- 1Department of Pathology, Zhongshan Hospital, Xiamen University, Xiamen, China
- 2Department of Pathology, School of Medicine, Sapporo Medical University, Sapporo, Japan
- 3Department of Radiation Oncology, National Disaster Medical Center, National Hospital Organization (NHO), Incorporated Administrative Agency 3256 Tachitawa City, Tokyo, Japan
- 4Department of Radiation Oncology, Xiamen Humanity Hospital Fujian Medical University, Xiamen, China
- 5Department of Radiation Oncology, Faculty of Medicine, Hokkaido University, Sapporo, Hokkaido, Japan
- 6Department of Radiation Oncology, Emeritus of The University of Texas M.D. Anderson Cancer Center, Baylor College of Medicine, Houston, TX, United States
- 7Department of Sapporo High Functioning Radiotherapy Center, Hokkaido Ohno Memorial Hospital, Sapporo, Japan
Purpose: This study is aimed to explore risk factors affect the therapy outcomes of adrenal metastases (AM) for stereotactic body radiation therapy (SBRT) and guide clinical dose selection.
Methods and materials: PubMed, Embase and Web of Science were searched in September 22, 2022 in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA). Subgroup analysis and meta-regression were used to search for sources of heterogeneity and identify risky outcomes factors. Publication bias test and sensitivity analysis were also conducted.
Results: Thirty-three studies with full text from 2009 to 2022 about AM with SBRT on 1483 patients were included. Pooled 1- and 2-year local control (LC) and overall survival(OS) were 81.7% (95% confidence interval [CI], 75.6%-86.5%), 62.8% (95% CI, 53.8%-71.8%), 67.4% (95%CI, 61.8%-73.1%) and 46.5% (95%CI, 40.4%-52.6%), respectively. Biological effective dose (BED, α/β=10Gy) and dose per fraction affected 1-year LC (Qm=23.89, 15.10; P<0.0001, 0.0001). In the range of 60-80Gy (BED10), the group of dose per fraction ≥ 9Gy achieved the excellent 1-year LC (< 9Gy: ≥ 9Gy =78%, 91%; χ2 = 10.16, P = 0.001). Tracking technology significantly affected 1- and 2-year OS (Qm = 5.73, 8.75; P = 0.017, 0.003) and high tracking adoption group showed excellent 1- and 2- year OS (78.7% [95%CI, 68.6%- 88.9%]; and 62.9% [95%CI, 53.1%-72.7%]).
Conclusion: Increasing the dose per fraction appropriately may help control locally AM lesious. Tracking technology might contribute to improve survival of advanced patients with AM. But these results need prospective studies to verify them.
Introduction
The adrenal glands are a common site of metastasis., accounting for up to 38% of metastastatic cancers (1), with melanomas (50%), lung and breast cancers (30-40%), and renal and gastrointestinal tumors (10-20%) (2) being commonly affected due to the adrenal glands’ rich blood supply (3). Advanced imaging and close follow-up programs for cancer patients have led to a rise in their detection, typically at the oligometastatic stage. Targeted local therapies are gaining popularity as ablative therapy may improve outcomes in patients with limited systemic disease burden (1, 2). A paired analysis of 62 patients with isolated AM found that SBRT and laparoscopic adrenalectomy had similar outcomes and survival rates (4), but complications occurred in 37.9% of patients, including ileus or gastroparesis, wound problems, pneumonia, and heart arrhythmia (5). Stereotactic body radiation therapy (SBRT) enables conformal delivery of ablative radiation doses using single or a small number of fractions, resulting in a more effective radio biologic dose (6). SBRT has become an important treatment option for adrenal metastases, with its low toxicity and ability to maintain adrenal function.
However, there are currently no specific clinical guidelines established for SBRT in AM. This has resulted in considerable diversity in prescription doses and fractionation plans in clinical settings (7), with notable fluctuations in 1-year and 2-year LC rates, spanning from 44% to 100% and 27% to 100%, respectively (8) Consequently, it is critical to investigate risk factors and develop reliable models to guide clinical dose selection.
Methods and materials
We conducted a systematic search for relevant studies in PubMed, Embase, and Web of Science, including those published up to July 2022 and updating the search until September 22, 2022. Thirty-three studies published between 2009 and 2022 were included, covering 1483 patients with 1660 lesions (3, 7, 9–38). The screening flow diagram was shown in Supplementary Figure A and clinicopathologic characteristics in Supplementary Table A. The search query used was “stereotactic OR radiosurgery OR sbrt OR sabr OR knife) AND (adrenal/exp OR adrenal) AND (metastasis/exp OR metastasis OR metastases/exp OR metastases OR metastatic)” (39). We excluded studies that did not report treatment outcomes or toxicity data specific to SBRT for AM, studies that did not grade toxicities according to Common Terminology Criteria for Adverse Events (CTCAE) criteria or define radiological responses according to the Response Evaluation Criteria in Solid Tumors criteria (RECIST) criteria, studies without BED10 information, redundant data or those reporting on fewer than 5 patients, or non-English reports were excluded. We followed PRISMA guidelines. Survival outcomes were calculated per patient, while the LC rate was calculated per metastatic lesion. Some outcome values were obtained from local control probability curves using Engauge Digitizer. SBRT was defined as the delivery of higher fractional doses of radiation than conventional fractionation (>1.8-2.5Gy) in a relatively small number of fractions (39). Local control was defined as the absence of progression at the treatment site. Oligometastatic disease defined as limited metastatic disease burden with 5 or fewer lesions in 2 or fewer organ sites. Synchronous metastases were defined as those present at the time of first diagnosis or appearing within 6 months. Since some criteria differed between studies, we performed simple calculations, such as calibrating technology adoption rates based on patients rather than lesions in some studies (7, 32). Biological effective doses were calculated by following quation:
where d is the single radiation dose, n is the number of fractions, and we picked up α/β =10Gy.
A pooled analysis based on maximum likelihood estimation was used to determine weighted study level rates of 1,2-year LC and 1,2-year OS, and subgroup analysis and meta-regression were conducted to explore possible sources of heterogeneity among study results. Qm was used to assess the degree of heterogeneity (variation) among the effect sizes of individual studies. Significant risk factors identified through meta-regression testing also need to undergo collinearity detection. We reported the total number of adverse events rather than estimating a pooled statistic because the reported rates of grade 3+ toxicity were uniformly low and frequently zero.
Statistical analysis
A pooled analysis based on maximum likelihood estimation was used to determine weighted study level rates of 1,2-year LC and 1,2-year OS. The metaprop function in the meta package (version 5.5-0) in R was used to perform a meta-analysis of binomial proportions (version 4.2.1). Subgroup analysis and meta-regression were respectively conducted using meta and metaregression function in the meta package. Variable correlation examination was performed by using scatterplotMatrix function in the car package (version3.1.0). Qm was used to assess the degree of heterogeneity (variation) among the effect sizes of individual studies. Cases with missing covariates were automatically excluded from regression analyses. The difference in BED10 between different groups was compared using an independent samples t-test by SPSS (version 22.0).
Results
Out of 915 records, we included 33 studies published between 2009 and 2022 that reported outcomes for 1483 patients with 1660 lesions of AM. The median follow-up was 13.4 months, ranging from 0 to 124 months, and the median OS was 19 months, ranging from 0.4 to 171 months. The median total dose was 36Gy(10-70Gy) and the median number of fraction was 4.5(1-18). The median dose per fraction was 7.5Gy, ranging from 2 to 30Gy, and the median BED10 was 71.4Gy, ranging from 20 to 180Gy. Among the patients, 61% had were primary lung cancer patients, and 91% were oligometastatic or oligoprogressive patients. Ninety-four percentage of the studies included data on the method of diagnosis, and only three studies reported biopsy tissue confirmation along with fiducial placement. Out of 1483 patients, only 19 (1.3%) patients reported grade3+ toxicity response, and out of 1401 patients, 22 patients (1.6%) experienced adrenal insufficiency. For more detailed information, please refer to Table A in the Supplement Tables. The pooled 1- and 2- year LC were 82% (95% CI, 76%-87%) and 63% (95% CI, 54%-72%), respectively, and the pooled 1- and 2- year OS were 67% (95%CI, 62%-73%) and 47% (95%CI, 40%-53%), respectively. The forest plots, funnel plots and sensitivity analysis were shown in Figures B–D in the Supplement Figures.
BED10 and dose per fraction were significant influence factors of LC
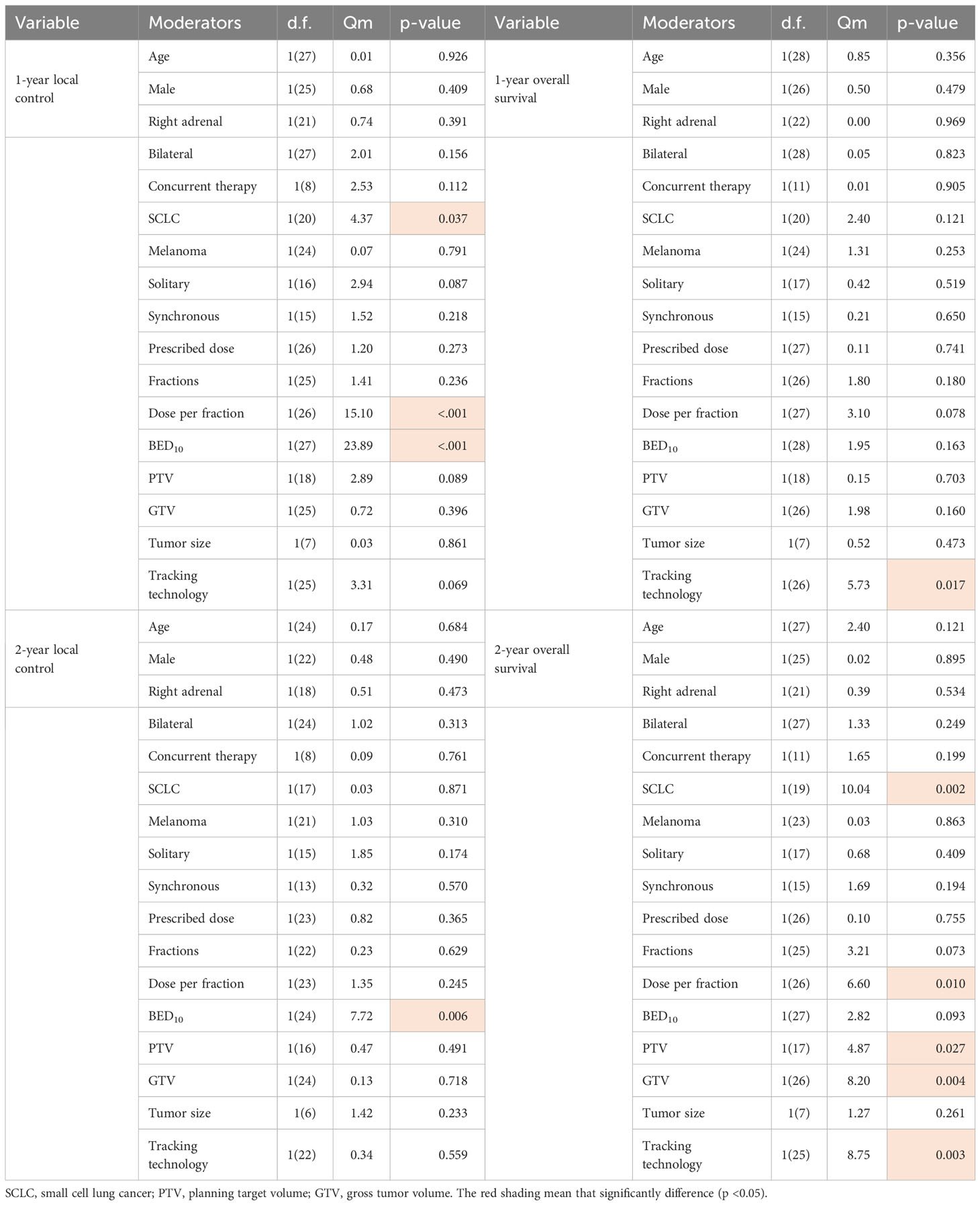
We analyzed the variables about technique, prescription dose and treatment method, or fractionation function to identify sources of heterogeneity for pooled 1- or 2-year LC. As shown in Table 1, BED10, dose per fraction and SCLC were significant influence factors of 1-year LC (Qm = 23.89, 15.10, 4.37; P<0.001,< 0.001, 0.037). Only BED10 play a crucial part for 2-year LC (Qm = 7.72; P = 0.006). We analyzed the correlation and showed there were moderate negative relation between SCLC and BED10 (The coefficient= -0.34, Figure 1), which might suggest the effect on 1-year LC by SCLC related to low BED10. On the other sides, BED10 have a low correlation with dose per fraction (The coefficient =0.24, Figure 1). In conclusion, total dose and dose per fraction should be most important factors for LC.
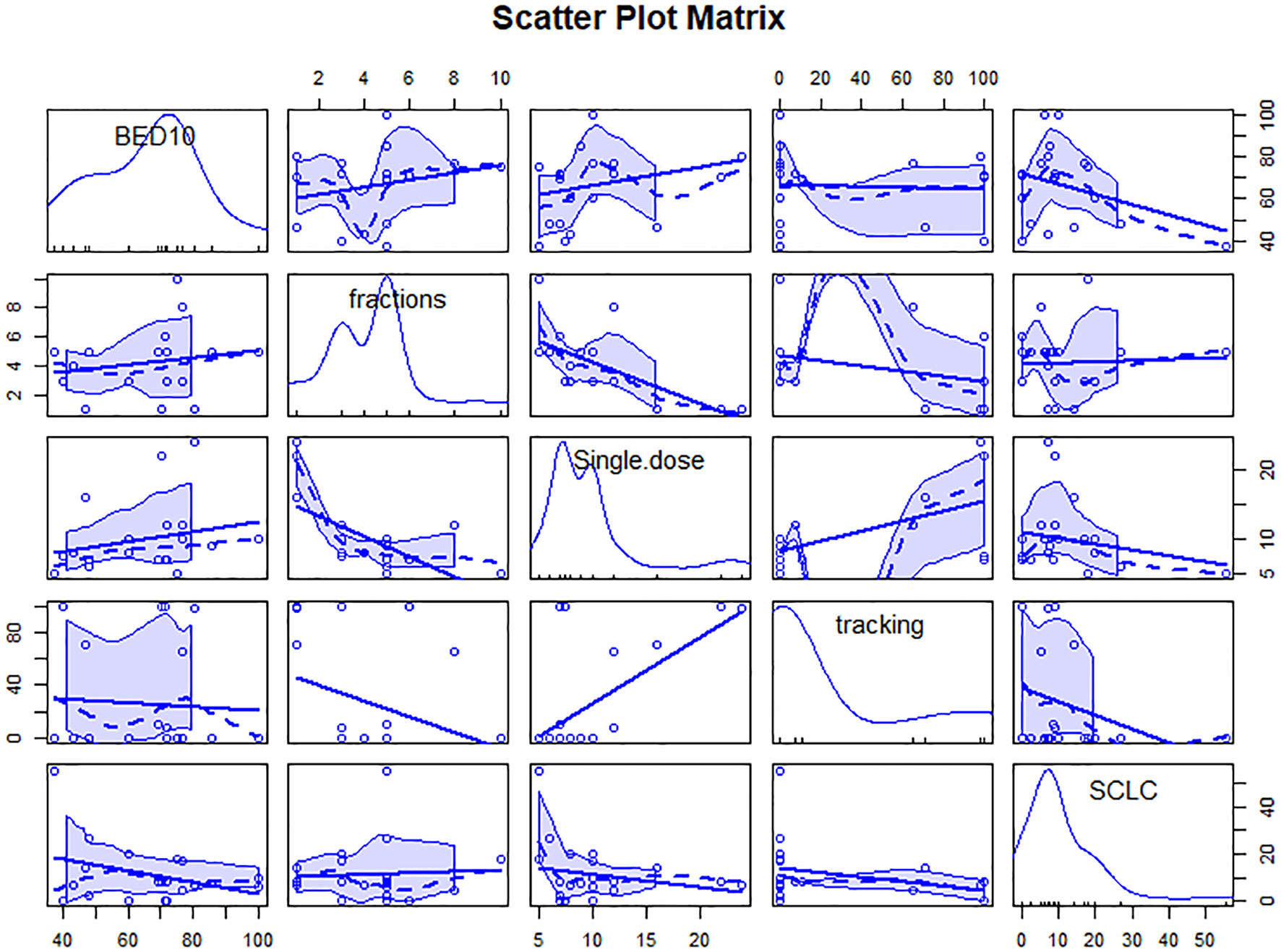
Figure 1 Scatter plot matrix of correlation between variables. The figure contains linear and smooth fitted curves, and corresponding marginal distributions (kernel density maps and axonal whisker maps). single.dose=dose per fraction.
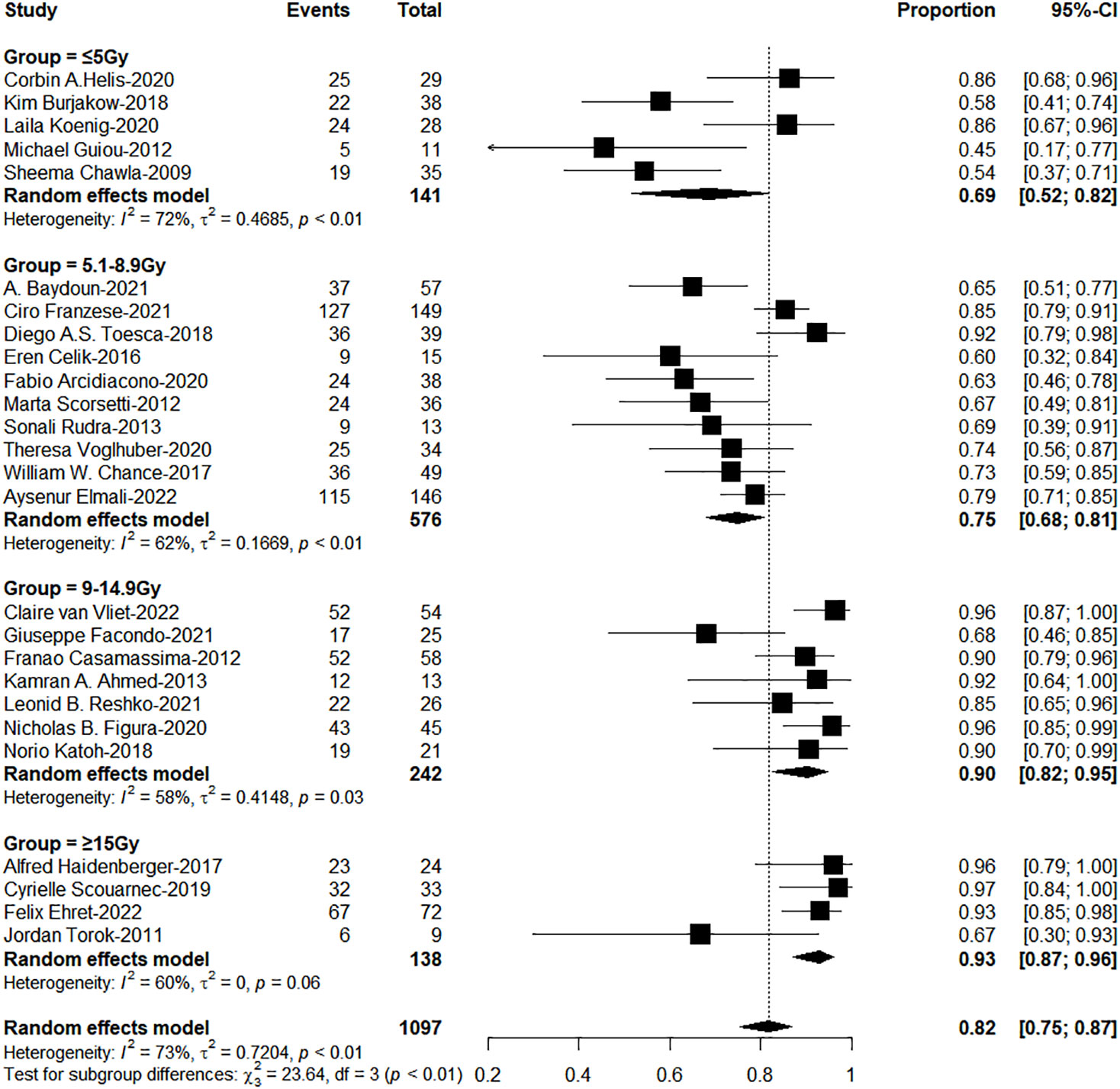
The subgroup analysis results showed the group of BED10 ≥ 100Gy have an excellent 1-year LC (<70Gy: 70-99Gy: ≥100Gy = 69%: 86%: 96%, χ2 = 33.74, P< 0.0001, Figure 2). In clinical practice, due to the presence of surrounding critical organs, we often cannot escalate the radiation dose without the support of tracking technology. The majority of studies still have a total prescription dose ranging from 60 to 80 Gy. Therefore, we further performed subgroup analysis in the range of 60-80Gy (BED10) (N=21 studies, accounting for 64%) and the results showed 1-year LC in the group of dose per fraction≥9Gy (91%: 78%, χ2 = 10.16, P=0.001, Figure 3). On the other hand, dose per fraction ≥ 9Gy have an excellent 1-year LC (≤5Gy: 5.1-8.9Gy: 9-14.9Gy: ≥ 15Gy = 68%: 75%: 90%: 94%, χ2 = 27.93, P =0.0001, Figure 4). Even in the low tracking adoption group (0-20% patients, most of them without tracking adoption (15/18)), dose per fraction still significantly affected 1-year LC (N=17 studies, Qm =13.93, P<0.001), which rules out the role of tracking technology in local control rates for AM.
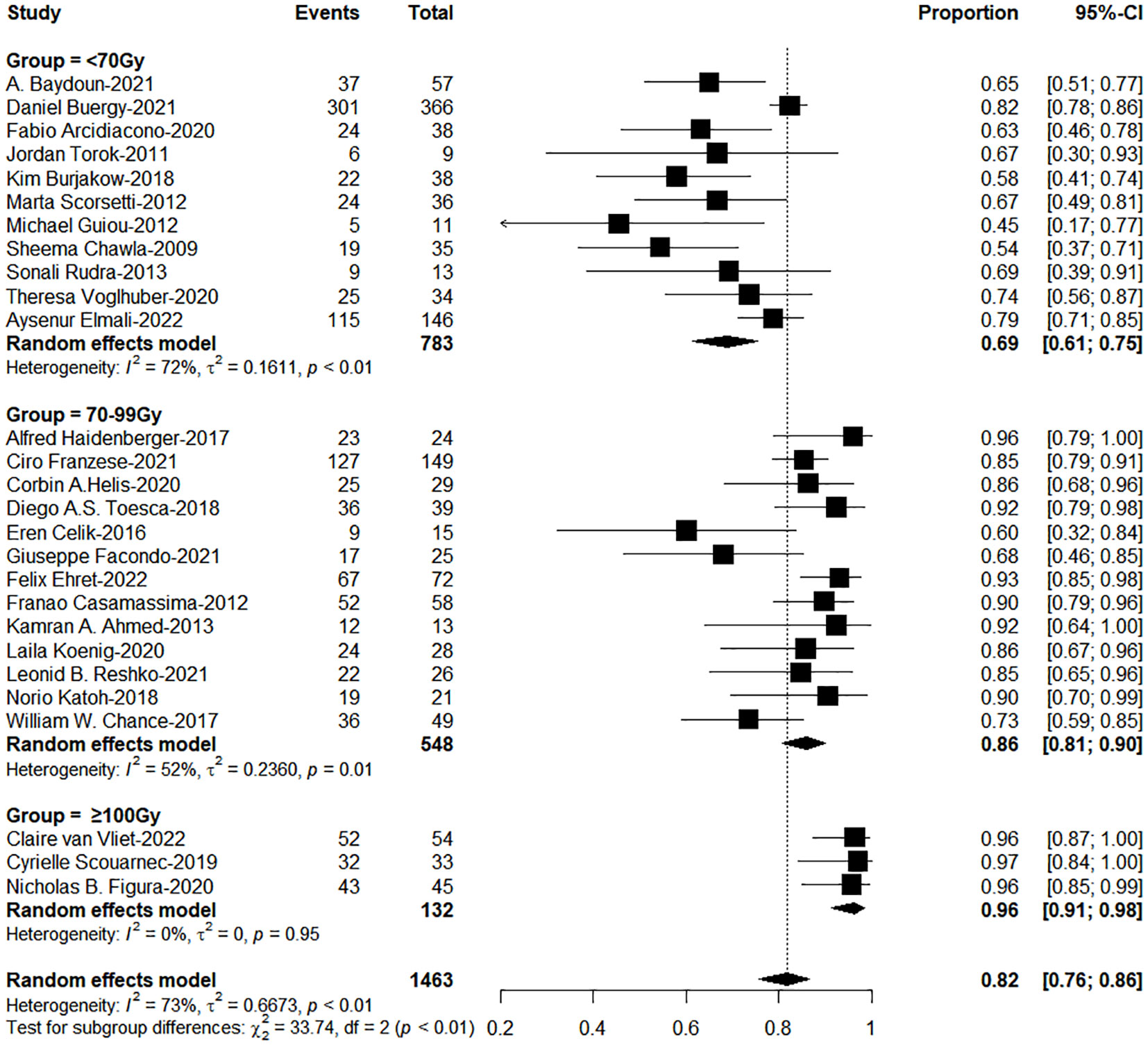
Figure 2 The forest plot of subgroup analysis for one-year local control by biological effective dose (BED, α/β=10Gy).
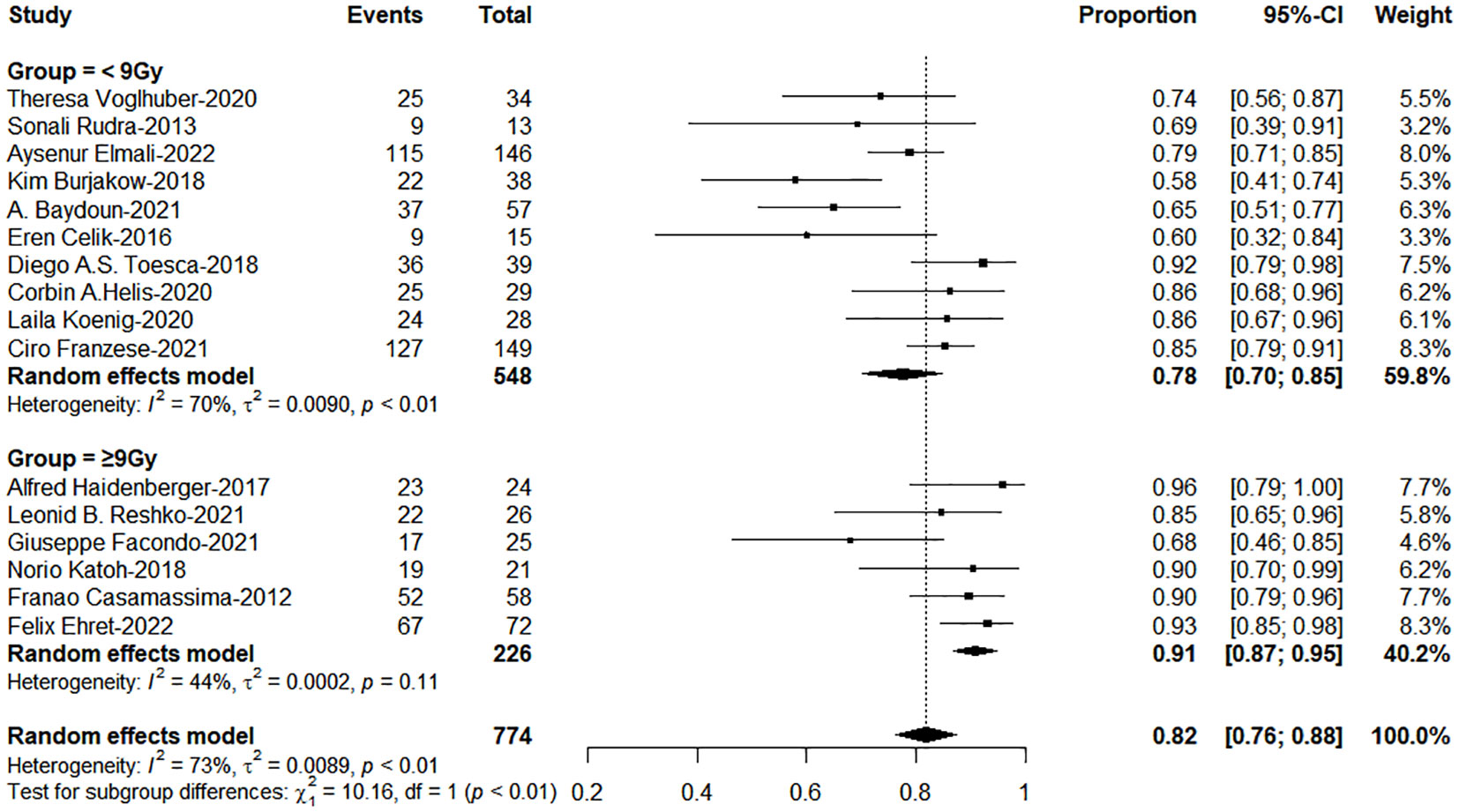
Figure 3 The forest plot of subgroup analysis for 1-year local control by dose per fraction with the range of Biological effective dose (α/β=10)(60-80Gy).
The application of tracking significantly improved overall survival
As shown in Table 1, only tracking technology adoption has a strong correlation with 1-year OS (Qm=5.73; P = 0.017). SCLC, dose per fraction, tumor volume (PTV, GTV), tracking technology had strong effects on 2-year OS (Qm=10.04, 6.60, 4.87, 8.20, 8.75; P = 0.002, 0.010, 0.027, 0.004, 0.003). As shown in Figure 2, tracking technology adoption had a strong correlation with dose per fraction (The coefficient =0.61). But in the low tracking adoption group (0-20% patients, most of them without tracking adoption(15/18)), dose per fraction didn’t significantly affect 2-year OS (N =17 studies, Qm = 0.30, P = 0.584). The high-tracking adoption group received a median BED10 of 75.5Gy (t= -0.837, P =0.410), while the low-tracking adoption group received a median BED10 of 69.4Gy. These results suggested that, with regard to overall survival, the impact of fractionation doses may be contingent upon the use of tracking technology rather than the tracking technology upon fractionation doses.
The total proportion of tracking technology is only 19.3% (257/1332) from published articles. High tracking adoption group got excellent 1-and 2-year OS (0-20% patients: 20%-80% patients: 80%-100% patients = 63%: 67%: 79%, c2 = 6.88; P=0.038; 41%: 46%: 63%, c2 = 12.72, P=0.002, Figures 5, 6). The side effects caused by the use of tracking technology accounted for 1.2% (3/257) of cases, all of which were associated with fiducial placement, including 1 case of spontaneous hematoma absorption, 1 case of pneumothorax, and 1 case requiring chest tube insertion, and these were concentrated in a single study (14). In high-tracking adoption group, the Grade 3+ toxicity response was 0.9% (2/213) and low-tracking adoption group, it was 1.1% (12/1091)(Differences were not statistically significant). Adrenal insufficiency morbidity was 1.8% (4/213) and 0.6% (6/979) separately because in high-tracking adoption group, three patients had only one remaining adrenal gland received SBRT.
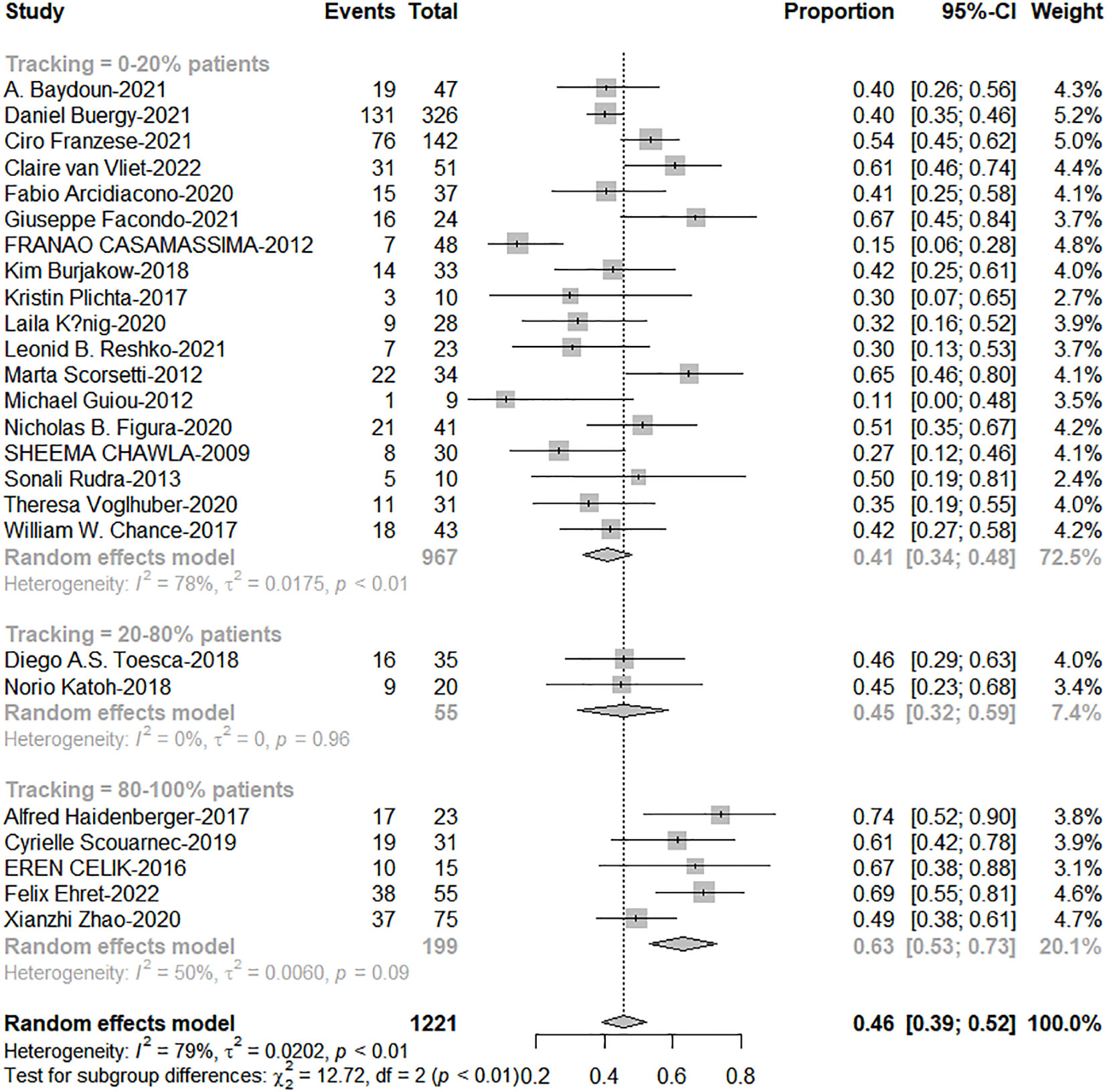
Figure 5 The forest plot of subgroup analysis for 1-year overall survival by tracking technology adoption rate.
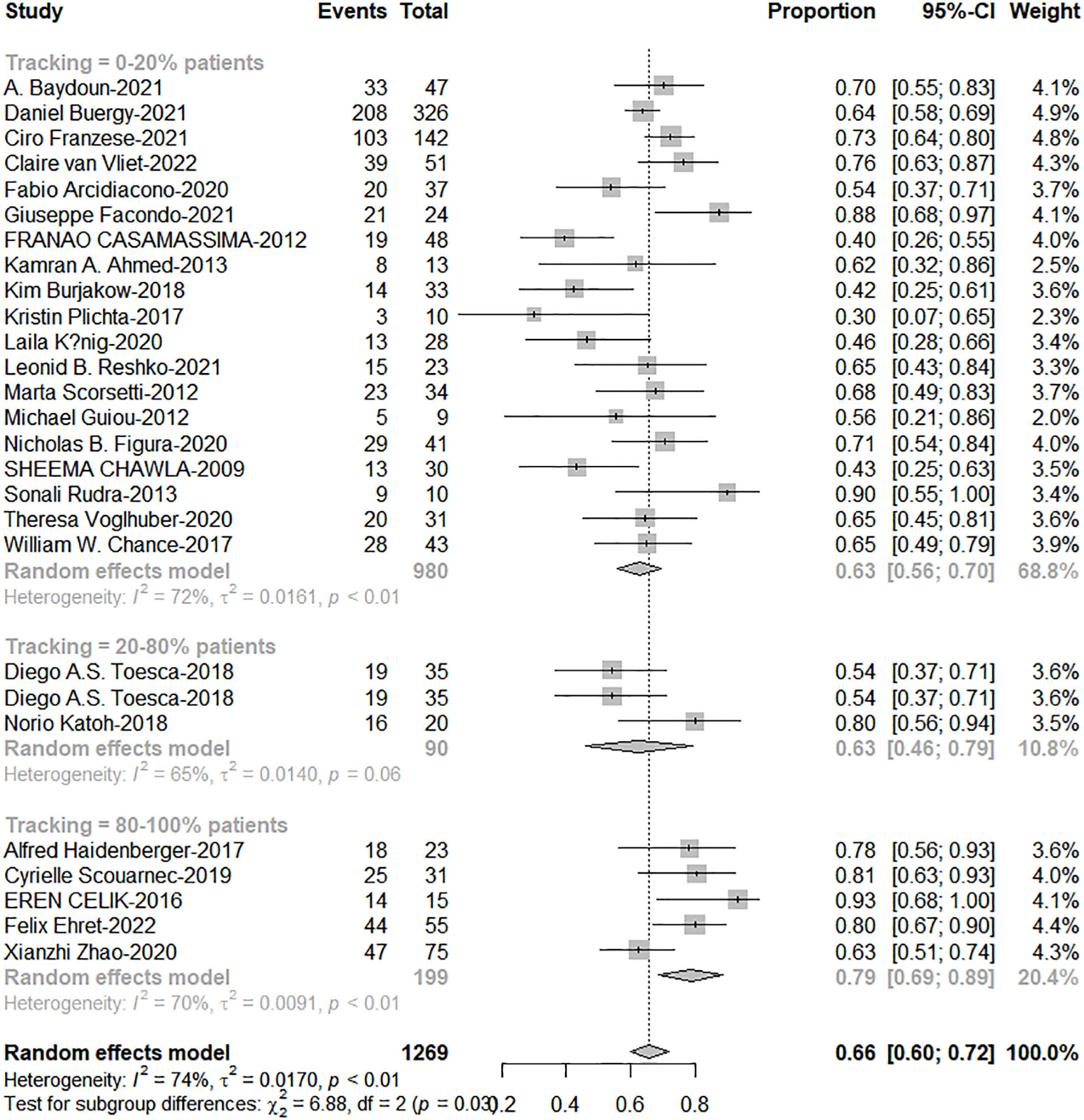
Figure 6 The forest plot of subgroup analysis for 2-year overall survival by tracking technology adoption rate.
Discussion
Delivery of high-dose on adrenal gland can be challenging due to the proximity of risk organs such as the duodenum, small intestine, and stomach, with dose limit violations potentially leading to life-threatening complications (40). In clinical practice, compromises on target doses are necessary (4, 8). Moreover, the overuse of radiation can trigger the expression of certain proteins, such as Transforming Growth Factor (TGF), Indoleamine-pyrrole 2,3-dioxygenase (IDO), and Programmed cell Death-Ligand 1 (PD-L1), which contribute to an increase in immune-suppressive cells like Tregs and tumor-associated macrophages (TAMs) (41), and have harmful effects on the immune system’s ability to fight cancer cells. Thus, seeking a dose scheme under 100Gy of BED10 not only helps to protect the surrounding organs at risk, but also helps to weaken the local immunosuppression around the tumor.
Our study underscores the importance of fractionated doses and emphasizes that fractionated doses should not be less than 9 Gy. When it is not feasible to achieve the currently recommended BED10 ≥ 100 Gy total dose, our study offers a solution by increasing the fractionated dose. This finding aligns with experimental data (42–44), as doses greater than 8-10 Gy, as opposed to doses greater than 5 Gy, can result in rapid endothelial cell apoptosis, leading to extensive tumor cell death and severe hypoxia in the microenvironment. Furthermore, the release of tumor antigens during the death or dying process of tumor cells post high fractionated radiation can trigger an anti-tumor immune response within weeks to months, contributing to the formation of a local immune environment (45, 46). There is evidence that in animal experiments, a dose of 8-10 Gy is optimal, as fractionated radiation at 8 Gy, combined with CTLA-4 inhibitors, can induce a distant effect (47).
Our research demonstrates that the utilization of tracking technology not only contributes to extending the survival of patients with AM but is also highly safe. Real-time monitoring and tracking of tumor location is an emerging technology to enhance the precision of tumor radiotherapy (48). This technology allows for high-dose, high-accuracy irradiation of the tumor, improving treatment effectiveness while avoiding “off-target” effects in the target area, and reducing adverse effects on surrounding tissues (48, 49). A study published in the journal Nature Cancer suggests that damage to tissues caused by “off-target” radiotherapy can locally activate neutrophils in the normal repair process and Notch signal transduction, which can promote cancer metastasis and reduce overall survival (50). For under-irradiated tumors cells, insufficient radiation fails to kill them; instead, it enhances its malignancy and promotes invasion and metastasis (51, 52).
Therefore, the application of real-time tracking technology is essential, especially for patients with AM. We found that 91% of AM patients fall within the current definition of oligometastasis. Multiple studies have shown that aggressive treatment in oligometastatic patients can significantly extend survival (53–55). However, regrettably, the use rate of tracking technology is only 19% (257/1332) and the importance of tracking technology urgently needs to be widely promoted. Of course, we also need to be vigilant about selection bias. Because this portion of patients in the high fiducial adoption group may also have better medical support, which could potentially influence the overall survival outcome. Nevertheless, we cannot deny the importance of adopting tracking radiation therapy, and we hope that prospective studies in the future will confirm our findings.
We are the first to propose a study on the focus of fractional dose and tracking technology in the treatment of AM using SBRT. William C. Chen et al (39) collected 39 studies between 2009 and 2019 to perform first meta-analyze on AM by SBRT. They only analyzed the relationship between the total dose (BED10) and local control, as well as overall survival, and reached the conclusion that dose escalation contributes to local control. They did not analyze and find the significance of fractionation dose and tracking techniques, perhaps due to the inclusion of a large number of abstracts with insufficient information in their study. To extract more data and ensure data accuracy, we excluded all studies that had only abstracts and lacked full texts. Furthermore, due to the recent advancements in tracking technology and the development of imaging techniques, many new articles (14/out of 33) on SBRT treatment for AM have emerged. Therefore, there is an urgent need for updated meta-analyses in this regard.
Our study has some limitations that need to be acknowledged. The inconsistencies in radiation techniques and dosimetry, and the inherent biological inaccuracies in calculating BED by linear-quadratic (LQ) formulas, mean that the reported doses are only informative and should be carefully considered. As our study is a retrospective analysis, it cannot establish causal relationships, and more prospective studies are needed to verify our findings. The results of meta model-based estimates should be interpreted with caution, given the heterogeneity of tumor control estimates extracted from the literature and the variability of diametric data reporting, as well as the definitions and statistical methods used to report tumor control. Despite our efforts to collect data of the same quality, there were still some differences in detail.
Conclusions
SBRT is a safe technique. Constrained by organs at risk, the clinical dose for treating AM often falls within the range of 40-80 Gy, especially in centers with low tracking adoption rates. But we recommend that the minimum dose per fraction should be set around 9 Gy to ensure treatment efficacy.Additionally, the use of tracking techniques may improve the survival rates of advanced AM patients and is strongly recommended. Prospective studies are needed to validate these discoveries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: KK. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: KK and RK. Statistical analysis: XL. Obtained funding: CP. Administrative, technical, or material support: KK, CP. Supervision: KK, CP, RK. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants 2021CXB022 from Medical Innovation Project of Fujian Province of China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1193574/full#supplementary-material
References
1. Franzese C, Franceschini D, Cozzi L, D’Agostino G, Comito T, De Rose F, et al. Minimally invasive stereotactical radio-ablation of adrenal metastases as an alternative to surgery. Cancer Res Treat (2017) 49:20–8. doi: 10.4143/crt.2016.057
2. Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: Final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer (2012) 118:2962–70. doi: 10.1002/cncr.26611
3. Desai A, Rai H, Haas J, Witten M, Blacksburg S, Schneider JG. A retrospective review of cyberKnife stereotactic body radiotherapy for adrenal tumors (Primary and metastatic): winthrop university hospital experience. Front Oncol (2015) 5:185. doi: 10.3389/fonc.2015.00185
4. Howell GM, Carty SE, Armstrong MJ, Stang MT, McCoy KL, Bartlett DL, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol (2013) 20:3491–6. doi: 10.1245/s10434-013-3050-2
5. Metman MJH, Viëtor CL, Seinen AJ, Berends AMA, Hemmer PHJ, Kerstens MN, et al. Outcomes after surgical treatment of metastatic disease in the adrenal gland; valuable for the patient? Cancers (2022) 14:156. doi: 10.3390/cancers14010156
6. Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol (2013) 2:215. doi: 10.3389/fonc.2012.00215
7. Baydoun A, Chen H, Poon I, Badellino S, Dagan R, Erler D, et al. Outcomes and toxicities in oligometastatic patients treated with stereotactic body radiotherapy for adrenal gland metastases: A multi-institutional retrospective study. Clin Transl Radiat Oncol (2021) 33:159–64. doi: 10.1016/j.ijrobp.2021.07.1319
8. Borghesi S, Casamassima F, Aristei C, Grandinetti A, Di Franco R. Stereotactic radiotherapy for adrenal oligometastases. Rep Pract Oncol Radiother (2022) 27:52–6. doi: 10.5603/RPOR.a2021.0104
9. Chance WW, Nguyen Q-N, Mehran R, Welsh JW, Gomez DR, Balter P, et al. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol (2017) 7:E195–203. doi: 10.1016/j.prro.2016.09.005
10. Ahmed KA, Barney BM, Macdonald OK, Miller RC, Garces YI, Laack NN, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol-cancer Clin Trials (2013) 36:509–13. doi: 10.1097/COC.0b013e3182569189
11. Arcidiacono F, Aristei C, Marchionni A, Italiani M, Fulcheri CPL, Saldi S, et al. Stereotactic body radiotherapy for adrenal oligometastasis in lung cancer patients. Br J Radiol (2020) 93. doi: 10.1259/bjr.20200645
12. Buergy D, Wuerschmidt F, Gkika E, Hoerner-Rieber J, Knippen S, Gerum S, et al. Stereotactic or conformal radiotherapy for adrenal metastases: Patient characteristics and outcomes in a multicenter analysis. Int J Cancer (2021) 149:358–70. doi: 10.1002/ijc.33546
13. Burjakow K, Fietkau R, Putz F, Achterberg N, Lettmaier S, Knippen S, et al. Fractionated stereotactic radiation therapy for adrenal metastases: contributing to local tumor control with low toxicity. Strahlentherapie Und Onkol (2019) 195:236–45. doi: 10.1007/s00066-018-1390-3
14. Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: University of florence experience. Int J Radiat Oncol Biol Phys (2012) 82:919–23. doi: 10.1016/j.ijrobp.2010.11.060
15. Celik E. Robot-assisted extracranial stereotactic radiotherapy of adrenal metastases in oligometastatic non-small cell lung cancer. AR (2017) 37. doi: 10.21873/anticanres.11954
16. Chawla S, Chen Y, Katz AW, Muhs AG, Philip A, Okunieff P, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys (2009) 75:71–5. doi: 10.1016/j.ijrobp.2008.10.079
17. Ehret F, Kaul D, Kufeld M, Vom Endt C, Budach V, Senger C, et al. Robotic stereotactic body radiotherapy for the management of adrenal gland metastases: a bi-institutional analysis. J Cancer Res Clin Oncol doi: 10.1007/s00432-022-03943-0
18. Elmali A, Akkus Yildirim B, Cengiz M, Cengiz M, Yuce Sari S, Onal HC, et al. Stereotactic radiotherapy for adrenal metastases: a multi-institutional review of patient characteristics and outcomes. Turkish Society for Radiation Oncology SBRT Group Study (TROD SBRT 10-004). Oncol Res Treat (2022). doi: 10.1159/000527052
19. Facondo G, Vullo G, Valeriani M, Ascolese AM, De Sanctis V, Osti MF. Stereotactic body radiation therapy (SBRT) for patients with oligometastatic/oligoprogressive adrenal metastases: Outcomes and toxicities profile in a monoinstitutional study. Cancer Treat Res Commun (2021) 29:100481–1. doi: 10.1016/j.ctarc.2021.100481
20. Figura NB, Oliver DE, Mohammadi H, Martinez K, Grass GD, Hoffe SE, et al. Novel dose escalation approaches for stereotactic body radiotherapy to adrenal oligometastases A single-institution experience. Am J Clin Oncol-Cancer Clin Trials (2020) 43:107–14. doi: 10.1097/COC.0000000000000634
21. Franzese C, Nicosia L, Facondo G, Lo Faro L, Cuccia F, Vullo G, et al. Stereotactic body radiation therapy for adrenal gland metastases: outcome and predictive factors from a multicenter analysis. Clin Exp Metastasis (2021) 38:511–8. doi: 10.1007/s10585-021-10124-9
22. Gamsiz H, Beyzadeoglu M, Sager O, Demiral S, Dincoglan F, Uysal B, et al. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori (2015) 101:98–103. doi: 10.5301/tj.5000222
23. Guiou M, Mayr NA, Kim EY, Williams T, Lo SS. Stereotactic body radiotherapy for adrenal metastases from lung cancer. J Radiat Oncol (2012) 1:155–63. doi: 10.1007/s13566-012-0037-8
24. Haidenberger A, Heidorn S-C, Kremer N, Muacevic A, Fuerweger C. Robotic radiosurgery for adrenal gland metastases. Cureus (2017) 9. doi: 10.7759/cureus.1120
25. Helis CA, Hughes RT, Nieto K, Ufondu A, Daugherty EC, Farris MK. Adrenal SBRT: a multi-institutional review of treatment outcomes and toxicity. Clin Exp Metastasis (2020) 37:585–92. doi: 10.1007/s10585-020-10052-0
26. Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic Body Radiation Therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlentherapie Und Onkol (2011) 187:245–51. doi: 10.1007/s00066-011-2192-z
27. Katoh N, Onishi H, UChinami Y, Inoue T, Kuriyama K, Nishioka K, et al. Real-time tumor-tracking radiotherapy and general stereotactic body radiotherapy for adrenal metastasis in patients with oligometastasis. Technol Cancer Res Treat (2018) 17. doi: 10.1177/1533033818809983
28. Koenig L, Haefner MF, Katayama S, Koerber SA, Tonndorf-Martini E, Bernhardt D, et al. Stereotactic body radiotherapy (SBRT) for adrenal metastases of oligometastatic or oligoprogressive tumor patients. Radiat Oncol (2020) 15. doi: 10.1186/s13014-020-1480-0
29. Plichta K, Camden N, Furqan M, Abu Hejleh T, Clamon GH, Zhang J, et al. SBRT to adrenal metastases provides high local control with minimal toxicity. Adv Radiat Oncol (2017) 2:581–7. doi: 10.1016/j.adro.2017.07.011
30. Reshko LB, Gaskins JT, Silverman CL, Dunlap NE. Stereotactic body radiation therapy (SBRT) of adrenal gland metastases in oligometastatic and oligoprogressive disease. Rep Pract Oncol Radiother (2021) 26:325–40. doi: 10.5603/RPOR.a2021.0055
31. Scorsetti M, Alongi F, Filippi AR, Pentimalli S, Navarria P, Clerici E, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: A retrospective analysis of 34 patients. Acta Oncol (2012) 51:618–23. doi: 10.3109/0284186X.2011.652738
32. Scouarnec C, Pasquier D, Luu J, le Tinier F, Lebellec L, Rault E, et al. Usefulness of stereotactic body radiation therapy for treatment of adrenal gland metastases. Front Oncol (2019) 9. doi: 10.3389/fonc.2019.00732
33. Shah MM, Isrow D, Fareed MM, Wen N, Ryu S, Ajlouni M, et al. Single institution experience treating adrenal metastases with stereotactic body radiation therapy. J Cancer Res Ther (2019) 15:S27–32. doi: 10.4103/jcrt.JCRT_655_16
34. Rudra S. Stereotactic body radiation therapy for curative treatment of adrenal metastases. (2013) 12:8. doi: 10.7785/tcrt.2012.500320
35. Toesca DAS, Koong AJ, von Eyben R, Koong AC, Chang DT. Stereotactic body radiation therapy for adrenal gland metastases: Outcomes and toxicity. Adv Radiat Oncol (2018) 3:621–9. doi: 10.1016/j.adro.2018.05.006
36. Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: a retrospective review of a noninvasive therapeutic strategy. Future Oncol (2011) 7:145–51. doi: 10.2217/fon.10.165
37. van Vliet C, Dickhoff C, Bahce I, Engelsman AF, Hashemi SMS, Haasbeek CJA, et al. Treatment patterns for adrenal metastases using surgery and SABR during a 10-year period. Radiother Oncol (2022) 170:165–8. doi: 10.1016/j.radonc.2022.02.023
38. Zhao X, Zhu X, Zhuang H, Guo X, Song Y, Ju X, et al. Clinical efficacy of Stereotactic Body Radiation Therapy (SBRT) for adrenal gland metastases: A multi-center retrospective study from China. Sci Rep (2020) 10:7836. doi: 10.1038/s41598-020-64770-2
39. Chen WC, Baal JD, Baal U, Pai J, Gottschalk A, Boreta L, et al. Stereotactic body radiation therapy of adrenal metastases: A pooled meta-analysis and systematic review of 39 studies with 1006 patients. Int J Radiat OncologyBiologyPhys (2020) 107:48–61. doi: 10.1016/j.ijrobp.2020.01.017
40. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann Oncol (2016) 27:v1–v27. doi: 10.1093/annonc/mdw326
41. Jayaraman P, Parikh F, Newton JM, Hanoteau A, Rivas C, Krupar R, et al. TGF-β1 programmed myeloid-derived suppressor cells (MDSC) acquire immune-stimulating and tumor killing activity capable of rejecting established tumors in combination with radiotherapy. OncoImmunology (2018) 7:e1490853. doi: 10.1080/2162402X.2018.1490853
42. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science (2003) 300:1155–9. doi: 10.1126/science.1082504
43. Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol Oncol (1984) 23:217–25. doi: 10.3109/02841868409136015
44. Song CW, Lee Y-J, Griffin RJ, Park I, Koonce NA, Hui S, et al. Indirect tumor cell death after high-dose hypofractionated irradiation: implications for stereotactic body radiation therapy and stereotactic radiation surgery. Int J Radiat Oncol Biol Phys (2015) 93:166–72. doi: 10.1016/j.ijrobp.2015.05.016
45. Kim M-S, Kim W, Park IH, Kim HJ, Lee E, Jung J-H, et al. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat Oncol J (2015) 33:265. doi: 10.3857/roj.2015.33.4.265
46. Miljanic M, Montalvo S, Aliru M, Song T, Leon-Camarena M, Innella K, et al. The evolving interplay of SBRT and the immune system, along with future directions in the field. (2022) 10:. doi: 10.3390/cancers14184530
47. Kabiljo J, Harpain F, Carotta S, Bergmann M. Radiotherapy as a backbone for novel concepts in cancer immunotherapy. Cancers (Basel) (2019) 12:79. doi: 10.3390/cancers12010079
48. Riboldi M, Orecchia R, Baroni G. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol (2012) 13:e383–391. doi: 10.1016/S1470-2045(12)70243-7
49. Zhang W, Oraiqat I, Litzenberg D, Chang K-W, Hadley S, Sunbul NB, et al. Real-time, volumetric imaging of radiation dose delivery deep into the liver during cancer treatment. Nat Biotechnol (2023) 41:1160–7. doi: 10.1038/s41587-022-01593-8
50. Nolan E, Bridgeman VL, Ombrato L, Karoutas A, Rabas N, Sewnath CAN, et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat Cancer (2022) 3:173–87. doi: 10.1038/s43018-022-00336-7
51. Carney DN, Mitchell JB. In vitro radiation and chemotherapy sensitivity of established cell. (1983) 43.
52. Katipally RR, Pitroda SP, Juloori A, Chmura SJ, Weichselbaum RR. The oligometastatic spectrum in the era of improved detection and modern systemic therapy. Nat Rev Clin Oncol (2022) 19:585–99. doi: 10.1038/s41571-022-00655-9
53. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol (2020) 38(25):2830–8. doi: 10.1200/JCO.20.00818
54. Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non–small cell lung cancer: a phase 2 trial. JAMA Oncol (2019) 5(9):1283–90. doi: 10.1001/jamaoncol.2019.1449
Keywords: adrenal metastases, SBRT (stereotactic body radiation therapy), fractionation dose, tracking, oligometastases
Citation: Liao X, Kishi K, Du K, Komaki R, Mizoe J, Aikawa G, Zheng W and Pan C (2023) Risk factors of local control in adrenal metastases treated by stereotactic body radiation therapy - a systematic review and meta-analysis. Front. Oncol. 13:1193574. doi: 10.3389/fonc.2023.1193574
Received: 25 March 2023; Accepted: 19 October 2023;
Published: 17 November 2023.
Edited by:
Sunil Krishnan, Mayo Clinic Florida, United StatesReviewed by:
Giuseppe Roberto D’Agostino, Humanitas Research Hospital, ItalyMihai Teodor Georgescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2023 Liao, Kishi, Du, Komaki, Mizoe, Aikawa, Zheng and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Pan, cGFuY2hhb0B4bXUuZWR1LmNu; Ritsuko Komaki, RHJLb21ha2lDb3hAZ21haWwuY29t
†These authors share first authorship
 Xuehong Liao
Xuehong Liao Kazushi Kishi
Kazushi Kishi Kaixin Du
Kaixin Du Ritsuko Komaki6*
Ritsuko Komaki6*