- 1Department of Endoscopy, Shijiazhuang Traditional Chinese Medicine Hospital, Shijiazhuang, China
- 2Department of Gastroenterology, Shijiazhuang Traditional Chinese Medicine Hospital, Shijiazhuang, China
- 3Institute of Traditional Chinese Medicine, North China University of Science and Technology, Tangshan, China
- 4Graduate School, Hebei North University, Zhangjiakou, China
The present study was to explore the association between lipoprotein(a) [Lp(a)] and colorectal cancer (CRC) among inpatients. This study included 2822 participants (393 cases vs. 2429 controls) between April 2015 and June 2022. Logistic regression models, smooth curve fitting, and sensitivity analyses were performed to investigate the relationship between Lp(a) and CRC. Compared with the lower Lp(a) quantile 1 (<79.6 mg/L), the adjusted odds ratios (ORs) in quantile 2 (79.6-145.0 mg/L), quantile 3 (146.0-299.0 mg/L), and quantile 4 (≥300.0 mg/L) were 1.41 (95% confidence interval [CI]: 0.95–2.09), 1.54 (95% CI: 1.04–2.27), 1.84 (95% CI: 1.25–2.7), respectively. A linear relationship between lipoprotein(a) and CRC was observed. The finding that Lp(a) has a positive association with CRC supports the “common soil” hypothesis of cardiovascular disease (CVD) and CRC.
1 Introduction
CRC is the third most prevalent and second most fatal cancer worldwide, responsible for approximately one in ten cancer cases and deaths in 2020, and is a significant burden on health systems (1). Current evidence supports the so-called “common soil” hypothesis in the pathogenesis of CVD and CRC (2–7), implying that the two conditions share several pathophysiological mechanisms and risk factors. Due to the thrombogenic and atherogenic properties of Lp(a) (8, 9), a prospective cohort study has shown that a high Lp(a) level is a risk factor for CVD. Therefore, we proposed the hypothesis that Lp(a) may be associated with CRC.
Lp(a) is made up of a low-density lipoprotein (LDL) core, which is produced by the liver, and an apolipoprotein B-100 molecule, which is covalently bonded to apolipoprotein(a) [Apo(a)] (10). The Lp(a) level is genetically determined, only slightly influenced by age, gender, and environmental factors, and is stable in healthy (11, 12). Recently, the role of Lp(a) in tumors has attracted increasing attention due to its potential role in tumor angiogenesis, which is a key step in tumor expansion and metastasis (13–18). However, experimental studies have also reported anti-angiogenic and anti-tumor effects (14, 19, 20).
The association between Lp(a) and CVD is well documented (21–25), while the relationship between Lp(a) and cancers (including breast, lung, prostate, colorectal, and liver cancers) has been reported (26–30), but is sparse and controversial. Furthermore, the relationship between lipoprotein(a) and CRC has not been reported in the study with a large sample size.
Given the unclear relationship between Lp(a) and CRC, we conducted the present study based on clinical data from a tertiary hospital in northern China, to explore the association between Lp(a) and CRC.
2 Materials and methods
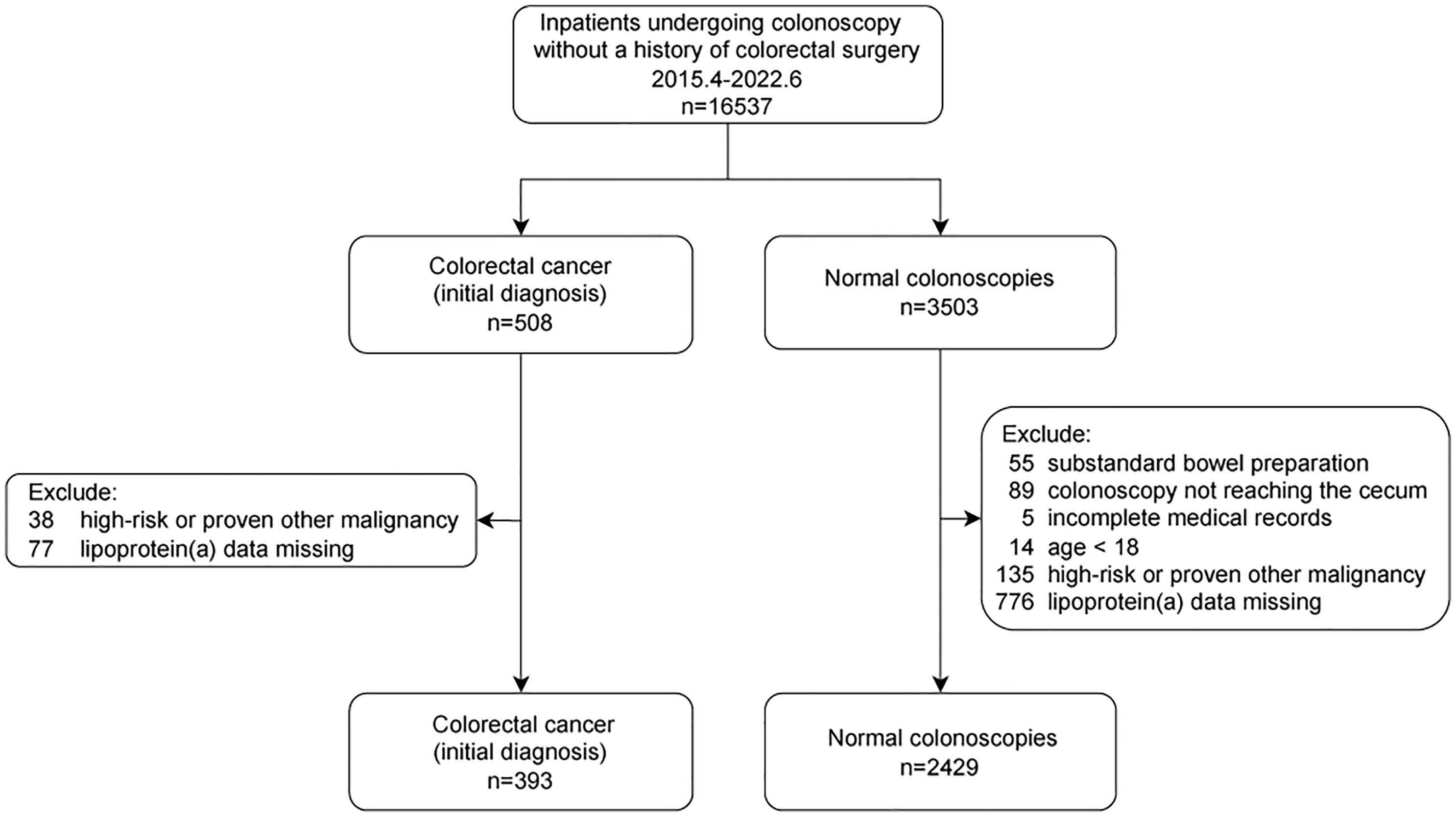
All consecutive inpatients who underwent colonoscopies were included between April 2015 and June 2022. Each patient was included only once. Subjects with CRC (initial diagnosis) or normal colonoscopy were considered cases or controls, respectively. The detailed inclusion and exclusion criteria are shown in Figure 1. Finally, 2822 individuals (393 individuals with CRC vs. 2429 controls) were enrolled.
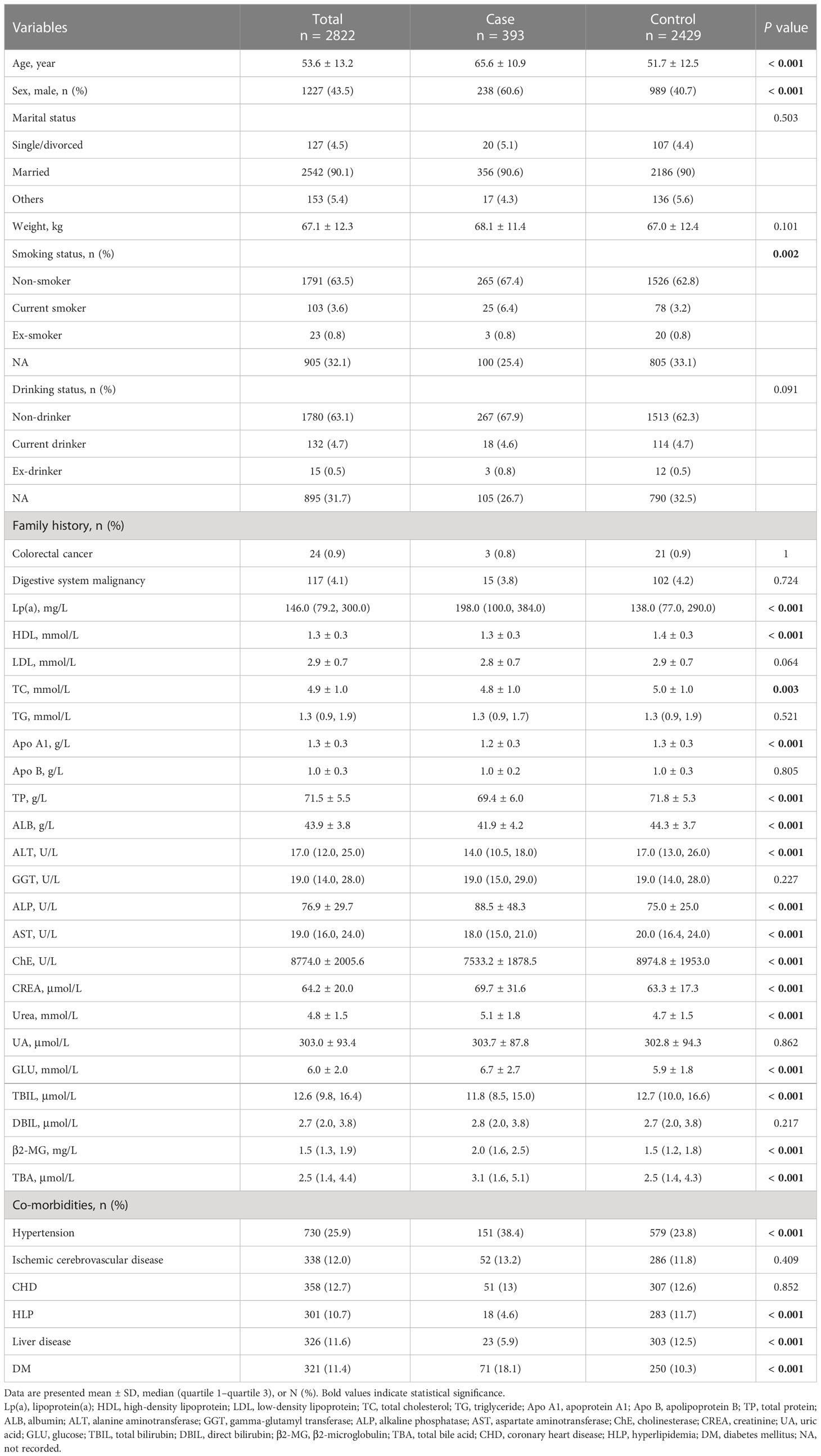
Several potential covariates were extracted from the laboratory information system (LIS) and hospital information system (HIS) at Shijiazhuang Traditional Chinese Medicine Hospital, including demography, co-morbidities, and laboratory data. Details are shown in Table 1. Classification of marital, drinking, and smoking statuses were described in our previous study, as well as liver disease (31). Laboratory indicators were extracted from the first test results during hospitalization.
Continuous data were presented as mean ± standard deviation or median (Q1–Q3) values. The Mann-Whitney U test or Student’s t test for continuous variables and the chi-squared test for categorical variables were performed.
The effect of Lp(a) on CRC was investigated using logistic regression models. To further evaluate the impact of Lp(a), the Lp(a) level (mg/L) was divided into quartiles: Q1 (<79.6), Q2 (79.6-145.0), Q3 (146.0-299.0), and Q4 (≥300.0). We constructed three models: (1) crude model; (2) adjusted for gender and age; and (3) adjusted for gender, age, weight, drinking status, smoking status, marital status, family history of CRC, albumin (ALB), alanine aminotransferase (ALT), β2-microglobulin (β2-MG), high-density lipoprotein (HDL), total cholesterol (TC), hypertension, and diabetes mellitus (DM). These potential confounders were selected based on previous studies or a change in effect estimate of more than 10%. Stratified binary logistic regression model and testing for interactions were used to analyze subgroups. Smooth curve fitting and propensity score matching (PSM) were performed to investigate the association between Lp(a) and CRC. Participants were matched for a fully model using a one-to-one nearest neighbor technique with a calliper width of 0.2. Furthermore, sensitivity analysis was conducted using all complete cases. Based on 5 replications and a chained equation approach method in the R mice procedure, multiple imputations were used to minimize bias and maximize statistical power that might occur to account for missing data.
Data analyses were performed with the statistical software packages R 3.3.2 (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.7.1. P<0.05 was considered statistically significant (two-tailed).
3 Results
3.1 Baseline characteristics
The present study enrolled 503 individuals with CRC and 3503 controls. 1189 individuals were excluded owing to incomplete medical records (n=5), substandard bowel preparation (n=55), colonoscopy note reaching the cecum (n=89), missing data (n=853), age<18 (n=14), high-risk or proven other malignancy (n=173). Consequently, 2822 individuals were included in this study (case: control = 393: 2429). The flowchart is shown (Figure 1).
The detailed characteristics are available in Table 1. Some significant differences were shown in some variables, including age, sex, smoking status, Lp(a), HDL, TC, apolipoprotein A1 (Apo A1), total protein (TP), ALB, ALT, alkaline phosphatase (ALP), aspartate aminotransferase (AST), cholinesterase (ChE), creatinine (CREA), urea, glucose (GLU), total bilirubin (TBIL), direct bilirubin (DBIL), β2-MG, total bile acid (TBA), hypertension, hyperlipidemia (HLP), liver disease, and DM.
3.2 Relationship between Lp(a) and CRC
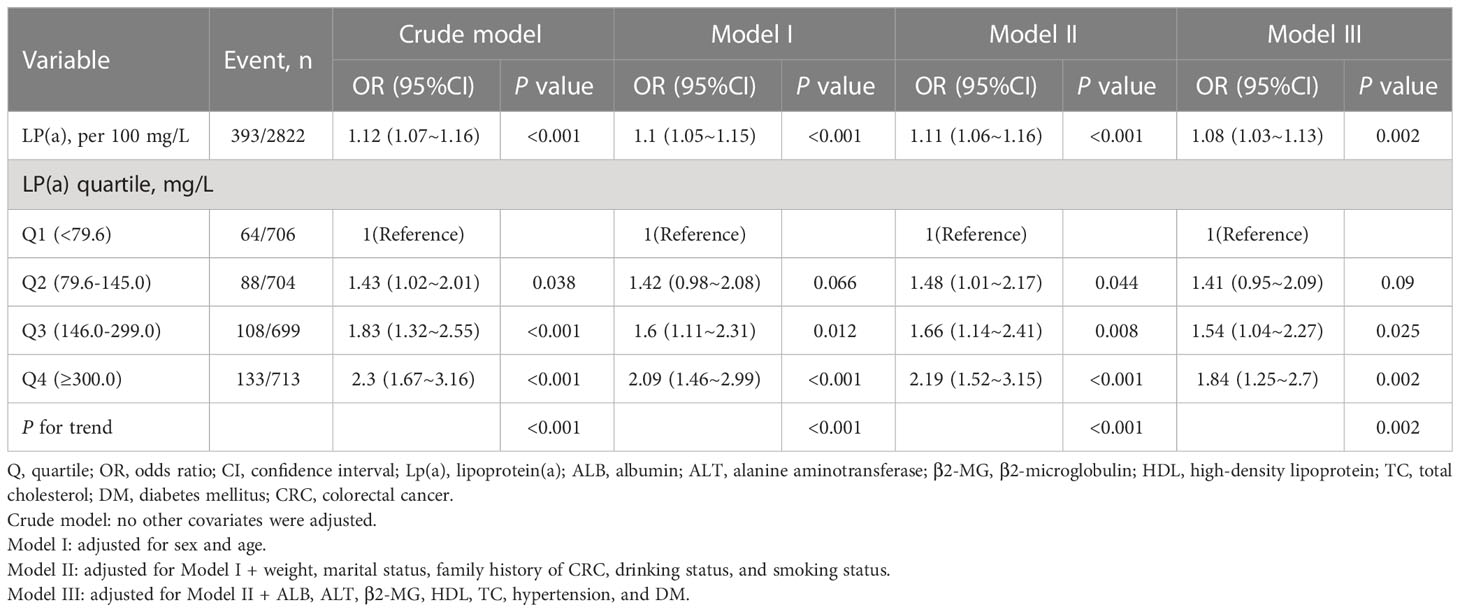
Multivariable logistic regression analyses were performed to assess the associations between Lp(a) and CRC (Table 2). When Lp(a) was a continuous variable, in Model 1 adjusted for gender and age, Lp(a) was positively related to CRC (Lp(a) per 100 mg/L, OR: 1.1, 95% CI: 1.05–1.15, P<0.001). Even after adjusting for more potential covariates (Model 2-3), the association remained stable (Lp(a) per 100 mg/L; Model 2: OR, 1.11 95% CI, 1.06–1.16, P<0.001; Model 3: OR, 1.08, 95% CI, 1.03–1.13, P=0.002). Overall, in all models (Crude model and Model 1-3), the risk of advanced colorectal adenomas increased as the level of Lp(a) increased.
When Lp(a) was analyzed in terms of quartiles, there was a positive association between Lp(a) and CRC. Compared with the lower Lp(a) Q1 (<79.6 mg/L), the adjusted ORs in Q2 (79.6-145.0 mg/L), Q3 (146.0-299.0 mg/L), and Q4 (≥300.0 mg/L) were 1.41 (95% CI: 0.95–2.09), 1.54 (95% CI: 1.04–2.27), 1.84 (95% CI: 1.25–2.7), respectively (Table 2). Furthermore, we observed a linear relationship between Lp(a) and CRC among inpatients after adjusting for several covariates (Figure 2, only 99% of the data is shown).
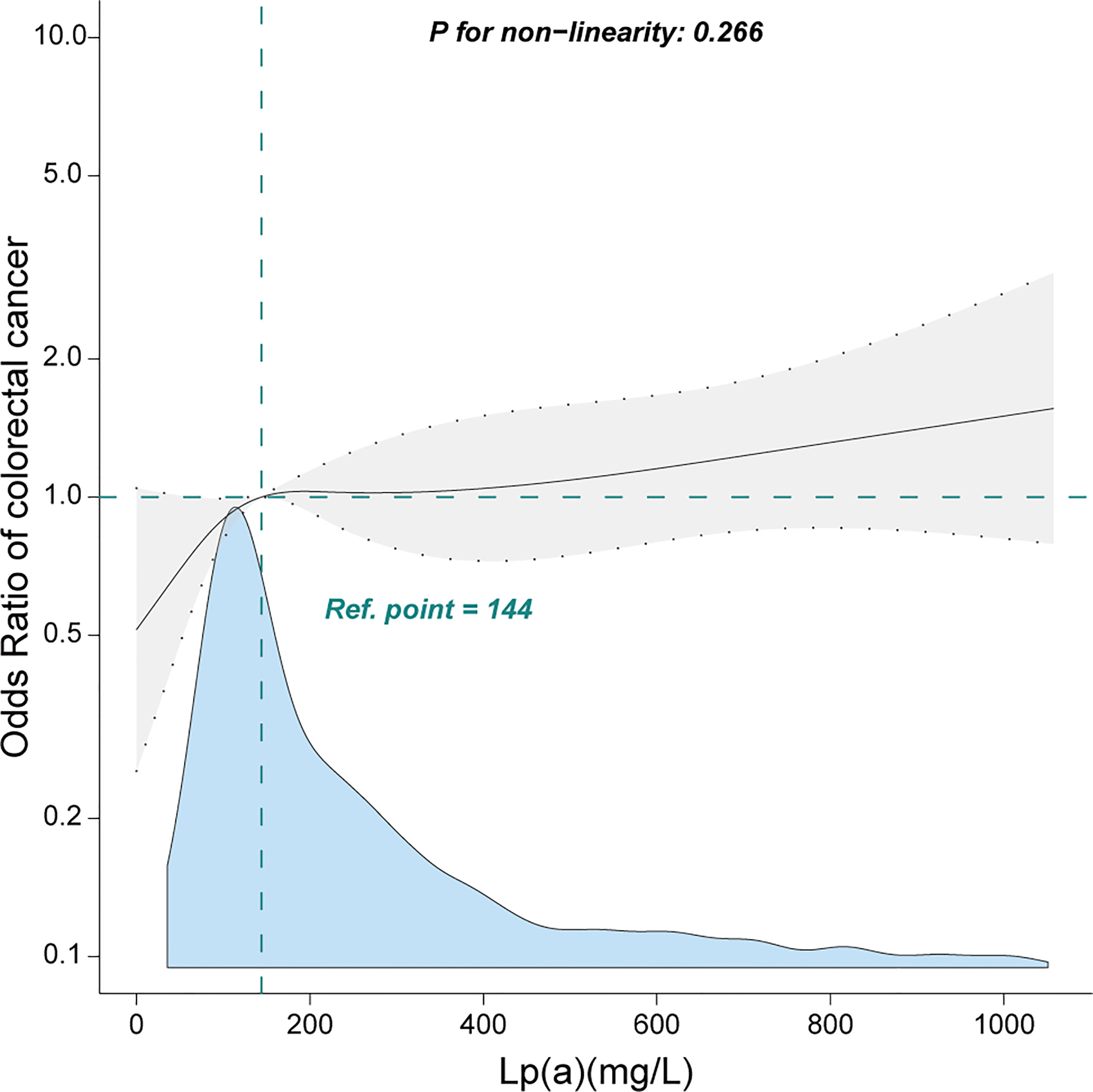
Figure 2 Linear relationship between lipoprotein(a) and colorectal cancer among inpatients. Adjustment factors included gender, age, weight, marital status, drinking status, smoking status, family history of CRC, albumin, alanine aminotransferase, β2-microglobulin, high-density lipoprotein, total cholesterol, hypertension, and diabetes mellitus. Only 99% of the data is shown.
3.3 Sensitivity analysis
Stratified analyses were conducted to investigate potential effects on the association between Lp(a) and CRC. No significant interactions were presented after stratifying by sex, age (<65 years and ≥65 years), drinking status, ischemic cerebrovascular disease, hypertension, liver disease, HLP, coronary heart disease (CHD), and DM (Figure 3). Given multiple testing, the P value of < 0.05 for the interaction in hypertension and DM subgroups may not be statistically significant. There remained 2604 participants after excluding those with missing data, and the relationship between Lp(a) and CRC remained stable in the sensitivity analysis (Supplementary Table 1). Furthermore, propensity score matching analysis was performed in this study (Supplementary Tables 2, 3) and indicated the relationship between Lp(a) and CRC remained stable.
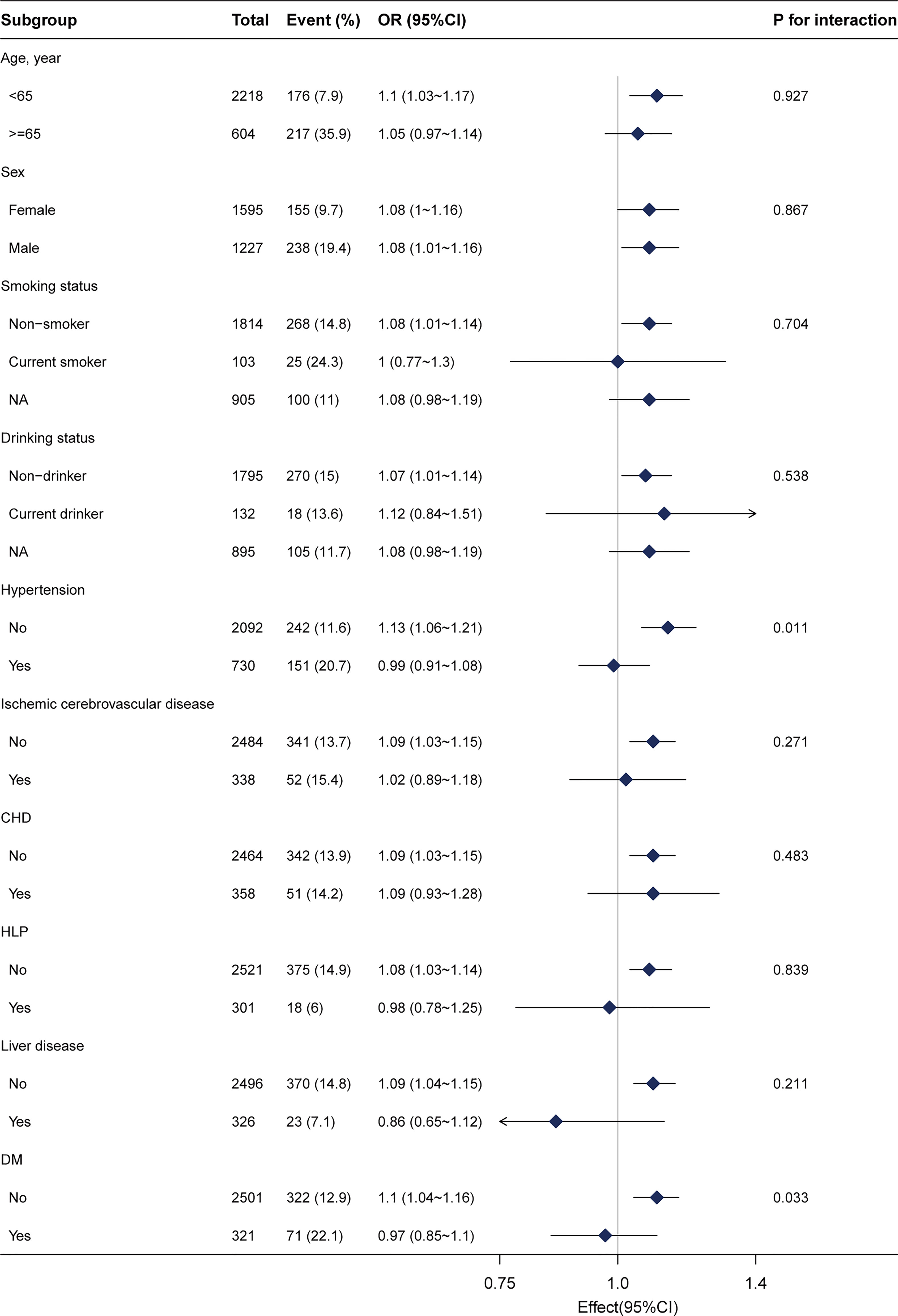
Figure 3 Subgroup analysis of the lipoprotein(a) and colorectal cancer among inpatients. Each stratification factor was adjusted for gender, age, weight, marital status, drinking status, smoking status, family history of CRC, albumin, alanine aminotransferase, β2-microglobulin, high-density lipoprotein, total cholesterol, hypertension, and diabetes mellitus. CHD, coronary heart disease; HLP, hyperlipidemia; DM, diabetes mellitus; NA, not recorded.
4 Discussion
This study demonstrated the positive relationship between Lp(a) and CRC. Both subgroup and sensitivity analyses indicated that the relationship remained robust.
There is no doubt that lipid parameters are related to CVD (32–34). Recently, the relationship between Lp(a) and tumors has received much attention. Current studies have shown that Lp(a) was related to certain tumors. Several studies have reported higher levels of Apo(a) or Lp(a) in patients with breast, lung, and prostate cancers [21-23]. While patients with liver cancer had relatively low levels of Lp(a) [24], the reason considered is that the liver being the main site of Apo(a) synthesis, liver cancer affects the expression of Apo(a) protein and consequently the synthesis of Lp(a).
Only one prospective study investigated the relationship between Lp(a) and CRC (n=58), it appeared that the highest levels of Lp(a) had the highest risk of CRC, although there was no significant difference; however, this could not be interpreted as a linear or quadratic relationship (28). In contrast, this present study, which included a larger sample size and adjusted for more covariates, found a linear relationship between Lp(a) and CRC. This may be because they analyzed all cancer sites, in which the effect of type-specific or site-specific cancers may be diluted. To our knowledge, this study is the first study with a larger sample size that found a positive association of Lp(a) with CRC.
The potential mechanisms underlying the association between high Lp(a) and CRC are unclear and require further study. One explanation is that high Lp(a) is more likely to induce the formation of fibrin networks and thrombi, promoting cancer cell adhesion, invasion, and metastasis. This is due to the structural similarity of Lp(a) to fibrinogen and tissue fibrinogen activator and the fact that it competes with fibrinogen for its binding site, leading to reduced fibrinolysis. Another explanation is quite different, as some animal studies have found that the proteolytic breakdown products of Lp(a) have anti-tumor properties both in vivo and in vitro (27, 29, 30). Considering the anti-tumor effects of Lp(a), we suggest that a high Lp(a) level may be a compensatory response to systemic chronic inflammation caused by aggressive and invasive tumors. Insight into the role of Lp(a) in cancer may shed light on strategies to prevent metastasis (27).
Our findings contribute further data to the common risk factors of CVD and CRC. Studies have shown that lowering Lp(a) levels reduces cardiovascular risk (35–37). We speculate that lowering Lp(a) may reduce the risk of CRC. Current research suggests that several therapies that may reduce cardiovascular risks, such as lifestyle modifications, aspirin, statins, fibrates, and ezetimibe drugs, have little effect on Lp(a) (38, 39). Recent studies have found that the therapeutic agents available to reduce Lp(a) levels are lipoprotein apheresis, small interfering RNA agents, antisense oligonucleotides, and PCSK9 inhibitors (35–37, 40, 41). However, more effects and cardiovascular benefits need to be further investigated.
The study had some limitations. First, missing data is common in observational studies. However, sensitivity analyses indicated that the results were stable. Second, repeated measurements of Lp(a) were lacking in this study and may not be representative of the relationship between long-term levels of Lp(a) and CRC. Third, confounding by unknown or unmeasured factors cannot be completely ruled out despite logistic regression and sensitivity analyses. Finally, these findings were based on clinical data from a tertiary hospital in northern China and require a multi-center study.
5 Conclusion
There was a positive relationship between Lp(a) and CRC among inpatients in China. This finding contributes further data to the common risk factors of CVD and CRC and may provide new ideas for the screening or diagnosis of CRC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Review Board of Shijiazhuang Traditional Chinese Medicine Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
HW and HuZ conceived and designed the study; HW, XC, PM, ZW, TZ, and HaZ collected and analyzed the data; HW and HuZ wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Hebei Administration of Traditional Chinese Medicine (grant number 2023145).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1181508/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Chan AO, Jim MH, Lam KF, Morris JS, Siu DC, Tong T, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA (2007) 298(12):1412–9. doi: 10.1001/jama.298.12.1412
3. Donati MB. The “common soil hypothesis”: evidence from population studies? Thromb Res (2010) 125:S92–5. doi: 10.1016/S0049-3848(10)70023-2
4. Kenzik KM, Balentine C, Richman J, Kilgore M, Bhatia S, Williams GR. New-onset cardiovascular morbidity in older adults with stage i to iii colorectal cancer. J Clin Oncol (2018) 36(6):609–16. doi: 10.1200/jco.2017.74.9739
5. Brown JC, Caan BJ, Prado CM, Weltzien E, Xiao J, Cespedes Feliciano EM, et al. Body composition and cardiovascular events in patients with colorectal cancer: a population-based retrospective cohort study. JAMA Oncol (2019) 5(7):967–72. doi: 10.1001/jamaoncol.2019.0695
6. Keramida K, Charalampopoulos G, Filippiadis D, Tsougos E, Farmakis D. Cardiovascular complications of metastatic colorectal cancer treatment. J Gastrointest Oncol (2019) 10(4):797–806. doi: 10.21037/jgo.2019.03.04
7. Marchetti M, Falanga A. Hemostatic biomarkers in occult cancer and cancer risk prediction. Thromb Res (2020) 191:S37–42. doi: 10.1016/S0049-3848(20)30395-9
8. Jones GT, Van Rij AM, Cole J, Williams MJ, Bateman EH, Marcovina SM, et al. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin Chem (2007) 53(4):679–85. doi: 10.1373/clinchem.2006.079947
9. Rasouli M, Kiasari AM. Interactions of lipoprotein(a) with diabetes mellitus, apolipoprotein b and cholesterol enhance the prognostic values for coronary artery disease. Clin Chem Lab Med (2008) 46(5):667–73. doi: 10.1515/cclm.2008.137
10. Kostner KM, Kostner GM. Lipoprotein(a): still an enigma? Curr Opin Lipidol (2002) 13(4):391–6. doi: 10.1097/00041433-200208000-00006
11. Sandholzer C, Hallman DM, Saha N, Sigurdsson G, Lackner C, Császár A, et al. Effects of the apolipoprotein(a) size polymorphism on the lipoprotein(a) concentration in 7 ethnic groups. Hum Genet (1991) 86(6):607–14. doi: 10.1007/bf00201550
12. Berglund L, Ramakrishnan R. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol (2004) 24(12):2219–26. doi: 10.1161/01.Atv.0000144010.55563.63
13. Schulter V, Koolwijk P, Peters E, Frank S, Hrzenjak A, Graier WF, et al. Impact of apolipoprotein(a) on in vitro angiogenesis. Arterioscler Thromb Vasc Biol (2001) 21(3):433–8. doi: 10.1161/01.atv.21.3.433
14. Kim JS, Chang JH, Yu HK, Ahn JH, Yum JS, Lee SK, et al. Inhibition of angiogenesis and angiogenesis-dependent tumor growth by the cryptic kringle fragments of human apolipoprotein(a). J Biol Chem (2003) 278(31):29000–8. doi: 10.1074/jbc.M301042200
15. Yu HK, Ahn JH, Lee HJ, Lee SK, Hong SW, Yoon Y, et al. Expression of human apolipoprotein(a) kringles in colon cancer cells suppresses angiogenesis-dependent tumor growth and peritoneal dissemination. J Gene Med (2005) 7(1):39–49. doi: 10.1002/jgm.638
16. Lee K, Yun ST, Kim YG, Yoon Y, Jo EC. Adeno-associated virus-mediated expression of apolipoprotein (a) kringles suppresses hepatocellular carcinoma growth in mice. Hepatology (2006) 43(5):1063–73. doi: 10.1002/hep.21149
17. Lippi G, Franchini M, Salvagno GL, Guidi GC. Lipoprotein[a] and cancer: anti-neoplastic effect besides its cardiovascular potency. Cancer Treat Rev (2007) 33(5):427–36. doi: 10.1016/j.ctrv.2007.02.006
18. Onn A, Herbst RS. Angiogenesis and lung cancer: implications for prognosis and treatment. Lancet Oncol (2007) 8(6):460–1. doi: 10.1016/S1470-2045(07)70153-5
19. Trieu VN, Uckun FM. Apolipoprotein(a), a link between atherosclerosis and tumor angiogenesis. Biochem Biophys Res Commun (1999) 257(3):714–8. doi: 10.1006/bbrc.1999.0519
20. Yu HK, Kim JS, Lee HJ, Ahn JH, Lee SK, Hong SW, et al. Suppression of colorectal cancer liver metastasis and extension of survival by expression of apolipoprotein(a) kringles. Cancer Res (2004) 64(19):7092–8. doi: 10.1158/0008-5472.Can-04-0364
21. Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, et al. Lipoprotein(a) levels and risk of future coronary heart disease: Large-scale prospective data. Arch Intern Med (2008) 168(6):598–608. doi: 10.1001/archinte.168.6.598
22. Jacobson TA. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin Proc (2013) 88(11):1294–311. doi: 10.1016/j.mayocp.2013.09.003
23. Gudbjartsson Daniel F, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol (2019) 74(24):2982–94. doi: 10.1016/j.jacc.2019.10.019
24. Kamstrup PR. Lipoprotein(a) and cardiovascular disease. Clin Chem (2021) 67(1):154–66. doi: 10.1093/clinchem/hvaa247
25. Miksenas H, Januzzi JL Jr., Natarajan P. Lipoprotein(a) and cardiovascular diseases. JAMA (2021) 326(4):352–3. doi: 10.1001/jama.2021.3632
26. Motta M, Giugno I, Ruello P, Pistone G, Di Fazio I, Malaguarnera M. Lipoprotein (a) behaviour in patients with hepatocellular carcinoma. Minerva Med (2001) 92(5):301–5.
27. Yang HH, Chen XF, Hu W, Lv DQ, Ding WJ, Tang LJ, et al. Lipoprotein(a) level and its association with tumor stage in male patients with primary lung cancer. Clin Chem Lab Med (2009) 47(4):452–7. doi: 10.1515/cclm.2009.094
28. Marrer É., Wagner A, Montaye M, Luc G, Amouyel P, Dallongeville J, et al. Lipoprotein(a) plasma levels and the risk of cancer: the prime study. Eur J Cancer Prev (2013) 22(3):286–93. doi: 10.1097/CEJ.0b013e328359cba7
29. Sharma A, Goswami B, Gupta N, Chakraborty B. Lipoprotein (a) plasma levels and risk of breast cancer. Hellenic J Surg (2015) 87(4):298–302. doi: 10.1007/s13126-015-0228-z
30. Wang FM, Zhang Y. High lipoprotein(a) level is independently associated with adverse clinicopathological features in patients with prostate cancer. Dis Markers (2019) 2019:9483935. doi: 10.1155/2019/9483935
31. Wang H, Zheng H, Cao X, Meng P, Liu J, Wang Z, et al. Relationship between fibrinogen level and advanced colorectal adenoma among inpatients: a retrospective case-control study. Front Med (2023) 10:1140185. doi: 10.3389/fmed.2023.1140185
32. Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J (2010) 31(23):2844–53. doi: 10.1093/eurheartj/ehq386
33. Alonso R, Andres E, Mata N, Fuentes-Jiménez F, Badimón L, López-Miranda J, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of ldl receptor mutation. J Am Coll Cardiol (2014) 63(19):1982–9. doi: 10.1016/j.jacc.2014.01.063
34. Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for cvd and cancer in the prospective epic-heidelberg cohort. BMC Med (2017) 15(1):218. doi: 10.1186/s12916-017-0976-4
35. Khan TZ, Hsu LY, Arai AE, Rhodes S, Pottle A, Wage R, et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J (2017) 38(20):1561–9. doi: 10.1093/eurheartj/ehx178
36. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med (2017) 376(18):1713–22. doi: 10.1056/NEJMoa1615664
37. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med (2018) 379(22):2097–107. doi: 10.1056/NEJMoa1801174
38. Duarte Lau F, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: a review. JAMA Cardiol (2022) 7(7):760–9. doi: 10.1001/jamacardio.2022.0987
39. Schwartz GG, Ballantyne CM. Existing and emerging strategies to lower lipoprotein(a). Atherosclerosis (2022) 349:110–22. doi: 10.1016/j.atherosclerosis.2022.04.020
40. Viney NJ, Van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet (2016) 388(10057):2239–53. doi: 10.1016/S0140-6736(16)31009-1
Keywords: lipoprotein(a), colorectal cancer, association, retrospective study, case-control study
Citation: Wang H, Zheng H, Meng P, Cao X, Liu J, Zhang T, Zuo H and Wang Z (2023) Relationship between lipoprotein(a) and colorectal cancer among inpatients: a retrospective study. Front. Oncol. 13:1181508. doi: 10.3389/fonc.2023.1181508
Received: 16 March 2023; Accepted: 24 April 2023;
Published: 05 May 2023.
Edited by:
Qun Zhang, Nanjing Medical University, ChinaReviewed by:
Hong Feng, Shandong Province Hospital Affiliated to Shandong First Medical University, ChinaPompilio Faggiano, Fondazione Poliambulanza Istituto Ospedaliero, Italy
Copyright © 2023 Wang, Zheng, Meng, Cao, Liu, Zhang, Zuo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanwei Zheng, MTMzMjMxMTkzMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Huijie Wang
Huijie Wang Huanwei Zheng
Huanwei Zheng Ping Meng2
Ping Meng2