
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 July 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1206059
 Michael Schmutzer1*
Michael Schmutzer1* Benjamin Skrap1
Benjamin Skrap1 Jun Thorsteinsdottir1
Jun Thorsteinsdottir1 Christoph Fürweger2
Christoph Fürweger2 Alexander Muacevic2
Alexander Muacevic2 Christian Schichor1
Christian Schichor1Objective: Treatment for meningiomas involving the superior sagittal sinus (SSS) is challenging and proved to be associated with higher risks compared to other brain locations. Therapeutical strategies may be either microsurgical (sub-)total resection or adjuvant radiation, or a combination of both. Thrombosis or SSS occlusion following resection or radiosurgery needs to be further elucidated to assess whether single or combined treatment is superior. We here present tumor control and side effect data of robotic radiosurgery (RRS) in combination with or without microsurgery.
Methods: From our prospective database, we identified 137 patients with WHO grade I meningioma involving the SSS consecutively treated between 2005 and 2020. Treatment decisions were interdisciplinary. Patients underwent RRS as initial/solitary treatment (group 1), as adjuvant treatment after subtotal resection (group 2), or due to recurrent tumor growth after preceding microsurgery (group 3). Positive tumor response was assessed by MRI and defined as reduction of more than 50% of volume. Study endpoints were time to recurrence (TTR), time to RRS, risk factors for decreased survival, and side effects. Overall and specific recurrence rates for treatment groups were analyzed. Side effect data included therapy-related morbidity during follow-up (FU).
Results: A total of 137 patients (median age, 58.3 years) with SSS meningiomas WHO grade I were analyzed: 51 patients (37.2%) in group 1, 15 patients (11.0%) in group 2, and 71 patients (51.8%) in group 3. Positive MR (morphological response) to therapy was achieved in 50 patients (36.4%), no response was observed in 25 patients (18.2%), and radiological tumor progression was detected in 8 patients (5.8%). Overall 5-year probability of tumor recurrence was 15.8% (median TTR, 41.6 months). Five-year probabilities of recurrence were 0%, 8.3.%, and 21.5% for groups 1–3 (p = 0.06). In multivariate analysis, tumor volume was significantly associated with extent of SSS occlusion (p = 0.026) and sex (p = 0.011). Tumor volume significantly correlated with TTR (p = 0.0046). Acute sinus venous thrombosis or venous congestion-associated bleedings did not occur in any of the groups.
Conclusion: RRS for grade I meningiomas with SSS involvement represents a good option as first-line treatment, occasionally also in recurrent and adjuvant scenarios as part of a multimodal treatment strategy.
Meningiomas are tumors of the arachnoid cap cells and contribute to nearly 14%–19% of all primary intracranial tumors (1). The majority of meningiomas is located supratentorially at the convexity (35%), parasagittally (20%), the sphenoid ridge (20%), intraventricularly (5%), and the tuberculum sellae (5%), whereas about 15% are found infratentorially (2, 3). Meningiomas are divided into three histological grades according to the World Health Organization (WHO) 2016 classification (4).
Since the seminal work of Simpson (5), a complete surgical resection, including the dural attachments whenever possible, has been considered to be the best treatment in regard to local disease control.
However, complete resection of meningiomas involving the major venous sinuses, e.g., the superior sagittal sinus (SSS), is challenging. Discontinuity of the venous drainage of the brain (due to tumor growth, surgical maneuvers, or radiation) may lead to venous congestion, resulting in severe neurological deficits (6–9). The Sindou classification (Table 1) was introduced to categorize the relationship between the tumor and the SSS and possibly to guide the surgical strategy (6–9). In the attempt to lower morbidity, alternative treatment strategies such as radiosurgery either alone or combined with a subtotal microsurgical resection have been proposed (10–14).
Data comparing radiosurgical treatment outcomes in case of solely stereotactic radiosurgery or in combination with microsurgical resection in this particular location are scarce and often combine multiple histological grades (15).
Therefore, the aim of this study was to evaluate tumor control rates and neurological outcome in the so far largest homogeneous cohort of radiosurgically treated grade I meningiomas involving the SSS. We also aimed to evaluate the role of RRS as a primary therapy or as an adjunct after initial partial resection either in an adjuvant or salvage role.
From our prospective database, all patients treated for a meningioma between 2005 and 2020 involving the superior sagittal sinus were screened. For each patient, clinical charts, treatment data, follow-up notes, and neuroimaging studies were reviewed.
Therapy decisions were interdisciplinary in all cases. For patients undergoing primary radiosurgery, MR morphological aspects (16) that highly suggest a grade I meningioma, such as homogeneous contrast enhancement, smooth margins to surrounding brain tissue, and no hints of invasive growth patterns, needed to be fulfilled.
Indications for primary RRS were in line with the EANO guidelines for meningioma treatment: patient’s preference, radiological tumor growth/progression, and mild neurological symptoms (16). For the final analysis, histologically proven grade II and III meningiomas were excluded. Indications for RRS were also traced and patients were divided into three groups according to the treatment sequence:
Primary radiosurgery without prior surgery
Adjuvant radiosurgery after subtotal microsurgical resection (without signs of progression)
Salvage radiosurgery due to radiological progression after subtotal microsurgical resection
The Cyberknife (Accuray Inc., Sunnyvale, CA, USA) used for RRS is a frameless, image-guided robotic system. The therapeutic radiation (photon) beam is generated by a 6-MV compact linear accelerator mounted on a six-axis robotic manipulator. In a typical RRS treatment, 100–200 non-isocentric, non-coplanar beams are used for radiosurgery. Intra-fraction patient motion is compensated by the automatic adaptation of beam directions based on stereoscopic X-ray images of the patient’s skull acquired periodically during treatment. Radiosurgical treatment data were reviewed, particularly the irradiated tumor volume and radiation doses. Radiation-induced complications such as edema or necrosis were also documented.
FU examinations [neurological as well as magnetic resonance imaging (MRI)] were performed after 6 months, every year for 2 years, and every 2 years thereafter.
All pre- and post-treatment neuroimaging studies were reviewed. In particular, the following parameters were analyzed: localization of the meningiomas (divided in anterior, middle, or posterior third of the SSS) (17), SSS invasion according to the Sindou classification (Table 1) (6–9, 18), and SSS occlusion rate (1) (Table 1) based on coronal contrast-enhanced T1 (CE-T1) and T2 sequences (divided into three groups). Tumor size was calculated and measured by volumetry. Manual segmentation of pre- and post-operative T2 and CE-T1 images was performed using the bumper tool of the Precision treatment planning software (Accuray Inc., Sunnyvale, CA, USA). Volume calculation of T2 and CE-T1 of meningiomas was performed by multiplying the sum of the tumor areas outlined on each transverse slice by the corresponding slice thickness.
Tumor response was assessed by MRI and divided into four categories (Table 2): tumor shrinkage and/or no change in size were scored as locally controlled disease. To exclude temporary tumor swelling after radiosurgery (19), a local recurrence was only scored after two consecutive follow-ups when an increase in size was observed.
Time to recurrence (TTR) was calculated as the time between treatment and the second positive MRI for tumor growth.
Perioperative morbidity was determined according to all documented medical, neurological, and approach-related adverse events and differentiated as transient or permanent deficit.
All data were collected in accordance with the World Medical Association Declaration of Helsinki (20). All patients expressed their consent for the treatment. For this study, we obtained an approval of the institutional review board of the Ludwig-Maximilians-University in Munich (reference number 20-479).
The reference point of this study was the date of first therapy. Primary endpoints were TTR, functional outcome, and treatment toxicity. The significance of time to event data was assessed using the Cox proportional hazards model and the log-rank test. Results were tested by using a 2-way analysis of variance (ANOVA) and Student’s t-test. Particular recurrence rates for each treatment arm (groups 1–3) were defined as number of recurrences of each treatment arm/number of total patients per treatment arm. The overall recurrence rate was specified as the number of all recurrences/total patient number. For risk factor analyses, uni- and multivariate tests were conducted. Variables tested for predictive significance concerning local recurrence were age, sex, side of the tumor, tumor volume, and radiosurgical prescription dose. Further, ROC analyses were performed to examine the risk of recurrence depending on tumor volume. GraphPad PRISM8.0d (GraphPad Prism, San Diego, CA, USA) and Excel (Microsoft, Redmond, WA, USA) software were used for statistical analysis. Differences were considered statistically significant at p < 0.05.
Table 3 summarizes patients’ characteristics in detail. In total, 1,293 patients with grade I meningiomas (n = 1162, 89.9%), grade II meningiomas (n = 123, 9.5%), and grade III anaplastic meningiomas (n = 8, 0.6%) were treated at our department. After exclusion of grade II and grade III tumors, 137 meningiomas (m:f = 1:2.3) involving the superior sagittal sinus were included in this study (Figure 1). Mean age at treatment was 57.4 years (median, 58.3 years; range, 25.7–87.1 years).
A total of 51 patients (37.2%) received a primary RRS (group 1), 15 patients (11.0%) were treated for a residual tumor after microsurgical resection (group 2), and 71 patients (51.8%) were treated due to relapse after surgery (group 3). Out of 86 patients of groups 2 and 3, 26 (30.2%) received surgery at the LMU University Hospital, whereas 60 patients (69.8%) underwent surgery at other institutions. Tumor volumes of patients operated at LMU University Hospital did not differ significantly from patients operated at external clinics (9.0 ± 5.4 cm3 vs. 7.1 ± 6.6 cm3, p = 0.17). In 18 (13.2%) patients, the tumor was located in the first third of the SSS, in 82 (59.8%) in the mid third, and in 37 (27.0%) in the last third of the SSS.
The median SSS occlusion ratio was subtotal both before treatment and at last FU (see Figure 2 for details). Sindou grade IV was median value for sinus invasion for both pre-treatment and at last FU (see Figure 3).
Table 4 summarizes anatomical morphological classifications for meningiomas according to the defined treatment groups. For all treatment arms, we found a cumulative tumor localization in the mid third of the superior sagittal sinus (p = 0.051) and further a subtotal occlusion of the coronal diameter (p = 0.29), but without statistical significance. A predominant accumulation of Sindou grade IV (p = 0.046) throughout the treatment arms was noticed.
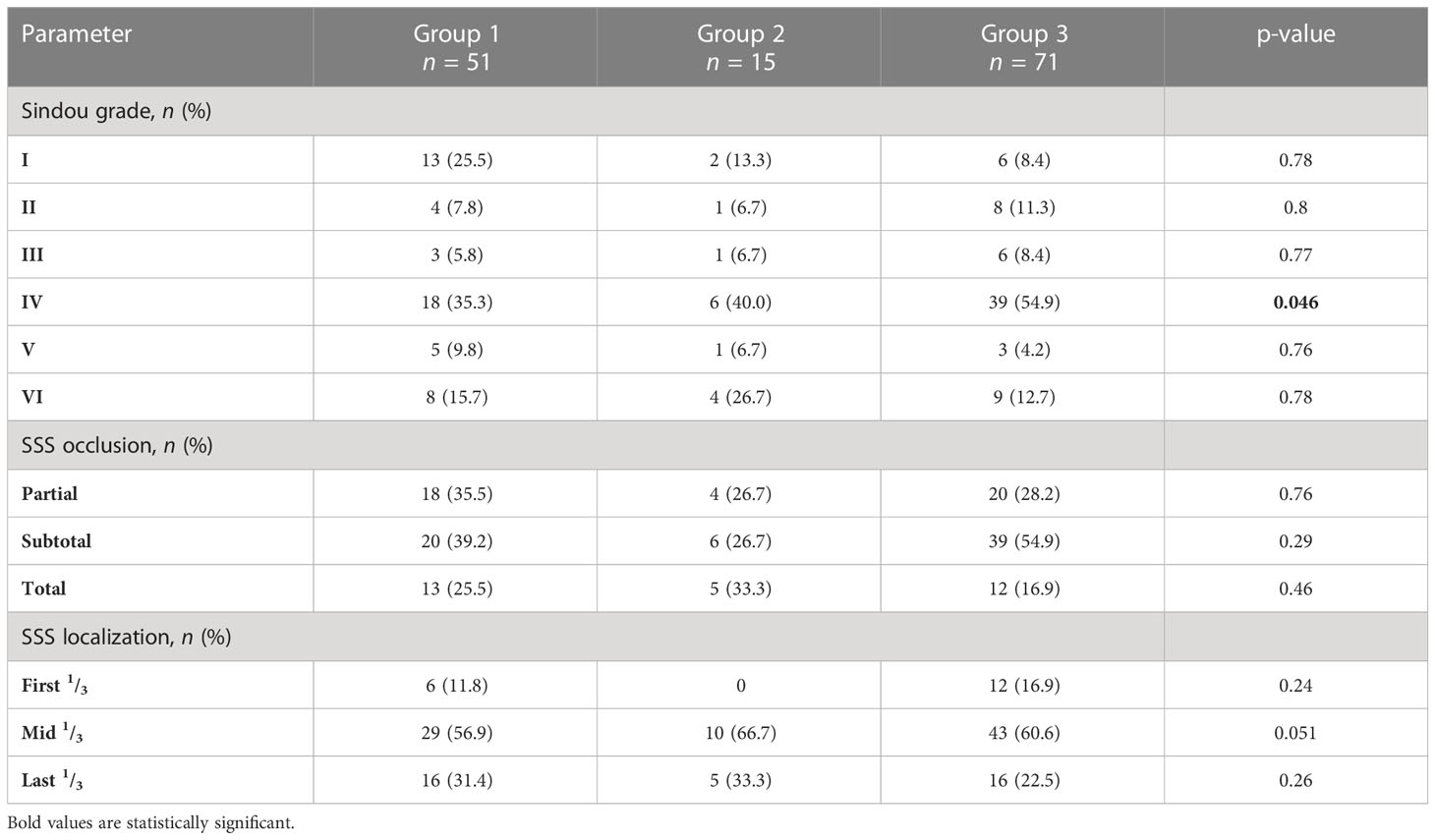
Table 4 Distribution of Sindou grades (I–VI), extent of SSS occlusion (<50%, 50%–99%, 100%), and SSS localization (first, middle, and last third) for patients of the three treatment groups.
Treatment of SSS meningiomas was either initial radiosurgery without prior surgical intervention (group 1), adjuvant RRS after subtotal resection without evidence of recurrence (group 2), or after local tumor recurrence of a surgical residue (group 3). All patients were treated with the CyberKnife® system (Accuray Inc., Sunnyvale, CA, USA). A total of 152 RRS sessions in 137 patients/tumors were performed. A total of 131 patients were treated with a single session and 6 patients underwent an average of 4.7 ± 0.8 therapy sessions (median, 5; range, 3–5). The mean target volume was 6.7 ± 5.3 cm3 (median, 5.81 cm3; range, 0.67–32.27 cm3) irradiated with an average dose of 15.5 ± 2.1 Gy (median, 15.0 Gy; range, 12.0–25.0 Gy). The applied dose and isodose on the three treatment groups did not differ significantly (p = 0.2 and 0.4).
Tables 3, 4 show detailed clinical parameters for the three treatment groups. Table 5 highlights radiometrical data according to the three subgroups.
Patients irradiated with RRS due to a recurrent tumor after microsurgery (mean age at radiosurgery of 60.2 ± 13.7 years) tended to be older (p = 0.1) compared to patients undergoing RRS following microsurgery due to a residual tumor (53.2 ± 12.7 years) and to patients receiving initially RRS therapy (54.7 ± 12.3 years, p = 0.04). Tumor volume of primarily RRS-treated meningiomas was significantly lower with 4.9 ± 4.2 cm3 compared to recurrent and residual tumors (7.0 ± 4.7 cm3, p = 0.016 and 10.8 ± 8.0 cm3, p = 0.0004). Moreover, volumes of meningiomas in the recurrent or residual situation did differ significantly (10.8 ± 8.0 vs. 7.0 ± 4.7 cm3, respectively; p = 0.013). The average time to recurrence after RRS was lower for solely initial irradiated tumors (group 1) with 43.9 ± 41.9 months compared to radiosurgery-treated meningiomas due to recurrence (group 3) or a residual tumor (group 2) with 55.7 ± 47.9 months and 57.7 ± 33.9 months, respectively, but not statistically significant.
Throughout the 157 RRS sessions, a transient peri-radiosurgical morbidity was observed in 8 patients (5.8%). These experienced perifocal post-radiation edema, which was symptomatic and required treatment with corticosteroids in 3 patients (2.2%) for a short period of time of less than 2 weeks. Two (1.4%) of these patients also developed radio-necrosis, but without the need of further interventions. These therapy-related morbidities did not differ significantly between groups 1 and 3. Detailed toxicity profile is listed in Table 6.
Ten (7.3%) patients reported headache during FU controls, three (2.2%) had seizures, which could be treated sufficiently with antiepileptic drugs, and two patients (1.4%) had vertigo and/or imbalance problems (see Table 6). Eight patients (5.8%) died during FU, but not due to meningioma- or RRS-related causes.
Interestingly, acute sinus venous thrombosis or venous congestion-associated bleedings did not occur in any of the groups, even if the sinus was already affected by the tumor, as classified by the Sindou- or the sinus occlusion criteria (Table 4).
The average FU period after RRS therapy was 55.2 ± 46.2 months (median, 42.0 months; range, 3.4–200.3 months). Average KPS at last FU was 97.3 (median, 100; range, 60–100).
A positive MRI morphological response to therapy was observed in 104 patients (75.9%). In 25 (18.2%) patients, no response was observed, and in 8 (5.8%) cases, there was evidence of progression (see Table 7).
The overall period between first diagnosis and RRS therapy initiation was 54.3 ± 49.8 months (median, 26.7 months; range, 0.4–305.3 months).
Patients with initial RRS-treated meningiomas (group 1) had an average period of 41.0 ± 35.4 months (median, 24.0 months; range, 1.1–194.5 months) between first diagnosis and RRS therapy initiation.
A total of 86 patients underwent 108 surgeries before RRS in the residual tumor (group 2) or local recurrent (group 3) (Table 8). For patients of group 2, the average time until RRS was 21.6 ± 15.8 months (median, 3.7 months; range, 0.4–63.9 months). In total, 15 patients received 18 microsurgical subtotal resections prior to radiosurgery.
All patients of group 3 with a recurrent meningioma (n = 71) had 90 surgical procedures in total before RRS treatment. The average period between microsurgery and RRS for patients of group 3 was 67.4 ± 61.0 months (median, 40.9 months; range, 8.2–305.3 months).
Kaplan–Meier estimates are summarized in Figure 4. Eight patients died during FU. The average time to recurrence (TTR) was 51.5 ± 44.5 months (median, 41.6 months; range, 3.4–200.3 months). Figure 4A shows an overall TTR analysis regardless of treatment arms for all included patients: 1-, 2-, 5-, and 10-year probabilities without any tumor recurrences are 100%, 97.6%, 84.2%, and 59.4%. The recurrence rate for all patients undergoing RRS is 12.4% (17 patients). Overall, survival curves do not differ significantly from each other (log rank, p = 0.06).
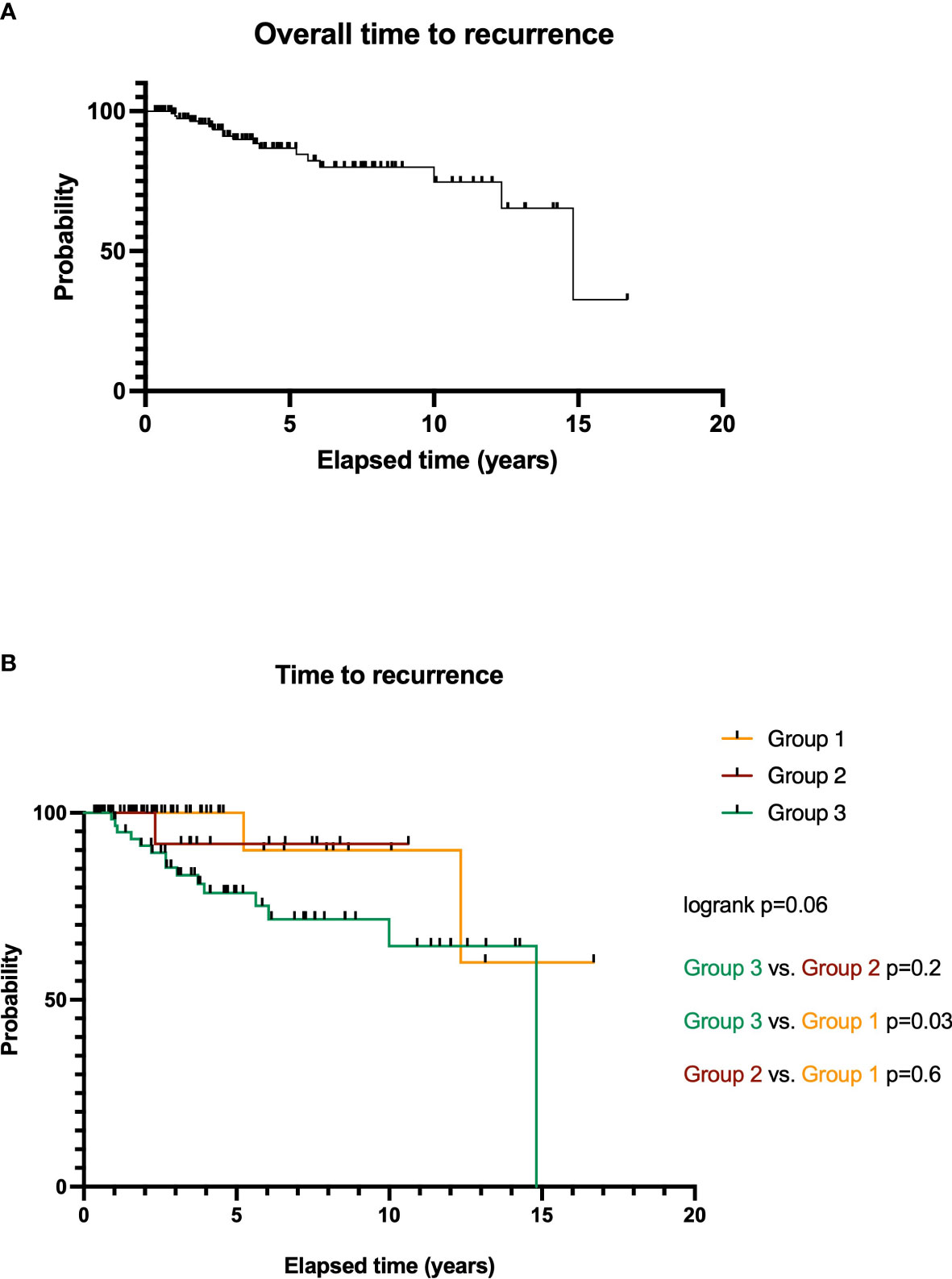
Figure 4 Overall time to recurrence (TTR) analysis for RRS-treated SSS meningiomas WHO grade I (A). TTR of meningioma patients depending on treatment options: microsurgery and RRS after local tumor recurrence (group 3), microsurgery of a residual tumor (group 2), and initial RRS therapy (group 1, (B)).
Further particular analysis of TTR of the treatment groups is illustrated in Figure 4B.
Meningiomas treated in group 1 show an average TTR of 43.9 ± 41.9 months (median, 34.4 months; range, 5.1–200.3 months) with a treatment-related recurrence rate of 2.2% (3 patients). The 1-, 2-, 5-, and 10-year survival rates are 100%, 100%, 100%, and 90.0%.
For patients of group 2, average TTR is 57.7 ± 33.9 months (median, 44.4 months; range, 8.0–127.5 months). Here, the 1-, 2-, and 5-year survival rates were 100%, 100%, and 91.7%. Comparison of both surgery groups (groups 2 vs. 3, p = 0.2, HR = 3.3) and to group 1 is not significant (p = 0.6, HR = 1.8). The recurrence rate for this treatment arm after RRS is 1.5% (2 patients).
Patients of group 3 had an average TTR of 55.7 ± 47.9 months (median, 44.9 months; range, 3.4–177.8 months). The 1-, 2-, 5-, and 10-year probabilities without tumor recurrence were 98.3%, 91.2%, 78.5%, and 64.4%. The particular recurrence rate for meningiomas of group 3 is 8.8% (12 patients).
Here, survival curves do show a significant difference compared to the initial surgery cases: p = 0.03 (groups 3 vs. 1, HR = 4.3).
The recurrence rate for all patients undergoing RRS of meningioma involving the SSS is 12.4% (17 patients). Overall, survival curves do not differ significantly from each other (log rank, p = 0.06).
In total, 17 patients suffered from new recurrences of their treated meningioma. All recurrent tumors were again treated by RRS. The average post-recurrence survival for all recurrent tumors was 60.5 ± 35.2 months (median, 59.3 months; range, 5.6–133.6 months).
Of the 17 meningioma recurrences, 3 showed up in group 1, 2 in group 2, and 12 in group 3. Patients with recurrent tumors in groups 1–3 received further RRS sessions. The average post-recurrence survival is 34.1 ± 27.6 months (median, 35.9 months; range, 5.6–60.8 months) in group 1. In group 2, the average post-recurrence survival is 54.9 months, and in group 3, it is 68.0 ± 32.0 months (median, 68.3 months; range, 30.4–133.6 months).
In the uni- and multivariate risk analysis, various factors correlating significantly with tumor volume and TTR were found (see Table 9). The occlusion of the SSS (p = 0.026), male sex (p = 0.011), and the comparison of groups 1 vs. 3 (p = 0.0003) significantly correlated with tumor volume in the multivariate analysis. Further, tumor volume (p = 0.0046) and initial RRS therapy (group 1 vs. 2, p = 0.001) significantly correlated with TTR in the multivariate analysis.
The ROC analysis suggests for primary RRS-treated meningiomas (group 1) a maximum tumor volume of 5.1 cm3 as the cutoff value (p = 0.0004) for radiosurgical treatment to further reduce (in recurrent situations) tumor recurrence. Similarly, for meningiomas, which underwent initial surgical treatment, a tumor volume of more than 5.2 cm3 was associated with higher recurrence rates (p = 0.0004).
The management of parasagittal meningiomas, especially those involving the SSS, can still be challenging today. Historically, the optimal treatment was considered as an aggressive total Simpson grade 1 resection. This is achievable in only a minority of cases (21) and requires complex reconstructive techniques of the draining veins and SSS (7) with relevant morbidity and mortality (22). Even after radical resection, recurrence rates ranging from 4% (7) to 13% (23, 24) are reported. Kondziolka et al. (11) introduced the concept of multimodal treatment for parasagittal meningiomas. They advocated radiosurgery as a first-line treatment for meningiomas smaller than 3 cm and subtotal surgery (leaving the sinus part) followed by radiosurgery for bigger lesions. More recently, less aggressive surgical strategies have gained popularity (25–27), where precedence is given to a lower mortality and morbidity over total resection (leaving parts that are invading the sinus or growing in the sinus, especially if the sinus is not completely occluded). In a recent systematic review, Giordan et al. (22) compared the two surgical strategies. They showed that the non-aggressive surgical strategy achieved better functional outcomes and that, in the aggressive strategy group, the rate of postoperative venous infarcts was doubled. The overall recurrence rate was 7% for an FU of 4.9 years in the aggressive group and 13% for an FU of 6 years in the non-aggressive group. However, the benefits of an aggressive surgical strategy also remain unclear on the other side, because a recently published study by Wang et al. (28) reported a higher KPS score of patients who underwent an aggressive surgical tumor resection.
In a context of multimodality treatment, several studies have shown the role of stereotactic radiosurgery both as a first and as an adjuvant treatment following surgery (29–34).
Many radiosurgical series present data regarding multiple locations and multiple histological (grading) diagnoses, and often many of the patients included could be considered “salvage” cases (35).
To our knowledge, this study presents the biggest consecutive series of WHO grade I (also presumptive) parasagittal meningiomas with SSS involvement treated at a single center in a 15-year interval. A total of 137 patients were analyzed. We allocated the patients into three treatment arms: primary RRS treatment (51 patients), RRS treatment after subtotal surgery (15 patients), and RRS treatment after post-surgical recurrence (71 patients).
RRS as a primary treatment has gained more and more popularity especially for smaller lesions in a variety of locations and is nowadays part of the neurosurgical armamentarium (11, 29, 30, 33, 36, 37). In our study, 51 patients received RRS as a first-line treatment. Their TTR was 43.9 ± 41.9 months with 5- and 10-year survival rates of 100% and 90.0%, respectively, and a recurrence rate of 2.2%.
A total of 86 patients received RRS post-surgery, either when growth of the residual tumor occurred (71 patients, group 3) or after sub-total resection as adjuvant treatment (15 patients, group 2).
Group 2 had a time to recurrence of 57.7 ± 33.9 months, and the 1-, 2-, and 5-year probabilities without tumor recurrence were 100%, 100%, and 91.7%. Group 3 had a time to recurrence of 55.7 ± 47.9 months, and the 1-, 2-, 5-, and 10-year survival rates were 98.3%, 91.2%, 78.5%, and 64.4%. In our series, there was no statistically significant difference between the three arms.
The overall TTR regardless of treatment arm for all included patients was 100%, 97.6%, 84.2%, and 59.4% at respectively 1, 2, 5, and 10 years. Kondziolka et al. (11) reported a similar tumor control rate at 5 years of 93 ± 5% in the primary RRS group but a lower control rate of 60 ± 10% in the group of patients with previous surgical treatment. Also, DiBiase et al. (10) reported a 5-year disease-free survival of 86.2% for benign meningiomas at multiple locations and Park et al. (38) reported a 3-year recurrence free rate of 95% for subtotally resected meningiomas (all locations and grades) with additional RRS treatment. In the study of Colombo et al. (33) and Hadelsberg et al. (39), the tumor control rate from benign primarily RRS-treated meningiomas (multiple locations) was 93.56% and 90.6%, respectively.
The role of adjuvant radiotherapy for grade II and III meningiomas is supported by a number of studies (40). There is also some evidence supporting upfront adjuvant radiotherapy (41). Its role for grade I meningiomas is still discussed. Some authors report no benefit of the adjuvant therapy, and therefore, the timing of the treatment remains unclear (42). In our series, a positive MRI morphological response to therapy was observed in 50 patients (36.4%). Two strategies are usually proposed: an early treatment of the residue or an initial wait-and-see approach with serial MRI follow-ups with secondary treatment (in this case RRS) reserved only when recurrence is observed. Some studies support the role of an early postoperative radiosurgical treatment in improving PFS compared to a treatment at recurrence (11, 43–45). Frostell et al. (46) published a study in which they confronted adjuvant radiosurgical treatment after subtotal resection of meningiomas involving the SSS. Their results showed better outcome for patients treated with upfront radiosurgery compared to those treated for recurrence. In their study, however, meningiomas of all three grades were included.
Also, our group (47) recently presented a study where early postoperative radiation treatment was advantageous in spheno-orbital grade I meningiomas. In our study, we found no statistically significant difference in PFS between the two postsurgical groups (post-recurrence and post-subtotal). It is important to notice that the non-significance of these data could be due to the relatively small number of patients that underwent RRS directly after subtotal resection (15 patients). However, Pikis et al. (48) showed that upfront stereotactic radiosurgery of grade I parasagittal meningioma leads to superior radiological tumor control compared to watch and wait. The question of the ideal timing for a post-surgical RRS therapy in a multimodal treatment remains open and needs to be further elucidated.
Tumor volume plays an important role in both rate of recurrence and complication rate post-radiosurgery (10, 49). This was also confirmed in our study.
ROC analysis regarding tumor volume and risk of recurrence showed that a volume greater than 5.1 cm3 and 5.2 cm3 in the primarily treated and postsurgical RRS groups did correlate to higher rate of recurrence. DiBiase et al. (10) and Kondziolka et al. (11) described bigger tumor volumes, >10 cm3 and >7.5 cm3, respectively, correlating with local recurrence.
In our opinion, the volume analysis represents a valuable tool in guiding the choice both of the primary treatment as of the timing of post-surgical RRS therapy. Furthermore, it should be used in the planning of the surgical strategy in a multimodal treatment environment.
Radiosurgical treatment can be associated with treatment-related morbidity. Former studies reported of patients with parasagittal meningiomas having higher risks of developing post-radiation edema compared to skull base meningiomas (50, 51). In our series, eight patients (5.8%) experienced perifocal post-radiation edema. Only three patients were symptomatic, requiring low-dose dexamethasone treatment, and two of these patients also developed radio-necrosis in follow-up MRI. This is less than what other studies reported (52). No patient needed a surgical treatment for a radiosurgical complication and no patient experienced an SSS thrombosis following RRS.
The main limitation of this study is its design as a retrospective cohort study. Furthermore, the three treatment arms are imbalanced due to different numbers of patients. Moreover, the allocation of patients to one of the arms was not randomized but based on clinical decision.
For the primary RRS-treated patients, the diagnosis of grade I meningioma was based solely on radiological criteria and not on a histological confirmation.
RRS for parasagittal grade I meningiomas with SSS involvement represents a good option as a first-line treatment, but also a second-line treatment in a recurrent and adjuvant (post-subtotal resection) scenario. Furthermore, it plays an important role in a multimodal strategy for the treatment of meningiomas involving the SSS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional review board of the Ludwig-Maximilians-University in Munich (reference number 20-479). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and design: MS, AM, and CS. Acquisition of data: MS and BS. Analysis and interpretation of data: MS, BS, JT, and CS. Drafting the article: MS. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: MS. Statistical analysis: MS. Study supervision: AM and CS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zeeshan Q, Patel A, Cheng CY, Zhao NH, Barber J, Ghodke BV, et al. Resection of meningiomas involving major dural venous sinuses: classification, technique, and long-term results. World Neurosurg (2019) 125:e521–36. doi: 10.1016/j.wneu.2019.01.128
2. Aghi M, Barker FG. Benign adult brain tumors: an evidence-based medicine review. Prog Neurological Surg (2006) 19:80–96. doi: 10.1159/000095184
3. al-Rodhan NR, Laws ER. Meningioma: a historical study of the tumor and its surgical management. Neurosurgery (1990) 26:832–46.
4. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
5. Simpson D. THE RECURRENCE OF INTRACRANIAL MENINGIOMAS AFTER SURGICAL TREATMENT. J Neurology Neurosurg Psychiatry (1957) 20:22–39. doi: 10.1136/jnnp.20.1.22
6. Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci (1997) 4:8–11. doi: 10.1054/jocn.2001.0868
7. Sindou MP, Alvernia JE. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg (2006) 105:514–25. doi: 10.3171/jns.2006.105.4.514
8. Sindou MP, Auque J, Jouanneau E. Neurosurgery and the intracranial venous system. In: New trends of surgery for stroke and its perioperative management. Vienna: Springer-Verlag. (2005) p. 167–75. doi: 10.1007/3-211-27911-3_27
9. Sindou M. Meningiomas involving major dural sinuses: should we attempt at radical removal and venous repair? World Neurosurg (2014) 81:46–7. doi: 10.1016/j.wneu.2013.07.119
10. DiBiase SJ, Kwok Y, Yovino S, Arena C, Naqvi S, Temple R, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys (2004) 60:1515–9. doi: 10.1016/j.ijrobp.2004.05.073
11. Kondziolka D, Flickinger JC, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Neurosurgery (1998) 43(3):405–13. doi: 10.1097/00006123-199809000-00001
12. Feigl GC, Samii M, Horstmann GA. VOLUMETRIC FOLLOW-UP OF MENINGIOMAS. Neurosurgery (2007) 61:281–7. doi: 10.1227/01.NEU.0000279999.95953.EA
13. Kollová A, Liščák R, Novotný J, Vladyka V, Šimonová G, Janoušková L. Gamma knife surgery for benign meningioma. J Neurosurg (2007) 107:325–36. doi: 10.3171/JNS-07/08/0325
14. Iwai Y, Yamanaka K, Ikeda H. Gamma knife radiosurgery for skull base meningioma: long-term results of low-dose treatment. J Neurosurg (2008) 109:804–10. doi: 10.3171/JNS/2008/109/11/0804
15. Pinzi V, Fariselli L, Marchetti M, Scorsetti M, Navarria P. Stereotactic radiotherapy for parasagittal and parafalcine meningiomas: patient selection and special considerations. Cancer Manage Res (2019) 11:10051–60. doi: 10.2147/CMAR.S187371
16. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol (2016) 17:e383–91. doi: 10.1016/S1470-2045(16)30321-7
17. Sindou M, Auque J. The intracranial venous system as a neurosurgeon’s perspective. Adv Tech Standards Neurosurg (2000) 26:131–216. doi: 10.1007/978-3-7091-6323-8_5
18. Han MS, Kim YJ, Moon KS, Lee KH, Yang JI, Kang WD, et al. Lessons from surgical outcome for intracranial meningioma involving major venous sinus. Med (United States) (2016) 95:1–7. doi: 10.1097/MD.0000000000004705
19. Chernov MF, Ono Y, Abe K, Usukura M, Hayashi M, Izawa M, et al. Differentiation of tumor progression and radiation-induced effects after intracranial radiosurgery. Acta Neurochirurgica Supplement (2013) 116:193–210. doi: 10.1007/978-3-7091-1376-9_29
20. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA (2013) 310:2191. doi: 10.1001/jama.2013.281053
21. Sheehan JP, Cohen-Inbar O, Ruangkanchanasetr R, Bulent Omay S, Hess J, Chiang V, et al. Post-radiosurgical edema associated with parasagittal and parafalcine meningiomas: a multicenter study. J Neuro-Oncology (2015) 125:317–24. doi: 10.1007/s11060-015-1911-1
22. Giordan E, Sorenson TJ, Lanzino G. Optimal surgical strategy for meningiomas involving the superior sagittal sinus: a systematic review. Neurosurgical Rev (2018) 43(2):525–35. doi: 10.1007/s10143-018-1026-1
23. Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochirurgica (1994) 126:53–8. doi: 10.1007/BF01476410
24. Mathiesen T, Pettersson-Segerlind J, Kihlström L, Ulfarsson E. Meningiomas engaging major venous sinuses. World Neurosurg (2014) 81:116–24. doi: 10.1016/j.wneu.2013.01.095
25. DiMeco F, Li KW, Casali C, Ciceri E, Giombini S, Filippini G, et al. Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery (2004) 55:1263–72. doi: 10.1227/01.NEU.0000143373.74160.F2
26. Raza SM, Gallia GL, Brem H, Weingart JD, Long DM, Olivi A. Perioperative and long-term outcomes from the management of parasagittal meningiomas invading the superior sagittal sinus. Neurosurgery (2010) 67:885–93. doi: 10.1227/NEU.0b013e3181ef2a18
27. Oyama H, Kito A, Maki H, Hattori K, Noda T, Wada K. Surgical results of parasagittal and falx meningioma. Nagoya J Med Sci (2012) 74:211–6.
28. Wang B, Zhang G-J, Wu Z, Zhang J-T, Liu P-N. Surgical outcomes and prognostic factors of parasagittal meningioma: a single-center experience 165 consecutive cases. Br J Neurosurg (2022) 36:756–61. doi: 10.1080/02688697.2020.1867825
29. Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of world health organization grade II and III intracranial meningiomas: treatment results on the basis of a 22-year experience. Cancer (2012) 118:1048–54. doi: 10.1002/cncr.26362
30. Santacroce A, Walier M, Régis J, Liščák R, Motti E, Lindquist C, et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery (2012) 70:32–9. doi: 10.1227/NEU.0b013e31822d408a
31. Gatterbauer B, Gevsek S, Höftberger R, Lütgendorf-Caucig C, Ertl A, Mallouhi A, et al. Multimodal treatment of parasagittal meningiomas: a single-center experience. J Neurosurg (2017) 127:1249–56. doi: 10.3171/2016.9.JNS161859
32. Manabe Y, Murai T, Ogino H, Tamura T, Iwabuchi M, Mori Y, et al. Cyber knife stereotactic radiosurgery and hypofractionated stereotactic radiotherapy as first-line treatments for imaging-diagnosed intracranial meningiomas. Neurologia Medico-Chirurgica (2017) 57:627–33. doi: 10.2176/nmc.oa.2017-0115
33. Colombo F, Casentini L, Cavedon C, Scalchi P, Cora S, Francescon P. CyberKnife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery (2009) 64:7–13. doi: 10.1227/01.NEU.0000338947.84636.A6
34. Flannery T, Poots J. Gamma knife radiosurgery for meningioma. In: Niranjan A, Lunsford LD, Kano H, editors. Progress in neurological surgery. S. Karger AG (2019). p. 91–9. doi: 10.1159/000493054
35. Malik I, Rowe JG, Walton L, Radatz MWR, Kemeny AA. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg (2005) 19:13–20. doi: 10.1080/02688690500080885
36. Zachenhofer I, Wolfsberger S, Aichholzer M, Bertalanffy A, Roessler K, Kitz K, et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery (2006) 58:28–36. doi: 10.1227/01.NEU.0000190654.82265.A3
37. Zada G, Pagnini PG, Yu C, Erickson KT, Hirschbein J, Zelman V, et al. Long-term outcomes and patterns of tumor progression after gamma knife radiosurgery for benign meningiomas. Neurosurgery (2010) 67:322–8. doi: 10.1227/01.NEU.0000371974.88873.15
38. Park CK, Jung NY, Chang WS, Jung HH, Chang JW. Gamma knife radiosurgery for postoperative remnant meningioma: analysis of recurrence factors according to world health organization grade. World Neurosurg (2019) 132:e399–402. doi: 10.1016/j.wneu.2019.08.136
39. Hadelsberg U, Nissim U, Cohen ZR, Spiegelmann R. LINAC radiosurgery in the management of parasagittal meningiomas. Stereotactic Funct Neurosurg (2015) 93:10–6. doi: 10.1159/000368440
40. Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, et al. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro-Oncology (2014) 16:628–36. doi: 10.1093/neuonc/nou025
41. Pant S, Tonse R, Kannan S, Moiyadi A, Shetty P, Epari S, et al. Impact of timing of radiation therapy on outcomes in atypical meningioma: a clinical audit. Pract Radiat Oncol (2018) 8:e275–84. doi: 10.1016/j.prro.2018.01.010
42. Sun SQ, Cai C, Murphy RKJ, Dewees T, Dacey RG, Grubb RL, et al. Radiation therapy for residual or recurrent atypical meningioma: the effects of modality, timing, and tumor pathology on long-term outcomes. Neurosurgery (2016) 79:23–32. doi: 10.1227/NEU.0000000000001160
43. Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys (2005) 61:809–16. doi: 10.1016/j.ijrobp.2004.07.669
44. Compter I, Zaugg K, Houben RMA, Dings JTA, Bosmans G, Buescher C, et al. High symptom improvement and local tumor control using stereotactic radiotherapy when given early after diagnosis of meningioma a multicentre study. Strahlentherapie und Onkologie (2012) 188:887–93. doi: 10.1007/s00066-012-0155-7
45. Bowden G, Faramand A, Mallella A, Wei Z, Patel K, Niranjan A, et al. Does the timing of radiosurgery after grade 1 meningioma resection affect long-term outcomes? Stereotact Funct Neurosurg (2021) 99:506–11. doi: 10.1159/000517427
46. Frostell A, Hakim R, Dodoo E, Sinclair G, Ohlsson M, Förander P, et al. Adjuvant stereotactic radiosurgery reduces need for retreatments in patients with meningioma residuals. World Neurosurg (2016) 88:475–82. doi: 10.1016/j.wneu.2015.10.062
47. Terpolilli NA, Ueberschaer M, Niyazi M, Hintschich C, Egensperger R, Muacevic A, et al. Long-term outcome in orbital meningiomas: progression-free survival after targeted resection combined with early or postponed postoperative radiotherapy. J Neurosurg (2019) 133(2):1–11. doi: 10.3171/2019.3.jns181760
48. Pikis S, Mantziaris G, Bunevicius A, Islim AI, Peker S, Samanci Y, et al. Stereotactic radiosurgery compared with active surveillance for asymptomatic, parafalcine, and parasagittal meningiomas: a matched cohort analysis from the IMPASSE study. Neurosurgery (2022) 90:750–7. doi: 10.1227/neu.0000000000001924
49. Karaaslan B, Celtici E, Bulduk EB, Borcek AO, Kurt G, Kaymaz M, et al. Stereotactic radiosurgery after subtotal resection of critically-located grade i meningioma: a single-center experience and review of literature. Turkish Neurosurg (2020) 31(4):519–29. doi: 10.5137/1019-5149.JTN.30181-20.2
50. Chang JH, Chang JW, Choi JY, Park YG, Chung SS. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry (2003) 74:226–30. doi: 10.1136/jnnp.74.2.226
51. Kalapurakal JA, Laske W, Thomas RM, Braitman E. Therapeutic intracranial the development stereotactic Meningiomas : of cerebral edema after and radiation influence therapy. Radiology (1997) 204(2):461–5. doi: 10.1148/radiology.204.2.9240536
Keywords: meningioma, superior sagittal sinus, robotic radiosurgery, microsurgery, sinus occlusion
Citation: Schmutzer M, Skrap B, Thorsteinsdottir J, Fürweger C, Muacevic A and Schichor C (2023) Meningioma involving the superior sagittal sinus: long-term outcome after robotic radiosurgery in primary and recurrent situation. Front. Oncol. 13:1206059. doi: 10.3389/fonc.2023.1206059
Received: 14 April 2023; Accepted: 19 June 2023;
Published: 11 July 2023.
Edited by:
Marcos Vinicius Calfat Maldaun, Hospital Sirio Libanes, BrazilReviewed by:
Alfredo Conti, University of Bologna, ItalyCopyright © 2023 Schmutzer, Skrap, Thorsteinsdottir, Fürweger, Muacevic and Schichor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Schmutzer, bWljaGFlbC5zY2htdXR6ZXJAbWVkLnVuaS1tdWVuY2hlbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.