
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 14 September 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1188772
This article is part of the Research Topic Innovative Strategies and New Insights for the Treatment of Stage III Non-Small Cell Lung Cancer View all 10 articles
Background: Squamous cell carcinoma (SCC) is one of the most common subtypes of non-small cell lung cancer, but its treatment options remain limited. Epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (TKIs) have limited efficacy in the treatment of lung SCC. Here, we report an SCC patient who developed EGFR-T790M mutation and showed gefitinib resistance achieved an extremely long survival by taking Osimertinib alternatively.
Case summary: A patient, 66-year-old non-smoking and drinking male with advanced SCC who was deemed inoperable at the time of diagnosis. The first genetic testing showed deletion mutation of exon 19 of EGFR. The patient was then treated with gefitinib with no significant efficacy. EGFR-T790M mutation was found in the second genetic test. The treatment regimen was changed to radiotherapy with Osimertinib, and the patient’s primary lesion and the brain metastases were well controlled.
Conclusion: This typical case highlights the important role of Osimertinib in patients with SCC carrying EGFR mutations.
Lung cancer is the second most common cancer and the most common cause of cancer death worldwide. In 2020, 2.2 million new cases of lung cancer, accounting for 11.4% of the total 18 million cancer cases, were reported and 1.8 million new cancer deaths were related to lung cancer, accounting for 18% of all cancer deaths (1). The treatment of squamous non-small cell lung cancer, which constitutes 25%–30% of NSCLC, is challenging because of its specific clinicopathologic characteristics and rare incidence of targetable mutations (2). NSCLC has a poor prognosis, especially in stage IIIB/IV patients, with a 5-year overall survival (OS) rate of less than 5% (3).
The median survival time of patients with squamous cell carcinoma (SCC) was approximately 30% shorter than that of patients with other NSCLC subtypes (2). Here, we report a case of stage Ⅳ SCC patient who had lost the opportunity for surgery at the initial diagnosis. He was treated with first- and third-generation epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (TKIs), and he is still alive over 5 years after treatment.
On 29 December 2016, a 66-year-old Chinese male patient with no history of smoking and drinking presented to the Department of Oncology, Shandong Cancer Hospital and Institute (Jinan, China) with a cough and chest tightness for 1 month. Space occupying lesions in the lower lobe of the right lung were found during CT examination, and then he was admitted to our hospital for further treatment. At this time, the ECOG score was 1, and the patient had no previous history of related drugs and surgery. CT showed an irregular soft tissue mass in the lower lobe of the right lung with a cross section of about 4.8 cm × 4.6 cm, multiple lung masses, right hilar and mediastinal lymph node, and abdominal lymph node involvement, with metastases of right pleural, rib, and the brain. The clinical stage was T4N3M1, IV, and the pathological biopsy showed non-small cell lung cancer combined with immunohistochemical tendency of SCC. A deletion mutation of exon 19 of EGFR was found in genetic testing. The patient received 14 cycles of targeted therapy with gefitinib (250 mg/d, qd) from January 2017 to March 2018. The best response was stable disease (SD) (Figure 1). In March 2018, the CT reexamination showed the progress of the disease, and EGFR T790M mutation was found after genetic testing of specimen acquired by pathological puncture (Figure 2). Then the patient was admitted to the hospital for radiotherapy of the lower lobe of the right lung and metastatic lymph nodes, DT = 6000 cGy (200 cGy dose per time). In the same month, patient received targeted therapy with Osimertinib (80 mg/d, qd) until now. The effect was evaluated as SD. At this time, the patient’s clinical stage was T4N3M1, IV. After taking Osimertinib for more than 4 years, the primary lesion in the right lung was well controlled (Figure 3) and the brain metastases almost disappeared (Figure 4).
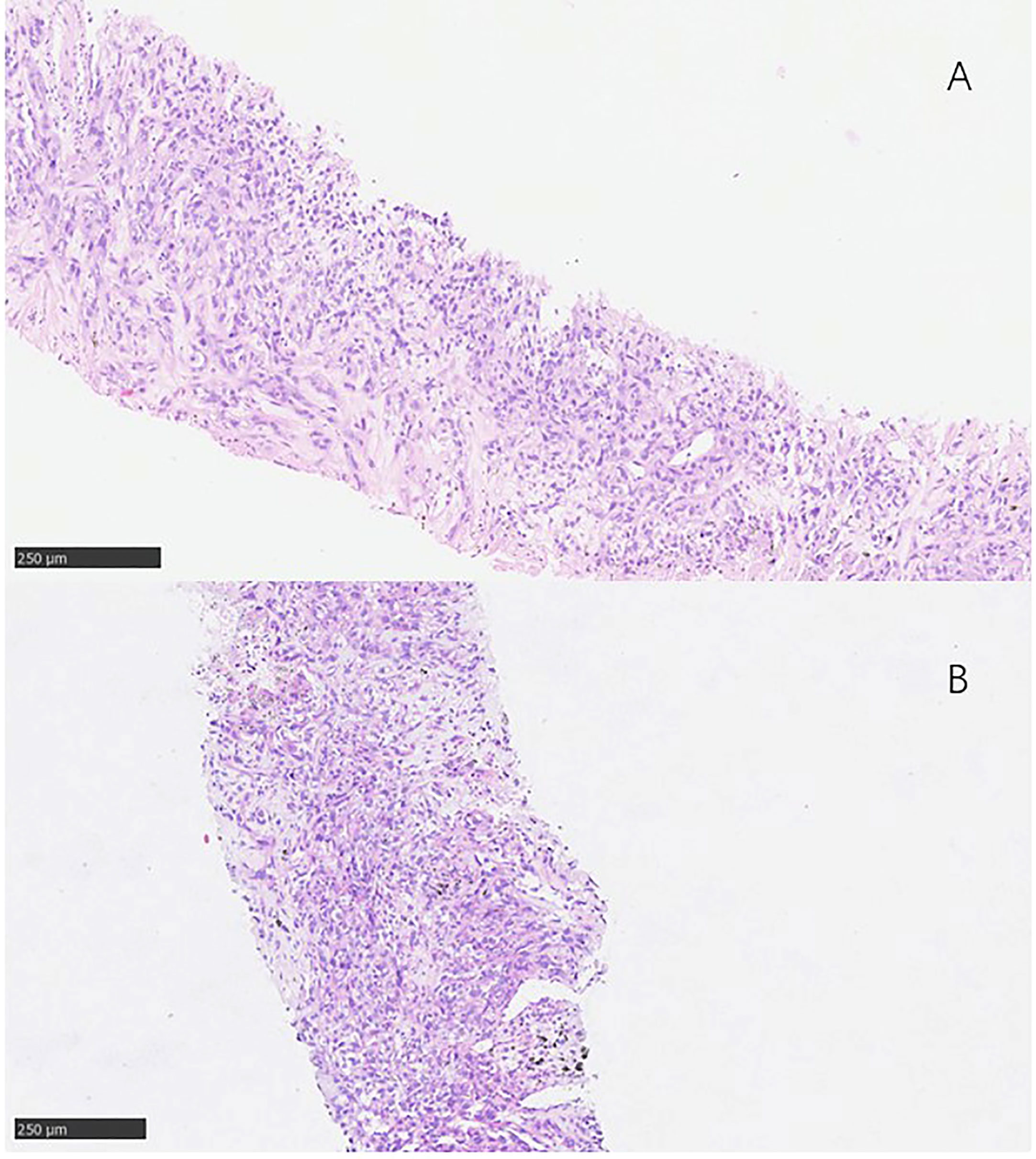
Figure 2 Pathological findings. (A) 3 January 2017, the pathological result was squamous cell carcinoma of non-small cell lung cance. (B) 17 January 2018, the pathological result was still lung squamous cell carcinoma.
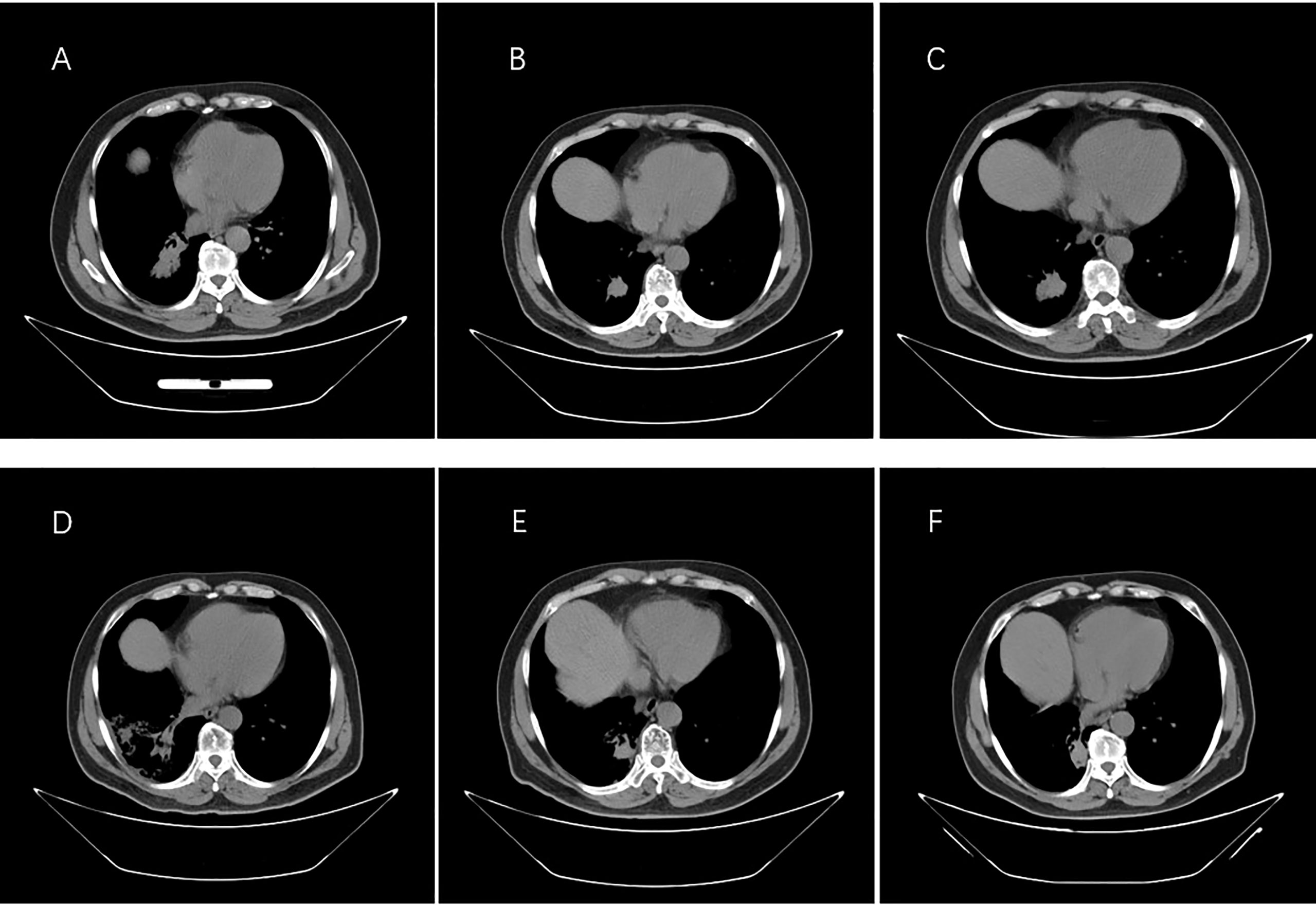
Figure 3 Images from computed tomography showing the tumor in the right lung in response to therapy. (A) Before therapy, imaging was performed on 30 December 2016. (B) Six months after taking gefitinib on 18 August 2017. (C) Resistance progresses on 20 January 2018. (D) After the radiotherapy on 5 June 2018. (E) One year after taking Osimertinib on 18 February 2019. (F) Last check on 20 May 2022.
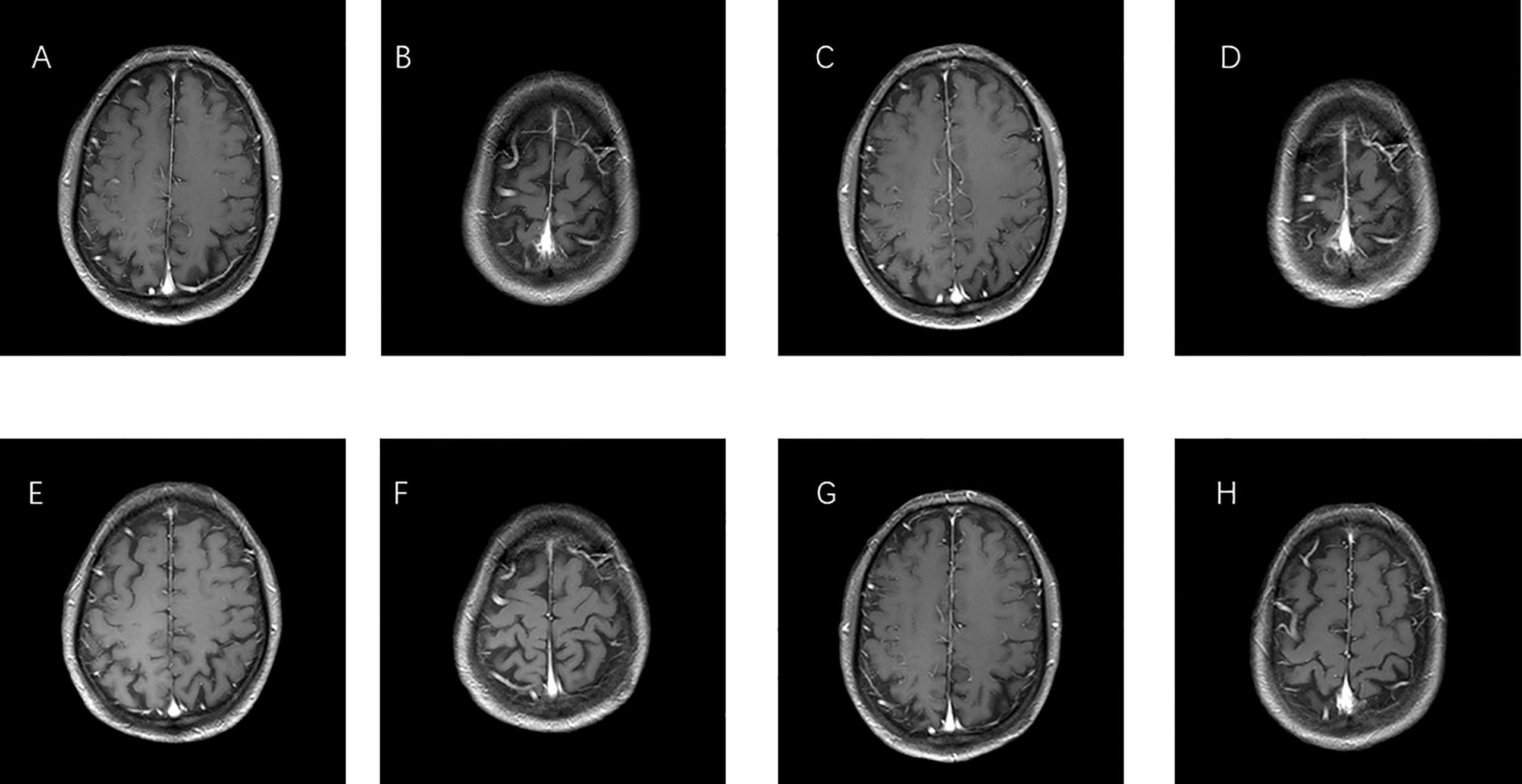
Figure 4 MRI images of brain metastases. (A, B) Before therapy, imaging performed on 30 December 2016. (C, D) One year after taking gefitinib on 19 March 2018. (E, F) One year after taking Osimertinib on 18 February 2019. (G, H) Last check on 20 May 2022.
Here, we described a patient with stage IV advanced SCC. At initial diagnosis, the mutation of EGFR exon 19del was found. After taking gefitinib for more than 1 year, the patient developed drug resistance. The result of second genetic test showed EGFR-T790M mutation, which is an acquired drug resistance mutation. When treatment was switched to Osimertinib with the combination of primary and metastatic lymph node radiotherapy resulted in a long progression-free survival (PFS).
The first-generation EGFR-TKIs Gefitinib (ZD1839) was approved as first-line therapy for the treatment of patients with NSCLC harboring EGFR sensitive mutation, specifically. EGFR exon 19 deletions (ex19del) and/or EGFR L858R mutation in exon 21. These mutations occur in 10%–40% of patients with NSCLC. However, the patient’s condition deteriorated after 10–14 months of treatment with gefitinib. Studies have indicated that approximately 50% of the progression of NSCLC was due to the additional EGFR T790M resistance mutation (4–7). The patient was consistent with clinical cohort data during gefitinib use. During this process, the patient developed drug resistance.
In patients with non-adenocarcinoma (ADC) non-small cell lung cancer carrying EFGR mutations, clinical studies have shown that the median OS of patients treated with EGFR-TKIs is significantly higher than patients not treated with EGFR-TKIs, and there is no significant difference in clinical characteristics between patients who respond to EGFR-TKIs and those who do not (8). Based on the literature review, the efficacy of EGFR-TKIs in lung SCC with EGFR mutant is lower than that in adenocarcinoma. According to a clinical study, 33 (13.3%) of 249 patients with SCC included in the study had EGFR mutations. Twenty of these patients received EGFR-TKI (erlotinib or gefitinib) with a response rate of 25% (95% confidence interval, 8.7%–49.1%). PFS was 1.4 months, and OS was 14.6 months. Approximately one-third of patients with EGFR-mutated lung SCC have PFS of more than 6 months (9). EGFR-T790M is a common drug resistance mutation, resulting in about 60% of NSCLC patients with EFGR mutation who are resistant to EGFR-TKIs (10). There are two types of T790M mutations: primary mutation and acquired mutation. The acquired T790M mutation is usually the resistance gene generated after the first- or second-generation EGFR-TKI treatment. Both the primary and the acquired mutations showed good response to the third generation EFGR-TKI Osimertinib (11, 12). In NSCLC patients with acquired (Chiang, Huang et al., 2020) resistance to first- or second-generation EGFR-TKIs, Osimertinib is an alternative choice of treatment. Osimertinib was superior to platinum doublet chemotherapy with a higher rate (71% vs. 31%), a longer PFS (10.1 vs. 4.4 months) and median OS (26.8 vs. 22.5 months) (13, 14). Compared with traditional chemotherapy, EGFR-TKI–targeted therapy enable patients of NSCLC with EGFR mutations to achieve longer progression free survival and OS (15). In the patient who acquired the EGFR-T790M resistance mutation after taking gefitinib, we selected Osimertinib for the treatment that was successful.
Among the metastatic sites of advanced lung cancer, the central nervous system (CNS) is the most common site, and 20%–65% of patients will develop brain metastases during the course of the disease (16). In advanced lung cancer, 20%–65% of patients will develop brain metastases. Up to 50% of Asian patients with NSCLC carry EGFR-gene mutations. The cumulative incidence of brain metastases was significantly high in patients with EGFR mutations, with 46%, 64%, and 71% at 1, 3, and 5 years, respectively (17). Preclinical studies of Osimertinib demonstrated a more homogeneous distribution in the brain than other TKIs (18, 19). Osimertinib can delay the development of symptomatic CNS metastases. After taking Osimertinib for more than 3 years, the brain metastases even disappeared, which indicates that Osimertinib has a good therapeutic effect on metastasis.
At present, there are few studies on the efficacy of EGFR-TKIs for lung SCC. SCC only accounts for less than 1% in FLURA and AURA trials to explore the efficacy of Osimertinib in lung cancer. No clinical studies of patients with lung SCC carrying EGFR mutations have been performed yet.
In the case of newly diagnosed advanced lung cancer with brain metastases and bone metastases, the patient received gefitinib or Osimertinib combined with radiotherapy extended a survival time to more than 60 months. The disease was stabilized, the primary lesion was well controlled, the brain metastases disappeared, and no tertiary or quaternary adverse reactions occurred after Osimertinib treatment. The results suggest that Osimertinib might be the choice of treatment for patients of lung SCC with EGFR mutations.
We present here a case with Osimertinib and radiotherapy treated advanced SCC. Throughout the course of treatment, patients showed significant responses to both Osimertinib and radiotherapy, with prolonged PFS and OS. The results suggest that Osimertinib might be a good choice for the treatment of patients with lung SCC accompanied by EGFR mutations.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Shandong Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZZ is responsible for thesis writing and picture editing. JL was responsible for case data collection. LY is responsible for the production and scanning of pathological sections. YL is responsible for reviewing articles and revising formats. All authors contributed to the article and approved the submitted version.
This manuscript was supported in part by the National Natural Science Foundation of China (Grants No. 81904186) and Shandong Traditional Chinese Medicine Science and Technology Project (Q-2022113). No writing assistance was utilized in the production of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Jr Bunn PA, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol (2018) 13:165–83. doi: 10.1016/j.jtho.2017.11.111
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553:446–54. doi: 10.1038/nature25183
4. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer (2007) 7:169–81. doi: 10.1038/nrc2088
5. Park K, Tan E-H, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol (2016) 17:577–89. doi: 10.1016/S1470-2045(16)30033-X
6. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res (2013) 19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246
7. Tanaka K, Nosaki K, Otsubo K, Azuma K, Sakata S, Ouchi H, et al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naïve patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget (2017) 8:68123–30. doi: 10.18632/oncotarget.19243
8. Kobayashi K, Soejima K, Fukunaga K, Shintani Y, Sekine I, Shukuya T, et al. Key prognostic factors for EGFR-mutated non-adenocarcinoma lung cancer patients in the Japanese Joint Committee of Lung Cancer Registry Database. Lung Cancer (2020) 146:236–43. doi: 10.1016/j.lungcan.2020.06.015
9. Hata A, Katakami N, Yoshioka H, Kunimasa K, Fujita S, Kaji R, et al. How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutations? J Thorac Oncol (2013) 8:89–95. doi: 10.1097/JTO.0b013e31827690b5
10. Li X, Lian Z, Wang S, Xing L, Yu J. Interactions between EGFR and PD-1/PD-L1 pathway: Implications for treatment of NSCLC. Cancer Lett (2018) 418:1–9. doi: 10.1016/j.canlet.2018.01.005
11. Wang S, Yan B, Zhang Y, Xu J, Qiao R, Dong Y, et al. Different characteristics and survival in non-small cell lung cancer patients with primary and acquired EGFR T790M mutation. Int J Cancer (2019) 144:2880–6. doi: 10.1002/ijc.32015
12. Chiang CL, Huang HC, Shen CI, Luo YH, Chen YM, Chiu CH. Post-progression survival in secondary EGFR T790M-mutated non-small-cell lung cancer patients with and without Osimertinib after failure of a previous EGFR TKI. Target Oncol (2020) 15:503–12. doi: 10.1007/s11523-020-00737-7
13. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
14. Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol (2020) 31:1536–44. doi: 10.1016/j.annonc.2020.08.2100
15. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
16. Page S, Milner-Watts C, Perna M, Janzic U, Vidal N, Kaudeer N, et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer (2020) 132:187–98. doi: 10.1016/j.ejca.2020.03.006
17. Han G, Bi J, Tan W, Wei X, Wang X, Ying X, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget (2016) 7:56998–7010. doi: 10.18632/oncotarget.10933
18. Soria JC, Ohe Y, Vansteenkiste J, John T, Grohe C, Majem M, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
Keywords: non-small cell lung carcinoma, squamous cell carcinoma, EGFR mutation, targeted therapy, cancer
Citation: Zhang Z, Lin J, Yang L and Li Y (2023) Osimertinib inhibits brain metastases and improves long-term survival in a patient with advanced squamous cell lung cancer: a case report and literatures review. Front. Oncol. 13:1188772. doi: 10.3389/fonc.2023.1188772
Received: 17 March 2023; Accepted: 07 August 2023;
Published: 14 September 2023.
Edited by:
Aakash Desai, Mayo Clinic, United StatesReviewed by:
Francesco Pepe, University of Naples Federico II, ItalyCopyright © 2023 Zhang, Lin, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Li, bHk2NjQ1NzgwOUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.