- 1Department of Neurosurgery, Jiange People’s Hospital, Jiange, Sichuan, China
- 2Department of Neurosurgery, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, Sichuan, China
- 3Department of Pathology, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, Sichuan, China
Background: Choroid plexus papilloma (CPP) is rare and even rarer in infants and young children, and it usually occurs in the ventricles. Due to the physical peculiarities of infants, tumor removal by microscopic or endoscopic surgery alone is difficult.
Case Presentation: A 3-month-old patient was found to have an abnormally enlarged head circumference for 7 days. Cranial magnetic resonance imaging (MRI) examination revealed a lesion in the third ventricle. The patient underwent combined microscopic and endoscopic “chopstick” technique to remove the tumor. He recovered well after the surgery. Postoperative pathological examination revealed CPP. Postoperative MRI suggested total resection of the tumor. Follow-up for 1 month showed no recurrence or distant metastasis.
Conclusions: Combined microscopic and endoscopic “chopstick” technique may be a suitable approach to remove tumors in infant ventricles.
Introduction
Choroid plexus papilloma (CPP) is a neuroectodermal tumor that accounts for less than 1% of intracranial tumors (1). CPP is even less frequent in infants. CPP is most often located in the lateral ventricles, with few reported cases in the third and fourth ventricles (2). Typical clinical manifestations of CPP include hydrocephalus, macrocephaly, and growth retardation (3, 4). The most common treatment for CPP is surgery. Transcallosal or transcortical approach are used to remove CPP located in the lateral ventricles. The corpus callosum approach is usually used to remove CPP located in the third ventricle. The blood supply of CPP is rich, and CPP is poorly demarcated from the surrounding tissues, which makes it difficult to remove the tumor with gross total resection. In particular, low tolerance to blood loss in infants makes surgical removal of CPP more difficult. With the advances in neurosurgical microscopy and improvement in endoscopic techniques, it has become possible for neurosurgeons to remove huge tumors from the ventricles minimally-invasively and completely. The resection of ventricular tumor was performed using microscopy or traditional endoscopic techniques. Here, we report a case of a 3-month-old infant with CPP in the third ventricle that was treated by combined microscopic and “chopstick” technique. We also conducted a systematic review of cases with CPP in infants reported after 2010.
Case presentation
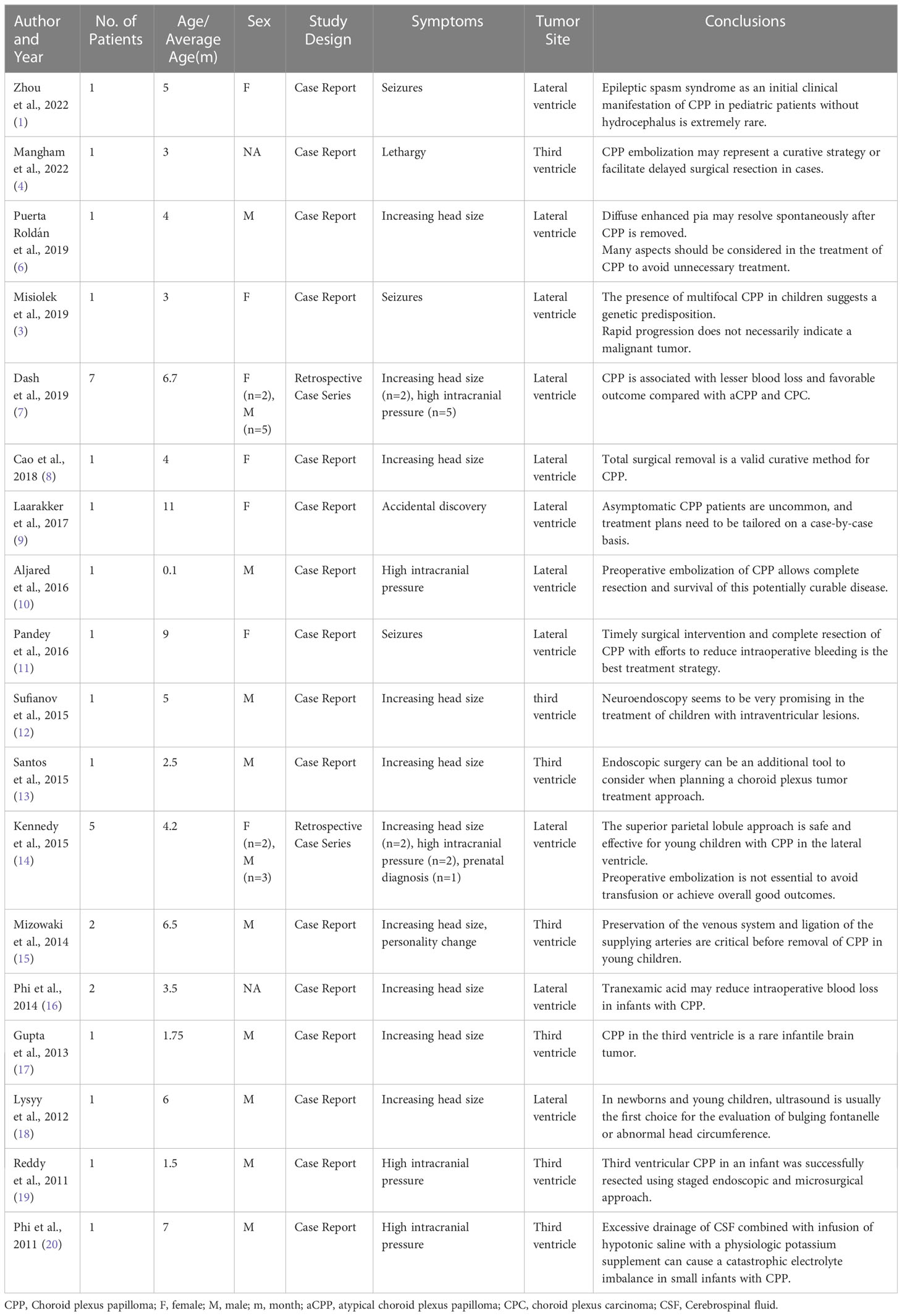
A 3-month-old patient presented with an abnormally large head circumference for 7 days. He was born vaginally at full term, without asphyxia or birth injuries, and was breastfed after birth. Growth and intelligence were appropriate for his age. On examination, the fontanelle pressure was high, the head circumference was 45 cm, and the eyes were gazing downward. Ultrasound examination performed at other hospitals suggested hydrocephalus. Cranial magnetic resonance imaging (MRI) showed the presence of a space-occupying lesion in the third ventricle, about 3.8×4.6×3.1 cm (3) in size, isosignal at T1 and mild high signal at T2-weighted images, accompanied with severe hydrocephalus (Figures 1A, B). Contrast-enhanced lesions showed “cauliflower-like” appearance on MRI (Figures 1C, D). Right frontal cortical fistula was performed to remove a part of the tumor under the microscope, and the tumor in the blind area of the microscope’s visual field was removed by the endoscopic “chopstick” technique (Figures 2A-C, E, F). The child recovered well after surgery (Figure 2D).Histopathological examination of the resected tumor papillary-like architecture that has fibrovascular cores, resulting in a diagnosis of CPP (WHO Grade 1) (Figure 3C). After 1 month of follow-up, MRI indicated no residual tumor (Figures 3A, B).
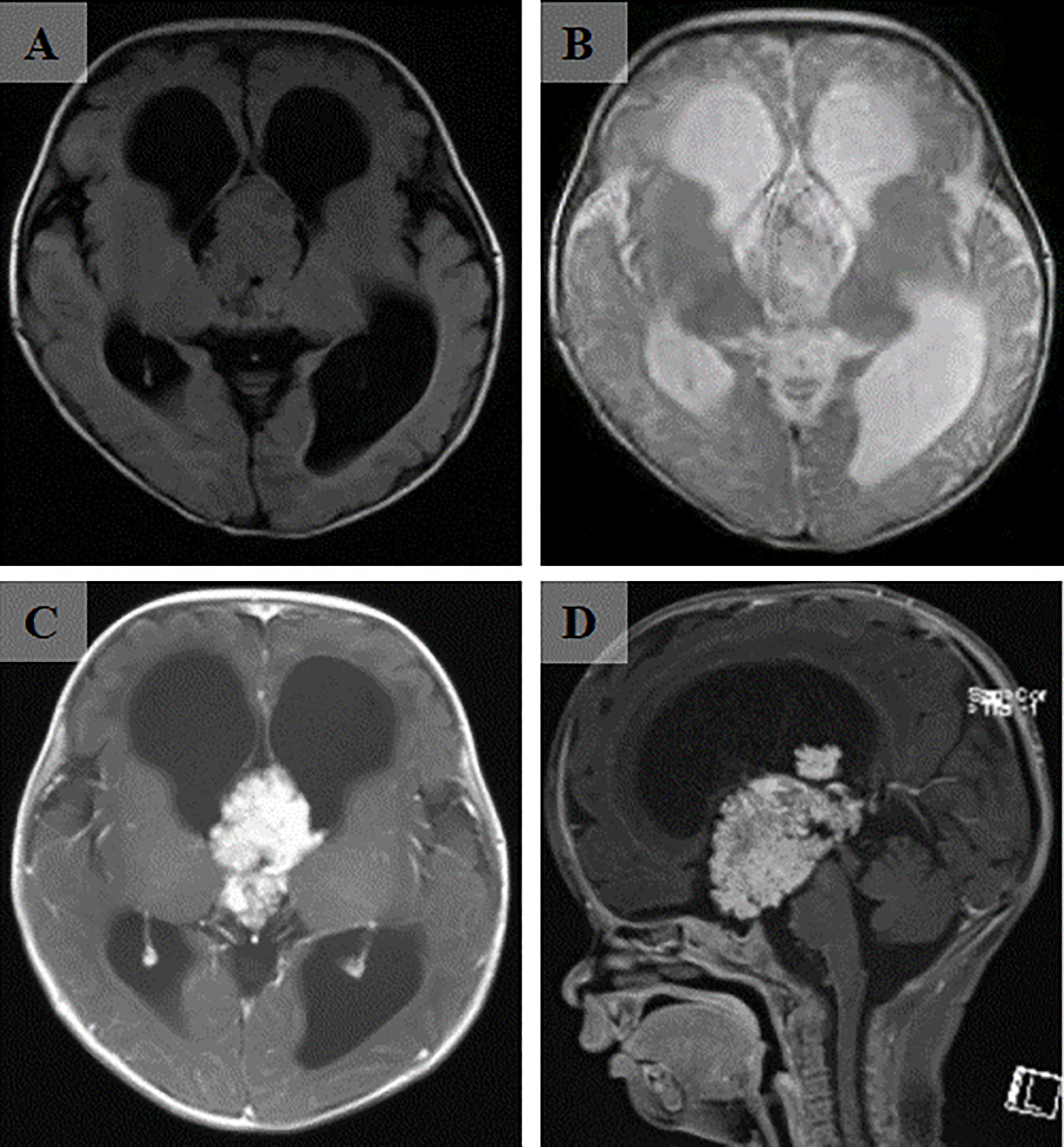
Figure 1 The cranial MRI of the head revealed a massive mass in the third ventricle. (A) The lesion was isointense on T1-weighted image. (B) The lesion was hyperintense on T2-weighted image. (C) After enhancement injection, the lesion in the third ventricle was significantly enhanced. (D) Sagittal view showed a “cauliflower-shaped” lesion after enhancement.
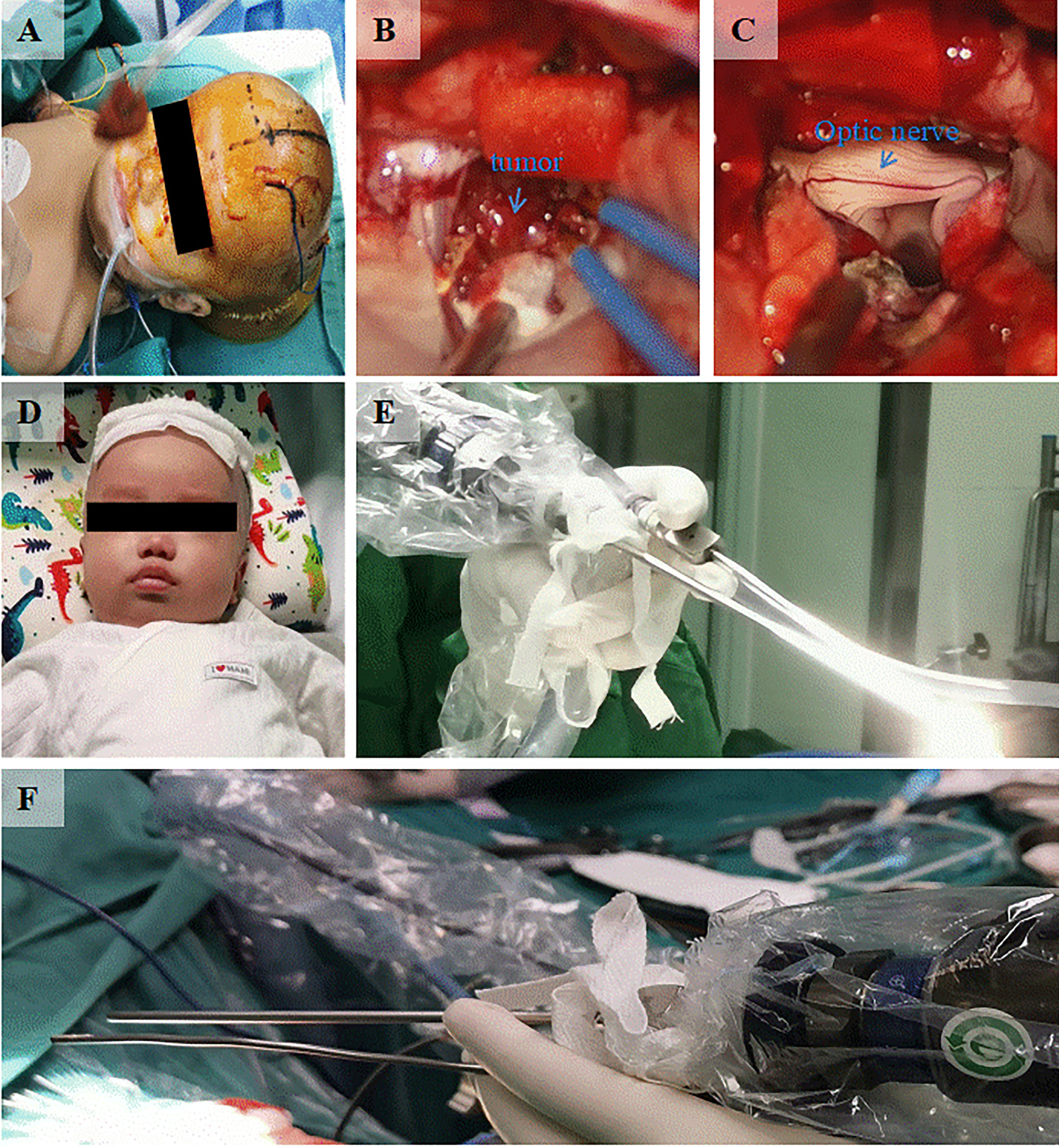
Figure 2 Intraoperative and postoperative conditions. (A) Appearance of the head during the surgical treatment. (B) Microscopic resection of the tumor by right frontal cortex fistula. (C) Exposure of optic nerve revealed after the tumor resection by microscope and endoscopic “chopstick” technique. (D) The recovery of the infant before discharge from the hospital. (E) Front view of the endoscopic “chopstick” technique. (F) Behind view of the endoscopic “chopstick” technique.
Figure 3 (A, B) Postoperative cranial MRI scan indicates total tumor resection. (C) Micrograph shows tumor papillary-like architecture that has fibrovascular cores (H and E, ×200).
Systematic review of literature
Our literature search was based on the Preferred Reporting Items for Systematic Evaluation and Meta-Analysis (PRISMA) (5). We systematically searched reports in the English language from PubMed and EMBASE databases since 2010 using the Boolean operators with the following keywords: “Choroid plexus papilloma,” “infant,” “baby,” “neonatal,” and “young.” Articles with sufficient information for postoperative pathological diagnosis of CPP in individuals under the age of 12 months were included. Articles that replicated, reviewed, or lacked detailed clinical data were excluded. Cases with postoperative pathological diagnoses of atypical CPP (aCPP) and choroid plexus carcinoma (CPC) were excluded. All identified articles were first screened by title and abstract, and then the full text was downloaded and assessed for eligibility. This process was carried out independently by three investigators. Any disagreements were resolved by consensus.
Results
Sixteen duplicate records were removed by database search. A total of 197 records were left for title and abstract checking. Forty full-text articles were evaluated. Eighteen articles were finally included for analysis (Table 1; Figure 4) (1, 3, 4, 6–20). The majority of the articles were case reports, and included a total of 30 cases with a pathological diagnosis of CPP. There were 18 males and nine females among the 30 patients, and sex was unknown in three cases. The minimum age was 3 days. The clinical manifestations of CPP were mainly enlarged head circumference (n=13) and high intracranial pressure (n=10). Twenty-two CPP cases were located in the lateral ventricle, while eight cases were located in the third ventricle. All 30 children were treated surgically, including 21 with microscopic resection alone, three with endoscopic resection alone, two with interventional embolization followed by microscopic resection, one with combined traditional endoscopic and microscopic treatment followed by cerebrospinal fluid shunt, and three with unspecified surgical modalities. The use of microscopy combined with the endoscopic “chopstick” technique had not been reported.
Discussion
CPP is a rare intracranial tumor that is classified as grade I by the WHO tumor classification. In pediatric patients, CPP usually occurs within 1 year of birth and is most commonly located in the lateral ventricle (64%) (21). According to our literature review, under the age of 1 year, CPP was located in the lateral ventricle in 73.3% of cases. Given that the location of CPP affects the cerebrospinal fluid circulation, hydrocephalus is often complicated in imaging. On computed tomography (CT) scans, CPP usually appears as isodense or hyperdense. MRI shows equal or low T1 signal and high T2 signal (2). In addition to surgical removal of the tumor, cerebrospinal fluid shunts or interventional embolization can be performed preoperatively (10). The most common surgical treatment for CPP is transcortical approach by a microscope (22). The survival rate after surgical resection of CPP is high, and it has been reported that the long-term survival rate ranges from 90% to 100% with gross total resection (11). For infant patients with a high surgical risk, preoperative tumor interventional embolization has been proposed (4, 10). Preparation of cerebrospinal fluid shunt alone may also complement CPP treatment (19). Because lesions are often located within the ventricle, microscope alone has a limited field of vision, so endoscopy can be used as a surgical supplement. Simple endoscopic resection of ventricular lesions alone is commonly used for tumors of smaller size. The endoscopic system is demanding for the operator and often requires skilled coordination and cooperation between the primary surgeon and assistant, and poor coordination may even increase the risk of additional procedures for the patient. Manickavasagam proposed the “chopstick” technique in 2010 (23). Labidi further elaborated the application of “chopstick” technique in 2018, in which the operator holds the endoscope and a suction device in one hand, while the other hand holds the instrument to perform various operations (24). This technique reduces the space required for surgery, alleviates the disadvantages of unstable holding of the mirror by the assistant for complex surgery, and avoids uncoordinated operation between the operator and assistant (24).
In our report, the lesion was first resected using the microscopic access through the frontal cortex, and then the tumor in the blind area of the microscope’s visual field was removed by endoscopic “chopstick” technique to achieve complete resection. In the “chopstick” technique, the endoscope and suction device are held in the left hand. The light source connector of the endoscope is placed between the thumb and ring fingers, and the direction can be changed as required during the operation. The suction device is placed between the index and middle fingers. A 0° or 30° endoscope can be used depending on the location of the lesion. This provides a new method for total resection of ventricle tumors with minimal trauma. Clearly, as this is a case report only, more studies with appropriate design and larger size are needed to provide stronger evidence level.
Conclusions
Microscope combined with endoscopic “chopstick” technique may be a new way to remove the tumors in the ventricle.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Investigation: WL, CW, XY. Methodology: HL, ZL. Project administration: HL, WL. Resources: HL, ZL. Supervision: HL. Histopathological examination: YJ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Foundation Program of Sichuan Provincial Health Commission (No. 21PJ181).
Acknowledgments
We would like to thank the parents of the child in this report for giving oral and written informed consent for the reporting and publication of the case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou F, Li Y, Shen L, Yao H, Hou X. Infantile epileptic spasms syndrome as an initial presentation in infantile choroid plexus papilloma: a case report. Front Pediatr (2022) 10:1035621. doi: 10.3389/fped.2022.1035621
2. Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int (2015) 6:151. doi: 10.4103/2152-7806.166167
3. Misiolek KA, Osborn ZG, Hauser N, Thomas D, Goodman JF, Fulkerson DH. Rapidly growing, multifocal, benign choroid plexus tumor in an infant: case report. J Neurosurg Pediatr (2019) 22:1. doi: 10.3171/2018.12.PEDS18453
4. Mangham WM, Elijovich L, Lee-Diaz JA, Orr BA, Gienapp AJ, Boop FA. Pre-operative embolization for staged treatment of infantile choroid plexus papilloma. Child’s nervous system (2022) 38(2):429–33. doi: 10.1007/s00381-021-05212-w
5. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Flow diagram template (2020). Available at: https://journalsplosorg/plosmedicine/article/figure?id=101371/journalpmed1003583g001.
6. Puerta Roldán P, Santa-María López V, Morales La Madrid A, Cruz O, Muchart J, Thomas C, et al. Vanishing diffuse leptomeningeal contrast enhancement in an infant with choroid plexus papilloma. Acta neurochirurgica (2019) 161(2):351–4. doi: 10.1007/s00701-018-03781-5
7. Dash C, Moorthy S, Garg K, Singh PK, Kumar A, Gurjar H, et al. Management of choroid plexus tumors in infants and young children up to 4 years of age: an institutional experience. World Neurosurg (2019) 121:e237–45. doi: 10.1016/j.wneu.2018.09.089
8. Cao LR, Chen J, Zhang RP, Hu XL, Fang YL, Cai CQ. Choroid plexus papilloma of bilateral lateral ventricle in an infant conceived by in vitro fertilization. Pediatr Neurosurg (2018) 53(6):401–6. doi: 10.1159/000491639
9. Laarakker AS, Nakhla J, Kobets A, Abbott R. Incidental choroid plexus papilloma in a child: a difficult decision. Surg Neurol Int (2017) 8:86. doi: 10.4103/sni.sni_386_16
10. Aljared T, Farmer JP, Tampieri D. Feasibility and value of preoperative embolization of a congenital choroid plexus tumour in the premature infant: an illustrative case report with technical details. Interventional neuroradiol (2016) 22(6):732–5. doi: 10.1177/1591019916665346
11. Pandey S, Sharma V, Singh K, Ghosh A, Gupta PK. Uncommon presentation of choroid plexus papilloma in an infant. J Pediatr Neurosci (2016) 11(1):61–3. doi: 10.4103/1817-1745.181254
12. Sufianov AA, Gaibov SS, Sufianov RA. Endoscopic monoportal removal of a choroid plexus papilloma in the posterior third ventricle in a child. J Neurosurg Pediatr (2015) 16(1):107–11. doi: 10.3171/2014.12.PEDS14306
13. Santos MM, Souweidane MM. Purely endoscopic resection of a choroid plexus papilloma of the third ventricle: case report. J Neurosurg Pediatr (2015) 16(1):54–7. doi: 10.3171/2014.12.PEDS14287
14. Kennedy BC, Cloney MB, Anderson RC, Feldstein NA. Superior parietal lobule approach for choroid plexus papillomas without preoperative embolization in very young children. J Neurosurg Pediatr (2015) 16(1):101–6. doi: 10.3171/2014.11.PEDS14281
15. Mizowaki T, Nagashima T, Yamamoto K, Kawamura A, Yoshida M, Kohmura E. Optimized surgical approach to third ventricular choroid plexus papillomas of young children based on anatomical variations. World Neurosurg (2014) 82(5):912.e15–9. doi: 10.1016/j.wneu.2013.03.011
16. Phi JH, Goobie SM, Hong KH, Dholakia A, Smith ER. Use of tranexamic acid in infants undergoing choroid plexus papilloma surgery: a report of two cases. Paediatric anaesthesia (2014) 24(7):791–3. doi: 10.1111/pan.12447
17. Gupta P, Sodhi KS, Mohindra S, Saxena AK, Das A, Khandelwal N. Choroid plexus papilloma of the third ventricle: a rare infantile brain tumor. J Pediatr Neurosci (2013) 8(3):247–9. doi: 10.4103/1817-1745.123696
18. Lysyy O, Puzhevsky A, Strauss S. Choroid plexus papilloma in an infant: ultrasound diagnosis. Eur J Pediatr (2012) 171(11):1717–8. doi: 10.1007/s00431-012-1842-1
19. Reddy D, Gunnarsson T, Scheinemann K, Provias JP, Singh SK. Combined staged endoscopic and microsurgical approach of a third ventricular choroid plexus papilloma in an infant. Minimally invasive neurosurgery: MIN (2011) 54(5-6):264–7. doi: 10.1055/s-0031-1287775
20. Phi JH, Shin CH, Wang KC, Park SH, Kim SK. Catastrophic electrolyte imbalance caused by excessive production and overdrainage of cerebrospinal fluid in an infant with choroid plexus papilloma. Child’s nervous system (2011) 27(7):1153–6. doi: 10.1007/s00381-011-1459-0
21. Basindwah SA, Alzahrani BS, Ajlan AM, Alkhalidi H. Persistence of communicating hydrocephalus post choroid plexus tumor resection: case reports and review of literature. Surg Neurol Int (2021) 12:483. doi: 10.25259/SNI_681_2021
22. Malomo TA, Okolo CA, Balogun JA. Endoscopic assisted, transfontanelle excision of a Large third ventricular atypical choroid plexus papilloma in an infant. J West Afr Coll Surgeons (2018) 8(4):136–50.
23. Manickavasagam J, Segaram S, Harkness P. Functional endoscopic sinus surgery chopstick technique. Laryngoscope (2010) 120(5):975–7. doi: 10.1002/lary.20862
Keywords: microscopic, “chopstick” technique, choroid plexus papilloma, infant, endoscopic
Citation: Li W, Li Z, Wei C, Yang X, Ji Y and Liu H (2023) Microscopic and endoscopic “chopstick” technique removal of choroid plexus papilloma in the third ventricle of an infant: a case report with systematic review of literature. Front. Oncol. 13:1182261. doi: 10.3389/fonc.2023.1182261
Received: 08 March 2023; Accepted: 12 June 2023;
Published: 26 June 2023.
Edited by:
Ignazio Gaspare Vetrano, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Cecilia Casali, IRCCS Carlo Besta Neurological Institute Foundation, ItalyElio Mazzapicchi, IRCCS Carlo Besta Neurological Institute Foundation, Italy
Copyright © 2023 Li, Li, Wei, Yang, Ji and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyuan Liu, dWpveTEwMEB0b20uY29t
†These authors have contributed equally to this work
 Weiwei Li1†
Weiwei Li1† Yuzhu Ji
Yuzhu Ji Hongyuan Liu
Hongyuan Liu