- 1Reproductive Medicine, Department of Obstetrics and Gynecology, Baruch-Padeh Medical Center, Poriya, Israel
- 2Azrieili Faculty of Medicine, Bar-Ilan University, Safed, Israel
- 3Department of Evolutionary and Environmental Biology, University of Haifa, Haifa, Israel
Introduction
Endometriosis-associated ovarian cancer (EAOC) is an evolving distinct clinical entity and challenge considered to develop from endometrioma (1). Several systematic reviews and meta-analyses corroborated the association between endometriosis and ovarian cancer (2–5). In the most recent and comprehensive, the summary relative risk of EAOC was estimated as 1.93 compared to women with no endometriosis (6). Furthermore, contemporary state-of-the-art methodologies have provided evidence of genetic correlation and causal relationship between endometriosis and EAOC (7).
Clear-cell and endometrioid ovarian epithelial carcinomas are the most intensely and reproducibly associated malignancies with endometriosis. While endometriosis may also be associated with low-grade serous ovarian carcinoma, this linkage is not well documented (8). Coexistence with endometriosis is observed in about 20%–50% of all women with clear-cell and endometrioid ovarian carcinomas (9–11). The increased risk of developing clear-cell and endometrioid ovarian carcinomas in women with endometriosis is 3.4 and 2.3-fold, respectively (6).
The rationale for the current opinion
Endometriosis is a widespread, chronic, inflammatory, and estrogen-dependent condition, and endometrioma is the most pathognomonic and diagnosed form of the disease. Their estimated prevalence is 1 in 10 and 1 in 18, respectively, in women of reproductive age (12). Therefore, the diagnosis of EAOC in women with endometriosis, particularly with an intact endometrioma, may raise much concern. Furthermore, the link and its translation into clinical practice regarding information to patients and early cancer detection still need to be clarified (13).
Overall, ovarian cancer is a postmenopausal disease, although, in about 12% of cases, it may develop in women < 44 years of age (14). This estimate includes numerous women with borderline and non-epithelial tumors, typically presenting at a young age (15, 16). Overall, women with EAOC are older than those with benign endometrioma but younger than women with non-EAOC ovarian carcinomas, such as high-grade serous ovarian carcinoma. Additionally, although there are several case reports of EAOC at a young age (17–20), the exact age distribution of EAOC diagnosis is still not well-expounded.
Furthermore, although age was suggested as a risk factor in cases with EAOC, many discrepancies exist between reports (21–24). For example, previous publications reported a wide range of EAOC premenopausal diagnosis rates between 30 and 70% of cases (21, 25–28). Moreover, due to the disease’s infrequency, publications exploring EAOC in the last decade were retrospective in design and included a modest number of women, the largest including 163 women (29).
Collectively, the age at EAOC diagnosis is still not well delineated. Although endometrioma is a prevalent manifestation in the reproductive age, EAOC may seem exceptional. A methodical assessment of age at EAOC diagnosis may have numerous implications on the clinical management of women with intact endometrioma, especially in planning future pregnancies. It may clarify the chance of EAOC diagnosis stratified by age and direct physicians on advising, following, and treating their patients relying on relevant medical evidence. Moreover, it may supplement essential information to reproductive endocrinologists and gynecological surgeons in their counseling for the best treatment approach, especially when atypical features of an endometrioma appear on transvaginal ultrasound (TVUS).
Methods
To reach the objective of this opinion paper, a search for cohort studies published in the English literature was performed on Pubmed.com from January 2011 to October 2022, addressing EAOC, clear-cell ovarian cancer, and endometrioid ovarian cancer. Cohort studies that targeted women with EAOC elaborating on the mean age ± standard deviation (SD) or median and interquartile range (IQR) or range (minimum and maximum) were included in the quantitative assessment. The diagnosis of EAOC should have been performed surgically and approved by pathology.
The keywords ‘endometriosis’, ‘endometrioma’, ‘ovarian endometriosis’, ‘endometriosis-associated ovarian cancer’, ‘clear-cell ovarian carcinoma’, ‘endometrioid ovarian carcinoma’, ‘age’, and ‘reproductive age’ were included. The relevance of reached publications was evaluated following reading the abstract. Case reports and reviews were excluded. Furthermore, publications that assessed only a specific age group of women were omitted from the evaluation. Articles with an inappropriate design were also excluded.
Statistical evaluation
We assumed normal distribution for the statistical analyses of women’s age since this information was not specified in the 25 studies included in our analysis. The mean and standard deviation of women’s age were reported in 17 studies. For the other studies where only median and IQR or median and minimum/maximum were given, mean and SD were approximated using the method of Wan et al. (30). The weighted mean and SD were calculated with the number of participants in each study as the weighting factor, using the formulas of N.I.S.T (31) for each of the four studied groups (all eligible studies combined, studies that targeted EAOC originating from ovarian endometriosis, studies that were conducted in countries with a high incidence of EAOC and studies that included women solely with clear-cell ovarian carcinoma). Using z-scores, we calculated the percentage of women in the four groups below 50, 45, 40, and 35 years.
Results
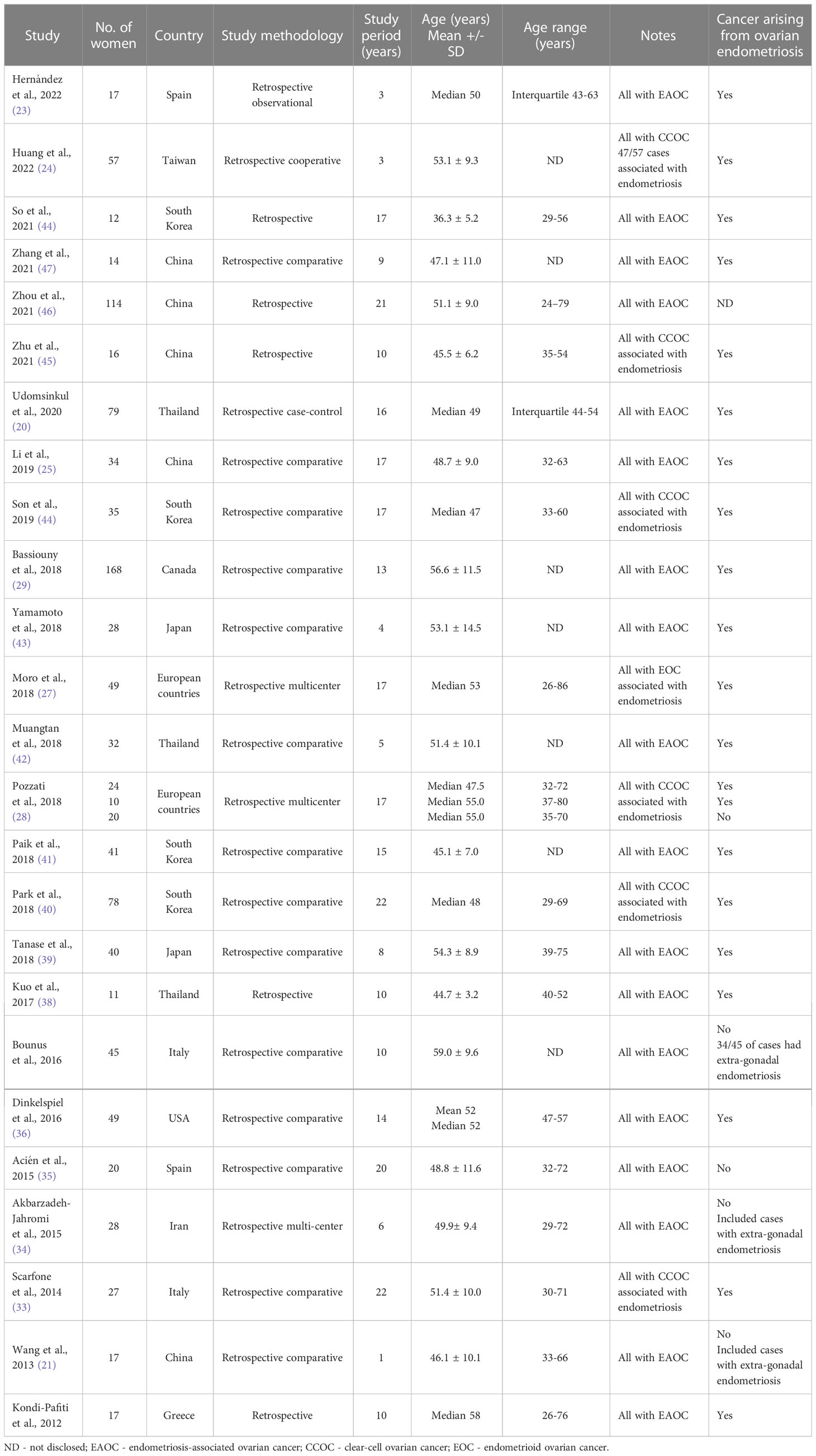
Twenty-five studies (27 cohorts) were eligible for the quantitative age evaluation at EAOC diagnosis, including 1082 cases (Table 1). The diagnosis of EAOC was performed surgically and examined by experienced pathologists in all studies (21–25, 27–29, 32–48).
Eleven studies were excluded from the quantitative evaluation. Five studies did not disclose EAOC patients’ age, SD, or range (49–53). Two studies assessed only EAOC women above 45 or below 40 years (26, 54). One study targeted women following endometrioma resection (55), and one more study evaluated only EAOC cases resistant to platinum (56). Other studies did not meet the inclusion criteria (57, 58).
The characteristics of eligible 25 studies are précised in Table 1. All studies were retrospective in design. The studies originated from countries around the globe in Asia, Europe, and North America. Most studies (19/25) summarized an extended institutional clinical experience of more than seven years. In all studies, the mean ( ± SD) duration invested in EAOC institutional evaluation was 12.26 ± 6.39 years.
Eighteen studies assessed all cases of EAOC combined (21–23, 25, 29, 32, 34–39, 41–43, 46–48), six evaluated only patients with clear-cell ovarian cancer associated with endometriosis (24, 28, 33, 40, 44, 45), and one study endometrioid ovarian cancer associated with endometriosis (27). In addition, six eligible studies were performed in countries with a higher prevalence of EAOC, in Japan, Thailand, and Taiwan (22, 24, 38, 39, 42, 43). Nineteen eligible studies included EAOC cases arising within the endometrioma following Sampson and Scott’s criteria (59, 60). In contrast, six studies included patients with extra-gonadal endometriosis (61), or the subtype of endometriosis was not disclosed (Table 1).
Assessing all eligible studies conjointly, the mean age of the 1082 women at EAOC diagnosis was 51.64 ± 3.24 years. Among these women, 30.68% and 2.10% were below 50 and 45 years, respectively, equivalent to 31:100 and 2:100 among all women assessed. On the other hand, EAOC diagnosis in women below 40 and 35 years resulted in much lower estimates corresponding to 0.017% and <0.001%, equivalent to 2:10,000 and <1:100,000, respectively.
Nineteen studies targeted EAOC originating from ovarian endometriosis and included 838 cases with a mean age of 51.51 ± 3.29 years. Among these women, 32.26% and 2.38% were below 50 and 45 years of age, equivalent to 32:100 and 2:100, respectively. However, EAOC diagnosis in this group of women below 40 and 35 years resulted in much lower estimates corresponding to 0.023% and <0.001%, equivalent to 2:10,000 and <1:100,000, respectively.
Six studies were conducted in countries with a high incidence of EAOC and included 247 cases with a mean age of 51.39 ± 3.30 years. Of these women, 33.71%, 2.66%, 0.028%, and <0.001% were below 50, 45, 40, and 35 years, equivalent to 34:100, 3:100, 3:10,000, and <1:100,000, respectively.
Six studies included women solely with clear-cell ovarian carcinoma and involved 267 cases with a mean age of 50.27 ± 3.58 years. Of these women, 46.95%, 7.02%, 0.20%, and 0.001% were below 50, 45, 40, and 35 years, equivalent to 47:100, 7:100, 2:1000, and 1:100,000, respectively.
Discussion
Our methodical assessment, summarizing 25 carefully chosen studies (27 cohorts) and including 1082 EAOC cases, shows that EAOC diagnosis is a disease of the menopausal age. These global results rely on an extended experience of 12.26 ± 6.39 years invested among various institutional practices. In our analysis of all eligible studies, the mean age of women with EAOC was 51.64 ± 3.24 years. Nonetheless, 31% of women were < 50 years of age, most probably peri- or premenopausal, and only 2% of patients were <45 years of age, apparently diagnosed during the reproductive period. Furthermore, the diagnosis of EAOC in young women <40 and <35 years of age was sporadic, equivalent to 1:5,000 and <1:100,000, respectively. These estimates did not change when EAOC cases in women with extra-gonadal endometriosis were excluded from the evaluation.
Estimates of premenopausal EAOC diagnosis in countries with a higher disease prevalence, Japan, Thailand, and Taiwan, seem to increase slightly. Almost 34:100, and 3:100, of women developing EAOC in those countries, were <50 and <45 years, respectively. However, only 3:10,000 and <1:100,000 were below <40 and <35 years, respectively.
Furthermore, studies that evaluated only clear-cell ovarian cancer associated with endometriosis showed a further increase in premenopausal diagnosis. Almost 47:100 and 7:100 of women with this type of cancer were diagnosed <50 and <45 years, respectively. However, no significant clinical change in this group was diagnosed in young women <40 and <35 years. Therefore, it is plausible that the slight increase in the premenopausal diagnosis of EAOC in Japan, Thailand, and Taiwan is caused by the predominance of clear-cell ovarian carcinoma in these countries (62).
Taken together, our results demonstrate that a malignant transformation of a benign endometrioma to EAOC in the reproductive age is a sporadic event, notably in women <40 years of age. In women of late reproductive age, between 40-45 years, EAOC is diagnosed in almost 2-3% of cases. In cases with clear-cell ovarian carcinoma associated with endometriosis, our results imply that up to 7% of cases are diagnosed in this age group. These estimates are clinically significant since endometriosis and endometrioma are prevalent at young ages, estimated at 1 in 10 and 1 in 18, respectively. They may be beneficial to ease the concerns of these patients and their attending practitioners. Furthermore, these estimates may be employed for proper guidance and counseling of women of reproductive age with an intact endometrioma planning for a future pregnancy.
Our results, assessing a total of 1082 cases, demonstrates the age diversity at diagnosis between patients with EAOC and non-EAOC, specifically high-grade serous ovarian cancer (HGSOC). The mean age of women with HGSOC, the most common and lethal type of ovarian cancer, is 63 years (63), almost 12 years older than EAOC.
Furthermore, our analyses substantiate previous studies, counting on modest numbers, indicating that age is an independent risk factor in cases of EAOC (21–24); and translating these risks into informatics that may be introduced into the clinical setting. Our calculations also imply that EAOC should not be an argument for surgical treatment of an intact endometrioma in the reproductive age, particularly in cases planning a future pregnancy. It is well recognized today that endometriotic cystectomy significantly reduces ovarian reserve (64, 65), estimated by 39% and 57%, in uni- and bilateral cases, 9-12 months following surgery, suggesting a long-standing impact on women’s reproductive life span (65).
Although EAOC diagnosis at the reproductive is infrequent and even sporadic in women <40 years, the index of suspicion should be directed into distinct clinical situations. Cases with relapsing or worsening pelvic pain, the rapid growth of an endometrioma, or alternately larger-sized endometrioma, particularly >9 cm, should be perused to investigate EAOC (12). In this setting, serum cancer antigen 125 has no added benefit (66, 67).
Clinically, imaging is vital in evaluating and differential diagnosing an endometrioma transformation to an EAOC, noticeably in reproductive age. Pelvic transvaginal ultrasound (TVUS) is the first-line imaging mode, while computerized tomography scan performs poorly. Magnetic resonance imaging (MRI) is a valuable adjunct for ovarian findings described as intermediate or atypical by TVUS (68, 69).
On TVUS, benign endometrioma appears as an ovarian mass with a homogenous ‘ground glass’ appearance, uni- or bilateral, without solid parts or papillations. However, as age increases, papillations and other solid parts become more frequent, and the ‘ground glass’ appearance becomes less common, while cyst diameter seems to stay the same (70). Conversely, EAOC, specifically clear-cell and endometrioid ovarian carcinomas, are large, above 9 cm, with a mean size of 11-13 cm, unilateral tumors with solid components, papillary projections, and vascularization (12, 71).
In clinical practice, about 5–25% of cases will have indeterminate or atypical adnexal findings by TVUS (72). Since most of these cases may be benign, MRI performance is crucial in managing and counseling these cases. In addition, MRI-supportive performance in such situations may reduce patient anxiety, repeat imaging, unnecessary follow-up, and avoid surgery. On MRI, benign endometriomas typically display features of T2-weighted image shading (72). A larger cyst and an enhanced solid portion of the endometrioma may suggest a malignant transformation (73). Shading disappearance within the endometrioma on T2-weighted images may also mean malignant transformation (39).
Endometrioma and deep infiltrating endometriosis (DIE) occasionally co-exist in the same women (74, 75). In a recent cross-sectional study of 1,191 women with subfertility aged 25–39 years, undergoing a systematic TVUS evaluation, both disease subtypes were found in almost 22% of women (75). Nevertheless, the available evidence supports EAOC rise mainly from endometrioma (1). Malignant transformation of extra-gonadal sites, as in superficial or deep endometriosis, is most unusual (76–78). In a recent narrative review of cases with DIE malignant transformation, only eight patients were collected in ten years (78). Thus, a high level of suspicion should be invested in DIE cases presenting with new clinical manifestations and appearing with a pelvic mass such as on the pelvic wall, pouch of Douglass, and recto-vaginal septum (78). Further studies are essential to explore the association between extra-gonadal endometriosis and malignant transformation.
Figure 1 summarizes in a flow chart our suggested way of clinical management of endometrioma (isolated or joined by DIE) developing manifestations of EAOC, including atypical TVUS endometrioma features. The risk factors for EAOC have been discussed earlier (12) and are summarized in Figure 1. MRI performance is mandatory in these cases, especially when atypical endometrioma features appear on TVUS. A definite diagnosis of EAOC necessitates surgery, most commonly by endoscopy. Definitive management should consider the patient’s age, the final pathological diagnosis, stage, and grade of disease. Since women with EAOC are diagnosed at an earlier stage and have a more favorable histological grade (12), conservative surgical management should be discussed in infertile cases or women of reproductive age desiring a future pregnancy.
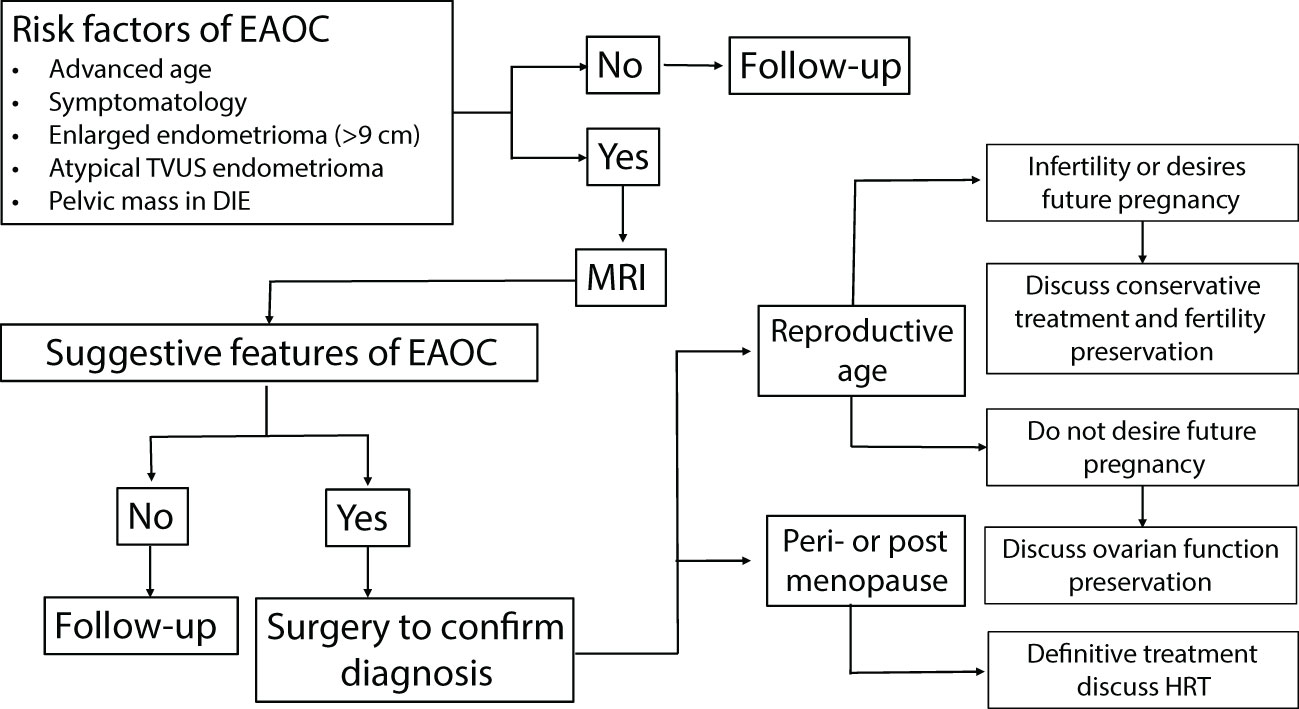
Figure 1 Clinical management of women with an isolated endometrioma or joint with deep infiltrating endometriosis, suspected to have EAOC, in the reproductive age and menopausal transition. EAOC, endometriosis-associated ovarian cancer; TVUS, transvaginal ultrasound; MRI, magnetic resonance imaging; HRT, hormone replacement therapy.
The time and intervals of follow-up in cases with isolated endometrioma, with typical or atypical TVUS features, is an intricate but essential demand and need more targeted studies to be addressed. Likewise, follow-up of cases with atypical TVUS features and reassuring MRI results need further investigation. Meanwhile, we recommend a multidisciplinary case-by-case consultation (adjoining a gynecological oncologist and reproductive medicine experts) contemplating, age, desire for future pregnancy, symptomatology, ovarian reserve, physical and TVUS findings, and MRI features.
In summary, this is the first report in the literature delineating and expounding the age at EAOC diagnosis, substantiating that age is an independent risk factor for the disease. Our results analyzing 1082 cases from 25 studies demonstrate that EAOC is a menopausal disease, with a mean age of 51.64 ± 3.24 years at diagnosis. About 30.68% of patients with EAOC are <50 years upon diagnosis, presumably premenopausal, equivalent to 31 in 100 women. However, only 2.1%, 0.017%, and <0.001%, apparently during the reproductive age, comparable to 1 in 50, 1 in 5,000, and < 1 in 10,000, are <45, <40, and <35 years, respectively. In cases with clear-cell ovarian carcinoma associated with endometriosis, 47 in 100 and 7 in 100 are below 50 and 45 years, with no clinically significant changes in women <40 and <35 years. Since endometrioma is widespread in women of reproductive age, these estimates are essential for guidance and counseling, especially in women planning for a future pregnancy. Our results imply that the likelihood of EAOC development during reproductive age is reassuring, and this should be translated into clinical practice regarding information to patients. At the same time, a high index of suspicion should remain in special situations when clinical, TVUS, and MRI features suggest EAOC. Conversely, an extended follow-up should be considered in women with large or atypical endometriomas not retracting following menopause.
Author contributions
JY conceived the idea of this perspective, contributed to study design and data extraction, performed the analyses and data interpretation, and drafted the manuscript; II contributed to study design and execution, contributed to analyses and interpretation of data, performed the statistical analysis, and revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saavalainen L, Lassus H, But A, Tiitinen A, Härkki P, Gissler M, et al. Risk of gynecologic cancer according to the type of endometriosis. Obstet Gynecol (2018) 131:1095–102. doi: 10.1097/AOG.0000000000002624
2. Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol (2012) 13:385–94. doi: 10.1016/S1470-2045(11)70404-1
3. Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer (2014) 110:1878–90. doi: 10.1038/bjc.2014.29
4. Wang C, Liang Z, Liu X, Zhang Q, Li S. The association between endometriosis, tubal ligation, hysterectomy and epithelial ovarian cancer: meta-analyses. Int J Environ Res Public Health (2016) 13:1138. doi: 10.3390/ijerph13111138
5. Li J, Liu R, Tang S, Feng F, Liu C, Wang L, et al. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: a meta-analysis. Arch Gynecol Obstet (2018) 299:35–46. doi: 10.1007/s00404-018-4968-1
6. Kvaskoff M, Mahamat-Saleh Y, Farland LV, Shigesi N, Terry KL, Harris HR, et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update (2021) 27:393–420. doi: 10.1093/humupd/dmaa045
7. Mortlock S, Corona RI, Kho PF, Pharoah P, Seo JH, Freedman ML, et al. A multi-level investigation of the genetic relationship between endometriosis and ovarian cancer histotypes. Cell Rep Med (2022) 3:100542. doi: 10.1016/j.xcrm.2022.100542
8. Guidozzi F. Endometriosis-associated cancer. Climacteric (2021) 24:587–92. doi: 10.1080/13697137.2021.1948994
9. Stamp JP, Gilks CB, Wesseling M, Eshragh S, Ceballos K, Anglesio MS, et al. BAF250a expression in atypical endometriosis and endometriosis-associated ovarian cancer. Int J Gynecol Cancer (2016) 26:825–32. doi: 10.1097/IGC.0000000000000698
10. Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol (2016) 34:2888–98. doi: 10.1200/JCO.2016.66.8178
11. Tanha K, Mottaghi A, Nojomi M, Moradi M, Rajabzadeh R, Lotfi S, et al. Investigation on factors associated with ovarian cancer: an umbrella review of systematic review and meta-analyses. J Ovarian Res (2021) 14:153. doi: 10.1186/s13048-021-00911-z
12. Younis JS. Endometriosis-associated ovarian cancer: what are the implications for women with intact endometrioma planning for a future pregnancy? a reproductive clinical outlook. Biomolecules (2022) 12:1721. doi: 10.3390/biom12111721
13. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: endometriosis. Hum Reprod Open (2022) 2022:hoac009. doi: 10.1093/hropen/hoac009
14. Kim SY, Lee JR. Fertility preservation option in young women with ovarian cancer. Future Oncol (2016) 12:1695–8. doi: 10.2217/fon-2016-0181
15. Cheung A, Shah S, Parker J, Soor P, Limbu A, Sheriff M, et al. Non-epithelial ovarian cancers: how much do we really know? Int J Environ Res Public Health (2022) 19:1106. doi: 10.3390/ijerph19031106
16. Della Corte L, Mercorio A, Serafino P, Viciglione F, Palumbo M, De Angelis MC, et al. The challenging management of borderline ovarian tumors (BOTs) in women of childbearing age. Front Surg (2022) 9:973034. doi: 10.3389/fsurg.2022.973034
17. Tagashira Y, Shimada M, Kigawa J, Iba T, Terakawa N. Ovarian endometrioid adenocarcinoma arising from endometriosis in a young woman. Gynecol Oncol (2003) 91:643–7. doi: 10.1016/j.ygyno.2003.08.005
18. Matalliotakis I, Mahutte NG, Koukoura O, Arici A. Endometriosis associated with stage IA clear cell ovarian carcinoma in a woman with IVF-ET treatments in the Yale series. Arch Gynecol Obstet (2006) 274:184–6. doi: 10.1007/s00404-006-0143-1
19. Saylam K, Devreker F, Simon P, Fayt I, Noël J-C. Ovarian clear cell carcinoma occurring in a young patient with endometriosis and long-term ovulation stimulations. Acta Obstet Gynecol Scand (2006) 85:1506–7. doi: 10.1080/00016340600603577
20. Woodard TL, Awonuga AO, Puscheck E. Malignant transformation of endometrioma in a woman with a history of ovulation induction and in vitro fertilization. Case Rep Med (2012) 2012:497362. doi: 10.1155/2012/497362
21. Wang S, Qiu L, Lang JH, Shen K, Yang JX, Huang HF, et al. Clinical analysis of ovarian epithelial carcinoma with coexisting pelvic endometriosis. Am J Obstet Gynecol (2013) 208:413.e1–5. doi: 10.1016/j.ajog.2012.12.004
22. Udomsinkul P, Triratanachart S, Oranratanaphan S. Risk factors for endometriotic-cyst associated ovarian cancer: a case controlled study. Taiwan J Obstet Gynecol. (2020) 59:269–74. doi: 10.1016/j.tjog.2020.01.016
23. Hernández A, Sanz A, Spagnolo E, Carbonell M, Rodríguez E, López A, et al. Evaluation of women’s age and ultrasonographic features to choose surgical treatment for endometriosis associated with ovarian cancer. J Clin Med (2022) 11:2414. doi: 10.3390/jcm11092414
24. Huang KJ, Li YX, Wu CJ, Chang WC, Wei LH, Sheu BC. Sonographic features differentiating early-stage ovarian clear cell carcinoma from endometrioma with atypical features. J Ovarian Res (2022) 15:84. doi: 10.1186/s13048-022-01019-8
25. Li Q, Sun Y, Zhang X, Wang L, Wu W, Wu M, et al. Endometriosis-associated ovarian cancer is a single entity with distinct clinicopathological characteristics. Cancer Biol Ther (2019) 20:1029–34. doi: 10.1080/15384047.2019.1595278
26. Cai Y, Yin J, Jin Y, Li Y, Wu M, Yang J, et al. Endometriosis-associated ovarian cancer is not a distinct clinical entity among young patients: a 12-year cohort study. Eur J Surg Oncol (2020) 46:876–82. doi: 10.1016/j.ejso.2019.11.517
27. Moro F, Magoga G, Pasciuto T, Mascilini F, Moruzzi MC, Fischerova D, et al. Imaging in gynecological disease (13): clinical and ultrasound characteristics of endometrioid ovarian cancer. Ultrasound Obstet Gynecol (2018) 52:535–43. doi: 10.1002/uog.19026
28. Pozzati F, Moro F, Pasciuto T, Gallo C, Ciccarone F, Franchi D, et al. Imaging in gynecological disease (14): clinical and ultrasound characteristics of ovarian clear cell carcinoma. Ultrasound Obstet Gynecol (2018) 52:792–800. doi: 10.1002/uog.19171
29. Bassiouny D, El-Baz MA, Gamil TM, Shams N, Ismiil N, Dubé V, et al. Endometriosis-associated ovarian cancer is a subset with a more favorable outcome and distinct clinical-pathologic characteristics. Int J Gynecol Pathol (2019) 38:435–42. doi: 10.1097/PGP.0000000000000533
30. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
31. N.I.S.T. Weighted Standard Deviation. DATAPLOT reference manual (1996). Available at: https://www.itl.nist.gov/div898/software/dataplot/refman2/ch2/weightsd.pdf.
32. Kondi-Pafiti A, Papakonstantinou E, Iavazzo C, Grigoriadis C, Salakos N, Gregoriou O. Clinicopathological characteristics of ovarian carcinomas associated with endometriosis. Arch Gynecol Obstet (2012) 285:479–83. doi: 10.1007/s00404-011-1957-z
33. Scarfone G, Bergamini A, Noli S, Villa A, Cipriani S, Taccagni G, et al. Characteristics of clear cell ovarian cancer arising from endometriosis: a two center cohort study. Gynecol Oncol (2014) 133:480–4. doi: 10.1016/j.ygyno.2014.03.017
34. Akbarzadeh-Jahromi M, Shekarkhar G, Sari Aslani F, Azarpira N, Heidari Esfahani M, Momtahan M. Prevalence of endometriosis in malignant epithelial ovarian tumor. Arch Iran Med (2015) 18:844–8.
35. Acién P, Velasco I, Acién M, Capello C, Vela P. Epithelial ovarian cancers and endometriosis. Gynecol Obstet Invest (2015) 79:126–35. doi: 10.1159/000367597
36. Dinkelspiel HE, Matrai C, Pauk S, Pierre-Louis A, Chiu YL, Gupta D, et al. Does the presence of endometriosis affect prognosis of ovarian cancer? Cancer Invest (2016) 34:148–54. doi: 10.3109/07357907.2016.1139716
37. Bounous VE, Ferrero A, Fuso L, Ravarino N, Ceccaroni M, Menato G, et al. Endometriosis-associated ovarian cancer: a distinct clinical entity? Anticancer Res (2016) 36:3445–9.
38. Kuo HH, Huang CY, Ueng SH, Huang KG, Lee CL, Yen CF. Unexpected epithelial ovarian cancers arising from presumed endometrioma: a 10-year retrospective analysis. Taiwan J Obstet Gynecol (2017) 56:55–61. doi: 10.1016/j.tjog.2015.09.009
39. Tanase Y, Kawaguchi R, Takahama J, Kobayashi H. Factors that differentiate between endometriosis-associated ovarian cancer and benign ovarian endometriosis with mural nodules. Magn Reson Med Sci (2018) 17:231–7. doi: 10.2463/mrms.mp.2016-0149
40. Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, et al. Significance of ovarian endometriosis on the prognosis of ovarian clear cell carcinoma. Int J Gynecol Cancer (2018) 28:11–8. doi: 10.1097/IGC.0000000000001136
41. Paik ES, Kim TJ, Choi CH, Kim BG, Bae DS, Lee JW. Clinical outcomes of patients with clear cell and endometrioid ovarian cancer arising from endometriosis. J Gynecol Oncol (2018) 29:e18. doi: 10.3802/jgo.2018.29.e18
42. Muangtan S, Suknikhom W, Sananpanichkul P, Bhamarapravatana K. Suwannarurk K.Epithelial ovarian cancer with endometriosis is not associated with menopausal status: a Co-association study at prapokklao hospital. Asian Pac J Cancer Prev (2018) 19:1337–41.
43. Yamamoto Y, Wakikawa A, Ueno A, Nagai R, Matsumoto M, Komatsu J, et al. Comparison of endometriotic cysts and ovarian cancer in association with endometriotic cysts. Cancer Treat Res Commun (2018) 14:26–9. doi: 10.1016/j.ctarc.2017.11.002
44. Son JH, Yoon S, Kim S, Kong TW, Paek J, Chang SJ, et al. Clinicopathologic characteristics of ovarian clear cell carcinoma in the background of endometrioma: a surveillance strategy for an early detection of malignant transformation in patients with asymptomatic endometrioma. Obstet Gynecol Sci (2019) 62:27–34. doi: 10.5468/ogs.2019.62.1.27
45. Zhu C, Zhu J, Qian L, Liu H, Shen Z, Wu D, et al. Clinical characteristics and prognosis of ovarian clear cell carcinoma: a 10-year retrospective study. BMC Cancer (2021) 21:322. doi: 10.1186/s12885-021-08061-7
46. Zhou L, Yao L, Dai L, Zhu H, Ye X, Wang S, et al. Ovarian endometrioid carcinoma and clear cell carcinoma: a 21-year retrospective study. J Ovarian Res (2021) 14:63. doi: 10.1186/s13048-021-00804-1
47. Zhang X, Li M, Tang Z, Li X, Song T. Differentiation between endometriosis-associated ovarian cancers and non- endometriosis-associated ovarian cancers based on magnetic resonance imaging. Br J Radiol (2021) 94:20201441. doi: 10.1259/bjr.20201441
48. So KA, Hong SR, Kim NR, Yang EJ, Shim SH, Lee SJ, et al. Association between atypical endometriosis and ovarian malignancies in the real world. J Ovarian Res (2021) 14:110. doi: 10.1186/s13048-021-00865-2
49. Kumar S, Munkarah A, Arabi H, Bandyopadhyay S, Semaan A, Hayek K, et al. Prognostic analysis of ovarian cancer associated with endometriosis. Am J Obstet Gynecol (2011) 204:63.e1–7. doi: 10.1016/j.ajog.2010.08.017
50. Garrett LA, Growdon WB, Goodman A, Boruta DM, Schorge JO, del Carmen MG. Endometriosis-associated ovarian malignancy: a retrospective analysis of presentation, treatment, and outcome. J Reprod Med (2013) 58:469–76.
51. Tay SK, Cheong MA. Evidence for ethnic and environmental contributions to frequency of ovarian clear cell carcinoma. Aust N Z J Obstet Gynaecol (2014) 54:225–30. doi: 10.1111/ajo.12188
52. Wang KC, Chang WH, Lee WL, Huang N, Huang HY, Yen MS, et al. An increased risk of epithelial ovarian cancer in Taiwanese women with a new surgico-pathological diagnosis of endometriosis. BMC Cancer (2014) 14:831. doi: 10.1186/1471-2407-14-831
53. Wang Q, Wang L LY, Ding X, Liu A. Expression and significance of ARID1A mRNA in endometriosis- associated ovarian cancer. J BUON (2017) 22:1314–21.
54. He ZX, Shi HH, Fan QB, Zhu L, Leng JH, Sun DW, et al. Predictive factors of ovarian carcinoma for women with ovarian endometrioma aged 45 years and older in China. J Ovarian Res (2017) 10:45. doi: 10.1186/s13048-017-0343-2
55. Haraguchi H, Koga K, Takamura M, Makabe T, Sue F, Miyashita M, et al. Development of ovarian cancer after excision of endometrioma. Fertil Steril (2016) 106:1432–1437.e2. doi: 10.1016/j.fertnstert.2016.07.1077
56. Ren T, Sun TT, Wang S, Sun J, Xiang Y, Shen K, et al. Clinical analysis of chemo-resistance risk factors in endometriosis associated ovarian cancer. J Ovarian Res (2018) 11:40. doi: 10.1186/s13048-018-0418-8
57. Tang H, Liu Y, Wang X, Guan L, Chen W, Jiang H, et al. Clear cell carcinoma of the ovary: clinicopathologic features and outcomes in a Chinese cohort. Med (Baltimore) (2018) 97:e10881. doi: 10.1097/MD.0000000000010881
58. Vassard D, Schmidt L, Glazer CH, Lyng Forman J, Kamper-Jørgensen M, Pinborg A. Assisted reproductive technology treatment and risk of ovarian cancer-a nationwide population-based cohort study. Hum Reprod (2019) 34:2290–6. doi: 10.1093/humrep/dez165
59. Sampson JA. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Arch Surg (1925) 10:1–72. doi: 10.1001/archsurg.1925.01120100007001
61. Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A Rev literature. Best Pract Res Clin Obstet Gynaecol. (2004) 18:349–71. doi: 10.1016/j.bpobgyn.2003.03.001
62. Machida H, Matsuo K, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, et al. Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: a JSGO–JSOG joint study. Gynecol Oncol (2019) 153:589–96. doi: 10.1016/j.ygyno.2019.03.243
63. SEER Ovarian Cancer. Available at: https://seer.cancer.gov/statfacts/html/ovary.html (Accessed December 20, 2022).
64. Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab (2012) 97:3146–54. doi: 10.1210/jc.2012-1558
65. Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: a systematic review and meta-analysis. Hum Reprod Update (2019) 25:375–91. doi: 10.1093/humupd/dmy049
66. Sölétormos G, Duffy MJ, Abu Hassan SO, Verheijen RH, Tholander B, Bast RC, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European group on tumor markers. Int J Gynecol Cancer (2016) 26:43–51. doi: 10.1097/IGC.0000000000000586
67. Sisodia RC, del Carmen MG. Lesions of the ovary and fallopian tube. N Engl J Med (2022) 387:727–36. doi: 10.1056/NEJMra2108956
68. Timmerman D, Ameye L, Fischerová D, Epstein E, Melis GB, Guerriero S, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. Br Med J (2010) 341:c6839. doi: 10.1136/bmj.c6839
69. Andreotti RF, Timmerman D, Strachowski LM, Froyman W, Benacerraf BR, Bennett GL, et al. O-RADS US risk stratification and management system: a consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology (2020) 294:168–85. doi: 10.1148/radiol.2019191150
70. Guerriero S, Van Calster B, Somigliana E, Ajossa S, Froyman W, De Cock B, et al. Age-related differences in the sonographic characteristics of endometriomas. Hum Reprod (2016) 31:1723–31. doi: 10.1093/humrep/dew113
71. Seidman JD, Cho KR, Ronnett BM, Kurman RJ. Urface epithelial tumors of the ovary. In: Kurman RJ, Ellenson LH, Ronnet BM, editors. Blaustein’s pathology of the female genital tract, 6th ed. Boston, MA, USA: Springer (2011). p. 679–784.
72. Robinson KA, Menias CO, Chen L, Schiappacasse G, Shaaban AM, Caserta MP, et al. Understanding malignant transformation of endometriosis: imaging features with pathologic correlation. Abdom Imaging (2019) 45:1762–75. doi: 10.1007/s00261-019-01914-7
73. Tanaka YO, Yoshizako T, Nishida M, Yamaguchi M, Sugimura K, Itai Y. Ovarian carcinoma in patients with endometriosis: MR imaging findings. Am J Roentgenol (2000) 175:1423–30. doi: 10.2214/ajr.175.5.1751423
74. Hajati A, Hajati O. A review of more than 2000 cases of site-specific pelvic endometriosis rates by MRI: a guide to minimizing under/overdiagnosis non-invasively. Insights Imaging (2022) 13:129. doi: 10.1186/s13244-022-01270-z
75. Alson S, Jokubkiene L, Henic E, Sladkevicius P. Prevalence of endometrioma and deep infiltrating endometriosis at transvaginal ultrasound examination of subfertile women undergoing assisted reproductive treatment. Fertil Steril (2022) 118:915–23. doi: 10.1016/j.fertnstert.2022.07.024
76. Abaid LN, Cupp JS, Chang M, Beanes SR, Goldestein BH. Clear cell carcinoma of the pelvic side wall arising from endometriosis. Gynecol Oncol Rep (2018) 25:24–6. doi: 10.1016/j.gore.2018.05.006
77. Cucinella G, Sozzi G, Di Donna MC, Unti E, Mariani A, Chiantera V. Retroperitoneal squamous cell carcinoma involving the pelvic side wall arising from endometriosis: a case report. Gynecol Obstet Invest (2022) 87:159–64. doi: 10.1159/000520983
Keywords: endometriosis, endometrioma, endometriosis-associated ovarian cancer, clear-cell ovarian carcinoma, endometrioid ovarian cancer, reproductive age, age at diagnosis.
Citation: Younis JS and Izhaki I (2023) At what age endometriosis-associated ovarian cancer is diagnosed? The implications for women in the reproductive age. Front. Oncol. 13:1193123. doi: 10.3389/fonc.2023.1193123
Received: 24 March 2023; Accepted: 15 May 2023;
Published: 23 May 2023.
Edited by:
Annamaria Ferrero, Mauriziano Hospital, ItalyReviewed by:
Emanuele Perrone, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2023 Younis and Izhaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnny S. Younis, anlvdW5pc0Bwb3JpYS5oZWFsdGguZ292Lmls
†ORCID: Johnny S. Younis, orcid.org/0000-0002-3793-6377
Ido Izhaki, orcid.org/0000-0002-4525-4683
 Johnny S. Younis
Johnny S. Younis Ido Izhaki
Ido Izhaki