
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 May 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1183227
Background: A comprehensive understanding of the anatomical variations in the pulmonary bronchi and arteries is particularly essential to the implementation of safe and precise left superior division segment (LSDS) segmentectomy. However, no report shows the relationship between the descending bronchus and the artery crossing intersegmental planes. Thus, the purpose of the present study was to analyze the branching pattern of the pulmonary artery and bronchus in LSDS using three-dimensional computed tomography bronchography and angiography (3D-CTBA) and to explore the associated pulmonary anatomical features of the artery crossing intersegmental planes.
Materials and methods: The 3D-CTBA images of 540 cases were retrospectively analyzed. We reviewed the anatomical variations of the LSDS bronchus and artery and assorted them according to different classifications.
Results: Among all 540 cases of 3D-CTBA, there were 16 cases (44.4%) with lateral subsegmental artery crossing intersegmental planes (AX3a), 20 cases (55.6%) Without AX3a in the descending B3a or B3 type, and 53 cases (10.5%) with AX3a, 451 cases (89.5%) Without AX3a in the Without the descending B3a or B3 type. This illustrated that the AX3a was more common in the descending B3a or B3 type (P < 0.005). Similarly, there were 69 cases (36.1%) with horizontal subsegmental artery crossing intersegmental planes (AX1 + 2c), 122 cases (63.9%) Without AX1 + 2c in the descending B1 + 2c type, and 33 cases (9.5%) with AX1 + 2c, 316 cases (90.5%) Without AX1 + 2c in the Without the descending B1 + 2c type. Combinations of the branching patterns of the AX1 + 2c and the descending B1 + 2c type were significantly dependent (p < 0.005). The combinations of the branching patterns of the AX1 + 2c and the descending B1 + 2c type were frequently observed.
Conclusions: This is the first report to explore the relationship between the descending bronchus and the artery crossing intersegmental planes. In patients with the descending B3a or B3 type, the incidence of the AX3a was increased. Similarly, the incidence of the AX1 + 2c was increased in patients with the descending B1 + 2c type. These findings should be carefully identified when performing an accurate LSDS segmentectomy.
With the widespread use of High-resolution computed tomography (HRCT), an increasing number of ground-glass opacities (GGOs) are being identified. Several studies have shown the same oncologic efficacy between a video-assisted thoracoscopic surgery (VATS) lobectomy and segmentectomy for GGO-dominant peripheral lung cancer (1–7).
The JCOG0804 study evaluates the efficacy and safety of sublobar resection for GGO-dominant peripheral lung cancer with consolidation tumor ratio ≤0.25 and maximum tumor diameter ≤2.0 cm (1). Based on the result of JCOG0804, sublobar resection with enough surgical margin offered sufficient local control and relapse-free survival (RFS) for GGO-dominant peripheral lung cancer (1). The JCOG0802 study is a randomized, controlled, non-inferiority trial to confirm whether segmentectomy is not inferior to lobectomy regarding prognosis (2, 3). The JCOG0802 study showed segmentectomy to be non-inferior and superior to lobectomy with regards to overall survival (OS) and concluded segmentectomy should be the standard surgical procedure, rather than lobectomy, for patients with small-sized (≤2 cm, consolidation-to-tumor ratio >0.5) peripheral non-small cell lung carcinoma (NSCLC) (2, 3).
The primary forms of sublobar resection currently contain wedge resection and anatomical segmentectomy. However, VATS segmentectomy is more complex than a standard lobectomy because of the anatomical sophistication of the lung, characterizing both segmental vessels and bronchi structures that diversify at different levels. Therefore, comprehensive knowledge of the pulmonary bronchovascular pattern by general thoracic surgeons has become more significant to the implementation of safe and precise left superior division segment (LSDS) surgery. However, only a few studies represent anatomic variations of the LSDS using three-dimensional computed tomography bronchography and angiographyy (3D-CTBA) (8–12). Moreover, we also found the aberrant artery crossing intersegmental planes in LSDS (13). The purpose of the present study was to classify the branching patterns of the left superior division bronchus (LSDB) and the left superior division artery (LSDA) by using data obtained from 3D-CTBA. Furthermore, we explore the associated anatomical features of artery crossing intersegmental planes in LSDS.
The inclusion and exclusion criteria of this study:
Inclusion criteria:
①. GGO, with a diameter of less than 2 cm and with a consolidation tumor ratio of less than 25%, situated in the left upper lobe (LUL);
②. Sublobar resection (segmentectomy or wedge resection) was implemented;
③. Patients underwent routine chest-enhanced CT examinations preoperatively.
④. Without a history of left lung surgery;
Exclusion criteria:
①. The images showed by enhanced CT lung examination were not distinct, which influenced the three-dimensional (3D) reconstruction of the lung;
②. The lesion size of the LUL exceeded 3cm.
From October 2020 to October 2022, 540 patients (248 men, 292 women; mean age, 56 years) were enrolled from the Department of Thoracic Surgery, Hebei General Hospital. After collecting CT data, the volume data from both arterial and venous phases were imported into a reconstruction software (Infer Operate Thorax Planning), which computed and processed the data before presenting it in 3D-CTBA images (14). All procedures involving human participants in this study were in accordance with the Declaration of Helsinki (revised in 2013). The Research Ethics Committee approved this retrospective study at Hebei General Hospital (no. 2022119). The need for patient consent was waived because of the retrospective nature of the study. Variations in the LSDA and LSDB were classified and summarized.
The LSDS and lingular segment (LS) form LUL. The LUL was sorted into the apicoposterior segment (S1 + 2), anterior segment (S3), superior lingular segment (S4), and inferior lingular segment (S5). S1 + 2 and S3 were subclassified into three pulmonary subsegments (S1 + 2a, S1 + 2b, S1 + 2c, S3a, S3b, S3c) respectively. The LSDS is consists of S1 + 2 and S3. The LS is comprised of S4 and S5.
Segmental and subsegmental bronchi of LSDB were nominated (10): B1 + 2 is the apicoposterior segmental bronchus that divides into apical (B1 + 2a), posterior (B1 + 2b) and horizontal ramus (B1 + 2c); B3 is the anterior segmental bronchus that is further sorted into lateral (B3a), medial (B3b) and superior ramus (B3c) (Figure 1). LSB divides into superior (B4) and inferior (B5) segmental bronchi (Figure 1).
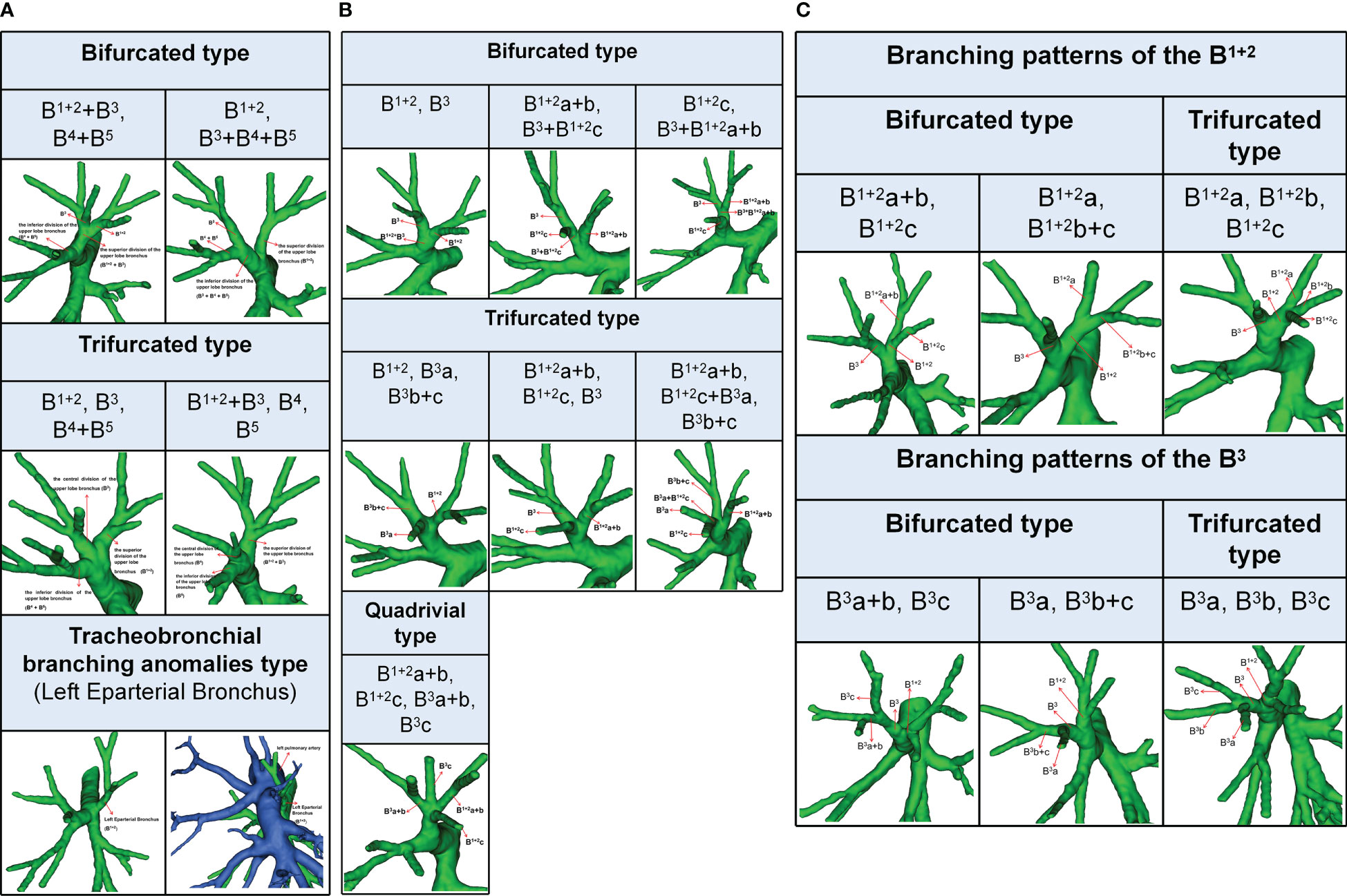
Figure 1 (A) 3D reconstruction model of branching patterns of the Left upper lobe bronchus.(B) 3D reconstruction model of branching patterns of the Left superior division bronchus (B1 + 2 + B3). (C) 3D reconstruction model of branching patterns of the B1 + 2 and B3.
According to the classification proposed by Dominique (15), the term left eparterial bronchus refers to any bronchus directed toward the LUL that originates from the left main bronchus (LMB) above the level where the left pulmonary artery (LPA) crosses the LMB (Figure 1A).
Segmental and subsegmental arteries of LSDA were named (10): A1 + 2 is the apicoposterior segmental artery that divides into apical (A1 + 2a), posterior (A1 + 2b) and horizontal ramus (A1 + 2c); A3 is the anterior segmental artery that is further sorted into lateral (A3a), medial (A3b) and superior ramus (A3c) (Figure 2A). The LSA was comprised of the superior lingular artery (A4) and inferior lingular artery (A5).
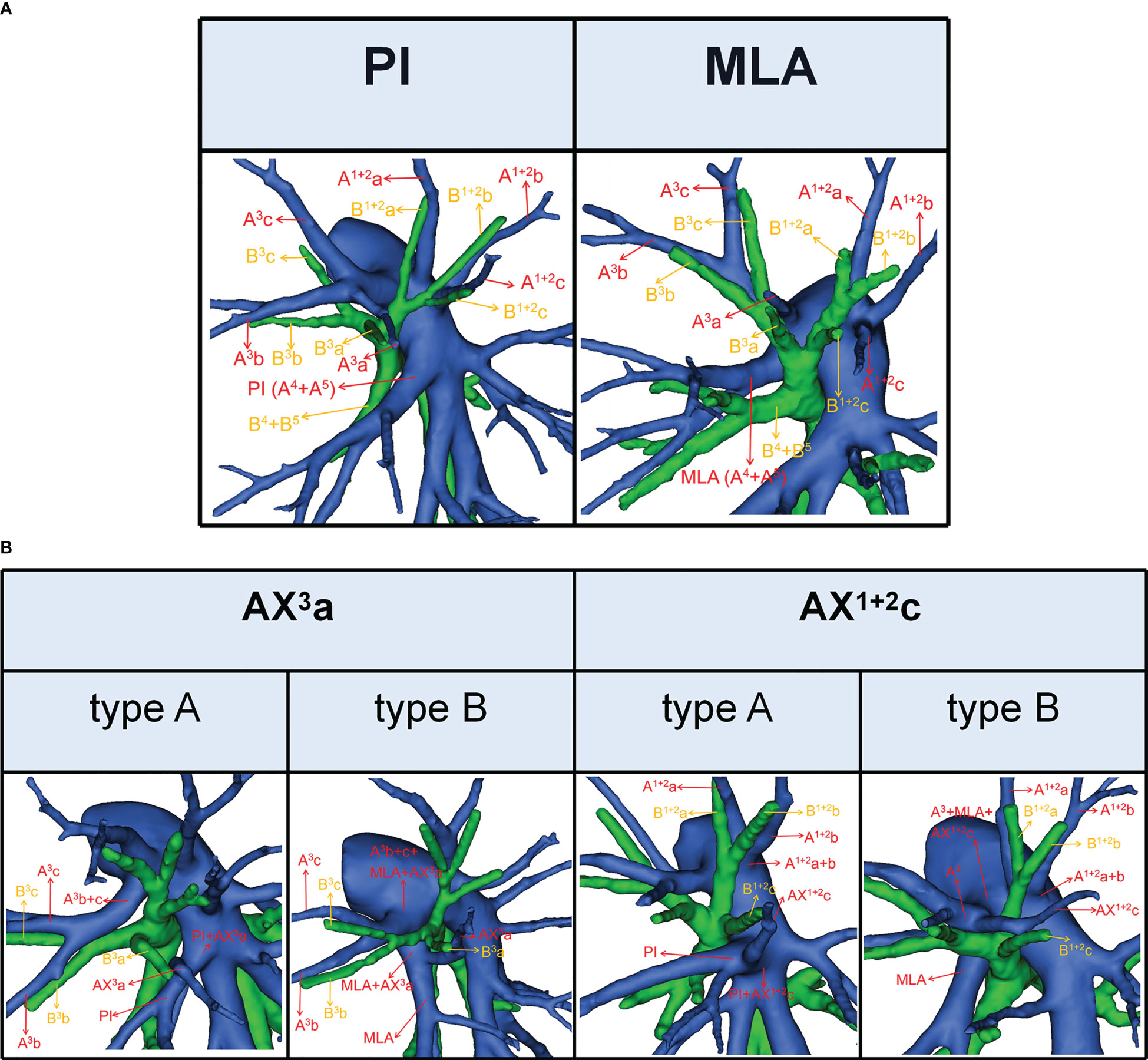
Figure 2 (A) 3D reconstruction model of PI and MLA. (B) 3D reconstruction model of AX3a: type A, AX3a originates from PI; type B, AX3a originates from MLA. 3D reconstruction model of AX1 + 2c: type A, AX1 + 2c originates from PI; type B, AX1 + 2c originates from A3.
According to the origin of LSA, the nomenclature of LSA was divided into two types (11): PI, which originates from the interlobar portion of the LPA; MLA, which originates from the mediastinal portion of the LPA (Figure 2A).
The lateral subsegmental artery crossing intersegmental planes was defined as the AX3a (16). And AX3a has two origins (Figure 2B). When AX3a originates from PI, it spans the intersegmental planes between S3 and S4 and supplies S3a (Figure 2B). Similarly, when AX3a originates from MLA, it also spans the intersegmental planes between S3 and S4 and supplies S3a (Figure 2B).
The horizontal subsegmental artery crossing intersegmental planes was named as AX1 + 2c. Moreover, AX1 + 2c has two origins (Figure 2B). When AX1 + 2c originates from PI, it spans the intersegmental planes between S3 and S4 and supplies S1 + 2c (Figure 2B). However, when AX1 + 2c originates from A3, it spans the intersegmental planes between S1 + 2 and S3 and supplies S1 + 2c (Figure 2B).
All statistical analyses were implemented using SPSS 23.0 (SPSS, Chicago, IL, USA). Qualitative data were presented as the number of cases (percentage). The Pearson Chi-Square test was used to evaluate the significance of dependencies between the groups. A P-value less than 0.05 was considered statistically significant.
The branching patterns of the LUL bronchus were classified into three types (Table 1; Figure 1A): bifurcated type (493/540, 91.3%), trifurcated type (45/540, 8.3%), and tracheobronchial branching abnormalities type (2/540, 0.4%). The bifurcated type was further divided into two subtypes (Table 1; Figure 1A): type 1, in which a common trunk of B1 + 2+B3 originates from the superior division of the upper lobe bronchus and a common trunk of B4+B5 originates from the inferior division of the upper lobe bronchus (90.9%); type 2, in which B1 + 2 originates from the superior division of the upper lobe bronchus and a common trunk of B3+B4+B5 originates from the inferior division of the upper lobe bronchus (0.4%). The trifurcated type was also further sorted into two subtypes (Table 1; Figure 1A): type 1, in which B1 + 2 comes from the superior division of the upper lobe bronchus, B3 comes from the central division of the upper lobe bronchus, and a common trunk of B4+B5 comes from the inferior division of the upper lobe bronchus (3.9%); type 2, in which a common trunk of B1 + 2+B3 comes from the superior division of the upper lobe bronchus, B4 comes from the central division of the upper lobe bronchus, and B5 comes from the inferior division of the upper lobe bronchus (4.4%). Moreover, B1 + 2 originating from the LMB was found in 2 cases in the Left Eparterial Bronchus. The LPA was on the ventral side of B1 + 2 and did not cross the dorsal side of the LMB (Figure 1A).
The branching patterns of LSDB were divided into three types (Table 2; Figure 1B): bifurcated type (305/491, 62.1%), trifurcated type (185/491, 37.7%), and quadrivial type (1/491, 0.2%). The bifurcated type was further separated into three subtypes (Table 2; Figure 1B): subtype I (B1 + 2, B3), subtype II (B1 + 2a+b, B3+B1 + 2c), subtype III (B1 + 2c, B3+B1 + 2a+b). These subtypes accounted for 58.7%, 2.0%, and 1.4%, respectively. The trifurcated type was also further subclassified into three subtypes: subtype I (B1 + 2, B3a, B3b+c) was observed in 2.2% of cases, subtype II (B1 + 2a+b, B1 + 2c, B3) was the most common (35.0%) and subtype III (B1 + 2a+b, B1 + 2c+B3a, B3b+c) was seen in 2 cases (0.4%). The quadrivial type (B1 + 2a+b, B1 + 2c, B3a+b, B3c) was the less common (0.2%).
The branching pattern of B1 + 2 included two types: 522 cases were bifurcated, while trifurcated was found only in 18 cases (Table 3; Figure 1C). The bifurcated type was further divided into subtype I (B1 + 2a+b, B1 + 2c), which was presented in 488 patients (90.4%) and subtype II (B1 + 2a, B1 + 2b+c), that was occurred in 34 patients (6.3%) (Figure 1C). Similarly, the branching pattern of B3 contained two types: bifurcated type (445/540, 82.4%) and trifurcated type (95/540, 17.6%) (Table 3; Figure 1C). The bifurcated type was further divided into subtype I (B3a+b, B3c), which was found in 70 patients (13.0%) and subtype II (B3a, B3b+c), which was the most common (69.4%) (Figure 1C).
According to the original location and the number of the A3, the branching patterns of the A3 were classified and summarized in detail (Table 4; Figure 3). When the composition of the A3 included a single branch, the branching patterns of the A3 were classified into two types: type A, A3 from the anterior portion of LPA (83.0%); type B, A3 from the interlobar portion of LPA (0.5%). When the composition of the A3 contained two branches, the branching patterns of the A3 were divided into four types: type A, A3b+c from the anterior portion of LPA and AX3a from MLA (4.6%); type B, A3b+c from the anterior portion of LPA and AX3a from PI (8.1%); type C, A3b+c from the anterior portion of LPA and A3a from the interlobar portion of LPA (Independent A3a) (3.0%); type D, A3b+c from the anterior portion of LPA and A3a from the interlobar portion of LPA (A3a and A1 + 2c share a common trunk) (0.7%). As detailed in Table 4, the incidence of AX3a was 12.8% (69/540). Furthermore, we summarized the distribution of AX3a in bronchus type (Table 5).
In the following types (Table 2; Figure 1B), B1 + 2, B3a, B3b+c (2.2%) and B1 + 2a+b, B1 + 2c+B3a, B3b+c (0.4%), the components of B3 comprised the descending B3a. Similarly, in the following types (Table 1; Figure 1A), B1 + 2, B3, B4+B5 (3.9%) and B1 + 2, B3+B4+B5 (0.4%), the components of B3 comprised the descending B3. As shown in Figure 4 and Table 6, the incidence of AX3a with and without the descending B3a or B3 type was 44.4% (16/36) and 10.5% (53/504), respectively. This indicated that the AX3a was more common in the descending B3a or B3 type (P < 0.005).
According to the original location and the number of the A1 + 2, the branching patterns of the A1 + 2 were also sorted and summarized in detail (Table 7; Figure 5). When the composition of the A1 + 2 consisted of a single branch (Figure 5A), two types were defined: type A, A1 + 2 from the posterolateral portion of LPA (7.4%); type B, A1 + 2 from A3 (0.7%). When the composition of the A1 + 2 involves two branches, patients can be divided into one of the following eight types (Figure 5A): type A, A1 + 2a from A3 and A1 + 2b+c from posterolateral portion of LPA (13.9%); type B, A1 + 2a+b from A3 and A1 + 2c from interlobar portion of LPA (12.4%); type C, A1 + 2a+b from A3 and A1 + 2c from interlobar portion of LPA (A3a and A1 + 2c share a common trunk) (0.7%); type D, A1 + 2a+b from posterolateral portion of LPA and AX1 + 2c from A3 (2.4%); type E, A1 + 2a+b from posterolateral portion of LPA and AX1 + 2c from PI (2.4%); type F, A1 + 2a+b from A3 and AX1 + 2c from PI (1.7%); type G, A1 + 2a from posterolateral portion of LPA and A1 + 2b+c from posterolateral portion of LPA (3.0%); type H, A1 + 2a+b from posterolateral portion of LPA and A1 + 2c from interlobar portion of LPA (16.5%). When the composition of the A1 + 2 contained three branches, there are five types (Figure 5B): type A, A1 + 2a from A3, A1 + 2b from posterolateral portion of LPA and A1 + 2c from interlobar portion of LPA (22.4%); type B, A1 + 2a from A3, A1 + 2b from posterolateral portion of LPA and AX1 + 2c from PI (7.4%); type C, A1 + 2a from A3, A1 + 2b from posterolateral portion of LPA and AX1 + 2c from A3 (2.8%); type D, A1 + 2a from posterolateral portion of LPA, A1 + 2b from posterolateral portion of LPA and AX1 + 2c from PI (2.2%); type E, A1 + 2a from posterolateral portion of LPA, A1 + 2b from posterolateral portion of LPA and A1 + 2c from interlobar portion of LPA (4.1%). As shown in Table 7, the incidence of AX1 + 2c was 18.9% (102/540). Furthermore, we summarized the distribution of AX1 + 2c in bronchus type (Table 8).
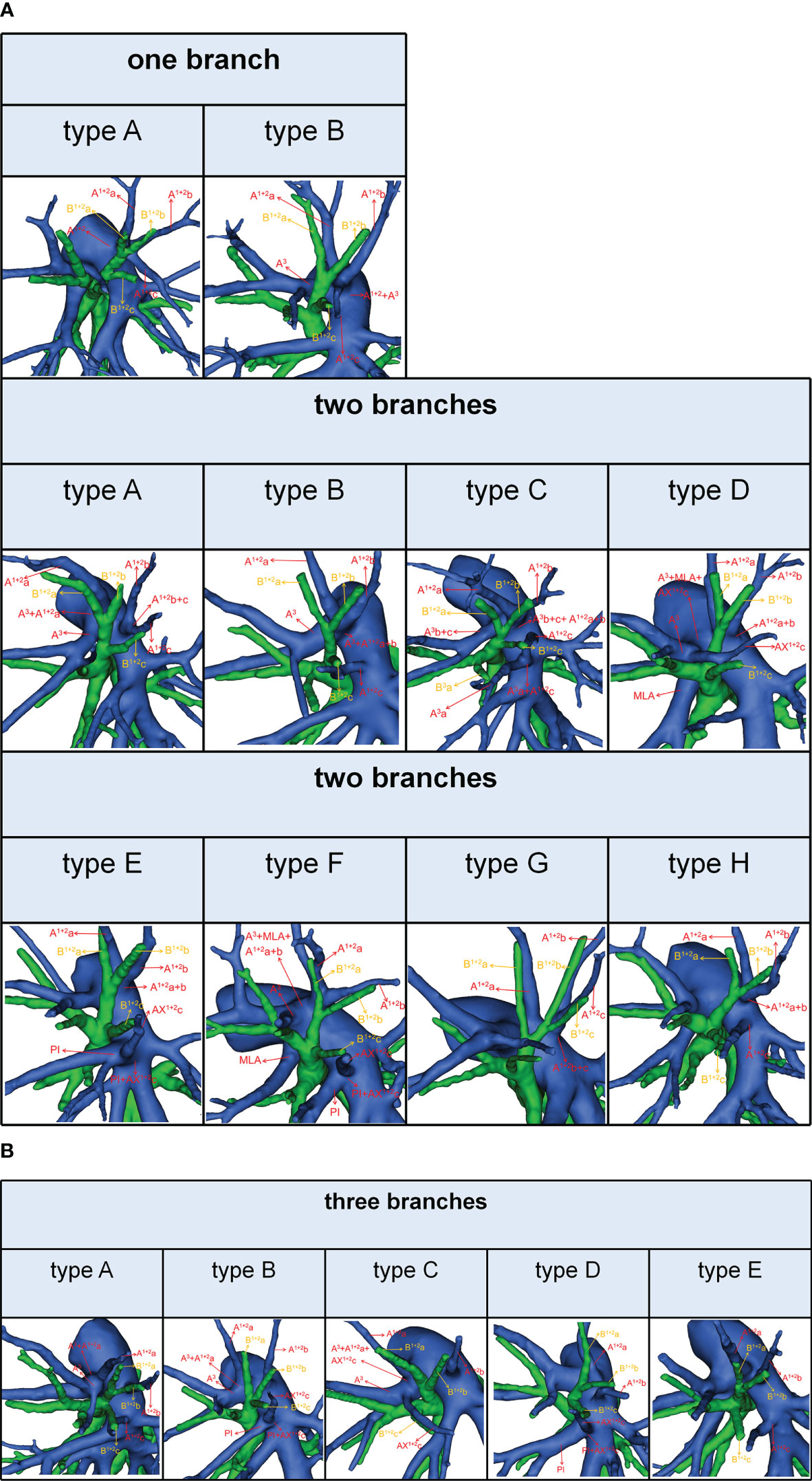
Figure 5 (A) 3D reconstruction model of branching patterns of the A1 + 2, when the composition of the A1 + 2 contained single branch and two branches. (B) 3D reconstruction model of branching patterns of the A1 + 2, when the composition of the A1 + 2 contained three branches.
In the following types (Table 2; Figure 1B), B1 + 2a+b, B3+B1 + 2c (2.0%), B1 + 2c, B3+B1 + 2a+b (1.4%), B1 + 2a+b, B1 + 2c, B3 (35.0%), and B1 + 2a+b, B1 + 2c+B3a, B3b+c (0.4%), the components of B1 + 2 included the descending B1 + 2c. As shown in Figure 6 and Table 9, the incidence of AX1 + 2c with and without the descending B1 + 2c type was 36.1% (69/191) and 9.5% (33/349), respectively. Combinations of the branching patterns of the AX1 + 2c and the descending B1 + 2c type were significantly dependent (p < 0.005). This indicates that the incidence of AX1 + 2c was increased in patients with the descending B1 + 2c type.
The low-dose thin-slice chest CT has extremely boosted the screening rate of GGOs. Some previous studies indicated that sublobar resection should be the standard surgical procedure for patients with GGO-dominant peripheral NSCLC (1–7). Identification and dissection of the branch of LSDA are significant parts of the LSDS anatomic segmentectomy. The anatomical variations of the branching pattern in the LSDA are diverse, which extraordinarily augments the challenge in the VATS segmentectomy of the LSDS. Intraoperative misunderstandings of the branch of LSDA can result in serious complications, such as uncontrollable intraoperative bleeding that convert to open thoracotomy. Moreover, pulmonary arterial injury, particularly the LUL, was a common source of hemorrhage (17). Therefore, thoracic surgeons must have a systemic and precise understanding of the branching pattern in the LSDA.
Fortunately, advances in HRCT and reconstruction techniques have allowed for the visualization of the branching pattern of the bronchus and vascular of the lungs (18, 19). The efficacy of blood vessel visualization is reportedly about 95.2% (20). The literature illustrated that 3D-CTBA is a valuable tool for thoracic surgeons to implement segmentectomy or subsegmentectomy procedures, which decreases operative-related complications and operation time and guarantees safe surgical margins (19).
However, there are only a few reports that have comprehensively summarized and sorted the branching pattern of LSDA and LSDB using 3D-CTBA (8–13). And the correlation of the descending bronchus and the artery crossing intersegmental planes in LSDS has not been reported in previous studies. In the present study, we detailedly analyzed the branching pattern of LSDA and LSDB and highlighted the differences between our results and those of previous reports (8–13). Furthermore, we explored the associated anatomical features of AX3a and AX1 + 2c.
In the present study, the branching patterns of the LUL bronchus were divided into three types (Table 1; Figure 1A). A common trunk of B3+B4+B5 originating from the inferior division of the upper lobe bronchus was a rare anomaly (0.4%), which was similar to the findings of Wang (0.6%) and Zhang (0.1%) (10, 21). When B3 originated from the B4+B5, the literature reported that it is often accompanied by the following two other variants: A, A3 comes from A4+A5 and V1 + 2c drained into the inferior pulmonary vein; B, additional fissure dividing the LUL into S1 + 2 and S3 + 4+5 (21). It is significant to note these malformations before the S1 + 2 segmentectomy is performed. Moreover, we found that the trifurcated type included two branching types (Table 1): B1 + 2, B3, B4+B5 type (3.9%), which incidence was higher than that of Maki (1.7%) and B1 + 2+B3, B4, B5 type, which has not been reported in the previous studies. When B3 originated the central division of the upper lobe bronchus, it is greatly easy to mistake the superior division of the upper lobe bronchus (B1 + 2) for B1 + 2+B3 during LSDS segmentectomy, which resulted in inadequate surgical safety margins. The Left Eparterial Bronchus was seen in 2 cases (0.4%), which incidence was similar to that of Maki (0.3%) (8). Previous reports demonstrated that the Left Eparterial Bronchus type has the following characteristics (1): the LPA does not cross the dorsal side of the displaced bronchus (2); incomplete lobulation exists between the LUL and left lower lobes (LLL) (22, 23). Therefore, whenever abnormalities of the pulmonary artery and incomplete lobulation are identified, it is crucial to keep an eye on the existence of the Left Eparterial Bronchus.
We found that the branching patterns of the LSDB had three types (Table 2; Figure 1B): bifurcated type (62.1%), which incidence was lower than that of Maki (76.0%) and Wang (65.7%); trifurcated type (37.7%), which was considerably higher than the frequency reported by Maki (24.0%) and Wang (34.3%); quadrivial type (0.2%), which was not found by Maki and Wang (8–10). In the bifurcated type, the B1 + 2, B3 type was the most common type (58.7%) and the B1 + 2c, B3+B1 + 2a+b type was first reported (8–10). However, we have not found the B1 + 2+B3c, B3a+b type (10). For the B1 + 2a+b, B3+B1 + 2c type, a mistaken ligation of the trunk of B3+B1 + 2c will result in lung volume loss in the S3 segmentectomy (Figure 1B). In trifurcated type, the B1 + 2a+b, B1 + 2c, B3 type was the most common type (35.0%), and the B1 + 2a+b, B1 + 2c+B3a, B3b+c type was not found in the previous literature. For the B1 + 2a+b, B1 + 2c+B3a, B3b+c type, a mistaken ligation of the trunk of B1 + 2c+B3a will lead to the enlarged intersegmental plane in the S1 + 2c segmentectomy (Figure 1B). Moreover, the B1 + 2a, B1 + 2b+c, B3 type and B1 + 2, B3a+b, B3c type were not detected in our study (8, 10).
We also observed that the branching patterns of the B1 + 2 had two types (Table 3; Figure 1C): the bifurcated type(96.7%), which incidence was similar to that of Wang (100.0%), and the trifurcated type (3.3%), which has not been reported in the literature (10). Moreover, the classification of the branching patterns of the B3 was the same as that of Wang (Table 3).
To our knowledge, the detailed classification of branching patterns of the A3 was first reported (Table 4; Figure 3). An understanding of the origin of the A3 branch is essential in clinical practice if a safe and precise S3 segmentectomy is to be implemented. When A3 originates from the interlobar portion of LPA, A3 can usually be identified by dissecting interlobar fissures. When AX3a arose from PI, AX3a should be carefully dissected from PI before it is ligated to avoid injuring PI. When a common trunk of A1 + 2c and A3a directly originated from the interlobar portion of the LPA, A3a should be resected without resecting A1 + 2c.
In the case of S3a segmentectomy, it is necessary to investigate the origin of A3a. In the present study, A3a arose from the interlobar portion of LPA in 20 cases (3.7%) (Table 4; Figure 3). This compares with the figures showed by Maki (3.9%) and Murota (8.4%) (8, 11). AX3a originating from the PI was observed in cases (8.1%), which incidence was similar to that of Maki (6.1%) and Murota (8.1%) (8, 11). Moreover, AX3a originating from MLA occurred in 25 patients (4.6%) and was the first reported (Figure 2B).
In our study, an interesting finding concerned the AX3a (Table 5; Figure 4). There was a significant correlation between the branching patterns of the AX3a and the descending B3a or B3 type (Table 6). Moreover, the incidence of AX3a was increased in patients with the descending B3a or B3 type (P < 0.005). This can be clarified by the paralleling correlation between pulmonary segmental arteries and pulmonary segmental bronchi. Thus, when the AX3a is identified preoperatively, the thoracic surgeons must investigate the possibility of the descending B3a or B3. For B1 + 2, B3a, B3b+c type, when S3a segmentectomy was planned and performed, it was practical and safe to ligate the AX3a originating from PI in the side of the oblique fissure, followed by the B3a (Figure 4). For B1 + 2a+b, B1 + 2c+B3a, B3b+c type, it was easier to dissect the B3a from the oblique fissure in the S3a segmentectomy (Figure 4). And AX3a originating MLA was identified after resection B3a. At the same time, it may be disturbed by the LUL vein.
It is significant to understand the branching patterns of A1 + 2 pre-operatively (Figure 5; Table 7). When A1 + 2 originated from the posterolateral portion of LPA (Figure 5A), during S1 + 2a segmentectomy, it is necessary to dissect the branches of A1 + 2 in a center-to-periphery direction to distinguish A1 + 2a, A1 + 2b, and A1 + 2c. However, it significantly increased the difficulty of dissection. When A1 + 2a+b originated from the posterolateral portion of LPA and A1 + 2c originated from the interlobar portion of LPA (type H), during S1 + 2 segmentectomy, we need to dissect the posterolateral portion and the interlobar portion of LPA to discriminate A1 + 2a+b and A1 + 2c (Figure 5A).
As shown in Figure 5 and Table 7, the branching patterns of the A1 + 2 in this study were somewhat different from the previous report (12). The main reason is the introduction of AX1 + 2c. In the present study, AX1 + 2c arose from the PI in 74 cases (13.7%) (Figure 2B). This compares with the figures revealed by Deng (6.8%) and Murota (3.8%) (9, 11). Moreover, AX1 + 2c originating from A3 was observed in 28 patients (5.2%) and was the first reported (Figure 2B).
AX1 + 2c is not uncommon in clinical practice; however, if it occurs, it can lead to significant difficulties for patients to perform S1 + 2c segmentectomy. No previous report has reported the distribution of AX1 + 2c branching patterns in the bronchus type (Table 8). Moreover, the combination of branching patterns of the AX1 + 2c and the descending B1 + 2c type showed significant dependence (Table 9). And it was first reported. This denoted that the AX1 + 2c was often combined with the descending B1 + 2c type. The paralleling relationship between pulmonary segmental arteries and pulmonary segmental bronchi may explain this phenomenon. Thus, when the AX1 + 2c was recognized preoperatively, it is crucial to keep an eye on the existence of the descending B1 + 2c type. For B1 + 2a+b, B1 + 2c, B3 type, when S1 + 2c segmentectomy was implemented, it was feasible and secured to dissect the AX1 + 2c originating from PI in the side of the oblique fissure, followed by the B1 + 2c (Figure 6). When AX1 + 2c arose from A3, it ran deep within the lung parenchyma of S3. For B1 + 2a+b, B3+B1 + 2c type, it was easier to identify the B1 + 2c from the oblique fissure in the S1 + 2c segmentectomy (Figure 6). However, the AX1 + 2c was exposed by lifting the B1 + 2c stump. And it was essential to avoid causing damage to the A3 when the AX1 + 2c was ligated. In sum, we defined the coexistence of the AX1 + 2c and the descending B1 + 2c, the AX3a and the descending B3a, and the AX3a and the descending B3 as “Hebei’s triad combinations”.
This is the first report to explore the associated pulmonary anatomical features of AX1 + 2c and AX3a. We found that the incidence of AX1 + 2c was increased in patients with the descending B1 + 2c type, and AX3a was increased in patients with the descending B3a or B3 type. This knowledge will assist in the preoperative planning of S1 + 2c segmentectomy and S3a segmentectomy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The Research Ethics Committee approved this retrospective study at Hebei General Hospital (no. 2022119). The need for patient consent was waived because of the retrospective nature of the study.
ZL: project design and initiation, data analysis, manuscript writing. QZ: project design and initiation, data analysis, manuscript writing. WW: project design and initiation, data analysis, manuscript writing. ZH: data collection. XZ: supervisor. All authors contributed to the article and approved the submitted version.
This work was supported by the Department of Hebei Provincial Finance (2018034).
The authors would like to thank Birong Li for polishing our paper and revising the pictures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
3D-CTBA, three-dimensional computed tomography bronchography and angiography; NSCLC, non-small cell lung carcinoma; GGOs, ground glass opacities; HRCT, high-resolution computed tomography; VATS, video-assisted thoracoscopic surgery; RFS, relapse-free survival; OS, overall survival; LMB, left main bronchus; LUL, left upper lobe; LLL, left lower lobe; LSDS, left superior division segment; LS, lingular segment; LSDB, left superior division bronchus; LSDA, left superior division artery; LSB, lingular segment bronchus; LSA, lingular segment artery; LPA, left pulmonary artery; 3D, three-dimensional.
1. Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg (2022) 163(1):289–301 e2. doi: 10.1016/j.jtcvs.2020.09.146
2. Suzuki K, Saji H, Aokage K, Watanabe SI, Okada M, Mizusawa J, et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg (2019) 158(3):895–907. doi: 10.1016/j.jtcvs.2019.03.090
3. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (2022) 399(10335):1607–17. doi: 10.1016/S0140-6736(21)02333-3
4. Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg (2018) 53(3):640–7. doi: 10.1093/ejcts/ezx357
5. Nakamura H, Taniguchi Y, Miwa K, Adachi Y, Fujioka S, Haruki T, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg (2011) 59(3):137–41. doi: 10.1055/s-0030-1250377
6. Moon MH, Moon YK, Moon SW. Segmentectomy versus lobectomy in early non-small cell lung cancer of 2 cm or less in size: a population-based study. Respirology (2018) 23(7):695–703. doi: 10.1111/resp.13277
7. Landreneau RJ, Normolle DP, Christie NA, Awais O, Wizorek JJ, Abbas G, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol (2014) 32(23):2449–55. doi: 10.1200/JCO.2013.50.8762
8. Maki R, Miyajima M, Ogura K, Tada M, Takahashi Y, Adachi H, et al. Pulmonary vessels and bronchus anatomy of the left upper lobe. Surg Today (2022) 52(4):550–8. doi: 10.1007/s00595-022-02471-1
9. Deng Y, Cai S, Huang C, Liu W, Du L, Wang C, et al. Anatomical variation analysis of left upper pulmonary blood vessels and bronchi based on three-dimensional reconstruction of chest CT. Front Oncol (2022) 12:1028467. doi: 10.3389/fonc.2022.1028467
10. He H, Wang F, Wang PY, Chen P, Li WWL, Perroni G, et al. Anatomical analysis of variations in the bronchus pattern of the left upper lobe using three-dimensional computed tomography angiography and bronchography. Ann Transl Med (2022) 10(6):305. doi: 10.21037/atm-22-598
11. Murota M, Yamamoto Y, Satoh K, Ishimura M, Yokota N, Norikane T, et al. An analysis of anatomical variations of the left pulmonary artery of the interlobar portion for lung resection by three-dimensional CT pulmonary angiography and thin-section images. Jpn J Radiol (2020) 38(12):1158–68. doi: 10.1007/s11604-020-01024-1
12. Gao C, Xu WZ, Li ZH, Chen L. Analysis of bronchial and vascular patterns in left upper lobes to explore the genesis of mediastinal lingular artery and its influence on pulmonary anatomical variation. J Cardiothorac Surg (2021) 16(1):306. doi: 10.1186/s13019-021-01682-w
13. Zhang C, Li J, Sun H, Liu H. An aberrant anterior ascending segmental pulmonary artery (A3aii) in a patient with lung cancer. Eur J Cardiothorac Surg (2022) 61(5):1201–3. doi: 10.1093/ejcts/ezab564
14. Li Z, Kong Y, Wu W, Chen S, Zhang X. What is the correlation between the defective and splitting posterior segmental bronchus and recurrent artery crossing intersegmental planes in the right upper lobe? Front Surg (2023) 10:1113783. doi: 10.3389/fsurg.2023.1113783
15. Chassagnon G, Morel B, Carpentier E, Le Pointe HD, Sirinelli D. Tracheobronchial branching abnormalities: lobe-based classification scheme. Radiographics (2016) 36(2):358–73. doi: 10.1148/rg.2016150115
16. Boyden EA, Hartmann JF. An analysis of variations in the bronchopulmonary segments of the left upper lobes of fifty lungs. Am J Anat (1946) 79(3):321–60. doi: 10.1002/aja.1000790302
17. Cao C, Cerfolio RJ, Louie BE, Melfi F, Veronesi G, Razzak R, et al. Incidence, management, and outcomes of intraoperative catastrophes during robotic pulmonary resection. Ann Thorac Surg (2019) 108(5):1498–504. doi: 10.1016/j.athoracsur.2019.05.020
18. Wu W-B, Xu X-F, Wen W, Xu J, Zhu Q, Pan X-L, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis (2016) 8:S710–S5. doi: 10.21037/jtd.2016.09.43
19. Tongxin L, Jing X, Runyuan W, Wei W, Yu Z, Dong W, et al. Application research of three-dimensional printing technology and three-dimensional computed tomography in segmentectomy. Front Surg (2022) 9:881076. doi: 10.3389/fsurg.2022.881076
20. Fukuhara K, Akashi A, Nakane S, Tomita E. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg (2008) 34(4):875–7. doi: 10.1016/j.ejcts.2008.07.014
21. Zhang M, Sun WJ, Wu QC, Ge MJ. Boyden’s triad in the left lung: an interesting phenomenon. Interact Cardiovasc Thorac Surg (2022) 35(2). doi: 10.1093/icvts/ivac082
22. Yaginuma H. Investigation of displaced bronchi using multidetector computed tomography: associated abnormalities of lung lobulations, pulmonary arteries and veins. Gen Thorac Cardiovasc Surg (2020) 68(4):342–9. doi: 10.1007/s11748-019-01223-2
Keywords: left superior division segment (LSDS), anatomical variation, artery crossing intersegmental planes, non-small cell lung carcinoma (NSCLC), segmentectomy, video-assisted thoracoscopic surgery (VATS)
Citation: Li Z, Zhao Q, Wu W, Hu Z and Zhang X (2023) Analysis of bronchovascular patterns in the left superior division segment to explore the relationship between the descending bronchus and the artery crossing intersegmental planes. Front. Oncol. 13:1183227. doi: 10.3389/fonc.2023.1183227
Received: 09 March 2023; Accepted: 15 May 2023;
Published: 24 May 2023.
Edited by:
Giuseppe Cardillo, San Camillo Forlanini Hospital, ItalyReviewed by:
Bilgin Kadri Aribas, Bülent Ecevit University, TürkiyeCopyright © 2023 Li, Zhao, Wu, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaopeng Zhang, bHprankxMjNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.