
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 23 June 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1208028
Parts of this article's content have been modified or rectified in:
Erratum: Characterization and outcome of post-transplant lymphoproliferative disorders within a collaborative study
 Philipp Lückemeier1
Philipp Lückemeier1 Aleksandar Radujkovic2
Aleksandar Radujkovic2 Udo Holtick3
Udo Holtick3 Lars Kurch4
Lars Kurch4 Astrid Monecke5
Astrid Monecke5 Uwe Platzbecker1
Uwe Platzbecker1 Marco Herling1
Marco Herling1 Sabine Kayser1,6,7*
Sabine Kayser1,6,7*Background: Post-transplant lymphoproliferative disorders (PTLD) are heterogeneous lymphoid disorders ranging from indolent polyclonal proliferations to aggressive lymphomas that can arise after solid organ transplantation (SOT) and allogeneic hematopoietic transplantation (allo-HSCT).
Methods: In this multi-center retrospective study, we compare patient characteristics, therapies, and outcomes of PTLD after allo-HSCT and SOT. Twenty-five patients (15 after allo-HSCT and 10 after SOT) were identified who developed PTLD between 2008 and 2022.
Results: Median age (57 years; range, 29-74 years) and baseline characteristics were comparable between the two groups (allo-HSCT vs SOT), but median onset of PTLD was markedly shorter after allo-HSCT (2 months vs. 99 months, P<0.001). Treatment regimens were heterogeneous, with reduction of immunosuppression in combination with rituximab being the most common first-line treatment strategy in both cohorts (allo-HSCT: 66%; SOT: 80%). The overall response rate was lower in the allo-HSCT (67%) as compared to the SOT group (100%). Consequently, the overall survival (OS) trended towards a worse outcome for the allo-HSCT group (1-year OS: 54% vs. 78%; P=0.58). We identified PTLD onset ≤150 days in the allo-HSCT (P=0.046) and ECOG >2 in the SOT group (P=0.03) as prognostic factors for lower OS.
Conclusion: PTLD cases present heterogeneously and pose unique challenges after both types of allogeneic transplantation.
Post-transplant lymphoproliferative disorders (PTLDs) are a heterogeneous group of lymphoid or plasmocytic proliferations occurring after transplantation. The new World Health Organization classification of hematolymphoid tumors categorizes them as any hyperplasia or lymphoma arising in the immune deficiency/dysregulation setting post-transplantation (1). They are associated with iatrogenic immunosuppression and Epstein-Barr virus (EBV) in recipients of solid-organ transplant (SOT) or hematopoietic stem cell transplantation (allo-HSCT) (2). EBV-association is particularly pronounced in PTLD after allo-HSCT (~83%) as compared to cases after SOT (~33%) (3), often coincides with rapidly rising EBV DNAemia and mostly occurs within 100 days after allo-HSCT (4). While PTLDs are rare with an incidence of 1%-3.2% in the allo-HSCT setting, their incidence has been increasing alongside the number of transplantations, unrelated or HLA-mismatched related donors, selective T-cell depletion, antithymocyte globulin use and donor as well as recipient age (4–7). The risk of PTLD increases in patients with two or more risk factors (8). Thus, prospective monitoring of EBV activation and early treatment intervention seems to be reasonable in patients with elevated risk of PTLD after allo-HSCT. Clinically, extra-nodal disease is common, including 10%-15% presenting with central nervous system (CNS) disease (9). Despite improvements in treatment, PTLD remains one of the most life-threatening complications of allo-HSCT with an overall survival (OS) rate of ~50% after 6 years and a PTLD-related mortality rate of ~30% in the first year after diagnosis (3, 4). Standard risk-stratified therapy in patients with PTLDs after SOT consists of rituximab with or without chemotherapy (10, 11). Non-destructive PTLDs, also termed early lesions, tend to regress with reduction of immunosuppression (RIS) (12). Their prognosis seems to be excellent, if immune suppression reduction can be achieved without graft rejection. However, elevated lactate dehydrogenase, organ dysfunction, multi-organ involvement, advanced stage, bulky disease, and older age have been reported to be associated with a lack of response to decreased immune suppression (13, 14).
To address the need for standardization in patients with PTLDs after allo-HSCT, the Sixth European Conference on Infections in Leukemia (ECIL-6) published guidelines for the management of these patients (15). However, randomized controlled trials are sparse and treatment strategies remain to be standardized.
The aim of this retrospective study from three academic centers was to evaluate the characteristics and outcomes of PTLD in adult patients after allo-HSCT in comparison to PTLD after SOT.
Information on 25 adult patients with PTLD diagnosed between 2008 and 2022 (2008-2015, n=8; after 2015, n=17) was collected within a multicenter cohort (University Hospital of Leipzig, n=18; University Hospital Heidelberg, n=5; University Hospital Cologne, n=2). To identify patients, keyword searches including ‘PTLD’, ‘post-transplant’, and ‘lymphoproliferative’ were performed on all doctor’s letters and coded diagnoses of patients that visited the centers since 2008. Detailed case report forms (including information on baseline characteristics, chemotherapy, allo-HSCT, response, and survival) were collected from all participating centers. Inclusion criteria were adult patients with a confirmed diagnosis of PTLD following allo-HSCT or SOT. All patients who fulfilled these criteria were included by the participating groups/institutions, respectively.
Morphologic response was routinely assessed by computed tomography (CT) scans or magnetic resonance imaging (MRI). Disappearance of lymphoma lesions is termed as complete response (CR) and regression of at least 50% as partial response (PR). Metabolic imaging by positron emission tomography (PET) using the radiotracer 18-fluorodeoxyglucose (18F-FDG) was capable of further distinguishing residual tumor tissue based on metabolic response. The Deauville score (DS) was used to graduate metabolic response in interim and end-of-treatment 18F-FDG PET scans (16).
Survival endpoints including overall survival (OS) and relapse-free survival (RFS) were defined according to the revised recommendations of the International Harmonization Project in Lymphoma and the 2014 Lugano classification (16, 17). Comparisons of characteristics of patients with PTLD after allo-HSCT as compared to those after SOT were performed with the t-test for continuous variables (age), the Mann-Whitney test (e.g. for ECOG) and Fisher’s exact test (for cause of transplantation, sex) for categorical variables. Log-rank tests were employed to compare survival curves between groups. Median follow-up was calculated using the reverse Kaplan-Meier method. To identify prognostic variables with respect to outcome, a univariate analysis of preselected factors was performed. Variables included age, ECOG, IPI, and PTLD onset. All statistical analyses were performed with GraphPad Prism 7.
Overall demographic and clinical data were collected from 25 patients diagnosed with PTLD between 2008 and 2022. Of those, 15 patients were diagnosed with PTLD after allo-HSCT and 10 patients with PTLD after SOT (6 liver, 3 kidney, 1 kidney and pancreas). There was no statistical difference in median age between the two groups (Table 1; P=0.70). In the allo-HSCT group, 11 of 15 (73%) patients were male as compared to 6 of 10 (60%) patients in the SOT group (P=0.67; Table 1). Ten (67%) patients in the allo-HSCT group were transplanted due to a hematological malignancy, whereas only one patient (10%) in the SOT group received a transplant due to a malignancy (hepato-cellular carcinoma; P=0.012). EBV association was detected in 13 of 15 (87%) patients in the allo-HSCT group and in 4 of 10 (40%) patients in the SOT group (P=0.03). Of the 14 allo-HSCT patients still receiving immunosuppressive agents at PTLD onset, 10 (71%) received cyclosporine, three (21%) received tacrolimus, and two (14%) received mycophenolic acid (one patient in conjunction with cyclosporine, ruxolitinib, and methylprednisolone). In the SOT group, all patients received immunosuppressive agents at PTLD onset: 8 (80%) patients received tacrolimus and the two other patients (20%) received cyclosporine. Moreover, 8 (80%) SOT patients additionally received mycophenolic acid and one SOT patient (10%) additionally received everolimus. Further baseline characteristics are summarized in Table 1.
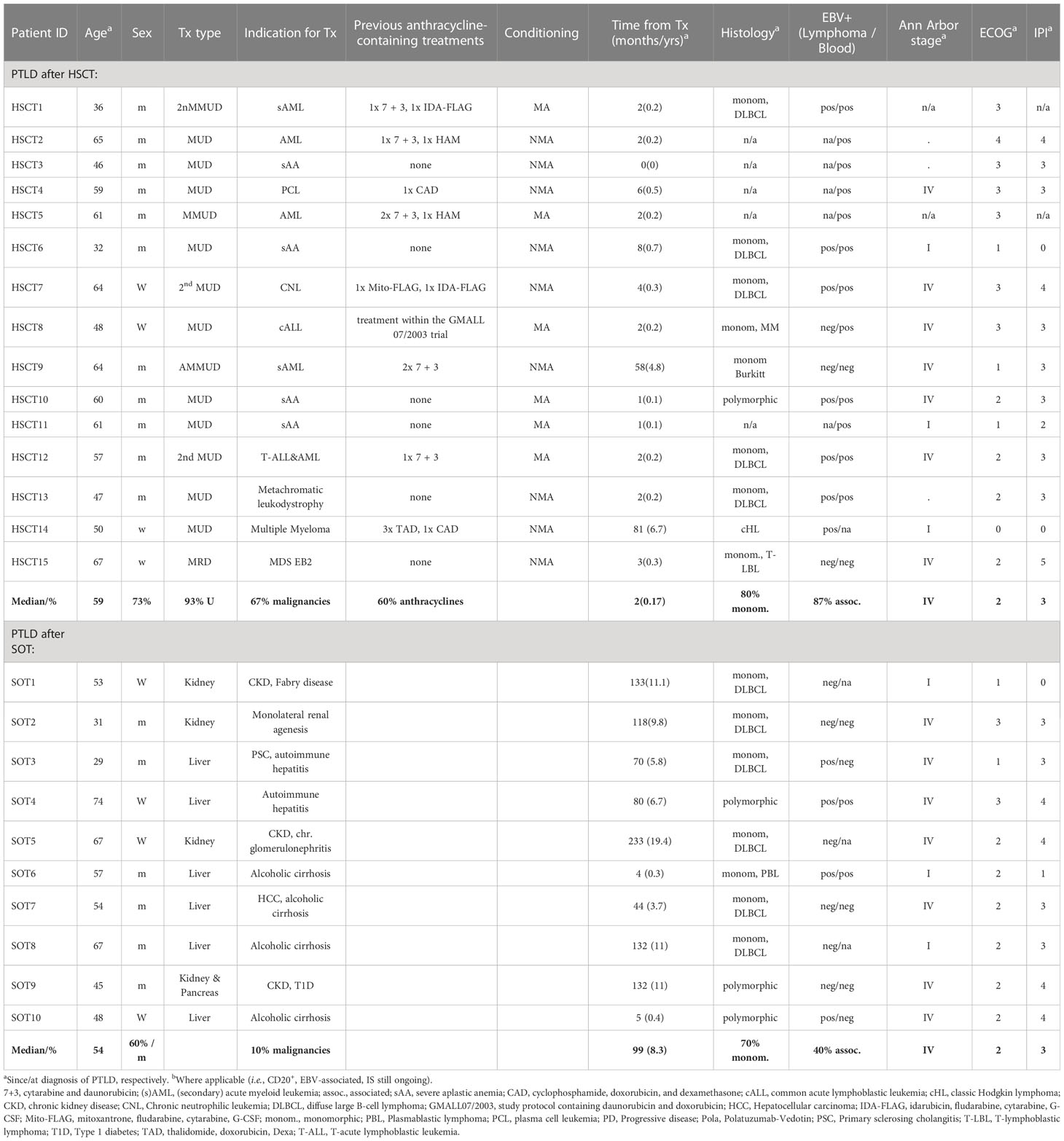
Table 1 Baseline characteristics of patients with post-transplant lymphoproliferative disorders (PTLD) at onset of PTLD.
Nine of ten (90%, 60% of the total allo-HSCT group) patients with malignancies in the allo-HSCT group had received an anthracycline-containing chemotherapy prior to transplantation as compared to none in the SOT group. In the allo-HSCT group, six (40%) patients received myeloablative (MA) and nine (60%) patients received non-myeloablative (NMA) conditioning regimens.
In five of 15 (33%) patients of the allo-HSCT group, PTLD was diagnosed merely by EBV transcripts in peripheral blood (PB) and concomitant lymphadenopathy. Histological confirmation of PTLD was omitted in these cases due to a poor performance status [ECOG ≥3 in patients HSCT2-5 (Table 1)] or difficult accessibility of the lesion [hilar lymphadenopathy, patient HSCT11 (Table 1)]. All PTLD cases in the SOT group were histologically confirmed (18). The predominant classification of PTLD in both groups was monomorphic PTLD (allo-HSCT group, n=8/10, 80%; SOT group, n=7/10, 70%), one case of classic Hodgkin-Lymphoma like PTLD was identified (allo-HSCT group, n=1; 10%); all others had a polymorphic PTLD (allo-HSCT, n=1, 10%; SOT, n=3; 30%).
In the SOT group, 4 of 10 (40%) PTLD patients showed EBV-positivity in the lymphoma tissue. Importantly, of those, only two (50%) had detectable levels of EBV in PB. In the allo-HSCT group, 13 of 15 (87%) cases were EBV-associated either by virtue of EBV-positivity in the lymphoma (70%, n=7/10 analyzable patients), or EBV-positivity in PB (86%, n=12/14 analyzable patients). Involvement of the CNS was diagnosed in three of 25 patients (allo-HSCT, n=2; 13%; SOT, n=1; 10%).
There was a strong difference in the latency period from transplantation to the occurrence of PTLD between the allo-HSCT group (median, 2 months; range, 0-81 months) as compared to the SOT group (median, 99 months; range, 4-233 months; P<0.001; Figure 1). In the allo-HSCT group, PTLD onset was strongly associated with ongoing immunosuppression: only one patient (HSCT14; Table 1) did not receive immunosuppressive therapy at the time of PTLD diagnosis. The second patient in the allo-HSCT group diagnosed >10 months after transplantation (HSCT9; Table 1) received long-term immunosuppressive therapy due to chronic graft-versus-host disease (GvHD).
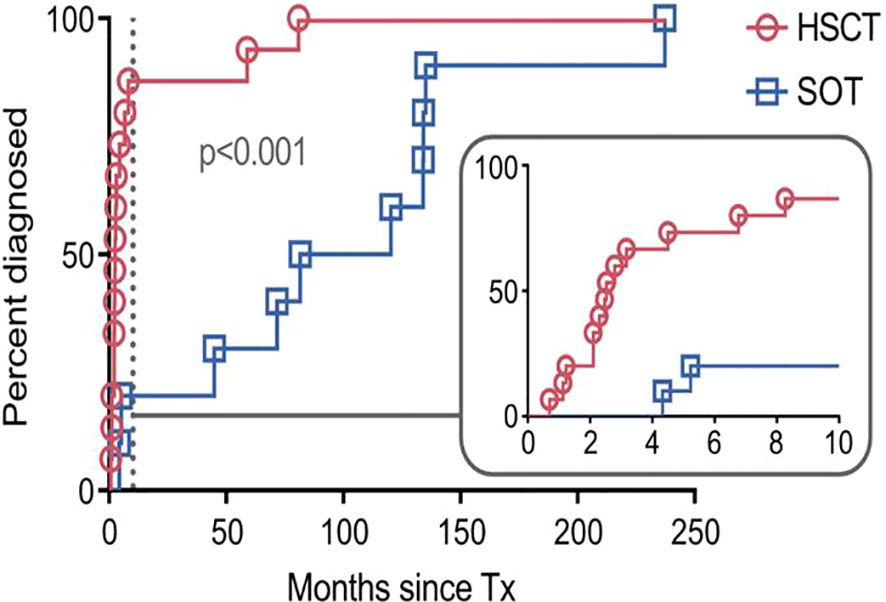
Figure 1 Time to diagnosis of post-transplant lymphoproliferative disorder (PTLD) after transplantation. Each symbol represents a PTLD diagnosis on a given day. The inset shows the same data up until month 10 (marked by dashed line) on an enlarged x-axis. SOT, recipients of solid organ transplantation; HSCT, recipients of hematopoietic stem cell transplantation; Tx, transplantation.
In our cohort, ECOG-PS did not differ between the allo-HSCT and the SOT group (P=0.58; Table 1). In addition, Ann Arbor stages as well as the international prognostic index (IPI) of PTLD at diagnosis were similar between the two groups (Table 1).
The most common first-line therapy was a switch in immunosuppression from cyclosporine A or tacrolimus to sirolimus or a rapid reduction of immunosuppression (both abbreviated ‘RIS’) in combination with rituximab +/- additional agents (RIS+R+X; Table 2). In the allo-HSCT group, 10 patients (66% of total; 77% of patients with CD20+ PTLD) received this form of first-line therapy. Of those, six (60%) responded with a CR and one (10%) patient responded with a partial response (PR). In the SOT group, eight patients (80%) received first-line RIS+R+X and 6 of them (75%) responded with a CR, while two patients (25%) responded with a PR.

Table 2 Treatment and response of patients with post-transplant lymphoproliferative disorders (PTLD) at onset of PTLD.
One patient of the allo-HSCT group (HSCT14) did not receive immunosuppressive therapy at the diagnosis of PTLD. Another patient (HSCT7) had severe chronic GvHD at PTLD onset and did, therefore, not undergo RIS. Two patients of the allo-HSCT group had CD20-negative PTLD subtypes (HSCT14, classic Hodgkin lymphoma [cHL] and HSCT15, T-lymphoblastic lymphoma [T-LBL]) and, therefore, did not receive rituximab. Treatment in the latter two cases consisted of doxorubicin, vinblastine, dacarbazine (AVD) + radiotherapy (RTx) or brentuximab-vedotin (BV) + donor lymphocyte infusions (DLI) + RTx + cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP), respectively.
Furthermore, many patients required chemotherapy. Five of 15 (33%) patients in the allo-HSCT group received anthracycline-containing regimens, of whom two (40%) achieved a CR. In the SOT group, 5 of 10 (50%) patients received anthracyclines of whom three (60%) achieved a CR and two (40%) achieved a PR.
Finally, three of 13 (23%) patients in the allo-HSCT group with EBV detection in their lymphoma and/or PB received virus-specific T cells (VST). All of them are in ongoing CR. None of the patients in the SOT group received VST.
The best response to treatment at any time was aggregated into an overall response rate (ORR; including CRs and PRs). This ORR was 67% (n=10/15) in the allo-HSCT group and 100% (n=10/10) in the SOT group (Figure 2). Of note, all responders in the allo-HSCT group achieved a CR. Eighty percent (n=8/10) of SOT patients achieved a CR and 20% (n=2/10) achieved a PR.
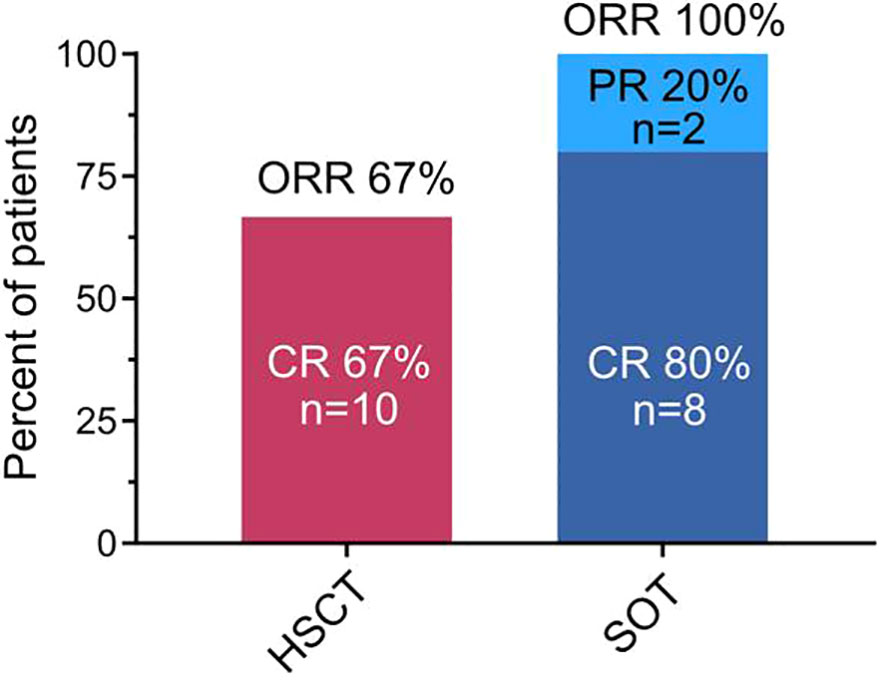
Figure 2 Overall response rate (ORR) as characterized by best response to treatment. Overall response rate included patients with achievement of complete response or partial response to any treatment after the diagnosis of post-transplant lymphoproliferative disorder. Percentages and numbers of patients achieving complete response (CR), partial response (PR), and that of the overall response rate are indicated group-wise. SOT, recipients of solid organ transplantation; HSCT, recipients of hematopoietic stem cell transplantation.
18F-FDG-PET for metabolic response assessment was available in 6 (HSCT9, HSCT12, HSCT14, HSCT15, SOT1, and SOT3) of the 25 patients. Of these, 5 had a complete metabolic response (CMR; Deauville 1-3) and one (HSCT15) had a morphologically stable disease with a partial metabolic response (PMR; Deauville 4-5). Representative 18F-FDG-PET/CT and -MR images of two patients who achieved CMR (Deauville 2) are shown in Figure 3.
Figure 3 Representative fluorodeoxyglucose positron emission tomography–computed tomography and magnetic resonance tomography (18F-FDG PET/CT and MRT) images of two patients who achieved a complete response. Upper panel: (A–F) 18F-FDG PET/CT of patient HSCT12 on the day of diagnosis of post-transplant lymphoproliferative disorder (PTLD). (G) 18F-FDG PET/CT of the same patient 49 days after PTLD diagnosis. The patient had received a 100% reduction of their immunosuppression and 4 cycles of rituximab in the meantime. Lower panel: (A–H) 18F-FDG PET/MRT of patient SOT3 on the day of start of PTLD treatment. (G) 18F-FDG PET/MRT of the same patient 44 days after start of PTLD treatment. The patient had received one cycle of R-CHOP and one cycle of intensive anthracycline-based chemotherapy in the meantime. (H) 8F-FDG PET/MRT of the same patient 151 days after start of PTLD treatment.
Data on response and outcome were available in all patients. Median follow-up after diagnosis of PTLD was 54.7 months (range, 0.5-97.5 months) after allo-HSCT and 33.7 months (range, 6.5-93.0 months) after SOT. Median OS was not reached for allo-HSCT patients and was 85.3 months for SOT patients. The estimated 1-year RFS and OS were 89% (95%-CI, 43-98%) and 54% (95%-CI, 24-77%) in the allo-HSCT and 86% (95%-CI, 33-98%) and 78% (95%-CI, 36-94%) in the SOT cohort, respectively (Figure 4).
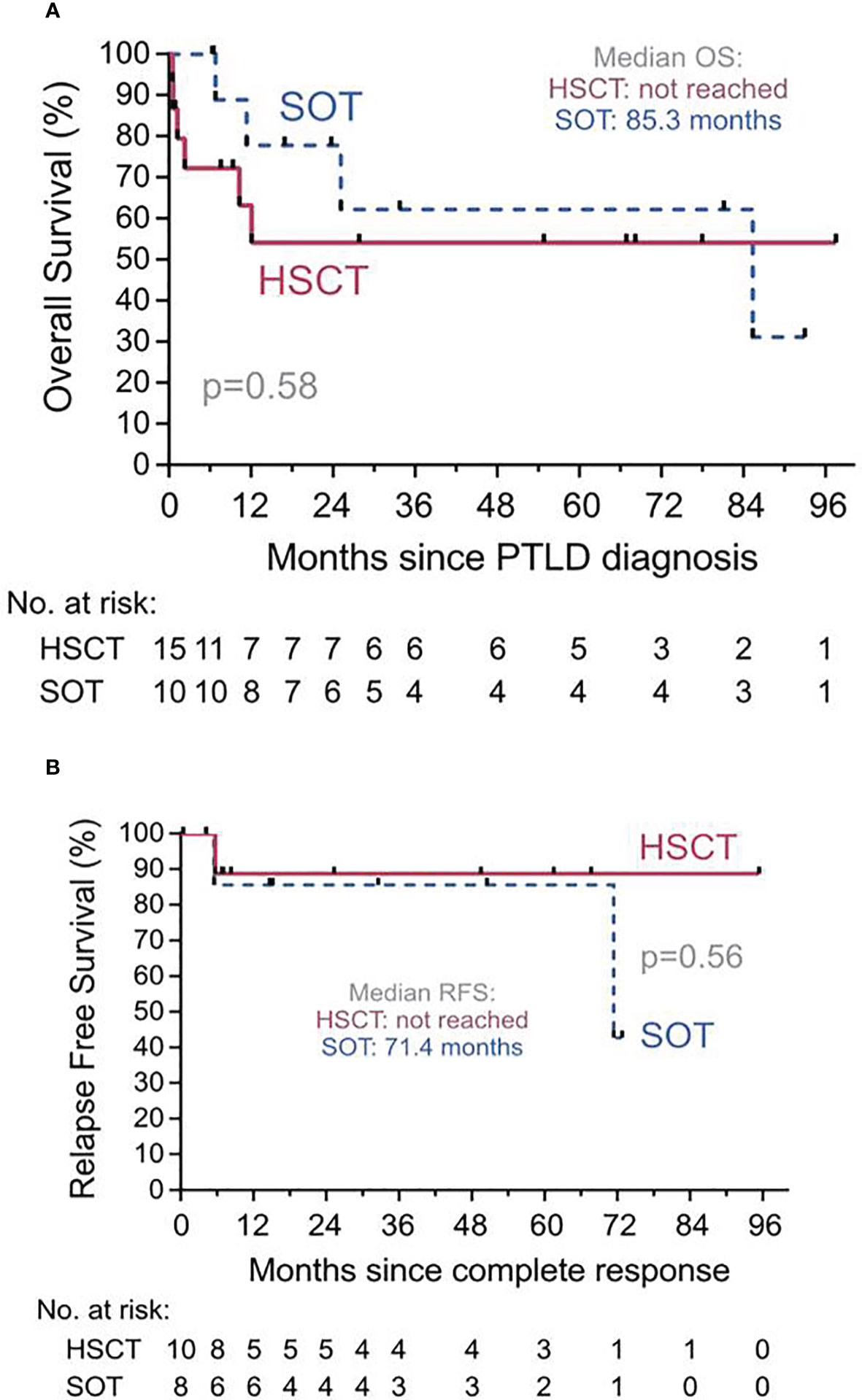
Figure 4 Overall Survival (OS) and Relapse Free Survival (RFS). (A) OS from diagnosis of PTLD for recipients of solid organ transplantation (SOT, dashed line) or of hematopoietic stem cell transplantation (HSCT, solid line). Median OS for allo-HSCT was not reached. PTLD, Post transplant lymphoproliferative disorder. (B) RFS since complete response for recipients of SOT (dashed line) or of allo-HSCT (solid line) who achieved a complete response of their PTLD at any point. Median OS for allo-HSCT was not reached.
One patient in the allo-HSCT and two patients in the SOT group relapsed after achievement of CR of the PTLD, respectively. Six (40%) patients died in the allo-HSCT group, (relapse of PTLD, n=5; VZV-encephalitis n=1) and 4 (40%) patients in the SOT group (relapse of PTLD, n=2; colorectal cancer n=1; sepsis, n=1).
In univariate analysis, time from transplantation to PTLD onset ≤150 days was significantly associated with a lower OS in the allo-HSCT group only (P=0.046). In the SOT group, ECOG-PS >2 was identified as significant prognostic factor of a lower OS (P=0.03). Age and IPI were not significantly associated with OS in either of the groups (Table 3).
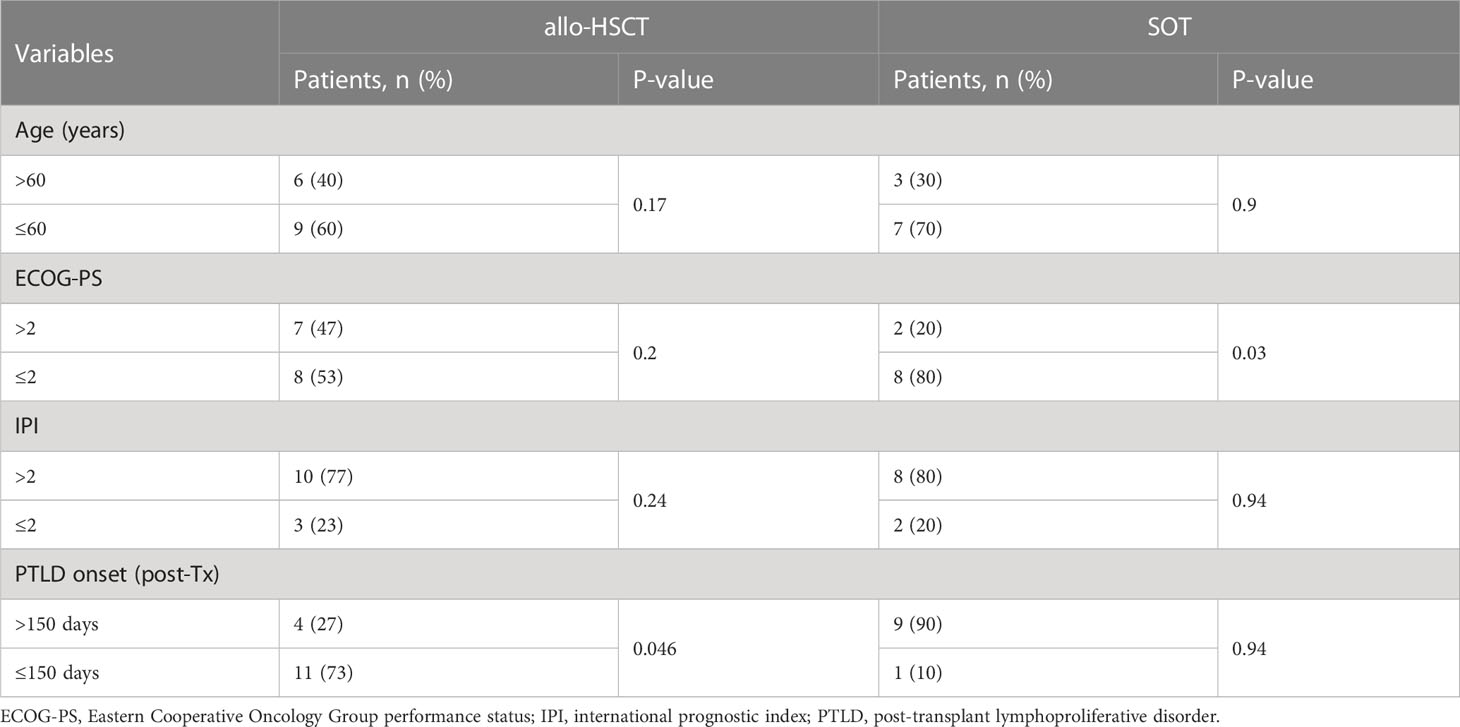
Table 3 Univariate analysis of prognostic factors for overall survival in patients with post-transplant lymphoproliferative disorders after hematopoietic stem cell or solid organ transplantation.
The focus of our study was to characterize adult patients with PTLD after allo-HSCT as well as SOT in a cohort study from three University hospitals and to compare differential outcomes.
Latency from transplantation to the onset of PTLD was shorter in the allo-HSCT group, similar to what is reported in the literature (11, 18). This is likely due to the strong immunosuppressive therapy after allo-HSCT. Both, the extent and duration of the immunosuppressive therapy are main risk factors for development of PTLD (2). This is corroborated by the fact that almost all patients of the allo-HSCT group were still on immunosuppressive therapy at the time of PTLD diagnosis. Tailored maintenance immunosuppressive regimens, however, may help to reduce the risk of PTLD onset (19).
In contrast to other reports (3), we provide comprehensive clinical characteristics including ECOG-PS, stage, and IPI at the time of PTLD diagnosis for allo-HSCT patients. Patient characteristics at time of PTLD diagnosis were comparable between both groups. Main differences were the frequency of malignancies as the indication for the transplantation and, in conjunction with that, the fraction of patients that previously received anthracycline-containing treatments. Surprisingly, this higher burden of pretreatment with chemotherapy and lower latency in the allo-HSCT group did not translate into a lower median ECOG-PS. There was, however, a larger fraction of patients with ECOG-PS >2. An ECOG-PS ≤2 was a prerequisite for inclusion in the phase II PTLD-1 trial (NCT01458548) and the phase II PTLD-2 trial (NCT02042391). These trials exclusively recruited patients with PTLD after SOT and demonstrated that a risk-stratified sequential treatment with rituximab +/- CHOP is safe and effective (10, 11). However, the higher fraction of patients with ECOG-PS >2, the high susceptibility to cytopenias after chemotherapy, and the common previous exposure to anthracyclines warrants caution when transferring the results of the safety of the CHOP regimen from these trials to patients with PTLD after allo-HSCT.
The reported treatment strategies of PTLD in our series involved RIS or IS-switch and rituximab in almost all cases in both groups. RIS is an established approach in PTLD after SOT and has been shown to be an independent prognostic factor in PTLD after allo-HSCT (4). However, RIS after allo-HSCT bears the risk of promoting GvHD and can be prohibitive in cases of coexisting extensive active GvHD, such as in one of our cases (HSCT7). How to balance the need for both decreased IS for the treatment of PTLD and increased IS for treatment of GvHD in such cases remains to be elucidated. The combined immunosuppressive and potential anti-lymphoma effects by mTOR inhibitors (i.e. Sirolimus, Everolimus) are hypothesized, but not systematically validated, rationales.
Fewer patients in the allo-HSCT group received CHOP regimens as compared to the SOT group. The relatively low percentage reflects the high prevalence of prior anthracycline-containing therapies for malignancies in the allo-HSCT group (60%). For example, concerns about the level of cumulative anthracycline doses are documented as reasons to omit them in the treatment of PTLD in HSCT9. Therefore, prior treatment with chemotherapy and cumulative toxicities are a major consideration when choosing PTLD treatments in this group.
EBV-specific T-cell therapy (VST) was used exclusively in allo-HSCT patients. However, adoptive transfer of EBV-specific T cells has shown great promise for the treatment of EBV-viremia and EBV+ PTLD in SOT as well (20, 21). We suspect that, because VST are an established treatment for viral infections after allo-HSCT, physicians were more experienced and inclined to provide VST to allo-HSCT PTLD patients. Moreover, the greater use of VST in the allo-HSCT group was probably due to the markedly higher EBV-association. Other works that defined EBV-positivity as the histological finding of EBV-encoded small RNAs (EBERs) have also found the frequency of EBV-positivity to be ~83% after allo-HSCT (3) and to be ~60% in a SOT dominated cohort (22). In our SOT cohort, only two of four patients with EBER-positive PTLD also had detectable EBV levels in the peripheral blood (PB). Data on the comparative detection of EBV genetic material in PTLD lesions and PB are lacking. Thus, EBER tests should always be performed so as not to overlook EBV-association and miss out on VST treatment options. However, EBV-positive PTLD can also develop in the absence of high viral loads. In addition, high viral loads are not predictive in all settings, and a decline in viral loads may not always predict treatment response. Nevertheless, a rapid decline in EBV load occurs almost invariably after anti-B cell monoclonal antibody treatment, irrespective of long term PTLD response (23). The lack of standardization of techniques and results for assessment of circulating EBV DNA has been an additional complicating factor. Therefore, an international WHO standard for determining EBV load was published in 2013 (23).
With the approval of tabelecleucel for relapsed or refractory EBV-associated PTLD after SOT or allo-HSCT (20), we expect this form of therapy to become more widespread.
Beyond these options, patients in both groups received a wide variety of treatments. These were usually chosen to address the specific subtype of PTLD: two patients (HSCT6 and SOT4) received local therapy and methotrexate for PTLD, while three patients received regimens that were more akin to those developed for the non-PTLD entity counterparts (HSCT9 with ‘GMALL-B-NHL 2002’ for Burkitt lymphoma, HSCT14 with AVD + RTx for cHL, HSCT15 with BV for T-cell PTLD). This aspect is often underreported in PTLD studies. PTLD subtypes themselves appear to be prognostic factors for OS (24–26). However, the heterogeneity in treatments even within PTLD subtypes hampers the identification of prognostic factors and optimal treatment algorithms from retrospective analyses. In addition, there is a concern that chemotherapy might result in relapses of original hematological malignancies or graft rejection. Therefore, more prospective studies are warranted towards a PTLD-specific prognostic model.
In our cohort, the ORR was lower in the allo-HSCT group. Consequently, the OS trended towards an inferior outcome for this group. However, a substantial fraction of patients in the SOT group eventually experienced progressive PTLD. Nevertheless, our data compare favorably with other published analyses (11, 27, 28). In the allo-HSCT group, time from transplantation ≤150 days was identified as a prognostic factor for lower OS in a univariate analysis. PTLD onset as a prognostic factor was observed to varying degrees in previous works (4, 10). One study by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation found a trend for lower OS for PTLD onset <100 days after allo-HSCT (4) and another found that later onset of PTLD after SOT had a longer time to progression (10). In our analysis on the total PTLD cohort, neither age, ECOG-PS or IPI were associated with lower OS. In the SOT group, however, ECOG-PS > 2 was significantly associated with lower OS. The low patient numbers of our cohort were prohibitive of a multivariable analysis and warrant caution when interpreting the significance of the identified prognosticators.
Only a very small number of studies directly compared cases of PTLD after allo-HSCT with that after SOT (29–32). These found a later onset, a higher ORR to treatment as well as a trend for a longer OS in the SOT group as compared to the allo-HSCT group. In contrast, the relapse rate was higher in the SOT as compared to the allo-HSCT group (24, 25, 27, 28). However, in most of these studies treatment is not comprehensively reported or patients from the pre-rituximab era are included. Particularly, in these series rituximab was given to only 36% (n=10) of PTLD patients after SOT and 38% (n=5) of PTLD patients after allo-HSCT (29, 31). This presumably influenced the rather poor median OS in the allo-HSCT groups of <12 months in the majority of studies. Therefore, our study provides important data on more recently treated PTLD patients.
Limitations of this study included its retrospective nature, the small size of the cohorts, and the low number of patients who received a PET-based response assessment. Recently, novel insights into pathogenesis (33) and treatment strategies for PTLD have emerged, including adoptive immunotherapy and targeted therapeutics, such as tabelecleucel, CAR-T cells, and CD30-based therapy (34), that could substantially improve outcomes.
In the near future, data from the phase-3 ALLELE study (NCT03394365) testing tabelecleucel vs. placebo in patients with PTLD after allo-HSCT or SOT might yield more insight into differential response patterns in a relapsed or refractory setting.
In conclusion, we show that PTLD after allo-HSCT has an earlier onset, a lower ORR, and tends to have a worse outcome when compared to PTLD after SOT in a multicenter cohort. We highlight that GvHD and pre-exposure to anthracyclines are important considerations in the treatment of PTLD after allo-HSCT and describe existing treatment heterogeneities. Refined prognostic models are needed. Finally, novel therapies for PTLD are being approved and new studies are warranted to analyze their safety and efficacy in a real-world setting.
The datasets presented in this article are not readily available because The raw data supporting the conclusions of this article are restricted in accordance with the local legislation. Requests to access the datasets should be directed t\o cy5rYXlzZXJAZGtmei1oZWlkZWxiZXJnLmRl.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
PL and SK were responsible for the concept of this paper, contributed to the literature search and data collection, analysed and interpreted data, and wrote the manuscript. PL, AR, UH, MH, UP and SK contributed patients and critically revised the manuscript. AM and LK performed research and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors acknowledge support from the University of Leipzig within the program of Open Access Publishing.
UH received consulting fees or honoraria from Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
2. Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med (2018) 378:549–62. doi: 10.1056/NEJMra1702693
3. Naik S, Riches M, Hari P, Kim S, Chen M, Bachier C, et al. Survival outcomes of allogeneic hematopoietic cell transplants with EBV-positive or EBV-negative post-transplant lymphoproliferative disorder, a CIBMTR study. Transpl Infect Dis (2019) 21:e13145. doi: 10.1111/tid.13145
4. Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the infectious diseases working party of the European group for blood and marrow transplantation. Clin Infect Dis (2013) 57:794–802. doi: 10.1093/cid/cit391
5. Kalra A, Roessner C, Jupp J, Williamson T, Tellier R, Chaudhry A, et al. Risk factors for post-transplant lymphoproliferative disorder after thymoglobulin-conditioned hematopoietic cell transplantation. Clin Transplant (2018) 32(1):e13150. doi: 10.1111/ctr.13150
6. Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood (2009) 113:4992–5001. doi: 10.1182/blood-2008-09-178046
7. Gao X-N, Lin J, Wang L-J, Li F, Li H-H, Wang S-H, et al. Risk factors and clinical outcomes of Epstein-Barr virus DNAemia and post-transplant lymphoproliferative disorders after haploidentical and matched-sibling PBSCT in patients with hematologic malignancies. Ann Hematol (2019) 98:2163–77. doi: 10.1007/s00277-019-03742-7
8. Fujimoto A, Hiramoto N, Yamasaki S, Inamoto Y, Uchida N, Maeda T, et al. Risk factors and predictive scoring system for post-transplant lymphoproliferative disorder after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2019) 25:1441–9. doi: 10.1016/j.bbmt.2019.02.016
9. Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep (2013) 8:173–83. doi: 10.1007/s11899-013-0162-5
10. Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult b-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol (2012) 13:196–206. doi: 10.1016/S1470-2045(11)70300-X
11. Zimmermann H, Koenecke C, Dreyling MH, Pott C, Dührsen U, Hahn D, et al. Modified risk-stratified sequential treatment (subcutaneous rituximab with or without chemotherapy) in b-cell post-transplant lymphoproliferative disorder (PTLD) after solid organ transplantation (SOT): the prospective multicentre phase II PTLD-2 trial. Leukemia (2022) 11:2468–78. doi: 10.1038/s41375-022-01667-1
12. Nelson BP, Wolniak KL, Evens A, Chenn A, Maddalozzo J, Proytcheva M. Early posttransplant lymphoproliferative disease: clinicopathologic features and correlation with mTOR signaling pathway activation. Am J Clin Pathol (2012) 138:568–78. doi: 10.1309/AJCPQYYE04AVGVYI
13. Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation (2001) 71:1076–88. doi: 10.1097/00007890-200104270-00012
14. Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transplant (2011) 11:336–47. doi: 10.1111/j.1600-6143.2010.03387.x
15. Styczynski J, van der Velden W, Fox CP, Engelhard D, La Camara Rd, Cordonnier C, et al. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European conference on infections in leukemia (ECIL-6) guidelines. Haematologica (2016) 101:803–11. doi: 10.3324/haematol.2016.144428
16. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol (2014) 32:3059–68. doi: 10.1200/JCO.2013.54.8800
17. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol (2007) 25:571–8. doi: 10.1200/JCO.2006.08.2305
18. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer (2017). p. 585.
19. Mucha K, Staros R, Foroncewicz B, Ziarkiewicz-Wróblewska B, Kosieradzki M, Nazarewski S, et al. Comparison of post-transplantation lymphoproliferative disorder risk and prognostic factors between kidney and liver transplant recipients. Cancers (Basel) (2022) 14(8):1953. doi: 10.3390/cancers14081953
20. Prockop S, Mahadeo KM, Beitinjaneh A, Choquet S, Stiff P, Reshef R, et al. Multicenter, open-label, phase 3 study of tabelecleucel for solid organ or allogeneic hematopoietic cell transplant recipients with Epstein-Barr virus-driven post transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE). Blood (2021) 138:301. doi: 10.1182/blood-2021-147274
21. Jiang W, Clancy LE, Avdic S, Sutrave G, Street J, Simms R, et al. Third-party CMV- and EBV-specific T-cells for first viral reactivation after allogeneic stem cell transplant. Blood Adv (2022) 6:4949–66. doi: 10.1182/bloodadvances.2022007103
22. Vergote VK, Deroose CM, Fieuws S, Laleman W, Sprangers B, Uyttebroeck A, et al. Characteristics and outcome of post-transplant lymphoproliferative disorders after solid organ transplantation: a single center experience of 196 patients over 30 years. Transpl Int (2022) 35:10707. doi: 10.3389/ti.2022.10707
23. Ruf S, Wagner HJ. Determining EBV load: current best practice and future requirements. Expert Rev Clin Immunol (2013) 9(2):139–51. doi: 10.1586/eci.12.111
24. Koff JL, Li J-X, Zhang X, Switchenko JM, Flowers CR, Waller EK. Impact of the posttransplant lymphoproliferative disorder subtype on survival. Cancer (2018) 124:2327–36. doi: 10.1002/cncr.31339
25. Pearse WB, Vakkalagadda CV, Helenowski I, Winter JN, Gordon LI, Karmali R, et al. Prognosis and outcomes of patients with post-transplant lymphoproliferative disorder: a single center retrospective review. Blood (2020) 136:9–10. doi: 10.1182/blood-2020-141286
26. Liu Y, Wang BC, Zuppan CW, Chau P, Fitts J, Chinnock R, et al. Relationship of post-transplant lymphoproliferative disorders (PTLD) subtypes and clinical outcome in pediatric heart transplant recipients: a retrospective single institutional Analysis/Experience of 558 patients. Cancers (Basel) (2023) 15(3):976. doi: 10.3390/cancers15030976
27. García-Cadenas I, Yáñez L, Jarque I, Martino R, Pérez-Simón JA, Valcárcel D, et al. Frequency, characteristics, and outcome of PTLD after allo-SCT: a multicenter study from the Spanish group of blood and marrow transplantation (GETH). Eur J Haematol (2019) 102:465–71. doi: 10.1111/ejh.13226
28. Zhu C-Y, Zhao S-S, Wang X-K, Wang L, Wang F-Y, Fang S, et al. Outcome of rituximab-based treatment for post-transplant lymphoproliferative disorder after allogeneic hematopoietic stem cell transplantation: a single-center experience. Ann Transplant (2019) 24:175–84. doi: 10.12659/AOT.914101
29. Romero S, Montoro J, Guinot M, Almenar L, Andreu R, Balaguer A, et al. Post-transplant lymphoproliferative disorders after solid organ and hematopoietic stem cell transplantation. Leuk Lymphoma (2019) 60:142–50. doi: 10.1080/10428194.2018.1474462
30. Tai R, Tirumani SH, Tirumani H, Shinagare AB, Hornick JL, Ramaiya NH. Is there a difference in post-transplant lymphoproliferative disorder in adults after solid organ and haematologic stem cell transplantation? experience in 41 patients. Br J Radiol (2015) 88:20140861. doi: 10.1259/bjr.20140861
31. Yoon J-H, Lee S, Kim H-J, Lee J-W, Min W-S, Chung BH, et al. Comparative analysis of post-transplant lymphoproliferative disorder after kidney transplantation versus hematopoietic stem cell transplantation. Transpl Int (2014) 27:721–32. doi: 10.1111/tri.12328
32. Yoon SO, Yu E, Cho YM, Suh C, Kim KM, Han DJ, et al. Post-transplant lymphoproliferative disorders: clinicopathological analysis of 43 cases in a single center, 1990-2009. Clin Transplant (2012) 26:67–73. doi: 10.1111/j.1399-0012.2010.01392.x
33. Butzmann A, Sridhar K, Jangam D, Song H, Singh A, Kumar J, et al. Mutations in JAK/STAT and NOTCH1 genes are enriched in post-transplant lymphoproliferative disorders. Front Oncol (2021) 11:790481. doi: 10.3389/fonc.2021.790481
Keywords: PTLD, Epstein - Barr virus, allogeneic hematopoietic stem cell transplantation, solid organ transplantation, outcome
Citation: Lückemeier P, Radujkovic A, Holtick U, Kurch L, Monecke A, Platzbecker U, Herling M and Kayser S (2023) Characterization and outcome of post-transplant lymphoproliferative disorders within a collaborative study. Front. Oncol. 13:1208028. doi: 10.3389/fonc.2023.1208028
Received: 18 April 2023; Accepted: 05 June 2023;
Published: 23 June 2023.
Edited by:
Onder Alpdogan, Thomas Jefferson University, United StatesReviewed by:
Takero Shindo, Kyoto University Hospital, JapanCopyright © 2023 Lückemeier, Radujkovic, Holtick, Kurch, Monecke, Platzbecker, Herling and Kayser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabine Kayser, cy5rYXlzZXJAZGtmei1oZWlkZWxiZXJnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.