
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 28 April 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1192792
This article is part of the Research Topic Radioligand Therapy in Prostate Cancer View all 5 articles
 David Parker1
David Parker1 Jessica Zambelli1
Jessica Zambelli1 Montana Kay Lara1
Montana Kay Lara1 Trevor Hamilton Wolf1
Trevor Hamilton Wolf1 Amber McDonald1
Amber McDonald1 Erica Lee1
Erica Lee1 Lotfi Abou-Elkacem1
Lotfi Abou-Elkacem1 Eva J. Gordon1*
Eva J. Gordon1* Richard P. Baum2*
Richard P. Baum2*Despite decades of research and clinical trials, metastatic castration-resistant prostate cancer (mCRPC) remains incurable and typically fatal. Current treatments may provide modest increases in progression-free survival but can come with significant adverse effects and are disaggregated from the diagnostic imaging needed to fully assess the spread of metastatic disease. A theranostic approach, using radiolabeled ligands that target the cell surface protein PSMA, simplifies the visualization and disease treatment process by enabling both to use similar agents. Here, we describe an exemplary case wherein a gentleman in his 70s with mCRPC on diagnosis was treated with 177Lu–PSMA-617 and abiraterone, and remains disease-free to date, over five years later.
The increasing cellular and molecular resolution of diagnostic tools for human disease reinforces the need for targeted, multimodal therapeutic approaches. Theranostics, the simultaneous use of diagnostics and therapeutics, leverages the molecular strengths of both. One implementation of theranostics utilizes non-toxic radiolabeling of diseased tissue combined with targeted radioactive drugs for treatment. Some of these treatments also have utility as imaging tools to monitor tracer uptake and guide treatment decisions. Originally pioneered in the 1940s using radioactive iodine for characterizing and treating thyroid cancer, theranostics has recently been applied to metastatic castration-resistant prostate cancer (mCRPC), among other cancers, with great success (1–5).
Indeed, on December 1, 2020, and March 23, 2022, the US Food and Drug Administration (FDA) approved 68Ga–PSMA-11 and 177Lu–PSMA-617 for imaging and treating mCRPC, respectively. These agents bind to prostate-specific membrane antigen (PSMA), a protein prevalent on the surface of prostate cancer cells, either enabling visualization or delivering toxic radiation, depending on the radioactive element employed (6). This approach takes advantage of the simultaneous diagnostic and therapeutic power of PSMA labeling.
Standard care for localized prostate cancer involves expectant management and monitoring for progression, followed by surgery and radiation. Upon metastasis, the first line of treatment is androgen deprivation therapy (ADT). However, metastatic prostate cancer can be unresponsive to ADT, termed castration-resistant, either at treatment outset or after initial sensitivity. To treat patients who are unresponsive to ADT, there have been several treatment options designed to re-sensitize patients to ADT or disrupt the androgen axis (7–9), in addition to therapeutic strategies such as systemic chemotherapy with taxane-based agents or radium-223 to mitigate bony metastases, the most common metastatic site in prostate cancer (10, 11). Nevertheless, PSMA-targeted radioligand therapy improves upon current strategies because unlike ADT, resistance to 177Lu–PSMA-617 does not readily emerge. Instead, PSMA expression increases with prostate tumor malignancy and metastases, potentially yielding greater efficacy from 177Lu–PSMA-617 (12, 13).
Here, we present an exemplary case wherein a 73-year-old male was treated for mCRPC in 2017 with 177Lu–PSMA-617. After progressing through initial standard of care treatment with chemotherapy and ADT, the patient underwent a gallium-68 (Ga-68) PSMA PET/CT scan revealing widespread, PSMA-expressing bony metastases. Remarkably, after four treatments with PSMA-targeting radioligand therapy over the course of ten months, while continuing abiraterone treatment, the patient continues to have no evidence of disease as of the writing of this article, over five years after initiating PSMA therapy.
We report a case of a now 80-year-old male who presented in May 2015 at a routine screening with highly elevated prostate specific antigen (PSA). Eight months prior, the patient had initiated testosterone therapy with Androgel to treat hypogonadism and fatigue. After discontinuing testosterone, repeat screening showed PSA levels remained elevated. In August 2015, a prostate core biopsy revealed prostatic adenocarcinoma originally classified as Gleason 7 (3 + 4). CT and bone scan revealed extensive lymph and bone metastases throughout the axial and appendicular skeleton. The patient began ADT with degarelix to quickly lower testosterone and PSA levels (Figure 1).
A second pathology review in September 2015 upstaged the patient to Gleason 7 (4 + 3), stage 4B, which has a five-year survival rate of ~30% (14). In October 2015, ADT was switched to leuprolide, and in the following month he began denosumab for bone support. Starting in November 2015, the patient underwent 6 cycles of chemotherapy with docetaxel, based on evidence from the CHARRTED trial, in which addition of docetaxel at the beginning of treatment with ADT was demonstrated to improve overall survival in men with metastatic prostate cancer (15–17). A post-chemotherapy CT and bone scan in March 2016 showed improved appearance of known bone metastases suggesting some degree of treatment response and no evidence of solid organ metastases or abdominopelvic lymphadenopathy. PSA was also well-controlled on ADT, and the patient continued with leuprolide therapy alone.
A rise in PSA level in October 2016 prompted a switch to ADT with degarelix as the patient’s testosterone was still above castrate level. In December, 2016, multiple imaging studies (CT, MRI, and 18-F bone imaging PET/CT) showed continued osteoblastic skeletal metastasis. At this time, the patient was considering multiple clinical trials and underwent a gallium-68 (Ga-68) PSMA PET/CT scan to evaluate his candidacy for PSMA-targeting radioligand therapy. The scan showed multiple PSMA avid lesions consistent with active bone metastasis and no evidence of PSMA avidity in soft tissue, confirming his candidacy for lutetium-177 (Lu-177) PSMA radioligand therapy. Moreover, persistent castration-resistant disease supported the addition of abiraterone, which was initiated in February 2017.
At this time, 177Lu–PSMA-617 was not approved in the U.S., so this patient travelled to Germany to receive treatment. While continuing with abiraterone, the patient completed four cycles of 177Lu–PSMA-617 therapy over the course of 10 months (due to the patient’s robust response, the interval between the 3rd and 4th cycles was extended) and tolerated treatment well without any adverse effects (Figure 1). Whole-body planar imaging (4, 24, 48, and 72 hours after radioligand therapy) and SPECT/CT (24 and 48 hours after radioligand therapy) were performed to monitor treatment uptake. Interval and follow-up scans showed continued response to treatment with near complete regression of disease and dramatic decrease of PSMA expression in the bone (Figure 2; Table 1). The patient continues on leuprolide every three months along with daily abiraterone, and remains stable as of the time of this publication, over five years after his last treatment in January, 2018.
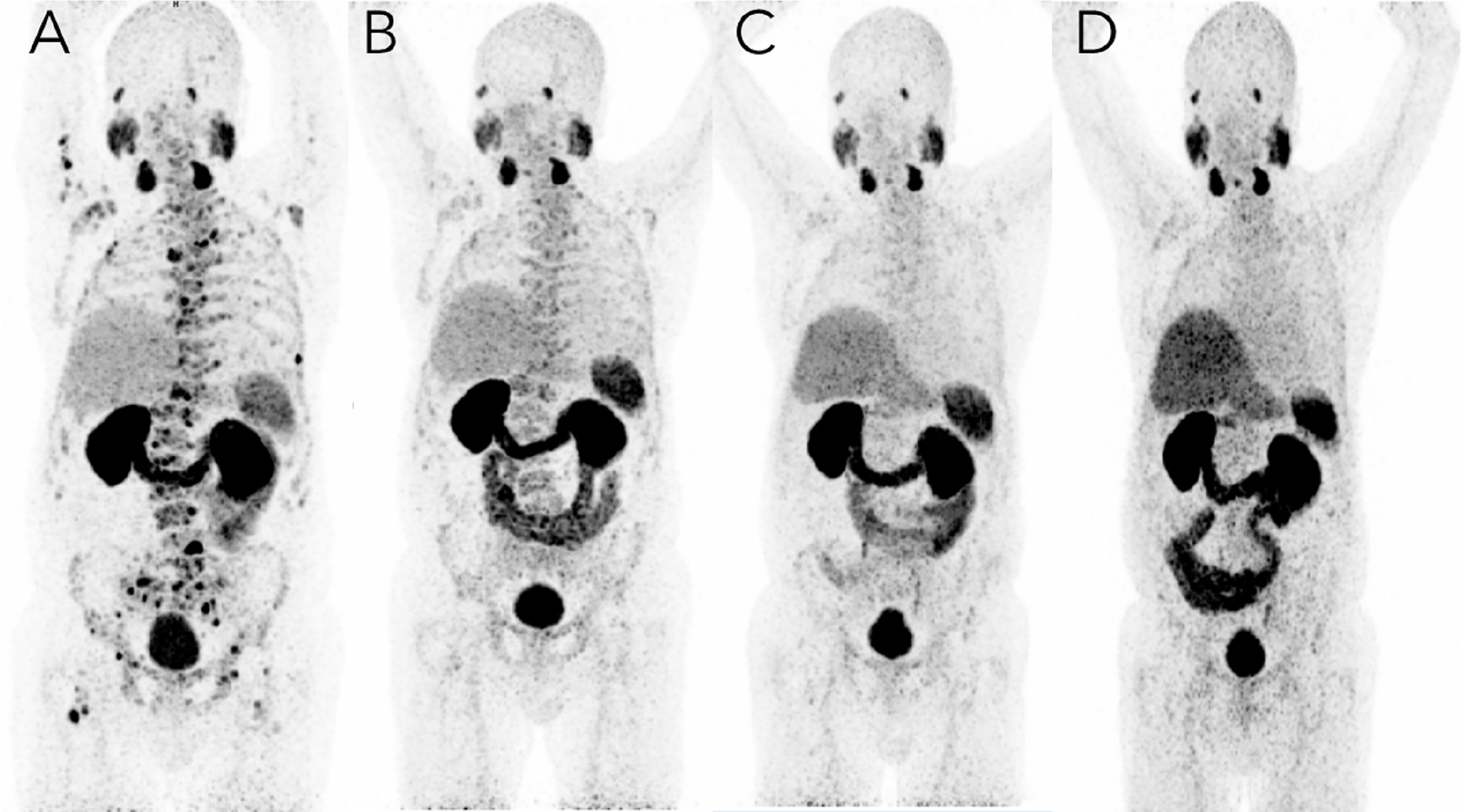
Figure 2 PSMA imaging before and after targeted radioligand therapy with 177Lu–PSMA-617. A 73-year-old male with mCRPC in 2017 with multiple bone and lymph node metastases with increased PSMA expression. (A) Imaging on March 14, 2017, just before the first dose of [177Lu]–PSMA-617 therapy; the baseline coronal PET [68Ga]-PSMA-11 PET maximum intensity projection (MIP) image shows bone metastases with significantly higher PSMA expression as well as the normal biodistribution of the tracer. (B) Imaging on July 24, 2017, just before the third dose of Lu-177 PSMA radioligand therapy, showed intense but decreasing uptake with an excellent target-to-background ratio, corresponding with molecular response to RLT therapy. Imaging four months (May 15, 2018) (C) and just over one year (February 11, 2019) (D) after the fourth dose of [177Lu]–PSMA-617, the [68Ga]-PSMA-11 PET MIP image shows multiple decreased or disappeared metastases. No evidence of PSMA-positive lymph nodes or new metastases and very mild uptake was noted in the central skeleton and sternum. Overall, no new and no intense PSMA-avid lesions were noted.
Prostate cancer is the fifth leading cause of death worldwide resulting in more than 350,000 deaths in 2018 alone (18–20). Additionally, mCRPC is generally incurable and fatal despite decades of research. While local or regional stages of prostate cancer have a nearly 100% five-year survival rate, the mCRPC five-year survival rate remains around 31%, demarcating a clear need for better diagnostic and therapeutic options (21).
The U.S. FDA approval of radiopharmaceutical 177Lu–PSMA-617 for the treatment of PSMA-positive mCRPC in March of 2022 marks an important advancement in mCRPC diagnostics and therapeutics. Approval follows the results of the VISION clinical trial (NCT03511664), where image-based progression-free survival was ~2.5 times longer when 177Lu–PSMA-617 was combined with standard care for adult patients with PSMA-positive mCRPC who had been treated with ADT and taxane-based chemotherapy (22). Similarly, the overall survival was four months longer, a 35% increase, when 177Lu–PSMA-617 was combined with standard care compared to standard care alone. These significant improvements in progression-free and overall survival for mCRPC patients demonstrate the powerful application of theranostics. While 177Lu–PSMA-617 in combination with abiraterone, the treatment course used in this patient, is not currently approved in the U.S., recent literature supports the potential for improved outcomes with this regimen (23).
Theranostics have shown great benefit in multiple settings, beginning with radioactive iodine for thyroid cancer in the 1940s, and more recent applications in gastroenteropancreatic neuroendocrine tumors (177lutetium DOTATATE), neuroblastomas (123I-metaiodobenzylguanidine), and melanoma (platinum nanoparticles, among others) (24–27). Combining imaging for better characterization, staging, and disease progression with targeted, localized radiation can decrease side effects and improve quality of life for specific patients. However, correctly identifying which patients will respond best remains a challenge. The use of PET/CT scanning in comparison with targeted radioligand imaging may help to highlight disease heterogeneity, providing more insight for patient eligibility.
For this patient, 177Lu–PSMA-617 was particularly successful likely at least in part due to the disease being limited to bony metastases and lymph nodes rather than soft tissue involvement. Interestingly, germline analysis in this patient had revealed a mutation in the TSC2 gene. TSC2 encodes the protein tuberin, which is involved in inhibiting mTOR signaling, a canonical pathway involved in cell growth and proliferation whose disfunction is related to oncogenic signaling, including in mCRPC (28, 29). Loss of function in TSC2 results in increased mTOR activity (30). Additionally, the PI3K-mTOR signaling pathway is often dysregulated in prostate cancer (31). This pathway interacts with multiple signal transduction cascades, including the androgen receptor, MAPK, and WNT pathways, that can promote tumor growth (28). Moreover, mechanistic studies suggest there is a correlation between PSMA expression and increased mTOR activity (32). The possible role of downstream aberrant activity in mTOR signaling in the presence of this patient’s high PSMA expressivity may provide some insight into his notable response to this therapy.
In the absence of curative therapy, 177Lu–PSMA-617 represents a promising theranostic approach for prostate cancer, and potentially other PSMA-expressing cancers as well (33, 34). Recent clinical studies have underscored the utility of this theranostic option showing it is non-inferior to docetaxel (35), may have potential for use as an earlier line treatment in chemo-naïve mCRPC due to its efficacy and low toxicity (36), and can also be used in heavily pretreated patients with similar effects (37). The remarkable response of our patient highlights the potential of theranostics to monitor and control extensive disease with excellent tolerability and restoration of quality of life.
I went for my annual physical with my primary care provider and found out that my PSA was 1800. I had scans and went to see an oncologist, and he told me I didn’t have much time - maybe 6 months to 1 year if I went on chemotherapy. So, I did (and lost my ponytail as a result), and responded pretty well at first, but then I got worse. My Private Health team told me about a new treatment available in Germany, so I went to see Dr. Baum, and that turned out quite well. After the first treatment, he showed me the scans and said the cancer had retreated a bit and he was encouraged. It got smaller and smaller with each subsequent treatment, and after the 4th treatment he couldn’t see any remaining disease. I go back every year for scans, but the disease looks like it is all gone. The treatments were not uncomfortable and didn’t hurt, and though the food at the clinic was terrible, the people were fun. I have kept in touch with one who also had a great response to the treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
RB: Treated the patient. DP, JZ, AM, EL, EG: Managed the case. ML, TW, LA-E, EG: Drafted and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors appreciate helpful guidance on therapeutic options for this patient from Channing Paller, MD from Johns Hopkins Sidney Kimmel Comprehensive Cancer Center and Paul G. Corn, MD, PhD from MD Anderson, and editorial support from Julie Nowicki, PhD from Private Health Management.
Authors DP, JZ, AM, EL, LA, and EG are employed by Private Health Management. Authors ML and TW were employed by Private Health Management. Author RB is employed by Curanosticum.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kulkarni HR, Singh A, Langbein T, Schuchardt C, Mueller D, Zhang J, et al. Theranostics of prostate cancer: From molecular imaging to precision molecular radiotherapy targeting the prostate specific membrane antigen. BJR (2018) 91:20180308. doi: 10.1259/bjr.20180308
2. Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med (2016) 57:1006–13. doi: 10.2967/jnumed.115.168443
3. Mansouri M, Shahbazi-Gahrouei D. A review on theranostic applications of iodine nanoparticles: Recent findings and perspectives. Nanomedicine J (2021) 8:234–40. doi: 10.22038/nmj.2021.56425.1575
4. Zhao L, Chen J, Pang Y, Fu K, Shang Q, Wu H, et al. Fibroblast activation protein-based theranostics in cancer research: A state-of-the-art review. Theranostics (2022) 12:1557–69. doi: 10.7150/thno.69475
5. Jokar N, Velikyan I, Ahmadzadehfar H, Rekabpour SJ, Jafari E, Ting HH, et al. Theranostic approach in breast cancer: a treasured tailor for future oncology. Clin Nucl Med (2021) 46:e410–20. doi: 10.1097/RLU.0000000000003678
6. Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med (2015) 56:914–20. doi: 10.2967/jnumed.114.147413
7. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med (2011) 364:1995–2005. doi: 10.1056/NEJMoa1014618
8. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. New Engl J Med (2013) 368:138–48. doi: 10.1056/NEJMoa1209096
9. Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med (2012) 367:1187–97. doi: 10.1056/NEJMoa1207506
10. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet (2011) 377:813–22. doi: 10.1016/S0140-6736(10)62344-6
11. Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New Engl J Med (2013) 369:213–23. doi: 10.1056/NEJMoa1213755
12. Wright GL, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urologic Oncology: Semin Original Investigations (1995) 1:18–28. doi: 10.1016/1078-1439(95)00002-Y
13. Ristau BT, O’Keefe DS, Bacich DJ. The prostate-specific membrane antigen: lessons and current clinical implications from 20 years of research. Urologic Oncology: Semin Original Investigations (2014) 32:272–9. doi: 10.1016/j.urolonc.2013.09.003
14. Cancer of the prostate - cancer stat facts . SEER. Available at: https://seer.cancer.gov/statfacts/html/prost.html (Accessed December 8, 2022).
15. Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol (2018) 36:1080–7. doi: 10.1200/JCO.2017.75.3657
16. Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
17. Sweeney C, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Eisenberger MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. JCO (2014) 32:LBA2–2. doi: 10.1200/jco.2014.32.18_suppl.lba2
18. Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol (2019) 75:88–99. doi: 10.1016/j.eururo.2018.03.028
19. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med (2018) 378:645–57. doi: 10.1056/NEJMra1701695
21. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA: A Cancer J Clin (2022) 72:403–502. doi: 10.3322/caac.21731
22. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med (2021) 385:1091–103. doi: 10.1056/NEJMoa2107322
23. Suman S, Parghane RV, Joshi A, Prabhash K, Talole S, Basu S. Combined 177 Lu-PSMA-617 PRLT and abiraterone acetate versus 177 Lu-PSMA-617 PRLT monotherapy in metastatic castration-resistant prostate cancer: an observational study comparing the response and durability. Prostate (2021) 81:1225–34. doi: 10.1002/pros.24219
24. Jia AY, Kashani R, Zaorsky NG, Spratt DE, Kiess AP, Michalski JM, et al. Lutetium-177 DOTATATE: A practical review. Pract Radiat Oncol (2022) 12:305–11. doi: 10.1016/j.prro.2022.02.002
25. Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, et al. Criteria for evaluation of disease extent by 123I-metaiodobenzylguanidine scans in neuroblastoma: A report for the international neuroblastoma risk group (INRG) task force. Br J Cancer (2010) 102:1319–26. doi: 10.1038/sj.bjc.6605621
26. Kraal KCJM, Tytgat GAM, van Eck-Smit BLF, Kam B, Caron HN, van Noesel M. Upfront treatment of high-risk neuroblastoma with a combination of 131I-MIBG and topotecan. Pediatr Blood Cancer (2015) 62:1886–91. doi: 10.1002/pbc.25580
27. Guan M, Zhu S, Li S. Recent progress in nanomedicine for melanoma theranostics with emphasis on combination therapy. Front Bioengineering Biotechnol (2021) 9:661214. doi: 10.3389/fbioe.2021.661214
28. Shorning BY, Dass MS, Smalley MJ, Pearson HB. The PI3K-AKT-mTOR pathway and prostate cancer: At the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21124507
29. Palamiuc L, Emerling BM. PSMA brings new flavors to PI3K signaling: a role for glutamate in prostate cancer. J Exp Med (2017) 215:17–9. doi: 10.1084/jem.20172050
30. Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-akt signaling through downregulation of PDGFR. J Clin Invest (2003) 112:1223–33. doi: 10.1172/JCI17222
31. Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell (2010) 18:11–22. doi: 10.1016/j.ccr.2010.05.026
32. Kaittanis C, Andreou C, Hieronymus H, Mao N, Foss CA, Eiber M, et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J Exp Med (2017) 215:159–75. doi: 10.1084/jem.20171052
33. Van de Wiele C, Sathekge M, de Spiegeleer B, de Jonghe PJ, Beels L, Maes A. PSMA-targeting positron emission agents for imaging solid tumors other than non-prostate carcinoma: a systematic review. Int J Mol Sci (2019) 20:E4886. doi: 10.3390/ijms20194886
34. de Galiza Barbosa F, Queiroz MA, Nunes RF, Costa LB, Zaniboni EC, Marin JFG, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging (2020) 20:23. doi: 10.1186/s40644-020-00300-7
35. Satapathy S, Mittal BR, Sood A, Das CK, Mavuduru RS, Goyal S, et al. 177Lu-PSMA-617 versus docetaxel in chemotherapy-naïve metastatic castration-resistant prostate cancer: A randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging (2022) 49:1754–64. doi: 10.1007/s00259-021-05618-3
36. Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med (2019) 60:955–62. doi: 10.2967/jnumed.118.216820
Keywords: case report, PSMA, radioligand therapy, prostate cancer, metastatic castration resistant prostate cancer, Lu-PSMA-617, theranostics
Citation: Parker D, Zambelli J, Lara MK, Wolf TH, McDonald A, Lee E, Abou-Elkacem L, Gordon EJ and Baum RP (2023) Case Report: Long-term complete response to PSMA-targeted radioligand therapy and abiraterone in a metastatic prostate cancer patient. Front. Oncol. 13:1192792. doi: 10.3389/fonc.2023.1192792
Received: 23 March 2023; Accepted: 14 April 2023;
Published: 28 April 2023.
Edited by:
Arun Azad, Peter MacCallum Cancer Centre, AustraliaReviewed by:
Nattakorn Dhiantravan, Queensland Health, AustraliaCopyright © 2023 Parker, Zambelli, Lara, Wolf, McDonald, Lee, Abou-Elkacem, Gordon and Baum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva J. Gordon, ZWdvcmRvbkBwcml2YXRlaGVhbHRoLmNvbQ==; Richard P. Baum, YmF1bXJwQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.