- Department of Urology, Shengzhou People’s Hospital, Shengzhou Branch of the First Affiliated Hospital of Zhejiang University, Shengzhou, Zhejiang, China
Introduction: Emerging evidence have suggested a potential relationship between hysterectomy and risk of kidney cancer with inconsistent results. We aimed to investigate the association of hysterectomy with kidney cancer risk based on a meta-analysis of all available cohort studies.
Methods: A comprehensive literature search was performed in the PubMed and Embase database, covering all the papers published by September 2022. The pooled relative risks (RRs) and 95% confidence intervals (CIs) were estimated using a DerSimonian and Laird random effects model.
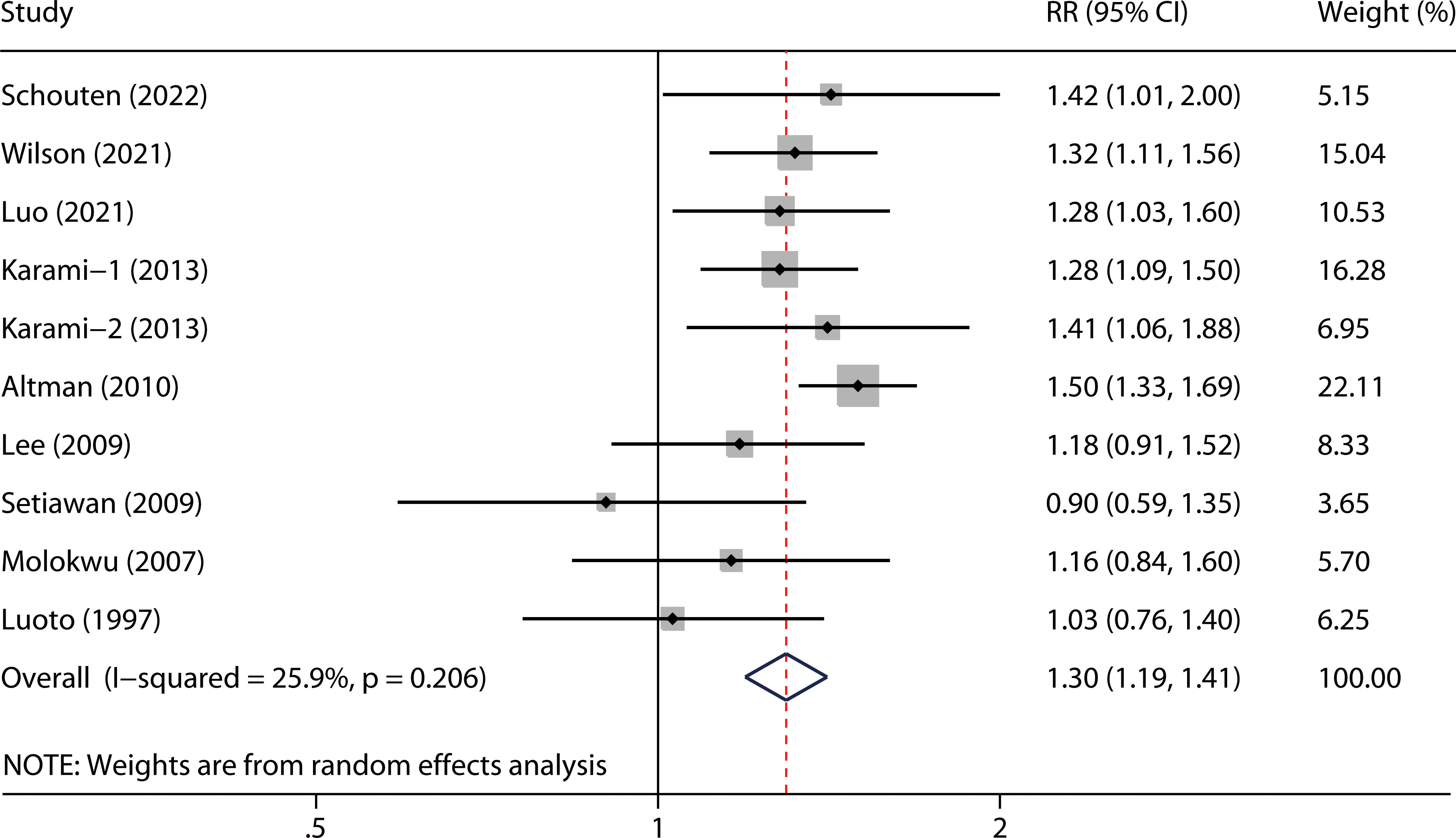
Results: Overall, our meta-analysis included 10 cohorts from 9 studies with approximately 240 million participants. The pooled RR with its 95% CI showed a significantly positive association between hysterectomy and risk of kidney cancer (RR 1.30, 95% CI 1.19-1.41). No obvious heterogeneity was observed across the studies (P = 0.206 for heterogeneity; I2 = 25.9%).
Conclusion: Findings from this meta-analysis of cohort studies indicated that hysterectomy was positively associated with subsequent kidney cancer risk. Further large prospective studies with long-term follow-up are warranted to verify these findings.
Background
Kidney cancer develops from the renal parenchyma, which ranks seventh among the most frequently diagnosed cancers in men and ninth in women (1). According to 2018 GLOBOCAN data, approximately 403,000 people developed kidney cancer, constituting 2.2% of all cancer diagnoses, and an estimated 175,098 people died from kidney cancer, which accounted for 1.8% of all cancer deaths globally (2). The incidence of kidney cancer is two-fold higher in men compared with women (3). There was a slightly growing trend for both new kidney cancer incidence and mortality since 2012 (4). The average annual percentage increase is approximately 2% to 3% in most countries (5). These findings suggest that additional investigations are required for better prevention and treatment (6).
Established risk factors for kidney cancer include tobacco smoking, body size, history of hypertension and chronic kidney disease (7, 8). The difference in incidence by sex has motivated epidemiologic studies into the etiologic relevance of hormonal and reproductive factors. Emerging evidence have suggested a potential relationship between hysterectomy and risk of kidney cancer with conflicting results. Four relatively small prospective studies (9–12) performed before 2010 found no statistically significant relationship between hysterectomy and risk of kidney cancer. However, several large-scale cohort studies (13–15) with longer follow-up published since 2010 reported a positive relationship on this topic. Given the potential impact of hysterectomy on kidney cancer incidence, and inconsistent findings from previous studies, we, therefore, aimed to investigate the association of hysterectomy with kidney cancer risk based on a meta-analysis of all available cohort studies.
Materials and methods
Publication search
We conducted a comprehensive literature search in the PubMed and Embase database, covering all the papers published from their inception to September 2022. The search strategy was used as follows: (hysterectomy or reproductive factors) and (kidney or renal) and (cancer or carcinoma) and (cohort or prospective). We also examined the cited references from retrieved articles and reviews to identify additional relevant studies. This systematic review and meta-analysis was planned, performed, and reported according to the PRISMA guidelines of quality for reporting meta-analyses (16, 17).
Study selection
Studies included in this meta-analysis met all of the following criteria: (a) one of the exposures of interest was hysterectomy history; (b) one of the outcomes of interest was kidney cancer incidence; (c) they had a cohort or prospective design; and (d) studies provided the rate ratios (RRs) or hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) or data to calculate them. Exclusion criteria: review or editorial paper; non-human studies; studies based on same population; cancer mortality as the outcome; and the exposure not including hysterectomy.
Data extraction
Two authors (YL and LY) independently extracted the data using a predefined extraction form with disagreements resolved by consensus. The following characteristics were collected from each study: the first author’s name, year of publication, the country in which the study was performed, cohort name, cohort size, kidney cancer cases, average participants’ age, follow-up time, methods of ascertainment of hysterectomy and kidney cancer, risk estimates with their 95% CIs, and adjusted covariates in the data analysis. For each study, we extracted the most-fully adjusted risk estimate.
Quality assessment
The same two authors (YL and LY) independently completed the quality assessment using the Newcastle-Ottawa Scale (NOS) (18), which was developed to assess the quality of observational studies with its design and content. NOS is an eight-item instrument with three broad perspectives and awards a maximum of nine points to each study. A higher score indicates better methodological quality. Any disagreements were resolved by consensus and discussion.
Statistical methods
The pooled RRs and 95% CIs were estimated using a DerSimonian and Laird random effects model (19), which took account of both within- and between-study variability. The heterogeneity across studies was assessed by the Q statistic and the I2 score (20). The Q statistic was used to determine the presence of heterogeneity with a significance level set at P ≤ 0.10. The value of I2 was used to calculate the proportion of variation (I2 < 25% indicated low heterogeneity, I2 = 25-50% moderate heterogeneity, and I2 > 50% high heterogeneity). Galbraith plot analysis was further used to detect potential sources of heterogeneity. A number of stratified analyses were performed according to publishing year (before 2010 versus 2010 and thereafter), geographical region (Europe vs. United States), number of cases (> 500 vs. ≤ 500), number of participants (> 100,000 vs. ≤ 100,000), and duration of follow-up (> 15 years vs. ≤ 15 years). A sensitivity analysis was performed by repeating the meta-analysis after ruling out of each included study in turn. Potential publication bias was assessed by Begg’s test (21) and Egger’s test (22). All of the statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX). A 2-sided P value < 0.05 was considered significant unless stated otherwise.
Results
Literature search
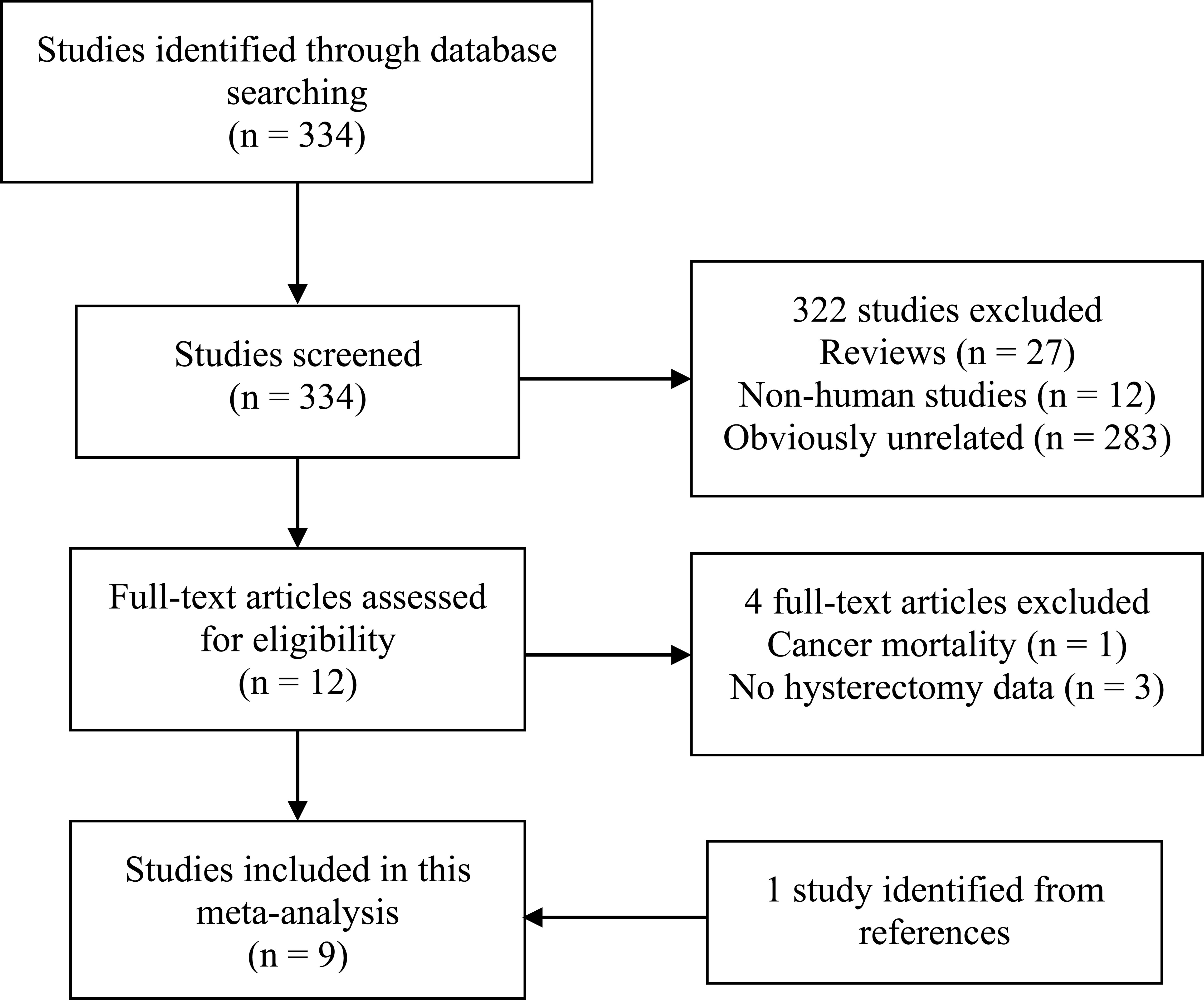
The detailed process of the literature search and study selection was presented in a flow diagram (Figure 1). Briefly, after removing duplications, the search strategy identified 334 articles. Of these, the majority were excluded after the first round of titles and abstract screening, mainly because they were reviews, non-human studies, or obviously not relevant to our analysis. After full-text review of 13 papers, 4 studies were excluded for the reasons as follows: cancer mortality as the outcome (23); the exposure not including hysterectomy (24–26). Thus, a total of 9 studies with 10 cohorts (9–15, 27, 28) were included in this meta-analysis. Of note, Karami et al’s study (14), based on NIH-AARP and PLCO cohorts, was regarded as two independent cohorts.
Study characteristics
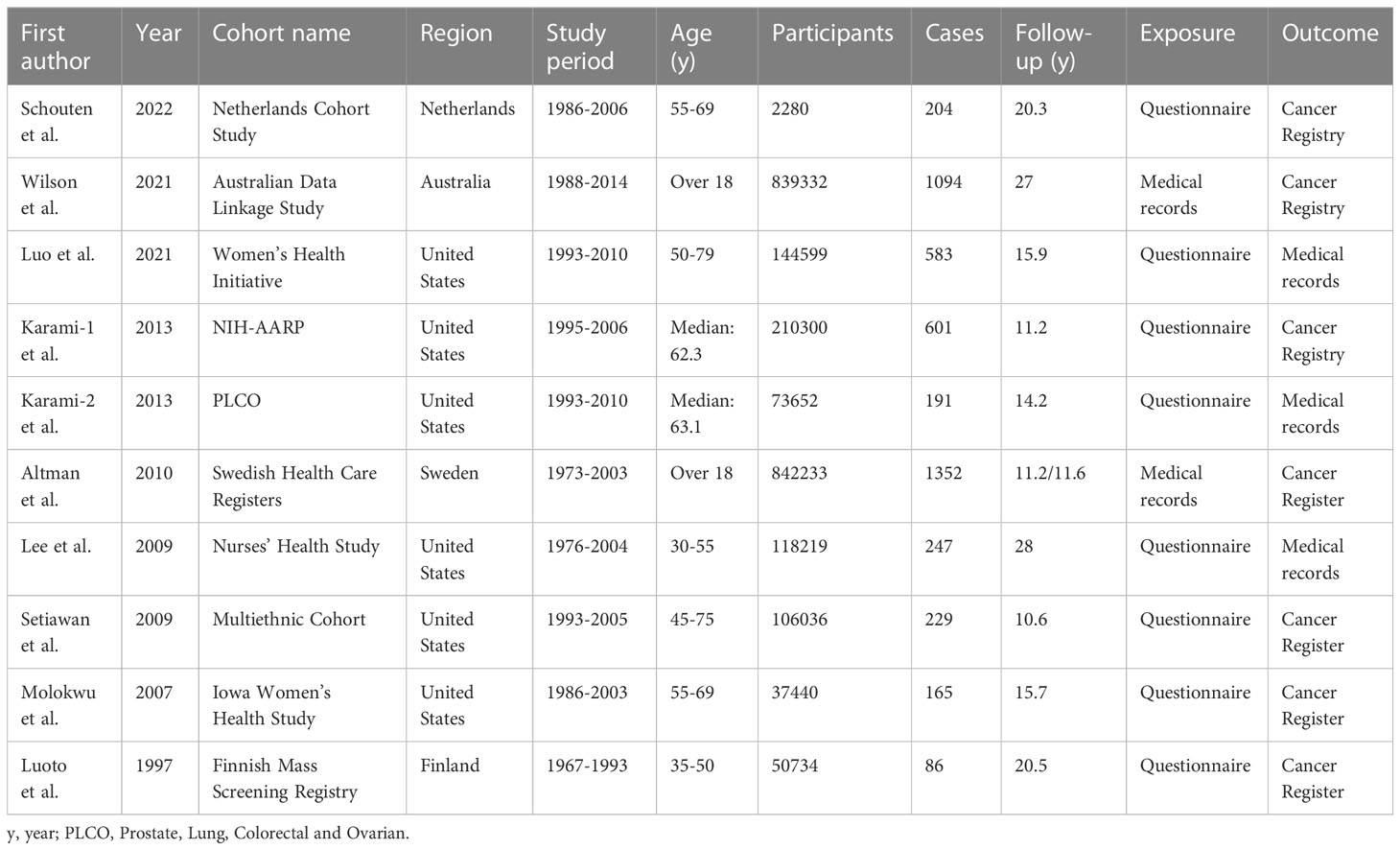
These cohorts were performed in the following regions: America (n = 6), Europe (n = 3), and Australia (n = 1). A total of approximately 240 million participants with 4752 cases were included in these studies. These cohort studies were published between 1997 and 2022, of which three large cohorts (15, 27, 28) were published in the recent two years, and were not included in a previous meta-analysis (29). Information on hysterectomy was obtained by self-administrated questionnaire or medical records. The outcome was confirmed by linking to cancer registry or medical records. Adjustments were made for potential confounding of one or more factors in all studies. The study quality, as assessed by the NOS, ranged from 6 to 8, with a mean value of 7.2 (Supplemental Table S1). The detailed information of the studies at baseline are presented in Table 1.
Hysterectomy and risk of kidney cancer
The overall RR with its 95% CI showed a statistically significant positive association between hysterectomy and kidney cancer risk (Figure 2, RR 1.30, 95% CI 1.19-1.41). No obvious heterogeneity was observed among the included studies (P = 0.206 for heterogeneity; I2 = 25.9%).
Subgroup analysis
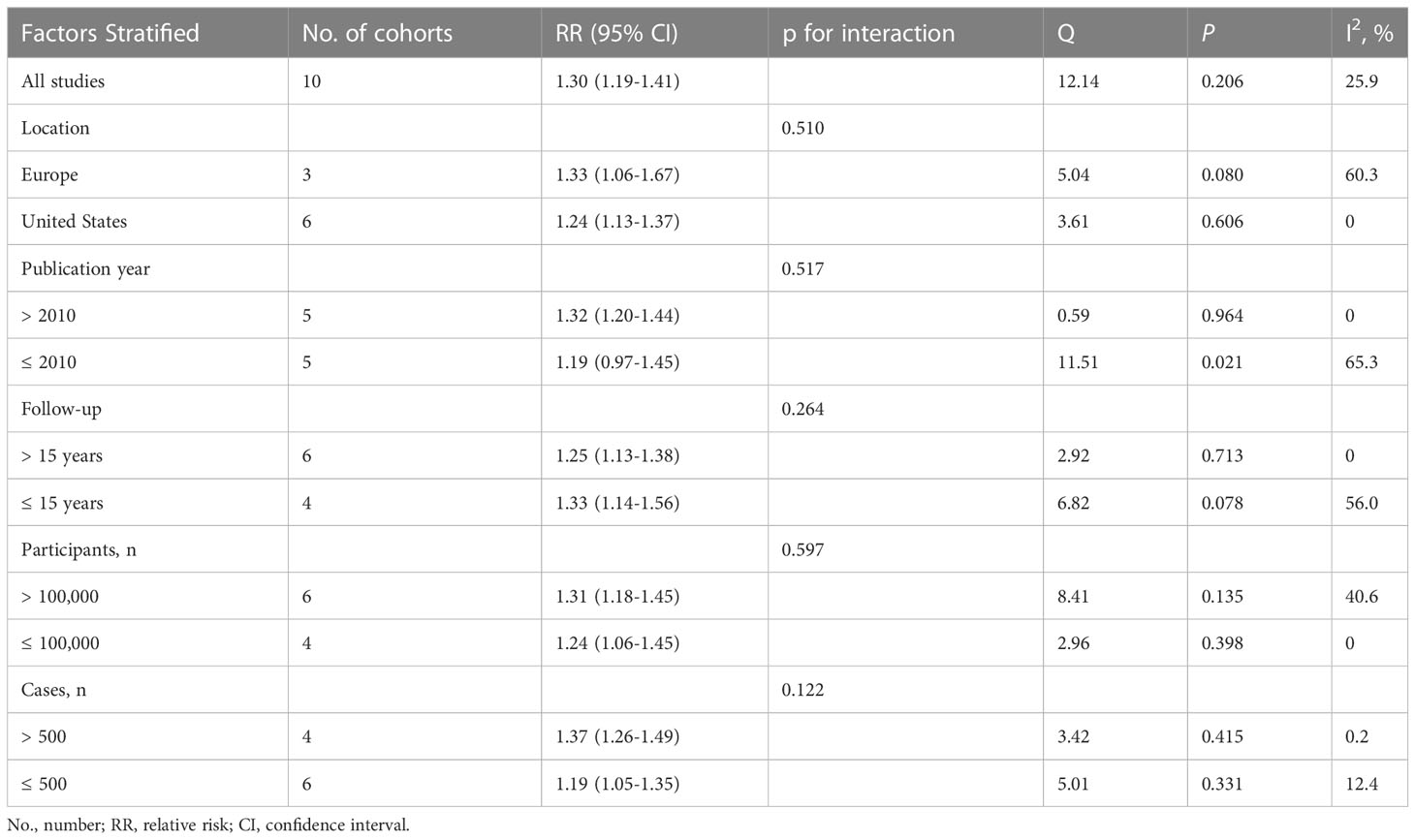
A significant association between hysterectomy and risk of kidney cancer was observed in most subgroups based on geographical region, publication year, duration of follow-up, number of participants and number of cases, except for studies published before 2010 (Table 2). No significant interactions were observed for these factors in all analyses based on meta-regression models (all P for interaction > 0.05).
Sensitivity analysis and publication bias
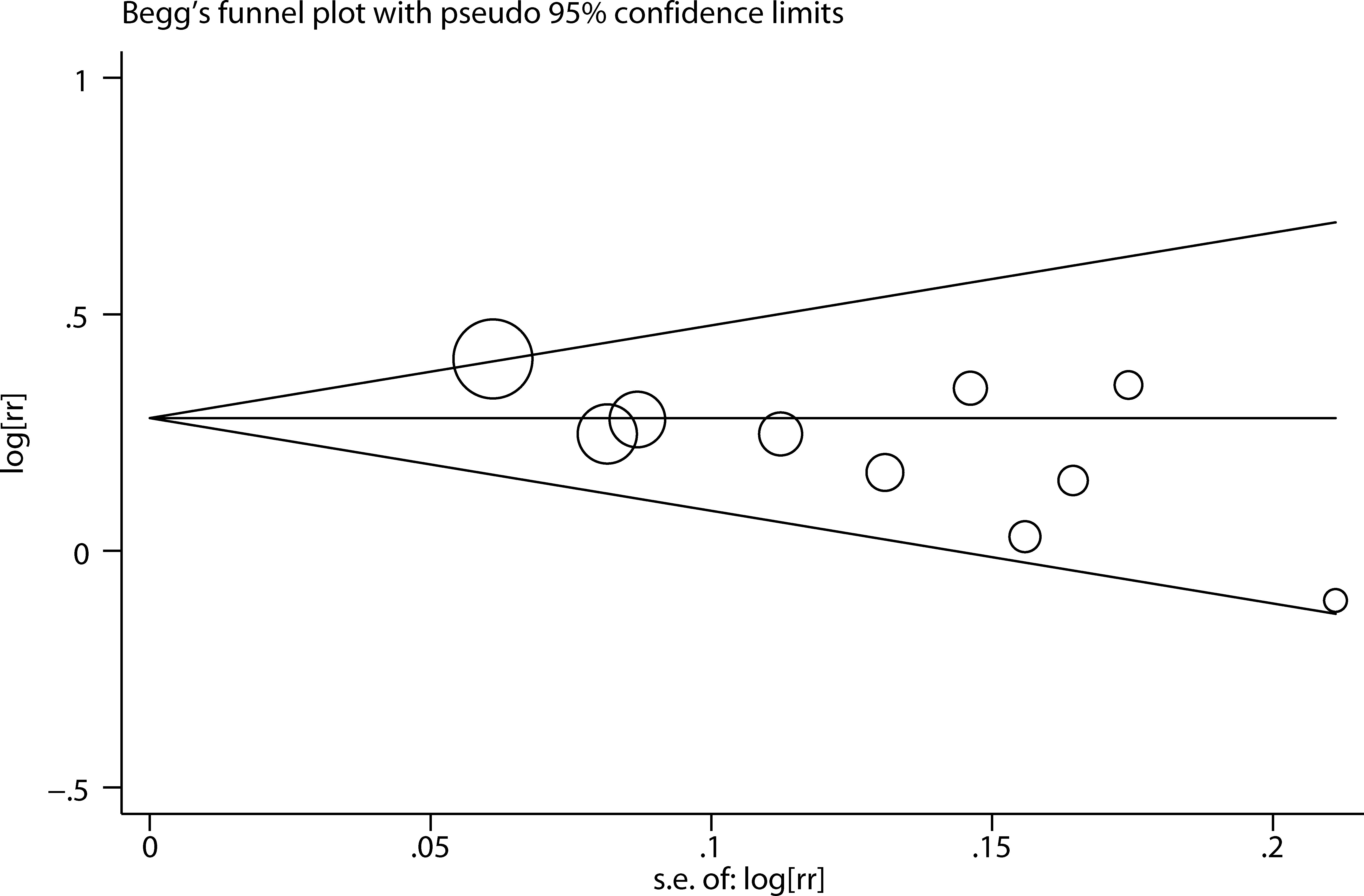
We performed two types of sensitivity analyses. First, the impact of each study on the summary RR was evaluated by repeating the meta-analysis after omitting one study at a time. The exclusion of any single study did not substantially alter the pooled RR (Figure 3). Second, Galbraith plot analysis indicated that Altman et al.’s study was the major source of heterogeneity (Figure 4). After excluding this study, the heterogeneity was dramatically reduced, with I2 of from 25.9% (P = 0.206) to 0.0% (P = 0.636), while the association maintained significant (RR = 1.25, 95% CI 1.16-1.36). There was some degree of publication bias with Egger’s test (P = 0.017), whereas no evidence of publication bias was identified from Begg’s test (P = 0.152, Figure 5).
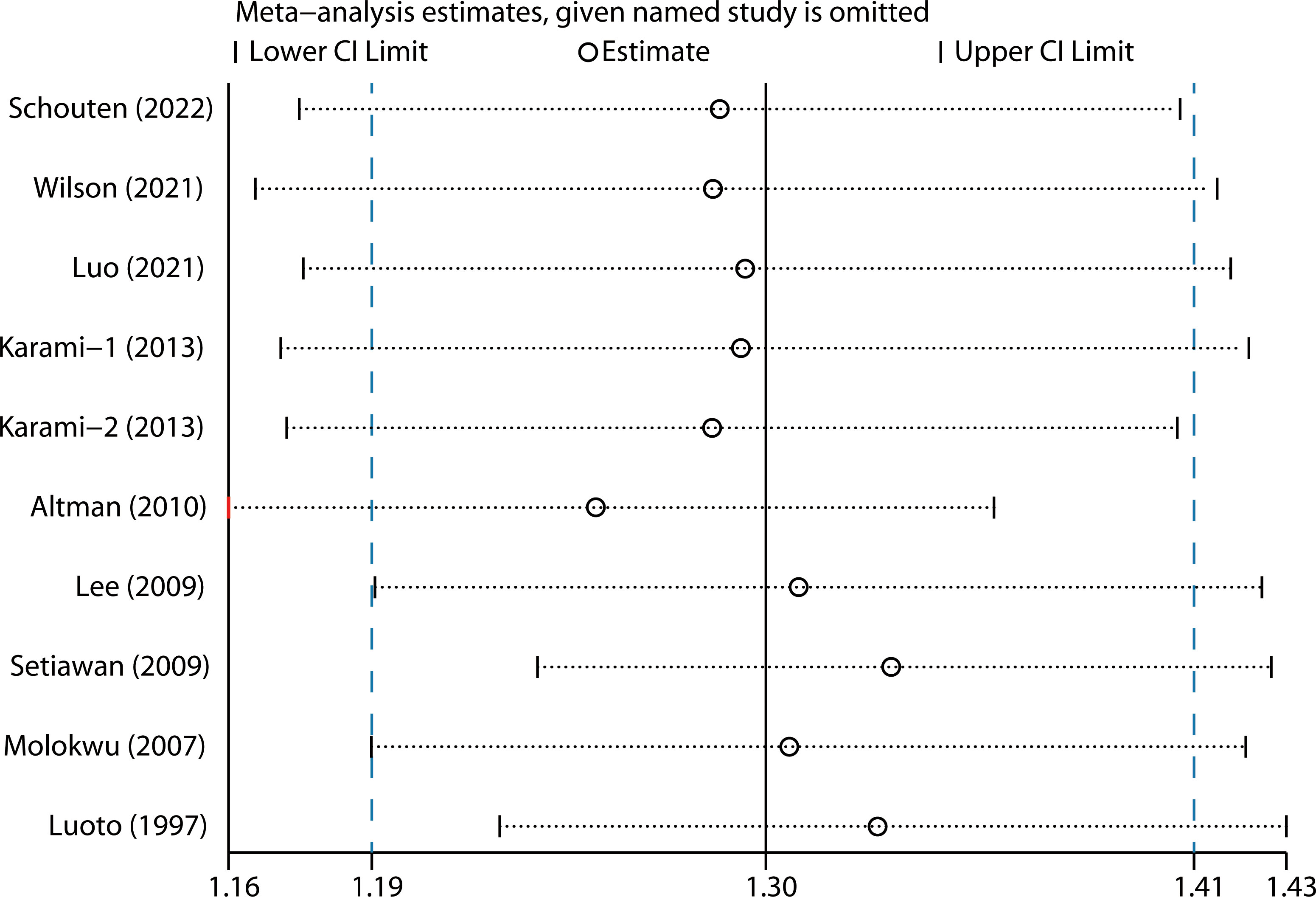
Figure 3 Sensitivity analysis by removing each study in turn and then repeating the pooled analysis.
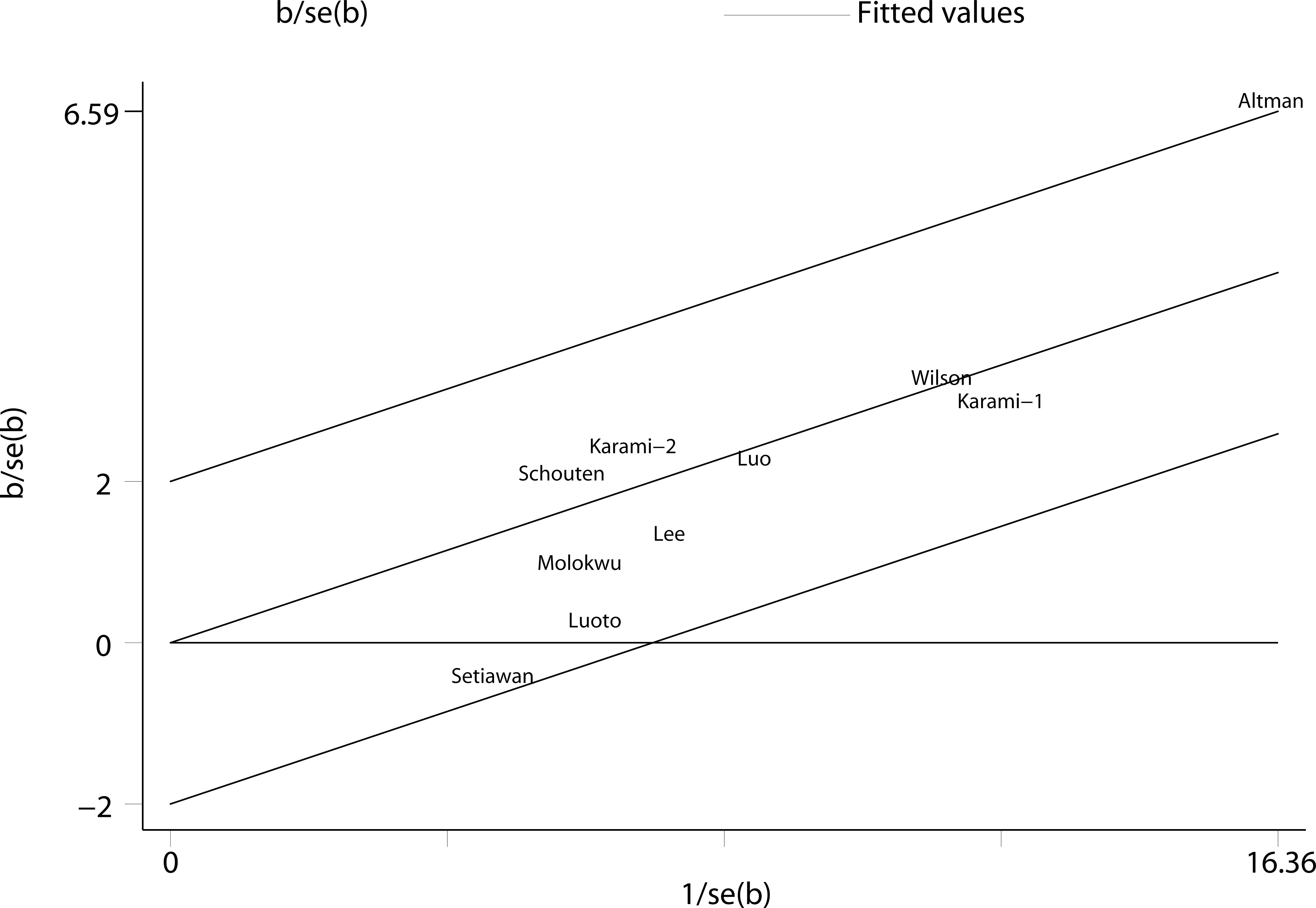
Figure 4 Galbraith plot analysis indicated that Altman et al.’s study was the major source of heterogeneity.
Discussion
The findings from this meta-analysis of cohort studies indicated that hysterectomy was significantly associated with a 30% higher risk of kidney cancer, and the result was robust with no obvious heterogeneity observed across the included studies. Sensitivity analyses also showed similar results.
The results of our study were consistent with a previous meta-analysis published in 2014 (29), which also reported that hysterectomy was significantly associated with kidney cancer (RR 1.29, 95% CI 1.16-1.43). However, this study included both case-control and cohort studies. Case-control studies may be prone to select and recall bias. In addition, only seven cohort studiers were available in this meta-analysis. By contrast, our study was based on cohort studies and included the recent three large-scale cohort studies (11–13) with longer follow-up, which enhanced the statistical power.
Previous studies which reported a positive association between hysterectomy and kidney cancer risk have been criticized for the issue of detection bias. Detection bias may partially explain the positive results since women with a history of hysterectomy may seek medical care and exanimation more actively than those without, and thus had a higher chance to be detected with kidney cancer. However, detection bias may contribute to a higher short-term cancer incidence relative to the surgical procedure, but this may not still come into play decades after the surgery. In fact, this association did not change substantially in studies which performed subgroup analyses according to the time since hysterectomy or age at hysterectomy (13, 27).
It remains unclear what biological mechanisms would mediate the association between hysterectomy and kidney cancer risk. Hysterectomy may cause renal damage or pelvic anatomy changes. A high incidence of post-renal obstruction following hysterectomy has been reported in previous studies. Hydronephrosis could appear after hysterectomy even without any obvious intraoperative ureteral injury (14, 30, 31). It may result from the twisting and constricting of the distal ureter caused by pelvic anatomy changes after a hysterectomy. Ureteropelvic junction obstruction and persistent subclinical hydronephrosis after hysterectomy may be involved in renal cell proliferation and cancer transition of the renal parenchyma (32). Another possible explanation for the association between hysterectomy and risk of kidney cancer may attribute to estrogen and progesterone replacement therapy, which was commonly used among women who have undergone a hysterectomy. Abnormal endocrine stimulation played a significant role in kidney cancer pathophysiology (33). Progesterone has been reported to inhibit the kidneys’ ability to filter out toxins (34). Estrogen receptor α (ERα) overexpression increased the transcriptional factor activity of HIF-1α and inhibition of ER-α signaling in VHL-deficient cancer cells could suppress tumor development (33, 35). Recent studies also indicated that the estrogen receptor beta (ERβ) could affect the progression of renal cancer (36).
This study had several limitations. First, the confounding bias that was inherent in the included studies cannot be excluded. Inadequate control or adjustment for confounders may bias the results in either direction, leading to underestimation or overestimation of the risk estimates (37). Second, because of the various types of risk estimates and time intervals used by the original studies (Supplemental Table S2), we were unable to assess the impact of time since hysterectomy and age at hysterectomy on the association between hysterectomy and kidney cancer risk. Future prospective studies with detailed data on these could provide further insight into the relationship. Third, significant publication bias was observed with the Egger’s test. Although we performed a throughout literature search, grey papers and studies with a small sample size or null result are less likely to be published.
Nevertheless, this study had some strengths. First, a total of approximately 240 million participants were included in this meta-analysis, which provided sufficient statistical power to evaluate the impact, if any, of hysterectomy on kidney cancer risk. Second, only cohort studies were included in this meta-analysis, which, to some extent, avoided the selection and recall bias typically existing in case-control studies. Lastly, no significant heterogeneity was found across the included studies, which indicated our findings were relatively robust.
Conclusions
In summary, findings from this meta-analysis of cohort studies indicated that hysterectomy was positively associated with the risk of kidney cancer. Future large prospective studies with long term follow-ups are warranted to verify these findings, and the potential underlying molecular mechanism that links hysterectomy and kidney cancer incidence needs further exploration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LY: project development, data collection and analysis, manuscript writing and editing. PY: data collection and analysis, manuscript writing. YL: project development, manuscript editing, and project supervising.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1181112/full#supplementary-material
References
1. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet (2009) 373(9669):1119–32. doi: 10.1016/s0140-6736(09)60229-4
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Scelo G, Li P, Chanudet E, Muller DC. Variability of sex disparities in cancer incidence over 30 years: the striking case of kidney cancer. Eur Urol Focus (2018) 4(4):586–90. doi: 10.1016/j.euf.2017.01.006
4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
5. Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol (2015) 67(3):519–30. doi: 10.1016/j.eururo.2014.10.002
6. Sung WW, Ko PY, Chen WJ, Wang SC, Chen SL. Trends in the kidney cancer mortality-to-incidence ratios according to health care expenditures of 56 countries. Sci Rep (2021) 11(1):1479. doi: 10.1038/s41598-020-79367-y
7. Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol (2018) 36(36):JCO2018791905. doi: 10.1200/jco.2018.79.1905
8. Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam J, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol (2022) 82(5):529–42. doi: 10.1016/j.eururo.2022.08.019
9. Luoto R, Auvinen A, Pukkala E, Hakama M. Hysterectomy and subsequent risk of cancer. Int J Epidemiol (1997) 26(3):476–83. doi: 10.1093/ije/26.3.476
10. Molokwu JC, Prizment AE, Folsom AR. Reproductive characteristics and risk of kidney cancer: Iowa women's health study. Maturitas (2007) 58(2):156–63. doi: 10.1016/j.maturitas.2007.07.003
11. Lee JE, Hankinson SE, Cho E. Reproductive factors and risk of renal cell cancer: the nurses' health study. Am J Epidemiol (2009) 169(10):1243–50. doi: 10.1093/aje/kwp030
12. Setiawan VW, Kolonel LN, Henderson BE. Menstrual and reproductive factors and risk of renal cell cancer in the multiethnic cohort. Cancer Epidemiol Biomarkers Prev (2009) 18(1):337–40. doi: 10.1158/1055-9965.epi-08-0790
13. Altman D, Yin L, Johansson A, Lundholm C, Grönberg H. Risk of renal cell carcinoma after hysterectomy. Arch Intern Med (2010) 170(22):2011–6. doi: 10.1001/archinternmed.2010.425
14. Karami S, Daugherty SE, Schonfeld SJ, Park Y, Hollenbeck AR, Grubb RL 3rd, et al. Reproductive factors and kidney cancer risk in 2 US cohort studies, 1993-2010. Am J Epidemiol (2013) 177(12):1368–77. doi: 10.1093/aje/kws406
15. Schouten LJ, van de Pol J, Kviatkovsky MJ, van den Brandt PA. Reproductive and external hormonal factors and the risk of renal cell cancer in the Netherlands cohort study. Cancer Epidemiol (2022) 79:102171. doi: 10.1016/j.canep.2022.102171
16. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer (2005) 93(4):387–91. doi: 10.1038/sj.bjc.6602678
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
23. Chiu HF, Kuo CC, Kuo HW, Lee IM, Yang CY. Parity, age at first birth and risk of death from kidney cancer: a population-based cohort study in Taiwan. Eur J Public Health (2014) 24(2):249–52. doi: 10.1093/eurpub/ckt057
24. Kabat GC, Silvera SA, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer (2007) 96(5):845–9. doi: 10.1038/sj.bjc.6603629
25. Högnäs E, Kauppila A, Hinkula M, Tapanainen JS, Pukkala E. Incidence of cancer among grand multiparous women in Finland with special focus on non-gynaecological cancers: a population-based cohort study. Acta Oncol (2016) 55(3):370–6. doi: 10.3109/0284186x.2015.1063775
26. Lambe M, Lindblad P, Wuu J, Remler R, Hsieh CC. Pregnancy and risk of renal cell cancer: a population-based study in Sweden. Br J Cancer (2002) 86(9):1425–9. doi: 10.1038/sj.bjc.6600263
27. Luo J, Rohan TE, Neuhouser ML, Liu N, Saquib N, Li Y, et al. Hysterectomy, oophorectomy, and risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev (2021) 30(3):499–506. doi: 10.1158/1055-9965.epi-20-1373
28. Wilson LF, Tuesley KM, Webb PM, Dixon-Suen SC, Stewart LM, Jordan SJ. Hysterectomy and risk of breast, colorectal, thyroid, and kidney cancer - an Australian data linkage study. Cancer Epidemiol Biomarkers Prev (2021) 30(5):904–11. doi: 10.1158/1055-9965.epi-20-1670
29. Karami S, Daugherty SE, Purdue MP. Hysterectomy and kidney cancer risk: a meta-analysis. Int J Cancer (2014) 134(2):405–10. doi: 10.1002/ijc.28352
30. Hazewinkel MH, Gietelink L, van der Velden J, Burger MP, Stoker J, Roovers JP. Renal ultrasound to detect hydronephrosis: a need for routine imaging after radical hysterectomy? Gynecol Oncol (2012) 124(1):83–6. doi: 10.1016/j.ygyno.2011.09.010
31. Mansour Ghanaie M, Asgari SA, Haghbin A, Mehdizade F, Asgari Ghalebin SM. Post-hysterectomy transient hydronephrosis: a prospective study. J Family Reprod Health (2021) 15(1):13–8. doi: 10.18502/jfrh.v15i1.6068
32. Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Increased risk of renal cell carcinoma subsequent to hysterectomy. Cancer Epidemiol Biomarkers Prev (1999) 8(11):999–1003.
33. Czarnecka AM, Niedzwiedzka M, Porta C, Szczylik C. Hormone signaling pathways as treatment targets in renal cell cancer (Review). Int J Oncol (2016) 48(6):2221–35. doi: 10.3892/ijo.2016.3460
34. Pechere-Bertschi A, Burnier M. Gonadal steroids, salt-sensitivity and renal function. Curr Opin Nephrol Hypertens (2007) 16(1):16–21. doi: 10.1097/MNH.0b013e328011d7f6
35. Jung YS, Lee SJ, Yoon MH, Ha NC, Park BJ. Estrogen receptor α is a novel target of the Von hippel-lindau protein and is responsible for the proliferation of VHL-deficient cells under hypoxic conditions. Cell Cycle (2012) 11(23):4462–73. doi: 10.4161/cc.22794
36. Ding J, Yeh CR, Sun Y, Lin C, Chou J, Ou Z, et al. Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene (2018) 37(37):5037–53. doi: 10.1038/s41388-018-0175-6
Keywords: hysterectomy, kidney cancer, cohort, meta-analysis, risk
Citation: Yu L, Yu P and Lu Y (2023) Is hysterectomy associated with kidney cancer risk? A meta-analysis of cohort studies. Front. Oncol. 13:1181112. doi: 10.3389/fonc.2023.1181112
Received: 07 March 2023; Accepted: 03 July 2023;
Published: 20 July 2023.
Edited by:
Aristotle Bamias, National and Kapodistrian University of Athens, GreeceReviewed by:
Hong Weng, Wuhan University, ChinaKimon Tzannis, National and Kapodistrian University of Athens, Greece
Copyright © 2023 Yu, Yu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Lu, MTMzNjI1NTA4NThAMTYzLmNvbQ==
 Ling Yu
Ling Yu Pengkui Yu
Pengkui Yu Yi Lu
Yi Lu