
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 22 June 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1209811
This article is part of the Research Topic Cervical Cancer Awareness – Volume I: Screening, Vaccines, and Prevention View all 8 articles
Background: The conization length for cervical precancerous lesions is essential for treatment but is left undetermined. This study aims to explore the reasonable and optimal conization length in patients with different types of cervical transformation zones (TZs) to reach the treatment outcome of margin negative in the surgery.
Methods: From July 2016 to September 2019, a multi-center prospective case–control study with or suspicion of cervical precancer was enrolled from five medical centers in Shanghai, China. The clinical characteristics, cytology, human papillomavirus (HPV), histopathology, and details of cervical conization were recorded.
Results: A total of 618 women were enrolled in this study; 6.8% (42/618) had positive internal (endocervical and stromal) margins and 6.8% (42/618) had positive external (ectocervical) margins of loop electrosurgical excision procedure (LEEP) specimen. Comparing the positive internal margin group with the negative group, age (p = 0.006) and cytology (p = 0.021) were significantly different. Multivariate logistic regression analysis showed that the risk factors for positive internal margin were cytology ≥ high-grade squamous intraepithelial lesion (HSIL) (odds ratio (OR) 3.82, p = 0.002) and age (OR 1.11, p < 0.001). The positive internal margin rate was 2.7%, 5.1%, and 6.9% in TZ1, TZ2, and TZ3, respectively, while the positive external margin was 6.7%, 3.4%, and 1.4%, respectively. In the TZ3 group, the HSIL positive internal margin of the 15–16-mm group (10.0%, 19/191) was significantly greater than in TZ1 (2.7%, 4/150) (p = 0.010) and TZ2 (5.0%, 9/179) (p = 0.092); when excision length increases to 17–25 mm, the positive internal margin rate dramatically decreased to 1.0% (1/98).
Conclusion: A cervical excision length of 10–15 mm is reasonable for TZ1 and TZ2 patients, while 17–25 mm is optimal for TZ3 excision with more negative internal margins.
Cervical cancer has high morbidity and mortality across the world, accounting for an estimated 604,000 new cancer cases and 342,000 deaths worldwide in 2018 (1, 2). For cervical precancerous lesion treatment, excision procedures comprise three methods, i.e., loop electrosurgical excision procedure [LEEP; also called large loop excision of the transformation zone (LLETZ)], laser, and cold knife (3, 4).
According to the 2011 International Federation of Cervical Pathology and Colposcopy (IFCPC) terminology, the cervical transformation zone (TZ) is categorized into three types, with the squamous–columnar junction (SCJ) also separated into three types, and it is proposed that the cervical conization length of each transformation zone is different (5). In the 2011 IFCPC colposcopy terminology, TZ3 patients’ conization requires longer and larger excision of cervical tissue than type 1 or type 2 with a significant amount of endocervical epithelium. However, the precise conization length of different TZs was not defined and thus needs further investigation. The 2017 American Society for Colposcopy and Cervical Pathology (ASCCP) terminology categorized SCJ into two types (namely, fully visible SCJ and incompletely visible SCJ) and two types of treatment principles for cervical lesions (6, 7). The British National Health Service (NHS) cervical screening program gives more detailed information for three types of TZs with a wide range of conization lengths, i.e., 7–10, 10–15, and 15–25 mm for TZ1, TZ2, and TZ3, respectively (8).
However, currently, the LEEP conization length has not been standardized (9–11). According to a previous retrospective study carried out in our center in 2019, comparing the different margin status of conization specimens, the persistence rate of endocervical and stromal margin status has no significant statistical difference for internal margins, while internal margin and other margins (ectocervical margins status and negative margin) differ significantly (12). Therefore, to clarify, we group the endocervical and stromal margins as internal margins while the ectocervical margin as external margins, which is also demonstrated in Figure 1.
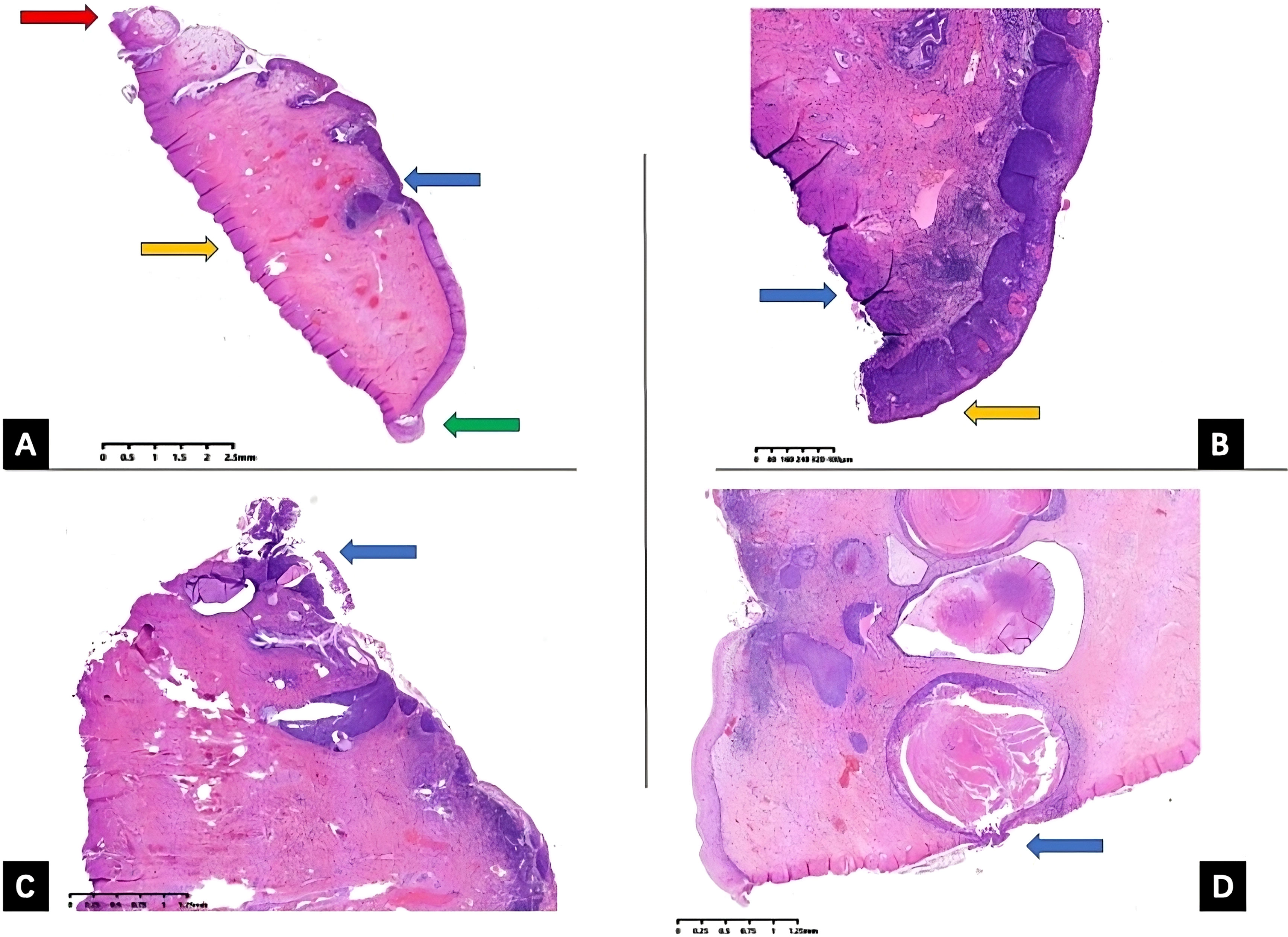
Figure 1 Uterine cervix LEEP specimen histopathology slides. Uterine cervix LEEP conization specimen hematoxylin and eosin (H&E) staining histopathology slides with different margin status. (A) All margins are negative, external (ectocervical) margin is demonstrated by green arrow, red arrow pointing at internal (endocervical) margin, orange arrow pointing at the stromal margin, and blue arrow pointing at the HSIL lesions. (B) External (ectocervical) margin positive, orange arrow pointing at external margin, blue arrow pointing at the stromal margin (C) internal (endocervical) margin positive, blue arrow pointing at the endocervical margin (D) internal (stromal) margin positive, and blue arrow pointing at the stromal margin. LEEP, loop electrosurgical excision procedure; HSIL, high-grade squamous intraepithelial lesion.
In this study, we investigated cervical conization lengths and margin status in LEEP patients to explore a reasonable excision length for different types of TZs and to help achieve better outcomes of treatment simultaneously.
From July 2016 to September 2019, we prospectively enrolled patients with or suspicious of cervical precancer, including 1) cytology high-grade squamous intraepithelial lesion (HSIL)/ASC-H with positive human papillomavirus (HPV) results; 2) colposcopy-directed punch biopsy histological HSIL, regardless of the results of cytology and colposcopy; 3) cytology atypical glandular cells (AGCs) with positive HPV results; 4) colposcopy impression of HSIL with biopsy histopathology low-grade squamous intraepithelial lesion (LSIL). Exclusion criteria: 1) biopsy HSIL with stromal invasion, with invasion depth not determined; 2) cytology HSIL with biopsy confirmed invasive cancer. Cases were included in strict accordance with the inclusion criteria and exclusion criteria.
A total of 618 cases were included from five medical centers, i.e., Obstetrics and Gynecology Hospital of Fudan University, Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai First Maternal and Child Health Hospital Affiliated to Tongji University, Cancer Hospital Affiliated to Fudan University, and Shanghai Tongji Hospital. According to the LEEP histopathological report, the negative excision margin group includes cervical chronic inflammation, LSIL, HSIL with a negative margin, and adenocarcinoma in situ (AIS) with a negative resection margin. The margin with LSIL belonged to a negative margin. Margins equal to or higher than HSIL (HSIL, AIS, and malignancy) belonged to the positive margins. Five types of margin status were analyzed, including positive external, positive internal (endocervical or stromal), positive undetermined, and negative margin.
Stata 15.0 statistical software was used to analyze the data. t-Test and chi-square test analysis were used to analyze the differences in the mean value and proportion, respectively. The logistic regression model was used for univariate and multivariate analyses. p < 0.05 was considered statistically significant.
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Obstetrics and Gynecology Hospital of Fudan University (IRB Number: 2016-03).
A total of 618 cases of women who underwent cervical LEEP were classified as reported in Table 1. The clinical characteristics, including age, parity, mode of delivery, smoking, age of first sexual intercourse, contraceptive methods, cytology, HPV testing, histopathological results, colposcopy examination (type of transformation zones and colposcopy impression), and details of cervical conization (excision length, width, cervical length, the proportion of excision) were all analyzed and compared between two groups (positive internal margins vs. negative external margins). Age and cytology were significantly different between the two groups (p = 0.006, p = 0.021).
According to the histopathological report of LEEP conization of the cervix, 90.0% of women had negative margins, including chronic inflammation 12.3%, LSIL 14.9%, HSIL 62.3%, AIS 0.3%, and HSIL combined with AIS 0.2%; 10.0% of women had positive margins, including HSIL with positive external margin 3.2%, HSIL with positive endocervical margin 4.2%, HSIL with positive stromal margin 1.1%, AIS margin positive 0.3%, AIS combined with HSIL (AIS and HSIL) margin positive 0.3%, squamous cell carcinoma 0.6%, and adenocarcinoma 0.2% (Table 2).
The patients were categorized into two groups according to the negative and positive cervical LEEP excision margins. The clinical characteristics of the two groups were compared by univariate logistic regression. The results showed the age of first sexual intercourse (p = 0.181), number of sexual partners (p = 0.483), parity (p = 0.292), delivery method (p = 0.692), HPV (p = 0.592), transformation zone (p = 0.140), colposcopy impression (p = 0.883), colposcopy-directed punch biopsy histopathology (p is omitted), cervical length (p = 0.821), conization length (p > 0.999), and the ratio of conization length to cervical length (p = 0.907) were not significantly different between the two groups. However, the difference in cytology (p = 0.021) and age (p = 0.006) in the two groups was statistically significant.
As shown in Table 3, multiple variables were then incorporated into the multivariate logistic regression model, including age, age of first sexual intercourse, number of sex partners, parity, vaginal delivery, condom, positive high-risk HPV (hrHPV), transformation zone, cytology ≥ HSIL (ASC-H, HSIL, AGC, AIS, squamous cell carcinoma, and adenocarcinoma), colposcopy impression ≥ HSIL (HSIL, AIS, and suspicious cancer), length of excision, length of the cervix, and proportion of excision. Multivariate logistic regression analysis showed that the risk factors for the positive internal LEEP margin were cytology ≥ HSIL (odds ratio 3.82, 95% confidence interval 1.62–8.97, p = 0.002) and age (odds ratio 1.11, 95% confidence interval 1.05–1.17, p < 0.001).
In this study, the conization length of the type 1 transformation zone was 13.5 ± 2.3 mm (7–15 mm), the conization length of the type 2 transformation zone was 13.7 ± 2.1 mm (10–15 mm), and the conization length of the type 3 transformation zone was 16.4 ± 2.0 mm (15–25 mm). Type 3 transformation zone patients were subdivided into two groups according to the length of cervical conization as 15 and 16–25 mm for data analysis. The total positive rate of excision margin was 10.7% (16/150), 10.6% (19/179), and 9.3% (27/289). Since positive external margin only has a close correlation with the width of the lesion and no correlation on the residual recurrence extended into the cervical canal, we analyzed the proportion of positive internal margins showing 2.7% (4/150), 5.1% (9/179), and 6.9% (20/289), respectively. Comparing the three groups, the difference was not statistically significant in the positive internal margin rate for TZ1 vs. TZ2 (p = 0.274) and TZ1 vs. TZ3 (p = 0.063). In TZ3 excision length groups of 15 and 16–25 mm, the positive internal margin positive rate was 9.7% (16/164) and 3.2% (4/125), respectively. When comparing the TZ3 (15 mm) group with the TZ1 group, the difference was statistically significant (p = 0.010). The difference between TZ3 (15 mm) vs. TZ2 was not significant (p = 0.092). Neither the difference for TZ3 (16–25 mm) vs. TZ1 (p = 0.764) nor TZ3 (16–25 mm) vs. TZ2 (p = 0.438) was statistically significant. When excision length was 15, 16, 17, 18, 19, 20, 21, and 25 mm in TZ3, the positive rate of internal resection margin was 9.8% (16/164), 11.1% (3/27), 0% (0/9), 0% (0/47), 0% (0/1), 2.7% (1/37), 0% (0/1), and 0% (0/3), respectively (Table 4).
In Figure 2, we evaluated LEEP margins according to the excision lengths of three transformation zones. This figure illustrated the positive rate of internal margins when we gradually added the excision length during conization surgery. In TZ1, when excision length reaches 15 mm, the detection rate of positive internal margin was still 0%; in TZ2, the positive rate was 8.11%, 8.11%, 6.38%, 7.69%, 7.41%, and 6.15% when excision length reaches 10, 11, 12, 13, 14, and 15 mm, respectively. In TZ3, when excision length increased from 15 to 25 mm, the positive internal margin rate decreased from 10.91% to 7.27%, except for one case of HSIL combined with AIS; even if the resection length was 15 mm, the resection margin was positive.
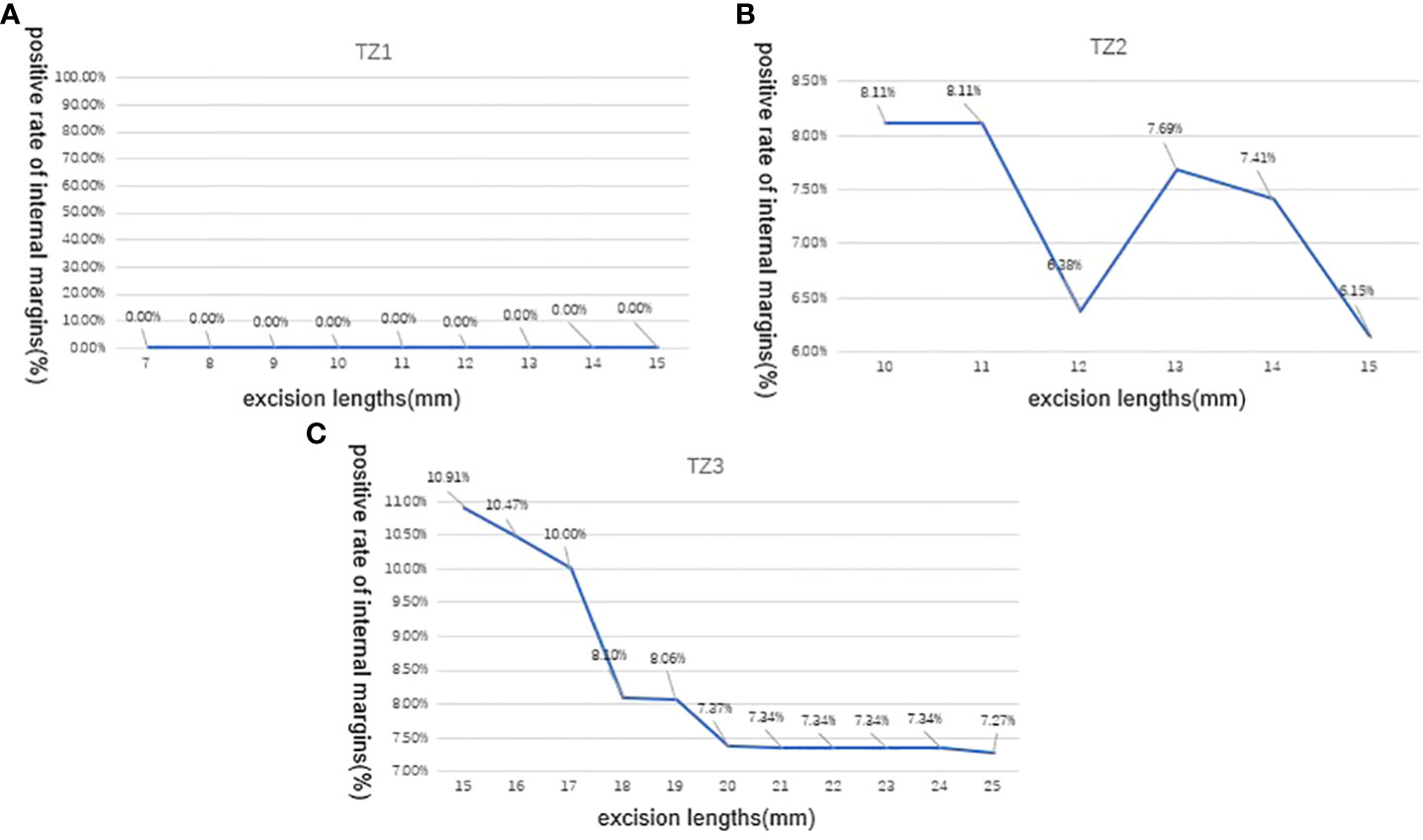
Figure 2 Positive rate of LEEP internal margins according to excision lengths of three transformation zones (TZs). (A) In TZ1, the detection rate of positive internal margin was still 0% When the excision length is gradually increased from 7 to 15mm; (B) In TZ2, the positive internal margin rate was 8.11%,8.11%, 6.38%, 7.69%, 7.41%, and 6.15% when the excision length is gradually increased from 10 to 15mm, respectively; (C) In TZ3, the positive internal margin rate decreased from 10.91% to 7.27% when the excision length is gradually increased from 15 to 25 mm.TZ, transformation zone; LEEP, loop electrosurgical excision procedure.
This multi-center prospective study recruited patients who underwent LEEP conization (13–15). After reviewing their histopathological results, the LEEP specimen margin was negative for 90.0% (556/618), and the margin was positive for 10.0% (62/618), 0.81% (5/618) of which were further diagnosed as invasive carcinoma. In this study, most patients with cervical lesions (90.0%) can obtain early diagnosis and precise treatment through a single LEEP conization. Patients with positive margins (9.2%) were triaged to follow-up within 3–6 months after surgery and repeat conization if necessary (16, 17). As a result, most patients can preserve their cervix through cervical conization, without the need for total hysterectomy. Red House Hospital’s single-center retrospective large sample data showed that LEEP specimen histopathology diagnosed 5.97% (759/12,713) as early cervical cancer (18), and the retrospective single-center data from Cancer Hospital affiliated to Fudan University of LEEP specimen histopathology diagnosed 8.60% (112/1,303) as early cervical cancer (19). LEEP specimen histopathology diagnosis rate of cervical cancer (0.81%) was lower than in that of two single centers. The reason might be that this study excluded patients with biopsy results suggesting cervical cancer with an uncertain depth of invasion, and this group of patients was found to have high proportions of invasive cervical cancer diagnosed by conization 61.9%.
Multivariate logistic regression analysis further confirmed that cytology ≥ HSIL and age are high-risk factors for LEEP positive internal margins, which indicates that the severity and duration of the disease might be related to the positive internal margin status. During the 3–6 months’ follow-up after conization, our previously published data and the cancer hospital team’s data showed that age, menopause, positive conization margin, diagnosis of microinvasive carcinoma by conization, abnormal cytology in postoperative follow-up, and positive HPV were high-risk factors for persistent HSIL. In the 3–6 months’ follow-up after conization, the proportion of persistent HSIL in patients with positive margin was 7.6% (46/609) and only 1.9% in patients with negative margin (55/2,964). The risk of persistent HSIL varies in the different positive margins, with positive external margin in 4.7% (13/278), positive undetermined (including stromal) margin in 9.7% (22/227), and positive endocervical margin in 13.2% (23/174). The data show that positive margins do not mean that lesions always persist after surgery, and positive internal margin had significantly higher risks of persistent HSIL when compared with positive external margin. During the 3–6 months’ follow-up after conization, only 7.6% of patients have persistent HSIL after conization on average, which means that up to 92.4% of patients with positive margins for HSIL lesion regressed naturally.
In this study, the LEEP margin of type 1, 2, and 3 transformation zones under different conization lengths was thoroughly investigated. In the condition of excision length of 7–15 mm for TZ1 and 10–15 mm for TZ2, this study showed that both the total positive margin rates and positive rate of external margin of TZ1 were similar to those of TZ2 (10.7% (16/150) vs. 10.6% (19/179), and 2.7% (4/150) vs. 5.1% (9/179), respectively). Hence, clinicians can simply choose a loop with an excision length of 10–15 mm for fully visible SCJ (TZ1 and TZ2) patients.
In 27 patients with TZ3 with positive margins, most (74.1%) were found when the excision length was 15 mm, and only 26.0% were found in the 16–25-mm group. Both the total positive margin rate and positive internal margin rate of the 15-mm group were significantly higher than those of the 16–25-mm group (12.2% (20/164) vs. 5.6% (7/125), 9.7% (16/164) vs. 2.7% (4/150)), showing that the excision length of TZ3 should be greater than 15 mm, and the clinician should choose loops with excision length greater than 15 mm. When excision length was 15, 16, 17, 18, 19, 20, 21, and 25 mm in TZ3, the positive rate of internal margin was 9.8% (16/164), 11.1% (3/27), 0% (0/9), 0% (0/47), 0% (0/1), 2.7% (1/37), 0% (0/1), and 0% (0/3), respectively. Hence, 17–20 mm can achieve more negative internal margins and retain a more normal cervix and thus might be optimal for TZ3 excision.
According to the trend line in Figure 2, excision of 7–10 mm of the type 1 transformation zone patients is reasonable and safe for HSIL, except for the case of HSIL combined with AIS. If cytology and colposcopy suggest glandular disease, the excision depth needs to be >10 mm. For women who have completed childbirth, the excision length can reach 18–20 mm. The treatment of AIS should follow the latest Society of Gynecologic Oncology Evidence-Based Review and Recommendations of Adenocarcinoma in Situ (20–22).
The limitation of the study is that the sample size of each excision length was not evenly distributed in TZ3, while relatively limited cases were in the 17-mm, 19-mm, and longer than 20-mm groups. In the future, more data from these groups will help to achieve a more accurate conclusion.
In conclusion, the cervical LEEP aims to resect cervical precancerous lesions. However, up to now, the study of conization length is scant. In this study, we explored the reasonable and optimal conization length in patients with different types of TZs. The ideal cervical conization length for patients with TZ1 and TZ2 is 10–15 mm, while 17–25 mm is optimal for TZ3 excision with more negative internal margins.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
QC and YY had full access to all data and take responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: LS. Acquisition, analysis, and interpretation of data: QC, YY, YX, and YL. Drafting of the manuscript: QC and YY. Critical revision of the manuscript for important intellectual content: all authors. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Commission of Shanghai Municipality (No. 18411963600).
The authors are grateful to the Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc (2020) 112(2):229–32. doi: 10.1016/j.jnma.2020.03.002
3. Moon H, Ro SW. MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers (Basel) (2021) 13(12):162–81. doi: 10.3390/cancers13123026
4. Basu P, Taghavi K, Hu SY, Mogri S, Joshi S. Management of cervical premalignant lesions. Curr Probl Cancer (2018) 42(2):129–36. doi: 10.1016/j.currproblcancer.2018.01.010
5. Khan MJ, Werner CL, Darragh TM, Guido RS, Mathews C, Moscicki AB, et al. ASCCP colposcopy standards: role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis (2017) 21(4):223–9. doi: 10.1097/LGT.0000000000000338
6. Bornstein J, Bentley J, Bösze P, Girardi F, Haefner H, Menton M, et al. 2011 colposcopic terminology of the international federation for cervical pathology and colposcopy. Obstetrics gynecology (2012) 120(1):166–72. doi: 10.1097/AOG.0b013e318254f90c
7. Chen T, Liu X, Feng R, Wang W, Yuan C, Lu W, et al. Discriminative cervical lesion detection in colposcopic images with global class activation and local bin excitation. IEEE J BioMed Health Inform (2022) 26(4):1411–21. doi: 10.1109/JBHI.2021.3100367
8. Hill E, Nemec M, Marlow L, Sherman SM, Waller J. Maximising the acceptability of extended time intervals between screens in the NHS cervical screening programme: an online experimental study. J Med screening (2021) 28(3):333–40. doi: 10.1177/0969141320970591
9. Macdonald M, Smith J, Tidy JA, Palmer JE. Conservative management of CIN2: national audit of British society for colposcopy and cervical pathology members' opinion. J obstetrics gynaecology (2018) 38(3):388–94. doi: 10.1080/01443615.2017.1316973
10. Gustafson LW, Hammer A, Bennetsen MH, Kristensen CB, Majeed H, Petersen LK, et al. Cervical intraepithelial neoplasia in women with transformation zone type 3: cervical biopsy versus large loop excision. BJOG (2022) 129(13):2132–40. doi: 10.1111/1471-0528.17200
11. Mosseri J, Hocquemiller R, Mergui JL, Uzan C, Canlorbe G. Laser conization for cervical intraepithelial neoplasia: effectiveness and obstetric outcomes. J Gynecol Obstet Hum Reprod (2022) 51(4):102341. doi: 10.1016/j.jogoh.2022.102341
12. Chen L, Liu L, Tao X, Guo L, Zhang H, Sui L. Risk factor analysis of persistent high-grade squamous intraepithelial lesion after loop electrosurgical excision procedure conization. J Low Genit Tract Dis (2019) 23(1):24–7. doi: 10.1097/LGT.0000000000000444
13. Abdulaziz A, You X, Liu L, Sun Y, Zhang J, Sun S, et al. Management of high-grade squamous intraepithelial lesion patients with positive margin after LEEP conization: a retrospective study. Med (Baltimore) (2021) 100(20):e26030. doi: 10.1097/MD.0000000000026030
14. Bogani G, DI Donato V, Sopracordevole F, Ciavattini A, Ghelardi A, Lopez S, et al. Recurrence rate after loop electrosurgical excision procedure (LEEP) and laser conization: a 5-year follow-up study. Gynecologic Oncol (2020) 159(3):636–41. doi: 10.1016/j.ygyno.2020.08.025
15. Przybylski M, Pruski D, Millert-Kalinska S, Zmaczynki A, Baran R, Horbaczewska A, et al. Remission of HPV infection after LEEP-conization - a retrospective study. Ginekologia Polska (2022) 93(2):99–104. doi: 10.5603/GP.a2021.0164
16. Aguiar TD, Valente RP, Figueiredo AR, Beires JM, Vieira-Baptista P. Risk factors for positive margins in high-grade cervical intraepithelial neoplasia after transformation zone excision. J Low Genit Tract Dis (2022) 26(3):207–11. doi: 10.1097/LGT.0000000000000668
17. Codde E, Munro A, Stewart C, Spilsbury K, Bowen S, Codde J, et al. Risk of persistent or recurrent cervical neoplasia in patients with 'pure' adenocarcinoma-in-situ (AIS) or mixed AIS and high-grade cervical squamous neoplasia (cervical intra-epithelial neoplasia grades 2 and 3 (CIN 2/3)): a population-based study. BJOG (2018) 125(1):74–9. doi: 10.1111/1471-0528.14808
18. Cong Q, Song Y, Wang Q, Zhang H, Gao S, Du M, et al. A Large retrospective study of 12714 cases of LEEP conization focusing on cervical cancer that colposcopy-directed biopsy failed to detect. BioMed Res Int (2018) 2018:5138232. doi: 10.1155/2018/5138232
19. Xiang L, Li J, Yang W, Xu X, Wang H, Li Z, et al. Conization using an electrosurgical knife for cervical intraepithelial neoplasia and microinvasive carcinoma. PloS One (2015) 10(7):e131790. doi: 10.1371/journal.pone.0131790
20. Nikolopoulos M, Athanasias P, Godfrey M, Nikolopoulos K, Maheshwari MK. Cervical glandular neoplasia referrals and the diagnosis of adenocarcinoma in situ: correlating cytology, colposcopy findings, and clinical outcomes. Cytopathology (2021) 32(6):751–7. doi: 10.1111/cyt.13027
21. Teoh D, Musa F, Salani R, Huh W, Jimenez E. Diagnosis and management of adenocarcinoma in situ: a society of gynecologic oncology evidence-based review and recommendations. Obstetrics gynecology (2020) 135(4):869–78. doi: 10.1097/AOG.0000000000003761
Keywords: LEEP, conization margins, length, transformation zone, cervical intraepithelial neoplasia
Citation: Cong Q, Yu Y, Xie Y, Li Y and Sui L (2023) Risk factors of LEEP margin positivity and optimal length of cervical conization in cervical intraepithelial neoplasia. Front. Oncol. 13:1209811. doi: 10.3389/fonc.2023.1209811
Received: 21 April 2023; Accepted: 05 June 2023;
Published: 22 June 2023.
Edited by:
Mwansa Ketty Lubeya, University of Zambia, ZambiaReviewed by:
Mwate Joseph Chaila, John Snow Health Zambia Limited, ZambiaCopyright © 2023 Cong, Yu, Xie, Li and Sui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Sui, c3VpbG9uZ0BmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.