
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 June 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1186311
Purpose: Accurate risk stratification can improve lymphoma management, but current volumetric 18F-fluorodeoxyglucose (FDG) indicators require time-consuming segmentation of all lesions in the body. Herein, we investigated the prognostic values of readily obtainable metabolic bulk volume (MBV) and bulky lesion glycolysis (BLG) that measure the single largest lesion.
Methods: The study subjects were a homogeneous cohort of 242 newly diagnosed stage II or III diffuse large B-cell lymphoma (DLBCL) patients who underwent first-line R-CHOP treatment. Baseline PET/CT was retrospectively analyzed for maximum transverse diameter (MTD), total metabolic tumor volume (TMTV), total lesion glycolysis (TLG), MBV, and BLG. Volumes were drawn using 30% SUVmax as threshold. Kaplan–Meier survival analysis and the Cox proportional hazards model assessed the ability to predict overall survival (OS) and progression-free survival (PFS).
Results: During a median follow-up period of 5.4 years (maximum of 12.7 years), events occurred in 85 patients, including progression, relapse, and death (65 deaths occurred at a median of 17.6 months). Receiver operating characteristic (ROC) analysis identified an optimal TMTV of 112 cm3, MBV of 88 cm3, TLG of 950, and BLG of 750 for discerning events. Patients with high MBV were more likely to have stage III disease; worse ECOG performance; higher IPI risk score; increased LDH; and high SUVmax, MTD, TMTV, TLG, and BLG. Kaplan–Meier survival analysis showed that high TMTV (p = 0.005 and < 0.001), MBV (both p < 0.001), TLG (p < 0.001 and 0.008), and BLG (p = 0.018 and 0.049) were associated with significantly worse OS and PFS. On Cox multivariate analysis, older age (> 60 years; HR, 2.74; 95% CI, 1.58–4.75; p < 0.001) and high MBV (HR, 2.74; 95% CI, 1.05–6.54; p = 0.023) were independent predictors of worse OS. Older age (hazard ratio [HR], 2.90; 95% CI, 1.74–4.82; p < 0.001) and high MBV (HR, 2.36; 95% CI, 1.15-6.54; p = 0.032) were also independent predictors of worse PFS. Furthermore, among subjects ≤60 years, high MBV remained the only significant independent predictor of worse OS (HR, 4.269; 95% CI, 1.03–17.76; p = 0.046) and PFS (HR, 6.047; 95% CI, 1.73–21.11; p = 0.005). Among subjects with stage III disease, only greater age (HR, 2.540; 95% CI, 1.22–5.30; p = 0.013) and high MBV (HR, 6.476; 95% CI, 1.20–31.9; p = 0.030) were significantly associated with worse OS, while greater age was the only independent predictor of worse PFS (HR, 6.145; 95% CI, 1.10–4.17; p = 0.024).
Conclusions: MBV easily obtained from the single largest lesion may provide a clinically useful FDG volumetric prognostic indicator in stage II/III DLBCL patients treated with R-CHOP.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive non-Hodgkin lymphoma (1). Although chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) achieves complete response in 70% of cases, prognosis remains poor in subjects who do not respond or develop relapse. There is thus a need to identify prognostic factors that stratify patients at greater risk so that treatment can be tailored for optimized outcomes (2).
Image-derived valuation of tumor burden has refined lymphoma staging and response assessment (3), and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has become a standard procedure (4). Furthermore, volumetric FDG parameters have significant prognostic value in various lymphoma subtypes including DLBCL (5–9). A simple measurement that has long been used to assess prognosis is the presence of bulky disease (10, 11), which denoted lesions exceeding a threshold size based initially on X-ray and, later, on computed tomography (CT). The prognostic significance of mediastinal bulky disease was also found to be retained in lymphomas located outside the mediastinum.
The routine employment of PET/CT in lymphoma patients offers a unique opportunity to refine the meaning of bulky disease from simple unidimensional measurement to a three-dimensional metabolic burden. However, current volumetric PET/CT variables of total metabolic tumor volume (TMTV) and total lesion glycolysis (TLG) require tedious and time-consuming segmentation of all FDG lesions across the entire body (12). If comparable prognostic information could be provided by the single largest (bulky) lesion, it would be much easier to apply in daily clinical practice. This points to the need to elucidate the prognostic value of metabolic variables of the single largest (bulky) lesion. Delaby and coworkers recently addressed this issue in DLBCL patients and observed a significant association between low metabolic bulky volume (MBV) and longer survival (13). Although encouraging, the study has significant drawbacks that need to be resolved before their conclusions can be asserted. Particularly important for survival analysis is the recruitment of a cohort that is homogeneous in the treatment regimen and established prognostic factors (14). The study mentioned above was limited by including subjects receiving several different first-line chemotherapies. Moreover, their small cohort included subjects with lymphoma stages ranging from I to IV, which resulted in only 11–22 cases in stages I–III. For stage I disease, segmentation of all lesions is not an issue, and better risk stratification is less urgent because DLBCL patients have excellent outcomes (15, 16). The inclusion of stage IV disease is problematic because it often includes bone marrow involvement (17), for which delineation (18) and segmentation on FDG PET/CT is difficult (8, 19, 20). Therefore, FDG volumetry is most practical and useful for patients with stage II and III lymphomas (21, 22).
Additionally requiring clarification is the relative prognostic value of MBV compared to the bulky lesion’s metabolic tumor burden (bulky lesion glycolysis, BLG). In this study, we thus measured MBV and BLG on baseline FDG PET/CT in a homogeneous cohort of 242 patients with stage II or III DLBCL who received R-CHOP as first-line chemotherapy. The associations of MBV and BLG with progression-free survival (PFS) and overall survival (OS) were analyzed and compared with major clinical prognostic factors.
Study candidates were 1,056 pathology-confirmed DLBCL patients who underwent FDG PET/CT at our institution between 2008 and 2015. Among these, we selected 303 candidates with Ann Arbor stage II or III disease who underwent pretreatment PET/CT. We excluded 12 patients who did not receive R-CHOP as first-line treatment and 49 who had PET/CT data errors. Consequently, data from 242 stage II or III DLBL patients who underwent baseline FDG PET/CT followed by R-CHOP first-line treatment were included in the final analysis. This retrospective study was approved by our institutional review board, and the requirement for informed consent was waived.
After fasting for at least 6 h to reach a blood glucose level <150 mg, PET/CT was performed 75 min after injecting 5 MBq/kg FDG without intravenous or oral contrast on a Discovery STe PET/CT scanner (GE Healthcare, Chicago, IL). Following continuous spiral CT with a 16-slice helical CT (140 keV; 30–170 mA), an emission scan was obtained from head to thigh for 2.5 min per frame. A three-dimensional mode reconstruction of attenuation-corrected images (3.9×3.9×3.3 mm) was conducted using an ordered-subset expectation maximization algorithm (20 subsets and two iterations).
Tomographic attenuation-corrected PET, CT, and fusion PET/CT images were reviewed in axial, coronal, and sagittal planes. MTD was defined as the largest lesion’s maximum transverse diameter on CT, and the SUVmax of the highest FDG uptake lesion was recorded.
Volumetric PET parameters were semiautomatically measured from transaxial PET tomographs on a GE Advantage Workstation 4.4 using an SUV-based automated contouring software (PET VCAR; GE Healthcare). The appropriateness of the segmented lesion volumes was determined by consensus between two experienced nuclear medicine physicians who were blinded to patient outcomes. Briefly, a cubical bounding volume of interest was drawn around each target lesion with care not to include areas of high physiological uptake (brain, heart, liver, kidney, or bladder). A threshold of the local tumor SUVmax was then applied for lesion segmentation, and any region of physiological uptake was manually excluded. When 30% and 40% thresholds of the local SUVmax were pilot tested, the 40% threshold method significantly underestimated the visual volume of large lesions with high FDG uptake, as previously reported (23, 24). In comparison, the 30% threshold method better delineated the tumor margins of bulky hypermetabolic DLBCL lesions and was therefore selected for analysis.
The MTV of a lesion was defined as the sum of all voxels with FDG uptake exceeding the threshold of the local SUVmax. The TMTV was the sum of the MTV of all VOIs; the MBV was defined as the largest MTV; the TLG was the sum of the product of MTV and SUVmean of all lesions; and the BLG was defined as the product of the MBV and its SUVmean.
Clinical information was obtained from our institutional information system. Medical records were reviewed for clinical characteristics including age, gender, and Eastern Cooperative Oncology Group (ECOG) performance status. Laboratory data within 1 week of the PET/CT included serum lactate dehydrogenase (LDH), hemoglobin, and cell counts. IPI risk scores were calculated from these data.
Patients underwent follow-up PET-CT at the interim and at the end of treatment. Disease progression was diagnosed based on PET/CT findings and clinical evidence of progression. Disease relapse after treatment was defined as clinical or imaging evidence of recurrence during follow-up.
Continuous variables are described as means and standard deviations (SD) and were compared with Student’s t-tests. Categorical and discrete data were compared by Pearson’s chi-square tests. p-values < 0.05 were considered significant.
The primary endpoint for survival analyses was OS or PFS. OS was the time from the day of baseline PET/CT (1–2 days before the start of chemotherapy) to the day of any cause death. PFS was the time from the day of baseline PET/CT to the day of primary progression, recurrence, or any cause death. Patients alive at the date of last follow-up were counted as censored observations. Receiver operating characteristic (ROC) curve analysis identified the optimal cutoff values of MTD, MBV, TMTV, TLG, and BLG for event prediction.
Survival curves were obtained from Kaplan–Meier estimates and compared using the log-rank test. Cox proportional hazards regression analysis was performed to identify univariate and multivariate factors predictive of PFS and OS. A p-value < 0.05 was accepted as the threshold for inclusion. Data were analyzed using Statistical Package for the Social Sciences version 23.0 (IBM Corp., Armonk, NY).
The demographic and clinical characteristics of the 242 DLBCL patients are summarized in Table 1. All subjects were treated by R-CHOP as first-line chemotherapy. The mean age was 56.2 ± 13.7 years, and 40.5% were over 60 years of age. The male-to-female ratio was 1.37:1. Among the subjects, 156 had Ann Arbor stage II (64.5%) and 86 had stage III disease (35.5%). ECOG performance was 1 or higher in 109 subjects (45.1%), and IPI risk score was 2 or higher in 126 subjects (52.1%). Blood LDH was >490 IU/L in 134 patients (55.4%) and averaged 623.9 ± 21.0 IU/L.
The largest (bulky) lesion had an MTD of 73.6 ± 42.6 mm. The lesion with the highest FDG uptake had a mean SUVmax of 21.0 ± 9.0. The mean TMTV was 215.3 ± 381.9 cm3; the mean MBV was 172.0 ± 350.1 cm3; the mean TLG was 1,890.9 ± 2,655.9; and the mean BLG was 1,640.9 ± 2,533.8.
ROC curve analyses revealed that the optimal cutoff for predicting events was 74 mm for MTD, 112 cm3 for TMTV, 88 cm3 for MBV, 950 for TLG, and 750 for BLG. When categorized into low (≤ 88 cm3) and high MBV (> 88 cm3) groups, the latter was more likely to have stage III disease (p <0.001), high ECOG (p <0.05), high IPI risk score (p <0.001), and high blood LDH (p <0.001) compared to the former (Table 1). Other clinical variables did not show significant differences between MBV groups.
Study subjects were followed up for a median of 5.41 years (range, 0.02–12.67 years). Progression, recurrence, or any cause death occurred in a total of 85 patients during follow-up. Death occurred in 65 patients at a median of 6.2 months.
Kaplan–Meier survival analyses with log-rank tests for clinical variables showed significantly worse OS for patients who were older (>60 years; p <0.001) and had increased ECOG score (≥1; p = 0.019), high IPI risk score (≥2; p <0.001), or stage III disease (p <0.001; Figure 1). PFS was significantly worse for patients who were older (p <0.001) and had increased ECOG score (p = 0.001), high IPI score (p <0.001), and stage III disease (p = 0.001).
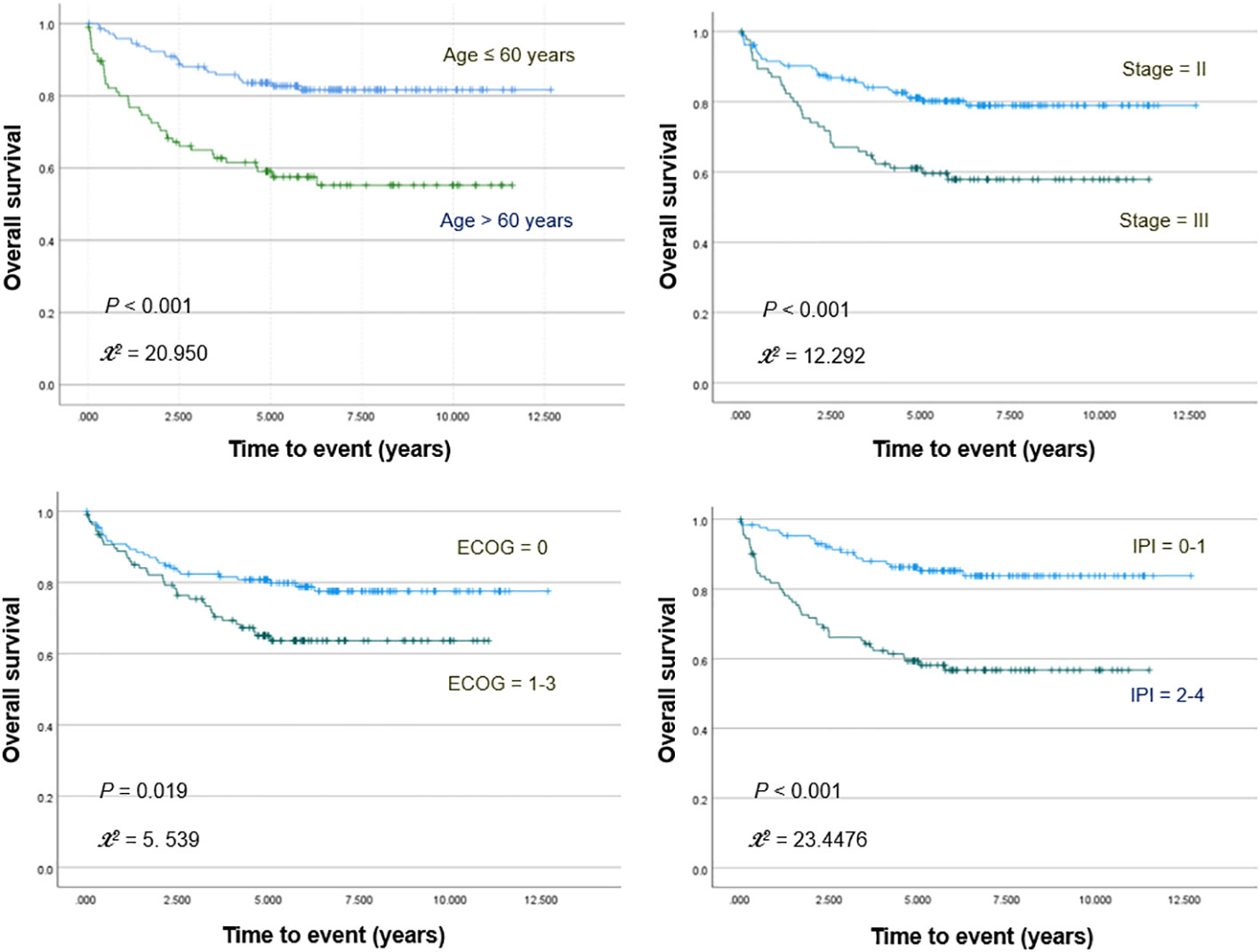
Figure 1 Kaplan–Meier curves for overall survival in patients stratified for age, Ann Arbor stage, ECOG stage, and IPI score.
PET/CT images of representative low and high MBV patients are illustrated in Figure 2. Among FDG parameters, greater TMTV (>112 cm3; p = 0.005), MBV (>88 cm3; p <0.001), TLG (>950; p <0.001), and BLG (>750; p = 0.018) were associated with significantly worse OS (Figures 3, 4). PFS was significantly worse for patients who had greater TMTV (p <0.001), MBV (p <0.001), TLG (p = 0.008), and BLG levels (p = 0.049; Figures 3, 4).
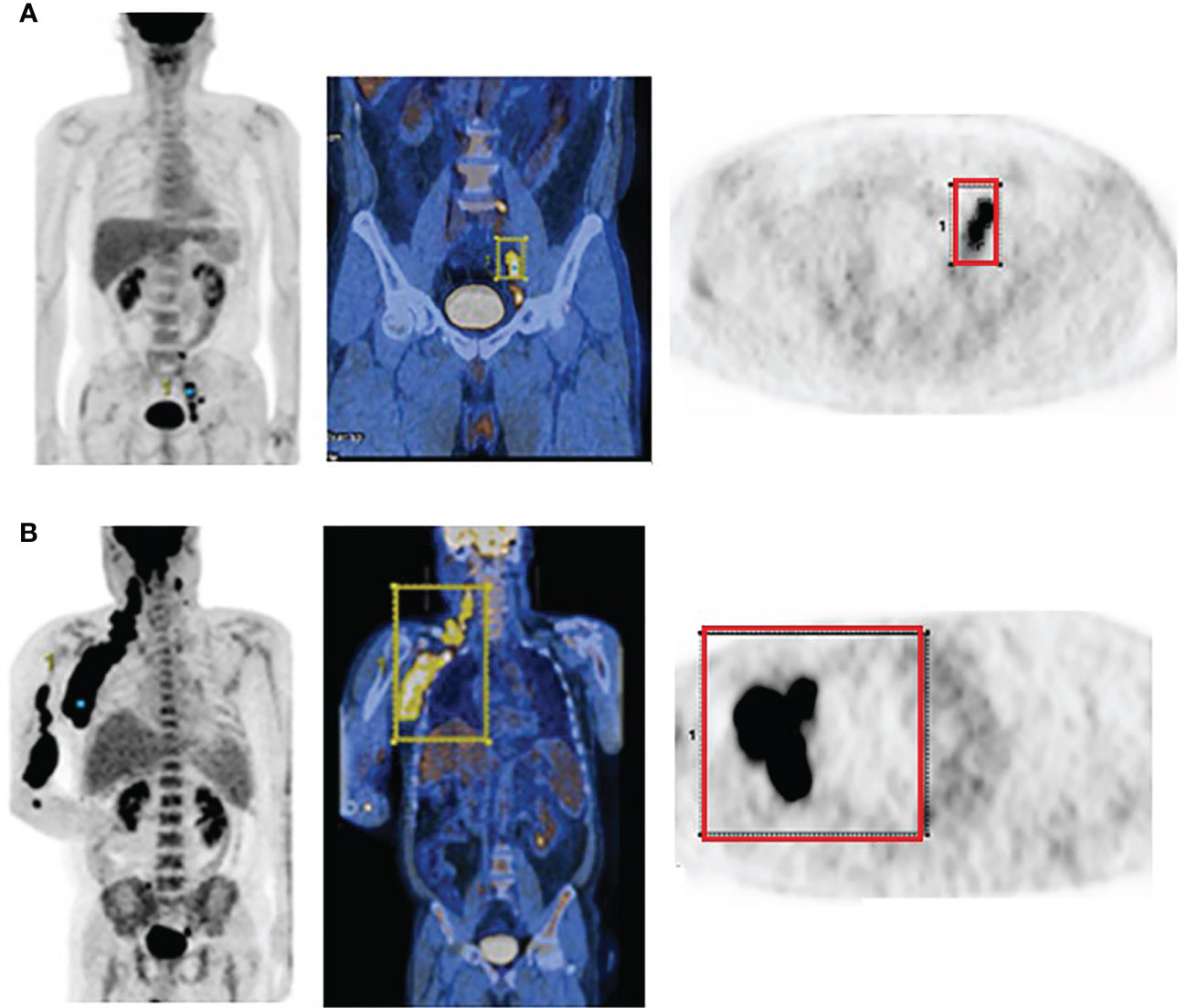
Figure 2 FDG PET and PET/CT maximum intensity projection images (left) and transaxial PET images (right) of representative DLBCL patients with a small (A) and large MBV (B). VOIs encompassing the lesions are also shown.
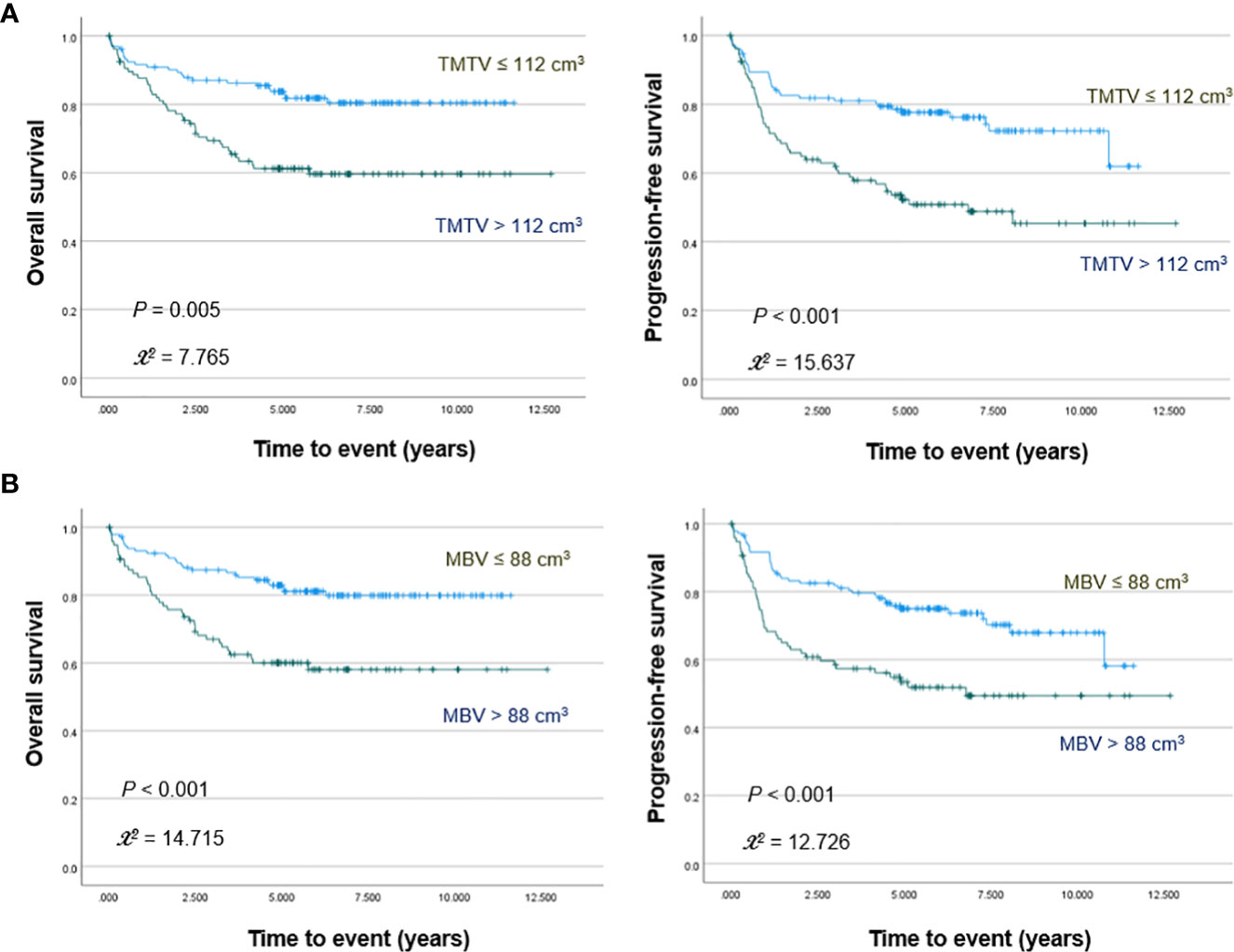
Figure 3 Kaplan–Meier curves for overall survival (left) and progression free survival (right) in patients stratified for TMTV (A) and MBV levels (B).
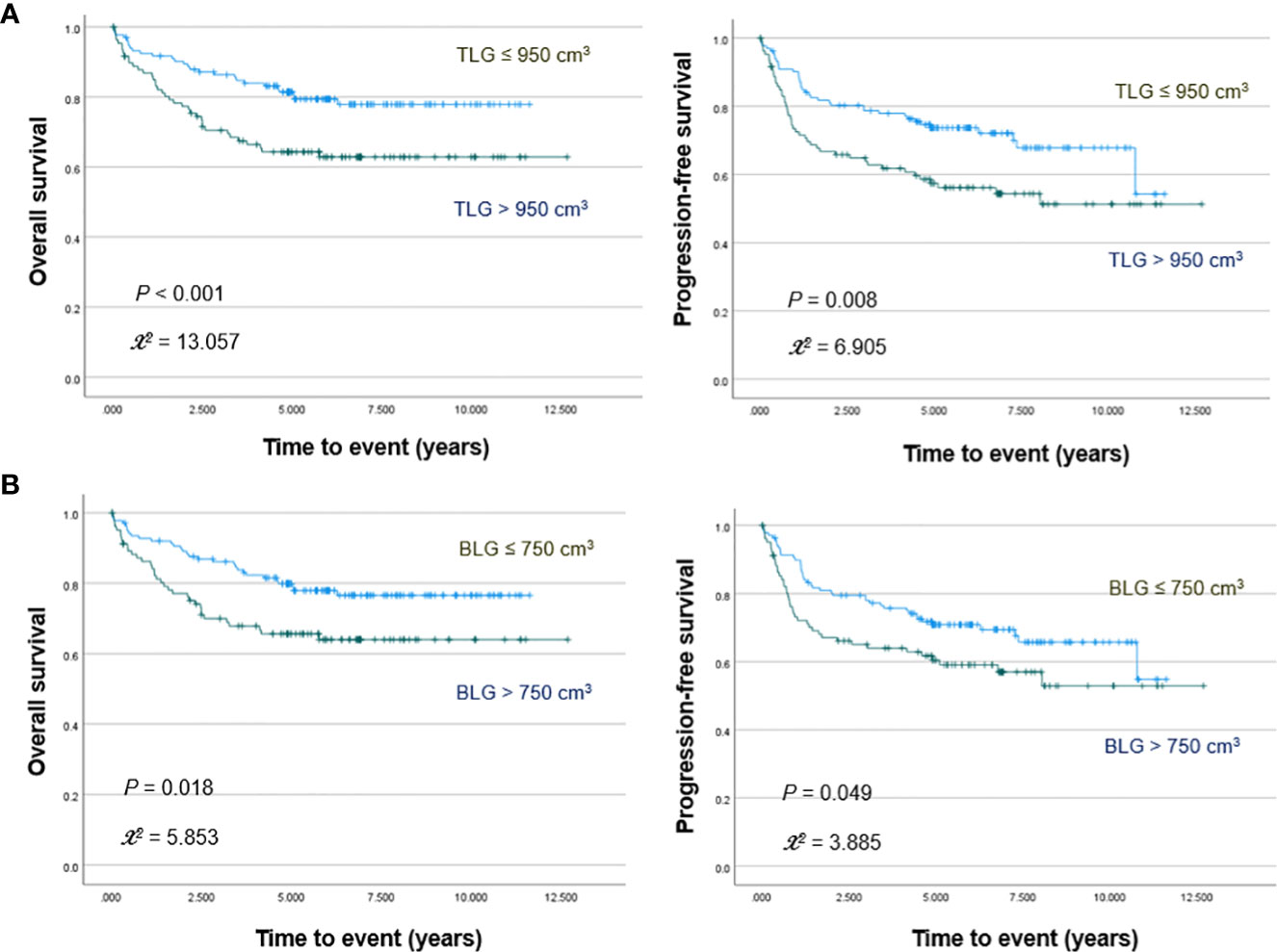
Figure 4 Kaplan–Meier curves for overall survival (left) and progression free survival (right) in patients stratified for TLG (A) and BLG (B) levels.
The results of the Cox univariable analysis for OS are summarized in Table 2. Among volumetric FDG parameters, TMTV (hazard ratio [HR], 2.464; 95% CI, 1.49–4.08; p = 0.000), MBV (HR, 2.523; 95% CI, 1.54–4.14; p = 0.000), TLG (HR, 1.995; 95% CI, 1.22–3.27; p = 0.006), and BLG (HR, 1.796; 95% CI, 1.10–2.93; p = 0.019) were significant predictors. Significant clinical predictors included age, stage, hemoglobulin, ECOG status, and IPI score.
The results of the Cox univariable analysis for PFS are summarized in Table 3. Volumetric parameters of TMTV (HR, 2.367; 95% CI, 1.53–3.67; p <0.001), MBV (HR, 2.127; 95% CI, 1.39–3.26; p = 0.001), and TLG (HR, 1.778; 95% CI, 1.16–2.73; p = 0.008) were significant predictors. BLG showed borderline significance (HR, 1.524; 95% CI, 1.00–2.33; p = 0.052). Age, stage, ECOG status, and IPI score were again significant clinical predictors.
Forward stepwise multivariate Cox proportional hazards analysis was performed with significant univariate predictors including either TMTV and TLG or replacing these parameters with MBV and BLG. As a result, high MBV (HR, 2.742; 95% CI, 1.15-6.54; p = 0.023) and older age (HR, 2.739; 95% CI, 1.58–4.75; p < 0.001) were significant independent predictors of worse OS, whereas TMTV, TLG, and BLG were not (Table 2). Moreover, only high MBV (HR, 2.357; 95% CI, 1.08–5.16; p = 0.032) and older age (HR, 2.898; 95% CI, 1.74–4.82; p < 0.001) were significant independent predictors of worse PFS (Table 3).
Given the major prognostic influence of age, we further performed multivariable analysis in subjects under 60 years of age. The results of this subgroup revealed that high MBV remained the only significant independent predictor of worse OS (HR, 4.269; 95% CI, 1.03–17.76; p = 0.046) and PFS (HR, 6.047; 95% CI, 1.73–21.11; p = 0.005).
In addition, since the significantly greater number of patients with stage II disease (that has better prognosis) might have influenced our survival analysis results, we further performed multivariable analysis in subjects with the more advanced stage III disease. The results in this subgroup revealed that only greater age (HR, 2.540; 95% CI, 1.22–5.30; p = 0.013) and high MBV (HR, 6.476; 95% CI, 1.20–31.9; p = 0.030) were significantly associated with worse OS, while greater age was the only independent predictor of worse PFS (HR, 6.145; 95% CI, 1.10–4.17; p = 0.024).
In this study, we investigated whether MBV and BLG of the single largest DLBCL lesion provide prognostic information comparable to that by TMTV and TLG measured from all lesions spread across the body. Significantly, this was tested in a homogeneous cohort of patients with stage II/III disease who received first-line R-CHOP therapy.
As a result, Kaplan–Meier and Cox univariable analyses demonstrated strong associations of high MBV and BLG with worse OS and PFS, similar to that of high TMTV and TLG. MTD, the traditional index of bulky disease, showed a weaker prognostic value, which was expected given its unidimensional attribute. In addition, treatment with rituximab has been suggested to reduce the prognostic value of MTD in DLBCL (25). Importantly, multivariable analyses revealed that MBV was the only FDG PET parameter remaining a significant independent predictor of worse OS and PFS.
For FDG volumetry, the lesion boundary was outlined by the % SUVmax threshold method, which provides indices highly concordant with actual tumor volume (26). Many previous investigations have verified that MTV and TLG obtained using different %SUVmax thresholds are highly reproducible with excellent inter-observer agreements (24, 27–29).
Although the EANM guidelines recommended a SUVmax threshold of 41% for measuring MTV (30), there is no real consensus on the best threshold for segmentation of DLBCL lesions. Indeed, the 41% SUVmax threshold tends to underestimate MTV of tumors with high heterogeneous FDG uptake {23,24}. DLBCLs have characteristically high glycolytic metabolism, and the largest DLBCL lesion in our study had high SUVmax that exceeded 20.0 in more than half of the cases. Consequently, we found that a 40% SUVmax threshold led to a significant underestimation of the metabolic volume for bulky DLBCL lesions. A 30% SUVmax threshold more closely fit the margins of large glycolytic tumors and was therefore selected for MBV determination. Other studies have also used a 30% SUVmax threshold for metabolic tumor segmentation (31–34). Furthermore, tumor MTV and TLG measurements using a 30% SUVmax threshold were also recently shown to have excellent intra- and inter-operator agreements and high correlation coefficients (31). Hence, while investigating the interobserver variability of MBV measurements was beyond the scope of the present study, previous reports firmly support good reproducibility for measuring this major TMTV component.
In our study, ROC analysis showed an optimal TMTV cutoff of 112 ml for predicting adverse events, which is slightly smaller than the 147–550 ml found optimal in previous studies. This could be due to dissimilar subject characteristics such as lower total tumor burden and divergent treatment or to differences in scanner type or acquisition protocol as previously described (35). In contrast, the optimal MBV cutoff of 88 ml in our study was greater than the 41.5 ml reported by Delaby et al. (13). This might be attributable to exclusion of stage I disease that have smaller lesions and the use of a 30% SUVmax threshold for lesion segmentation.
Among our subjects, those with high MBV of the largest lesion were more likely to have stage III disease, elevated ECOG stage, greater IPI risk score, and higher LDH. Kaplan–Meier and Cox analysis confirmed that high MBV was among significant univariate predictors of worse PFS and among those of worse OS. High TMTV, which has established prognostic value in DLBCL [22.23], was also included among significant univariate predictors of worse PFS and OS.
On Cox multivariate analysis, MBV was the only FDG parameter that remained a significant independent predictor of worse PFS and OS. This might indicate that lymphomas with tumor burden concentrated to a large single mass respond poorer to treatment compared to lymphomas with a similar total tumor burden spread across multiple smaller lesions. Hence, our study in a homogeneous cohort of DLBCL patients treated with R-CHOP demonstrates that MBV is an important predictor of worse PFS and OS, even though bulky disease in DLBCL has been suggested to be less clearly associated with outcomes in the rituximab era (25, 36). In our results, it was noted that the HRs for TMTV and MBV showed rather wide confidence intervals. Previous multivariable analysis studies that established the prognostic value of lymphoma TMTV have also shown HRs with comparable or even wider 95% confidence intervals (37), suggesting that volumetric FDG PET parameters may have such a tendency.
Interestingly, the IPI score that incorporates several clinical prognostic factors did not show a significant independent prognostic association on multivariate analysis. On the other hand, older age was a strong independent predictor of worse OS and PFS. Age is one of the most powerful indicators of a poor prognosis, and shorter survival is associated with advancing age in numerous clinical trials (38),. In many of these studies, the prognostic association was shown using 60 years as the threshold (39). Furthermore, the International Prognostic Index (IPI), which remains a robust prognostic tool for DLBCL, identifies age >60 as a predictor of survival. In this era of rituximab, the revised IPI also uses age >60 as a major prognostic factor (2). For this reason, we selected a cutoff of 60 years as a clinical prognostic variable and additionally investigated whether MBV had the capacity to stratify risk among younger or older subjects. The results demonstrated that high MBV was the single independent predictor for worse PFS and OS in patients under 60 years of age.
In addition, there were predominantly more patients with stage II compared to stage III disease in our subjects. Given the more favorable prognosis of earlier disease stage, this asymmetry in patient number might potentially bias survival analysis results. Although Cox multivariable analysis partly adjusts for asymmetries in the number of patients with different characteristics, we performed an additional analysis in patients with the more advanced stage III disease. The results in this sub-group showed that only greater age and high MBV were significant independent predictors of worse OS, further supporting the prognostic value of MBV.
Progress in FDG volumetry has led to the exploration of TLG as an additional parameter that combines MTV with SUVmean (35). TLG predicted the outcome of DLBCL patients better than IPI score (6), and some studies showed a prognostic value of TLG that was similar to TMTV (35, 40), whereas others demonstrated lower performance (41). In our study, we defined BLG as the product of the largest lesion MTV and its SUVmean, which we believe is the first of its description. In our subjects, both high TLG and high BLG were univariate predictors of worse OS and PFS. Interestingly, the prognostic significance of BLG was inferior to that of TLG, contrasting with the superiority of MBV over TMTV. However, neither TLG nor BLG were independent predictors on multivariable analysis.
Given the difficulty of TMTV measurements in patients with numerous lesions, our results support the use of MBV as a conveniently obtainable volumetric FDG parameter with significant prognostic value in DLBCL. However, because of the limitations associated with retrospective studies, the encouraging results of our study need to be confirmed by future prospective investigations.
Limitations of this study include the sample size, although it is larger than that of the previous report on MBV prognosis. Second, while cutoff values of volumetric PET parameters were selected by ROC analysis, other cutoff points may have led to different prognostic associations. Finally, the follow−up time was relatively short in some cases. The results of this study will thus require external verification by future investigations.
MBV is a convenient FDG volumetric parameter that provides useful prognostic information in patients with stage II/III DLBCL treated with R-CHOP, in a manner independent of other clinical prognostic factors. This supports an opportunity to refine bulky disease to indicate the greatest metabolic tumor volume that is attained without tedious segmentation of all lesions in the entire body.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Samsung Medical Center institutional review board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
K-HL and CL conceived of the idea. MJ and K-HL performed the volumetric measurements. K-HL and HJ performed the statistical analysis. S-JK analyzed the clinical data. K-HL and JH worte the manuscript. JC provided critical feedback on the analysis. K-HL supervised the findings of this work. All authors discussed the results and contributed to the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2021M2E8A1044740).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Caimi PF, Hill BT, Hsi ED, Smith MR. Clinical approach to diffuse large b cell lymphoma. Blood Rev (2016) 30:477–91. doi: 10.1016/j.blre.2016.06.003
2. Vaidya R, Witzig TE. Prognostic factors for diffuse large b-cell lymphoma in the R(X)CHOP era. Ann Oncol (2014) 25:2124–33. doi: 10.1093/annonc/mdu109
3. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol (2014) 32:3048–58. doi: 10.1200/JCO.2013.53.5229
4. Barrington SF, Trotman J. The role of PET in the first-line treatment of the most common subtypes of non-Hodgkin lymphoma. Lancet Haematol (2021) 8:e80–93. doi: 10.1016/S2352-3026(20)30365-3
5. Song MK, Yang D-H, Lee G-W, Kun S-N, Shin S, Pak KJ, et al. High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large b cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res (2016) 42:1–6. doi: 10.1016/j.leukres.2016.01.010
6. Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee S-H. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the international prognostic index for patients with diffuse large b cell lymphoma. Cancer (2013) 119:1195:1202–13. doi: 10.1002/cncr.27855
7. Shagera QA, Cheon GJ, Koh Y, Yoo MY, Kang KW, Lee DS, et al. Prognostic value of metabolic tumour volume on baseline 18F-FDG PET/CT in addition to NCCN-IPI in patients with diffuse large b-cell lymphoma: further stratification of the group with a high-risk NCCN-IPI. Eur J Nucl Med Mol Imaging (2019) 46:1417–27. doi: 10.1007/s00259-019-04309-4
8. Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas R-O, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large b-cell lymphoma. Eur J Nucl Med Mol Imaging (2014) 41:2017–22. doi: 10.1007/s00259-014-2822-7
9. Mikhaeel NG, Smith D, Dunn JT, Phillips M, Møller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging (2016) 43:1209–19. doi: 10.1007/s00259-016-3315-7
10. Milgrom SA, Dabaja BS, Mikhaeel NG. Advanced-stage Hodgkin lymphoma: have effective therapy and modern imaging changed the significance of bulky disease? Leuk Lymphoma (2021) 62:1554–62. doi: 10.1080/10428194.2021.1881515
11. Buteau JP, Seymour JF, Hofman MS. The evolving definition of bulky disease for lymphoma. Leuk Lymphoma (2020) 61:1525–8. doi: 10.1080/10428194.2020.1797014
12. Laffon E, Marthan R. Could we avoid computing TMTV of DLBCL patients in routine practice? Eur J Nucl Med Mol Imag (2018) 45:2235–7. doi: 10.1007/s00259-018-4097-x
13. Delaby G, Hubaut M-A, Morschhauser F, Besson A, Huglo D, Herbaux C, et al. Prognostic value of the metabolic bulk volume in patients with diffuse large b-cell lymphoma on baseline 18F-FDG PET-CT. Leuk Lymphoma (2020) 61:1584–91. doi: 10.1080/10428194.2020.1728750
14. Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer (2003) 89:232–8. doi: 10.1038/sj.bjc.6601118
15. Bobillo S, Joffe E, Lavery JA, Sermer D, Ghione P, Noy A, et al. Clinical characteristics and outcomes of extranodal stage I diffuse large b-cell lymphoma in the rituximab era. Blood (2021) 137:39–48. doi: 10.1182/blood.2020005112
16. Chihara D, Oki Y, Fanale MA, Westin JR, Nastoupil LJ, Neelapu S, et al. Stage I non-Hodgkin lymphoma: no plateau in disease-specific survival? Ann Hematol (2019) 98:1169–76. doi: 10.1007/s00277-018-3571-7
17. Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-b-cell lymphoma. N Engl J Med (2002) 346:235–42. doi: 10.1056/NEJMoa011795
18. Yamanaka S, Miyagawa M, Sugawara Y, Hasebe S, Fujii T, Takeuchi K, et al. The prognostic significance of whole-body and spleen MTV (metabolic tumor volume) scanning for patients with diffuse large b cell lymphoma. Int J Clin Oncol (2021) 26:225–32. doi: 10.1007/s10147-020-01807-6
19. Barrington SF, Meignan M. Time to prepare for risk adaptation in lymphoma by standardizing measurement of metabolic tumor burden. J Nucl Med (2019) 60:1096–102. doi: 10.2967/jnumed.119.227249
20. Vercellino L, Cottereau A-S, Casasnovas O, Tilly H, Feugier P, Chartier L, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood (2020) 135:1396–405. doi: 10.1182/blood.2019003526
21. Kim J, Hong J, Kim SG, Hwang KH, Kim M, Ahn HK, et al. Prognostic value of metabolic tumor volume estimated by 18F-FDG positron emission tomography/computed tomography in patients with diffuse large b-cell lymphoma of stage II or III disease. Nucl Med Mol Imaging (2014) 48:187–95. doi: 10.1007/s13139-014-0280-6
22. Song MK, Chung JS, Shin HJ, Lee S-M, Lee S-E, Lee H-S, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large b cell lymphoma without extranodal site involvement. Ann Hematol (2012) 91:697–703. doi: 10.1007/s00277-011-1357-2
23. Ceriani L, Milan L, Johnson PWM, Martelli M, Presilla S, Giovanella L, et al. Baseline PET features to predict prognosis in primary mediastinal b cell lymphoma: a comparative analysis of different methods for measuring baseline metabolic tumour volume. Eur J Nucl Med Mol Imaging (2019) 46:1334–44. doi: 10.1007/s00259-019-04286-8
24. Ilyas H, Mikhaeel NG, Dunn JT, Rahman F, Møller H, Smith D, et al. Defining the optimal method for measuring baseline metabolic tumour volume in diffuse large b cell lymphoma. Eur J Nucl Med Mol Imaging (2018) 45:1142–54. doi: 10.1007/s00259-018-3953-z
25. Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-b-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera international trial group (MInT) study. Lancet Oncol (2008) 9:435–44. doi: 10.1016/S1470-2045(08)70078-0
26. Meignan M, Sasanelli M, Casasnovas RO, Luminari S, Fioroni F, Coriani C, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging (2014) 41:1113–22. doi: 10.1007/s00259-014-2705-y
27. Choi JW, Dean EA, Lu H, Thompson Z, Qi J, Krivenko G, et al. Repeatability of metabolic tumor burden and lesion glycolysis between clinical readers. Front Immunol (2023) 14:994520. doi: 10.3389/fimmu.2023.994520
28. Ferrández M, Eertink JJ, Golla SSV, Wiegers SE, Zwezerijnen GJC, Pieplenbosch S, et al. Combatting the effect of image reconstruction settings on lymphoma 18F-FDG PET metabolic tumor volume assessment using various segmentation methods. EJNMMI Res (2022) 12:44. doi: 10.1186/s13550-022-00916-9
29. Tutino F, Puccini G, Linguanti F, Puccini B, Rigacci L, Kovalchuk S, et al. Baseline metabolic tumor volume calculation using different SUV thresholding methods in Hodgkin lymphoma patients: interobserver agreement and reproducibility across software platforms. Nucl Med Commun (2021) 42:284–91. doi: 10.1097/MNM.0000000000001324
30. Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging (2015) 42:328–54. doi: 10.1007/s00259-014-2961-x
31. Iwasa H, Nagamachi S, Nakayama S, Yamamoto T, Yoshimitsu K. The reproducibility of MTV and TLG of soft tissue tumors calculated by FDG-PET: comparison between the lower limit by the fixed value SUV 2.5 and that value by 30% of SUVmax. Jpn J Radiol (2023) 41:531–40. doi: 10.1007/s11604-022-01378-8
32. Choi S, Ryeong H, Cho HY, Pahk K, Lee S-H, Chung J-H, et al. Predictive value of preoperative volume-based 18F-2-Fluoro-2-Deoxy-d-Glucose positron emission Tomography/Computed tomography parameters in patients with resectable lung adenocarcinoma. Nucl Med Mol Imaging (2018) 52(6):453–61. doi: 10.1007/s13139-018-0555-4
33. Lai AYT, Perucho JAU, Xu X, Hui ES, Lee EYP. Concordance of FDG PET/CT metabolic tumour volume versus DW-MRI functional tumour volume with T2-weighted anatomical tumour volume in cervical cancer. BMC Cancer (2017) 17(1):825. doi: 10.1186/s12885-017-3800-9
34. Kitao T, Hirata K, Shima K, Hayashi T, Sekizawa M, Takei T, et al. Reproducibility and uptake time dependency of volume-based parameters on FDG-PET for lung cancer. BMC Cancer (2016) 16:576. doi: 10.1186/s12885-016-2624-3
35. Kostakoglu L, Chauvie S. Metabolic tumor volume metrics in lymphoma. Semin Nucl Med (2018) 48:50–66. doi: 10.1053/j.semnuclmed.2017.09.005
36. Başcı S, Yiğenoğlu TN, Bakırtaş M, Ulu BU, Yaman S, Dal MS, et al. The effect of bulky mass on prognosis in diffuse large-b-cell lymphoma: still poor? Leuk Res (2021) 102:106521. doi: 10.1016/j.leukres.2021.106521
37. Gou B, Tan X, Ke Q, Cen H. Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: a meta-analysis. PloS One (2019) 14:e0210224. doi: 10.1371/journal.pone.0210224
38. Bertini M, Boccomini C, Calvil R. The influence of advanced age on the treatment and prognosis of diffuse large-cell lymphoma (DLCL). Clin Lymphoma (2001) 1:278–84. doi: 10.3816/clm.2001.n.002
39. Vose JM, Armitage JO, Weisenburger DD, Bierman PJ, Sorensen S, Hutchins M, et al. The importance of age in survival of patients treated with chemotherapy for aggressive non-hodgkin's lymphoma. J Clin Oncol (1988) 6:1838–44. doi: 10.1200/JCO.1988.6.12.1838
40. Esfahani SH, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with 18F-FDG PET/CT as a predictor of progression-free survival in diffuse large b-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging (2013) 3:272–81.
Keywords: lymphoma, DLBCL, PET/CT, (18)F-FDG, bulky, metabolic tumor volume
Citation: Jin H, Jin M, Lim CH, Choi JY, Kim S-J and Lee K-H (2023) Metabolic bulk volume predicts survival in a homogeneous cohort of stage II/III diffuse large B-cell lymphoma patients undergoing R-CHOP treatment. Front. Oncol. 13:1186311. doi: 10.3389/fonc.2023.1186311
Received: 14 March 2023; Accepted: 24 May 2023;
Published: 13 June 2023.
Edited by:
Alberto Fabbri, Siena University Hospital, ItalyReviewed by:
Laura Evangelista, University of Padua, ItalyCopyright © 2023 Jin, Jin, Lim, Choi, Kim and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung-Han Lee, khleenm@naver.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.