
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 May 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1190327
This article is part of the Research Topic Advances in the Surgical Management of Gastric and Colorectal Cancers View all 35 articles
Background: The purpose of this study is to construct a novel and practical nomogram and risk stratification system to accurately predict cancer-specific survival (CSS) of early-onset locally advanced rectal cancer (EO-LARC) patients.
Methods: A total of 2440 patients diagnosed with EO-LARC between 2010 and 2019 were screened from the Surveillance, Epidemiology, and End Results (SEER) database. The pool of potentially eligible patients was randomly divided into two groups: a training cohort (N=1708) and a validation cohort (N=732). The nomogram was developed and calibrated using various methods, including the coherence index (C-index), receiver operating characteristic curve (ROC), calibration curves, and decision curves (DCA). A new risk classification system was established based on the nomogram. To compare the performance of this nomogram to that of the American Joint Committee on Cancer (AJCC) staging system, DCA, net reclassification index (NRI), and integrated discrimination improvement (IDI) were employed.
Result: Seven variables were included in the model. The area under the ROC curve (AUC) for the training cohort was 0.766, 0.736, and 0.731 at 3, 6, and 9 years, respectively. Calibration plots displayed good consistency between actual observations and the nomogram’s predictions. The DCA curve further demonstrated the validity of the nomination form in clinical practice. Based on the scores of the nomogram, all patients were divided into a low-risk group, a middle-risk group, and a high-risk group. NRI for the 3-, 6-, and 9-year CSS(training cohort: 0.48, 0.45, 0.52; validation cohort: 0.42, 0.37, 0.37), IDI for the 3-, 6-, and 9-year CSS (training cohort: 0.09, 0.10, 0.11; validation cohort: 0.07, 0.08, 0.08). The Kaplan-Meier curve revealed that the new risk classification system possesses a more extraordinary ability to identify patients in different risk groups than the AJCC staging.
Conclusion: A practical prognostic nomogram and novel risk classification system have been developed to efficiently predict the prognosis of EO-LARC. These tools can serve as a guide to individualize patient treatment and improve clinical decision-making.
Colorectal cancer is a common malignancy of the gastrointestinal system, with the third-highest global incidence and mortality rate among malignant tumors (1). Due to insidious disease, poor specificity of clinical symptoms, and lack of widely used screening tools, a significant proportion of patients are diagnosed with locally advanced rectal cancer (LARC) at the time of diagnosis (2, 3). LARC has garnered considerable attention from researchers domestically and internationally due to its high risk of recurrence and distant metastasis. Early-onset rectal cancer(EORC), which refers to rectal cancer diagnosis in individuals under 50 years, is still not fully understood in terms of its causes and underlying mechanisms (4). In recent years, investigators have studied the clinical and molecular biological features of EORC and found that it may be a separate disease rather than a subgroup of rectal cancer (5, 6). The prognosis of early-onset locally advanced rectal cancer(EO-LARC)may differ from EORC, and a separate survival analysis of EO-LARC is warranted (7). Radical surgical resection is still one of the main treatments for EO-LARC.
The current National Comprehensive Cancer Network (NCCN) clinical practice guidelines primarily rely on the AJCC TNM staging system for predicting prognosis and guiding treatment of EO-LARC patients (8). However, the TNM staging system still has certain limitations, such as age, gender, histological type, degree of tumor differentiation, serum biomarkers, and treatment-related factors affecting patient prognosis (9). Compared with the traditional TMN staging system or other staging systems, nomograms have demonstrated accurate predictive value for many types of tumors and are widely used in clinical applications (10, 11). However, there is no nomogram model to predict the postoperative survival of EO-LARC patients.
In this study, we investigated the factors that affect the postoperative survival of EO-LARC patients based on a large sample dataset from multiple centers in the SEER database and created a nomogram and a novel risk stratification system based on these data to help clinicians make personalized predictions of patient prognosis and guide clinical decisions.
Clinically relevant data for patients diagnosed with EO-LARC between 2010 and 2019 were extracted from the SEER registry database (2010–2019) using SEER*Stat 8.3.9.2 software. This study meets the requirements of the Declaration of Helsinki, and SEER is a publicly available database. The patients’ records and information included in this study were anonymous before analysis. Therefore, institutional ethics committee approval was not required for this study.
International Classification of Diseases in Oncology (ICD) (C20.9) and ICD code O-3 morphology (8140) were used for differentiation. Inclusion criteria: (a) age <50; (b) confirmed diagnosis of locally progressive rectal cancer (T1-2 N+/T3-4 N0/T3-4 N+); (c) radical surgery; (d) known cause of death. Exclusion criteria: (a) incomplete clinicopathological information; (b) lack of follow-up information; (c) occurrence of distant metastases or undetermined distant metastases; (d) missing treatment options; The process of selection by brush is shown in the flow chart Figure 1.
Thirteen clinically relevant variables for EO-LARC were downloaded with the seer database, including age, gender, race, pathology, tumor size, T-stage, N-stage, tumor size, lymph node ratio (LNR), CEA, radiotherapy, and survival data were extracted. The main terminal point of the study was the time until cancer-specific death. Tumor staging was performed using the 8th edition AJCC TNM staging criteria.
All eligible cases were randomly divided into a training cohort (n=1708, 70% of the total cases) and a validation cohort (n=732, 30% of the total cases). The training cohort was used to build the prognostic model of the nomogram. Meanwhile, the validation cohort was used to test the stability of the model. All variables included in the study were analyzed using univariate Cox regression analysis and multivariate Cox regression analysis to screen for variables that significantly affect postoperative CSS in patients with EO-LARC.
Based on the training and validation cohorts, the models were validated using C-index, receiver operating characteristics (ROC), calibration curves, and decision curve analysis (DCA). The C-index showed the nomogram’s performance and prediction accuracy, while the ROC showed its sensitivity and specificity. 3-, 6-, and 9-year calibration curves were produced to assess the degree to which model predictions and actual data agreed. The analyses above were done 1,000 times by Bootstrap rerun to lessen bias.
Using the net reclassification index (NRI), C-index, integrated discrimination improvement (IDI), and DCA compared to the AJCC staging system, the nomogram model’s net benefit and risk stratification were evaluated. DCA evaluated the nomogram’s clinical usefulness. Using the best threshold of total score chosen by X-Tile, all eligible patients were separated into low-risk, middle-risk, and high-risk groups. Log-rank tests and Kaplan-Meier curves were used to compare the CSS of patients in various groups.
All study variables are presented as the number of cases and percentages. Univariate and multi-factor Cox regression analyses, C-index, calibration plots, ROC curves, and DCA curves were generated using R version 3.6.3 and correlation packages. Kaplan-Meier and log-rank tests were applied for survival analysis. Differences in the distributions of the training and validation cohorts were detected by the chi-square test. A two-tailed p-value of less than 0.05 was considered statistically significant.
A total of 2440 patients were diagnosed with early-onset locally advanced rectal cancer and randomized in a 7:3 ratio to the training cohort (1708, 70%) and the validation cohort (732, 30%) (Figure 1). The population and clinical features of patients with early-onset locally advanced rectal cancer were summarized below in Table 1. Of all patients eligible for inclusion in the research, 1371 (56.19%) were male, 1069 (43.18%) were female, 1910 (78.28%) were white, and 192 (7.87%) were black. Most patients received chemotherapy treatment (90.12%) and radiotherapy (73.98%). The training and validation cohorts were not statistically different in the
distribution of the 13 variables (P >0.05).
Univariate analysis of the training cohort showed that age, race, sex, pathology, radiotherapy, grade, T-stage, N-stage, CEA, LNR, and radiation were promotional factors for patients with early-onset locally advanced rectal cancer (P<0.05). The findings of multivariate Cox regression analysis showed that sex, pathology, radiotherapy, grade, T-stage, CEA, and LNR were independently prognostic factors affecting CSS in patients with early-onset locally advanced rectal cancer (P<0.05) and were therefore included in the build-up of the nomogram (Table 2).
The results of the nomogram, which incorporates all the independent prognostic factors in the multivariate Cox regression model, including sex, pathology, radiotherapy, grade, T-stage, CEA, and LNR, for the prediction of CSS at 3, 6, and 9 years for patients with EO-LARC are shown in Figure 2. This nomogram can be used to predict individual CSS according to different clinicopathological characteristics of patients. The internal validation C-index of the model assesses the model’s accuracy; the calibration curve assesses the consistency of the predicted values with the actual survival. The C-indexes for the training and validation cohorts were 0.747 (95% CI:0.735-0.752) and 0.744 (95% CI: 0.731-0.756), respectively (Figure 3). The ROC curves, DCA curves, and calibration curves are shown in Figures 3–5. The results of the ROC curve analysis showed that the AUCs for the training cohort at 3, 6, and 9 years were 0.766, 0.736, and 0.731, respectively. The AUCs for the validation cohort at 3, 6, and 9 years was 0.791, 0.751, and 0.746, respectively. The calibration curves all showed that the 3, 6, and 9-year predicted CSS probabilities strongly agreed with the actual observations. In addition, the DCA curves at 3, 6, and 9 years showed outstanding positive clinical net benefits in both the training and validation cohorts. e validation cohort. DCA, decision curve analysis; CSS, cancer-specific survival.
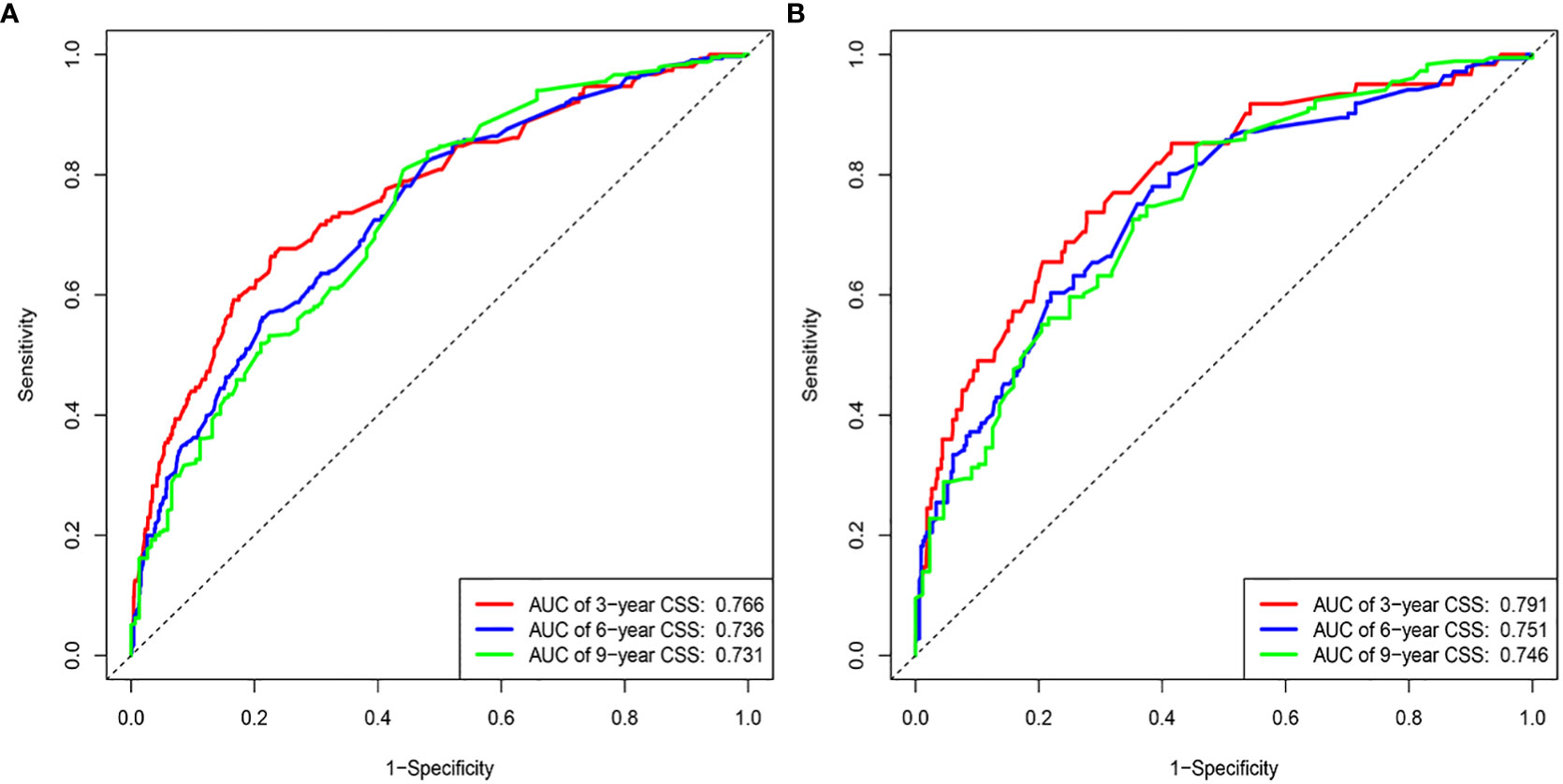
Figure 3 ROC curves. (A) Training cohorts based on the nomogram. (B) Validation cohorts based on nomogram.
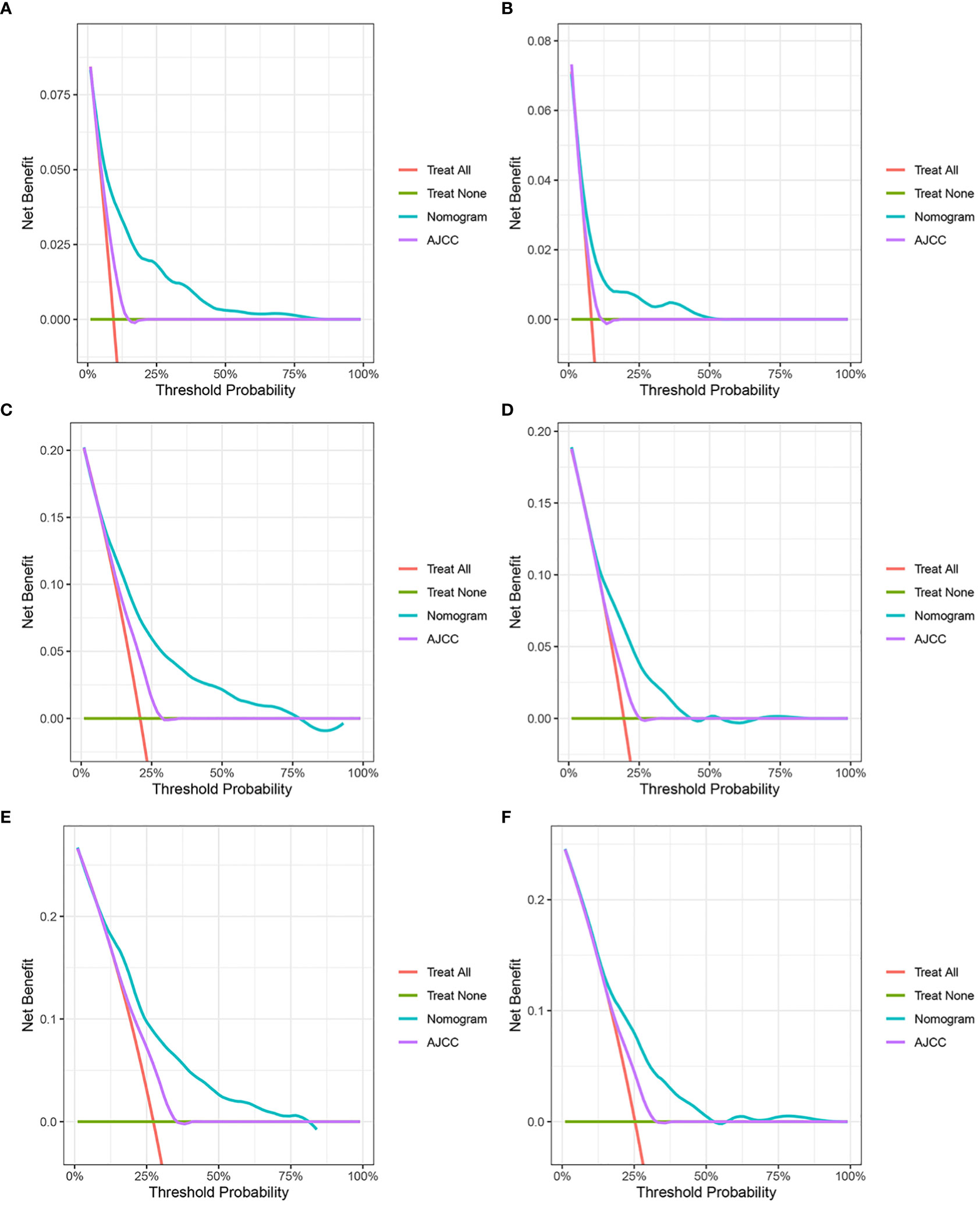
Figure 4 Decision curve analysis. (A, C, E) DCA curves of 3-year, 6-year, and 9-year CSS in the training cohort. (B, D, F) DCA curves of 3-year, 6-year, and 9-year CSS in the validation cohort. DCA, decision curve analysis; CSS, cancer-specific survival.
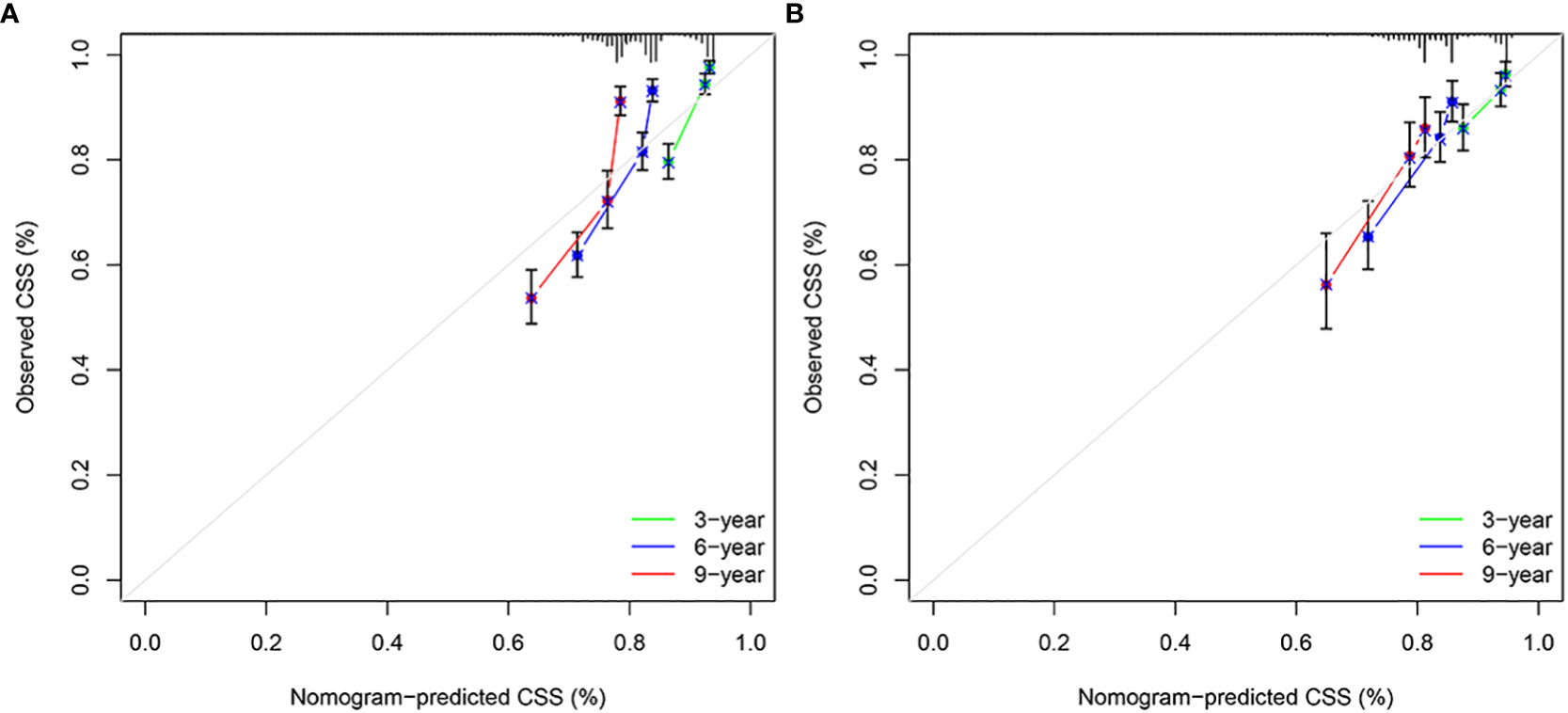
Figure 5 Calibration plots of 3-year, 6-year, and 9-year CSS for EO-LARC patients. (A) Calibration plots of 3-year, 6-year, and 9-year CSS in the training cohort. (B) Calibration plots of 3-year, 6-year, and 9-year CSS in the validation cohort. CSS, cancer-specific survival.
In the training and validation cohort, the C-index of the nomogram was all higher than that of the AJCC staging system (Figure 6). The 3-, 6-, and 9-year NRIs were 0.48 (95% CI=0.40-0.65), 0.45 (95% CI=0.36-0.66), and 0.52 (95% CI=0.37-0.71), respectively (Table 3). IDI (training cohort: 3-, 6-, 9-year CSS: 0.09, 0.10, 0.11; validation cohort: 3-, 6-, 9-year CSS: 0.07, 0.08, 0.08) indicated that the established nomogram significantly outperformed AJCC TNM staging system (P<0.05) (Table 3). The net benefit of the nomogram was compared to that of the AJCC staging system. The DCA curves showed that the nomogram had a higher net benefit and clinical validity than the 8th edition of the AJCC TNM staging system in the training and validation cohorts (Figure 4).
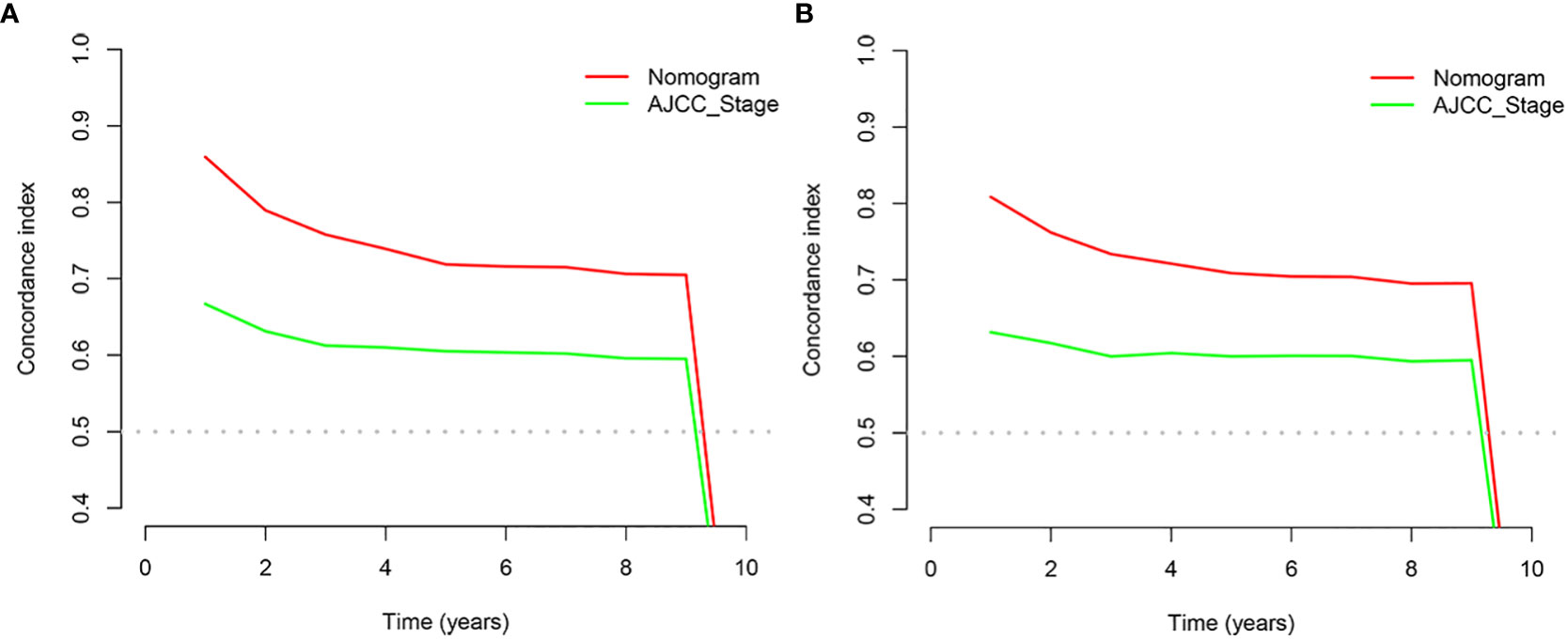
Figure 6 C-index analysis. (A) Nomogram-related C-index and AJCC staging criteria-related C-index in the training cohort. (B) Nomogram-related C-index and AJCC staging criteria-related C-index in the validation cohort.
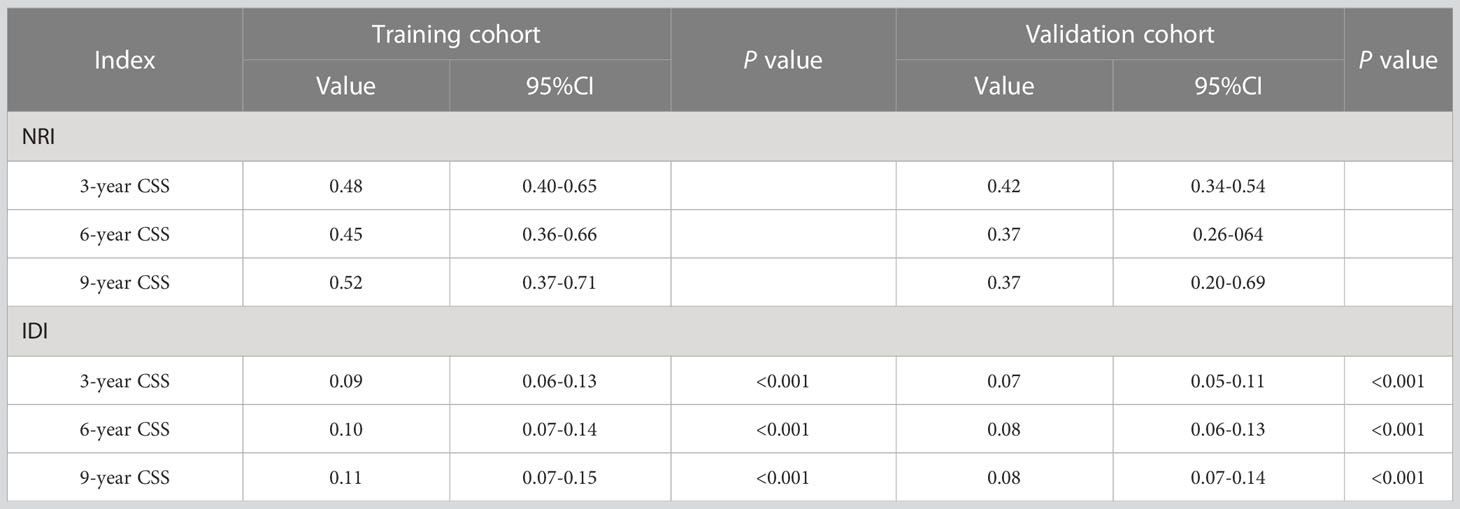
Table 3 The nomogram and AJCC staging criteria for NRI and IDI are in the CSS projections for EO-LARC.
Finally, using the total points determined by the nomogram, we created a risk stratification system. Three risk groups of EO-LARC patients were created: low risk (total points < 134), middle risk (134 ≤ total points <162), and high risk (total points ≥ 162). (Figure 7). The AJCC staging approach had a limited ability to identify high-risk patients in both the training and validation cohorts, but the Kaplan-Meier CSS curves demonstrated excellent differentiation among the three risk groups (Figure 8).
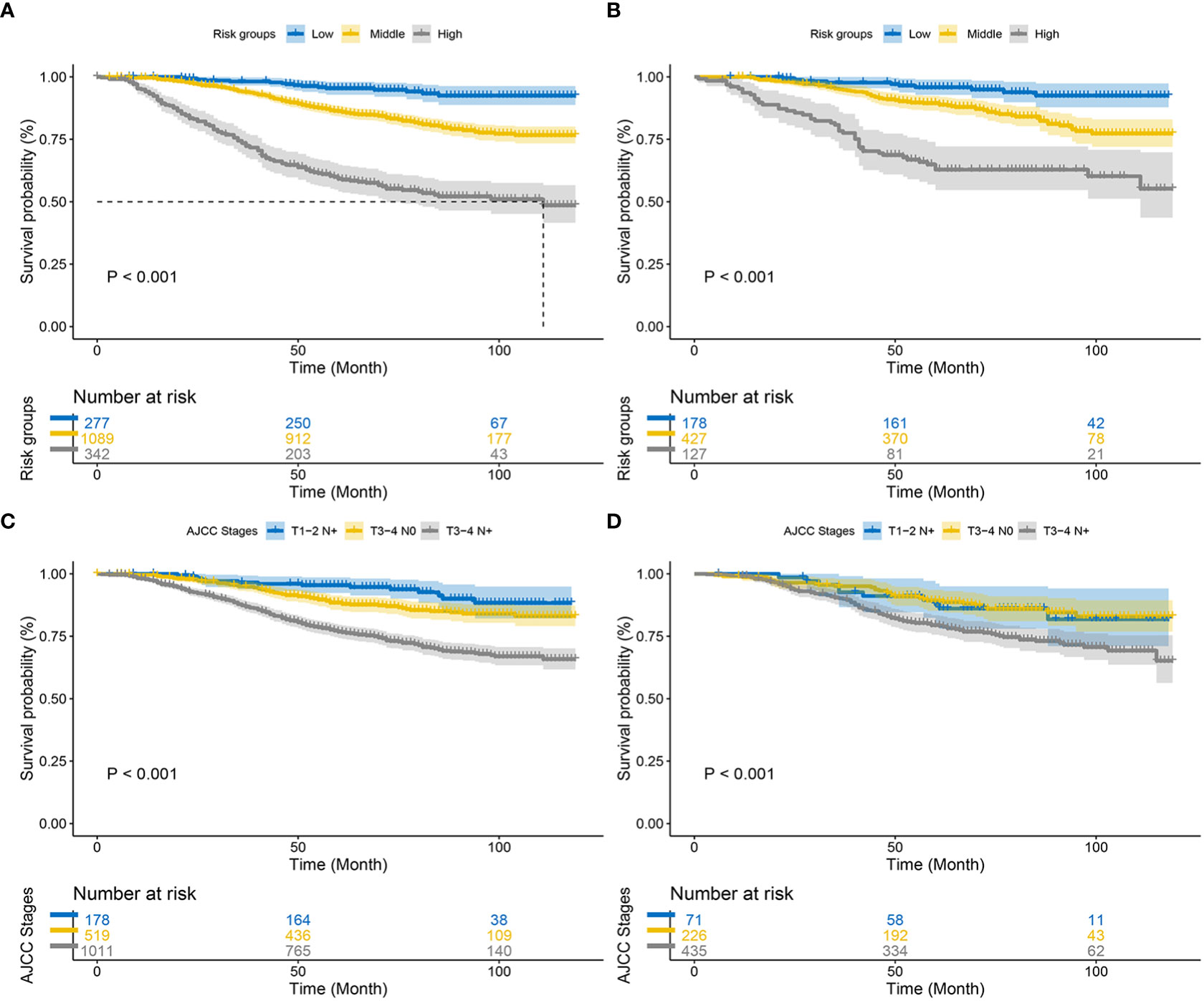
Figure 8 Kaplan–Meier CSS curves of patients with EO-LARC based on different criteria. (A, B) Kaplan–Meier CSS curves of the training and validation cohorts based on the new risk stratification system. (C, D) Kaplan–Meier CSS curves of the training and validation cohorts based on AJCC staging criteria.
The incidence of EORC patients is increasing annually, and the results of their survival analysis have been reported successively (12, 13). Accurate prediction of patient survival prognosis can assist medical personnel in making individualized treatment and follow-up decisions. While the AJCC TNM staging system is currently the most commonly used prognostic assessment system, relying solely on anatomic invasion and metastasis of tumors may impact the accuracy of survival prediction. In recent years, several clinical prediction models for predicting tumor prognosis have emerged and shown the superior predictive ability of the AJCC TNM staging system (14, 15). Clinical prediction models for projecting EO-LARC patients are relatively rare.
In this study, based on univariate and multifactorial COX proportional risk regression analysis, sex, pathology, radiotherapy, grade, T-stage, CEA, and LNR were independent risk factors affecting the postoperative outcome of EO-LARC patients. Gender differences had a significant effect on postoperative EO-LARC. This study found a worse prognosis in male patients (HR=1.45; 95% CI=1.16-1.82; P<0.01), which aligns with most of the literature. The protective effect of estrogen and differences in pregnancy, birth, anatomy, and physiology may be associated with a relatively lower incidence and better prognosis of CRC in women (16).
Mucinous and signet ring cell carcinoma were identified as independent risk factors for postoperative EO-LARC patients (HR=1.49; 95% CI=1.03-2.15; P<0.01), consistent with many recent studies on the relationship between tumor type and prognosis (17). Signet ring carcinoma is a rare type of colorectal cancer with a low incidence. It belongs to a particular kind of invasive adenocarcinoma along with mucinous adenocarcinoma, which has a high incidence of tumor infiltration depth, lymph node metastasis, vascular tumor embolism, and combined intestinal obstruction, with a late stage of disease at the time of patient presentation, a low surgical resection rate, and a poor prognosis.
Radiotherapy is an essential tool in the treatment of EO-LARC, and the European Society for Medical Oncology (ESMO) guidelines recommend two preoperative radiotherapy modalities: long-course radiotherapy combined with concurrent chemotherapy. Still, short-course preoperative radiotherapy is the predominant treatment in some European countries (18). This study showed that patients could benefit from radiotherapy (HR=0.56; 95% CI=0.41-0.77; P<0.01). The effect of tumor differentiation on the postoperative outcome of colorectal cancer patients has been studied more frequently, and the lower the degree of tumor cell differentiation tends to be more malignant, less sensitive to treatment such as radiotherapy, and less favorable overall treatment prognosis. The College of American Pathologists used the degree of tumor differentiation as a class IIA prognostic factor for colorectal cancer (19). The results of the present study also showed that high-grade (low/undifferentiated) was an independent risk factor for postoperative CSS in patients with EO-LARC (P<0.001), and the degree of differentiation was significant for the prognosis of rectal cancer.
This study found that T-stage was an independent risk factor for postoperative CSS in EO-LARC patients by multifactorial analysis (P<0.01). That is, the more extensive invasion of the primary focus within a specific range, the more involvement of nearby lymph nodes, or the more distant metastasis of the tumor, the worse the prognosis of patients. A study on colon cancer noted that the 5-year survival rate of patients with high T-stage was much lower than that of patients with T1-stage (20).
Carcinoembryonic antigen (CEA) is a standard tumor marker in colorectal cancer. It can, to some extent, provide a basis for tumor diagnosis, recurrence, and metastasis and is most effective when patients have high preoperative serum CEA levels (21). The results of this study are consistent with previous studies (22, 23), where elevated preoperative CEA was an independent risk factor for postoperative EO-LARC patients (HR=1.24; 95% CI=1.05-1.47; P<0.001), which is also consistent with clinical reality.
Lymph node metastasis is a common form of metastasis in colorectal cancer, which can lead to disease recurrence and even death. The rate of lymph node metastasis is calculated by dividing the number of metastatic lymph nodes by the number of pathologically examined lymph nodes. Compared with the traditional number of lymph node metastases, it can effectively avoid differences due to individual patient factors. It can be used for lymph node staging and prognosis assessment of colorectal patients (24, 25). The study results indicated that lymph node metastasis rate (LNR) (HR=1.24; 95% CI=1.05-1.47; P<0.001) had a high predictive value for the survival of EO-LARC patients.
Nomograms are an intuitive and easy-to-understand statistical tool that can consider multiple risk factors and provide individualized assessments of patients. This study conducted a multifactorial survival analysis, which included seven objective clinical and pathological factors (sex, pathology, radiotherapy, grade, T-stage, CEA, and LNR) to construct a nomogram that predicts CSS at 3, 6, and 9 years in patients with EO-LARC. The C-index, NRI, ROC, and IDI demonstrated that the nomogram had better clinical value than AJCC staging. Furthermore, EO-LARC patients were classified into low, medium, and high-risk groups based on the total score of the nomogram. The results of Kaplan-Meier and Cox risk ratio models, showed significant differences in CSS between these three groups.
Although the EO-LARC patients included in this study were rigorously screened, several limitations remain: (i) The SEER database does not contain detailed treatment protocols, gene expression information, immunotherapy, and other indicators, which may affect the accuracy and comprehensiveness of the prediction model. (ii) Retrospective studies may lead to inherent bias, and direct deletion of patients with missing data may introduce selection bias. (iii) The lack of independent external validation in the study may affect the practical generalizability of the prediction model. The selection of predictors still needs to be optimized in the future and confirmed based on prospective randomized clinical trials.
In conclusion, this study constructed and validated a prognostic nomogram, which provides a simple and reliable tool for survival prediction of EO-LARC patients after surgery. Meanwhile, the new risk stratification model can conveniently screen patients with different risks, which is important for the individualized treatment of EO-LARC cancer patients.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
All methods have adhered to the relevant official SEER database guidelines and regulations.
Conceptualization: YS. Data curation: DY. Formal analysis: YS and YL. Writing-original draft: YS. Writing-review and editing: LL and JQ. All authors contributed to the article and approved the submitted version.
National Key R&D Program Projects (Number: 2022YFC2407304).
We would like to thank those who have been involved in the establishment of the SEER database and making this database available on line.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Bigness A, Imanirad I, Sahin IH, Xie H, Frakes J, Hoffe S, et al. Locally advanced rectal adenocarcinoma: treatment sequences, intensification, and rectal organ preservation. CA Cancer J Clin (2021) 71(3):198–208. doi: 10.3322/caac.21661
4. Nfonsam V, Wusterbarth E, Gong A, Vij P. Early-onset colorectal cancer. Surg Oncol Clin N Am (2022) 31(2):143–55. doi: 10.1016/j.soc.2021.11.001
5. Silla IO, Rueda D, Rodríguez Y, García JL, de la Cruz Vigo F, Perea J. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol (2014) 20(46):17288–96. doi: 10.3748/wjg.v20.i46.17288
6. Kirzin S, Marisa L, Guimbaud R, De Reynies A, Legrain M, Laurent-Puig P, et al. Sporadic early-onset colorectal cancer is a specific sub-type of cancer: a morphological, molecular and genetics study. PloS One (2014) 9(8):e103159. doi: 10.1371/journal.pone.0103159
7. Xiao TX, Hou WY, Mei SW, Liu Q.. [Survival analysis of early-onset locally advanced rectal cancer: a retrospective study based on the surveillance, epidemiology, and end results (SEER) database]. Zhonghua Wei Chang Wai Ke Za Zhi (2023) 26(1):75–83. doi: 10.3760/cma.j.cn441530-20220704-00291
8. Greene FL. Current TNM staging of colorectal cancer[J]. Lancet Oncol (2007) 8(7):572–3. doi: 10.1016/S1470-2045(07)70185-7
9. Hueman M, Wang H, Henson D, Chen D. Expanding the TNM for cancers of the colon and rectum using machine learning: a demonstration. ESMO Open (2019) 4(3):e000518. doi: 10.1136/esmoopen-2019-000518
10. Yang D, Su Y, Zhao F, Hu Y, Zhao K, Xiong X, et al. Low-grade hepatocellular carcinoma characteristics, a practical nomogram and risk stratification system: a SEER population-based study. Expert Rev Gastroenterol Hepatol (2022) 16(11-12):1115–23. doi: 10.1080/17474124.2022.2150610
11. Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond) (2020) 40(7):301–12. doi: 10.1002/cac2.12067
12. McClelland PH, Liu T, Ozuner G. Early-onset colorectal cancer in patients under 50 years of age: demographics, disease characteristics, and survival. Clin Colorectal Cancer (2022) 21(2):e135–44. doi: 10.1016/j.clcc.2021.11.003
13. Wu J, Lu L, Chen H, Lin Y, Zhang H, Chen E, et al. Prognostic nomogram to predict the overall survival of patients with early-onset colorectal cancer: a population-based analysis. Int J Colorectal Dis (2021) 36(9):1981–93. doi: 10.1007/s00384-021-03992-w
14. Muneoka Y, Akazawa K, Ishikawa T, Ichikawa H, Nashimoto A, Yabusaki H, et al. Nomogram for 5-year relapse-free survival of a patient with advanced gastric cancer after surgery. Int J Surg (2016) 35:153–9. doi: 10.1016/j.ijsu.2016.09.080
15. Zhao K, Yang D, Zhou Y, Ding Y. Clinical features and prognostic models in patients with intrahepatic cholangiocarcinoma: a population-based analysis. J Gastrointest Surg (2023) 27(5):945–55. doi: 10.1007/s11605-023-05602-2
16. Haller DG, O’Connell MJ, Cartwright TH, Twelves CJ, McKenna EF, Sun W, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol (2015) 26(4):715–24. doi: 10.1093/annonc/mdv003
17. Deans GT, Parks TG, Rowlands BJ, Spence RA. Prognostic factors in colorectal cancer. Br J Surg (1992) 79(7):608–13. doi: 10.1002/bjs.1800790706
18. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34(1):10–32. doi: 10.1016/j.annonc.2022.10.003
19. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. college of American pathologists consensus statement 1999. Arch Pathol Lab Med (2000) 124(7):979–94. doi: 10.5858/2000-124-0979-PFICC
20. Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg (1995) 180(6):705–12.
21. Yuan SQ, Zhou ZW, Wan DS, Chen G, Lu ZH, Wang GQ, et al. The role of half-life of carcinoembryonic antigen (CEA) in prognosis prediction of colorectal cancer patients with preoperatively elevated CEA. Ai Zheng (2008) 27(6):612–7.
22. Nakamura Y, Shida D, Tanabe T, Takamizawa Y, Imaizumi J, Ahiko Y, et al. Prognostic impact of preoperatively elevated and postoperatively normalized carcinoembryonic antigen levels following curative resection of stage I-III rectal cancer. Cancer Med (2020) 9(2):653–62. doi: 10.1002/cam4.2758
23. Yang KL, Yang SH, Liang WY, Kuo YJ, Lin JK, Lin TC, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat Oncol (2013) 8:43. doi: 10.1186/1748-717X-8-43
24. Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg (2010) 251(6):1070–8. doi: 10.1097/SLA.0b013e3181d7789d
Keywords: early-onset rectal cancer, risk stratification, clinical prediction model, cancer-specific survival, AJCC staging
Citation: Su Y, Yang DS, Li Yq, Qin J and Liu L (2023) Early-onset locally advanced rectal cancer characteristics, a practical nomogram and risk stratification system: a population-based study. Front. Oncol. 13:1190327. doi: 10.3389/fonc.2023.1190327
Received: 20 March 2023; Accepted: 26 April 2023;
Published: 16 May 2023.
Edited by:
Ye Zhou, Fudan University, ChinaCopyright © 2023 Su, Yang, Li, Qin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Liu, aGFsZXNhbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.