
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 13 July 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1216394
 Guiming Fu1,2†
Guiming Fu1,2† Xiaoyi Li3†
Xiaoyi Li3† Fengli Guo1,4†
Fengli Guo1,4† Xianhui Ruan1
Xianhui Ruan1 Wei Zhang1,5
Wei Zhang1,5 Weijing Zhang6
Weijing Zhang6 Yaping Zhang6
Yaping Zhang6 Yibo Chen2
Yibo Chen2 Chunhua Li2
Chunhua Li2 Jin Chen2
Jin Chen2 Xiangqian Zheng1*
Xiangqian Zheng1* Zhaohui Wang2*
Zhaohui Wang2* Ming Gao1,7,8*
Ming Gao1,7,8*Background: At present, there are some controversies in the formulation of surgical protocol for small medullary thyroid carcinoma(s-MTC). We wanted to explore the feasibility of normal thyroid gland retention in small medullary thyroid carcinoma based on different tumor diameters and its prognostic impact on the tumor.
Methods: The data of patients with stage T1 MTC treated at Tianjin Cancer Hospital and Sichuan Cancer Hospital from 2006 to 2021 were analyzed. The tumor diameters of 0.5 cm and 1.0 cm were used as dividing points. The outcomes were tumor recurrence, metastasis, or patient death. Survival was estimated by the Kapan–Meier curve.
Results: A total of 121 T1 s-MTC patients were included, including 55 with total thyroidectomy (TT) and 66 with subthyroidectomy (Sub-TT). There were eleven cases of tumor recurrence and metastasis, and four patients died. When the tumor diameter was 1.0 cm as the cut-off point, tumor diameter (p = 0.010), TT (p = 0.028), unilateral and bilateral type (p = 0.009), and TNM staging (p = 0.007) had significant effects on progression-free survival (PFS). The tumor diameter, unilateral and bilateral type, and TT were risk factors for the prognosis of T1 MTC (p < 0.05).
Conclusion: The tumor diameter of 1.0 cm can be used as a cut-off point for stage T1 MTC. Alt-hough there was no significant difference in overall survival (OS) between T1a and T1b in patients, tumor diameter significantly influenced PFS. TT is not necessary for patients with sporadic MTC with T1a.
MTC was first proposed by Hazand et al. in 1959 as a single pathological type of thyroid neuroendocrine carcinoma. It originates from parafollicular thyroid C cells and can be divided into sporadic and hereditary types according to their heredity. Although the incidence of MTC is less than 5% of all thyroid cancers, it accounts for 13.0% of all thyroid cancer-related deaths (1–3) and is mainly treated by surgical removal of all tumor tissue (4). In 2005, the National Comprehensive Cancer Network (NCCN) published an article that advocated at least TT and bilateral central dissection for all patients with MTC (5). Revised guidelines from the American Thyroid Association (ATA) in 2015 recommended TT and central lymph node dissection for patients with MTC in whom cervical lymph node metastasis or distant metastasis was not found during ultrasound (3, 4). In 2019, the European Society for Medical Oncology (ESMO) recommended that surgical regimens be based on calcitonin levels in patients with MTC. It is considered that only partial normal thyroid tissue can be retained for unilateral lobular MTC with negative cervical lymph nodes, indicated by preoperative color ultrasound, undetectable serum calcitonin after surgery, and no strain RET mutation (6). As of 2022, the NCCN thyroid cancer guidelines still recommend at least TT and bilateral central resection for all patients with tumors ≥1.0 cm in diameter or with cancer foci in bilateral thyroid lobes. TT and central cervical dissection are also recommended for patients with only unilateral thyroid lesions and tumor diameter <1.0 cm (7). Thus, the world’s leading guidelines for the treatment of MTC are cautious about whether to retain part of the thyroid gland, preferring to perform at least TT and bilateral central cervical lymph node dissection, regardless of the stage of the primary tumor. In the past decade or more, we have found that an increasing number of patients with MTC are accidentally discovered during physical examination. According to our experience, these patients tend to have small tumor lesions, and most of them are not willing to accept lifelong medication after TT, but the overall prognosis of the disease is good. At the same time, the concept of thyroid microcarcinoma is widely accepted and some reports indicate that TT may not be necessary for early MTC with a small tumor diameter (8, 9). Therefore, in search of relevant information, we retrospectively analyzed and summarized the data of patients with stage T1 MTC treated at two major cancer centers in southern China and northern China over the past 15 years.
Clinical and pathological data of patients with stage T1 MTC treated at Tianjin Cancer Hospital and Sichuan Cancer Hospital from November 2006 to November 2021 were retrospectively analyzed, and data of eligible cases were collected, sorted, and statistically analyzed according to inclusion and exclusion criteria. Inclusion criteria: (1) postoperative pathological findings confirmed MTC; (2) all patients were newly diagnosed and initially treated, and had no previous history of thyroid surgery; (3) color Doppler ultrasound indicated that the maximum diameter of cancer nodules was less than 2.0 cm, with or without cervical lymph node metastasis; (4) no extraocular invasion of cancer nodules was found by preoperative color Doppler ultrasound and intraoperatively; (5) all surgeons had more than 10 years of experience in thyroid surgery; (6) the scope of surgery at least included the removal of the affected glandular lobe and isthmus, with or without the dissection of the neck lymph node; (7) complete clinical and pathological data of patients and long-term follow-up. Exclusion criteria: (1) postoperative pathology indicated papillary carcinoma, follicular carcinoma, or mixed carcinoma of other pathological types; (2) other malignant tumors in the past; (3) refusal to be studied; (4) lost to follow-up.
Lymph nodes in the central cervical region were dissected (zones VI and VII): upper boundary—lower margin of the hyoid; lower boundary—superior sternal fossa; outer boundary—lateral margins of the carotid sheath; bottom boundary—prevertebral fascia. Lymph nodes in the lateral cervical region were dissected in areas II–V: upper boundary—mastoid plane; lower boundary—supraclavicular fossa; inner boundary—sternocleidomastoid medial border; outer boundary—trapephalus anterior border; bottom boundary—prevertebral fascia.
All patients were followed up at the outpatient clinic after surgery, and the examination items mainly included blood tests (thyroid function, serum calcitonin, carcinoembryonic antigen) and imaging examinations (neck color Doppler ultrasound, chest thin layer computed tomography). Blood tests and color Doppler ultrasonography of the neck were performed every three months for the first year after surgery and then every six months. Thin-section computed tomography of the chest was performed semiannually or annually as needed. Total thyroidectomy and central or lateral cervical lymph node dissection were performed for patients with significantly elevated serum calcitonin in a short period of time or with definite recurrent lesions confirmed by color Doppler ultrasound. Multidisciplinary treatment should be made for patients with distant metastasis such as lung metastasis.
We used the tumor diameters of 0.5 cm and 1.0 cm as the cut-off points for two groups. OS and PFS were the main outcome events. Classification variables using frequency and percentage were described, using the χ2 test or Fisher’s exact test for comparison. Continuous variables were described using mean ± standard deviation. The Shapiro–Wilk test was used to verify normal distribution, and the independent sample t-test was used for comparison between groups. Survival was estimated by the Kaplan–Meier curve and compared with the log-rank test. Univariate analysis of different disease characteristics was carried out with the Cox hazards model. Disease characteristics with p ≤ 0.20 were included in multivariate prognostic hazard ratio analysis to determine independent risk factors. Bilateral p < 0.05 indicated statistical significance. All analyses were performed with SPSS software version 25.0 (SSPS, Inc., Chicago, Illinois, US).
A total of 121 patients in the T1 stage met the inclusion criteria, including 22 patients with the familial type (18.18%) and 99 patients with the sporadic type (81.82%). There were 44 males (36.36%) and 77 females (63.64%), with a male-to-female ratio of 1:1.75. The age range was 24 to 78 years old, with an average age of 49.79 ± 11.38 years old. The maximum diameter of cancer foci was 0.30–1.90 cm, with an average of 1.18 ± 0.51 cm. There were 92 cases (76.03%) of single-lobe carcinoma and 29 cases (23.97%) of multi-lobe carcinoma, of which 20 cases were single-lobe multifocal carcinoma and 9 cases were double-lobe multifocal carcinoma. TT was performed in 55 cases (45.45%) and Sub-TT in 66 cases (54.55%). Central cervical lymph nodes were dissected in 103 cases (85.12%), including 60 unilateral cases and 43 bilateral cases. There were 53 cases (43.80%) with lateral cervical lymph node dissection, 47 with unilateral lymph node dissection, and 6 with bilateral lymph node dissection. Twenty patients (16.53%) with central cervical lymph node metastasis and forty-three patients (35.54%) with lateral cervical lymph node metastasis were diagnosed postoperatively. Tumor staging was performed according to the International Union for Cancer Control (UICC) Version 8 staging system, including stage I (39 cases, 32.23%), stage III (19 cases, 15.70%), stage IV (44 cases, 36.36%), and uncertain stage (19 cases, 15.70%) (Table 1). The follow-up time was 3 to 178 months. Eleven patients (9.10%) developed tumor recurrence and metastasis, and four patients (3.31%) died. The 5-year, 10-year, and 15-year OS rates of stage T1a patients were all 100.00%, and the PFS rates were 98.11%, 96.23%, and 96.23%, respectively. The 5-year, 10-year, and 15-year OS rates of stage T1b patients were 95.59%, 95.59%, and 94.12%, respectively. The PFS rates of stage T1b patients were 88.24%, 85.29%, and 85.29%, respectively.
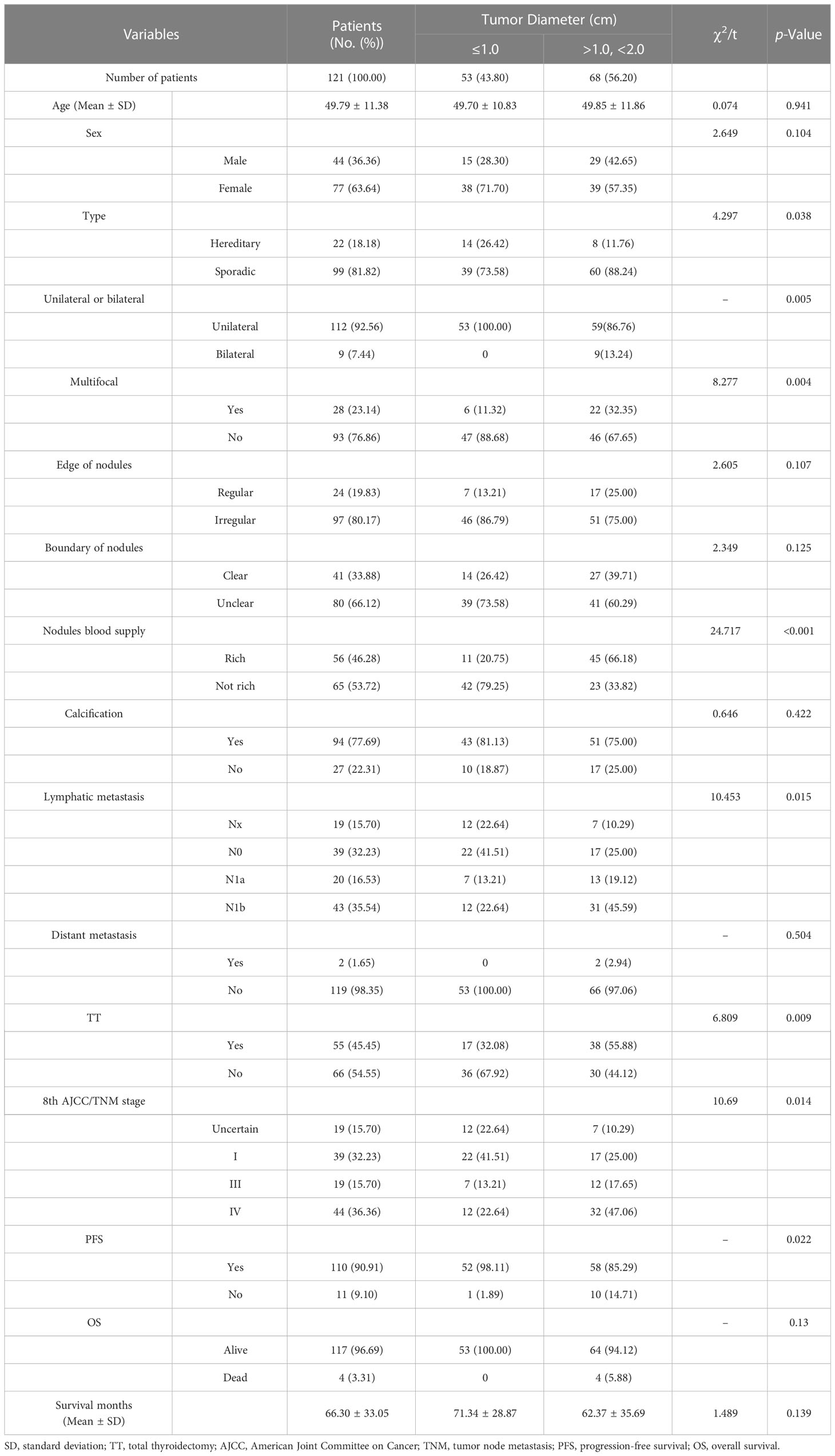
Table 1 Univariate analysis of groups was performed using 1.0 cm as the tumor diameter cut-off point.
When the tumor diameter of 1.0 cm was taken as the cut-off point, χ2 inspections or t-tests showed that for cancer patients with T1a and T1b, the onset type (p = 0.038), single and double side (p = 0.005), multifocal carcinoma (p = 0.004), focal blood supply (p < 0.001), lymph node metastasis (p = 0.015), the total removal of the thyroid (p = 0.009), TNM staging (p = 0. 014), and PFS (p = 0.022) were statistically significant for OS (Table 1). Kaplan–Meier curve estimated survival rates showed that tumor diameter (p = 0.102), TT (p = 0.817), and TNM staging (p = 0.232) had no significant impact on OS in patients with MTC at T1 (Figure 1). At the same time, however, we found that tumor diameter (p = 0.010), TT (p = 0.028), unilateral and bilateral type (p = 0.009), and TNM staging (p = 0.007) all had significant effects on PFS (Figure 2). Univariate analysis showed that tumor diameter (p = 0.034), unilateral and bilateral type (p = 0.018), and TT (p = 0.042) were prognostic risk factors for T1 MTC. All factors with p ≤ 0.20 were included in the Cox hazards model for multi-factor analysis, but no independent risk factors were found (p > 0.05) (Table 2). With a tumor diameter of 0.5 cm as the boundary point, χ2 inspections or t-tests showed that between the two groups of patients, there was no significant difference (p > 0.05) in the onset type, single and double side, multifocal carcinoma, calcification, or lymph node metastasis (Table 3). Kaplan–Meier curves did not identify disease characteristics that were significant for OS and PFS. No positive or independent risk factors were found in univariate analysis and multivariate analysis using the Cox hazards model.
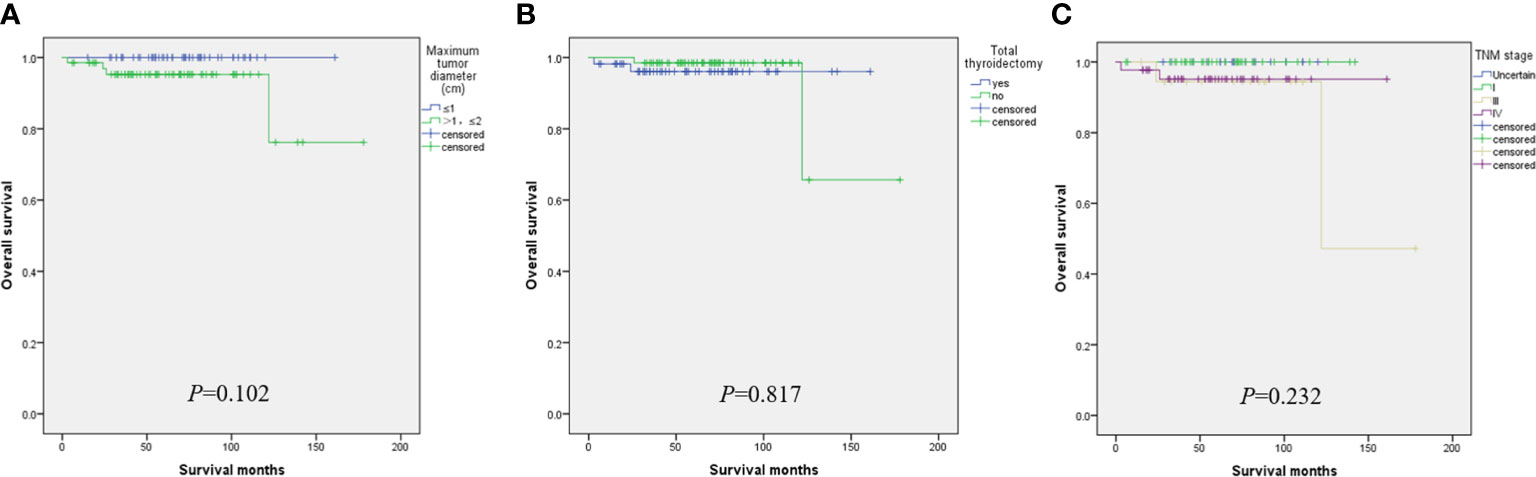
Figure 1 When the maximum tumor diameter was 1.0 cm as the cut-off point, Kaplan–Meier curve survival analysis showed that maximum tumor diameter (A), total thyroidectomy (B), and TNM staging (C) had no significant effect on OS.
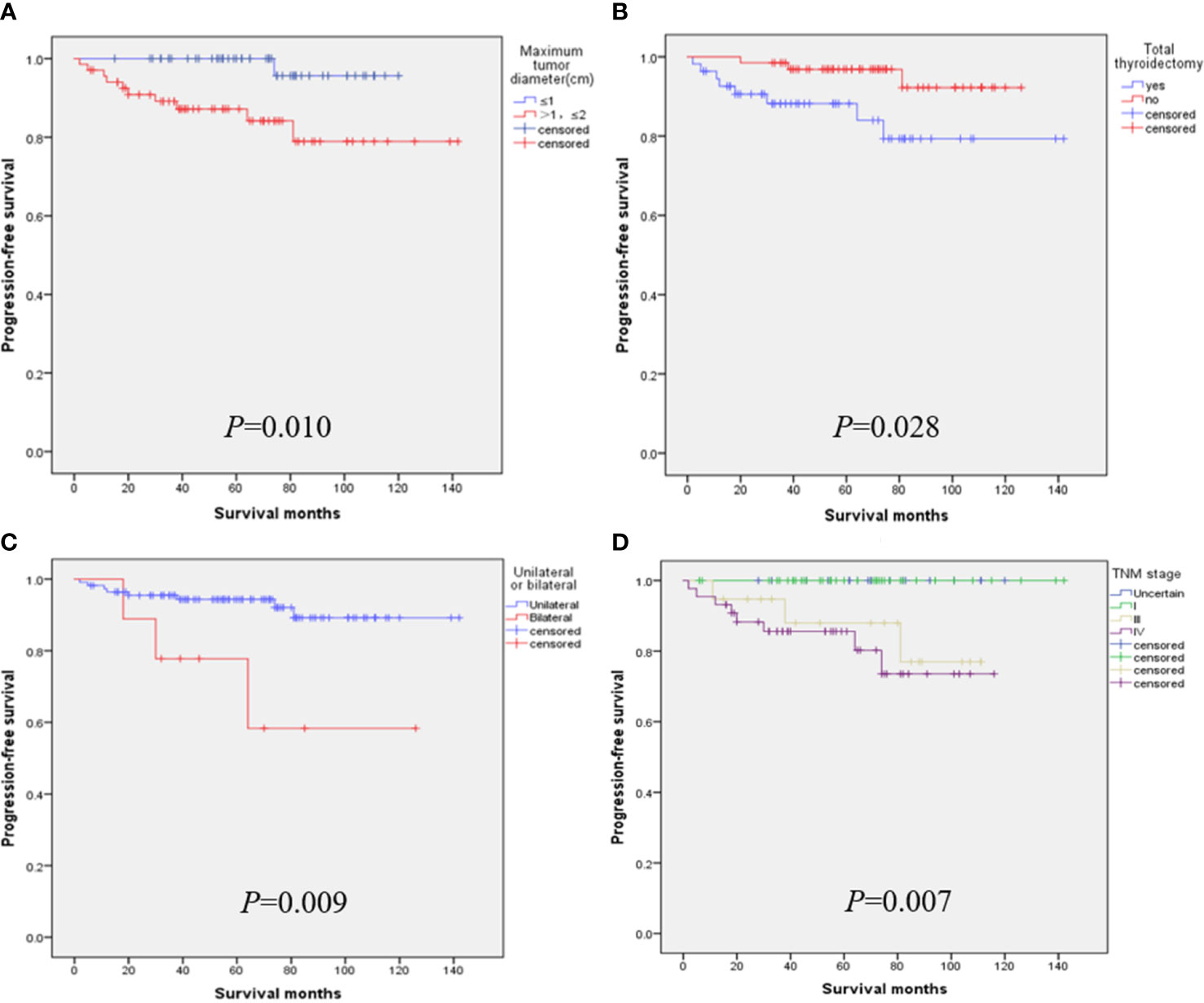
Figure 2 When the maximum tumor diameter was 1.0 cm as the cut-off point, Kaplan–Meier curve survival analysis showed that maximum tumor diameter (A), total thyroidectomy (B), unilateral and bilateral type (C), and TNM staging (D) all had significant effects on PFS.
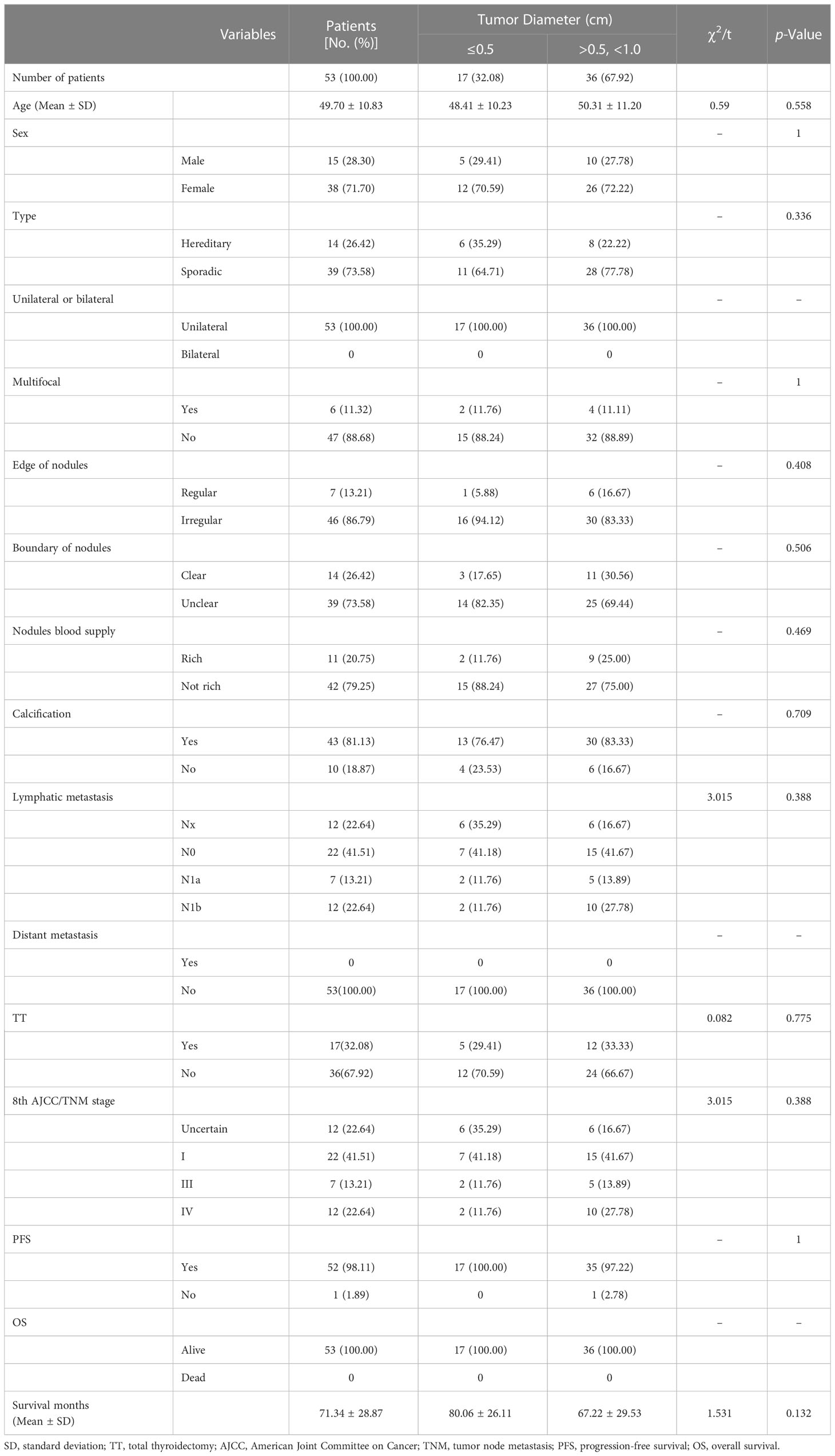
Table 2 Univariate analysis of groups was performed using 0.5 cm as the tumor diameter cut-off point.
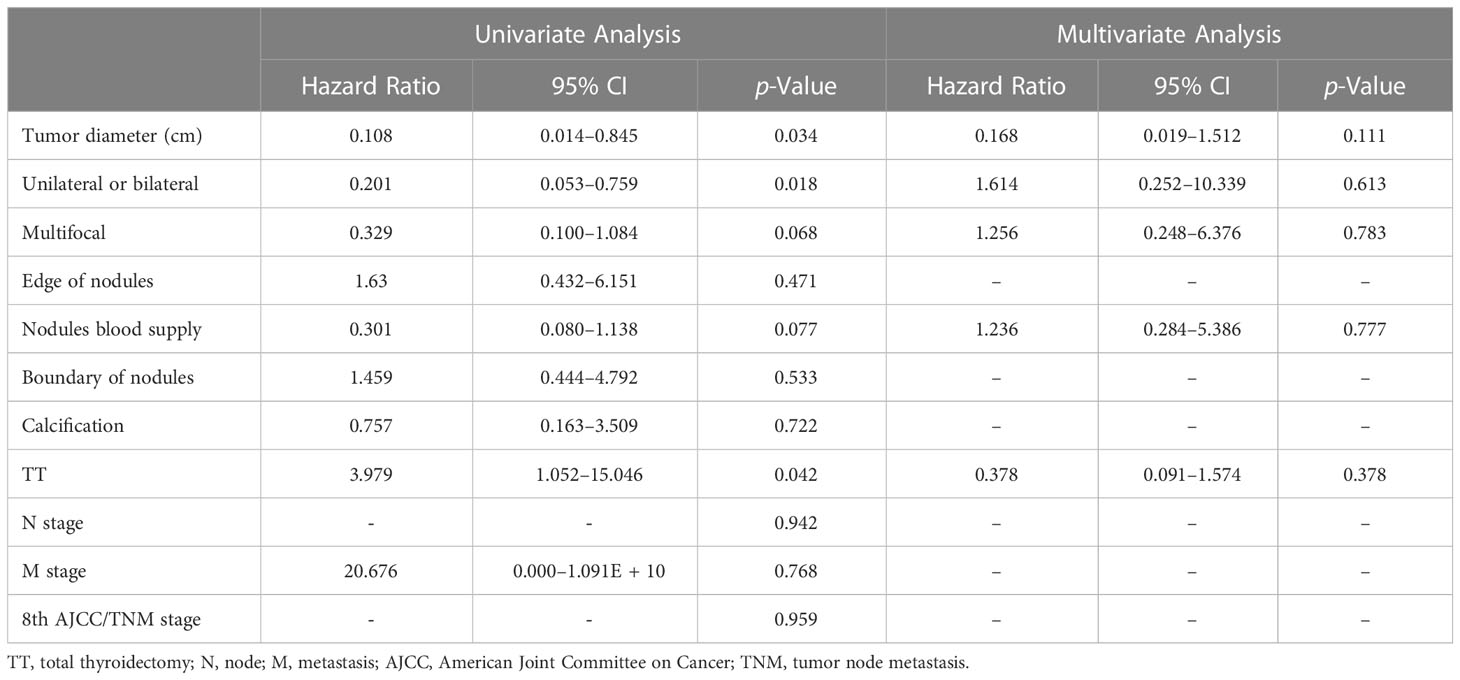
Table 3 Multivariate prognostic hazard ratio analysis was performed using the Cox proportional hazards model.
Since 1988, when the World Health Organization defined thyroid cancer with the largest tumor diameter ≤1.0 cm as microcarcinoma, extensive research on thyroid micro-carcinoma has been carried out worldwide. As early as 1998, Beressi N. et al. reported that the proportion of sporadic micro MTC in all sporadic MTC increased rapidly, among which the proportion was 3.6% before 1984, 14.3% in 1984–1989, and 22.0% in 1990–1996 (10). Kazaure H.S. et al. analyzed 310 cases of medullary thyroid micro-carcinomas detected between 1988 and 2007 and found that the proportion of micro MTC increased by 39.0% (11). Similarly, Machens et al. found that the incidence of micro MTC increased from 19.0% to 39.0% between 2011 and 2015, and Kwon H et al. reported similar findings (12, 13). Our study showed that T1 MTC accounted for 1.33% of the total MTC from 2006 to 2010, 12.26% from 2011 to 2015, and 26.25% from 2016 to 2021, and morbidity also showed the same trend. It can be seen that the incidence of patients with small-sized MTC continues to rise.
Overall, the incidence of MTC in thyroid cancer is consistently below 5%, with a 5-year relative survival rate of about 93.0% for stage I–III patients and 28% for stage IV patients (14–17). Kim S.J. reported a 95.0% OS rate after 71 months of follow-up in patients with minimal MTC (18). Beressi N. reported a 10-year survival rate of 93.9% ± 4.4% for micro MTC (10). Kazaure H.S. et al. reported that overall 10-year survival rates were 96.0%, 87%, and 50% for patients with focal lesions, regional metastases, and distant metastases in minimal MTC, respectively (11). However, Saltiki K.’s study showed that in patients with tumor sizes of 0.1–1.0 cm and 1.1–1.5 cm, the probability of no disease progression at 10 years was 96.6% and 81.3%, respectively, and suggested that tumor size may only be clinically significant in patients with MTC of 1.0 cm (19). In this study, survival analysis of patients with stage T1 MTC was conducted with tumor diameters of 0.5 cm and 1.0 cm as the cut-off points. The results showed that the 5-year, 10-year, and 15-year OS rates of T1a patients with T1 MTC were all 100.00%, and PFS rates were 98.11%, 96.23%, and 96.23%, respectively. Meanwhile, the 5-year, 10-year, and 15-year OS rates of stage T1b patients were 95.59%, 95.59%, and 94.12%. The PFS rates of stage T1b patients were 88.24%, 85.29%, and 85.29%.
Thus, it is highly effective to use a 1.0 cm tumor diameter as the cut-off point. Although it is not significant for the OS of patients, it is critical for the PFS of the disease. It can be seen that the PFS of patients with T1a is significantly higher than that of patients with T1b (p = 0.010). When the tumor diameter of 0.5 cm was used as the cut-off point, we did not obtain valuable information on disease survival, but we believe that this may be related to the small number of patients with a tumor diameter of less than 0.5 cm. Generally, the prognosis of MTC at this stage is still good, and patients with s-MTC still have high OS and PFS rates.
Although the idea of TT and bilateral central cervical lymph node dissection for MTC patients has long been accepted by most surgeons, an increasing number of patients with early MTC are hoping to retain a normal thyroid gland. We may have overstated the benefits of TT for patients with early MTC and overlooked the potential risks of surgical complications and lifelong levothyroxine tablets, especially in poor populations. Raffel A. et al. performed a second surgery on an s-MTC patient who was found by chance in postoperative pathology and who was more likely to develop temporary and permanent hypoparathyroidism after surgery (5.6%; 3.5–8.8%), vocal cord paralysis (3.8–8.5%; 2.8–8.4%), and transient Horner syndrome (3.8–5.6%) (20). Follow-up results showed a 100% biochemical cure rate within 1.5–10.0 years after Sub-TT. Therefore, it is suggested that TT and neck dissection should not be mandatory for patients with sporadic and isolated T1 MTC as long as the genetic background is excluded (20). Three different reports by Miyauchi A., Randle R.W., and Shabina R. analyzed a total of 927 cases of sporadic s-MTC, and all concluded that unilateral thyroidectomy was acceptable for sporadic isolated s-MTC patients with normal contra-lateral thyroid and no RET mutation detected in the reproductive cell line. Dis-ease-specific survival rates did not change (21–23).
Our team has also reached similar conclusions in previous studies on surgical selection and prognosis of unilateral sporadic medullary thyroid carcinoma, i.e., that patients with total thyroidectomy have little benefit in terms of biochemical cure/OS (24). Hamy A. and Zhang D. even argued that sporadic minimal MTC was almost 100% located in the thyroid gland, lymph node metastasis was rare, calcitonin could hardly be detected after surgery, and the value of central cervical dissection was questionable (16, 25). After a follow-up of 233 patients with MTC for 7 to 445 months, Ito et al. also considered it controversial to perform TT and lymph node dissection for all MTCS with lesions confined to the thyroid, especially for T1a (26). Interestingly, studies from the Korean NHIS database showed that a total of 34.6% of patients with MTC underwent adeno lobectomy alone, and this proportion did not change significantly between 2004 and 2016 (17). Similarly, our results also showed that TT had no significant effect on OS in T1a and T1b MTC patients, but it did significantly affect PFS (p = 0.028). Patients who underwent TT had a lower PFS rate (Figure 1B), which we believe is related to cervical lymph node metastasis. TT was performed for the included T1 patients mainly because most of them had already developed cervical lymph node metastasis or even distant metastasis when the disease was discovered. Therefore, even after TT, they still had a lower PFS rate, which was consistent with the lower PFS rate for the later TNM stage (Figure 2D). In addition, after TT, patients need to take levothyroxine tablets for lifelong replacement therapy, and the long-term potential risk of cardiovascular dis-ease and the economic burden should be considered before surgery.
Compared with differentiated thyroid carcinoma, MTC appears to be more prone to cervical lymph node metastasis and distant metastasis and may be associated with many other factors in addition to tumor diameters, such as male sex, calcitonin, and carcinoembryonic antigen levels, RET mutations, bilateral carcinoma, multifocal carcinoma, neoplasmic thyroid extracapsular invasion, marginal burrs, morphological irregularities, calcification, and blood supply (26–31). The level of calcitonin before and after surgery and the doubling time of calcitonin after surgery is now widely believed to be associated with the prognosis of MTC and determines whether the surgeon decides to perform elective lateral cervical lymph node dissection at the time of initial surgery (32). For disease progression, postoperative calcitonin has been shown to be a more important predictor than tumor size (19). Due to the limitations of hospital testing levels, the cancer centers involved in this study did not carry out the detection of calcitonin levels 15 years ago. Meanwhile, since most of the small medullary thyroid carcinomas were accidentally found during the pathological examination after surgery, the level of calcitonin was not routinely detected before surgery.
For advanced MTC patients with unresectable tumor recurrence or distant metastasis, patients with RET mutation or fusion will likely receive RET inhibitor therapy and other measures (33, 34). Bilateral carcinoma also appears to be a factor in poor prognosis. Bilateral cancers tend to be more prone to cervical lymph node and blood route metastasis than unilateral cancers. The results of this study were further confirmed in stage T1 MTC, where bilateral carcinoma had significantly lower PFS (Figure 2C). Multifocal carcinoma and nodular blood supply may be other factors affecting the PFS rate of s-MTC. Although the results of our study were negative, this may be due to the small number of patients analyzed (Figure 3). Cervical lymph node metastasis is another prognostic factor, and the later the stage of lymph node metastasis, the higher the recurrence rate. In particular, stage N1b patients had a significantly higher recurrence rate than those without cervical lymph node metastasis (13). Finally, the adhesion hyperplasia found in the tumor during the histopathological examination may also indicate that MTC has high invasive and metastatic potential. If adhesion hyperplasia is absent or only slightly visible, the risk of metastasis may be considered low (35, 36).
The incidence of s-MTC in patients with MTC continues to increase, and high cure rates can be achieved by early surgical excision. The tumor diameter of 1.0 cm could be used as the cut-off point for T1 MTC. Although there was no significant difference in OS rates between T1a and T1b patients, the former had a higher PFS rate. TT is not necessary for patients with sporadic s-MTC, but the preservation of part of the normal gland is careful, especially when the tumor diameter is greater than 1.0 cm, and the patient must be evaluated comprehensively before surgery.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Conceptualization, GF and FG; methodology, GF and XL; software, XL; formal analysis, GF; investigation, GF, FG, WZ, WJZ, YZ, and YC; resources, ZW and XZ; data curation, XL; writing—original draft preparation, GF, XL, and FG; writing—review and editing, XR; visualization, XR; supervision, CL, JC, and XZ; project administration, ZW and XZ; funding acquisition, MG and XZ. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (82172821,82103386,82272721), the Tianjin Municipal Science and Technology Project (19JCYBJC27400,21JCZDJC00360) and the Beijing–Tianjin–Hebei Basic Research Cooperation Project(20JCZXJC00120), the Science and Technology Development Fund of the Tianjin Education Commission for Higher Education(2021ZD033), the Tianjin Medical Key Discipline (Specialty) Construction Project (TJYXZDXK-058B), and the Tianjin Health Research Project (TJWJ2022XK024).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
2. Ceolin L, Duval MADS, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer (2019) 26:R499–518. doi: 10.1530/ERC-18-0574
3. Gosnell JE, Duh QY. Medullary thyroid carcinoma-we should do better. JAMA Surg (2018) 153:59. doi: 10.1001/jamasurg.2017.3894
4. Ilanchezhian M, Khan S, Okafor C, Glod J, Del Rivero J. Update on the treatment of medullary thyroid carcinoma in patients with multiple endocrine neoplasia type 2. Horm Metab Res (2020) 52:588–97. doi: 10.1055/a-1145-8479
5. Kouvaraki MA, Shapiro SE, Lee JE, Evans DB, Perrier ND. Surgical management of thyroid carcinoma. J Natl Compr Cancer Netw (2005) 3:458–66. doi: 10.6004/jnccn.2005.0022
6. Filetti S, Durante C, Hartl D, Leboulleux S, Locati L, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 30:1856–83. doi: 10.1093/annonc/mdz400
7. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw (2022) 20:925–51. doi: 10.6004/jnccn.2022.0040
8. Baloch ZW, LiVolsi VA. Microcarcinoma of the thyroid. Adv Anat Pathol (2006) 13:69–75. doi: 10.1097/01.pap.0000213006.10362.17
9. Gui Z, Wang Z, Xiang J, Sun W, He L, Dong W, et al. Incidental T1 stage medullary thyroid carcinoma: the effect of tumour diameter on prognosis and therapeutic implications. Clin Endocrinol (2022) 97:355–62. doi: 10.1111/cen.14702
10. Beressi N, Campos J, Beressi J, Franc B, Niccoli-Sire P, Conte-Devolx B, et al. Sporadic medullary microcarcinoma of the thyroid: a retrospective analysis of eighty cases. Thyroid (1998) 8:1039–44. doi: 10.1089/thy.1998.8.1039
11. Kazaure HS, Roman SA, Sosa JA. Medullary thyroid microcarcinoma. Cancer (2012) 118:620–7. doi: 10.1002/cncr.26283
12. Machens A, Dralle H. Surgical cure rates of sporadic medullary thyroid cancer in the era of calcitonin screening. Eur J Endocrinol (2016) 175:219–28. doi: 10.1530/EJE-16-0325
13. Kwon H, Kim WG, Sung TY, Jeon MJ, Song DE, Lee YM, et al. Changing trends in the clinicopathological features and clinical outcomes of medullary thyroid carcinoma. J Surg Oncol (2016) 113:152–8. doi: 10.1002/jso.24126
14. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer (1998) 83:2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638:aid-cncr31>3.0.co;2-1
15. Wells SA Jr, Asa S, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid (2015) 25:567–610. doi: 10.1089/thy.2014.0335
16. Zhang D, Colombo C, Sun H, Kim HY, Pino A, De Leo S, et al. Unilateral surgery for medullary thyroid carcinoma: seeking for clinical practice guidelines. Front Endocrinol (2022) 13:875875. doi: 10.3389/fendo.2022.875875
17. Ahn HY, Chae JE, Moon H, Noh J, Park YJ, Kim SG. Trends in the diagnosis and treatment of patients with medullary thyroid carcinoma in Korea. Endocrinol Metab (2020) 35:811–9. doi: 10.3803/EnM.2020.709
18. Kim SJ, Yun HJ, Shin SJ, Lee YS, Chang HS. Serum calcitonin-negative medullary thyroid carcinoma: a case series of 19 patients in a single center. Front Endocrinol (2021) 12:747704. doi: 10.3389/fendo.2021.747704
19. Saltiki K, Rentziou G, Stamatelopoulos K, Georgiopoulos G, Stavrianos C, Lambrinoudaki E, et al. Small medullary thyroid carcinoma: post-operative calcitonin rather than tumour size predicts disease persistence and progression. Eur J Endocrinol (2014) 171:117–26. doi: 10.1530/EJE-14-0076
20. Raffel A, Cupisti K, Krausch M, Wolf A, Schulte KM. Incidentally found medullary thyroid cancer: treatment rationale for small tumors. World J Surg (2004) 28:397–401. doi: 10.1007/s00268-003-7121-6
21. Miyauchi A, Matsuzuka F, Hirai K, Yokozawa T, Kobayashi K, Kuma S, et al. Unilateral surgery supported by germline RET oncogene mutation analysis in patients with sporadic medullary thyroid carcinoma. World J Surg (2000) 24:1367–72. doi: 10.1007/s002680010226
22. Randle RW, Bates MF, Schneider DF, Sippel RS, Pitt SC. Survival in patients with medullary thyroid cancer after less than the recommended initial operation. J Surg Oncol (2018) 117:1211–6. doi: 10.1002/jso.24954
23. Ahmed SR, Ball DW. Incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab (2011) 96:1237–45. doi: 10.1210/jc.2010-2359
24. Zhang J, Gu P, Huang D, Zhao J, Zheng X, Gao M. Surgical selection and prognostic analysis in patients with unilateral sporadic medullary thyroid carcinoma. Langenbecks Arch Surg (2022) 407:3013–23. doi: 10.1007/s00423-022-02591-9
25. Hamy A, Pessaux P, Mirallié E, Mucci-Hennekinne S, Gibelin H, Mor-Martinez C, et al. Central neck dissection in the management of sporadic medullary thyroid microcarcinoma. Eur J Surg Oncol (2005) 31:774–7. doi: 10.1016/j.ejso.2005.03.007
26. Ito Y, Miyauchi A, Kihara M, Higashiiyama T, Fukushima M, Miya A. Static prognostic factors and appropriate surgical designs for patients with medullary thyroid carcinoma: the second report from a single-institution study in Japan. World J Surg (2018) 42:3954–66. doi: 10.1007/s00268-018-4738-z
27. Pazaitou-Panayiotou K, Chrisoulidou A, Mandanas S, Tziomalos K, Doumala E, Patakiouta F. Predictive factors that influence the course of medullary thyroid carcinoma. Int J Clin Oncol (2014) 19:445–51. doi: 10.1007/s10147-013-0588-8
28. Kim JH, Pyo JS, Cho WJ. Clinicopathological significance and prognosis of medullary thyroid microcarcinoma: a meta-analysis. World J Surg (2017) 41:2551–8. doi: 10.1007/s00268-017-4031-6
29. Oh HS, Kwon H, Song E, Jeon MJ, Song DE, Kim TY, et al. Preoperative clinical and sonographic predictors for lateral cervical lymph node metastases in sporadic medullary thyroid carcinoma. Thyroid (2018) 28:362–8. doi: 10.1089/thy.2017.0514
30. Li X, Zhou W, Zhan W. Clinical and ultrasonographic features of medullary thyroid microcarcinomas compared with papillary thyroid microcarcinomas: a retrospective analysis. BMC Med Imaging (2020) 20:49. doi: 10.1186/s12880-020-00444-9
31. Huang S, Zhong J, Chen H. Prediction model of cervical lymph node metastasis in medullary thyroid carcinoma. Mod Tumor Med (2022) 30:416–21. doi: 10.3969/j.issn.1672-4992.2022.03.010
32. Niederle MB, Riss P, Selberherr A, Koperek O, Kaserer K, Scheuba C. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br J Surg (2021) 108:174–81. doi: 10.1093/bjs/znaa047
33. Hadoux J, Pacini F, Tuttle RM, Schlumberger M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol (2016) 4:64–71. doi: 10.1016/S2213-8587(15)00337-X
34. Kiesewetter B, Riss P, Scheuba C, Raderer M. How I treat medullary thyroid cancer. ESMO Open (2021) 6:100183. doi: 10.1016/j.esmoop.2021.100183
35. Kaserer K, Scheuba C, Neuhold N, Weinhäusel A, Haas OA, Vierhapper H, et al. Sporadic versus familial medullary thyroid microcarcinoma. Am J Surg Pathol (2001) 25:1245–51. doi: 10.1097/00000478-200110000-00004
Keywords: thyroid malignancy, medullary thyroid carcinoma, tumor diameter, surgery, prognosis
Citation: Fu G, Li X, Guo F, Ruan X, Zhang W, Zhang W, Zhang Y, Chen Y, Li C, Chen J, Zheng X, Wang Z and Gao M (2023) Partial preservation of the normal thyroid gland based on tumor diameter may be possible in small medullary thyroid carcinoma: a two-center 15-year retrospective study. Front. Oncol. 13:1216394. doi: 10.3389/fonc.2023.1216394
Received: 03 May 2023; Accepted: 28 June 2023;
Published: 13 July 2023.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandCopyright © 2023 Fu, Li, Guo, Ruan, Zhang, Zhang, Zhang, Chen, Li, Chen, Zheng, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangqian Zheng, eHpoZW5nMDVAdG11LmVkdS5jbg==; Zhaohui Wang, NDkxOTQzNDMwQHFxLmNvbQ==; Ming Gao, aGVhZGFuZG5lY2syMDA3QGFsaXl1bi5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.