- 1Department of Medical Oncology, Radboud University Medical Center, Nijmegen, Netherlands
- 2Department of Research, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht, Netherlands
- 3Department of Medical Oncology, Amsterdam University Medical Center location University of Amsterdam, Amsterdam, Netherlands
- 4Department of Intensive Care, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, Netherlands
Introduction: Nowadays nearly every patient with cancer is discussed in a multidisciplinary team meeting (MDTM) to determine an optimal treatment plan. The growth in the number of patients to be discussed is unsustainable. Streamlining and use of computerised clinical decision support systems (CCDSSs) are two major ways to restructure MDTMs. Streamlining is the process of selecting the patients who need to be discussed and in which type of MDTM. Using CCDSSs, patient data is automatically loaded into the minutes and a guideline-based treatment proposal is generated. We aimed to identify the pros and cons of streamlining and CCDSSs.
Methods: Semi-structured interviews were conducted with Dutch MDTM participants. With purposive sampling we maximised variation in participants’ characteristics. Interview data were thematically analysed.
Results: Thirty-five interviews were analysed. All interviewees agreed on the need to change the current MDTM workflow. Streamlining suggestions were thematised based on standard and complex cases and the location of the MDTM (i.e. local, regional or nationwide). Interviewees suggested easing the pressure on MDTMs by discussing standard cases briefly, not at all, or outside the MDTM with only two to three specialists. Complex cases should be discussed in tumour-type-specific regional MDTMs and highly complex cases by regional/nationwide expert teams. Categorizing patients as standard or complex was found to be the greatest challenge of streamlining. CCDSSs were recognised as promising, although none of the interviewees had made use of them. The assumed advantage was their capacity to generate protocolised treatment proposals based on automatically uploaded patient data, to unify treatment proposals and to facilitate research. However, they were thought to limit the freedom to deviate from the treatment advice.
Conclusion: To make oncological MDTMs sustainable, methods of streamlining should be developed and introduced. Physicians still have doubts about the value of CCDSSs.
Introduction
To provide high-quality, comprehensive and holistic oncological care, it has become common practice to discuss nearly all patients with cancer at least once in oncological multidisciplinary team meetings (MDTMs) (1). In these (usually weekly) meetings lasting one to two hours, outcomes of diagnostics and patient and disease characteristics are discussed, with the intention of determining an optimal multidisciplinary treatment plan (2). MDTMs are attended by medical specialists, and in university/training hospitals also by residents (defined as graduate doctors, in training to become medical specialists), from all involved specialties, including medical, radiation and surgical oncology, pathology, radiology, and nuclear radiology. In addition, MDTMs are often attended by clinical nurse specialists and have administrative support (3).
Discussing a patient in the MDTM is an important step in the treatment process, since it improves tumour staging, decision-making, and possibly survival (4–6). In addition, MDTMs can also have educational aspects and are used for quality assurance (7, 8). Several national guidelines therefore require that every patient with cancer should be discussed at least once in a MDTM (9–12). Not infrequently, however, a patient is discussed several times: preoperatively to discuss diagnosis and treatment plan, before and after neo-adjuvant treatment, postoperatively to determine the Tumour-Node-Metastasis (TNM) stage, additional therapies and follow-up plan (13). Patients may also be discussed in the setting of recurrent disease and in the palliative phase (9). New complex treatment options, multimodality treatments and more aggressive approaches to oligometastatic disease all increase the need to discuss patients in MDTMs more frequently than before (14). Moreover, as cancer incidence continues to rise, the number of new patients to be discussed is also increasing (15).
In most western countries, there are different types of oncological MDTMs (e.g. local MDTMs, regional MDTMs, regional/nationwide expert teams). Local MDTMs are attended by medical specialists from one (non-teaching) peripheral hospital, sometimes with the participation of an academic or regional expert specialist as an advisor. At the beginning of this century there were many general MDTMs (where different tumour-types are discussed within one MDTM), but nowadays the majority of them have been replaced by tumour-type-specific MDTMs. Many hospitals have established interhospital collaborations, which has led to regional networks of collaborating hospitals who jointly participate in one tumour-type-specific MDTM. Communication mainly takes place via videoconferencing, sometimes via audioconferencing. The composition of regional MDTMs varies, although a regional or academic expert hospital is always affiliated. Developing regional MDTM networks is common practice in many countries worldwide and this process has only accelerated in the COVID-19 era (16–18). Regional/nationwide expert teams consist of a number of super-specialised specialists from across the country or broader region, brought together via videoconference, who are consulted if the local/regional MDTM does not reach consensus. This can be on a regular basis (i.e. every two to four weeks) or on demand. Patients are often discussed in several MDTMs (8).
A number of studies investigated the amount of discussion time per case and found different results, with a median discussion time per case ranging from two to four minutes (19–22). Shorter discussion time results in decreased sharing of patient-centred information (e.g. psychosocial information and patient’s view) (22). Lack of patient-centeredness leads to greater inability to reach decisions (23, 24). The decision-making process is also found to be negatively affected by prolonged MDTMs and time/workload pressure (25–27). In addition, time pressure forces MDTMs into a more business-like atmosphere, which harms team dynamics between participants (23, 28). Previously, we reported facilitators and barriers on high quality and well-functioning MDTMs (29).
There is much concern about how MDTMs currently work. The perceived time pressure is not yet at the expense of good quality patient care (30–32). Therefore, there is an urgent need to reduce the time commitment for participants in MDTMs to continue providing optimal care for patients with cancer in the near future. After an extensive and systematic literature search we identified two major ways to reduce the time constraints on oncological MDTMs: streamlining and using computerised clinical decision support systems (CCDSSs) (33). Streamlining is the process of selecting the patients who need to be discussed and in which type of MDTM (8). CCDSSs are interactive software systems that are designed to help clinicians with decision-making tasks, such as determining a diagnosis based on automatically uploaded patient data into the minutes, or recommending for a guideline-based treatment proposal for the patient (34, 35). These CCDSSs aim to reduce unjustified practice variations and improve the quality of care (36). For example, the use of OncoDoc2, a CCDSS implemented in breast cancer MDTMs in France, was found to improve the decision compliance rate with the reference guidelines from 73% to 93% after its implementation (37).
In this study we aim to identify the pros and cons of a number of suggestions for streamlining and use of CCDSSs, as perceived by medical specialists and residents participating in MDTMs on a regular basis. Our results will further shape the debate on ways to restructure and future-proof MDTMs.
Methods
Study design
Between May 2018 and May 2019 a qualitative semi-structured interview study was conducted among participants in oncological MDTMs. We followed the COREQ (consolidated criteria for reporting qualitative research) checklist (Supplement A). The study was approved by the local ethics committee (CMO Arnhem – Nijmegen: registration number ECSW-L T-2022-5-11-24356). All interviewees agreed to participate after reading written information about the project and its aims, and their consent was formally recorded.
Interviewees
Interviewees participated in oncological MDTMs on a regular (e.g. weekly) basis. In order to maximise variation in interviewees’ professional and demographic characteristics, we purposively sampled (38) interviewees based on five criteria: 1) sex; 2) medical specialist versus residents; 3) type of hospital (peripheral or academic medical centre) 4) region of hospital (coded to A-B-C-D, based on the provinces in the Netherlands) and 5) specialty (surgical, medical and radiation oncology, radiology, nuclear radiology and pathology). Interviewees were approached by email by two researchers (ID and JW) to participate in our study.
Data collection
The primary researcher (JW) conducted semi-structured interviews. JW is a medical oncologist who has been attending two MDTMs per week for over five years and received interview training prior to the study from an experienced researcher in the field of qualitative research (GH). Interviews were conducted using a topic guide, which was evaluated and adjusted if necessary after each interview. The main topics that guided question development were: the current MDTM setting, MDTM workload and ideas on future-proofing MDTMs with emphasis on streamlining and CCDSSs (Supplement B).
During the interviews JW used probes, took notes and summarised statements to fully comprehend and validate interviewees’ perspectives. All interviewees gave their consent prior to each interview and were given the opportunity to comment and reflect on the accuracy and validity of the information obtained. All interviews were audiotaped and transcribed verbatim. Interviews had a median duration of 38.7 minutes and lasted between 27 and 72 minutes. It is worth noting that the interviews were also conducted to gain insight into the current perceived quality of MDTMs and whether they serve educational purposes for residents (29, 39). These findings are beyond the scope of this article. The transcripts were loaded and stored on secure servers at the hospital where the researchers work, using ATLAS.ti software version 8.0, a software program for detailed coding in qualitative data analysis.
Data analysis
The data was analysed using thematic analysis, with the unit of analysis being the recorded interview. In thematic analysis researchers familiarise themselves with the data through a process of reading and re-reading, generating initial codes, finding overarching themes and revising those themes (40). Three researchers (JW, AO, RM) were involved in reviewing and analysing the interview transcripts. AO and RM had different backgrounds from JW to ensure different reflexive positions (AO is a health scientist, RM is a student of biomedicine). Relevant data was identified and structured using open, axial and selective coding. Coding is the interpretive process by which conceptual labels are given to the data (41). Initially, all three researchers independently read the transcripts and coded relevant fragments (related to identifying the pros and cons of various suggestions for streamlining and using CCDSSs) to minimise the subjectivity of findings (open coding). After each interview, the transcript was coded before the next interview took place. During the iterative analysis process, researchers regularly shared and discussed the uniqueness and meaning of generated open codes. After discussion, codes were reformulated and those with the same meaning were grouped into one unique code (axial coding). After the open and axial coding of the first 15 interviews, all three researchers reached consensus on a list of codes (codebook) that guided the further coding of the rest of the interviews performed by one researcher (RM). New codes and related text fragments were then discussed with at least one of the other researchers. Finally, in the latter transcripts only data that provided additional insights were coded (selective coding). Data saturation was reached after 35 interviews: i.e. new data no longer provided additional insights in relation to the research question (42). During the iterative analysis process, researchers regularly shared and discussed the meaning and uniqueness of generated open codes. Throughout the analysis JW grouped codes belonging to the same concept into categories and finally identified themes from the data in consultation with other research members involved (RV, ID, GH). Data analysis was supported using a qualitative analysis software program (ATLAS.ti version 8.0).
Results
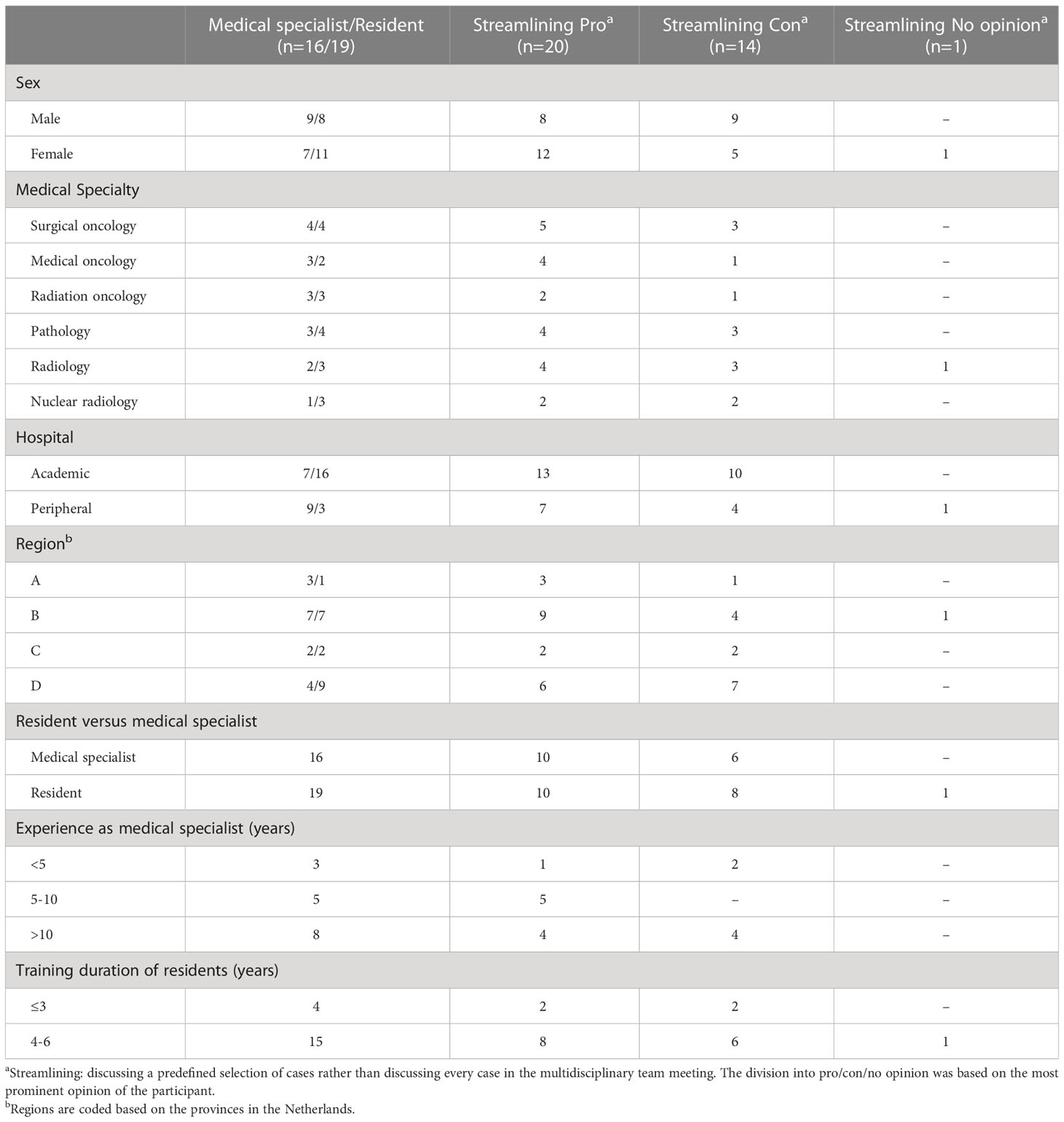
Thirty-five individual semi-structured telephone interviews were analysed. Interviewees were evenly divided according to sex, medical specialist versus resident and medical specialties. The distribution of the interviewees across the regions was slightly skewed. (Table 1). Furthermore, Table 1 also shows that the main opinion of the interviewees towards streamlining was slightly more positive (n=20) than negative (n=14), and that more female interviewees were in favour of streamlining (n=12 versus 5), while the pro-con distribution among the male interviewees was evenly distributed (n=8 versus 9). There were no differences in the pro-con streamlining distribution in the other subgroups (i.e. medical specialty, type of hospital, region, resident versus medical specialist, years of experience as specialist, or years in training as resident).
All interviewees noted that attending and preparing MDTMs is very time-consuming and indicated that the current MDTM workflow needs to be changed in the near future to keep the implementation of MDTMs feasible.
The analysis resulted in the emergence of streamlining suggestions that were thematised based on standard and complex cases and the location of the MDTM (i.e. local MDTM, regional MDTM or regional/nationwide expert teams). (Table 2) Furthermore, Table 2 also lists the associated six categories, with pros and cons per category and corresponding quotes.

Table 2 Pros and cons of suggestions for streamlininga oncological multidisciplinary team meetings.
Suggestions for streamlining
Theme 1: local or regional MDTM – standard cases
A standard case was defined as one involving a low-complex patient with a high-volume tumour type that can be treated according to existing protocols. Three categories were found in this theme: 1) discuss standard cases within local panels of two to three specialists outside the MDTM, 2) refer briefly to standard cases during the MDTM without discussion, 3) do not discuss standard cases in MDTMs at all.
The interviewees had different opinions about whether a standard case can be defined. Opponents indicated that each patient case is unique and therefore complex in its own way. They argued that every patient is entitled to a multidisciplinary discussion. Excluding standard cases from MDT discussion entails a risk of missing important information, which can ultimately lead to the formulation of incorrect treatment proposals. In addition, MDTMs have a role in recognising opportunities for patients to participate in clinical trials.
However, those interviewees in favour of distinguishing standard cases from complex cases agreed that a standard case can be defined. They proposed establishing in advance a set of criteria that a patient must meet in order to be classified as a standard case. Time saved by not discussing the standard cases could be used to discuss complex cases in more depth.
Some interviewees identified a middle ground: discussing a standard case in a local panel of two to three specialists outside the MDTM instead of with the complete MDT. Another suggestion was to introduce the standard case in the MDTM briefly without discussion: the time investment would be low, while facilitating a check for details that have been overlooked and producing MDTM minutes for practical and legal considerations.
Theme 2: regional MDTM
Two categories were found in this theme: 1) discuss complex cases in regional MDTMs, not in local MDTMs and 2) discuss all cases in regional MDTMs; abolish local MDTMs.
A complex case was defined as one involving a high-complex patient or a low-volume tumour type, both requiring multidisciplinary discussion in order to decide on the individual treatment proposal. Again, the same disagreement among interviewees was noted regarding the feasibility of distinguishing between standard and complex cases. Opponents were concerned that the local MDTM would lose expertise if complex cases were no longer discussed there. They were afraid of authority issues between participants from different hospitals in regional MDTMs. They argued that the number of patients to be discussed in regional MDTMs would increase, forcing them to participate in the discussion of patients from another hospital rather than their own hospital, fearing inefficiency and an increased workload. Besides, they questioned whether there would be appropriate financial compensation for this increase in the workload.
Those interviewees in favour of centralising complex cases to regional MDTMs believed this would create scope for pooling specialist expertise and increasing uniformity of treatment or clinical trial proposals across hospitals. They applauded the fact that regional MDTMs would contribute to further subspecialisation of specialists from peripheral hospitals within tumour types, arguing that the field of oncology is too extensive for medical professionals to be able to undertake in its full breadth.
Theme 3: regional/nationwide expert teams
One category was found in this theme: discuss highly complex cases with a regional/nationwide expert team. Most interviewees agreed that for highly complex cases, where the regional MDTM cannot reach consensus, it should be possible to consult a regional or nationwide expert team. However concerns were raised about time delay when consulting such a super-specialised team of experts. Furthermore, interviewees were unable to indicate how often such an expert team should meet and whether this should apply to every tumour type.
Including the use of CCDSSs
In Table 3 the pros and cons with associated quotes for the use of CCDSSs were listed.
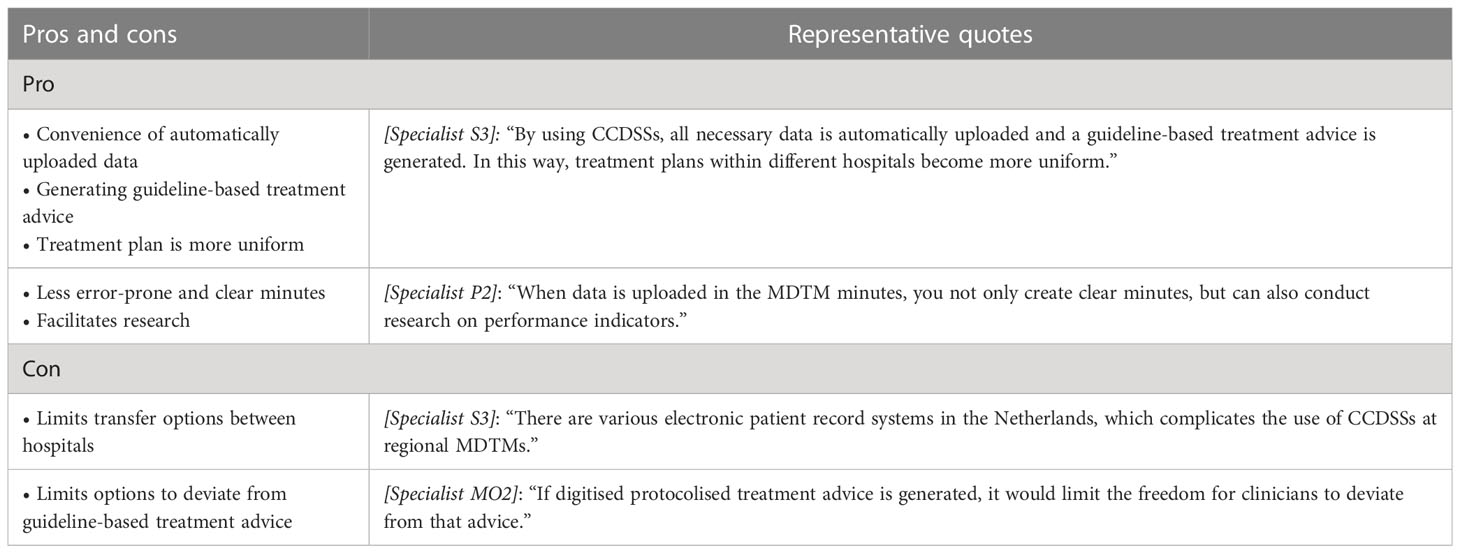
Table 3 Pros and cons of using computerised clinical decision support systems (CCDSSs) within oncological multidisciplinary team meetings (MDTMs).
Most interviewees were unfamiliar with CCDSSs. Those who had heard of CCDSSs all indicated that they expected them to be an improvement for MDTMs. None of them had actual experience with using CCDSSs. The main assumed benefits mentioned were: these systems would create greater uniformity in treatment proposals (since guideline-based treatment advice is automatically uploaded), facilitate research and result in complete and error-free minutes. However, there were also concerns about limiting the freedom for specialists to deviate from automatically generated advice and about technical problems transferring patient data between hospitals working with different electronic patient record systems.
Discussion
The current way of discussing oncological patients in MDTMs requires a considerable time investment from its participants and puts them under substantial time pressure. All interviewees agreed that there needs to be a change in the current workflow in order to guarantee the quality of MDTMs in the near future. Through streamlining, the MDTM workload can be reduced. Using CCDSSs could help standardise treatment proposals and facilitate research, but whether it would reduce the MDTM workload is unclear. We report on the pros and cons of streamlining and CCDSSs.
The increasing pressure on MDTMs and concerns regarding their sustainability are not unique to the Netherlands. Several other countries (i.e. the UK, USA, France and Australia) have reported on the need to change the current workflow of MDTMs to ensure they will be future-proof (8, 32, 43–46). In a recent national survey in the UK, the majority (69%) of the 1220 participants agreed that streamlining cases would be beneficial, although 25% of participants were against it (32). Different options for performing streamlining were explored in this survey: discuss patients in smaller teams rather than the full MDT (56% agreed), do not discuss patient cases that fit into protocolised treatment pathways at all (45% agreed), and select cases with a few participants in a pre-MDTM for discussion in the main MDTM (63% agreed) (32). These streamlining options are in line with the suggested options we found in this study. Furthermore, we noted a suggestion to improve selection of patients for local or regional MDTMs, and a suggestion regarding the next step for expert team consultation in highly complex cases. An example of a virtual on-demand expert MDTM is an advisory board for pregnant patients with cancer (47).
The most frequently mentioned objection to streamlining was a concern regarding the difficulty of distinguishing a standard case from a complex case in advance, as a result of which patients might receive incorrect treatment advice. Guidelines on how to perform this categorisation were drawn up by Winters et al. in 2021 (8). They described 11 steps to ensure the quality of the streamlining process, including the requirement that the case selection method be robust, coherent and evidence-based, the role of the chairperson strengthened, and the selection criteria audited regularly and frequently (8). Furthermore, in 2020 Soukup et al. developed and validated the tool ‘Measure of case-Discussion Complexity’ (MeDiC), which defined 27 factors that contribute to the complexity of a patient case. These factors, for example increased size (T3/T4), nodes affected or diagnostic uncertainty, can be scored by using the tool. The higher the score, the more complex the case is defined. As part of the streamlining process it was proposed to use the tool in a pre-MDTM in which the (very) high complex cases could be distinguished from the low/moderate complex cases (48). Both the guidelines by Winters et al. and the MeDiC tool are useful implementing strategies for streamlining.
The usefulness of streamlining needs to be monitored from the moment it is introduced. The emphasis should be on its impact on reducing inter-hospital differences with regard to treatment proposals and participation in clinical trials, the frequency with which patients are discussed, changes in the type of MDTM where a patient is discussed, clinical patient outcomes, effects on tumour-type-specific specialisation among specialists, feasibility of the organisation of regional/nationwide MDTM networks, stakeholder satisfaction and of course the perceived effects on time pressure.
Further future improvements must be sought in expanding the opportunities offered by CCDSSs (49, 50). A systematic review of 148 randomised controlled trials investigating the effects and clinical outcomes of CCDSSs in a variety of clinical settings concluded that CCDSSs are effective in improving health-care processes. However, evidence regarding economic, workload and efficiency outcomes were sparse (51). Another systematic review of the evaluation of CCDSS, by Van de Velde et al. (52), mentioned that clinical decision support (CDS) should be loaded automatically rather than on demand and the advice should be displayed on screens. However, adherence to CDS advice proved limited (52). In our study, interviewees were unfamiliar with CCDSSs and were afraid that automatically generated protocolised treatment proposals would limit the freedom to deviate from that treatment advice. In 2020, Klarenbeek et al. analysed the barriers and facilitators for implementing CCDSS in a lung-cancer MDTM and agreed that exposing discrepancies between formal guidelines and contextualised decisions would make specialists legally more vulnerable to criticism from patients. Furthermore, they reported a potential lack of confidence among specialists in the accuracy of system algorithms (50). Nonetheless, numerous facilitators for using CCDSSs were listed, including a more structured way of working and reduced MDTM preparation time and duration (50). Strikingly, the majority of those interviewed in our study had neither heard of CCDSSs nor had a good idea of how the use of CCDSSs could contribute to improving MDTMs, while as MDTM participants they are expected to further roll out the implementation of CCDSSs. The possibilities for CCDSSs are likely to continue to increase in the coming years. We believe that when attention is paid to informing physicians about the opportunities offered by CCDSSs, they will gradually be incorporated into MDTMs. Good IT support is mandatory.
Limitations
Our results must be interpreted in light of some limitations. First, we conducted our interview study solely in the Netherlands. However, as previously stated, several other countries also reported on the need to change the MDTM workflow. We believe therefore that our findings are relevant worldwide.
Second, we only interviewed medical specialists and residents, as they actively contribute to the MDTM discussion. However, it would be valuable to also include insights from the clinical nurse specialist, administrator or other participants such as pharmacists or genetic counsellors, with regard to future-proofing MDTMs. Moreover, the current research was focused on tumour-type specific MDTMs. It is unsure whether our findings are applicable for cancer generic molecular tumour board meetings. Further research is needed.
Third, since being in favour or against streamlining was asked in all interviewees, frequencies could be reported on that item. On the other identified pros and cons we could not report frequencies due to the semi-structured interview method of this study.
Fourth, we conducted only telephone interviews and are aware that face-to-face interviews might have created a different dynamic or depth. The primary researcher maintained an open attitude at all times, so we believe that this potential disadvantage was minimalised. Using telephone interviews increased the scope for making appointments and possibly even the willingness of the interviewees to participate, as they have busy schedules.
Lastly, due to the medical background of the interviewer as medical oncologist, it is possible that the direction of interviews or the interpretation of the data was unintentionally steered. However, this potential bias was mitigated by extensive interview training and having multiple researchers from different backgrounds involved to analyse the data.
Conclusions
The pressure on oncological MDTMs has increased dramatically in recent years due to the large number of patients that needs to be discussed. This will lead to an untenable situation in the near future. One way to address this problem is to restructure MDTMs through streamlining: discuss standard cases only briefly, not at all, or outside the MDTM with a local panel of two to three specialists, while more complex cases are discussed in tumour-type-specific regional MDTMs and highly complex cases with regional/nationwide expert teams. We identified several pros and cons in connection with streamlining. The use of CCDSSs is another way that can help future-proof MDTMs, although physicians have some doubts about whether CCDSSs reduce the MDTM workload. CCDSSs can help standardise treatment proposals and facilitate research. Further studies should focus on the implementation of streamlining and of CCDSSs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the local ethics committee (CMO region Arnhem – Nijmegen) (registration number ECSW-LT-2021-11-19-79410). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JW identified the research question, the topic guide, and the research design which was corrected and checked by ID, RM, GH, JH, RV, and VL. JW received extensive interview training from GH; JW and ID invited interviewees to participate. JW performed semi-structured telephone interviews. JW and RM analysed interview transcripts. The codebook was developed and refined and categories and themes emerged in consultation with ID, GH, and RV. JW wrote the manuscript. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The assistance of A. Oude-Bos (AO) in coding the data of this study was greatly appreciated. The grammatical assistance of A. Mills is highly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1178165/full#supplementary-material
References
1. Horlait M, Baes S, De Regge M, Leys M. Understanding the complexity, underlying processes, and influencing factors for optimal multidisciplinary teamwork in hospital-based cancer teams: a systematic integrative review. Cancer nursing (2021) 44(6):E476–e92. doi: 10.1097/NCC.0000000000000923
2. El Saghir NS, Keating NL, Carlson RW, Khoury KE, Fallowfield L. Tumor boards: optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Meeting (2014), e461–6. doi: 10.14694/EdBook_AM.2014.34.e461
3. Ottevanger N, Hilbink M, Weenk M, Janssen R, Vrijmoeth T, de Vries A, et al. Oncologic multidisciplinary team meetings: evaluation of quality criteria. J Eval Clin practice (2013) 19(6):1035–43. doi: 10.1111/jep.12022
4. Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev (2016) 42:56–72. doi: 10.1016/j.ctrv.2015.11.007
5. Kočo L, Weekenstroo HHA, Lambregts DMJ, Sedelaar JPM, Prokop M, Fütterer JJ, et al. The effects of multidisciplinary team meetings on clinical practice for colorectal, lung, prostate and breast cancer: a systematic review. Cancers (Basel) (2021) 13(16):4159. doi: 10.3390/cancers13164159
6. Peng D, Cheng YX, Cheng Y. Improved overall survival of colorectal cancer under multidisciplinary team: a meta-analysis. BioMed Res Int (2021) 2021:5541613. doi: 10.1155/2021/5541613
7. Rankin NM, Lai M, Miller D, Beale P, Spigelman A, Prest G, et al. Cancer multidisciplinary team meetings in practice: results from a multi-institutional quantitative survey and implications for policy change. Asia-Pacific J Clin Oncol (2018) 14(1):74–83. doi: 10.1111/ajco.12765
8. Winters DA, Soukup T, Sevdalis N, Green JSA, Lamb BW. The cancer multidisciplinary team meeting: in need of change? history, challenges and future perspectives. BJU Int (2021) 128(3):271–9. doi: 10.1111/bju.15495
9. Stichting Oncologische Samenwerking. SONCOS normeringrapport 6; multidisciplinaire oncologische zorg in nederland. (2018) (Commission on cancer). Available at: https://demedischspecialist.nl/sites/default/files/Soncos_norm_versie6_rapp2018.pdf.
10. Cannell E. The French cancer plan: an update. Lancet Oncol (2005) 6(10):738. doi: 10.1016/S1470-2045(05)70369-7
11. American college of surgeons C, IL. Commission on cancer. cancer program standards 2012, ensuring patient-centered care. (2012).
12. Victorian Cancer plan 2016-2020; improving cancer outcomes for all victorians . Available at: www.healthvic.gov.au/cancer.
13. Freytag M, Herrlinger U, Hauser S, Bauernfeind FG, Gonzalez-Carmona MA, Landsberg J, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC cancer (2020) 20(1):355. doi: 10.1186/s12885-020-06809-1
14. Askelin B, Hind A, Paterson C. Exploring the impact of uro-oncology multidisciplinary team meetings on patient outcomes: a systematic review. Eur J Oncol Nurs (2021) 54:102032. doi: 10.1016/j.ejon.2021.102032
15. Registry NpbNC . Available at: https://www.cijfersoverkanker.nl/.
16. Shamash J, Ansell W, Alifrangis C, Thomas B, Wilson P, Stoneham S, et al. The impact of a supranetwork multidisciplinary team (SMDT) on decision-making in testicular cancers: a 10-year overview of the anglian germ cell cancer collaborative group (AGCCCG). Br J cancer (2021) 124(2):368–74. doi: 10.1038/s41416-020-01075-1
17. Perlmutter B, Simon R, Joyce D, Walsh RM, Augustin T. Benefits and challenges of virtual tumor board conferences in the COVID-19 era. J Am Coll Surg (2021) 233(5 Supplement 1):S243. doi: 10.1016/j.jamcollsurg.2021.07.502
18. To N, Bekker HL, Henry K, Melling P, Turley J, Lodge JPA, et al. COVID-19 restrictions on multidisciplinary team meeting decision-making: service evaluation in a major UK cancer centre. Br J surg (2021) 108(4):e162–e3. doi: 10.1093/bjs/znab009
19. Yuan Y, Ye J, Ren Y, Dai W, Peng J, Cai S, et al. The efficiency of electronic list-based multidisciplinary team meetings in management of gastrointestinal malignancy: a single-center experience in southern China. World J Surg Oncol (2018) 16(1):146. doi: 10.1186/s12957-018-1443-1
20. Mullan BJ, Brown JS, Lowe D, Rogers SN, Shaw RJ. Analysis of time taken to discuss new patients with head and neck cancer in multidisciplinary team meetings. Br J Oral Maxillofac Surg (2014) 52(2):128–33. doi: 10.1016/j.bjoms.2013.10.001
21. Gandamihardja TAK, Soukup T, McInerney S, Green JSA, Sevdalis N. Analysing breast cancer multidisciplinary patient management: a prospective observational evaluation of team clinical decision-making. World J surg (2019) 43(2):559–66. doi: 10.1007/s00268-018-4815-3
22. Festen S, Nijmeijer H, van Leeuwen BL, van Etten B, van Munster BC, de Graeff P. Multidisciplinary decision-making in older patients with cancer, does it differ from younger patients? Eur J Surg Oncol (2021) 47(10):2682–8. doi: 10.1016/j.ejso.2021.06.003
23. Dew K, Stubbe M, Signal L, Stairmand J, Dennett E, Koea J, et al. Cancer care decision making in multidisciplinary meetings. Qual Health Res (2015) 25(3):397–407. doi: 10.1177/1049732314553010
24. Geerts PAF, van der Weijden T, Savelberg W, Altan M, Chisari G, Launert DR, et al. The next step toward patient-centeredness in multidisciplinary cancer team meetings: an interview study with professionals. J Multidiscip Healthc (2021) 14:1311–24. doi: 10.2147/JMDH.S286044
25. Soukup T, Lamb BW, Morbi A, Shah NJ, Bali A, Asher V, et al. A multicentre cross-sectional observational study of cancer multidisciplinary teams: analysis of team decision making. Cancer Med (2020) 9(19):7083–99. doi: 10.1002/cam4.3366
26. Soukup T, Gandamihardja TAK, McInerney S, Green JSA, Sevdalis N. Do multidisciplinary cancer care teams suffer decision-making fatigue: an observational, longitudinal team improvement study. BMJ Open (2019) 9(5):e027303. doi: 10.1136/bmjopen-2018-027303
27. Soukup T, Lamb BW, Weigl M, Green JSA, Sevdalis N. An integrated literature review of time-on-Task effects with a pragmatic framework for understanding and improving decision-making in multidisciplinary oncology team meetings. Front Psychol (2019) 10:1245. doi: 10.3389/fpsyg.2019.01245
28. Delaney G, Jacob S, Iedema R, Winters M, Barton M. Comparison of face-to-face and videoconferenced multidisciplinary clinical meetings. Australas Radiol (2004) 48(4):487–92. doi: 10.1111/j.1440-1673.2004.01349.x
29. Walraven JEW, Verhoeven RHA, Meulen RV, Hoeven J, Lemmens V, Hesselink G, et al. Facilitators and barriers to conducting an efficient, competent and high-quality oncological multidisciplinary team meeting. BMJ Open Qual (2023) 12(1). doi: 10.1136/bmjoq-2022-002130
30. Maharaj AD, Evans SM, Zalcberg JR, Ioannou LJ, Graco M, Croagh D, et al. Barriers and enablers to the implementation of multidisciplinary team meetings: a qualitative study using the theoretical domains framework. BMJ Qual safety (2021) 30(10):792–803. doi: 10.1136/bmjqs-2020-011793
31. Field KM, Rosenthal MA, Dimou J, Fleet M, Gibbs P, Drummond K. Communication in and clinician satisfaction with multidisciplinary team meetings in neuro-oncology. J Clin Neurosci (2010) 17(9):1130–5. doi: 10.1016/j.jocn.2010.03.001
32. Hoinville L, Taylor C, Zasada M, Warner R, Pottle E, Green J. Improving the effectiveness of cancer multidisciplinary team meetings: analysis of a national survey of MDT members’ opinions about streamlining patient discussions. BMJ Open Qual. (2019) 8(2):e000631. doi: 10.1136/bmjoq-2019-000631
33. Walraven JEW, van der Hel OL, van der Hoeven JJM, Lemmens V, Verhoeven RHA, Desar IME. Factors influencing the quality and functioning of oncological multidisciplinary team meetings: results of a systematic review. BMC Health Serv Res (2022) 22(1):829. doi: 10.1186/s12913-022-08112-0
34. Klarenbeek SE, Weekenstroo HHA, Sedelaar JPM, Fütterer JJ, Prokop M, Tummers M. The effect of higher level computerized clinical decision support systems on oncology care: a systematic review. Cancers (Basel). (2020) 12(4):1032. doi: 10.3390/cancers12041032
35. O’Sullivan D, Fraccaro P, Carson E, Weller P. Decision time for clinical decision support systems. Clin Med (London England). (2014) 14(4):338–41. doi: 10.7861/clinmedicine.14-4-338
36. Peleg M. Computer-interpretable clinical guidelines: a methodological review. J Biomed inform (2013) 46(4):744–63. doi: 10.1016/j.jbi.2013.06.009
37. Séroussi B, Bouaud J, Gligorov J, Uzan S. Supporting multidisciplinary staff meetings for guideline-based breast cancer management: a study with OncoDoc2. AMIA Annu Symp Proc (2007) 2007:656–60.
38. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health (2015) 42(5):533–44. doi: 10.1007/s10488-013-0528-y
39. Walraven JEW, van der Meulen R, van der Hoeven JJM, Lemmens V, Verhoeven RHA, Hesselink G, et al. Preparing tomorrow’s medical specialists for participating in oncological multidisciplinary team meetings: perceived barriers, facilitators and training needs. BMC Med Educ (2022) 22(1):502.
40. Braun V CV. Using thematic analysis in psychology. Qual Res Psychol (2006) 3:77–101. doi: 10.1191/1478088706qp063oa
41. Corbin JSA. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol (1990) 3–21. doi: 10.1007/BF00988593
42. Sawatsky AP, Ratelle JT, Beckman TJ. Qualitative research methods in medical education. Anesthesiology (2019) 131(1):14–22. doi: 10.1097/ALN.0000000000002728
43. Soukup T, Lamb BW, Sevdalis N, Green JS. Streamlining cancer multidisciplinary team meetings: challenges and solutions. Br J Hosp Med (London England: 2005). (2020) 81(3):1–6. doi: 10.12968/hmed.2020.0024
44. Field KM, Rosenthal MA, Dimou J, Kaye A, Gibbs P, Drummond K. Neuro-oncology multidisciplinary team (MDT) meetings: an effective method of documentation and information dissemination. Asia-Pacific J Clin Oncol (2009) 5:A194.
45. Rollet Q, Bouvier V, Moutel G, Launay L, Bignon AL, Bouhier-Leporrier K, et al. Multidisciplinary team meetings: are all patients presented and does it impact quality of care and survival - a registry-based study. BMC Health Serv Res (2021) 21(1):1032. doi: 10.1186/s12913-021-07022-x
46. Harzstark AL, Altschuler A, Amsden LB, Alavi M, Liu L, Presti JC, et al. Implementation of a multidisciplinary expert testicular cancer tumor board across a Large integrated healthcare delivery system Via early case ascertainment. JCO Clin Cancer Inform. (2021) 5:187–93. doi: 10.1200/CCI.20.00114
47. Heimovaara JH, Boere IA, de Haan J, van Calsteren K, Amant F, van Zuylen L, et al. Ten-year experience of a national multidisciplinary tumour board for cancer and pregnancy in the Netherlands. Eur J Cancer (Oxford England: 1990). (2022) 171:13–21. doi: 10.1016/j.ejca.2022.04.040
48. Soukup T, Morbi A, Lamb BW, Gandamihardja TAK, Hogben K, Noyes K, et al. A measure of case complexity for streamlining workflow in multidisciplinary tumor boards: mixed methods development and early validation of the MeDiC tool. Cancer Med (2020) 9(14):5143–54. doi: 10.1002/cam4.3026
49. Patkar V, Acosta D, Davidson T, Jones A, Fox J, Keshtgar M. Using computerised decision support to improve compliance of cancer multidisciplinary meetings with evidence-based guidance. BMJ Open (2012) 2(3). doi: 10.1136/bmjopen-2011-000439
50. Klarenbeek SE, Schuurbiers-Siebers OCJ, van den Heuvel MM, Prokop M, Tummers M. Barriers and facilitators for implementation of a computerized clinical decision support system in lung cancer multidisciplinary team meetings-a qualitative assessment. Biol (Basel). (2020) 10(1):9. doi: 10.3390/biology10010009
51. Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Ann Internal Med (2012) 157(1):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450
Keywords: multidisciplinary team meeting, oncology, streamlining, computerized clinical decision support systems, oncology care
Citation: Walraven JEW, Verhoeven RHA, van der Hoeven JJM, van der Meulen R, Lemmens VEPP, Hesselink G and Desar IME (2023) Pros and cons of streamlining and use of computerised clinical decision support systems to future-proof oncological multidisciplinary team meetings. Front. Oncol. 13:1178165. doi: 10.3389/fonc.2023.1178165
Received: 02 March 2023; Accepted: 27 April 2023;
Published: 18 May 2023.
Edited by:
Francesco Bianco, University of Illinois Chicago, United StatesReviewed by:
Tayana Soukup, King’s College London, United KingdomPamala Pawloski, HealthPartners Institute for Education and Research, United States
Copyright © 2023 Walraven, Verhoeven, van der Hoeven, van der Meulen, Lemmens, Hesselink and Desar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janneke E. W. Walraven, SmFubmVrZS53YWxyYXZlbkByYWRib3VkdW1jLm5s
†ORCID: Janneke E. W. Walraven, orcid.org/0000-0001-7161-5369
Rob H. A. Verhoeven, orcid.org/0000-0001-9678-1611
Valery E. P. P. Lemmens, orcid.org/0000-0002-4109-5734
Gijs Hesselink, orcid.org/0000-0003-2532-0724
Ingrid M. E. Desar, orcid.org/0000-0003-0945-8299
 Janneke E. W. Walraven
Janneke E. W. Walraven Rob H. A. Verhoeven2,3†
Rob H. A. Verhoeven2,3† Ingrid M. E. Desar
Ingrid M. E. Desar