- 1Breast and Endocrine Division, Department of Surgery, Korea University Medical Center, Seoul, Republic of Korea
- 2Center for Breast Cancer, National Cancer Center, Research Institute and Hospital, Goyang, Gyeonggi, Republic of Korea
- 3Department of Surgery, Breast Service, Changi General Hospital, Singapore, Singapore
Background: Recent data from the ACOSOG Z0011 trial suggest that axillary lymph node dissection (ALND) may not be necessary for patients with positive sentinel lymph node biopsy (SLNB) receiving breast-conserving surgery (BCS) with irradiation. However, consensus statements and guidelines have recommended that patients undergoing mastectomy with tumor-positive sentinel node undergo completion ALND. In this study, we compared the locoregional recurrence rate of patients with tumor-positive sentinel nodes among three groups: mastectomy with SLNB, mastectomy with ALND and BCS with SLNB.
Method: We identified 6,163 women with invasive breast cancer who underwent surgical resection at our institution between January 2000 and December 2011. Clinicopathologic data obtained from the prospectively collected medical database were analyzed retrospectively. Among the patients with sentinel node positive, mastectomy with SLNB was performed in 39 cases, mastectomy with ALND in 181 cases, and BCS with SLNB in 165 cases. The primary end point was the loco-regional recurrence rate.
Results: Clinicopathologic characteristics were similar among the groups. There were no cases of loco-regional recurrence in the sentinel groups. At a median follow-up of 61.0 months (last follow-up May 2013), the loco-regional recurrence rate of each group was 0% for BCS with SLNB and mastectomy with SLNB only, and 1.7% for mastectomy with ALND (p=0.182).
Conclusion: In our study, there was no significant difference in loco-regional recurrence rates between groups. This result lends weight to the argument that SLNB without ALND may be a reasonable management for selected patients with appropriate surgery and adjuvant systemic therapy.
Background
In the early 1990s, introduction of the sentinel lymph node (SLN) concept revolutionized the axillary surgery in breast cancer (1). As a consequence, patients with a negative SLN can now avoid unnecessary axillary lymph node dissection (ALND) and its attendant morbidity (2). In 2001, an international multicenter phase III trial was initiated by the European Organization for Research and Treatment of Cancer (EORTC). The After Mapping of the Axilla: Radiotherapy of Surgery? (AMAROS) study compared ALND and axillary radiation therapy (ART) in patients with early breast cancer with tumor-positive sentinel nodes (3). Results from this study showed that axillary dissection had no influence on the administration of adjuvant treatment in the first 566 patients assessed. More recently, in 2004 the American College of Surgeons Oncology Groups (ACOSOG) started the Z0011 Trial, a multicenter randomized trial investigating loco-regional recurrence after sentinel lymph node biopsy (SLNB) with or without axillary dissection in patients with sentinel lymph node metastasis (4). This study enrolled patients with cancers 5 cm or smaller and 1-2 positive SLNs who were treated with breast-conserving surgery (BCS). These patients were randomized to receive ALND and breast radiation therapy (BRT) or BRT alone (5). The results were published in 2010, and the authors concluded that ALND was not necessary for all women with one or two positive axillary sentinel lymph nodes who undergo breast conserving surgery, adjuvant systemic therapy, and opposing tangential field whole breast irradiation (6).
Many breast oncologists consider that the findings of Z0011 shifted the surgical paradigm in breast cancer. The 2012 NCCN guidelines (7) now recommend considering no further surgery for clinically node-negative patients with tumors under 5 cm in size and one or two positive SLNs who will receive BRT (7, 8). Based on this evidence, a number of experts at the St. Gallen Consensus Conference in 2013 voted that axillary dissection can be omitted in patients with macrometastases in 1-2 sentinel nodes who undergo breast conserving surgery and planned radiotherapy. However, they did not agree that axillary dissection can safely be omitted in patients with macrometastases in 1-2 sentinel nodes who undergo mastectomy without planned radiotherapy (9).
Fisher previously argued that breast cancer is a systemic disease, and providing systemic therapy in accordance with the hypothesis is generating good results in breast cancer outcomes (10). For patients with axillary node metastases who undergo mastectomy, there is no definite proof that complete axillary dissection or radiotherapy is required as a local control. In this preliminary study, we compared patients with axillary node metastasis who underwent mastectomy and did not undergo ALND against patients who received BCS and irradiation with SLNB only and those who received mastectomy with ALND. We analyzed the locoregional recurrence rate and found no differences in outcomes among the three groups.
Methods
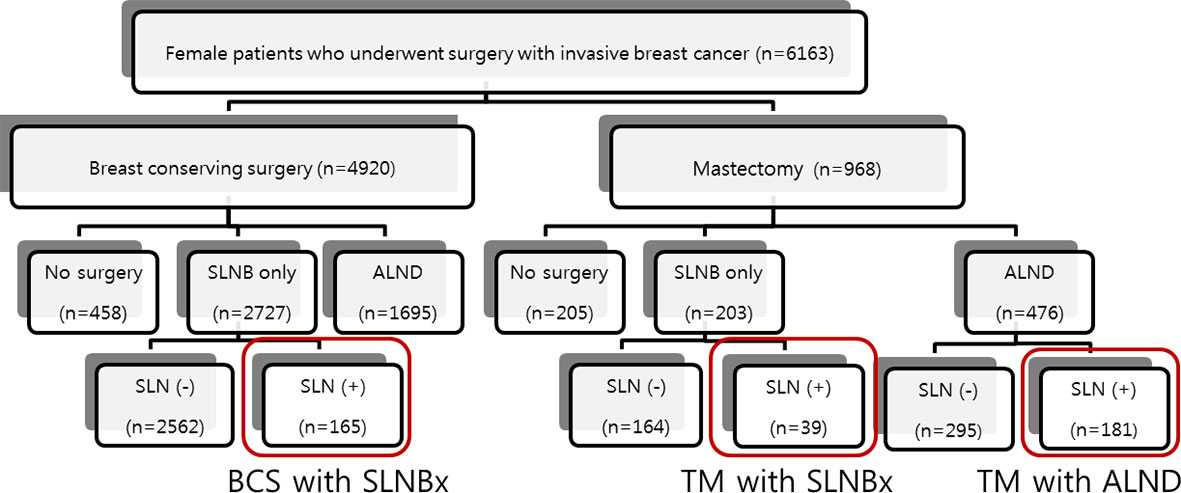
Our study is a retrospective study based on an existing prospective breast cancer database of the National Cancer Center, Goyang-si, Gyeonggi-do, Korea. We identified 6,163 consecutive women with invasive breast cancer who underwent surgery at our institution between January 2000 and December 2011. We excluded patients who had bilateral breast cancer or had no axillary surgery. All patients who were node-positive on sentinel biopsy were initially enrolled in this study. To observe the effects of different types of surgery on the breast and axilla, we compared the outcome of patients who underwent mastectomy with SLNB (n=39) only with that of patients who underwent BCS with SLNB (n=165) and those who underwent mastectomy with ALND (n=181). Patients who underwent BCS with ALND (n=104) were excluded from this study. The patient flow is outlined in Figure 1. We collected clinicopathologic information on these 385 patients by reviewing the prospective database of our institution for data on patient and tumor characteristics, breast surgery, sentinel-node biopsy, axillary lymph node dissection, radiotherapy, systemic adjuvant therapy, disease recurrence, and death during follow-up. The primary end point was the loco-regional recurrence rate.
Written informed consent was provided before surgery by all patients and this study was approved by the Institutional Review Board of our institution.
Patients were divided into three groups as above to perform statistical analysis. Associations among categorical variables were analyzed using chi-square test and one-way ANOVA. ER and PgR expression levels were divided into binary covariates based on Allred score (low score ≤6 and high score ≥7). Stepwise multivariate logistic regression analysis was performed to identify the significant factors associated with each surgery. Multivariate Cox regression analysis was used to compare recurrence-free survival according to surgery and to represent survival curves. A p-value less than or equal to 0.05 was considered to be statistically significant. All statistical analyses were performed with STATA Version 10 (StataCorp LP, College Station, TX, USA).
Results
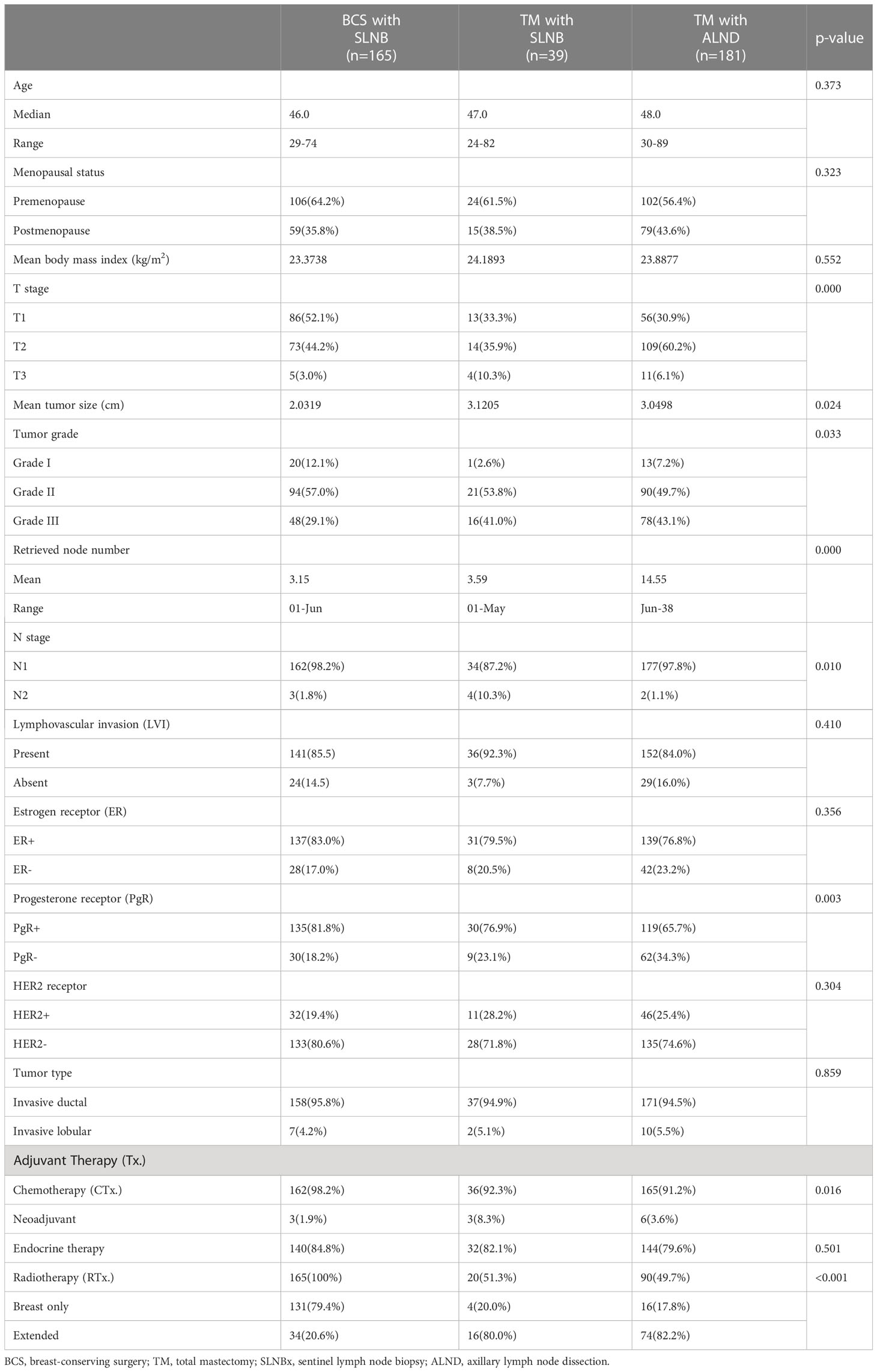
Of the 6,163 patients, 4,920 underwent breast-conserving surgery and 968 underwent mastectomy. Table 1 lists basic patient, tumor, and treatment characteristics. In general, there were no significant differences in clinical and tumor characteristics among the three groups. The median ages were 47.0 years for the mastectomy with SLNB group, 48.0 years for the mastectomy with ALND group, and 46.0 years for the BCS group (p=0.373). The mean tumor size was 3.1 cm for the mastectomy with SLNB group, 3.0 cm for the mastectomy with ALND group, and 2.0 cm for the BCS group, representing a statistically significant difference (p=0.024). The number of patients treated with radiotherapy (RTx.) was significantly different among the groups (p<0.05). The extent of RTx. also differed; patients in the mastectomy group were more likely to have chest wall RTx. including supraclavicular nodes or internal mammary nodes, whereas most of the patients in the BCS group underwent breast radiation only (Table 1). Other than tumor size, T stage, and radiotherapy, other factors including lymphovascular invasion, hormonal receptor and HER2 receptor status, and tumor type, were not significantly different among the groups. The median number of nodes removed in the two SLNB groups was approximately 3 (3.5 in the mastectomy group and 3.1 in the BCS group).
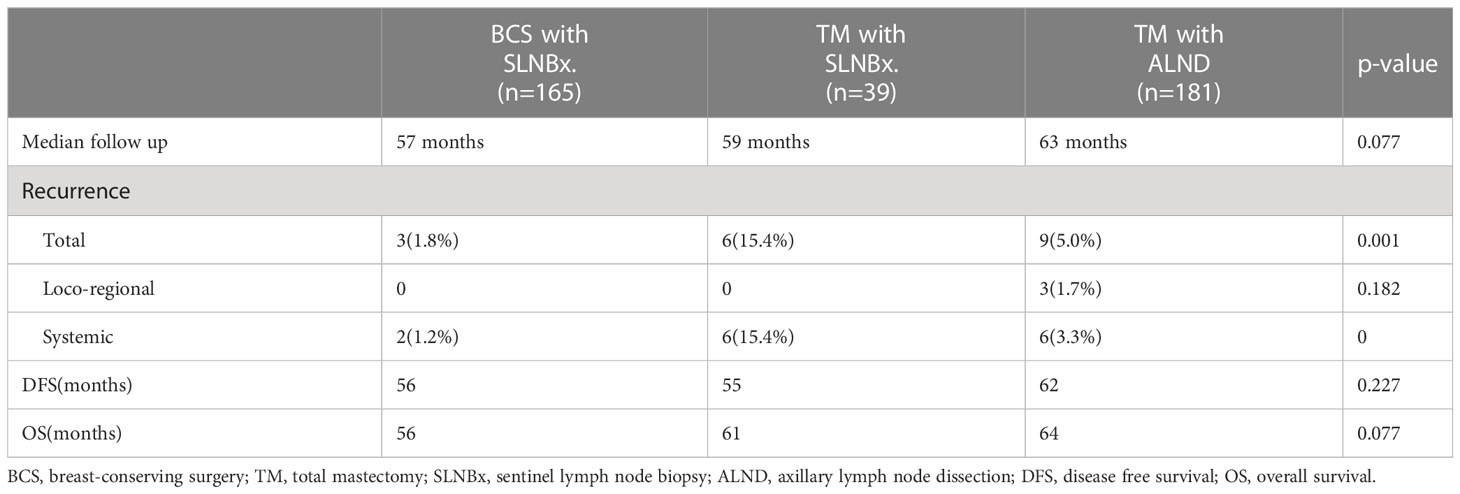
At a median follow-up of 61.0 months, the loco-regional recurrence rates were 0% in the mastectomy with sentinel lymph node biopsy only group, 0% in the breast conserving surgery with SLN group and 1.7% (3 cases) in the mastectomy with axillary dissection group; however, the difference was not statistically significant (p-value=0.182). The total and systemic recurrence rates were higher in the mastectomy group than the breast-conserving group and this difference was statistically significant (p < 0.05). The length of disease-free survival and overall survival was not significantly different among the three groups (Table 2). However, using the log-rank test, the mastectomy with sentinel biopsy group showed a worse prognosis than the other two groups for both disease-free survival (Figure 2A, p=0.018) and overall survival (Figure 2B), p=0.017).
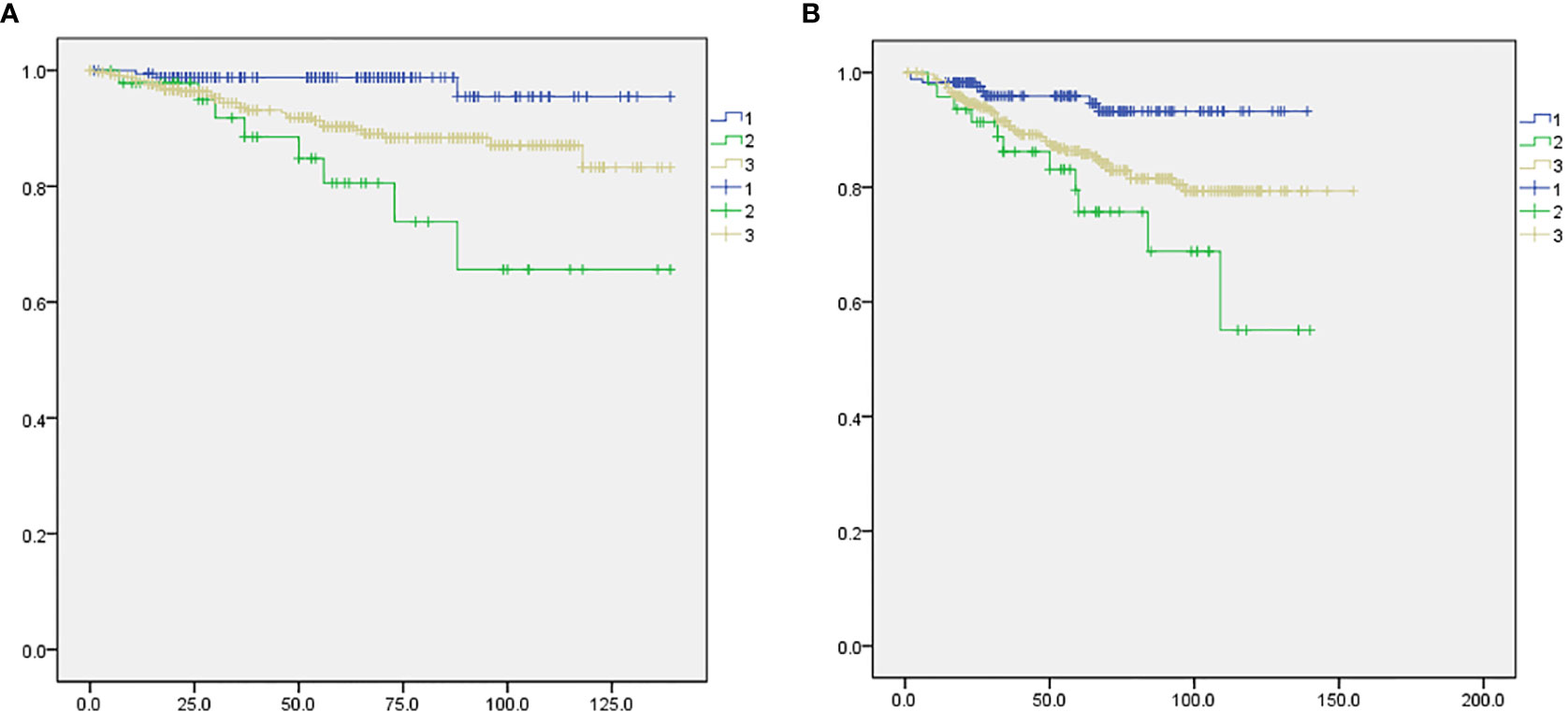
Figure 2 Comparison of disease-free survival and overall survival among the three study groups. 1: BCS with SLNB (blue), 2: mastectomy with SLNB (green), 3: mastectomy with ALND (gray). (A) Disease-free survival, p=0.018. (B) Overall survival, p=0.017.
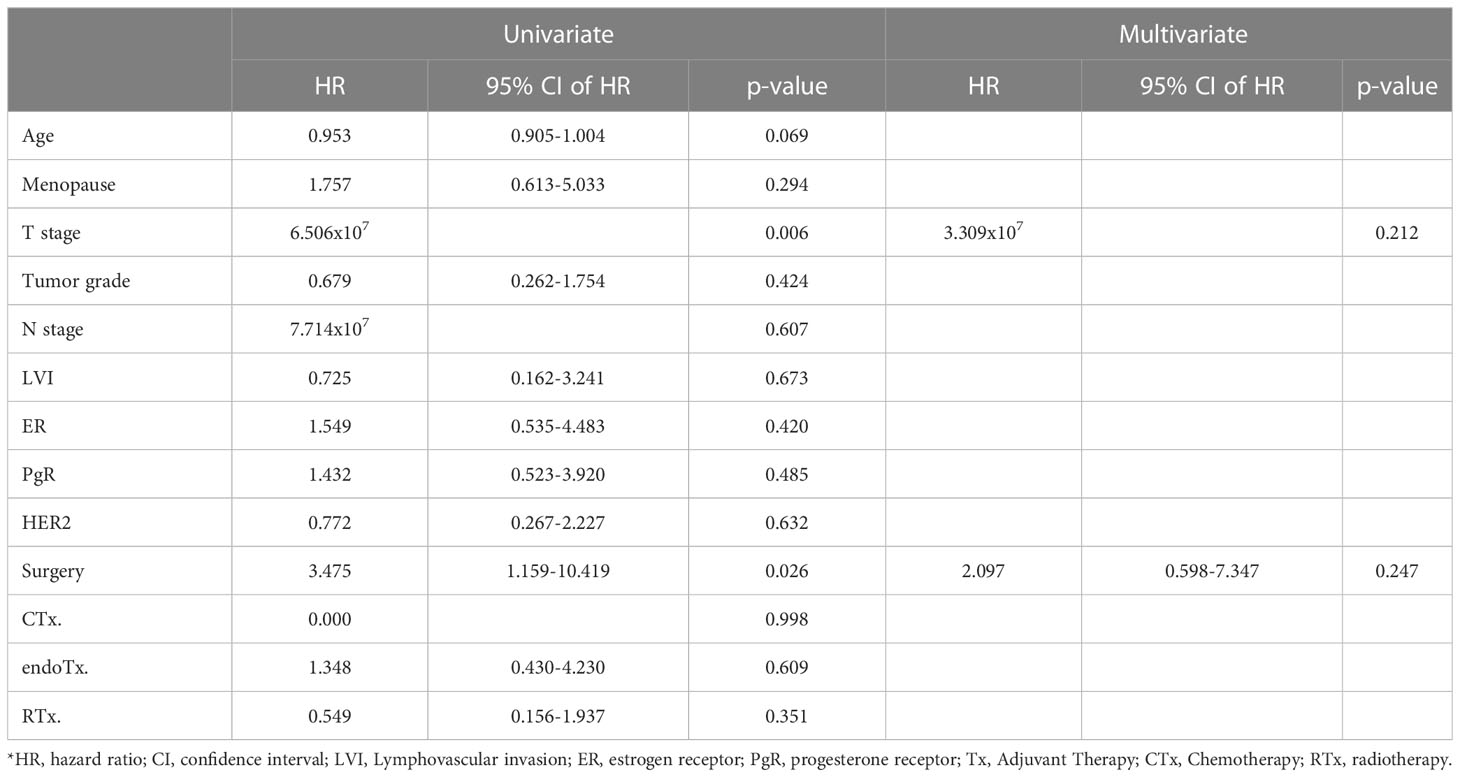
Table 3 shows the logistic regression analysis for loco-regional recurrence. Univariate analysis including all patients revealed that T stage (p=0.006, HR=6.506x10-7) and surgery type (p=0.026, HR=3.475 [1.159-10.419]) were statistically significant factors. However, in multivariate analysis neither factor was independently associated with recurrence (T stage: p=0.212, surgery: p=0.247).
Discussion
In the American College of Surgeons Oncology Group Z0011 randomized trial, ALND did not significantly affect overall survival or disease-free survival of patients with clinical T1-T2 breast cancer and a positive SLN who were treated with lumpectomy, adjuvant systemic therapy, and tangential-field whole breast radiation therapy (11, 12). No significant benefit in loco-regional control was seen with completion ALND despite the removal of additional tumor-involved lymph nodes (4). Yet despite increasing evidence that many women will not have additional nodal metastasis upon completion ALND, management of the patient with clinically negative, histologically positive lymph nodes undergoing mastectomy has not changed, and ALND remains the gold standard because of relatively insufficient evidence for mastectomy patients compared with BCS patients (13).
In this study, at a median follow-up of 61.0 months, we noted no difference among patients undergoing breast-conserving surgery with sentinel lymph node biopsy, mastectomy with axillary dissection, or mastectomy with sentinel lymph node biopsy groups with respect to the primary endpoint of loco-regional recurrence rate (panel). Although the number of enrolled patients was small, the protocol-specified criterion of non-inferiority of the mastectomy with sentinel lymph node biopsy only group was fulfilled. However, both disease-free survival and overall survival of the mastectomy with sentinel biopsy group were worse than the other two groups (Figure 2), possibly because of the higher initial tumor stage (Table 1). In addition, the radiotherapy rate in each group was different (Table 1). Due to the retrospective nature of this study, as well as the small number of case patients and limited data, we could not control for the rate and extent of radiotherapy.
Some previously published studies had a similar concept to ours. Crawford et al. (14) reported that routine completion axillary lymph node dissection for positive sentinel nodes in patients undergoing mastectomy was not associated with improved local control. They performed a retrospective review of women with stage 1 to 3 breast cancer and found that survival curves showed no significant difference in recurrence-free survival between the sentinel biopsy only group and axillary dissection group. Spiguel et al. (15) performed a retrospective analysis of a sentinel node-positive group staged as N1micro or N1. Their study included 123 node-positive patients who underwent SLNB alone with no completion axillary dissection for invasive breast cancer, among which approximately 30% of the patients underwent mastectomy. These patients showed a locoregional recurrence rate of 0.8%, and only one axillary recurrence, which is consistent with our results.
Several retrospective studies have reported low axillary recurrence rates in women with positive sentinel nodes who did not have completion ALND for various reasons (16–18). These retrospective studies are limited by their small numbers, limited knowledge of the reasons for not performing ALND, small number of sentinel node metastases, and lack of controls. Lannin et al. discussed some limitations of the results of ACOSOG Z0011 study in their essay (19). They showed that the Yale data confirmed the accuracy of the two Louisville models and reported that tumor size, number of positive sentinel nodes, and proportion of positive sentinel nodes were all significant predictors of prognosis. As differences in T stage or tumor size can be confounders, we compared groups of breast-conserving surgery with sentinel node biopsy, mastectomy with sentinel node biopsy, and mastectomy with axillary node dissection. We believe that comparing different surgery types reduces the fore-mentioned confounding effects.
Regardless of some limitations due to its retrospective nature, our study can be regarded as a preliminary study with sufficient value because we performed treatment with a relatively consistent policy at a single institution. The results of our study suggest that we can safely omit ALND in some patients with positive sentinel nodes who undergo mastectomy.
Conclusion
Our study showed no difference in loco-regional recurrence rates among these three groups. Our results lend weight to the argument that SLNB without ALND may be reasonable management for selected patients who can be treated with appropriate surgery and adjuvant systemic therapy. Further validation through a well-designed prospective randomized clinical trial with a larger study population and using a standard protocol is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The studies involving human participants were reviewed and approved by National Cancer Center. Written informed consent to participate in this study was provided by the patients (IRB No.NCC2016-0130)
Author contributions
JY and EL conceived and designed the study. JY and SL wrote the paper. JY and S-YJ designed the figures. JY and YK summarized the data, analyzed the data and created the tables. EL, SL and S-YJ reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Cancer Center of Korea Grant (CDA2014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1181069/full#supplementary-material
References
1. Krag Dn, Weaver Dl, Alex Jc, Fairbank Jt. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol (1993) 2(6):335–9; Discussion 340. doi: 10.1016/0960-7404(93)90064-6
2. Kell Mr, Burke Jp, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta-analysis. Breast Cancer Res Treat (2010) 120(2):441–7. doi: 10.1007/s10549-009-0705-6
3. Straver Me, Meijnen P, Van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Of Clin Oncol (2010) 28(5):731–7. doi: 10.1200/JCO.2008.21.7554
4. White Rl Jr., Wilke Lg. Update on the nsabp and acosog breast cancer sentinel node trials. Am Surgeon May (2004) 70(5):420–4. doi: 10.1177/000313480407000509
5. Giuliano Ae, Hunt Kk, Ballman Kv, McCall L, Beitsch PD, Brennan MB, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA (2011) 305(6):569–75. doi: 10.1001/jama.2011.90
6. Giuliano Ae, Mccall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American college of surgeons oncology group Z0011 randomized trial. Ann Surg Sep (2010) 252(3):426–32; Discussion 432-423. doi: 10.1097/SLA.0b013e3181f08f32
7. Carlson Rw, Allred Dc, Anderson Bo, Burstein HJ, Edge SB, Farrar WB, et al. Metastatic breast cancer, version 1.2012: featured updates to the nccn guidelines. J Of Natl Compr Cancer Network (2012) 10(7):821–9. doi: 10.6004/jnccn.2012.0086
8. Krishnan Ms, Recht A, Bellon Jr, Punglia Rs. Trade-offs associated with axillary lymph node dissection with breast irradiation versus breast irradiation alone in patients with a positive sentinel node in relation to the risk of non-sentinel node involvement: implications of acosog Z0011. Breast Cancer Res And Treat (2013) 138(1):205–13. doi: 10.1007/s10549-013-2418-0
9. Untch M, Gerber B, Harbeck N, Jackisch C, Marschner N, Mobus V, et al. 13th st. gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a German team of experts (Zurich 2013). Breast Care (2013) 8(3):221–9. doi: 10.1159/000351692
10. Fisher B. Systemic chemotherapy as an adjuvant to surgery in the treatment of breast cancer. Cancer (1969) 24(6):1286–9. doi: 10.1002/1097-0142(196912)24:6<1286::AID-CNCR2820240639>3.0.CO;2-7
11. Fisher B, Jeong Jh, Anderson S, Bryant J, Fisher Er, Wolmark N. Twenty-Five-Year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. New Engl J Of Med (2002) 347(8):567–75. doi: 10.1056/NEJMoa020128
12. Ainsworth Rk, Kollias J, Le Blanc A, De Silva P. The clinical impact of the American college of surgeons oncology group z-0011 trial–results from the breastsurganz national breast cancer audit. Breast (2013) 22(5):733–5. doi: 10.1016/j.breast.2012.11.005
13. Van Iterson V, Leidenius M, Krogerus L, Von Smitten K. Predictive factors for the status of non-sentinel nodes in breast cancer patients with tumor positive sentinel nodes. Breast Cancer Res And Treat Nov (2003) 82(1):39–45. doi: 10.1023/B:BREA.0000003918.59396.e4
14. Crawford Jd, Ansteth M, Barnett J, Glissmeyer M, Johnson Ng. Routine completion axillary lymph node dissection for positive sentinel nodes in patients undergoing mastectomy is not associated with improved local control. Am J Surg (2013) 205(5):581–4; Discussion 584. doi: 10.1016/j.amjsurg.2013.02.001
15. Spiguel L, Yao K, Winchester Dj, Gorchow A, Du H, Sener SF, et al. Sentinel node biopsy alone for node-positive breast cancer: 12-year experience At a single institution. J Am Coll Surg Jul (2011) 213(1):122–8; Discussion 128-129. doi: 10.1016/j.jamcollsurg.2011.03.034
16. Fant Js, Grant Md, Knox Sm, Livingston SA, Ridl K, Jones RC, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Of Surg Oncol (2003) 10(2):126–30. doi: 10.1245/ASO.2003.04.022
17. Jeruss Js, Winchester Dj, Sener Sf, Brinkmann EM, Bilimora MM, Barrera E Jr, et al. Axillary recurrence after sentinel node biopsy. Ann Of Surg Oncol (2005) 12(1):34–40. doi: 10.1007/s10434-004-1164-2
18. Hwang Rf, Gonzalez-Angulo Am, Yi M, Buchholz TA, Meric-Bernstam F, Kuerer HM, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer Aug 15 (2007) 110(4):723–30. doi: 10.1002/cncr.22847
Keywords: sentinel lymph node biopsy, mastectomy, node positive breast cancer, ACOSOG Z0011, axilla dissection
Citation: You JY, Lee ES, Lim SK, Kwon Y and Jung S-Y (2023) Could axillary lymph node dissection be omitted in the mastectomy patient with tumor positive sentinel node? Front. Oncol. 13:1181069. doi: 10.3389/fonc.2023.1181069
Received: 07 March 2023; Accepted: 19 April 2023;
Published: 22 June 2023.
Edited by:
Alejandro Martin Sanchez, Multidisciplinary Breast Center - Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyReviewed by:
Flavia De Lauretis, Agostino Gemelli University Polyclinic (IRCCS), ItalyAngela Bucaro, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2023 You, Lee, Lim, Kwon and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eun Sook Lee, ZXNsZWVAbmNjLnJlLmty
 Ji Young You
Ji Young You Eun Sook Lee
Eun Sook Lee Siew Kuan Lim3
Siew Kuan Lim3 Youngmee Kwon
Youngmee Kwon So-Youn Jung
So-Youn Jung