
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 August 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1200286
This article is part of the Research Topic Musculoskeletal Oncology: Recent Advances and Innovation View all 8 articles
 Christopher M. LaPrade1
Christopher M. LaPrade1 Logan M. Andryk2
Logan M. Andryk2 Joshua L. Christensen2
Joshua L. Christensen2 John C. Neilson2
John C. Neilson2 Adam N. Wooldridge2
Adam N. Wooldridge2 Donald A. Hackbarth2
Donald A. Hackbarth2 Manpreet Bedi3*
Manpreet Bedi3* David M. King2
David M. King2Introduction: Enchondromas and grade 1 chondrosarcomas are commonly encountered low-grade chondroid tumors in the proximal humerus. While there is a concern for malignant transformation, few studies have evaluated the natural history of these lesions. The purpose of this study is to evaluate the natural history of proximal humerus low-grade chondroid lesions managed both conservatively and surgically, and to define management criteria using clinical and radiographic findings for these low-grade chondroid lesions.
Methods: The patient population included 90 patients intended for conservative treatment and 22 patients proceeding directly to surgery. Data collection was based on a combination of chart review and patient imaging and descriptive statistics were calculated for each group.
Results: No malignant transformations were noted amongst any group. In the conservative treatment group, 7 of 64 (11%) progressed to surgery after an average of 20.3 months of conservative treatment due to persistent pain unexplained by other shoulder pathology. Importantly, 71% experienced continued pain at a mean of 53.1 months post-operatively. The group that went directly to surgery also demonstrated pain in 41% at an average follow-up of 57.3 months.
Discussion: Low-grade cartilaginous lesions of the proximal humerus without concerning imaging findings can be managed with conservative treatment and the risk of malignant transformation is very low. Patients with a clear source of their shoulder pain unrelated to their tumor and without concerning characteristics on imaging can be managed with serial annual radiographic imaging. Patients undergoing surgery for these indolent tumors are likely to experience persistent pain even after surgery.
Enchondromas (ECs) and low-grade (grade 1) chondrosarcomas (CSs) are commonly encountered low-grade chondroid tumors. Enchondromas are benign cartilage tumors reported to account for 20% of all bone tumors in a series of 3,607 bone tumors (1), and are likely underreported due to many ECs being asymptomatic (1–5). Chondrosarcomas are malignant cartilage tumors and were reported to account for 37% of malignant bone tumors when including low- and high-grade CSs (1).
Distinguishing between an EC and low-grade CS is a difficult endeavor for orthopaedic surgeons, pathologists, and radiologists; however, it is an important one because these low-grade chondroid tumors are typically managed differently. EC lesions are often incidentally identified on imaging, with characteristics of minimal or absent endosteal scalloping or surrounding bone and soft tissue edema, as well as absent cortical breakthrough or soft tissue masses (1, 2, 6–9). Histologically, low-grade CSs show slightly more cellularity and myxoid changes than ECs, but accurate diagnosis remains challenging (8). Asymptomatic enchondromas throughout the body can be managed conservatively with serial imaging, while symptomatic tumors may require curettage. Additionally, one study suggested that tumors greater than seven centimeters in one dimension may require surgical curettage as the risk of malignant transformation is increased in these larger lesions (1). As opposed to ECs, low-grade CSs are often characterized by pain and more aggressive imaging findings including endosteal scalloping, surrounding marrow, or soft tissue edema. Low-grade CS’s typically require surgical treatment ranging from intralesional excision and curettage to wide excision depending on location (1, 2, 6–8, 10–13). While many low-grade chondroid lesions are managed without surgical intervention, there is a worry of malignant transformation into higher-grade tumors, with studies reporting a transformation rate of ECs ranging from 2% to 4% (1, 4, 10).
Low-grade chondroid tumors may develop anywhere in the skeleton, though they are commonly found in the proximal humerus, with Hong et al. (14) reporting that 2% of shoulder Magnetic Resonance Imaging (MRI) studies demonstrated an incidental proximal humerus EC, while Woltsche et al. (15) showed an EC prevalence of 0.39% in 21,550 patients receiving shoulder MRIs. In addition, studies have reported that 18-28% of ECs and 14% of low-grade CSs are located in the humerus (1, 3, 16). While multiple studies have advocated that low-grade chondroid lesions throughout the body may be managed conservatively with observation through serial imaging (1, 2, 6, 7, 12, 13, 17), there is a paucity of studies examining the natural history of these lesions, particularly in the proximal humerus.
The purpose of this study is to examine the natural history of proximal humerus low-grade chondroid lesions managed both conservatively and surgically at a single academic institution. The aim was to better define management criteria using clinical and radiographic findings for these low-grade chondroid lesions that are often discovered incidentally. We hypothesize that the vast majority of incidentally noted low-grade chondroid lesions in the proximal humerus can be observed and have a minimal risk of malignant transformation in the short-term follow-up period.
This was a level III retrospective cohort study of patients seen by four orthopaedic oncologists at a single academic institution from 2000 to 2022. Following Institutional Review Board approval, all patients with diagnoses of ECs or CSs (low- and high-grade) of the proximal humerus were identified (n=119) (Figure 1). All patients with high-grade, grade 2 or 3 CSs (n=1, grade 2) and multiple hereditary enchondromatosis (n=2, one grade 2 CS and one EC) were excluded to yield a study population of 116 ECs and grade 1 CSs (Table 1). All patients underwent a diagnostic evaluation, which included a clinical examination and diagnostic imaging (radiographs and axial imaging, consisting of either computed tomography (CT) or magnetic resonance imaging (MRI)).
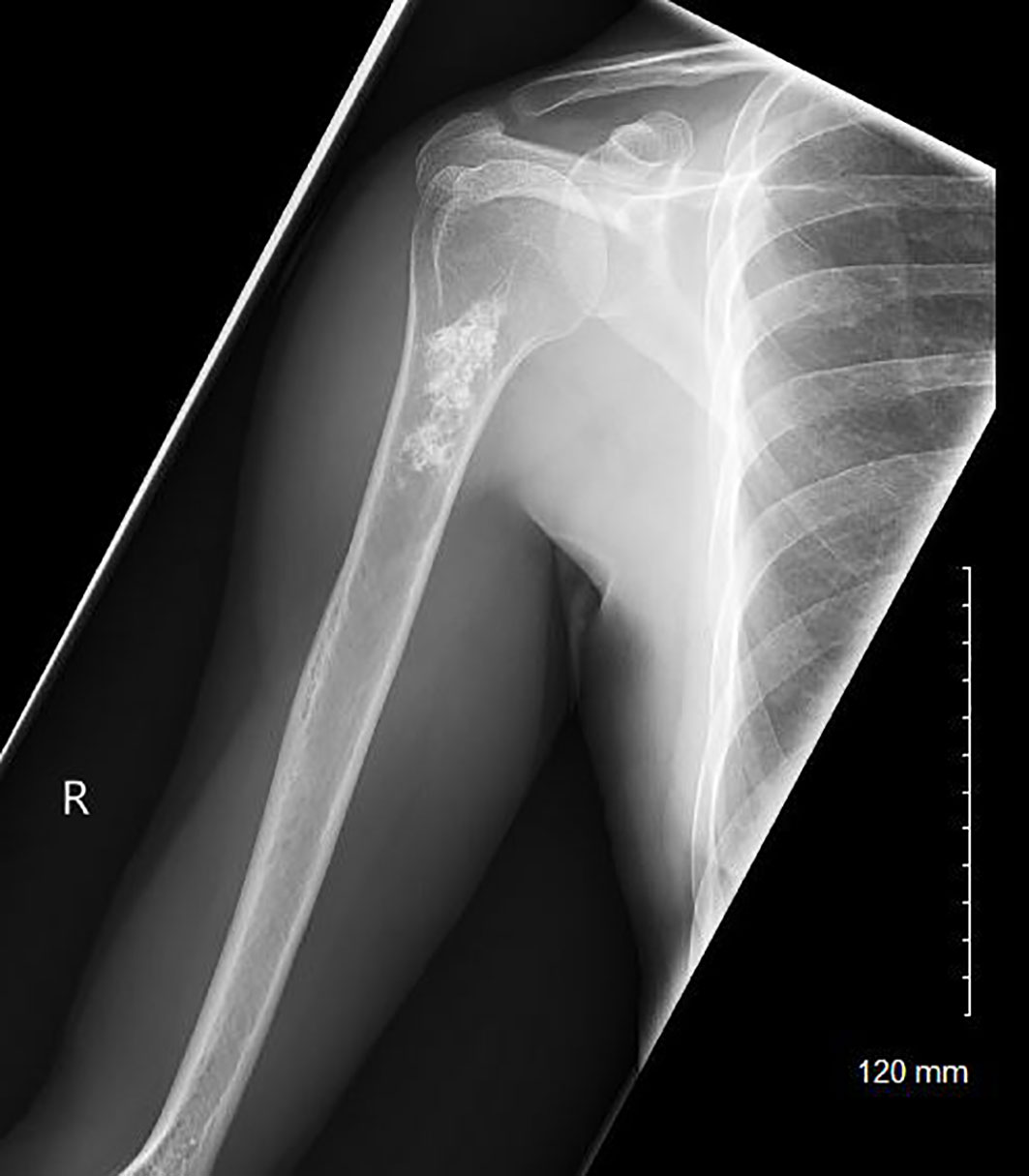
Figure 1 A radiograph of a patient with a low-grade chondroid lesion of the metadiaphyseal region of her right proximal humerus.
Data collection was based on a combination of chart review, imaging reports, and the original radiographs, CTs, and MRIs. The length of follow-up period was calculated from the first documentation of the lesion on imaging to the last follow-up radiograph for each patient. The size of the lesions at initial and final follow-up was determined by measuring the lesion using the Picture Archiving and Communication System (PACS) (McKesson Corporation, San Francisco, CA) at the maximal distance in the proximal/distal direction. The measurement was made in parallel to the cortex of the diaphysis, and measurements were confirmed with the imaging report in the patient record if it was available. Endosteal scalloping, surrounding bone and soft tissue edema, and the presence or absence of soft tissue masses were obtained from CT or MRI on the initial presentation of the patient. Other clinical information was collected from clinic notes. Tumors were considered incidentally found if other plausible explanations for the patient’s symptoms were identified on physical exam or imaging.
Data collection was performed using Microsoft Excel (Microsoft, Redmond, WA). Descriptive statistics were calculated for each of the final groups, with means and standard deviations calculated for size, age, follow-up time, and time to surgery. All other characteristics were reported as percentages of the overall group data. Fisher’s Exact Testing was used to generate statistical significance for various qualitative characteristics of biopsy-proven ECs versus CS, while t-testing was utilized for quantitative characteristics such as lesion size. The statistical significance threshold was set at p<0.05.
The study population was divided into two groups: patients intended for conservative treatment (n=90) and patients proceeding directly to surgery (n=26) (Figure 2). The conservative patient group consisted of patients who were originally intended for observation of the tumors with serial radiographic imaging at 3 months, 6 months and then yearly, per the guidelines established by Marco et al. (7). Patients without at least 3 months of follow-up with radiographs were excluded (n=26), leaving 64 in the final group intended for conservative treatment. The patients that proceeded directly to surgery included patients who displayed concerning radiographic findings or clinical findings in which conservative treatment was not recommended, such as in patients with evidence of endosteal scalloping or surrounding bone or soft tissue edema. To evaluate a more homogenous patient population, four of the original 26 patients in the surgical treatment group were excluded from the current study because the surgeries were performed for reasons unrelated to the concern for malignancy or symptoms from the lesion. These surgeries included two open reduction and internal fixation procedures for pathologic fractures, one rotator cuff repair with intended anchor placement over the tumor location, and one shoulder arthroplasty (Figure 2).
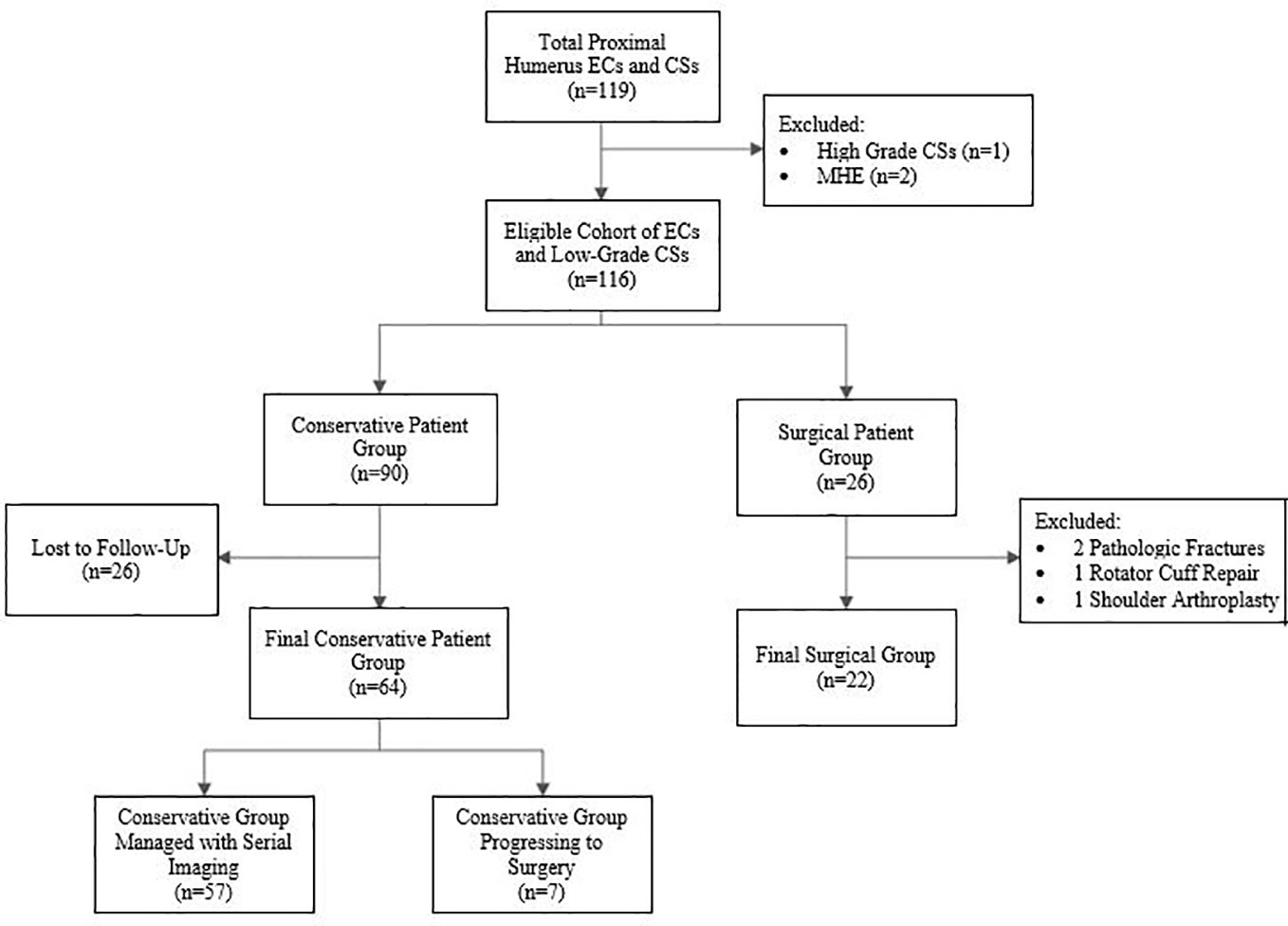
Figure 2 Flow chart of the inclusion and exclusion criteria for the final patient groups. ECs, enchondromas; CSs, chondrosarcomas; MHE, Multiple Hereditary Enchondromatosis.
Table 2 contrasts clinical and radiographic characteristics of ECs and low-grade CSs that were confirmed on pathology or biopsy. Overall, 79% of low-grade chondroid tumors in the proximal humerus were incidentally found secondary to work-up for other shoulder pathology (Table 3).
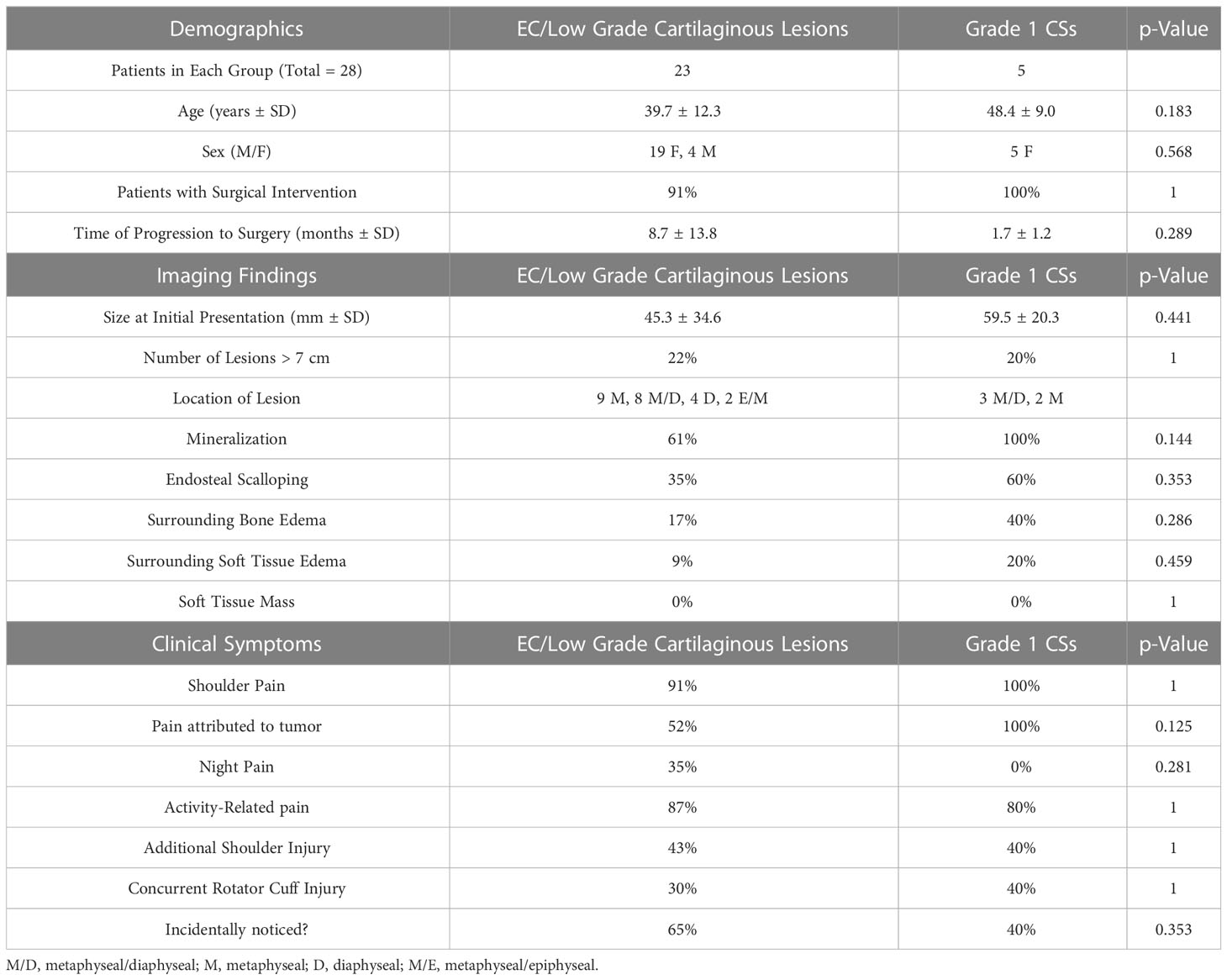
Table 2 Clinical and radiographic characteristics of ECs and low-grade CSs that were confirmed on pathology or biopsy.
64 patients were initially conservatively managed (Figure 2, Table 1). 57 of these patients (89%) continued with conservative treatment and did not progress to surgery, and the average follow-up was 55.3 months. Average size of the 57 lesions at initial identification was 4.68 ± 3.0cm, while average size at most recent radiologic follow-up was 4.83 ± 3.11cm. 9/57 lesions (18%) were larger than 7cm in length on initial finding. Almost all lesions remained stable in length at final follow-up, with only 4/57 (7%) total lesions increasing in length by ≥1cm at an average of 105.25 months of follow-up, and only 1/9 (11%) lesions >7cm increasing in length. None of these conservatively managed lesions were identified to have radiologic characteristics suggestive of transformation to a more malignant state over the course of their care.
Seven patients originally intended for conservative management (11%) progressed to surgery after a mean conservative treatment time of 20.3 months. All 7 underwent curettage with bone grafting, with 3/7 involving prophylactic plating. At a mean of 53.1 months of follow-up, 5 of the 7 surgical patients (71%) experienced persistent pain, but there were no other notable post-operative complications noted. Indications (some patients had multiple) for eventual surgical intervention were persistent pain (100%) and growth of tumor (29%). All lesions were diagnosed as enchondromas or well differentiated chondroid lesions following pathology review, except for one patient whose pathology data was inaccessible. Further evaluation of these 7 tumors did not reveal any malignant transformation to intermediate or high-grade CSs.
The surgical treatment group consisted of 22 patients indicated for surgery without preceding conservative treatment. Indications for surgery (some patients had multiple) were pain (59%), concern for CS or CS identified on biopsy (40%), endosteal scalloping (9%), lesion breaching the majority of the cortex (5%), and concern for chondroblastoma (5%).
All 22 patients were surgically treated with curettage and bone grafting (Figure 3), 9 of which also involved prophylactic plating. At an average follow-up of 57.3 months, 41% of patients noted continued pain at their most recent follow-up. Four post-operative complications were noted (18%): 2 patients required hardware removal, 1 patient experienced post-operative adhesive capsulitis, and 1 patient experienced a pathologic fracture through the curettage site secondary to multiple post-operative falls. Pathology noted 15 ECs or well-differentiated cartilaginous lesions (68%) and 5 low-grade CSs (23%). Pathology results were not available for two patients. At most recent follow-up, no tumor recurrences or malignant transformations were identified in the surgical treatment group.
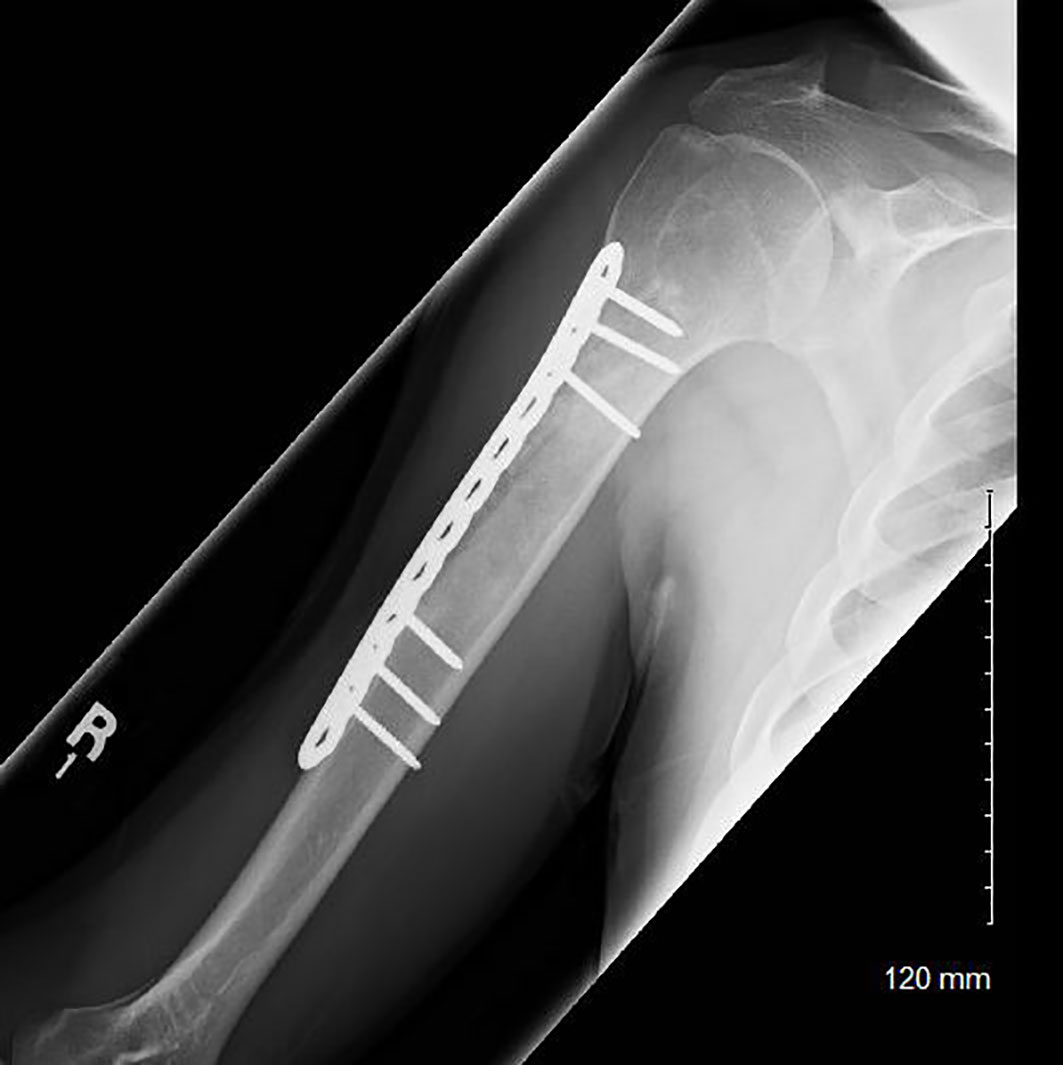
Figure 3 Post-operative radiograph of a patient who underwent curettage, grafting, and plating of a low-grade chondroid lesion of his proximal humerus.
28 of the 90 patients met inclusion criteria and had lesions with a biopsy-proven diagnosis. 8 biopsies were performed prior to a potential surgery (4 open core biopsies and 4 image-guided percutaneous core biopsies), and 20 were sent for pathologic diagnosis at the time of operative intervention. 23 of the biopsied patients (82%) were diagnosed with ECs or well-differentiated cartilaginous lesions, while 5 patients (18%) were diagnosed with low-grade CSs. While more patients with biopsy-proven CSs showed radiologic characteristics like endosteal scalloping, mineralization, and surrounding bone or soft tissue edema, there were no statistically significant differences between the two types of lesions.
This study was a retrospective review of 86 patients with low grade chondroid lesions of the proximal humerus, and none of the 86 lesions ultimately transformed to a more malignant state. Given the exceedingly low risk of malignant transformation combined with the long-term stability of these lesions and low potential for significant growth (exemplified by only 7% of conservatively managed lesions increasing in length by >1cm at final multi-year follow-up), we believe that the majority of low-grade chondroid lesions in the proximal humerus should be initially managed with conservative treatment and serial radiographic imaging. Although patients may experience small growth of their lesion over time, many remain asymptomatic and do not show other concerning findings for malignant transformation on imaging. Because of this relative stability, we believe that small growth of lesions without development of significant symptoms or other concerning imaging findings is not a direct indication for surgical intervention. Similarly, while a length >7 cm has previously been cited as a surgical indication due to concerning potential for malignant transformation (1), our study showed continued stability of almost all lesions over this size and we do not believe that this alone should warrant surgery without the presence of significant symptoms or additional concerning imaging findings.
There was a small percentage (11%) of patients initially indicated for conservative management who progressed to surgery after an average of 20.3 months; however, none of these patients had malignant transformation of their lesions and the majority progressed to surgery because of persistent pain. This is consistent with one other study (18) which reported a 16% surgical rate following initial conservative treatment of cartilaginous tumors of the long bones. While pain was often our primary surgical indication, 71% of these patients still experienced persistent post-operative pain at an average of 53.1 months. While multiple studies have proposed that low-grade chondroid lesions throughout the body may be managed conservatively with serial imaging (1, 2, 6, 7, 12, 13, 19), we believe our current study is one of the first to follow these patients with proximal humerus tumors in the short-term to determine the possibility of malignant transformation or failure of conservative treatment.
Few studies have evaluated the clinical outcomes after surgical intervention of primary bone tumors in the proximal humerus, and many of these studies focus on higher-grade tumors, including osteosarcomas, Ewing’s sarcomas, giant cell tumors, and chondrosarcomas, which are often treated with allograft implants (20–23). One study highlighting atypical cartilaginous tumors of the proximal humerus (24) showed that resection and cementing of these lesions led to excellent patient satisfaction and low post-operative pain, though another study by the same author (25) showed that surgery did not provide superior outcomes to clinical observation for cartilaginous tumors of long bones. The latter study appears to be more consistent with our study findings, as many patients achieved excellent outcomes with clinical observation and serial radiographic follow-up. In our study, 26% of our final patient population went directly to surgery for their low-grade proximal humerus cartilaginous lesions. In comparison to the conservative group, these patients had higher rates of concerning findings on imaging, including endosteal scalloping (50% versus 14%), surrounding bone edema (23% versus 9%), and size greater than 7cm (23% versus 16%). Despite these concerning imaging features, only 23% of the surgical group demonstrated low-grade CSs on pathology.
At a mean follow-up of 57.3 months post-operatively, the immediate surgical group also demonstrated persistent pain in 41% of patients and complications in 18%. The group of patients who were originally intended for conservative management but progressed to surgery also experienced persistent pain in 71%; however no notable complications were experienced in these patients. We hypothesize that the reports of high post-operative pain are at least partially related to concomitant shoulder pathology in the patients, which were present in 45% of the entire surgical population. This hypothesis is consistent with the findings of other studies (6, 25) which show that ECs usually present in conjunction with additional shoulder pathology.
Currently, literature has reported that certain clinical and radiographic criteria may help in distinguishing between ECs and CSs; nevertheless, there is controversy and most studies have not distinguished between low- and high-grade CSs. In this study, Table 2 illustrates the different characteristics of ECs and low-grade CSs which were confirmed on pathology or biopsy. Qualitatively, albeit with a small sample size, our data indicated ECs are more common than low-grade CSs and may be less likely to present with concerning findings on imaging, such as a larger size, endosteal scalloping, mineralization, or surrounding bone or soft tissue edema. These findings do correlate with the 2020 WHO classification of bone tumors (8) and previous studies reporting that CSs may be significantly increased in size in comparison to ECs (3, 16). Murphey et al. (16) also reported that CSs (low- and high-grade were not differentiated) displayed deeper and more extensive scalloping, pathologic fracture, more cortical destruction, and presence of soft tissue masses. However, it should be noted that a more recent study reported a low reliability in differentiating benign from malignant cartilaginous tumors, even for experienced radiologists and pathologists (12).
The presence of pain has often been cited as an indicator of CS in comparison to ECs, but this is controversial and some studies have shown significant differences in pain between the two lesions (16, 26, 27) while others have not (2). In one of the few studies comparing low-grade CSs to ECs, Welkering et al. (27) reported that low-grade CSs demonstrated significantly increased pain compared to ECs. Qualitatively in our study, pain was similar for these low-grade lesions that underwent surgery or biopsy (91% and 100% for ECs and low-grade CSs, respectively). However, our conservative treatment group, whose lesions were infrequently biopsied and theoretically would be more likely to be of a lower grade, did show decreased overall pain (79%) as compared to the surgical group.
This study has some notable limitations. Importantly, 29% of patients did not receive follow-up imaging at 3 months following the initial visit. Many of these patients who did not follow up may have been initially referred for clearance to undergo other shoulder procedures. It is likely that a number of these patients had routine follow up with their referring provider following surgery and were not sent back to us given the likely stability of these lesions as demonstrated in this study. While this is a reasonably large group of patients, we believe that many of these patients ultimately had incidentally found low-grade chondroid lesions which were asymptomatic and did not require long-term follow-up. All patients progressing with conservative treatments were informed that their lesions were likely to be benign, but the potential for malignant transformation was discussed with all patients. As the primary musculoskeletal oncology providers in the region, we would have expected to see any patients lost to follow-up whose lesions would have ultimately become symptomatic and transformed to higher-grade lesions. Additionally, our study was a short-term follow-up of patients with low-grade cartilaginous lesions, and we cannot use the lack of malignant transformation to assume that these lesions will be stable in the long-term follow-up period. Our mean follow-up for the conservative group was 55.3 months, and our final study group consisted of all patients who returned for at least one radiographic follow-up at only 3 months in order to get the most accurate sense of how many patients would fail conservative treatment. We therefore recommend further studies to explore the stability of these lesions in the long-term.
Based on our results, we believe there is a clear group of patients who do not require surgical intervention for low-grade chondroid lesions. We recommend a treatment algorithm as shown in Figure 4. Those patients with a clear source of their shoulder pain unrelated to their tumor and without concerning radiologic characteristics, such as endosteal scalloping or bone or soft tissue edema, likely will not benefit from surgical intervention in the short-term. In these lesions without concerning imaging findings, consideration should also be taken regarding whether a biopsy is truly necessary. Given the stability and low risk nature of these low-grade chondroid lesions, biopsy is often not necessary and may increase the risk for complications on its own (28–30). As the lesion is very likely to remain stable overall, we would instruct these patients to follow-up clinically for yearly physical examination with radiographs, with the caveat that they should present for further evaluation if their pain significantly increases. In general, we would recommend that after 2 to 3 years of annual radiographic follow-up without significant change and a lack of concerning features on imaging, patients should present to clinic as needed if they experience new or increasing symptoms. We would also recommend initiating physical therapy or offering steroid injection under fluoroscopic guidance for patients with continued pain, especially in those with an additional unrelated shoulder injury identified on imaging or clinical exam.
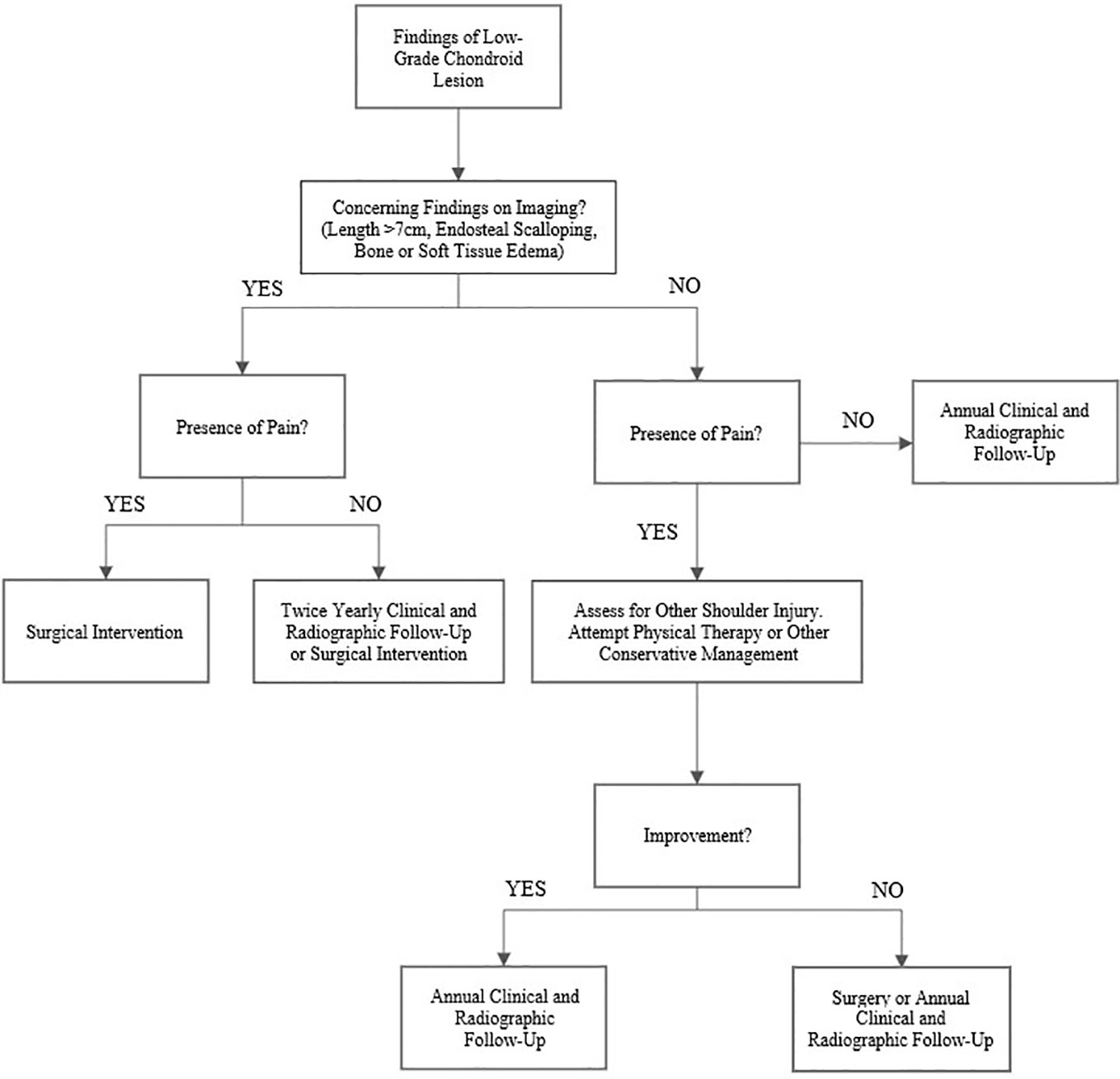
Figure 4 Proposed treatment algorithm for low-grade chondroid tumors in the proximal humerus. In general, we would recommend that after 2 to 3 years of yearly radiographic follow-up without significant change and a lack of concerning findings on imaging, patients should be instructed to present to clinic as needed if their pain increases.
For those patients with pain and potentially concerning imaging findings such as significant endosteal scalloping or bone or soft tissue edema, we would recommend proceeding to surgery directly as their lesion is more likely to be contributing directly to their pain. This is particularly true for patients without evidence of other concomitant shoulder pathology such as rotator cuff injury. Although surgical curettage and grafting may help with overall symptoms, it is important to communicate that there is a high likelihood that patients will still experience some level of persistent pain post-operatively. Asymptomatic patients with incidentally noted lesions that have concerning features on imaging may attempt conservative treatment, but these patients should have more frequent clinical and radiographic follow-up to ensure that their lesions do not progress to a more malignant state.
Most low-grade cartilaginous lesions of the proximal humerus can be managed with conservative treatment. Patients who present with more concerning findings such as larger lesion size, endosteal scalloping, persistent pain, the presence of bone or soft tissue edema, or a lack of another explanation for their pain may be considered for surgical curettage. We propose that patients with a clear source of their shoulder pain unrelated to their tumor and without concerning characteristics on imaging likely do not require surgical intervention in the short-term and can be managed with serial annual radiographic imaging. In the absence of significant pain or functional changes, given the low risk of malignant conversion of these lesions, patients do not require persistent follow-up imaging following demonstrated imaging stability documented over 2-3 years. Lastly, we would counsel patients with significant pain that surgery may not completely alleviate their pain post-operatively.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical College of Wisconsin Institutional Review Board #5. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
JN, AW, DH, MB, and DK conceived and planned the research, and performed the patient care as described in the manuscript. CL, LA, and JC planned the project, carried out the retrospective review, and analyzed the data. CL and LA took the lead in writing the manuscript, while all authors provided critical feedback and helped shape the final research project and manuscript. All authors interpreted and analyzed the results. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brien EW, Mirra JM, Kerr R. Benign and Malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I. The intramedullary cartilage tumors. Skeletal Radiol (1997) 26(6):325–53. doi: 10.1007/s002560050246
2. Chang CY, Garner HW, Ahlawat S, Amini B, Bucknor MD, Flug JA, et al. Society of Skeletal Radiology- white paper. Guidelines for the diagnostic management of incidental solitary bone lesions on CT and MRI in adults: bone reporting and data system (Bone-RADS). Skeletal Radiol (2022) 51(9):1743–64. doi: 10.1007/s00256-022-04022-8
3. Geirnaerdt MJ, Hermans J, Bloem JL, Kroon HM, Pope TL, Taminiau AH, et al. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. AJR Am J Roentgenol (1997) 169(4):1097–104. doi: 10.2214/ajr.169.4.9308471
4. Herget GW, Strohm P, Rottenburger C, Kontny U, Krauß T, Bohm J, et al. Insights into Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, Malignant transformation and the follow up. Neoplasma (2014) 61(4):365–78. doi: 10.4149/neo_2014_046
5. Ryzewicz M, Manaster BJ, Naar E, Lindeque B. Low-grade cartilage tumors: diagnosis and treatment. Orthopedics (2007) 30(1):35–46; quiz 47-8. doi: 10.3928/01477447-20070101-08
6. Levy JC, Temple HT, Mollabashy A, Sanders J, Kransdorf M. The causes of pain in benign solitary enchondromas of the proximal humerus. Clin Orthop Relat Res (2005) 431):181–6. doi: 10.1097/01.blo.0000150114.19489.c4
7. Marco RA, Gitelis S, Brebach GT, Healey JH. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg (2000) 8(5):292–304. doi: 10.5435/00124635-200009000-00003
8. Choi JH, Ro JY. The 2020 WHO classification of tumors of bone: an updated review. Adv Anat Pathol (2021) 28(3):119–38. doi: 10.1097/PAP.0000000000000293
9. Donthineni R, Ofluoğlu O. Solitary enchondromas of long bones: pattern of referral and outcome. Acta Orthop Traumatol Turc (2010) 44(5):397–402. doi: 10.3944/AOTT.2010.2121
10. Altay M, Bayrakci K, Yildiz Y, Erekul S, Saglik Y. Secondary chondrosarcoma in cartilage bone tumors: report of 32 patients. J Orthop Sci (2007) 12(5):415–23. doi: 10.1007/s00776-007-1152-z
11. Brown MT, Gikas PD, Bhamra JS, Skinner JA, Aston WJ, Pollock RC, et al. How safe is curettage of low-grade cartilaginous neoplasms diagnosed by imaging with or without pre-operative needle biopsy? Bone Joint J (2014) 96-B(8):1098–105. doi: 10.1302/0301-620X.96B8.32056
12. Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am (2007) 89(10):2113–23. doi: 10.2106/JBJS.F.01530
13. Wodajo FM. Top five lesions that do not need referral to orthopedic oncology. Orthop Clin North Am (2015) 46(2):303–14. doi: 10.1016/j.ocl.2014.11.012
14. Hong ED, Carrino JA, Weber KL, Fayad LM. Prevalence of shoulder enchondromas on routine MR imaging. Clin Imaging (2011) 35(5):378–84. doi: 10.1016/j.clinimag.2010.10.012
15. Woltsche JN, Smolle M, Szolar D, Bergovec M, Leithner A. Prevalence and characteristics of benign cartilaginous tumours of the shoulder joint. An MRI-based study. Skeletal Radiol (2023). doi: 10.1007/s00256-023-04375-8
16. Murphey MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics (1998) 18(5):1213–37; quiz 1244-5. doi: 10.1148/radiographics.18.5.9747616
17. Akoh CC, Craig E, Troester AM, Miller BJ. Radiographic enchondroma surveillance: assessing clinical outcomes and costs effectiveness. Iowa Orthop J (2019) 39(1):185–93.
18. Deckers C, Schreuder BH, Hannink G, de Rooy JW, van der Geest IC. Radiologic follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. J Surg Oncol (2016) 114(8):987–91. doi: 10.1002/jso.24465
19. Ahmed S, Jubouri S, Mulligan M. Incidental long bone cartilage lesions: is any further imaging workup neede. Skeletal Radiol (2021) 50(6):1189–96. doi: 10.1007/s00256-020-03664-w
20. Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am (2009) 91(10):2406–15. doi: 10.2106/JBJS.H.00815
21. Black AW, Szabo RM, Titelman RM. Treatment of Malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J Shoulder Elbow Surg (2007) 16(5):525–33. doi: 10.1016/j.jse.2006.12.006
22. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am (1999) 81(8):1138–46. doi: 10.2106/00004623-199908000-00009
23. Mourikis A, Mankin HJ, Hornicek FJ, Raskin KA. Treatment of proximal humeral chondrosarcoma with resection and allograft. J Shoulder Elbow Surg (2007) 16(5):519–24. doi: 10.1016/j.jse.2006.10.010
24. Omlor GW, Lohnherr V, Lange J, Gantz S, Merle C, Fellenberg J, et al. Enchondromas and atypical cartilaginous tumors at the proximal humerus treated with intralesional resection and bone cement filling with or without osteosynthesis: retrospective analysis of 42 cases with 6 years mean follow-up. World J Surg Oncol (2018) 16(1):139. doi: 10.1186/s12957-018-1437-z
25. Omlor GW, Lohnherr V, Lange J, Gantz S, Mechtersheimer G, Merle C, et al. Outcome of conservative and surgical treatment of enchondromas and atypical cartilaginous tumors of the long bones: retrospective analysis of 228 patients. BMC Musculoskelet Disord (2019) 20(1):134. doi: 10.1186/s12891-019-2502-7
26. Mirra JM, Gold R, Downs J, Eckardt JJ. A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones. A clinicopathologic analysis of 51 cases. Clin Orthop Relat Res (1985) 201):214–37. doi: 10.1097/00003086-198512000-00035
27. Welkerling H, Kratz S, Ewerbeck V, Delling G. A reproducible and simple grading system for classical chondrosarcomas. Analysis of 35 chondrosarcomas and 16 enchondromas with emphasis on recurrence rate and radiological and clinical data. Virchows Arch (2003) 443(6):725–33. doi: 10.1007/s00428-003-0896-x
28. Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am (1996) 78(5):656–63. doi: 10.2106/00004623-199605000-00004
Keywords: cancer/tumors, clinical outcomes, diagnostic imaging, shoulder, chondroid
Citation: LaPrade CM, Andryk LM, Christensen JL, Neilson JC, Wooldridge AN, Hackbarth DA, Bedi M and King DM (2023) Natural history of intraosseous low-grade chondroid lesions of the proximal humerus. Front. Oncol. 13:1200286. doi: 10.3389/fonc.2023.1200286
Received: 04 April 2023; Accepted: 21 July 2023;
Published: 11 August 2023.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Johanna Patricia Adevoso Canal, University of the Philippines Manila, PhilippinesCopyright © 2023 LaPrade, Andryk, Christensen, Neilson, Wooldridge, Hackbarth, Bedi and King. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manpreet Bedi, bWJlZGlAbWN3LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.