- 1Department of Medicine: Hematology/Oncology, Vanderbilt University Medical Center, Nashville, TN, United States
- 2Department of Pharmacy, Vanderbilt University Medical Center, Nashville, TN, United States
Colorectal cancer results in the deaths of hundreds of thousands of patients worldwide each year, with incidence expected to rise over the next two decades. In the metastatic setting, cytotoxic therapy options remain limited, which is reflected in the meager improvement of patient survival rates. Therefore, focus has turned to the identification of the mutational composition inherent to colorectal cancers and development of therapeutic targeted agents. Herein, we review the most up to date systemic treatment strategies for metastatic colorectal cancer based on the actionable molecular alterations and genetic profiles of colorectal malignancies.
Introduction
Within the United States, colorectal cancer (CRC) continues to be a substantial source of morbidity and mortality, with an estimated 153,000 new cases diagnosed and over 52,000 deaths projected in 2023 alone (1). Nearly a quarter of patients are afflicted with metastatic disease (mCRC) at disease presentation, while another 20% of patient initially diagnosed with localized disease, progressing to stage IV disease (2, 3). Stage IV disease portends a very poor prognosis, with an estimated 5-year survival rate of only 14%. While survival rates have improved within the United States and globally over the past several decades for CRC of all stages, mCRC survival rates have remained stable without significant progress (3–5). Therefore, extensive comprehension of the varying molecular and genetic profiles within mCRC and development of associated anti-neoplastic targets is pivotal to treatment advancement and improving patient outcomes. We present a review of the most current, trial-based evidence of the treatment of mCRC based on unique molecular and genetic profiles that allow for refinement and strengthening of therapeutic options for patients limited cytotoxic therapy options.
EGFR inhibitors
The role of EGFR in cellular signaling and its inhibition
The propagation of many known human neoplasms are driven by activation of epidermal growth factor receptor (EGFR) and its subsequential signaling pathways (6). Binding of an activating ligand to EGFR results in phosphorylation of EGFR tyrosine kinase, triggering downstream signaling pathways involved in cellular proliferation and metabolism. EGFR is involved in several pathways, including the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway as well as the RAS/RAF/mitogen-activated protein kinase (MAPK) pathway (7). Activation or dysregulation of the of these pathways or imbalance of the sensitive feedback loops results in transcription of genes promoting cell survival, anti-apoptosis, proliferation, angiogenesis and metastatic potential (6, 8).
Cetuximab and panitumumab are monoclonal antibodies used in the treatment of metastatic colon cancer, directed against EGFR. Cetuximab is a chimeric IgG-1 monoclonal antibody while panitumumab is a recombinant humanized IgG-2 kappa monoclonal antibody, both working to competitively inhibit the extracellular ligand of EGFR, limiting the aforementioned abnormal cellular signaling that result in tumorigenesis (9). Although considered equivalent in their efficacy, cetuximab has been shown to have a higher incidence of hypersensitivity reactions, with an estimated risk ratio of 5.47. This hypersensitivity was shown likely to be secondary to previously developed IgE antibodies against galactose-alpha-1,3-galactose present on the Fab portion of the cetuximab heavy chain. The prevalence of this pre-existing IgE antibody is higher in the Southeastern United states, thought related to regional exposure (10).
EGFR inhibitors and efficacy based on RAS mutational status
Mutations in genes (notably KRAS, NRAS, and BRAF) that encode proteins involved in EGFR-mediated cellular signaling pathways are associated with a lack of response to anti-EGFR therapy in mCRC (11–17). Mutations in the RAS family of genes result in protein expression that lead to inappropriate constitutive activation of the RAS/RAF/MAPK signaling that is less likely to be affected by inhibition of the upstream interaction of EGFR with an activating ligand. Thus, testing for these mutations is essential to ensure patients whose tumors harbor these mutations are not subjected to ineffective therapy with potentially severe toxicity and expense.
The first study to evaluate the use of EGFR inhibition in mCRC was in 2008, comparing the cetuximab use of cetuximab versus best supportive care (Table 1). The authors found that patients with wild type KRAS tumors had a significantly improved OS (9.5 vs 4.8 months HR0.55; 95% CI 0.41-0.74) with the use of cetuximab, versus no difference in survival or PFS for those with KRAS mutated tumors (Table 1). This was followed by studies investigating EGFR inhibition in combination with cytotoxic chemotherapy (14).
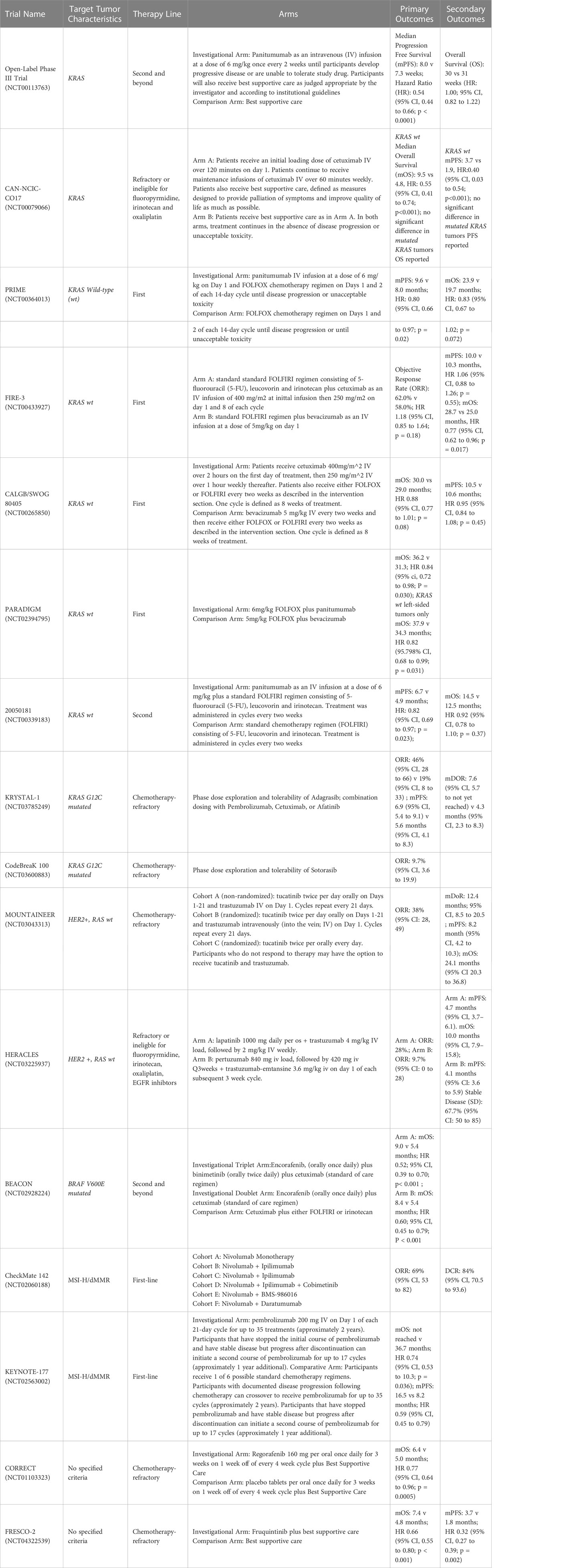
Table 1 Pivotal Clinical Trials in Metastatic Colorectal Cancer categorized by tumor characteristics.
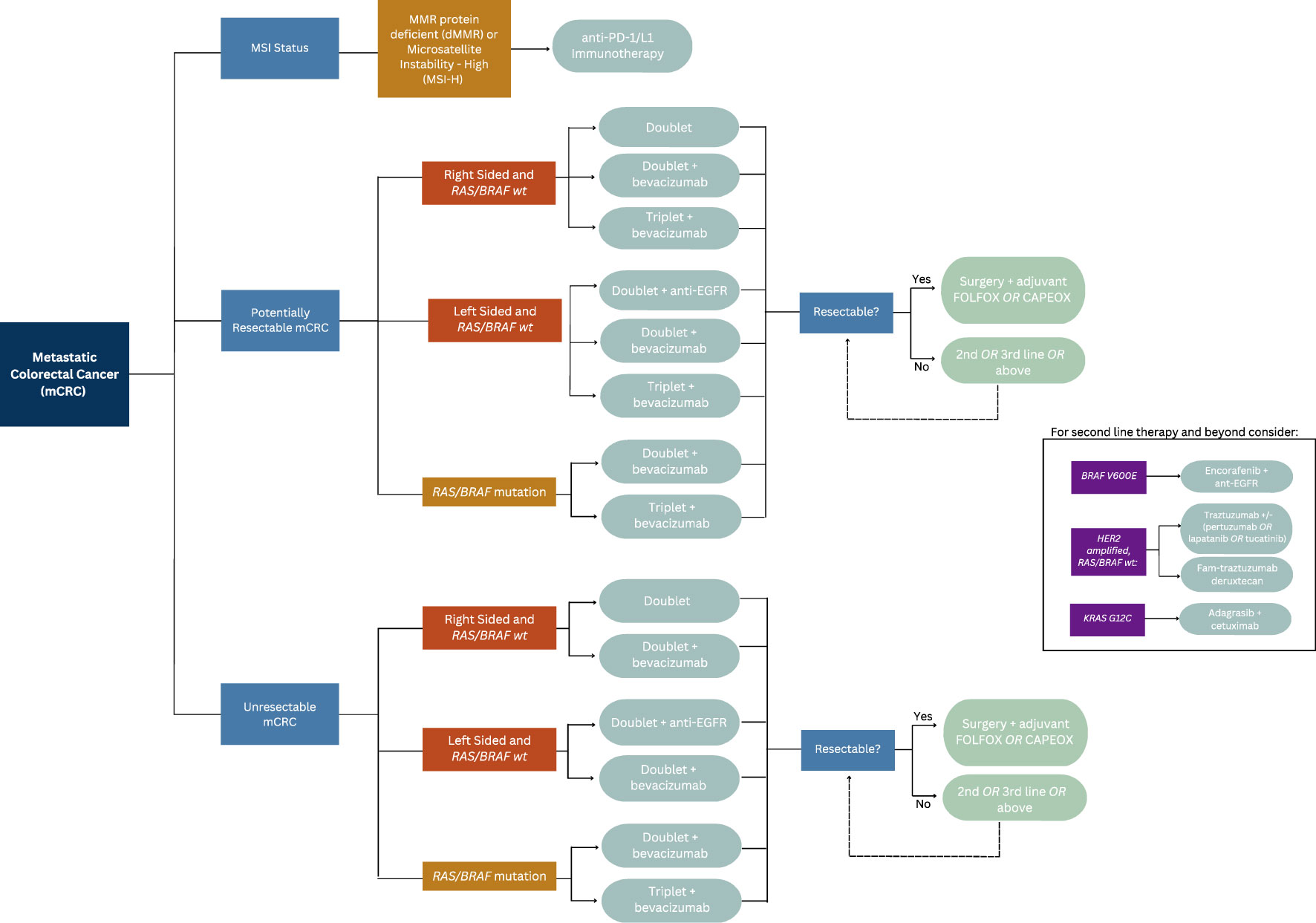
A retrospective analysis of three randomized controlled trials compared the outcomes of patients with mCRC who received chemotherapy or best supportive care with or without panitumumab in various lines of therapy, concluding patients with KRAS mutated mCRC were unlikely to benefit from EGFR inhibitors (Table 1) (18). Similarly, a subset analysis of patients enrolled in the CRYSTAL trial, which randomized untreated patients with mCRC to receive FOLFIRI either with or without cetuximab, found that patients with KRAS wild-type exon 2 tumors who received FOLFIRI and cetuximab experienced a longer median PFS compared to those who received FOLFIRI alone (9.9 vs 8.7 months; HR 0.68; 95% CI 0.50–0.94; p = 0.02) (12). An updated analysis of data from the CRYSTAL trial showed longer overall survival (OS) in patients who received cetuximab (23.5 vs 20.0 months; p = 0.009), a benefit largely derived by patients with RAS wild-type tumors (HR 0.69; 95% CI 0.54–0.88). Those with RAS-mutated tumors did not derive a survival benefit (1.05; 95% CI 0.86–1.28) (19, 20). This effect was also observed in the phase III PRIME trial, which compared groups of patients with untreated mCRC who received FOLFOX with or without panitumumab (Table 1). Among patients with wild-type KRAS and NRAS mCRC, improvements in PFS (HR 0.72; 95% CI 0.58–0.90; p = 0.004) and OS (HR 0.77; 95% CI 0.64–0.94; p = 0.009) were seen in patients who received FOLFOX plus panitumumab. Importantly, PFS was found to be worse in patients whose tumor harbored a KRAS/NRAS mutation (11, 21). The results reflect the current clinical practice of ensuring patients with RAS wild type tumors are provided anti-EGFR therapy, and that these therapies are avoided in those with a RAS mutated tumors due to lack efficacy or potentiation of worse outcomes. Please reference Figure 1 for the suggested treatment algorithm based on mutational status.
EGFR inhibition and primary tumor sidedness
The impact of EGFR inhibitors and the side of primary colon tumor is associated with treatment response, or lack thereof. This is believed to be a result of tumor sidedness being a surrogate for differing cumulative molecular subtypes (22).
In a multicenter analysis of 75 patients with RAS and BRAF wild-type mCRC who received cetuximab alone, panitumumab alone, or irinotecan plus cetuximab (in any line of therapy), no responses were observed in patients who had right-sided primary tumors while a response rate of 41% was seen in patients with left-sided primary tumors (p = 0.003). Progression free survival (PFS) (2.3 vs 6.6 months) was also longer in patients with left-sided tumors (HR 3.97; 95% CI 2.09–7.53; p < 0.0001) (23).
This phenomenon was demonstrated in retrospective evaluations of landmark CRC trials. First, in the CRYSTAL trial, referenced above, which randomized patients to FOLFIRI plus cetuximab versus FOLFIRI alone, and the FIRE-3 trial comparing FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab, patients with RAS wild type left sided tumors had had superior objective response rate (ORR), PFS and OS with the addition of cetuximab, in contrast to minimal efficacy seen in right sided wild type tumors (Table 1) (12, 24, 25). The CALGB/SWOG 80405 not only demonstrated poorer prognosis associated with right sided CRC, but KRAS wild type right sided tumors had significantly worse median OS relative to left sided KRAS wild type tumors when treated with cetuximab and chemotherapy (16.7 months (95% CI 13.1-19.4) vs 36 months (95% CI 32.6-40.3) (Table 1) (26). Most recently, the phase III, open-label multicenter PARADIGM trial was designed to determine the superiority of anti-EGFR therapy or anti-vascular endothelial growth factor (anti-VEGF) therapy when added to modified FOLFOX6 in RAS wild type mCRC (Table 1). This showed an improvement in overall survival by 3.6 months in patients with left sided tumors that were treated with panitumumab compared to bevacizumab (27).
EGFR inhibitors and conversion to resectable disease
EGFR inhibitors, when combined with chemotherapy, have also shown an ability to increase the possibility of liver metastases resection in patients with RAS wild-type mCRC. In a randomized trial from China, the addition of cetuximab to chemotherapy resulted in 20 of 70 (29%) of patients becoming eligible for hepatic resection compared to 9 of 58 (13%) of patients who did not receive cetuximab. R0 resection rates were 25.7% in cetuximab arm compared to 7.4% in those who didn’t receive cetuximab (p <0.01). Additionally, surgery improved median survival compared to those who did not receive surgery in the cetuximab arm (46.6 vs 25.7 months; p = 0.007) and the control arm (36.0 vs 19.6 months; p = 0.016) (28). In the VOLFI phase II trial, 75% of patients with RAS wild-type mCRC and liver metastases deemed potentially resectable were successfully converted to resectable disease upon receiving FOLFOXIRI with panitumumab compared to 36.4% in group of patients who received FOLFOXIRI alone. ORR was higher in the panitumumab arm, while PFS and OS were similar between both arms, with OS trend in favor of the arm that received panitumumab (29).
EGFR inhibitors in patients with refractory disease
For patients with wild-type KRAS/NRAS/BRAF mCRC whose disease progressed on a therapeutic regimen that contained an EGFR inhibitor, the use of an EGFR inhibitor as part of therapy in the next line is generally not recommended. However, if these patients’ first-line regimen did not include an EGFR inhibitor, there is evidence that use of an EGFR inhibitor in the subsequent line of therapy is beneficial. For example, in a phase III trial analyzing wild-type KRAS exon 2 tumors that exhibited disease progression on oxaliplatin-based and irinotecan-based chemotherapy, panitumumab monotherapy was compared to best supportive care. This trial demonstrated an overall survival benefit of nearly 3 months with the use of panitumumab (10.0 vs 7.4 months; HR 0.73; 95% CI 0.57–0.93; p < 0.01) (30). Not all studies evaluating EGFR inhibitors in this setting have improved overall survival, however. In Study 20050181, the addition of panitumumab to FOLFIRI compared to FOLFIRI alone in patients with mCRC who had wild-type KRAS exon 2 tumors resulted in an improvement in median PFS (6.7 vs 4.9 months; HR 0.82; 95% CI 0.69–1.10; p = 0.023) but no difference in OS (median 14.5 vs. 12.5 months; HR 0.92; 95% CI 0.78–1.10; p = 0.37) (Table 1) (31, 32). In the EPIC trial, which compared irinotecan plus cetuximab to irinotecan alone as second line treatment in patients with mCRC who progressed on first-line fluoropyrimidine and oxaliplatin based therapy, both ORR and median PFS, were significantly improved in the combination group (PFS 5.4 vs 2.6 months (95% CI 0.46-0.69), ORR 29.4% vs 5.0% (95% CI 4.04-17.40) respectively). There was no statically significant difference in median OS between arms, however, a post study treatment analysis indicated improvement in OS in those who received post-study cetuximab relative to those who received subsequent therapy without cetuximab or no therapy at all. Importantly, quality of life was found to be improved in the combination arm, including improvement in physical functioning, nausea, vomiting, appetite loss and pain (33, 34).
Chemotherapy choice when used in conjunction with EGFR inhibitors
There is conflicting data to suggest that oxaliplatin-based chemotherapy regimens reduce the efficacy of cetuximab in patients with untreated RAS wild-type mCRC. In the phase II OPUS trial, patients with mCRC and KRAS wild-type exon 2 tumors who received FOLFOX plus cetuximab in the first-line setting did not derive a statistically significant benefit with regard to OS compared to patients who received FOLFOX alone (22.8 vs 18.5 months; HR 0.85; p = 0.39) (16, 35). The lack of survival benefit when cetuximab is added to oxaliplatin-based regimens was also observed in the phase III MRC COIN trial, in which patients with KRAS wild-type mCRC or locally advanced disease who received cetuximab and either FOLFOX or CAPEOX did not have longer OS relative to patients who received chemotherapy alone (17.9 vs 17.0 months; HR 1.04; 95% CI 0.87–1.23; p = 0.67). However, a subgroup analysis of this trial indicated that those who received FOLFOX, rather than CAPEOX, might have experienced a benefit (36).
In contrast to the above findings, the results found by the phase III TAILOR trial, showed prolonged PFS (9.2 vs 7.4 months; p = 0.004) and OS (20.7 vs 17.8 months p = 0.02), and ORR (61.1% vs 39.5%; p < 0.001) among patients with untreated RAS wild-type mCRC who received cetuximab with FOLFOX compared to those who received FOLFOX alone (37). The results of the PARADIGM trial discussed above also demonstrated opposing results, with significantly improved OS in RAS wild type, left sided tumors with the use of oxaliplatin based therapy with the addition of panitumumab. Given these mixed results, suggested clinical practice is such that in the treatment of left sided RAS wild type tumors, the use of anti-EGFR therapy in conjunction with irinotecan or oxaliplatin based chemotherapy backbone is standard, with backbone choice based on individual patient co-morbidities and side effect profile.
Efficacy of EGFR inhibition in patients with BRAF mutations
BRAF encodes a protein that functions downstream of RAS in the EGFR-mediated signaling pathway and, when mutated, is constitutively active (3, 27). Therefore, upstream EGFR inhibition alone is not thought to prevent abnormal signaling mediated by BRAF mutations.
Specifically, BRAF V600E mutations result in the inappropriate activation of MAPK independently of RAS (38). Abnormal regulation of these pathways is invariably linked to carcinogenesis (4). Given this downstream effect, inhibition of EGFR presents little utility in the setting of concurrent RAS wild type and BRAF mutated CRC.
Approximately five to nine percent of patients with mCRC have BRAF V600E mutations, which do not typically occur in co-existence with RAS mutations (39). In subset analyses of patients in the aforementioned PRIME trial, as well as the COIN trial, BRAF mutations were not found to be predictive of response to the combination of chemotherapy and an EGFR inhibitor in patients with untreated mCRC (24). One meta-analysis that included 463 patients with BRAF mutant mCRC including nine phase III trials and one phase II trial with mCRC concluded that the addition of an EGFR inhibitor did not improve PFS (HR 0.88; 95% CI 0.67–1.14; p = 0.33) or OS (HR 0.91; 95% CI 0.62–1.34; p = 0.63) (29). Another meta-analysis of seven randomized control trials (RCT) found EGFR inhibitors did not improve PFS (HR 0.86; 95% CI 0.61–1.21) or OS (HR 0.97; 95% CI 0.67–1.41) in patients with BRAF mutations (30). Therefore, the use of ant-EGFR therapy in the setting of BRAF mutations is of little to no efficacy.
VEGF inhibitors
The role of VEGF in cellular signaling
Vascular endothelial growth factor (VEGF) is a protein that, upon binding to VEGF receptors 1 and 2 on the surface of endothelial cells, promotes tumor angiogenesis by promoting permeability, survival, and proliferation of endothelial cells. VEGF is expressed in the majority of human malignancies, while having little role in normal physiological angiogenesis. The activity of VEGF is inhibited by bevacizumab, a humanized monoclonal antibody against circulating VEGF-A, that has become a mainstay adjunctive therapy in the treatment of mCRC (40, 41).
Bevacizumab as part of first-line therapy
Several trials have investigated the efficacy of adding bevacizumab to chemotherapy in patients with untreated mCRC and have displayed varying results. Pooled results from several phase II trials of patients with untreated mCRC have indicated that OS was prolonged by the addition of bevacizumab to 5-flurouracil (5-FU)/leucovorin with or without irinotecan (42–44). A combined analysis of the results of these trials showed that adding bevacizumab to 5-FU/leucovorin improved median survival compared to 5-FU/leucovorin or irinotecan without bevacizumab (17.9 vs 14.6 months; p = 0.008) (45). In patients 70 years and older with untreated mCRC, the addition of bevacizumab to capecitabine prolonged PFS compared to capecitabine alone (9.1 vs 5.1 months; HR 0.53; 95% CI 0.41–0.69; p < 0.0001) in the AVEX trial (46).
Chemotherapy choice when used in conjunction with bevacizumab
A meta-analysis of six RCTs encompassing a total of 3,060 patients showed that the addition of bevacizumab to chemotherapy in the first line setting prolonged PFS (HR 0.72; 95% CI 0.66–0.78; p < 0.00001) and OS (HR 0.84; 95% CI 0.77–0.91; p < 0.00001) relative to chemotherapy alone (47). Subgroup analyses, however, indicated that this addition was largely limited to patients who received irinotecan-based regimens. This result was also reflected in a SEER analysis which showed the addition of bevacizumab to oxaliplatin-based chemotherapy did not improve OS but did improve OS for patients who received irinotecan (48). Additionally, in a large phase III trial, PFS, but not OS, was prolonged by 1.4 months by addition of bevacizumab to oxaliplatin-based chemotherapy (HR 0.83; 97.5% CI 0.72–0.95; p = 0.0023) in patients with untreated mCRC, yet a subset analysis suggested that those who received CAPEOX (rather than FOLFOX) were most likely to experience that benefit (49). To date, no trials have compared FOLFIRI to FOLFIRI plus bevacizumab or FOLFOXIRI to FOLFOXIRI plus bevacizumab. Despite the results discussed above, clinical practice prioritizes the use of bevacizumab in those with RAS mutant or right sided RAS wild type metastatic colon cancers in patients without contraindications to its use.
Bevacizumab and conversion to resectable disease
Few trials have been conducted to investigate the utility of bevacizumab in the peri-operative setting. The BECOME trial specifically evaluated the role of bevacizumab, in conjunction with FOLFOX, in the conversion of unresectable mCRC to resectable disease in patients with unresectable liver-limited mCRC. This trial found that the addition of bevacizumab to FOLFOX improved the rate at which patients underwent R0 hepatic resection (22.3% vs 5.8%; p < 0.01) (50). The multinational phase II OLIVIA trial sought to evaluate if the role of bevacizumab to either FOLFOX or FOLFOXIRI to facilitate oligometastatic resection in patients initially determined to have unresectable liver metastasis. The combination of FOLFOXIRI with bevacizumab resulted in higher ORR (81% (95% CI 65-91) vs 62% (95% CI 45-77), rate of resection (61% (95% CI 45-76) vs 49% (95% CI 32-65)), R0 resection rate (49% vs 23%) and median PFS (18.6 (95% CI 12.9-22.3) vs 11.5 months (95% CI 9.6-13.6)) relative to bevacizumab plus FOLFOX. These response rates were at the expense of higher grade ≥3 adverse events, including neutropenia (50% vs 35%), febrile neutropenia (13% vs 8%), and diarrhea (30% vs 14%) (51).
In the post-operative setting, the HEPATICA trial was designed to evaluate DFS in patents with mCRC who received CAPEOX with or without bevacizumab after resection of liver metastases. Unfortunately, due to low accrual and subsequent study closure no statistically significant conclusion was able to be drawn. However, the group who received CAPEOX and bevacizumab demonstrated higher scores related to quality of life than patients who received CAPEOX alone (52).
Bevacizumab as maintenance therapy
The utility of administering bevacizumab after disease stability has been achieved with chemotherapy-based regimens has been studied in several large trials with conflicting results. The CAIRO3 trial, analyzing patients with mCRC deemed to have at least stable disease after first-line treatment with CAPEOX and bevacizumab, were assigned to receive either maintenance capecitabine plus bevacizumab or observation. At time of progression, patients in both groups subsequently received CAPEOX plus bevacizumab until their disease progressed further. The study found that time to second progression was improved in patients who received maintenance capecitabine plus bevacizumab compared to those who were randomized to observation (8.5 vs 11.7 months; HR 0.67; 95% CI 0.56–0.81; p < 0.0001). No significant difference in OS was observed, although a trend towards improved OS was seen in patients who received maintenance capecitabine plus bevacizumab (53, 54). AIO 0207 trial showed bevacizumab alone was non-inferior to fluorouracil plus bevacizumab in time to first progression (HR 1.08; 95% CI 0.85–1.37; p = 0.53). Additionally, this study indicated that no treatment in the maintenance setting was not non-inferior to either bevacizumab alone or fluorouracil plus bevacizumab in patients who previously received induction therapy with oxaliplatin-based chemotherapy plus bevacizumab (55).
The previously mentioned data supporting maintenance bevacizumab conflicts with the outcome of PRODIGE9, which found that bevacizumab did not improve tumor control duration (15.08 vs 14.98 months HR 1.09; 95% CI 0.87–1.37), PFS (9.20 vs 8.90 months; HR 0.92; 95% CI 0.76–1.10), or OS (21.65 vs 21.98 months; HR 1.05; 95% CI 0.86–1.28) relative to no maintenance treatment among patients initially treated with FOLFIRI and bevacizumab (56). Similarly, the SAKK 41/06 trial found that non-inferiority in time to progression was not reached when comparing maintenance bevacizumab to no maintenance treatment in patient previously receiving previous chemotherapy plus bevacizumab (4.1 vs 2.9 months; HR 0.74; 95% CI 0.58–0.96) (57).
Maintenance bevacizumab was compared to maintenance bevacizumab plus erlotinib, an EGFR inhibitor, in the GERCOR DREAM; OPTIMOX3 trial. Median PFS from maintenance was not significantly different but trended towards use of both drugs (5.4 vs 4.9 months; stratified HR 0.81; 95% CI 0.66–1.01; p = 0.059) while median OS from maintenance was longer in patients that received both bevacizumab and erlotinib (24.9 vs 22.1 months; stratified HR 0.79; 95% CI 0.63–0.99; p = 0.036). However, Grade 3-4 adverse effects occurred in 21% of patients who received bevacizumab plus erlotinib compared to 0% of patients who received bevacizumab alone (58). Due to these significantly higher adverse effects of this combination in the setting of non-curative disease, the erlotinib is not routinely used in the maintenance setting. In clinical practice, largely based on the CAIRO3 study, de-escalated chemotherapy plus bevacizumab is safely and effectively used in the maintenance setting.
Bevacizumab in patients with refractory disease
Single agent bevacizumab is not recommended after progression on chemotherapy is generally not recommended due to inferior efficacy compared to chemotherapy alone or chemotherapy plus bevacizumab. Several trials have evaluated the efficacy of bevacizumab, in conjunction with chemotherapy, in patients with mCRC who experienced progression on first-line chemotherapy. In the ML18147 trial, patients with mCRC who progressed on first-line chemotherapy and bevacizumab were subsequently randomized to a different chemotherapy backbone with or without bevacizumab. Patients who were provided bevacizumab saw a statistically significant OS benefit (11.2 vs 9.8 months; HR 0.81; 95% CI 0.69–0.94; p = 0.0062) (59). The benefit of continuing bevacizumab, with a different chemotherapeutic regimen, in the second-line setting after progression on a regimen containing bevacizumab was also observed in the BEBYP trial, noting a longer PFS in patients who were continued on a regimen that contained bevacizumab (6.8 vs 5.0 months; HR 0.70; 95% CI 0.52–0.95; p = 0.001) (60). Further, adding bevacizumab to second-line FOLFOX for patients with mCRC who progressed on first-line irinotecan-based therapy that did not include bevacizumab was the focus of Study E3200. An improvement in median duration of survival was seen in the patients treated with second line FOLFOX plus bevacizumab compared to FOLFOX alone (12.9 vs 10.8 months; HR 0.75; p = 0.0011) (61). Retrospective and observational analyses also concur that continuation of bevacizumab after progression first-line chemotherapy containing bevacizumab provides a survival benefit (62, 63).
Ziv-aflibercept
Ziv-aflibercept is a recombinant protein designed to inhibit angiogenesis by preventing VEGF -A, B and placental growth factor from activating VEGF receptors. This novel drug evaluated in the phase III VELOUR trial studying its use in conjunction with FOLFIRI in patients with mCRC who had prior disease progression on oxaliplatin-based chemotherapy. OS was longer in patients who received FOLFIRI and ziv-aflibercept compared to FOLFIRI alone (13.5 vs 12.1 months; HR 0.82; 95% CI 0.71–0.94; p = 0.003) (64). Overall, clinical practice favors bevacizumab use in this setting due to its superior toxicity profile and lower cost.
Ramucirumab
Ramucirumab, a human IgG-1 monoclonal antibody against the extracellular portion of the VEGF receptor 2, has been studied in the chemotherapy refractory setting combined with cytotoxic regimens. In the phase III RAISE trial, patients with mCRC who had disease progression on FOLFOX and bevacizumab were randomized to FOLFIRI with or without ramucirumab. Patients in the ramucirumab arm experienced longer OS (13.3 vs 11.7 months; HR 0.84; 95% CI 0.73–0.98; p = 0.02) although therapy was discontinued more frequently in the group that received ramucirumab (11.5% vs 4.5%), most frequently secondary to neutropenia, thrombocytopenia, stomatitis and diarrhea (65). As a result of this study, the addition of ramucirumab to irinotecan or FOLFIRI for patients with refractory mCRC not previously exposed to irinotecan-based therapy is considered an acceptable regimen. However, bevacizumab remains most utilized clinically.
Regorafenib
Regorafenib is a multi-targeted tyrosine kinase inhibitor (TKI) that blocks interactions of ligands with VEGF, PDGF, BRAF, KIT, and RET and has been studied primarily in patients with refractory mCRC. Its broad receptor influence modulates downstream pathways involved in angiogenesis, cell growth, differentiation, and survival. The CORRECT trial evaluated the administration of regorafenib or placebo to patients with refractory mCRC whose disease had progressed on several lines of chemotherapy (Table 1). The study indicated prolonged OS in patients who received regorafenib (6.4 vs 5.0 months; HR 0.77; 95% CI 0.64–0.94); p = 0.005) (66). The CONCUR trial conducted in Asia observed this similar outcome, with prolonged OS with use of regorafenib compared to placebo in the refractory setting (8.8 vs 6.3 months; HR 0.55; 95% CI 0.40–0.77; p < 0.001) (67). Hand-foot skin reaction was the most frequent grade 3 (or higher) adverse effect and occurred in 17% of patients who received regorafenib in this trial. Other, but less common grade 3 (or higher) adverse effects included fatigue, hypertension, diarrhea, rash/desquamation. The ReDos trial utilized a dose-escalation of regorafenib to mitigate toxicity, while maintaining efficacy, however, adverse events remained significant (68). Due to the findings in these two trials, regorafenib is considered an accepted treatment regimen for patients with mCRC whose disease has progressed on chemotherapy, but its side effect profile warrants careful monitoring while on therapy.
Fruquintinib
Fruquintinib is a highly selective TKI that blocks VEGFR-1, VEGFR-2, and VEGFR-3 which was recently evaluated in the phase III FRESCO 2 trial, which randomized patients with refractory, previously treated mCRC (Table 1). Patients were allowed to have received prior trifluridine/tiparicil and/or regorafenib (median lines of therapy 5) to receive either best supportive care with or without fruquintinib. Patients who received fruquintinib experienced prolonged OS (7.4 vs 4.8 months; HR 0.66; 95% CI 0.55–0.80; p < 0.001 and PFS (3.7 vs 1.8 months; HR 0.32; 95% CI 0.27–0.39; p < 0.001). Grade 3 or higher adverse effects were seen in 62.7% of patients who received fruquintinib compared to 50.4% in patients who received placebo. Specific side effects seen in over 5% of patients were hand-foot syndrome, asthenia, and hypertension (69). Importantly, 97% of enrolled patients had received prior bevacizimab. Fruquitinib can be used after progression on other VEGF inhibitors including bevacizumab and regorafenib.
EGFR inhibitors versus bevacizumab
RAS mutational status and tumor sidedness impact the efficacy of bevacizumab and EGFR inhibitors in the first-line setting. As previously mentioned, in the CALGB/SWOG 80405 trial, no statistically significant OS benefit (30.0 vs 29.0 months; HR 0.88; 95% CI 0.77–1.01; p = 0.08) was seen among patients with wild-type KRAS exon 2 mCRC who received first-line chemotherapy (either FOLFOX or FOLFIRI) with cetuximab versus bevacizumab (70). However, patients with RAS wild-type, right-sided mCRC who received bevacizumab in the first-line setting showed a trend toward longer OS than those who received cetuximab (HR 1.36; 95% CI 0.93–1.99; p = 0.10). Conversely, patients with RAS wild-type, left-sided primary tumors who received cetuximab had significantly longer overall survival than those who received bevacizumab (HR 0.77; 95% CI 0.59–0.99; p = 0.04) (71).
In contrast, the FIRE-3 trial found an improvement in OS among patients who received first line FOLFIRI plus cetuximab compared to FOLFIRI plus bevacizumab (28.7 vs 25.0 months; HR 0.77; 95% CI 0.62–0.96; p = 0.017) in patients with KRAS exon 2 wild type mCRC (24, 72). However, trial has been criticized for its lack of third-party review and low rate of administration of second-line therapy (70). Improved efficacy with an EGFR inhibitor was also seen in the phase II PEAK trial, in which patients with wild-type RAS who received FOLFOX with panitumumab had longer PFS (12.8 vs 10.1 months; HR 0.68; 95% CI 0.48–0.96; p = 0.029) than patients who received FOLFOX and bevacizumab, although some have argued the small sample size limit its generalizability (73, 74). The more recent PARADIGM trial, discussed above, which compared FOLFOX plus panitumumab to FOLFOX plus bevacizumab in the first line for patients with RAS wild-type mCRC, showed longer OS for patients with left sided tumors using panitumumab (37.9 vs 34.3 months; HR 0.82; 95% CI 0.68–0.99; p =. 0.031) (27).
In the second-line setting, there is a paucity of data comparing bevacizumab and EGFR inhibitors. In the phase II SPIRITT trial, treatment with FOLFIRI plus panitumumab did not yield longer PFS survival compared to FOLFIRI plus bevacizumab in patients with KRAS wild type mCRC whose disease progressed on first-line oxaliplatin-based chemotherapy and bevacizumab (7.7 months vs 9.2 months; HR 1.01; 95% CI 0.68–1.50; p = 0.97) (75).
Combination EGFR and VEGF inhibition
The combination of EGFR and VEGF inhibition has shown efficacy in preclinical setting, finding improved survival and tumor inhibition in mouse models (76, 77). Given these findings and the proven benefit of the addition of EGFR or VEGF to cytotoxic therapy, investigators sought to determine the utility of VEGF in conjunction EGFR therapies in the metastatic setting.
The addition of bevacizumab and panitumumab to chemotherapy in first-line treatment of patients with mCRC (of all KRAS mutational subtypes) was studied in the phase III PACCE trial. Patients received chemotherapy and bevacizumab with or without panitumumab. The addition of panitumumab resulted in higher toxicity and shorter PFS (10.0 vs 11.4 months; HR 1.27; 95% CI 1.06–1.52), regardless of KRAS mutational status (78). The CAIRO2 trial came to a similar conclusion, with the addition of cetuximab to CAPEOX plus bevacizumab yielded a higher incidence of grade 3-4 toxicity (81% vs 72%; p = 0.03) and shorter PFS (9.4 vs 10.7 months; HR 1.22; 95% CI 1.04–1.43) (79). No difference in PFS between groups was observed among patients with wild-type KRAS tumors.
Conversely, the phase II randomized BOND-2 study investigated the use of cetuximab and bevacizumab in irinotecan-refractory mCRC. This study indicated that the addition of cetuximab and bevacizumab to irinotecan in this patient population resulted in improved time to progression (7.3 vs 4.9 months), improved response rate (37% vs 20%) and an overall survival benefit (14.5 vs 11.4 months) relative to cetuximab and bevacizumab alone, and without unexpected or higher rates of toxicity (80).
Due to the incidence of adverse effects experienced by patients in the PACCE and CAIRO2 trials, as well as the lack of efficacy, it is not recommended to combine these two drug classes within the same line of therapy.
BRAF inhibitors
Treatment for BRAF V600E mutation positive disease in non-first line setting
Inhibition of BRAF has been primarily studied in second line or greater setting. For patients with mCRC whose tumors contain BRAF V600E mutations with progression on first or second-line therapy, a triplet of therapy comprising encorafenib, a BRAF inhibitor, plus binimetinib, a MEK inhibitor, and cetuximab was compared to the doublet of encorafenib and cetuximab as well as to cetuximab plus either irinotecan or FOLFIRI in the BEACON trial (Table 1). Treatment with the triplet or doublet led to an OS benefit relative to treatment with cetuximab plus either irinotecan or FOLFIRI (9.3 vs 9.3 vs 5.9 months, respectively). Grade 3 adverse effects occurred more commonly in patients who received the triplet than those who received the doublet (58% vs 50%). Therefore, to limit toxicity while maintaining efficacy, doublet therapy (encorafenib plus either cetuximab or panitumumab) is recommended (81).
Irinotecan plus cetuximab and vemurafenib, a BRAF inhibitor, was evaluated in the treatment refractory setting, indicating improvement in PFS and disease control rate compared to irinotecan plus cetuximab alone in this population (82). To mitigate EGFR-mediated adaptive feedback reactivation of MAPK signaling, different combinations of dabrafenib, a BRAF inhibitor, panitumumab, and trametinib, a MEK inhibitor, were studied in patients with BRAF V600E mutation positive mCRC, with variable response rates. The triplet combination of these therapies was found to have the highest response rate (21%), but has not been adopted as a standard of care (83).
BRAF inhibitors in the first-line setting
Due to the significantly worse OS and limited response to standard first line therapy of BRAF mutated mCRC, BRAF inhibitors are also being studied in the first-line systemic therapy for patients with BRAF V600E mutated mCRC. The BREAKWATER trial (NCT040607421) is a phase 3 trial investigating the efficacy and safety of encorafenib, cetuximab, and either FOLFIRI or FOLFOX in patients with untreated BRAF V600E mutated mCRC. Additionally, the SEAMARK trial (NCT05217446) is a phase 2 trial comparing the combination of encorafenib, cetuximab, and pembrolizumab, an inhibitor of programmed death-1 receptor, to pembrolizumab alone in patients with untreated deficient mismatch repair (dMMR) and BRAF V600E mutated mCRC. Results are still pending for both trials.
Anti-HER2 therapy
HER2 in colorectal cancer
Human epidermal growth factor receptor 2 (HER2), which is encoded by the proto-oncogene ErbB2 (also known as HER2), is a member of the same family of signaling kinase receptors as EGFR. Dimerization of HER2 with other members of the EGFR family results in activation of several downstream signaling pathways, including RAS/RAF/ERK, PI3K/AKT/mTOR, and JAK/STAT3 (84, 85). HER2 is not commonly amplified or overexpressed in CRC with a prevalence estimated at 3 to 5%, however, is more frequently amplified or overexpressed in RAS/BRAF wild type tumors (86). HER2 has become one of the latest areas of study in targeted medicine within colorectal cancer. HER2 amplification or overexpression may predispose to the development of resistance upon treatment with an EGFR inhibitor for patients with RAS/BRAF wild type mCRC (83, 87). The prognostic value of HER2 expression or amplification is not well defined, however attempts to understand its impact have been performed. Specifically, In a cohort of patients with RAS/BRAF wild type mCRC whose treatment regimen included an EGFR inhibitor, median PFS was shorter among those with HER2 amplification compared to those without HER2 amplification (2.8 vs 8.1 months; HR 7.05; 95% CI 3.4–14.9; p < 0.001) (88). At this time, HER2-directed therapy is generally recommended in patients with HER2-amplified mCRC whose disease has progressed on systemic cytotoxic therapy, only to be considered first-line for patients who are not appropriate for cytotoxic therapy.
Trastuzumab-based therapy
The combination of two HER2-directed monoclonal antibodies, trastuzumab and pertuzumab, has been studied in two basket studies of patients with HER2-amplified cancers. In refractory HER2-amplified mCRC, an ORR of 23.1% (95% CI 18.1%–28.7%) and DCR of 44.2% (95% CI 38.1%–50.5%) was observed among 57 patients in the MyPathway study while an ORR of 14% (90% CI 4%–33%) and disease control rate of 50% (90% CI 36%–60%) was seen in 28 patients in the TAPUR study. Grade 3 or 4 AEs were limited, noted in up to 37% of patients in the MyPathway study while two patients in the TAPUR study developed grade 3 AEs (86, 89).
Trastuzumab has also been studied in combination with several other agents in this setting. The phase II HERACLES trial studied 27 patients with refractory HER2-positive, KRAS wild type mCRC who received trastuzumab plus the oral tyrosine kinase inhibitor lapatinib targeting EGFR1 and HER2 (Table 1). Nearly one third of patients had an object response (30% 95% CI 14%–50%), with 22% of patients experiencing grade 3 AEs, without any grade 4 events (90–92). Additionally, the efficacy of fam-trastuzumab deruxtecan, an antibody drug conjugate containing anti-HER2 antibody and a cytotoxic topoisomerase I inhibitor linked by a cleavable tetrapeptide linker, was the focus of the phase II DESTINY-CRC01 trial. 78 patients with refractory HER2-expressing, BRAFV600E and RAS wild type mCRC were stratified into three groups based on HER2 expression. Responses were only seen in patients with high tumoral HER2 expression (IHC3+ or IHC2+/ISH+), with an ORR of 45.3% (95% CI 31.6%–59.6%) and PFS 6.9 months (95% CI 4.1–8.7 months). Importantly, these responses were seen regardless of previous exposure to HER2 directed therapy. Unfortunately, 65.1% of the studied patients experienced grade 3 or higher AEs. Specifically, 9% of patients developed life threatening interstitial lung disease, with 3 fatalities (93).
More recently, The MOUNTAINEER trial evaluated the combination of trastuzumab and the HER2 selective tyrosine kinase inhibitor tucatinib (Table 1). Over 100 patients with refractory HER2-positive, RAS wild type mCRC were stratified to receive trastuzumab plus tucatinib or tucatinib monotherapy, with cross over permitted to the combination arm upon progression. 84 patients received trastuzumab and tucatinib, with an ORR of 38.1%, median duration of response of 12.4 months, median PFS of 8.2 months, and median OS of 24.1 months. Tucatinib monotherapy had a limited objective response of 3%, with no PFS or OS reported due to extensive cross over into the combination arm. This regimen had a superior side effect profile relative to other HER2 directed strategies, noting minimal grade 3 events, only 5 patients discontinuing therapy due to adverse effects, and no treatment related deaths (94). The results led to expedited FDA approval for this combination in refractory mCRC, and the phase III MOUNTAINEER-03 trial (NCT05253651), is ongoing, comparing trastuzumab plus FOLFOX to either FOLFOX, FOLFOX plus bevacizumab, or FOLFOX plus cetuximab for patients with untreated HER2-positive mCRC.
KRAS G12C
With the recognition of inferior outcomes utilizing EGFR inhibition in KRAS mutated CRC, it has become standard of care to test for RAS mutations via next generation sequencing prior to initiation of systemic therapy if possible. It is estimated that half of CRC harbor a KRAS mutation, varying in frequency amongst ethnicities KRAS mutation, with multiple studies suggesting associated worse prognosis (95–99).
A specific mutation within this family, KRASG12C, found in an estimated 3% of metastatic CRC, has shown to have poorer OS relative to other KRAS mutated CRC by up 10 months (99). However, this mutation has recently been found to be a valuable target for systemic therapy across various histologies and within CRC. CodeBreaK100, a phase II single arm trial published in 2021, used the irreversible KRASG12C protein inhibitor sotorasib in solid tumors harboring the KRASG12C mutation, including 62 CRC patients previously treated with 5-FU, oxaliplatin and irinotecan. In the CRC cohort, a modest 9.7% of patients had an objective response, not reaching primary endpoint of an 20% objective response rate (100).
This lack of response in the CRC relative to other histologies such as non-small lung cancer, is related to several factors including upstream basal receptor tyrosine kinase activation interfering with KRASG12C inhibitors and feedback suppression of the MAPK signaling with KRAS inhibition. Most clinically relevant, however, is the downstream activation of KRASG12C from high levels of EGFR signaling. Therefore, it was postulated, and shown in KRAS CRC cell line analysis, that concomitant EGFR and KRAS G12C blockade overcomes secondary resistance to anti-EGFR antibodies, increasing cell death rate (101). This concept led to the KRYSTAL-1 trial, a phase 1-2 open label non-randomized trial of patients with pre-treated KRAS G12C mutated CRC in which patients were provided adagrasib, an oral small molecule inhibitor of KRAS G12C protein in combination with cetuximab or adagrasib monotherapy (Table 1). The combination therapy had a statistically significant higher response rate (46% vs 19%), median duration of response (7.6 vs 4.3 months), and median PFS (6.9 vs 5.6 months), with a lower percentage of grade 3 or 4 treatment related adverse events (102). Additionally, the currently ongoing phase II clinical trial CodeBreaK 101, subprotocol H is attempting to combine sotorasib with panitumumab (Table 1) (100). Targeted therapy of KRASG12C in combination with ant-EGFR therapy appears to be a promising late-line therapy in patients harboring this mutation, improving response rates and PFS in patients that otherwise would be very limited in remaining effective treatment options.
DNA mismatch repair and microsatellite unstable tumors
The advent of immune checkpoint and its application in tumors deficient in mismatch repair (dMMR) has resulted in significant improvement not only in the treatment efficacy but quality of life of the estimated 15% of colorectal cancer patients with this alteration. Mismatch repair genes including MLH1 (human mutL homolog 1), MSH2 (human mutS homolog 2), MSH6 (humab mutS homolog 6) and PMS2 (human postmeiotic segregation 2) are committed to mending errors during DNA replication such as incorrect base pairing, deletions or insertions (103–105). Up to eighty percent of cases are sporadic in etiology, secondary to epigenetic influences via the lack of methylation or excess methylation of DNA or DNA promotor regions respectively (106–110). This is in contrast to germline mutations within MMR genes, seen in hereditary forms of dMMR, leading to lack gene expression as seen in Lynch syndrome (111, 112). MMR deficiency lends tumor cells to amass large amounts of errors within DNA, developing microsatellites of repeated nucleotide bases that can result in significant abnormalities in DNA promoters responsible for cell proliferation, hence the term high microsatellite instability or MSI-H (108, 113).
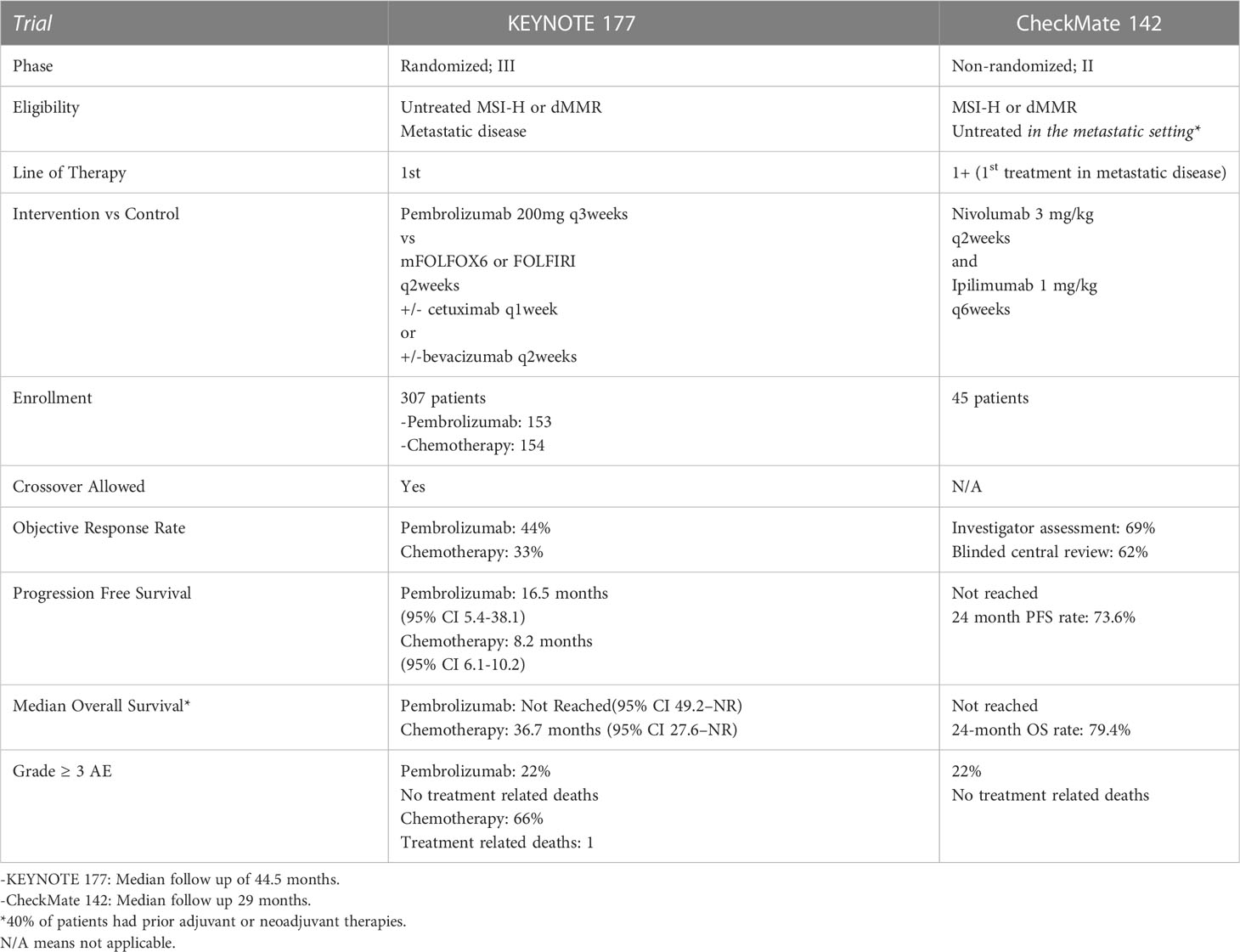
The use of immunotherapy, specifically, anti-programmed cell death 1 monoclonal antibodies (anti-PD-1) in mCRC was first demonstrated in the treatment refractory setting. Specifically, in the 2015 phase II study of pembrolizumab monotherapy at 10mg/kg every 2 weeks in patients with treatment refractory dMMR mCRC, dMMR metastatic noncolorectal and MMR proficient (pMMR) mCRC, those with dMMR mCRC demonstrated an 89% DCR, and 50% ORR, relative to pMMR patients who had 16% DCR and 0% ORR. At a nearly 6-month treatment duration, PFS and OS were not reached in the dMMR group vs a PFS and OS of 2.3 months and 7.6 months respectively in the pMMR group (114). Based on these results, the authors opened the phase II open label, multicenter KEYNOTE 164 trial (Tables 1, 2). In this study, patients with treatment refractory dMMR mCRC were provided pembrolizumab at 200mg every 3 weeks. OR was 33% in patients with ≥2 lines of therapy (cohort A) or ≥ 1 line of therapy (cohort B), with median OS of 31.4 months (95% CI 21.4 to 8.1months) in cohort A and not reached (95% CI 19.2 to not reached) in cohort B at a median follow up of 31.3 months (115). These results significantly contributed to the FDA approval of pembrolizumab for patients with dMMR or MSI-H disease that progressed on prior cytotoxic chemotherapy.
Similarly, the PD-1 inhibitor, nivolumab, received expedited approval the same year for treatment refractory dMMR or MSI-H mCRC based on the CheckMate 142 trial, in addition to its combination with ipilimumab (cytotoxic T-lymphocyte associated antigen-4 inhibitor) the following year (Tables 1, 2). In this phase II, non-randomized multicohort study, patients with progressive dMMR mCRC were provided 3mg/kg nivolumab every 3 weeks and ipilimumab (CTLA-4 inhibitor) 1mg/kg every 3 weeks for 4 doses followed by nivolumab 3mg/kg every 2 weeks until disease progression, death or unacceptable toxicity, or nivolumab monotherapy 3mg/kg every 2 weeks. First analyzed and reported were the results from the nivolumab monotherapy arm, indicating that at a median follow up of 12 months, 69% (95% CI 57-79) of the 74 patients had disease control for 12 weeks or longer and 31.1% (CI 20.8-42.9) had objective response (116). In the cohorts that received both nivolumab and ipilimumab, a 4 year follow up has been reported. At a median follow up of 50.9 months, OR was seen in 65% of patients (95% CI 55%-73%), and a disease control of greater than or equal to 12 weeks was seen in 81% of patients (95% CI 72%-87%). Although median PFS and OS were not reached, 48-month PFS and OS percentage were 53% (95% CI 43-62) and 71% (95% CO 61-78) respectively (117). Notably, responses mentioned in both CheckMate 142 analyses responses were seen regardless of PD-L1 status, BRAF or KRAS status. Although no direct comparison has been made between dual checkpoint inhibitors versus immunotherapy monotherapy, risks and benefits must be weighed in this treatment refractory setting given the higher frequency of immune related toxicity with combination therapy (118).
Importantly, however, it has been concluded that early identification of MSI-H/dMMR tumors and subsequent first line treatment with immunotherapy in mCRC has improved responses relative to first line cytotoxic chemotherapy. First, the use of pembrolizumab monotherapy was analyzed in the phase III open label, randomized trial, assigning untreated patients with dMMR/MSI-H mCRC to pembrolizumab 200mg every 3 weeks or standard of care chemotherapy with 5-FU based therapy with oxaliplatin or irinotecan. Of note, cross over to pembrolizumab was allowed after disease progression. At a median follow up of 32.4 months, OR was seen in 43.8% in the pembrolizumab cohort vs 33.1% in those treated with chemotherapy. PFS was significantly longer in the pembrolizumab cohort versus chemotherapy at 16.5 months vs 8.2 months respectively (HR 0.6, 95% CI 0.95 0.45 to 0.80). Those patients that had complete or partial response to therapy, 83% of patients in the pembrolizumab arm had continued response at 24 months relative to 35% of patients in the chemotherapy arm. Importantly, pembrolizumab resulted in less grade 3-5 adverse events relative to standard chemotherapy (22% vs 66%), and improved health related quality of life (119, 120). There was a trend toward overall survival benefit with the use of pembrolizumab, but this result was skewed due to 60% of patients treated with chemotherapy crossing over to pembrolizumab (121). Due to these results, the American Society of Clinical Oncology 2022 guidelines recommended that patients with dMMR mCRC should be offered pembrolizumab monotherapy as first line therapy if eligible (122).
A subset of CheckMate 142 analyzed 45 patients with MSI-H/dMMR mCRC that were treatment naive. These patients were treated with nivolumab 3mg/kg every 2 weeks plus ipilimumab 1mg/kg every 6 weeks, with both drugs continued until disease progression. At a median follow up of 29 months, disease control rate was 84% (95 CI 70.5 vs 93.5), and ORR was 69% (95% CI 53-82), with 13% of patients having a complete response. Median PFS and OS was not reached (123). With these results, nivolumab with or without ipilimumab are considered first line therapy options in patients with dMMR/MSI-H mCRC, however, pembrolizumab remains the preferred regimen.
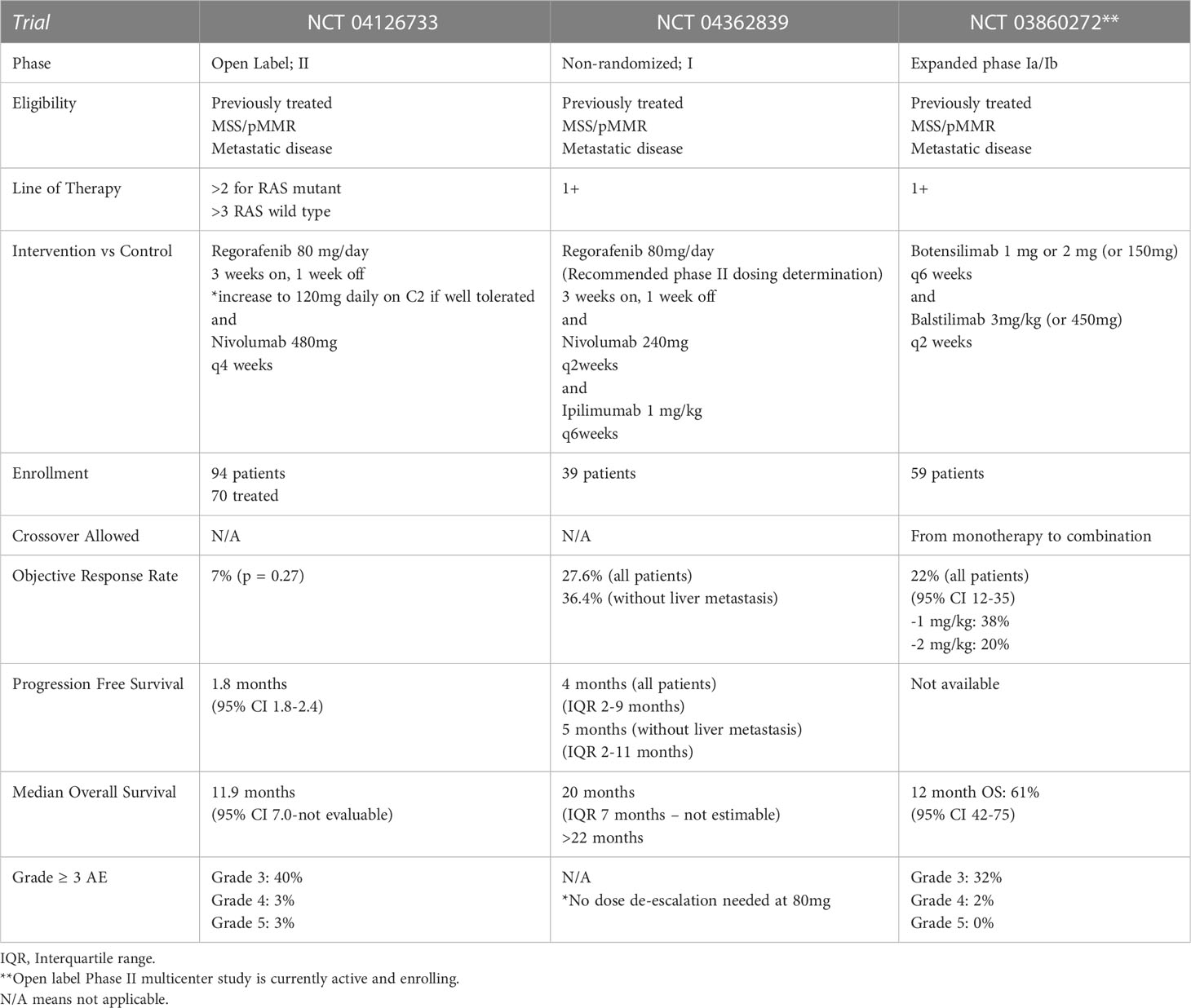
Under active study is the use of immunotherapy for patients with metastatic, chemo-refractory, microsatellite stable (MSS) disease. Early phase studies suggest that combination of the multikinase inhibitor regorafenib with immunotherapy provide objective response and improvement in PFS and OS. Table 3 compares completed phase I and II studies of this combination along with a phase Ia/Ib study of the novel therapy botensilimab, an antibody directed against T-cell receptor cytotoxic T-lymphocyte-associated antigen 4 in combination with the novel monoclonal PD-1 antibody balstilimab (124–127).
Discussion
The utilization of molecular and genetic tumor analysis of patients with mCRC has become increasingly paramount to optimize first line treatment, allow for thoughtful pursuit of subsequent line therapy, and improve overall survival for patients with mCRC. It has become evident that proper use of adjunctive therapies added to established cytotoxic chemotherapy, particularly monoclonal antibodies, can provide meaningful impact on the survival to patients with mCRC. Continued investigation of novel mutational targets is necessary to further the quality of life and survival benefits already demonstrated by harnessing the inhibition of HER2, KRAS G12C, BRAF, VEGF and EGFR. As additional therapeutic molecular and genetic targets are discovered, easily accessible and rapidly resulting testing modalities, such as next generation sequencing, need to be made available for all oncology centers to provide optimal and equitable oncology care to all patients.
Author contributions
All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep (2016) 6:29765. doi: 10.1038/srep29765
3. National Cancer Institute. SEER cancer stat facts: colorectal cancer (2022). Available at: https://seer.cancer.gov/statfacts/html/colorect.html.
4. Jiang Y, Yuan H, Li Z, Ji X, Shen Q, Tuo J, et al. Global pattern and trends of colorectal cancer survival: a systematic review of population-based registration data. Cancer Biol Med (2021) 19(2):175–86. doi: 10.20892/j.issn.2095-3941.2020.0634
5. Crooke H, Kobayashi M, Mitchell B, Nwokeji E, Laurie M, Kamble S, et al. Estimating 1- and 5-year relative survival trends in colorectal cancer (CRC) in the united states: 2004 to 2014. J Clin Oncol (2018) 36(4_suppl):587–. doi: 10.1200/JCO.2018.36.4_suppl.587
6. Krasinskas AM. EGFR signaling in colorectal carcinoma. Patholog Res Int (2011) 2011:932932. doi: 10.4061/2011/932932
7. Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin R, et al. Anti-EGFR therapy in metastatic colorectal cancer: mechanisms and potential regimens of drug resistance. Gastroenterol Rep (2020) 8(3):179–91. doi: 10.1093/gastro/goaa026
8. Samatar AAP, Poulikos I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discovery (2014) 13(12):928–42. doi: 10.1038/nrd4281
9. Li S, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell (2005) 7(4):301–11. doi: 10.1016/j.ccr.2005.03.003
10. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med (2008) 358(11):1109–17. doi: 10.1056/NEJMoa074943
11. Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. New Engl J Med (2013) 369(11). doi: 10.1056/NEJMoa1305275
12. Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med (2009) 360(14):1408–17. doi: 10.1056/NEJMoa0805019
13. Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol (2012) 30(29):3570–7. doi: 10.1200/JCO.2012.42.2592
14. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-Ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med (2008) 359(17):1757–65. doi: 10.1056/NEJMoa0804385
15. De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol (2008) 19(3):508–15. doi: 10.1093/annonc/mdm496
16. Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol (2009) 27(5):663–71. doi: 10.1200/JCO.2008.20.8397
17. Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol (2008) 26(3):374–9. doi: 10.1200/JCO.2007.12.5906
18. Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol (2013) 31(6):759–65. doi: 10.1200/JCO.2012.45.1492
19. Láng I, Köhne CH, Folprecht G, Rougier P, Curran D, Hitre E, et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer (2013) 49(2):439–48. doi: 10.1016/j.ejca.2012.08.023
20. Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol (2011) 29(15):2011–19. doi: 10.1200/JCO.2010.33.5091
21. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol (2010) 28(31):4697–705. doi: 10.1200/JCO.2009.27.4860
22. Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, et al. Clinical sequencing defines genomic landscape metastatic colorectal cancer. Cancer Cell (2018) 33(1):125–36.e3. doi: 10.1016/j.ccell.2017.12.004
23. Moretto R, Cremolini C, Rossini D, Pietrantonio F, Battaglin F, Mennitto A, et al. Location of primary tumor and benefit from anti-epidermal growth factor receptor monoclonal antibodies in patients with RAS and BRAF wild-type metastatic colorectal cancer. Oncologist. (2016) 21(8):988–94. doi: 10.1634/theoncologist.2016-0084
24. Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(10):1065–75. doi: 10.1016/S1470-2045(14)70330-4
25. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol (2017) 3(2):194–201. doi: 10.1001/jamaoncol.2016.3797
26. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA (2017) 317(23):2392–401. doi: 10.1001/jama.2017.7105
27. Yoshino T, Uetake H, Tsuchihara K, Shitara K, Yamazaki K, Watanabe J, et al. PARADIGM study: a multicenter, randomized, phase III study of mFOLFOX6 plus panitumumab or bevacizumab as first-line treatment in patients with RAS (KRAS/NRAS) wild-type metastatic colorectal cancer. J Clin Oncol (2021) 39(3_suppl):85–. doi: 10.1200/JCO.2021.39.3_suppl.85
28. Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol (2013) 31(16):1931–8. doi: 10.1200/JCO.2012.44.8308
29. Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschutz A, Wessendorf S, et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol (2019) 37(35):3401–11. doi: 10.1200/JCO.19.01340
30. Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer (2016) 115(10):1206–14. doi: 10.1038/bjc.2016.309
31. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol (2010) 28(31):4706–13. doi: 10.1200/JCO.2009.27.6055
32. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI +/- panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol (2014) 25(1):107–16. doi: 10.1093/annonc/mdt523
33. Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol (2008) 26(14):2311–9. doi: 10.1200/JCO.2007.13.1193
34. Sobrero A, Lenz HJ, Eng C, Scheithauer W, Middleton G, Chen W, et al. Extended RAS analysis of the phase III EPIC trial: irinotecan + cetuximab versus irinotecan as second-line treatment for patients with metastatic colorectal cancer. Oncologist (2021) 26(2):e261–e9. doi: 10.1002/onco.13591
35. Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol (2011) 22(7):1535–46. doi: 10.1093/annonc/mdq632
36. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet (2011) 377(9783):2103–14. doi: 10.1016/S0140-6736(11)60613-2
37. Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol (2018) 36(30):3031–9. doi: 10.1200/JCO.2018.78.3183
38. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature00766
39. Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of b-RAF. Cell. (2004) 116(6):855–67. doi: 10.1016/S0092-8674(04)00215-6
40. Diaz-Rubio E. Vascular endothelial growth factor inhibitors in colon cancer. Adv Exp Med Biol (2006) 587:251–75. doi: 10.1007/978-1-4020-5133-3_20
41. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discovery (2004) 3(5):391–400. doi: 10.1038/nrd1381
42. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med (2004) 350(23):2335–42. doi: 10.1056/NEJMoa032691
43. Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol (2005) 23(16):3697–705. doi: 10.1200/JCO.2005.05.112
44. Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol (2005) 23(16):3697–705. doi: 10.1200/JCO.2005.05.112
45. Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol (2005) 23(16):3706–12. doi: 10.1200/JCO.2005.00.232
46. Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol (2013) 14(11):1077–85. doi: 10.1016/S1470-2045(13)70154-2
47. Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer (2012) 12:89. doi: 10.1186/1471-2407-12-89
48. Meyerhardt JA, Li L, Sanoff HK, Wt C, Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol (2012) 30(6):608–15. doi: 10.1200/JCO.2011.38.9650
49. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol (2008) 26(12):2013–9. doi: 10.1200/JCO.2007.14.9930
50. Tang W, Ren L, Liu T, Ye Q, Wei Y, He G, et al. Bevacizumab plus mFOLFOX6 versus mFOLFOX6 alone as first-line treatment for RAS mutant unresectable colorectal liver-limited metastases: the BECOME randomized controlled trial. J Clin Oncol (2020) 38(27):3175–84. doi: 10.1200/JCO.20.00174
51. Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol (2015) 26(4):702–8. doi: 10.1093/annonc/mdu580
52. Snoeren N, van Hillegersberg R, Schouten SB, Bergman AM, van Werkhoven E, Dalesio O, et al. Randomized phase III study to assess efficacy and safety of adjuvant CAPOX with or without bevacizumab in patients after resection of colorectal liver metastases: HEPATICA study. Neoplasia (2017) 19(2):93–9. doi: 10.1016/j.neo.2016.08.010
53. Goey KKH, Elias SG, van Tinteren H, Lacle MM, Willems SM, Offerhaus GJA, et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol (2017) 28(9):2128–34. doi: 10.1093/annonc/mdx322
54. Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch colorectal cancer group. Lancet (2015) 385(9980):1843–52. doi: 10.1016/S0140-6736(14)62004-3
55. Hegewisch-Becker S, Graeven U, Lerchenmuller CA, Killing B, Depenbusch R, Steffens CC, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol (2015) 16(13):1355–69. doi: 10.1016/S1470-2045(15)00042-X
56. A T, B J, LM K, G F, B V, T J, et al. Final results of PRODIGE 9, a randomized phase III comparing no treatment to bevacizumab maintenance during chemotherapy-free intervals in metastatic colorectal cancer. J Clin Oncol (2016) 34(15_supplemental):3531–31. doi: 10.1200/JCO.2016.34.15_suppl.3531
57. Koeberle D, Betticher DC, von Moos R, Dietrich D, Brauchli P, Baertschi D, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol (2015) 26(4):709–14. doi: 10.1093/annonc/mdv011
58. Tournigand C, Chibaudel B, Samson B, Scheithauer W, Vernerey D, Mesange P, et al. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): a randomised, open-label, phase 3 trial. Lancet Oncol (2015) 16(15):1493–505. doi: 10.1016/S1470-2045(15)00216-8
59. Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol (2013) 14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1
60. Masi G, Salvatore L, Boni L, Loupakis F, Cremolini C, Fornaro L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol (2015) 26(4):724–30. doi: 10.1093/annonc/mdv012
61. Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern cooperative oncology group study E3200. J Clin Oncol (2007) 25(12):1539–44. doi: 10.1200/JCO.2006.09.6305
62. Grothey A, Flick ED, Cohn AL, Bekaii-Saab TS, Bendell JC, Kozloff M, et al. Bevacizumab exposure beyond first disease progression in patients with metastatic colorectal cancer: analyses of the ARIES observational cohort study. Pharmacoepidemiol Drug Saf (2014) 23(7):726–34. doi: 10.1002/pds.3633
63. Cartwright TH, Yim YM, Yu E, Chung H, Halm M, Forsyth M. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer (2012) 11(4):238–46. doi: 10.1016/j.clcc.2012.05.005
64. Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol (2012) 30(28):3499–506. doi: 10.1200/JCO.2012.42.8201
65. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol (2015) 16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0
66. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (2013) 381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X
67. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2015) 16(6):619–29. doi: 10.1016/S1470-2045(15)70156-7
68. Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM, Ciombor KK, Heying EN, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol (2019) 20(8):1070–82. doi: 10.1016/S1470-2045(19)30272-4
69. Dasari A, Sobrero A, Yao J, Yoshino T, Schelman W, Yang Z, et al. FRESCO-2: a global phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Future Oncol (2021) 17(24):3151–62. doi: 10.2217/fon-2021-0202
70. Modest DP, Stintzing S, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol (2015) 33(32):3718–26. doi: 10.1200/JCO.2015.61.2887
71. Venook AP, Ou F-S, Lenz H-J, Kabbarah O, Qu X, Niedzwiecki D, et al. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB / SWOG 80405 (Alliance). J Clin Oncol (2017) 35(15_suppl):3503–. doi: 10.1200/JCO.2017.35.15_suppl.3503
72. Heinemann V, von Weikersthal LF, Decker T, Kiani A, Kaiser F, Al-Batran SE, et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer (2021) 124(3):587–94. doi: 10.1038/s41416-020-01140-9
73. Wolpin BM, Bass AJ. Managing advanced colorectal cancer: have we reached the PEAK with current therapies? J Clin Oncol (2014) 32(21):2200–2. doi: 10.1200/JCO.2014.55.6316
74. Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis (2017) 32(8):1179–90. doi: 10.1007/s00384-017-2800-1
75. Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt M, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer (2015) 14(2):72–80. doi: 10.1016/j.clcc.2014.12.009
76. Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Pomatico G, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res (2000) 6(9):3739–47.
77. Shaheen RM, Ahmad SA, Liu W, Reinmuth N, Jung YD, Tseng WW, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer (2001) 85(4):584–9. doi: 10.1054/bjoc.2001.1936
78. Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol (2009) 27(5):672–80. doi: 10.1200/JCO.2008.19.8135
79. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med (2009) 360(6):563–72. doi: 10.1056/NEJMoa0808268
80. Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol (2007) 25(29):4557–61. doi: 10.1200/JCO.2007.12.0949
81. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med (2019) 381(17):1632–43. doi: 10.1056/NEJMoa1908075
82. Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol (2021) 39(4):285–94. doi: 10.1200/JCO.20.01994
83. Sartore-Bianchi A, Amatu A, Porcu L, Ghezzi S, Lonardi S, Leone F, et al. HER2 positivity predicts unresponsiveness to EGFR-targeted treatment in metastatic colorectal cancer. Oncologist. (2019) 24(10):1395–402. doi: 10.1634/theoncologist.2018-0785
84. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol (2001) 2(2):127–37. doi: 10.1038/35052073
85. Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol (2001) 12 Suppl 1:S9–13. doi: 10.1093/annonc/12.suppl_1.S9
86. Gupta R, Garrett-Mayer E, Halabi S, Mangat PK, D'Andre SD, Meiri E, et al. Pertuzumab plus trastuzumab (P+T) in patients (Pts) with colorectal cancer (CRC) with ERBB2 amplification or overexpression: results from the TAPUR study. J Clin Oncol (2020) 38(4_suppl):132. doi: 10.1200/JCO.2020.38.4_suppl.132
87. Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer (2013) 108(3):668–75. doi: 10.1038/bjc.2013.4
88. Raghav K, Loree JM, Morris JS, Overman MJ, Yu R, Meric-Bernstam F, et al. Validation of HER2 amplification as a predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis Oncol (2019) 3:1–13. doi: 10.1200/PO.18.00226
89. Meric-Bernstam F, Hainsworth J, Bose R, Burris Iii HA, Friedman CF, Kurzrock R, et al. MyPathway HER2 basket study: pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol (2021) 39(15_suppl):3004–. doi: 10.1200/JCO.2021.39.15_suppl.3004
90. Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol (2016) 17(6):738–46. doi: 10.1016/S1470-2045(16)00150-9
91. Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-b trial. ESMO Open (2020) 5(5):e000911. doi: 10.1136/esmoopen-2020-000911
92. Federica T, Sartore-Bianchi A, Lonardi S, Amatu A, Leone F, Ghezzi S, et al. Long-term clinical outcome of trastuzumab and lapatinib for HER2-positive metastatic colorectal cancer. Clin Colorectal Cancer (2020) 19(4):256–62. doi: 10.1016/j.clcc.2020.06.009
93. Yoshino T, Di Bartolomeo M, Raghav KPS, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): final results from a phase 2, multicenter, open-label study (DESTINY-CRC01). J Clin Oncol (2021) 39(15_suppl):3505–. doi: 10.1200/JCO.2021.39.15_suppl.3505
94. Strickler JH, Ng K, Cercek A, Fountzilas C, Sanchez FA, Hubbard JM, et al. MOUNTAINEER:open-label, phase II study of tucatinib combined with trastuzumab for HER2-positive metastatic colorectal cancer (SGNTUC-017, trial in progress). J Clin Oncol (2021) 39(3_suppl):TPS153–TPS. doi: 10.1200/JCO.2021.39.3_suppl.TPS153
95. Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst (1998) 90(9):675–84. doi: 10.1093/jnci/90.9.675
96. Yoon HH, Tougeron D, Shi Q, Alberts SR, Mahoney MR, Nelson GD, et al. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin Cancer Res (2014) 20(11):3033–43. doi: 10.1158/1078-0432.CCR-13-3140
97. Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol (2016) 27(9):1746–53. doi: 10.1093/annonc/mdw261
98. Taieb J, Le Malicot K, Shi Q, Penault-Llorca F, Bouche O, Tabernero J, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst (2017) 109(5):djw272. doi: 10.1093/jnci/djw272
99. Henry JT, Coker O, Chowdhury S, Shen JP, Morris VK, Dasari A, et al. Comprehensive clinical and molecular characterization of KRAS (G12C)-mutant colorectal cancer. JCO Precis Oncol (2021) 5:613–21. doi: 10.1200/PO.20.00256
100. Fakih MG, Kopetz S, Kuboki Y, Kim TW, Munster PN, Krauss JC, et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol (2022) 23(1):115–24. doi: 10.1016/S1470-2045(21)00605-7
101. Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discovery (2020) 10(8):1129–39. doi: 10.1158/2159-8290.CD-20-0187
102. Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med (2023) 388(1):44–54. doi: 10.1056/NEJMoa2212419
103. Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. (1994) 263(5153):1625–9. doi: 10.1126/science.8128251
104. Baker SM, Bronner CE, Zhang L, Plug AW, Robatzek M, Warren G, et al. Male Mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. (1995) 82(2):309–19. doi: 10.1016/0092-8674(95)90318-6
105. Chung DC, Rustgi AK. DNA Mismatch repair and cancer. Gastroenterology. (1995) 109(5):1685–99. doi: 10.1016/0016-5085(95)90660-6
106. Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer (2009) 100(2):266–73. doi: 10.1038/sj.bjc.6604867
107. Arnold CN, Goel A, Compton C, Marcus V, Niedzwiecki D, Dowell JM, et al. Evaluation of microsatellite instability, hMLH1 expression and hMLH1 promoter hypermethylation in defining the MSI phenotype of colorectal cancer. Cancer Biol Ther (2004) 3(1):73–8. doi: 10.4161/cbt.3.1.590
108. Goel A, Boland CR. Epigenetics of colorectal cancer. Gastroenterology. (2012) 143(6):1442–60.e1. doi: 10.1053/j.gastro.2012.09.032
109. Veigl ML, Kasturi L, Olechnowicz J, Ma AH, Lutterbaugh JD, Periyasamy S, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A (1998) 95(15):8698–702. doi: 10.1073/pnas.95.15.8698
110. Cui H, Horon IL, Ohlsson R, Hamilton SR, Feinberg AP. Loss of imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nat Med (1998) 4(11):1276–80. doi: 10.1038/3260
111. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res (1998) 58(22):5248–57.
112. Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol (2019) 37(4):286–95. doi: 10.1200/JCO.18.00283
113. Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. (1993) 363(6429):558–61. doi: 10.1038/363558a0
114. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
115. Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-High/Mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol (2020) 38(1):11–9. doi: 10.1200/JCO.19.02107
116. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/S1470-2045(17)30422-9
117. Andre T, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol (2022) 33(10):1052–60. doi: 10.1016/j.annonc.2022.06.008
118. Zhou S, Khanal S, Zhang H. Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther Clin Risk Manage (2019) 15:211–21. doi: 10.2147/TCRM.S193338
119. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-Instability-High advanced colorectal cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699
120. Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(5):665–77. doi: 10.1016/S1470-2045(21)00064-4
121. Diaz LA Jr., Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol (2022) 23(5):659–70. doi: 10.1016/S1470-2045(22)00197-8
122. Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol (2023) 41(3):678–700. doi: 10.1200/JCO.22.01690
123. Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-High/Mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol (2022) 40(2):161–70. doi: 10.1200/JCO.21.01015
124. El-Khoueiry AB, Fakih M, Gordon MS, Tsimberidou AM, Bullock AJ, Wilky BA, et al. Results from a phase 1a/1b study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC). J Clin Oncol (2023) 41(4_suppl):LBA8–8. doi: 10.1200/JCO.2023.41.4_suppl.LBA8
125. Fakih M, Sandhu J, Lim D, Li X, Li S, Wang C. Regorafenib, ipilimumab, and nivolumab for patients with microsatellite stable colorectal cancer and disease progression with prior chemotherapy; a phase 1 nonrandomized clinical trial. JAMA Oncol (2023) 9(5):627–34. doi: 10.1001/jamaoncol.2022.7845
126. Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicenter phase 2 study. eClinicalMedicine (2023) 58(101917):2589–5370. doi: 10.1016/j.eclinm.2023.101917
127. Agenus Inc. A study of botensilimab and balstilimab for the treatment of colorectal cancer. ClinicalTrials (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05608044.
Keywords: EGFR inhibitor, KRAS, metastatic colorectal cancer, HER2, mismatch repair
Citation: Cann CG, LaPelusa MB, Cimino SK and Eng C (2023) Molecular and genetic targets within metastatic colorectal cancer and associated novel treatment advancements. Front. Oncol. 13:1176950. doi: 10.3389/fonc.2023.1176950
Received: 01 March 2023; Accepted: 30 May 2023;
Published: 20 June 2023.
Edited by:
Jorge Melendez-Zajgla, National Institute of Genomic Medicine (INMEGEN), MexicoReviewed by:
Antonella Argentiero, National Cancer Institute Foundation (IRCCS), ItalyEleonora Lai, University Hospital and University of Cagliari, Italy
Copyright © 2023 Cann, LaPelusa, Cimino and Eng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher G. Cann, Y2hyaXN0b3BoZXIuZy5jYW5uQHZ1bWMub3Jn
 Christopher G. Cann
Christopher G. Cann Michael B. LaPelusa1
Michael B. LaPelusa1 Cathy Eng
Cathy Eng