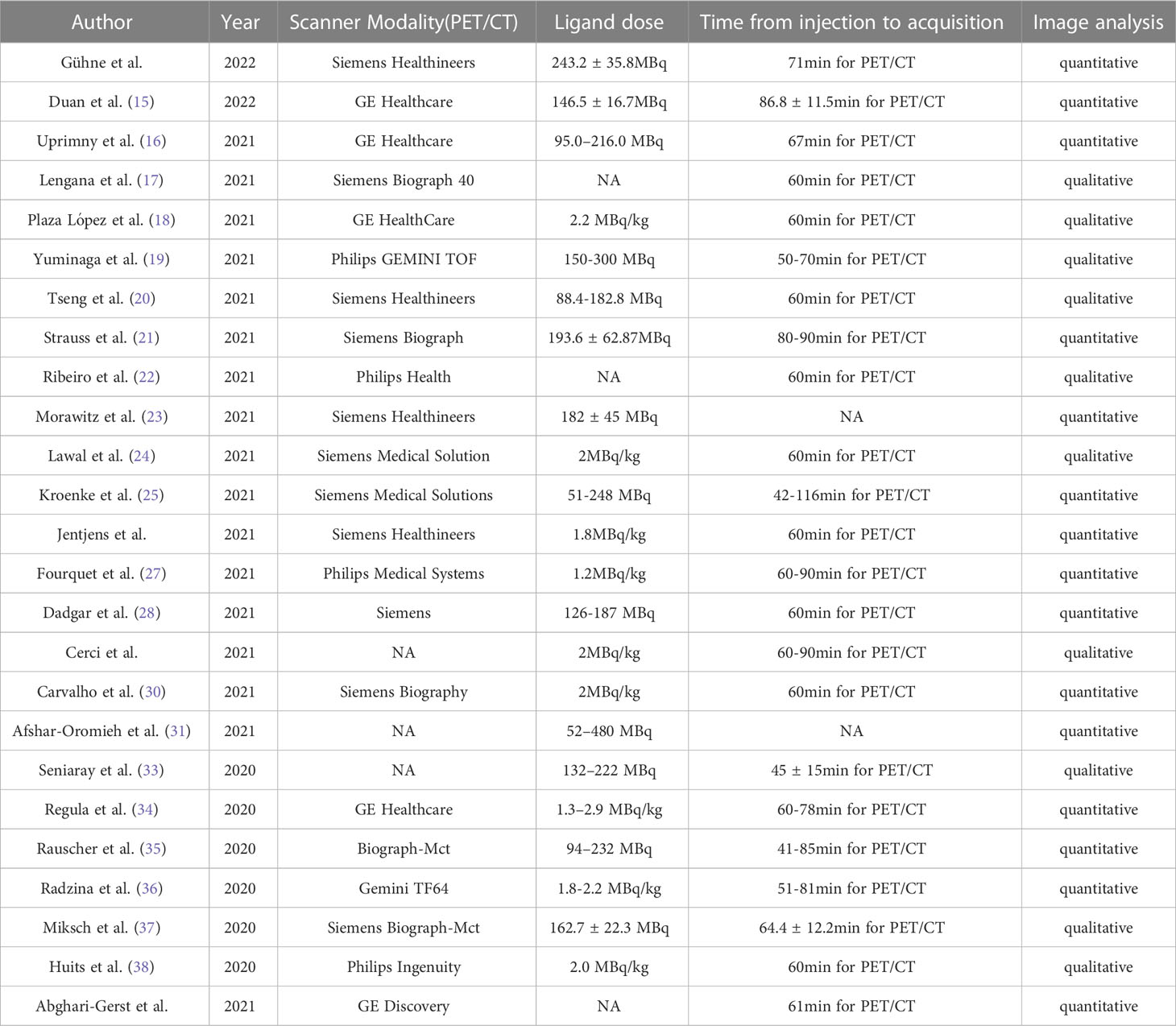
Two researchers independently extracted data for each of the included articles. The information that was extracted included the following: (1) the author, year of publication; (2) study characteristics, such as country, study design, analysis, and reference standard; (3) patient characteristics, such as number of patients, clinical indication, mean/median age, chemotherapy before PET; (4) technical characteristics, such as imaging test types, scanner modality, ligand dose, and time from injection to acquisition. When not explicitly mentioned, data were manually extracted from the literature, tables, and figures. We emailed the respective authors for more information when the paper lacked the information. Two researchers reached an accord to resolve their disagreements.
2.2.1 Data synthesis and statistical analysis
Heterogeneity was assessed using the I2 statistic. A forest plot was constructed in the random-effect model if the significant heterogeneity was observed (I2 > 50%), otherwise, the fixed model would be applied. All of them used DerSimonian and Laird method. Proportions were transformed with the Freeman-Tukey double inverse sine transformation, and confidence intervals were calculated using the Jackson method. For the presence of heterogeneity (I2>50%), we used meta-regression and sensitivity analysis to find out the source of heterogeneity.
Publication bias was evaluated using Egger’s test. A statistically significant P value was two-tailed and with the threshold of 0.05. Statistical analyses were performed in R software environment for statistical computing and graphics version 4.2.2
2.3 Results
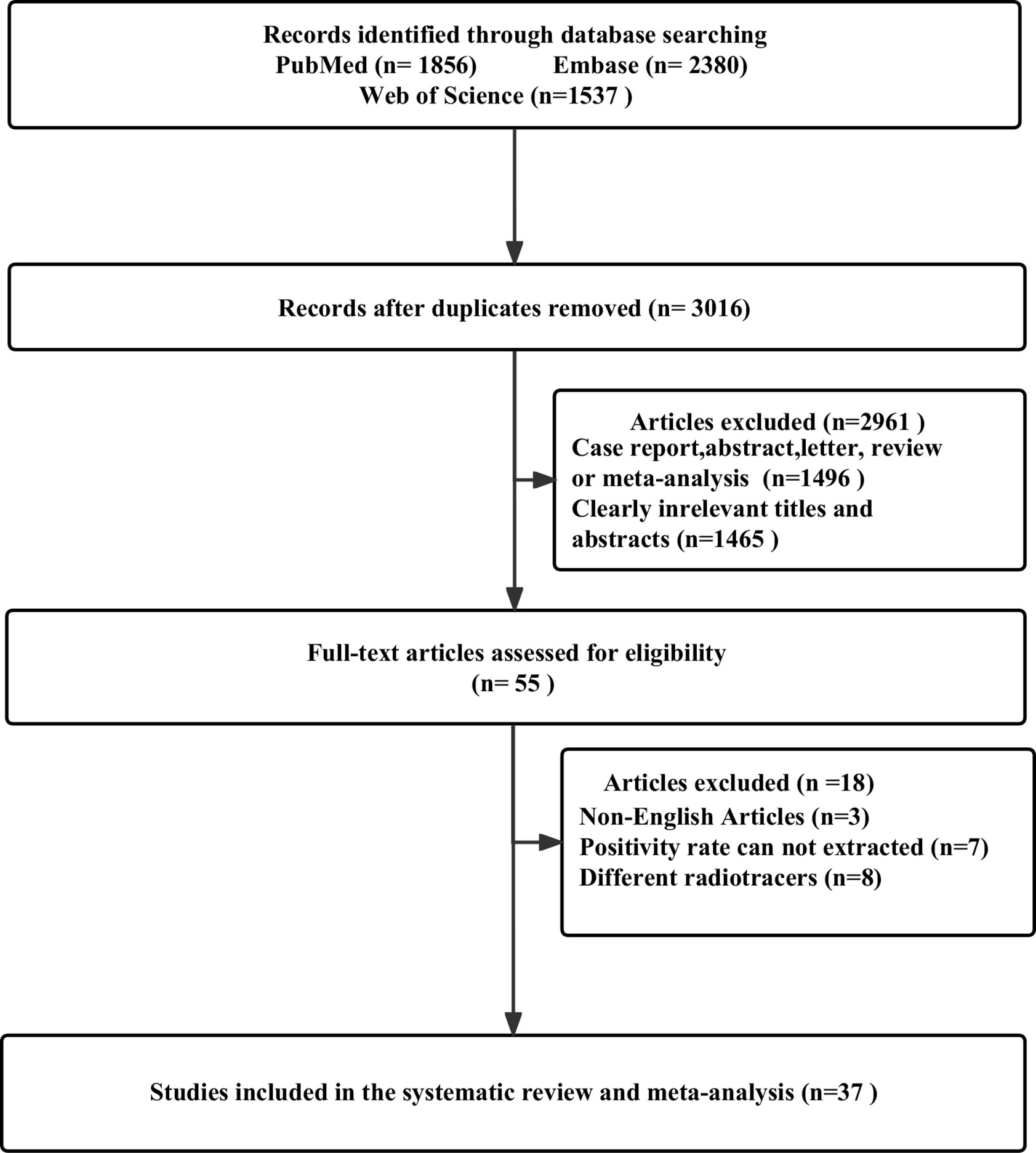
2.3.1 Literature search and study selection
After removing 2757 duplicate studies from the primary search, 3016 studies were identified out of the 5773 articles that were initially found. Based on the study’s title or abstract, 1465 papers were disqualified. A total of 1496 investigations were collectively omitted from case reports, abstracts, letters, reviews, or meta-analyses. There were still 55 studies for full-text screening, and another 18 were disqualified due to the following reasons: non-English studies (n = 3); cannot extract positivity rate data (n = 7); and different radiotracers (n = 8). 37 studies that satisfied the criteria for the meta-analysis were finally included, including 25 articles for 68Ga-PSMA-11 PET/CT (14–38) and 13 articles for 68Ga-PSMA-11 PET/MRI (4, 26, 39–49). One of the studies included not only 68Ga-PSMA-11 PET/CT but also 68Ga-PSMA-11 PET/MRI. Figure 1 illustrates the PRISMA flow chart of the study selection procedure.
2.3.2 Study description and quality assessment study description and quality assessment
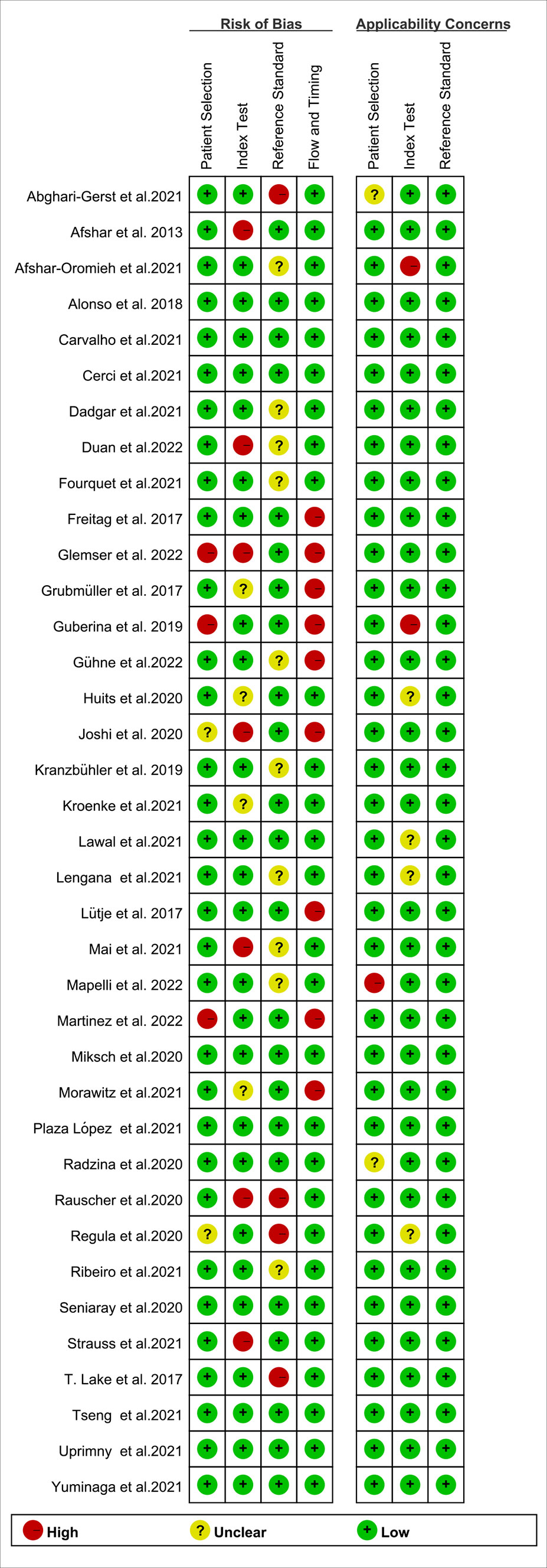
The study and patient characteristics from the 37 studies covering 8409 patients were listed in Table 1. Tables 2, 3 showed the technical the parts. Additionally, using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool, a quality assessment of the relevant studies was conducted. The quality evaluation chart reveals that flow and timing are the key areas where there is a high risk of bias (Figure 2). This is due to the fact that most studies did not analyze all of the enrolled patients, which caused this issue. Overall, the risk of bias of the included articles was satisfactory.
2.3.3 Diagnostic performance of 68Ga-PSMA-11 PET/CT and PET/MRI for biochemically recurrent prostate cancer
In comparison to 68Ga-PSMA-11 PET/CT, which had a positivity rate of 0.70 (95% Cl: 0.65-0.75), 68Ga-PSMA-11 PET/MRI had a positivity rate of 0.71 (95% Cl: 0.67-0.75). The analysis included 8409 patients from 37 studies. There was no statistically significant difference in the overall detection rate between 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI (P=0.58) (Figure 3).
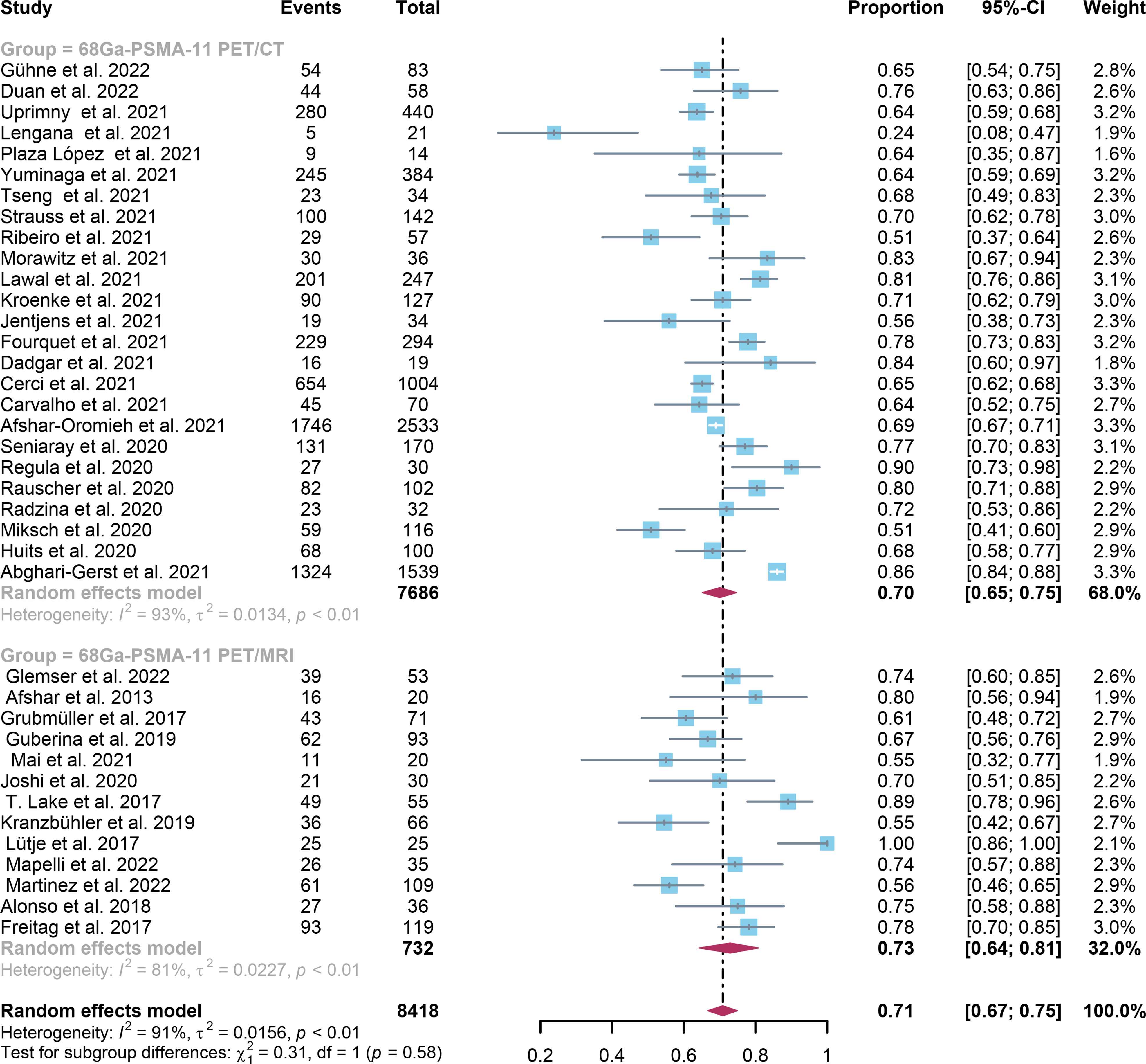
Figure 3 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI forest plots for biochemically recurrent prostate cancer. In each study, positive results were represented by squares, and the 95% confidence interval was shown by horizontal bars.
Regarding the pooled overall detection rate of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI for BCR, the I2 was 93% and 81%, respectively. For 68Ga-PSMA-11 PET/CT, the subgroup analysis and meta-regression analysis showed that the data analysis (qualitative vs. quantitative) was the possible cause of heterogeneity, while the study design (The number of patients: Greater than 56 vs. less than or equal to 56) was identified as the potential cause of heterogeneity for the 68Ga-PSMA-11 PET/MRI studies Tables 4, 5. There were no potential sources of heterogeneity found by the sensitivity analysis. The result revealed only slight variations in the data, with values ranging from 0.70 to 0.74 for the 68Ga-PSMA-11 PET/MRI and from 0.69 to 0.71 for the 68Ga-PSMA-11 PET/CT. Overall, the detection rates remained consistent and stable after sensitivity analysis. (Supplementary Tables 1, 2).
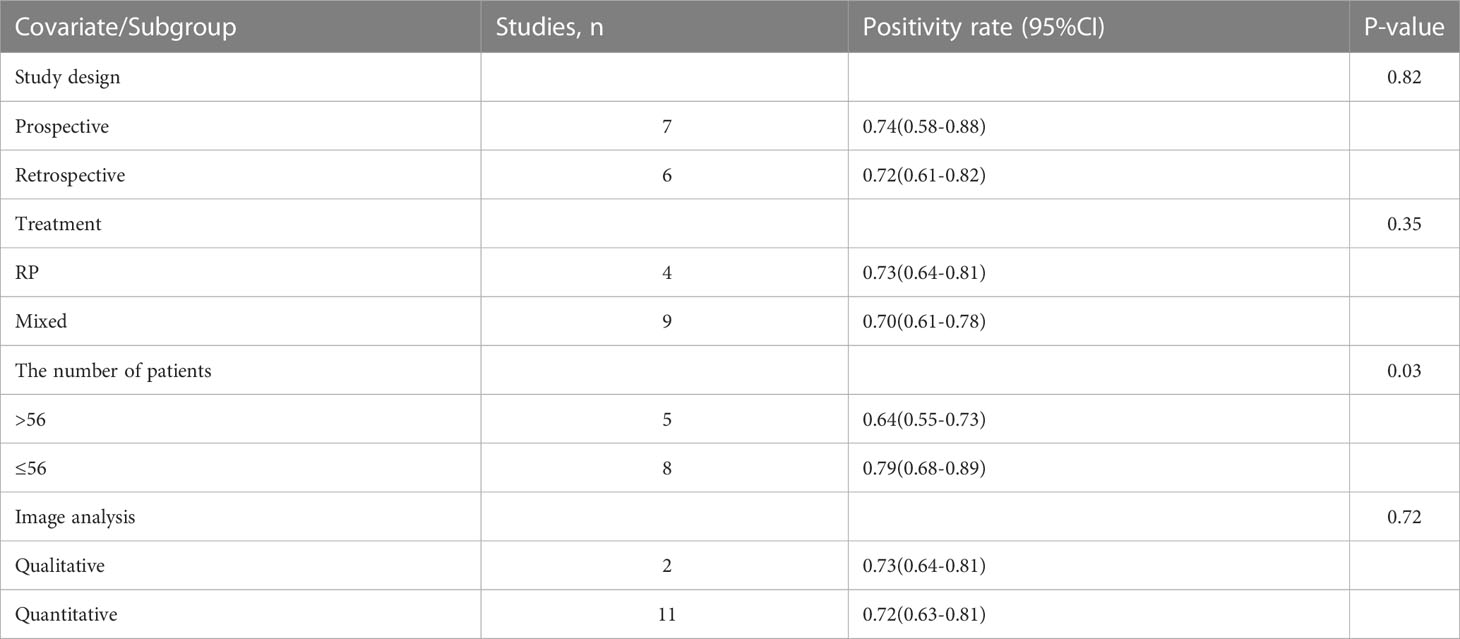
Table 4 Subgroup analysis and meta-regression analysis of diagnostic performance of 68Ga-PSMA-11 PET/MRI.
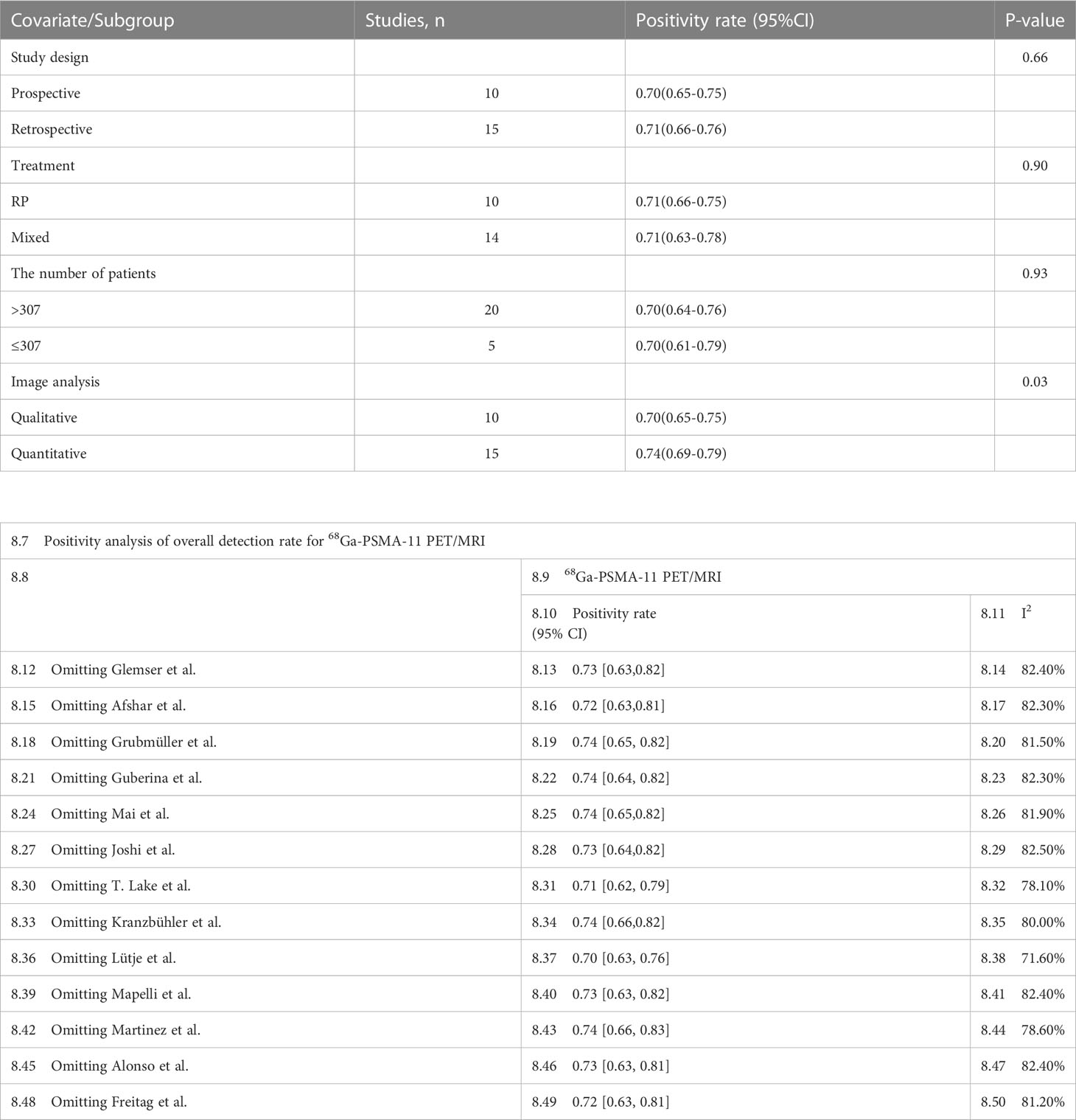
Table 5 Subgroup analysis and meta-regression analysis of diagnostic performance of 68Ga-PSMA-11 PET/CT.
According to the funnel plot and Egger’s test, both the 68Ga-PSMA-11 PET/CT (P = 0.39) and the 68Ga-PSMA-11 PET/MRI (P = 0.28) showed no sign of publication bias (Supplementary Tables 1 and 2).
2.3.4 BCR positivity rate for 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI according to the different PSA subgroups
For 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI, the detection rates were 0.47 (95% CI: 0.42-0.51) and 0.45 (95% CI: 0.24-0.67) for PSA levels <0.5 ng/ml, and when PSA levels were > 0.5 ng/ml, the detection rates were 0.77 (95% CI: 0.72-0.82) and 0.90 (95% CI: 0.79-0.98); Detection rates at PSA levels <0.2 ng/ml were 0.42 (95% CI: 0.36-0.47) and 0.13 (95% CI: 0.00-0.51); For PSA levels 0.2-0.5 ng/ml, the detection rates were 0.51 (95% CI: 0.41-0.62) and 0.46 (95% CI: 0.23-0.69); For PSA levels 0.5-1.0 ng/mL, detection rates were 0.63 (95% CI: 0.55-0.71) and 0.73 (95% CI: 0.45-0.95); For PSA values 1.0-2.0 ng/mL, the detection rates were 0.76 (95% CI: 0.69-0.82) and 0.63 (95% CI: 0.30-0.92); the detection rates for PSA levels > 2.0 ng/ml were 0.90 (95% CI: 0.85-0.93) and 0.89 (95% CI: 0.77-0.98). The only significant difference 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI was at PSA levels > 0.5 ng/ml (P=0.04) (Figures 4–10).
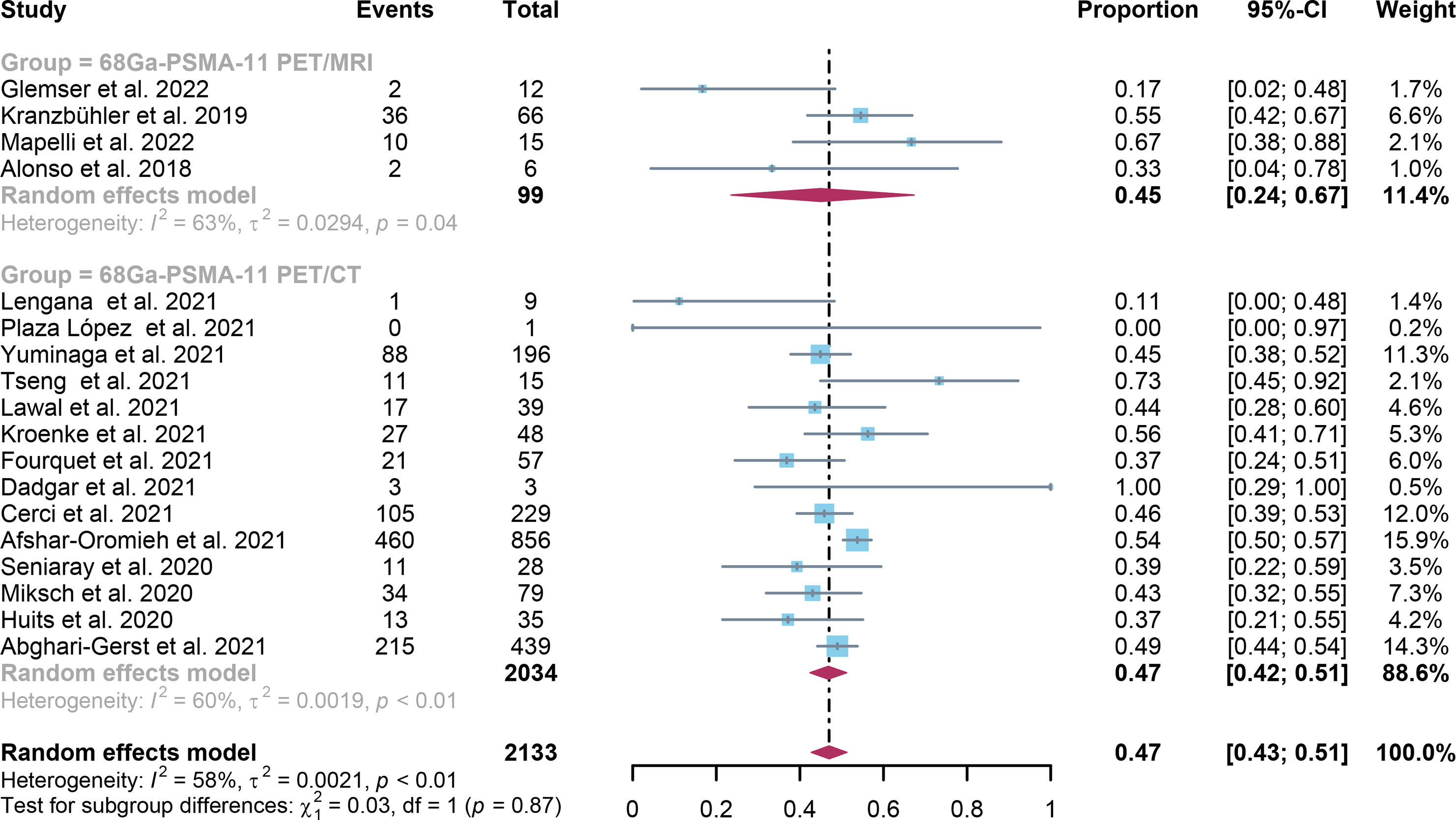
Figure 4 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with PSA<0.5.
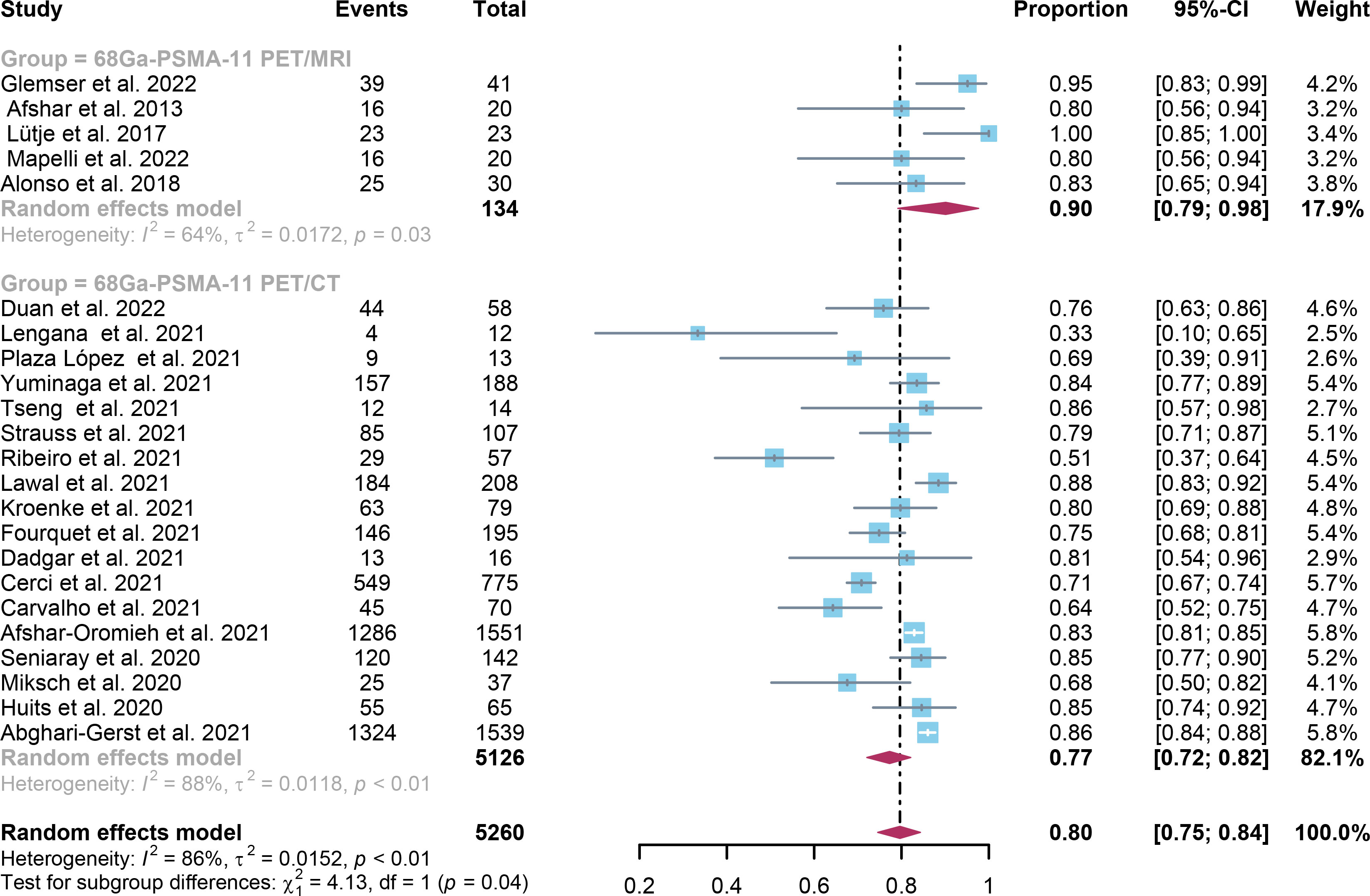
Figure 5 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with PSA>0.5.
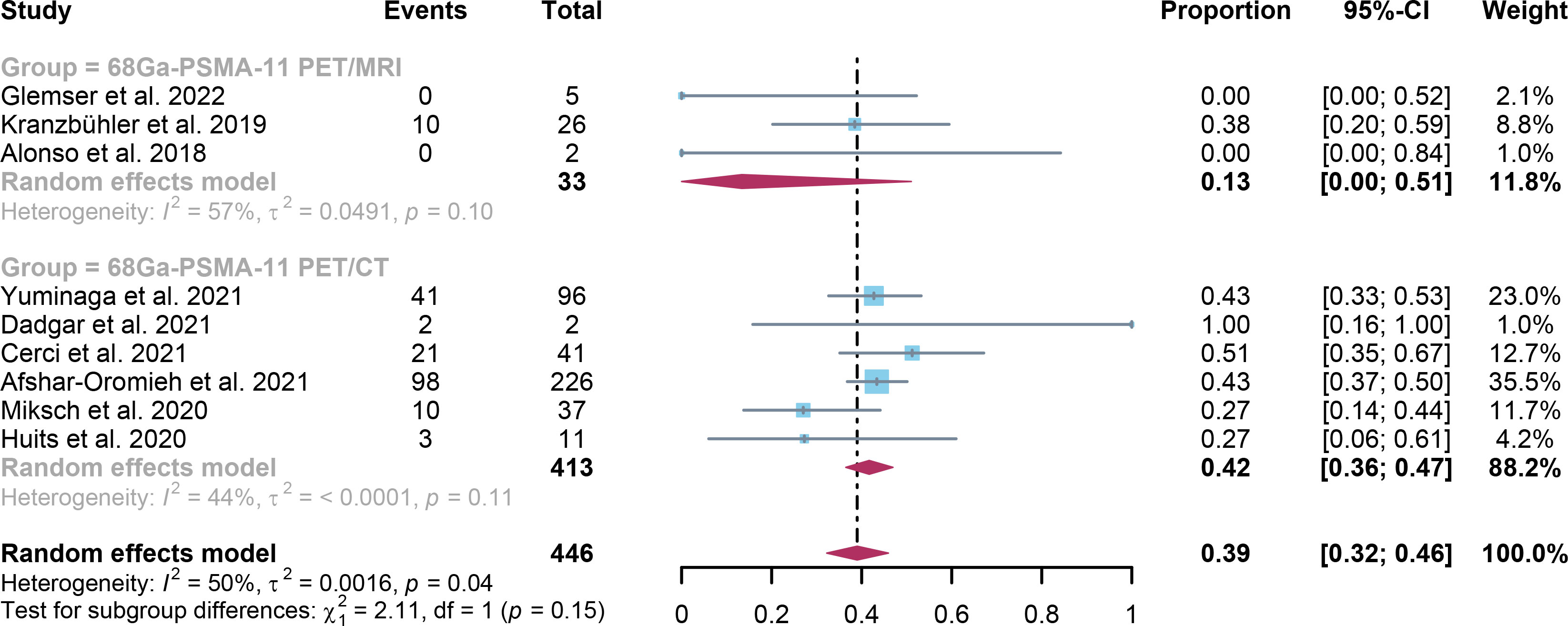
Figure 6 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with PSA≤ 0.2.
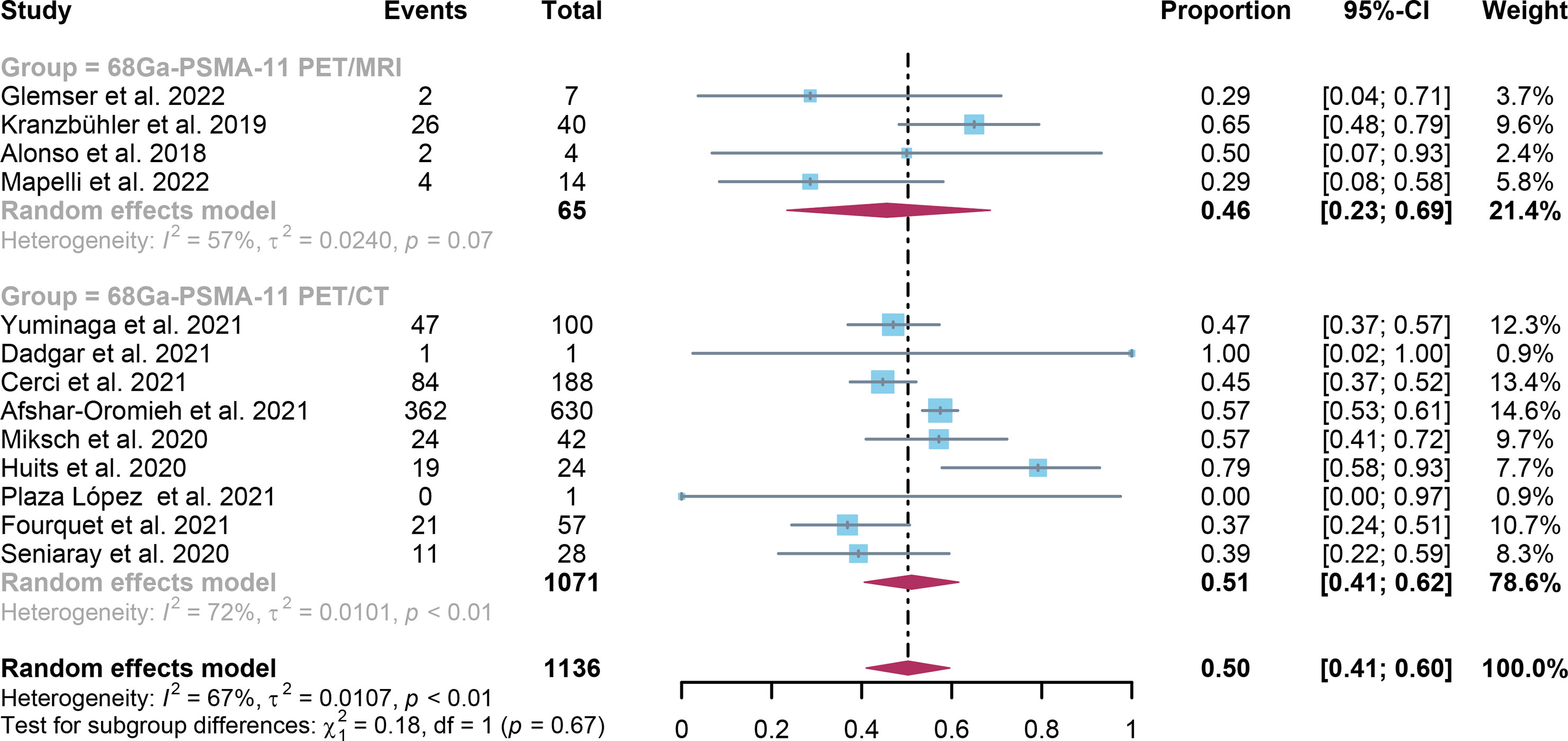
Figure 7 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with 0.2<PSA<0.5.
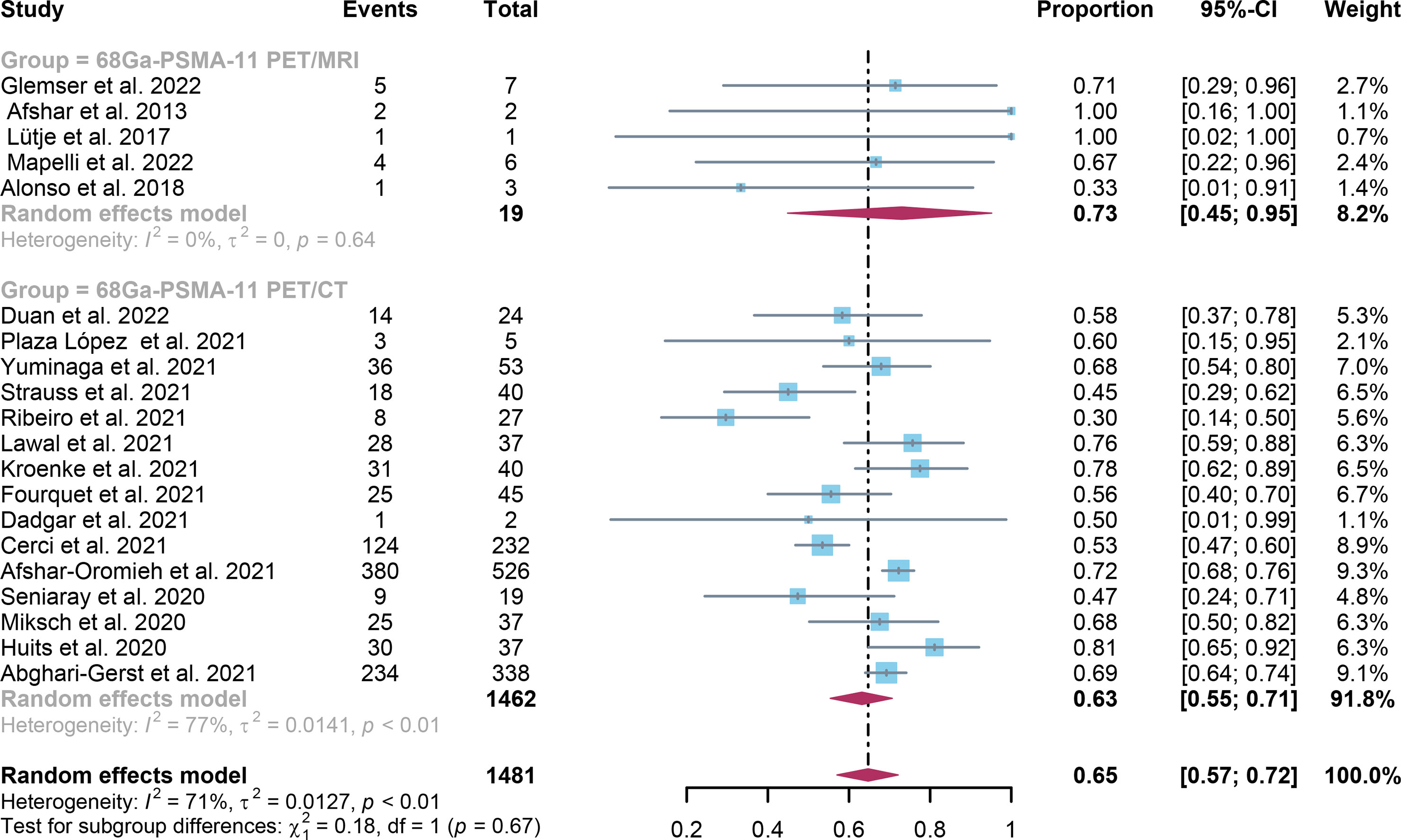
Figure 8 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with 0.5≤PSA<1.0.
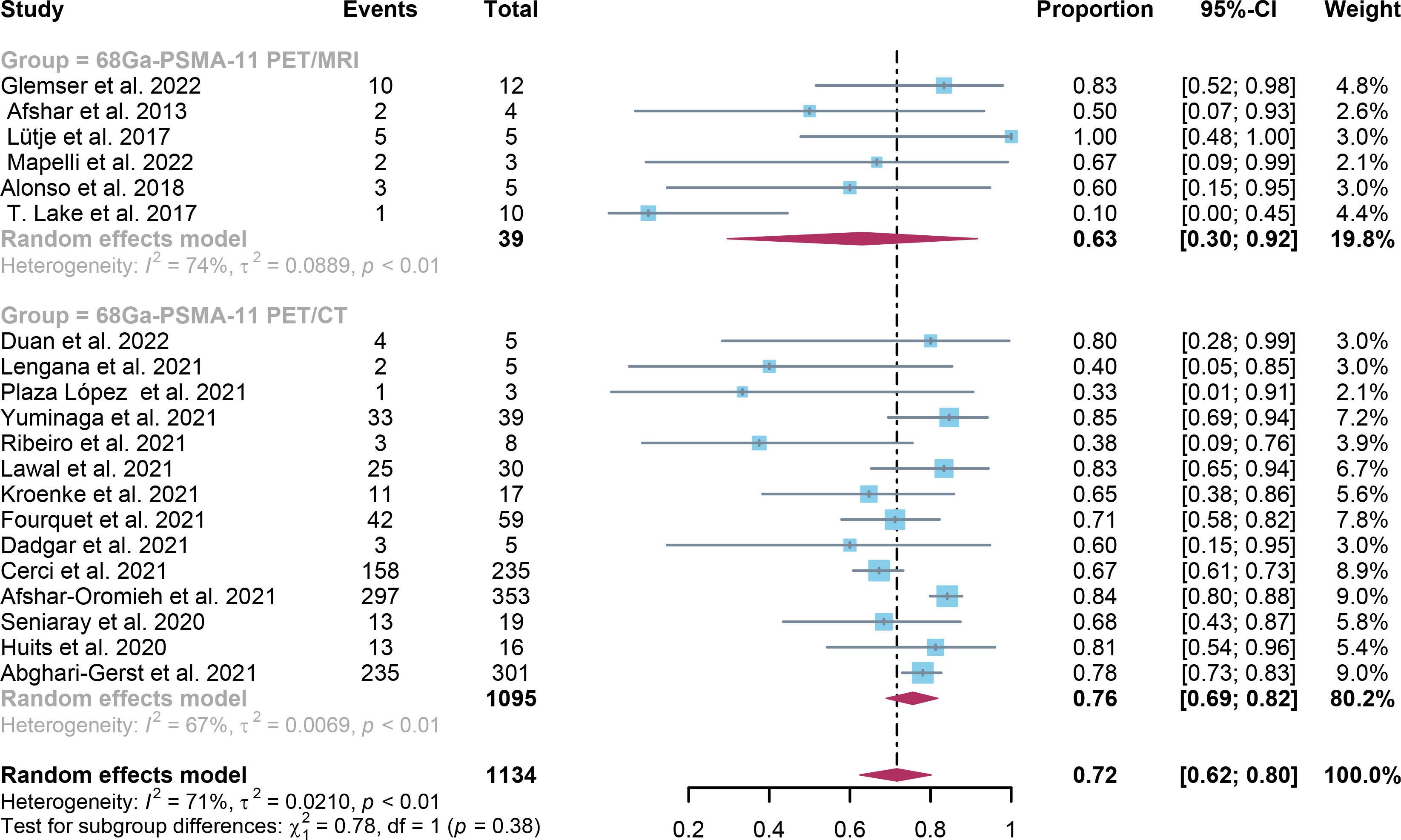
Figure 9 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with 1.0≤PSA<2.0.
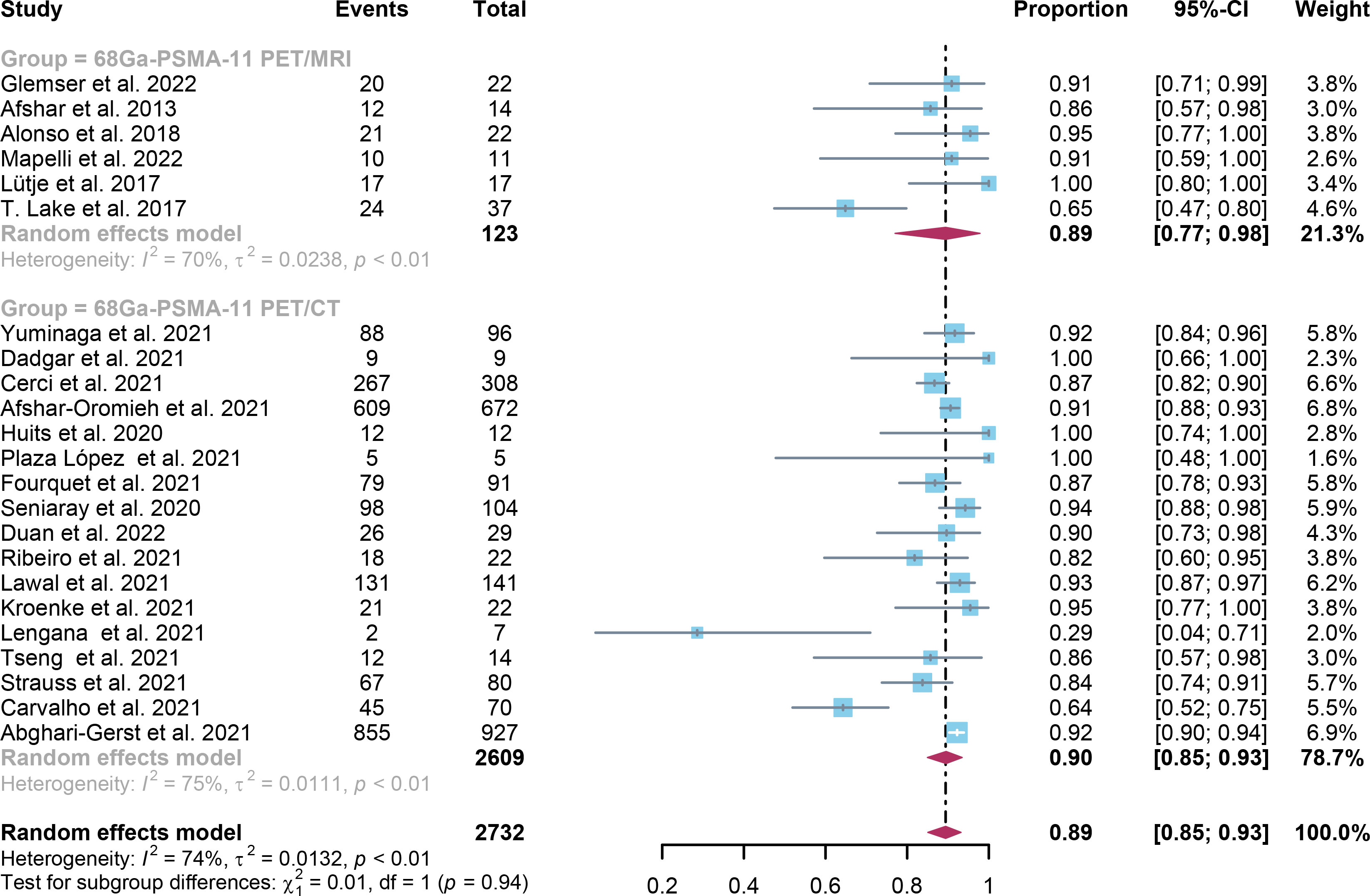
Figure 10 Forest plot of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI detection rates in patients with PSA≥2.0.
2.4 Discussion
According to previous studies, PET/MRI with PSMA imaging agent has a slightly higher diagnostic performance than PET/CT for local recurrence and lymph node recurrence (10, 44, 46, 49). However, according to Huo et al. and Glemser et al., there is no significant difference between the overall detection rates of the two imaging modalities (13, 47). Thus controversy remains regarding the diagnostic performance of both imaging modalities for biochemical recurrent prostate cancer. The aim of this study was to quantitatively compare the diagnostic performance of the two diagnostic modalities for biochemical recurrent prostate cancer.
In the present study, the capability of two imaging modalities to identify BCR was comprehensively reviewed and assessed. The detection rates in patient-based analysis for 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI were 0.70 (95% Cl: 0.65; 0.75) and 0.73 (95% Cl: 0.64; 0.81) accordingly. Between these two imaging modalities, there was no significant difference (P=0.58). A significant difference between these two imaging modalities for PSA levels aspect only existed when PSA was higher than 0.5, according to the study (P=0.04) (Figure 7).
PET/MRI provides metabolic, anatomical, and functional information in a single modality by combining the strengths of PET and MRI. While MRI offers precise anatomical and functional information through techniques like perfusion and diffusion-weighted imaging, PSMA-11 PET provides metabolic information by detecting PSMA expression in prostate cancer cells. With the use of this extensive information, clinicians can make a more thorough evaluation of the kind and severity of prostate cancer. PET/MRI detection rates may be superior to PET/CT for PSA levels higher than 0.5 due to the fact that PET/MRI provides more precise and detailed anatomical data, particularly in terms of soft tissue contrast (48). In order to more precisely localize probable tumor lesions, PSMA-11 PET/MRI can provide detailed anatomical information on the prostate region, including its shape, location, and size (36).
In addition, compared to PET/CT, PET/MRI often has lower radiation doses, which may be advantageous for younger patients or those who need numerous follow-up exams, lowering the risk of radiation exposure. It’s important to remember that clinical practices may vary between different medical facilities, even though PSMA-11 PET/MRI may be advantageous when PSA is greater than 0.5. It is important to stress that this is only an observation or trend and does not always mean that PSMA-11 PET/MRI is always preferable than PSMA-11 PET/CT.
Compared to previous meta-analyses (13), the current meta-study found that 68Ga-PSMA-11 PET CT and 68Ga-PSM A-11 PET-MRI had similar results in terms of diagnostic performance and detection rates for the detection of biochemically recurrent prostate cancer. This shows that for the same detection performance, PET/CT is more cost-effective. These findings are consistent with previous meta-analyses. The main disadvantage of the previous meta-analysis is the small sample size, while the main advantage of the meta-analysis in this article is the large sample size(including 37 studies). However, due to the recent development of 68Ga-PSMA-11 PET/MRI, there is limited study in this field and a scarcity of comparable evidence available. Future head-to-head studies that systematically assesses both modalities might produce novel findings.
The findings of the meta-analysis contrasting 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI for the identification of biochemically recurring prostate cancer can have significant repercussions for future study in the field as well as for policy and practice. To make the best use of various imaging modalities in clinical practice, these findings can inspire future study paths, help decision-making, and enhance patient management. The diagnosis of PSMA-PET has a significant impact on the management of recurrent patients, allowing clinicians to select better treatment options to treat them, such as the treatment of recurrent M1a prostate cancer, the MDT approach of targeting PSMA-positive lesions according to the pattern of recurrence (sLND, SBRT, combination of sLND and SBRT). Based on the PSMA-PET method, these treatments were chosen (50–53).
Both 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI demonstrated high heterogeneity in terms of overall detection rates. In an attempt to find out the source of this heterogeneity, we conducted sensitivity analysis and meta-regression. Our findings showed that for PET/CT, the primary cause of heterogeneity was image analysis (P=0.03). On the other hand, for PET/MRI, the primary cause of heterogeneity appeared to be the number of patients involved in the studies (P=0.03). The sensitivity analysis did not identify any potential sources of heterogeneity.
It is also important to note the limitations of our meta-analysis. First of all, the gold standard for pathology was not available for all of the patients. Secondly, many of the included studies were retrospective studies, further lager prospective studies are needed. Finally, the included study used various protocols, such as different methods for administering contrast agents, different contrast procedures, and varied standards for interpretation, which may cause heterogeneity.
2.5 Conclusion
The diagnostic efficacy of 68Ga-PSMA-11 PET/CT appears to be equivalent to that of 68Ga-PSMA-11 PET/MRI in detecting biochemically recurrent prostate cancer. Nonetheless, it should be noted that not all studies have used pathological biopsies as the gold standard. Therefore, additional larger head-to-head prospective studies are needed to address this issue.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
The study was conceptualized and designed by RH and ZZ, and it was verified using information gathered and examined by YL, HW, BL, and XZ. The manuscript was written by RH. The article’s submission was reviewed and approved by all of the writers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1216894/full#supplementary-material
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Cary KC, Punnen S, Odisho AY, Litwin MS, Saigal CS, Cooperberg MR. Nationally representative trends and geographic variation in treatment of localized prostate cancer: the urologic diseases in America project. Prostate Cancer Prostatic Dis (2015) 18(2):149–54. doi: 10.1038/pcan.2015.3
3. Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am (2001) 28(3):555–65. doi: 10.1016/s0094-0143(05)70163-4
4. Martinez J, Subramanian K, Margolis D, O'Dwyer E, Osborne J, Jhanwar Y, et al. 68ga-Psma-Hbed-Cc Pet/Mri is superior to multiparametric magnetic resonance imaging in men with biochemical recurrent prostate cancer: a prospective single-institutional study. Transl Oncol (2022) 15(1):101242. doi: 10.1016/j.tranon.2021.101242
5. Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. Eau-Estro-Siog guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol (2017) 71(4):630–42. doi: 10.1016/j.eururo.2016.08.002
6. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the Oriole phase 2 randomized clinical trial. JAMA Oncol (2020) 6(5):650–9. doi: 10.1001/jamaoncol.2020.0147
7. Ceci F, Rovera G, Iorio GC, Guarneri A, Chiofalo V, Passera R, et al. Event-Free Survival after (68) ga-Psma-11 Pet/Ct in Recurrent Hormone-Sensitive Prostate Cancer (Hspc) Patients Eligible for Salvage Therapy. Eur J Nucl Med Mol Imaging (2022) 49(9):3257–68. doi: 10.1007/s00259-022-05741-9
8. Fendler WP, Eiber M, Beheshti M, BOmanji J, Calais J, Ceci F, et al. Psma Pet/Ct: joint Eanm procedure guideline/Snmmi procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging (2023) 50(5):1466–86. doi: 10.1007/s00259-022-06089-w
9. Rovera G, Grimaldi S, Dall'Armellina S, Passera R, Oderda M, Iorio GC, et al. Predictors of bone metastases at (68)Ga-Psma-11 Pet/Ct in hormone-sensitive prostate cancer (Hspc) patients with early biochemical recurrence or persistence. Diagnostics (Basel) (2022) 12(6):1309. doi: 10.3390/diagnostics12061309
10. Liu FY, Sheng TW, Tseng JR, Yu KJ, Tsui KH, Pang ST, et al. Prostate-specific membrane antigen (Psma) fusion imaging in prostate cancer: Pet-Ct vs Pet-Mri. Br J Radiol (2022) 95(1131):20210728. doi: 10.1259/bjr.20210728
11. Sun J, Lin Y, Wei X, Ouyang J, Huang Y, Ling Z. Performance of 18f-Dcfpyl Pet/Ct imaging in early detection of biochemically recurrent prostate cancer: a systematic review and meta-analysis. Front Oncol (2021) 11:649171. doi: 10.3389/fonc.2021.649171
12. Kesch C, Vinsensia M, Radtke JP, Schlemmer HP, Heller M, Ellert E, et al. Intraindividual comparison of (18)F-Psma-1007 Pet/Ct, multiparametric Mri, and radical prostatectomy specimens in patients with primary prostate cancer: a retrospective, proof-of-concept study. J Nucl Med (2017) 58(11):1805–10. doi: 10.2967/jnumed.116.189233
13. Huo H, Shen S, He D, Liu B, Yang F. Head-to-head comparison of (68)Ga-Psma-11 Pet/Ct and (68)Ga-Psma-11 Pet/Mri in the detection of biochemical recurrence of prostate cancer: summary of head-to-head comparison studies. Prostate Cancer Prostatic Dis (2023) 26(1):16–24. doi: 10.1038/s41391-022-00581-y
14. Gühne F, Radke S, Winkens T, Kühnel C, Greiser J, Seifert P, et al. Differences in distribution and detection rate of the [(68)Ga]Ga-Psma ligands Psma-617, -I&T and -11-Inter-Individual comparison in patients with biochemical relapse of prostate cancer. Pharm (Basel) (2021) 15(1):9. doi: 10.3390/ph15010009
15. Duan H, Baratto L, Hatami N, Liang T, Mari Aparici C, Davidzon GA, et al. (68)Ga-Psma11 Pet/Ct for biochemically recurrent prostate cancer: influence of dual-time and Pmt- vs Sipm-based detectors. Transl Oncol (2022) 15(1):101293. doi: 10.1016/j.tranon.2021.101293
16. Uprimny C, Bayerschmidt S, Kroiss AS, Fritz J, Nilica B, Svirydenka H, et al. Early injection of furosemide increases detection rate of local recurrence in prostate cancer patients with biochemical recurrence referred for (68)Ga-Psma-11 Pet/Ct. J Nucl Med (2021) 62(11):1550–7. doi: 10.2967/jnumed.120.261866
17. Lengana T, Lawal IO, Rensburg CV, Mokoala KMG, Moshokoa E, Ridgard T, et al. A comparison of the diagnostic performance of (18)F-Psma-1007 and (68)Ga-Psma-11 in the same patients presenting with early biochemical recurrence. Hell J Nucl Med (2021) 24(3):178–185. doi: 10.1967/s002449912401
18. Plaza López PJ, Puertas E, Aguiló JJ, Suarez-Piñera M, Domenech B, Mestre-Fusco A, et al. (68)Ga-Psma-11 Pet/Ct in patients with occult biochemical recurrence of prostate carcinoma and negative (18)F-CholinePet/Ct. Prelim Assess Its Clin Use Actas Urol Esp (Engl Ed) (2021) 45(5):353–8. doi: 10.1016/j.acuroe.2021.04.008
19. Yuminaga Y, Rothe C, Kam J, Beattie K, Arianayagam M, Bui C, et al. (68)Ga-Psma Pet/Ct versus Ct and bone scan for investigation of Psa failure post radical prostatectomy. Asian J Urol (2021) 8(2):170–5. doi: 10.1016/j.ajur.2020.02.001
20. Tseng JR, Yu KJ, Liu FY, Yang LY, Hong JH, Yen TC, et al. Comparison between (68)Ga-Psma-11 Pet/Ct and multiparametric magnetic resonance imaging in patients with biochemically recurrent prostate cancer following robot-assisted radical prostatectomy. J Formos Med Assoc (2021) 120:688–96. doi: 10.1016/j.jfma.2020.07.029
21. Strauss DS, Sachpekidis C, Kopka K, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A. Pharmacokinetic studies of [(68) ga]Ga-Psma-11 in patients with biochemical recurrence of prostate cancer: detection, differences in temporal distribution and kinetic modelling by tissue type. Eur J Nucl Med Mol Imaging (2021) 48(13):4472–82. doi: 10.1007/s00259-021-05420-1
22. Ribeiro AMB, Lima ENP, de Cássio Zequi S. Evaluation of the clinical use of Pet/Ct with 68ga-Psma for the assessment of biochemical recurrence of low or intermediate-risk prostate cancer. Urol Oncol: Semin Original Invest (2021) 39(1):73.e9–73.e18. doi: 10.1016/j.urolonc.2020.07.010
23. Morawitz J, Kirchner J, Lakes J, Bruckmann NM, Mamlins E, Hiester A, et al. Psma Pet/Ct vs. Ct alone in newly diagnosed biochemical recurrence of prostate cancer after radical prostatectomy: comparison of detection rates and therapeutic implications. Eur J Radiol (2021) 136:109556. doi: 10.1016/j.ejrad.2021.109556
24. Lawal IO, Lengana T, Popoola GO, Orunmuyi AT, Kgatle MM, Mokoala KMG, et al. Pattern of prostate cancer recurrence assessed by (68)Ga-Psma-11 Pet/Ct in men treated with primary local therapy. J Clin Med (2021) 10(17):3883. doi: 10.3390/jcm10173883
25. Kroenke M, Mirzoyan L, Horn T, Peeken JC, Wurzer A, Wester H-J, et al. Matched-pair comparison of 68ga-Psma-11 and 18f-Rhpsma-7 Pet/Ct in patients with primary and biochemical recurrence of prostate cancer: frequency of non–tumor-related uptake and tumor positivity. J Nucl Med (2021) 62(8):1082–8. doi: 10.2967/jnumed.120.251447
26. Jentjens S, Mai C, Ahmadi Bidakhvidi N, De Coster L, Mertens N, Koole M, et al. Prospective comparison of simultaneous [(68)Ga]Ga-Psma-11 Pet/Mr versus pet/Ct in patients with biochemically recurrent prostate cancer. Eur Radiol (2022) 32(2):901–11. doi: 10.1007/s00330-021-08140-0
27. Fourquet A, Lahmi L, Rusu T, Belkacemi Y, Créhange G, de la Taille A, et al. Restaging the biochemical recurrence of prostate cancer with [(68)Ga]Ga-Psma-11 Pet/Ct: diagnostic performance and impact on patient disease management. Cancers (Basel) (2021) 13(7):1594. doi: 10.3390/cancers13071594
28. Dadgar H, Seyedi Vafaee M, Norouzbeigi N, Jafari E, Gholamrezanezhad A, Assadi M. Dual-Phase 68ga-Psma-11 Pet/Ct may increase the rate of detected lesions in prostate cancer patients. Urologia (2021) 88(4):355–61. doi: 10.1177/0391560321993544
29. Cerci JJ, Fanti S, Lobato EE, Kunikowska J, Alonso O, Medina S, et al. Diagnostic performance and clinical impact of (68)Ga-Psma-11 Pet/Ct imaging in early relapsed prostate cancer after radical therapy: a prospective multicenter study (Iaea-Psma study). J Nucl Med (2022) 63(2):240–7. doi: 10.2967/jnumed.120.261886
30. Carvalho J, Nunes P, Da Silva ET, Silva R, Lima J, Quaresma V, et al. [68ga]Ga-Psma-11 Pet-Ct: local preliminary experience in prostate cancer biochemical recurrence patients. Arch Ital Urol Androl (2021) 93(1):21–5. doi: 10.4081/aiua.2021.1.21
31. Afshar-Oromieh A, da Cunha ML, Wagner J, Haberkorn U, Debus N, Weber W, et al. Performance of [(68)Ga]Ga-Psma-11 Pet/Ct in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur J Nucl Med Mol Imaging (2021) 48(9):2925–34. doi: 10.1007/s00259-021-05189-3
32. Abghari-Gerst M, Armstrong WR, Nguyen K, Calais J, Czernin J, Lin D, et al. A comprehensive assessment of (68)Ga-Psma-11 Pet in biochemically recurrent prostate cancer: results from a prospective multicenter study on 2,005 patients. J Nucl Med (2022) 63(4):567–72. doi: 10.2967/jnumed.121.262412
33. Seniaray N, Verma R, Khanna S, Belho E, Pruthi A, Mahajan H. Localization and restaging of carcinoma prostate by (68)Gallium prostate-specific membrane antigen positron emission tomography computed tomography in patients with biochemical recurrence. Indian J Urol (2020) 36(3):191–9. doi: 10.4103/iju.IJU_275_19
34. Regula N, Kostaras V, Johansson S, Trampal C, Lindström E, Lubberink M, et al. Comparison of (68)Ga-Psma-11 Pet/Ct with (11)C-Acetate Pet/Ct in re-staging of prostate cancer relapse. Sci Rep (2020) 10(1):4993. doi: 10.1038/s41598-020-61910-6
35. Rauscher I, Krönke M, König M, Gafita A, Maurer T, Horn T, et al. Matched-pair comparison of (68)Ga-Psma-11 Pet/Ct and (18)F-Psma-1007 Pet/Ct: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med (2020) 61(1):51–7. doi: 10.2967/jnumed.119.229187
36. Radzina M, TIrane M, Roznere L, Zemniece L, Dronka L, Kalnina M, et al. Accuracy of (68)Ga-Psma-11 Pet/Ct and multiparametric Mri for the detection of local tumor and lymph node metastases in early biochemical recurrence of prostate cancer. Am J Nucl Med Mol Imaging (2020) 10(2):106–18.
37. Miksch J, Bottke D, Krohn T, Thamm R, Bartkowiak D, Solbach C, et al. Interobserver variability, detection rate, and lesion patterns of (68)Ga-Psma-11-Pet/Ct in early-stage biochemical recurrence of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging (2020) 47(10):2339–47. doi: 10.1007/s00259-020-04718-w
38. Huits TH, Luiting HB, van der Poel HG, Nandurkar R, Donswijk M, Schaake E, et al. Distribution of prostate cancer recurrences on gallium-68 prostate-specific membrane antigen ((68) Ga-Psma) positron-emission/computed tomography after radical prostatectomy with pathological node-positive extended lymph node dissection. BJU Int (2020) 125(6):876–83. doi: 10.1111/bju.15052
39. Mapelli P, Ghezzo S, Samanes Gajate AM, Preza E, Palmisano A, Cucchiara V, et al. (68)Ga-Psma and (68)Ga-Dota-Rm2 Pet/Mri in recurrent prostate cancer: diagnostic performance and association with clinical and histopathological data. Cancers (Basel) (2022) 14(2):334. doi: 10.3390/cancers14020334
40. Lütje S, Cohnen J, Gomez B, Grüneisen J, Sawicki L, Rübben H, et al. Integrated (68)Ga-Hbed-Cc-Psma-Pet/Mri in patients with suspected recurrent prostate cancer. Nuklearmedizin (2017) 56(3):73–81. doi: 10.3413/Nukmed-0850-16-09
41. Lake ST, Greene KL, Westphalen AC, Behr SC, Zagoria R, Small EJ, et al. Optimal Mri sequences for (68)Ga-Psma-11 Pet/Mri in evaluation of biochemically recurrent prostate cancer. EJNMMI Res (2017) 7(1):77. doi: 10.1186/s13550-017-0327-7
42. Kranzbühler B, Müller J, Becker AS, Garcia Schüler HI, Muehlematter U, Fankhauser CD, et al. Detection rate and localization of prostate cancer recurrence using (68)Ga-Psma-11 Pet/Mri in patients with low Psa values ≤ 0.5 ng/ml. J Nucl Med (2020) 61(2):194–201. doi: 10.2967/jnumed.118.225276
43. Joshi A, Roberts MJ, Perera M, Williams E, Rhee H, Pryor D, et al. The clinical efficacy of Psma Pet/Mri in biochemically recurrent prostate cancer compared with standard of care imaging modalities and confirmatory histopathology: results of a single-centre, prospective clinical trial. Clin Exp Metastasis (2020) 37(4):551–60. doi: 10.1007/s10585-020-10043-1
44. Guberina N, Hetkamp P, Ruebben H, Fendler W, Grueneisen J, Suntharalingam S, et al. Whole-body integrated [(68)Ga]Psma-11-Pet/Mr imaging in patients with recurrent prostate cancer: comparison with whole-body Pet/Ct as the standard of reference. Mol Imaging Biol (2020) 22(3):788–96. doi: 10.1007/s11307-019-01424-4
45. Grubmüller B, Baltzer P, D'Andrea D, Korn S, Haug AR, Hacker M, et al. (68)Ga-Psma 11 ligand pet imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging (2018) 45(2):235–42. doi: 10.1007/s00259-017-3858-2
46. Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of Pet/Ct and Pet/Mri hybrid systems using a 68ga-labelled Psma ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging (2014) 41(5):887–97. doi: 10.1007/s00259-013-2660-z
47. Glemser PA, Rotkopf LT, Ziener CH, Beuthien-Baumann B, Weru V, Kopp-Schneider A, et al. Hybrid imaging with [(68)Ga]Psma-11 Pet-Ct and Pet-Mri in biochemically recurrent prostate cancer. Cancer Imaging (2022) 22(1):53. doi: 10.1186/s40644-022-00489-9
48. Alonso O, Dos Santos G, García Fontes M, Balter H, Engler H. (68)Ga-Psma and (11)C-Choline comparison using a tri-modality Pet/Ct-Mri (3.0 t) system with a dedicated shuttle. Eur J Hybrid Imaging (2018) 2(1):9. doi: 10.1186/s41824-018-0027-1
49. Freitag MT, Radtke JP, Afshar-Oromieh A, Roethke MC, Hadaschik BA, Gleave M, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in (68)Ga-Psma-11-Pet of Pet/Ct and Pet/Mri: comparison with Mpmri integrated in simultaneous Pet/Mri. Eur J Nucl Med Mol Imaging (2017) 44(5):776–87. doi: 10.1007/s00259-016-3594-z
50. Bianchi L, Ceci F, Balestrazzi E, Costa F, Droghetti M, Piazza P, et al. Psma-pet guided treatment in prostate cancer patients with oligorecurrent progression after previous salvage treatment. Cancers (Basel) (2023) 15(7):2027. doi: 10.3390/cancers15072027
51. Bianchi L, Ceci F, Costa F, Balestrazzi E, Droghetti M, Piazza P, et al. The impact of Psma-Pet on oncologic control in prostate cancer patients who experienced Psa persistence or recurrence. Cancers (Basel) (2022) 15(1):247. doi: 10.3390/cancers15010247
52. Bravi CA, Droghetti M, Fossati N, Gandaglia G, Suardi N, Mazzone E, et al. Definition and impact on oncologic outcomes of persistently elevated prostate-specific antigen after salvage lymph node dissection for node-only recurrent prostate cancer after radical prostatectomy: clinical implications for multimodal therapy. Eur Urol Oncol (2022) 5(3):285–95. doi: 10.1016/j.euo.2021.06.003
Keywords: PET/CT, PET/MRI, prostate cancer, biochemically recurrence, meta-analysis
Citation: Huang R, Li Y, Wu H, Liu B, Zhang X and Zhang Z (2023) 68Ga-PSMA-11 PET/CT versus 68Ga-PSMA-11 PET/MRI for the detection of biochemically recurrent prostate cancer: a systematic review and meta-analysis. Front. Oncol. 13:1216894. doi: 10.3389/fonc.2023.1216894
Received: 04 May 2023; Accepted: 24 July 2023;
Published: 14 August 2023.
Edited by:
Fabio Grizzi, Humanitas Research Hospital, ItalyReviewed by:
Matteo Droghetti, University of Bologna, ItalyGiuseppe Carlo Iorio, University of Turin, Italy
Copyright © 2023 Huang, Li, Wu, Liu, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxi Zhang, emh6aHhpQDEyNi5jb20=
 Ruizhe Huang
Ruizhe Huang Yizhen Li
Yizhen Li