
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 28 June 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1182558
This article is part of the Research TopicNovel Biomarkers for Potential Clinical Applications in Lung CancerView all 41 articles
 Lionel Michaux1*
Lionel Michaux1* Alexandre Perrier2
Alexandre Perrier2 Camille Mehlman1
Camille Mehlman1 Hussa Alshehhi3
Hussa Alshehhi3 Antonin Dubois4
Antonin Dubois4 Roger Lacave2
Roger Lacave2 Florence Coulet2
Florence Coulet2 Jacques Cadranel1
Jacques Cadranel1 Vincent Fallet1
Vincent Fallet1Introduction: ALK tyrosine kinase inhibitors (ALK TKIs) have improved prognosis in ALK-rearranged (ALK+) non-small-cell lung cancer (NSCLC). However, drug resistance mechanisms occur inevitably during the course of treatment leading to disease progression. Activation of epidermal growth factor receptor (EGFR) bypass signaling pathway is an uncommon cause of acquired resistance to ALK TKIs.
Method: We present two patients with EML4-ALK rearranged NSCLC, developing an acquired EGFR resistance mutation after receiving multiple lines of ALK TKIs.
Results: While preclinical models have showed encouraging data, there is a critical need for clinical studies on treatment strategies to overcome this drug resistance. Three real-life therapeutic approaches were used in this report: i) using brigatinib, an inhibitor targeting both ALK and EGFR tyrosine kinases; ii) combining two ALK TKIs together; and iii) delivering doublet platinum chemotherapy. In case 1, time to treatment failure (TTF) was 9.5 months with brigatinib; in case 2, TTF was 10 months with combined TKIs (osimertinib and brigatinib), whereas TTF with chemotherapy was only 2 months. Tolerability profile TKIs combotherapy was acceptable.
Conclusion: These case reports underline the therapeutic complexity of EGFR-acquired resistance mutation in ALK+ NSCLC and offers some leads to solve this real-life clinical challenge.
Giving an ALK tyrosine kinase inhibitor (ALK TKI) is the standard of care for first-line treatment of patients with advanced ALK rearrangement driven non-small-cell lung cancer (ALK+ NSCLC).
However, nearly all patients develop acquired resistance to ALK TKIs. Resistance can be divided into two categories: in-target and off-target resistance mechanisms. Among these, the activation of alternative bypass signaling pathways have been described. We report two cases of rare resistance mechanisms involving EGFR pathway occurring after sequential treatment by ALK TKIs. We also aimed to describe our therapeutic approaches to overcome these EGFR-driven resistances.
A then 38-year-old, non-smoker woman, presented to the hospital with chronic cough and asthenia in June 2016. Computed tomography (CT) scan showed a middle lobe consolidation with infradiaphragmatic lymphatic nodes. The patient was subsequently diagnosed with a metastatic lung adenocarcinoma. ALK immunohistochemistry (IHC) was positive with a strong intensity score (clone D5F3), and fluorescence in situ hybridization (FISH) confirmed an EML4-ALK fusion (Vysis LSI ALK Break Apart FISH Probe Kit from Abbott Molecular®). No additional somatic gene alteration, especially EGFR mutation, was found (EGFR genotyping was done using RT-PCR on QX200 system, BIO RAD® system and TaqMan® probes from Life Technologies® for exons 18, 20, and 21 and fragment analysis by capillary gel electrophoresis on 3130 ABI system™ from Applied Technologies® for exon 19). As first-line treatment, the patient received crizotinib (250 mg twice daily) for 13 months until brain magnetic resonance imaging (MRI) revealed brain metastases. After 5 months of treatment with alectinib (600 mg twice daily), CT scan showed progression of lung lesions (Figure 1). Next-generation sequencing (NGS) of tumoral lung tissue DNA revealed an EGFR L861Q mutation (c.2582T>A, p.(Leu861Gln))—variant allelic frequency (VAF) of 1.7% (Tumor Hotspot MASTR™ Plus, Multiplicom®). While EML4-ALK rearrangement was still found, no ALK-dependent resistance mutation was identified. Alectinib was thus discontinued, and brigatinib (90 mg once daily for 1 week, then 180 mg once daily) was given to the patient. The CT scan showed reduction in lung opacities after 8 weeks. Nevertheless, a differential response was observed after 6 months of treatment with maintained lung response but a progression of brain lesions on MRI. Brigatinib was then increased to 240 mg (once daily), and with this dose regimen, the patient had grade 1 myalgia and increased levels of blood creatinine phosphokinases, aspartate aminotransferases, and lipases. After 3 months with brigatinib increased dose, brain metastases progressed, and brigatinib was discontinued. No mutation was shown on plasma circulating tumor DNA, and the patient received a combination of alectinib 600 mg (twice daily) and osimertinib 80 mg (once daily) as fourth-line therapy, which was discontinued after 2 months because of progression. Seven additional lines of treatment were given to the patient, including carboplatin pemetrexed doublet chemotherapy (fifth line) and lorlatinib (sixth line), with 12 and 8 months of response to treatment, respectively. ALK resistance compound mutations (ALK G1202R and ALK G1269A) were first detected after lorlatinib treatment and were still identified until the patient’s death 74 months after the diagnosis.
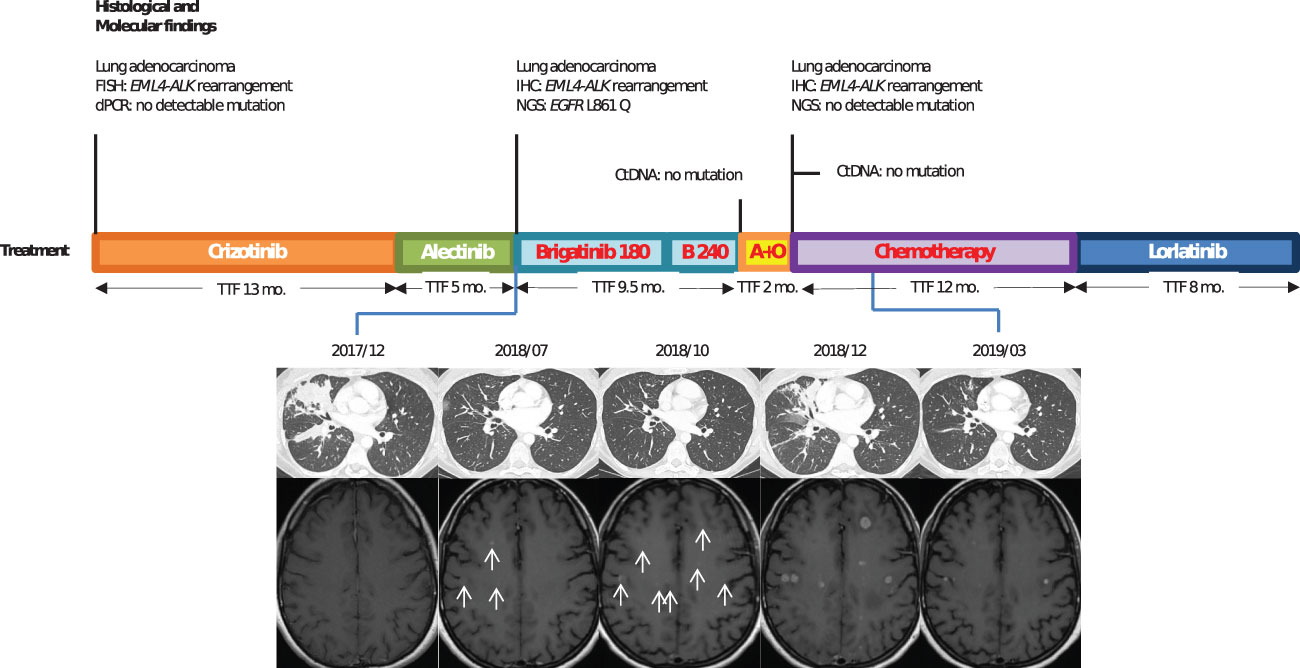
Figure 1 Case 1: timeline illustrating the changes in therapeutic regimen in correlation with molecular and radiological findings. FISH, fluorescence in situ hybridization; dPCR, digital polymerase chain reaction; TTF, time to treatment failure; Mo., months; ctDNA, circulating tumor DNA; IHC, immunohistochemestry; NGS, next-generation sequencing; Brigatinib 180, brigatinib with a 180-mg daily dose regimen; B240, brigatinib with a 240-mg daily dose regimen; A+O, combined treatment with alectinib and osimertinib. In red, the treatments used when EGFR resistance mutation was identified.
In June 2020, a 42-year-old, non-smoker woman was admitted to the hospital for chronic cough and dyspnea. Imaging tests showed a right upper lobe lung mass with secondary bones and liver lesions. CT-guided liver biopsies revealed a muco-epidermoid lung carcinoma. An immunohistological analysis revealed that TTF1 was negative, while the tumor cells without mucus were positive for p40. Tumor cells were also strongly positive for ALK IHC (clone D5F3). EML4-ALK fusion variant 1 was detected by RNA sequencing (Archer® FusionPlex™ Lung panel). No additional somatic gene alteration (such as TP53, KRAS, and KEAP1) was shown in the NGS DNA analysis (SOPHIA Solid Tumor Solution™, SOPHIA Genetics®). Alectinib 600 mg twice daily was started, and the patient experienced a partial response for 12 months. When imaging revealed progression with pelvic bone metastases, lorlatinib was initiated with a 100-mg daily dose regimen. After 2 months, the CT scan revealed new lung opacities that were attributed to a lymphangitic carcinomatosis (Figure 2). Lorlatinib was discontinued, and a carboplatin–paclitaxel doublet chemotherapy was started but was rapidly stopped because of radiological evidences of lung progression. Meanwhile, DNA-based NGS (AmpliSeq™ for Illumina® Focus Panel) in the tissue specimen from bronchus biopsies performed during progression under lorlatinib revealed an exon 20 EGFR T790M mutation (VAF, 1.3%) and the RNA sequencing the EML4-ALK rearrangement. The patient then started on a combination of brigatinib (90 mg once daily for 1 week, then 180 mg once daily) and osimertinib (80 mg once daily). Partial response in the lung was achieved after 6 weeks. The patient experienced, as treatment emergent adverse events, grade 2 vomiting and grade 1 thrombopenia. The patient remained on these combined therapies for 10 months until the CT scan showed pleural progression. As additional line of treatment, brigatinib and pemetrexed were given to the patient with a response of 6 months. The patient finally died 33 months after the diagnosis.
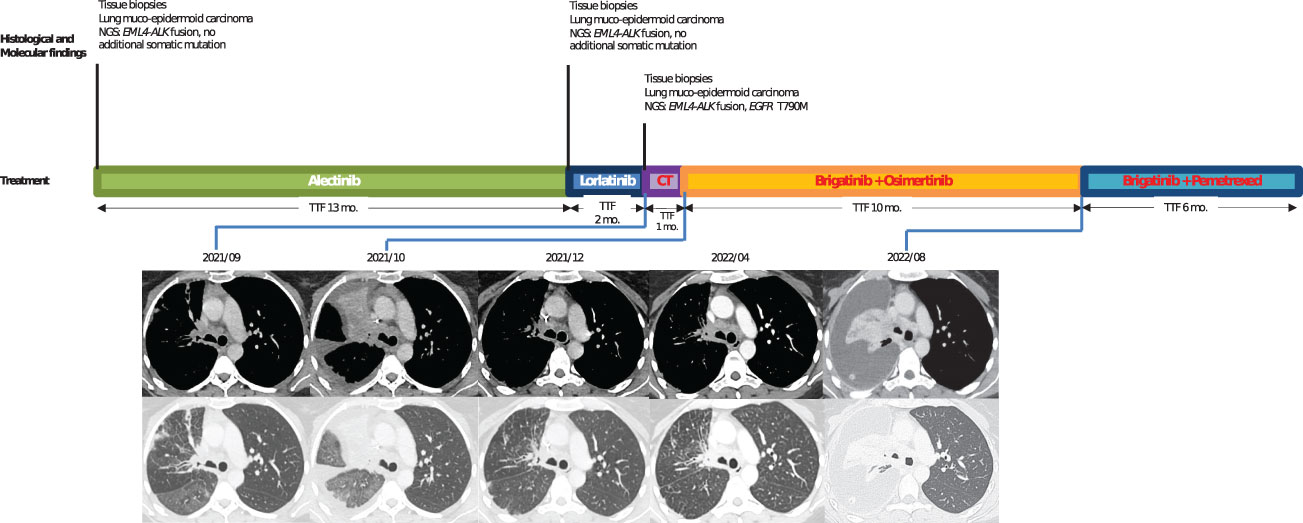
Figure 2 Case 2: timeline illustrating the changes in therapeutic regimen in correlation with molecular and radiological findings. NGS, next-generation sequencing; TTF, time to treatment failure; Mo., months; CT, chemotherapy. In red, the treatments used when EGFR resistance mutation was identified.
We reported here two cases of well-documented EGFR mutations linked to acquired resistance to ALK TKIs in ALK+ NSCLC. To our knowledge, neither L861Q nor T790M EGFR mutants have been described yet as EGFR bypass ALK resistance mechanism. These EGFR mutations were not detected at diagnosis even using different highly sensitive detection methods, making the possibility of de novo co-alterations unlikely.
Most frequently, ALK+ NSCLCs are adenocarcinomas, but in case 2, it was a very rare subtype of NSCLC: mucoepidermoid carcinoma. EML4-ALK rearrangements in this rare subtype have been described in small series, including a case report with durable response to alectinib (1, 2).
Initially described in preclinical studies, the activation of EGFR signaling was identified as an infrequent post-ALK TKIs bypass resistance mechanism (3). In the largest clinical cohort that evaluated the mechanisms of resistance post-ALK TKIs, the most frequent alteration reported was the acquisition of an ALK resistance mutation in 56% of biopsies post second-generation ALK inhibitors, and no EGFR acquired resistance was identified is this cohort (4). In smaller cohorts, EGFR mutation as a mechanism of resistance to ALK TKIs has been previously shown (5–7). In the four described patients, activating EGFR mutations (L858R or exon 19 deletion) were found in tissue biopsies.
Therapeutic strategy proposed in this context has not been clarified. In the present report, we experienced three clinical therapeutic strategies trying to overcome the activation of EGFR signaling pathway. As a first strategy, we switched alectinib for brigatinib, which was of interest because of brigatinib dual ALK and EGFR activity (8). In cellular assays, brigatinib has been reported to have a substantial activity against EGFR exon 19 deletion, with potency only sevenfold reduced compared to ALK (8). Moreover, preclinical (9) and clinical (10) data demonstrated that a combined targeted therapy of brigatinib and cetuximab could be beneficial to overcome the triple EGFR mutant (EGFR T790M and cis-C797S) resistance to osimertinib. However, brigatinib exhibits more modest activity against EGFR L858R or variants with T790M mutation (8), and there is no preclinical data supporting such an activity in case of rare L861Q EGFR mutation harbored by our patient. This strategy of using brigatinib to overcome EGFR-acquired resistance raises some limit in our first case with EGFR co-alteration. Even if no ALK resistance mutation was detected with the EGFR mutation after alectinib, the impact of brigatinib on this bypass resistance mutation remains uncertain, since this TKI also overcomes ALK-dependent resistance post-alectinib (11, 12). Finally, because of the isolated and asymptomatic brain progression, we chose to escalate brigatinib dose regimen to 240 mg daily. Despite a good tolerance, this strategy appeared to be ineffective. In the Phase 2 ALTA-2 trial, 13 patients escalated their brigatinib dosage from 180 to 240 mg daily after experiencing disease progression. While this higher dosage demonstrated an acceptable safety profile, the clinical benefit was disappointing, as no confirmed tumoral response was observed, and the progression-free survival was <2 months (13).
As an alternative therapeutic strategy, we experienced a combination treatment with both ALK and EGFR TKIs (alectinib + osimertinib in case 1 and brigatinib + osimertinib in case 2). No grade 3 toxicity was observed in both cases. An encouraging response was reported in case 2 in the fourth line after a rapid progression with both lorlatinib then chemotherapy. Concurrent inhibition of both EGFR and ALK is therapeutically effective in all of the ALK-resistant preclinical models, which have acquired resistance through EGFR pathway activation (3, 14) and has been described to be safe in several case reports in EGFR-resistant lung cancer with acquired ALK fusion co-alteration (15). However, in case 1, patient progressed dramatically after 2 months of combined therapies perhaps because the tumor was no longer EGFR dependent at this time.
Finally, a platinum-based doublet chemotherapy might be beneficial after failure of second- or third-generation ALK TKI in ALK rearranged NSCLC (16). Interestingly, our second patient rapidly progressed after 1 month of chemotherapy, while a prolonged (12 months) partial response occurred in the first patient. Nevertheless, this last result must be interpreted with caution given that the EGFR mutation was not present after treatment with brigatinib.
In conclusion, we reported two cases of EGFR mutant emerging after at least two lines of ALK TKIs in ALK-rearranged NSCLC. In order to overcome drug resistance, we adopted different strategies with variable efficacy. These case reports highlight both the complexity of drug resistance mechanisms and the therapeutic challenges in developing strategies to overcome drug resistance in oncogenic-driven NSCLC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LM: Conceptualization, Methodology, Investigation, Writing – Original draft. AP: Writing – Reviewing and Editing. CM: Writing – Reviewing and Editing. HA: Writing – Reviewing and Editing. AD: Writing – Reviewing and Editing. RL: Writing – Reviewing and Editing. FC: Writing – Reviewing and Editing. JC: Writing – Reviewing and Editing, Supervision. VF: Conceptualization, Methodology, Investigation, Writing – Original draft. All authors contributed to the article and approved the submitted version.
LM received support for attending meetings and travels from Bristol Myers Squibb and Olympus, all outside of the submitted work. CM received support for attending meeting and travel from ISIS Medical; outside of the submitted work. AD received support for attending meetings and travels from Astellas, Janssen, Bristol Myers Squibb, and Amgen, all outside of the submitted work. FC received payment for her institution for educational events from Astra Zeneca, outside of the submitted work. JC received grants for his institution from Pfizer, AbbVie, Sanofi, and Sophia Genetics; payment for participation to boards of experts from Amgen, Aztra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, and Takeda; all outside of the submitted work. VF received payment for lectures from Pfizer, Takeda, Bristol Myers Squibb, Aztra Zeneca, Roche, and Boehringer Ingelheim; support for attending meetings and travels from Takeda, Janssen, Pfizer, Aztra Zeneca, and Novartis; all outside of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huo Z, Wu H, Li J, Li S, Wu S, Liu Y, et al. Primary pulmonary mucoepidermoid carcinoma: histopathological and moleculargenetic studies of 26 cases. PloS One (2015) 10(11):e0143169. doi: 10.1371/journal.pone.0143169
2. Sakatani T, Masuda Y, Morikawa T, Usui K. Anaplastic lymphoma kinase-positive lung cancer with mucoepidermoid carcinoma differentiation: a case report. CRO (2020) 13(2):1037–41. doi: 10.1159/000510042
3. Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res (2011) 71(18):6051–60. doi: 10.1158/0008-5472.CAN-11-1340
4. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discovery (2016) 6(10):1118–33. doi: 10.1158/2159-8290.CD-16-0596
5. Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res (2012) 18(5):1472–82. doi: 10.1158/1078-0432.CCR-11-2906
6. Wang W, Jiang X, Song Z, Zhang Y. Patients harboring EGFR mutation after primary resistance to crizotinib and response to EGFR-tyrosine kinase inhibitor. Onco Targets Ther (2016) 9:211–5. doi: 10.2147/OTT.S97100
7. McCoach CE, Le AT, Gowan K, Jones K, Schubert L, Doak A, et al. Resistance mechanisms to targeted therapies in ROS1+ and ALK+ non–small cell lung cancer. Clin Cancer Res (2018) 24(14):3334–47. doi: 10.1158/1078-0432.CCR-17-2452
8. Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res (2016) 22(22):5527–38. doi: 10.1158/1078-0432.CCR-16-0569
9. Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun (2017) 8:14768. doi: 10.1038/ncomms14768
10. Wang Y, Yang N, Zhang Y, Li L, Han R, Zhu M, et al. Effective treatment of lung adenocarcinoma harboring EGFR-activating mutation, T790M, and cis-C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol 15(8):1369–75. doi: 10.1016/j.jtho.2020.04.014
11. Stinchcombe TE, Doebele RC, Wang X, Gerber DE, Horn L, Camidge DR. Preliminary clinical and molecular analysis results from a single-arm phase 2 trial of brigatinib in patients with disease progression after next-generation ALK tyrosine kinase inhibitors in advanced ALK+ NSCLC. J Thorac Oncol (2021) 16(1):156–61. doi: 10.1016/j.jtho.2020.09.018
12. Nishio M, Yoshida T, Kumagai T, Hida T, Toyozawa R, Shimokawaji T, et al. Brigatinib in Japanese patients with ALK-positive NSCLC previously treated with alectinib and other tyrosine kinase inhibitors: outcomes of the phase 2 J-ALTA trial. J Thorac Oncol (2021) 16(3):452–63. doi: 10.1016/j.jtho.2020.11.004
13. Ou SI, Nishio M, Ahn MJ, Mok T, Barlesi F, Zhou C, et al. Efficacy of brigatinib in patients with advanced ALK-positive NSCLC who progressed on alectinib or ceritinib: ALK in lung cancer trial of brigAtinib-2 (ALTA-2). J Thorac Oncol (2022) 17(12):1404–14. doi: 10.1016/j.jtho.2022.08.018
14. Miyawaki M, Yasuda H, Tani T, Hamamoto J, Arai D, Ishioka K, et al. Overcoming EGFR bypass signal-induced?acquired resistance to ALK tyrosine kinase inhibitors in ALK-translocated lung cancer. Mol Cancer Res (2017) 15(1):106–14. doi: 10.1158/1541-7786.MCR-16-0211
15. Hou H, Sun D, Zhang C, Liu D, Zhang X. ALK rearrangements as mechanisms of acquired resistance to osimertinib in EGFR mutant non-small cell lung cancer. Thorac Cancer (2021) 12(6):962. doi: 10.1111/1759-7714.13817
Keywords: non-small cell lung cancer, ALK rearrangement, tyrosine kinase inhibitors, resistance mutation, EGFR
Citation: Michaux L, Perrier A, Mehlman C, Alshehhi H, Dubois A, Lacave R, Coulet F, Cadranel J and Fallet V (2023) Therapeutic strategies to overcome EGFR mutations as acquired resistance mechanism in ALK-rearranged non-small-cell lung cancer: Case Reports. Front. Oncol. 13:1182558. doi: 10.3389/fonc.2023.1182558
Received: 08 March 2023; Accepted: 30 May 2023;
Published: 28 June 2023.
Edited by:
Hongda Liu, Nanjing Medical University, ChinaReviewed by:
Alessandro Morabito, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyCopyright © 2023 Michaux, Perrier, Mehlman, Alshehhi, Dubois, Lacave, Coulet, Cadranel and Fallet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lionel Michaux, bGlvbmVsbWljaGF1eDg5QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.