- Breast Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Background: Currently, it remains unclear regarding the association between tumor-infiltrating lymphocytes (TILs) and the efficacy of postoperative radiotherapy in primary tumors. Here we attempted to investigate the effect of TILs depending on the presence of postmastectomy radiotherapy (PMRT) on the prognosis in pT1-2N1M0 breast cancer.
Methods: The clinical data of pT1-2N1M0 breast cancer patients undergoing mastectomy and axillary lymph node dissection were retrospectively analyzed. The effect of TILs on the prognosis was assessed based on the infiltration degree (low: TILs ≤10%, high: TILs >10%), and then the prognosis of patients with low and high infiltration of TILs was analyzed based on presence or absence of PMRT.
Results: Totally 213 patients were eligible for the study, including 162 cases of low infiltration and 51 of high infiltration. High-infiltration patients tended to be ER/PR-negative, HER2-positive, and have high histological grade. The infiltration in triple-negative and HER2-positive subtypes was higher compared with Luminal A subtype. Regarding local-regional recurrence-free survival, recurrence-free survival, and overall survival (OS) rates, the differences were all inapparent whether in high- and low-infiltration patients or in high-infiltration patients with/without PMRT. Compared with those without PMRT, low-infiltration patients with PMRT showed a significantly increased OS rate (92.8% vs. 80.0%, p=0.023). Multivariate analysis further confirmed PMRT as an independent predicator of OS in low-infiltration patients (HR: 0.228, 95%CI: 0.081-0.644, p=0.005).
Conclusion: High infiltration of TILs in pT1-2N1M0 breast cancer may be associated with clinicopathological factors. Low-infiltration patients, but not high-infiltration patients, may derive survival benefits from PMRT.
1 Introduction
Carcinogenesis is associated with dysfunction of immune cells in the body (1). The immunity of patients with malignant tumors is usually low, which makes immune cells unable to recognize and kill the tumor cells. Tumor microenvironment (TME) comprising the cells around the tumor and non-cellular components plays a crucial role in tumor growth, migration, and metastasis. Tumor-infiltrating lymphocytes (TILs), an important component of the TME, are composed of immune cells infiltrating the tumor cells and have been identified in a variety of solid tumors, including breast cancer, colon cancer, cervical cancer, lung cancer and melanoma (2, 3). Previous studies have demonstrated that the TILs in breast cancer tissue are mainly composed of cytotoxic T (CD8+) cells, helper T (CD4+) cells, B (CD19+) cells and natural killer (NK) cells (4, 5), and the subtypes of TILs can affect the tumor cells and immune cells in multiple ways, consequently contributing to either an anti-tumor or pro-tumor effect (6–8).
There are studies suggesting that TILs are associated with the prognosis of patients with early-stage triple negative breast cancer (TNBC), the higher lymphocytic infiltration, the better the prognosis (9–11). However, this association is not identified in estrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative and HER2-positive breast cancers (11). In breast cancer patients treated with neoadjuvant chemotherapy, TILs are linked to the pathological complete response (pCR) and long-term prognosis (12–15). Additionally, TILs are predictive of the response to anthracycline- and platinum-based chemotherapy drugs, as well as anti-HER2 agent trastuzumab (10, 11, 15).
Radiotherapy, one of the important adjuvant therapies for breast cancer, can eliminate the dormant residual lesions to reduce the risk of local recurrence (16). It can not only result in cell necrosis and apoptosis by damaging the double-strand DNA (17, 18), but also can affect the tumorigenesis and progression by modulating the immune system and TME (19, 20). The evidence showed that the tumor necrosis induced by local radiotherapy could activate the immune system, leading to tumor shrinkage (21–23). However, this pathogenesis is still undefined. Regarding the association of TILs with the effect of postoperative radiotherapy on ipsilateral breast tumor recurrence, Kovacs et al. demonstrated that breast cancer patients with high TILs had a lower risk of local recurrence after breast-conserving surgery, and those with low TILs might derive larger benefits from radiotherapy (24). Tramm et al. found that high TILs were conductive to predicting the improvement of overall survival (OS) from postmastectomy radiotherapy (PMRT) in breast cancer, which was very significant in ER- tumors (25). Notably, there are still lack of studies on the association between TILs and the effect of postoperative radiotherapy in primary tumors.
For pT1-2N1M0 breast cancer patients, it remains controversial about whether PMRT should be performed. The current guidelines, such as National Comprehensive Cancer Network guidelines, have offered that for N1 breast cancer patients, clinical or pathological high-risk factors should be considered to determine whether PMRT is performed (26). However, in clinical practice, there is lack of unified standards for determination of clinical or pathological high-risk factors due to complexity, leading to frequently occurring biases from different physicians in radiotherapy decision-making of pT1-2N1M0 patients. Hence, in the present study, we attempted to investigate the effect of TILs depending on the presence of PMRT on the prognosis of pT1-2N1M0 breast cancer patients, with the aim of providing more evidence for clinical decision-making.
2 Materials and methods
2.1 Study population
The pT1-2N1M0 breast cancer patients who underwent mastectomy and axillary lymph node dissection (ALND) in The Fourth Hospital of Hebei Medical University between March 2011 and December 2015 were enrolled into the study. Inclusion criteria included (1): age ≥18 years; (2) primary invasive breast cancer initially diagnosed with pT1-2N1M0. According to pathological results, the largest diameter of the primary invasive lesion was 5 cm at most, and there were 1-3 axillary lymph node metastases but no distant metastases; (3) patients undergoing mastectomy combined with ALND, but not receiving any neoadjuvant therapies like chemotherapy, endocrine therapy, targeted therapy, or radiotherapy; (4) patients without the history of any other malignant tumors, including previous history of breast cancer. Those with insufficient clinicopathological information and follow-up data were excluded.
Written informed consent was obtained from each patient. This study was approved by the Institutional Review Board of The Fourth Hospital of Hebei Medical University (approval No.: 2020115) and was performed in accordance with the principles of Declaration of Helsinki and local regulations.
2.2 Collection of clinical data
The clinical data of patients enrolled in this study were extracted through consultation of the electronic medical record, involving age, histological grade, tumor stage, number of axillary lymph nodes, ER, progesterone receptor (PR) and HER2 status, molecular subtype, as well as presence or absence of lymphovascular invasion (LVI).
2.3 Evaluation of TILs
According to the evaluation criteria of TILs in breast cancer recommended by an International TILs Working Group 2014, TILs are divided into intratumoral and stromal types. Intratumoral TILs are defined as the lymphocytes in tumor nests with cell-to-cell contact but no intervention of stroma, as well as directly interacting with the tumor cells. Stromal TILs are defined as the lymphocytes dispersed in the stroma that do not directly contact the tumor cells, which is recommended as the optimal parameter by an International TILs Working Group because the growth pattern of tumor cells may affect the distribution of lymphocytes in tumor nests, leading to a higher heterogeneity and fewer numbers (26).
In this study, TILs were assessed based on the evaluation criteria of TILs recommended by an International TILs Working Group 2014 (27). Two pathologists were responsible for reviewing hematoxylin and eosin (H&E)-stained tumor sections of all patients and interpreting the infiltration degree of stromal TILs. The mean value assessed by two pathologists was considered as the final score. As shown in Figure 1, TILs ≤10% were defined as low infiltration (Figure 1A), while TILs >10% were considered as high infiltration (Figure 1B).
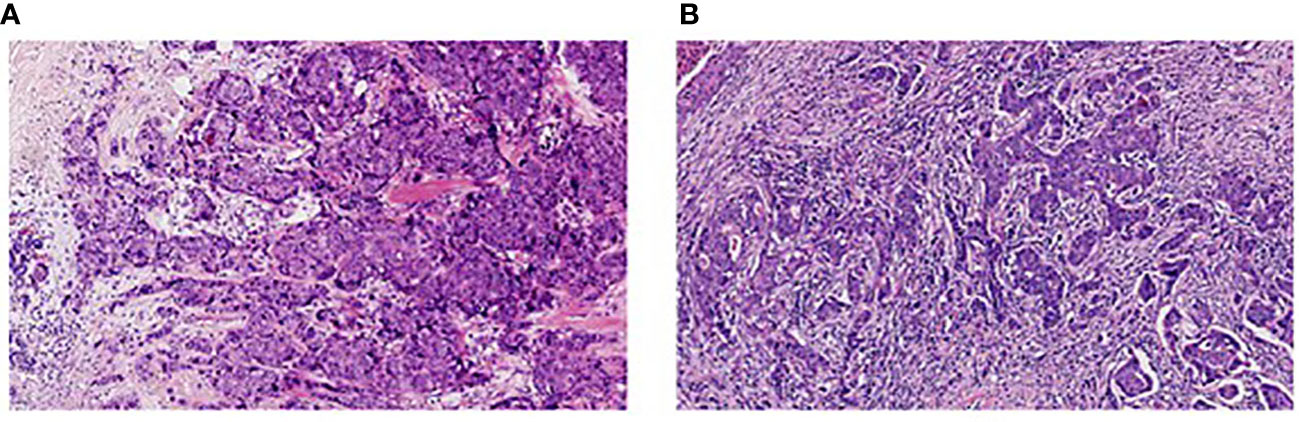
Figure 1 Infiltration of stromal tumor-infiltrating lymphocytes in breast cancer (H&E, 200×). (A) Low infiltration; (B) High infiltration.
2.4 Follow-up
All the patients were followed-up after mastectomy through further consultations and telephones, and the follow-up deadline was February 2020. The effect of TILs on the patients’ prognosis was first assessed based on the infiltration degree. Then, according to presence or absence of PMRT, the prognosis of patients with low and high infiltration of TILs was analyzed.
The primary observational endpoints included local-regional recurrence-free survival (LRFS), recurrence-free survival (RFS) and OS. LRFS was defined as the duration of time from surgery until any recurrence of ipsilateral chest, breast, regional lymph node recurrence, or death occurred from any cause. RFS was defined as the duration of time from surgery until any recurrence of ipsilateral chest, breast, regional lymph node recurrence, distant metastases, or death occurred from any cause. OS was defined as the time from surgery to death from any cause or the last date of contact for a surviving patient.
2.5 Statistical analysis
All the data were analyzed using SPSS 24.0 statistical software (SPSS Inc., Chicago, IL, USA). χ2 or Fisher’s exact test was utilized to analyze the categorical data, expressing as the case number and percentage [n(%)]. Survival curves were drawn using the Kaplan-Meier method and compared by Log-rank test. Univariate and multivariate Cox proportional-hazards models were employed to determine the influencing factors for the prognosis, and hazards ratios (HR) and 95% confidence interval (CI) were calculated, respectively. All statistical tests were two-sided. The value of p<0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
From March 2011 to December 2015, a total of 213 cases were eligible for the study and enrolled into the analysis, with the mean infiltration value of 6% in TILs (range: 3%, 10%), among whom 162 cases were assessed to have low infiltration of TILs (≤10%) and 51 had high infiltration (>10%) of TILs. The baseline characteristics of patients with high and low infiltration of TILs were compared in Table 1. It could be observed that by comparison to those with low infiltration, patients with high infiltration of TILs tended to be ER/PR-negative (49.0% vs. 16%, p=0.000), HER2-positive (41.2% vs. 21.0%, p=0.004), and grade III (66.7% vs. 33.3%, p<0.001). No significant differences were shown in age, tumor stage and number of axillary lymph nodes (p>0.05).

Table 1 Relationship between TILs infiltration in breast cancer tissue and clinicopathological characteristics, n(%).
According to St. Gallen consensus for molecular classification of breast cancer 2013 (26), breast cancer was classified into Luminal A subtype, Luminal B subtype, TNBC and HER2-positive subtype. In terms of different subtypes, the difference was pronounced between the patients with low and high infiltration of TILs (p<0.001). Additionally, the infiltration in triple-negative and HER2-positive subtypes was higher compared with Luminal A subtype (p=0.002, p<0.001), and the infiltration of TILs in HER2-positive breast cancer was significantly higher than that in Luminal B subtype (p=0.001; Table 1).
3.2 Association of TILs infiltration with prognosis
Postoperatively, all the patients were followed up for 7 years. Among 162 patients with low infiltration of TILs, 14, 33 and 20 cases experienced local-regional recurrence (LRR), recurrence and death, respectively, while in 51 patients with high infiltration of TILs there were 9 cases with LRR, 10 cases with recurrence and 6 deaths. The differences were all inapparent between the patients with high and low infiltration of TILs regarding the LRFS rate (91.4% vs. 82.4%, p=0.075; Figure 2A), RFS rate (79.6% vs. 80.4%, p=0.978; Figure 2B) and OS rate (87.7% vs. 88.2%, p=0.918; Figure 2C).
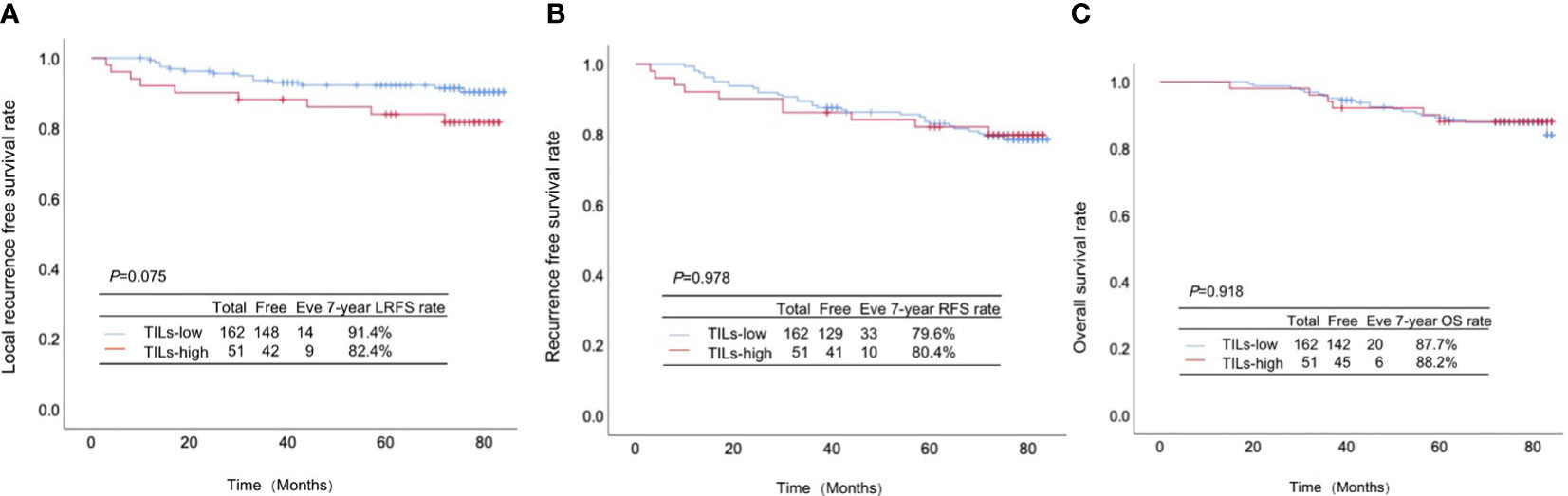
Figure 2 Association between TILs infiltration and the prognosis in pT1-2N1M0 breast cancer. (A) Local-regional recurrence-free survival rate, (B) recurrence-free survival rate and (C) overall survival rate between the patients with high and low infiltration of TILs.
3.3 PMRT-based subgroup analysis
Among 51 patients with high infiltration of TILs, 35 cases underwent PMRT, while 16 didn’t. There were no significant differences between the high-infiltration patients with and without PMRT in LRFS rate (85.7% vs. 75.0%, p=0.334, Figure 3A), RFS rate (82.9% vs. 75.0%, p=0.469, Figure 3B), and OS rate (88.6% vs. 87.5%, p=0.903, Figure 3C).
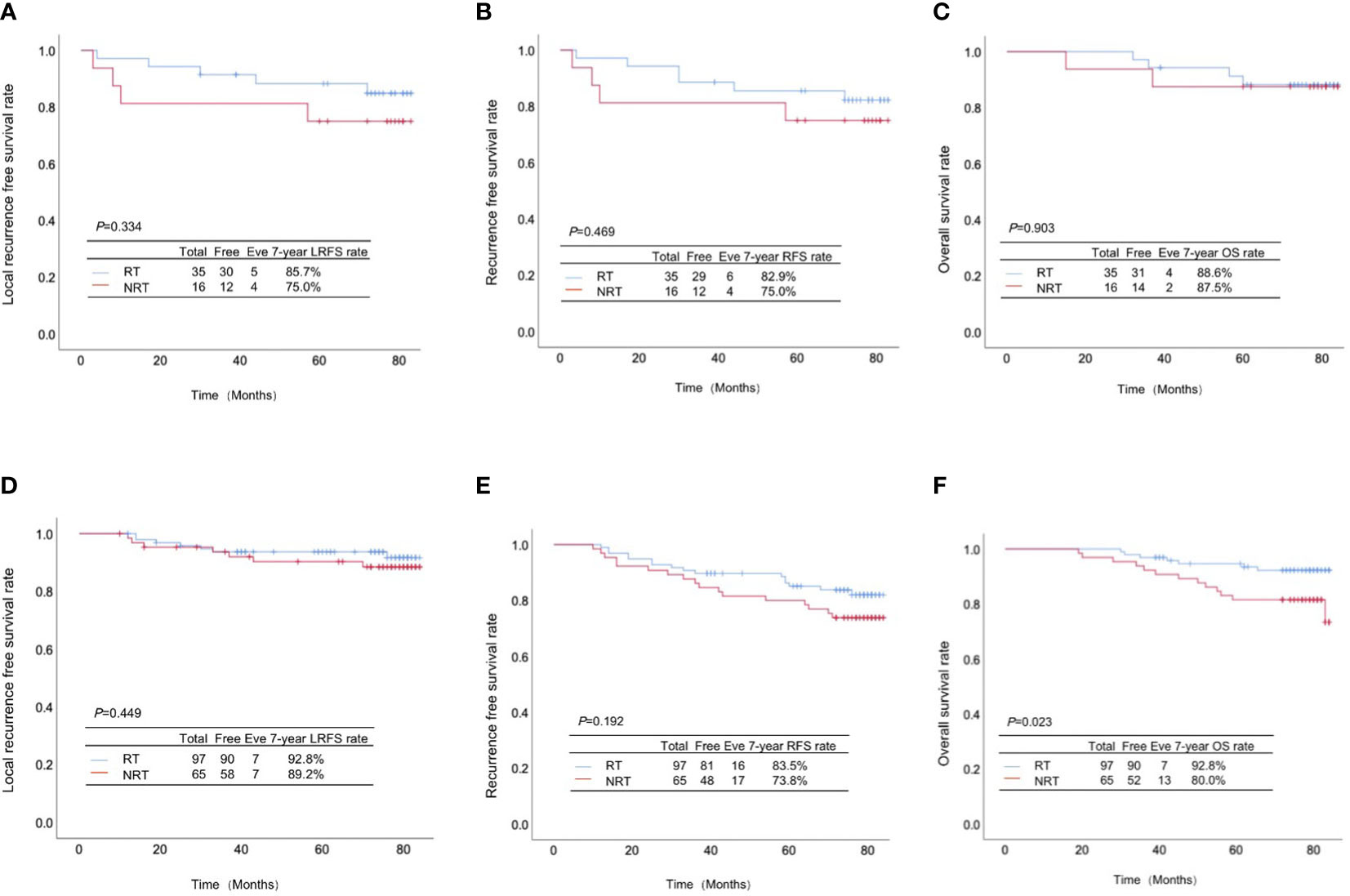
Figure 3 Association of TILs infiltration with the effect of postmastectomy radiotherapy (PMRT) on the prognosis of pT1-2N1M0 breast cancer patients. (A) Local-regional recurrence-free survival rate, (B) recurrence-free survival rate and (C) overall survival rate between the high-infiltration patients with and without PMRT; (D) Local-regional recurrence-free survival rate, (E) recurrence-free survival rate and (F) overall survival rate between the low-infiltration patients with and without PMRT.
Totally 97 cases out of 162 patients with low infiltration of TILs received PMRT, while 65 didn’t. Despite absence of significant differences in LRFS rate (92.8% vs. 89.2%, p=0.449, Figure 3D) and RFS rate (83.5% vs. 73.8%, p=0.192, Figure 3E), the OS rate of low-infiltration patients receiving PMRT was significantly higher than those without PMRT (92.8% vs. 80.0%, p=0.023, Figure 3F).
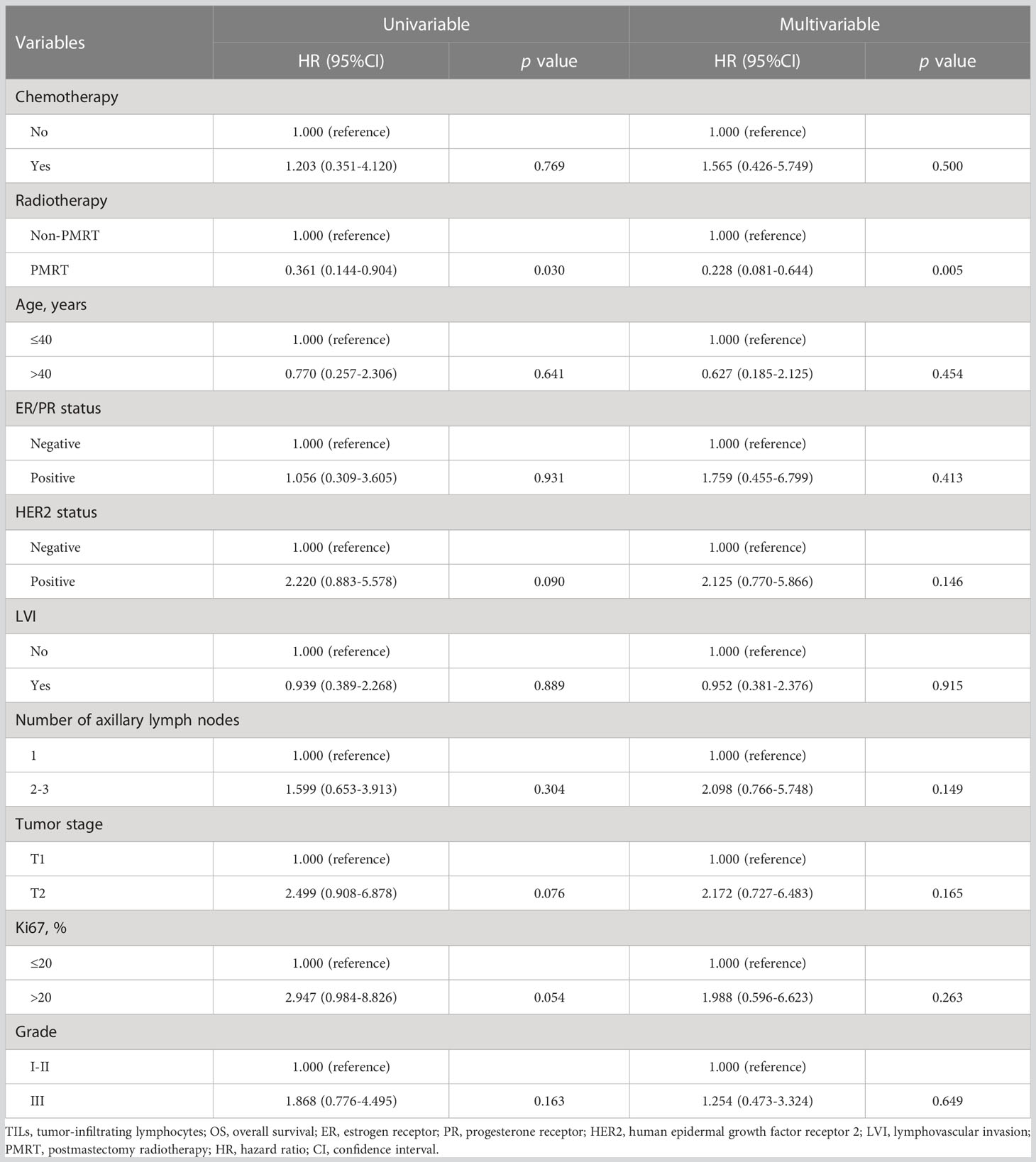
Univariate and multivariate Cox models of OS in 162 patients with low infiltration of TILs were established in Table 2. It could be observed that PMRT was an independent predicator for OS in patients with low infiltration of TILs whether through univariate analysis (HR: 0.361, 95%CI: 0.144-0.904, p=0.030) or through multivariate analysis (HR: 0.228, 95%CI: 0.081-0.644, p=0.005).
4 Discussion
Cytotoxic therapies including chemotherapy and radiotherapy can activate the body’s immune system (28–30). As a component of the TME, TILs are important immune predictors in the process of tumorigenesis and progression. In breast cancer with specific molecular subtypes, TILs can not only predict the prognosis and response to neoadjuvant chemotherapy, but also can predict the efficacy of anthracycline- and platinum-based chemotherapy drugs. However, it remains conflicting about the relationship between TILs and the effect of PMRT on the prognosis in pT1-2N1M0 breast cancer. In the present study, we demonstrated that patients with low infiltration of TILs may derive survival benefits from PMRT, with a lower risk of death compared with those not receiving PMRT, while for patients with high infiltration of TILs this positive effect of PMRT on the prognosis was not identified. Interestingly, Kovacs et al. also exhibited radiotherapy could decrease the risk of LRR and any recurrence in patients with low infiltration of TILs after breast-conserving surgery (24). Although our study did not confirm the association of PMRT with the risk of LRR and any recurrence in patients with low infiltration of TILs, our findings suggested that low TIL infiltration might be associated with sensitivity to PMRT, and patients with low infiltration of TILs were more likely to benefit from PMRT.
Currently, the relationship between TILs and the effect of radiotherapy is not fully understood. Our results indicated that the effect of radiotherapy was better in patients with low infiltration of TILs, which might be associated with a higher proportion of ER-positive patients who usually showed a better prognosis due to less invasion than ER-negative patients. A previous study has demonstrated that radiotherapy is more effective for breast cancers with less invasion, especially for Luminal-type (ER positive) breast cancer, and radiotherapy can decrease the cumulative incidence of ipsilateral breast cancer recurrence (31). ER can induce the production of reactive oxygen species, leading to genomic instability. It has a synergistic effect with radiotherapy on DNA damage of cancer cells to increase the effect of radiotherapy (32). Additionally, the impact of ER on cell cycles can also enhance the effect of radiotherapy on DNA damage (33, 34). Importantly, the benefits of patients with low infiltration of TILs from radiotherapy are more attributable to the changes in immune microenvironment caused by radiotherapy. Several studies have suggested that radiotherapy can induce the variations in the body’s immune cells and microenvironment and activate the anti-tumor effect of immune system (35–37).
Previous studies indicated the higher the TILs infiltration, the better the prognosis of TNBC patients (10, 11). In our study, however, all the differences were indistinctive in the LRFS rate, RFS rate and OS rate whether in high- or low-infiltration patients, which might be attributed to a higher proportion of patients with luminal-type breast cancer. Among 213 cases of pT1-2N1M0 breast cancer, 77.9% of patients were luminal-type breast cancer, while only 12.2% were TNBC. Additionally, pT1-2N1M0 breast cancer patients included in this study had similar TNM staging, leading to an analogical prognosis to some extent. Notably, patients with high infiltration of TILs tended to be ER/PR-negative, HER2 positive, and have high histological grade, and the infiltration in triple-negative and HER2-positive subtypes was higher than Luminal A subtype, which were supported by the previous research results (10, 11). Compared with the luminal subtype, the infiltration degree of TILs in triple-negative and HER2-positive subtypes was higher, but the prognosis was worse, which may lead to the presence of multiple unfavorable prognostic factors in patients with high infiltration of TILs. Hence, all these findings suggest that high infiltration of TILs may predict poor oncological characteristics. Notably, we observed that PMRT could improve the OS of patients with low infiltration of TILs, but not RFS and LRFS. However, in clinical practice the long-term benefits caused by PMRT are often transformed from local benefits. This may be related to the small sample size. In subsequent studies, we will further expand the sample size to explore the impact of TILs on the prognosis of this population and its possible mechanisms.
A major strength of this study was that it first investigated the effect of TILs depending on the presence of PMRT on the prognosis in pT1-2N1M0 breast cancer. For pT1-2N1M0 breast cancer, clinicians often make decisions on adjuvant radiotherapy based on whether the patient is combined with other clinical risk factors. Our results unveil that TILs may serve as a potential indicator for implementation of adjuvant radiotherapy in breast cancer, especially in the luminal-type breast cancer. Nevertheless, it was a retrospective study with the small sample size, and the association of PMRT with the risk of LRR and any recurrence was not identified whether in high- or low-infiltration patients. Additionally, the classification of luminal and non-luminal breast cancers was not included in univariate and multivariate COX analyses due to a great overlap between luminal and HR-positive breast cancers. In the future, we will continue to conduct more large-scale, prospective studies to validate our findings, hoping to find the key indicators that can accurately predict radiotherapy sensitivity and efficacy to aid in clinical decision-making.
In conclusion, high infiltration of TILs in pT1-2N1M0 breast cancer may be associated with clinicopathological factors, such as negative ER/PR, positive HER2 status, and high histological grade. Patients with low infiltration of TILs may derive survival benefits from PMRT, an independent predictor of OS, while these survival benefits are absent in patients with high infiltration of TILs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from each patient. This study was approved by the Institutional Review Board of The Fourth Hospital of Hebei Medical University (approval No.: 2020115) and was performed in accordance with the principles of Declaration of Helsinki and local regulations.
Author contributions
LZ: Conceptualization, and writing-original draft, TT and LL: Methodology, and formal analysis, CL and YL: Investigation, data curation and formal analysis, CG: Conceptualization, writing-reviewing, supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet (2011) 377:127–38. doi: 10.1016/S0140-6736(10)62231-3
2. Badalamenti G, Fanale D, Incorvaia L, Barraco N, Listì A, Maragliano R, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol (2019) 343:103753. doi: 10.1016/j.cellimm.2018.01.013
3. Li B. Why do tumor-infiltrating lymphocytes have variable efficacy in the treatment of solid tumors? Front Immunol (2022) 13:973881. doi: 10.3389/fimmu.2022.973881
4. Coppola L, Smaldone G, D’aiuto M, D’aiuto G, Mossetti G, Rinaldo M, et al. Identification of immune cell components in breast tissues by a multiparametric flow cytometry approach. Cancers (Basel) (2022) 14:3869. doi: 10.3390/cancers14163869
5. Goff SL, Danforth DN. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer (2021) 21:e63–73. doi: 10.1016/j.clbc.2020.06.011
6. Schwartzentruber DJ, Solomon D, Rosenberg SA, Topalian SL. Characterization of lymphocytes infiltrating human breast cancer: specific immune reactivity detected by measuring cytokine secretion. J Immunother (1992) 12:1–12. doi: 10.1097/00002371-199207000-00001
7. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
8. Nelson MA, Ngamcherdtrakul W, Luoh SW, Yantasee W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev (2021) 40(2):519–36. doi: 10.1007/s10555-021-09968-0
9. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor- infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol (2014) 32:2959–66. doi: 10.1200/JCO.2013.55.0491
10. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol (2014) 25:1544–50. doi: 10.1093/annonc/mdu112
11. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol (2013) 31:860–7. doi: 10.1200/JCO.2011.41.0902
12. Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: A secondary analysis of the neoALTTO trial. JAMA Oncol (2015) 1:448–54. doi: 10.1001/jamaoncol.2015.0830
13. Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol (2010) 28:105–13. doi: 10.1200/JCO.2009.23.7370
14. Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PLoS One (2013) 8:e79775. doi: 10.1371/journal.pone.0079775
15. Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol (2015) 33:983–91. doi: 10.1200/JCO.2014.58.1967
16. Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med (1995) 333:1444–55. doi: 10.1056/NEJM199511303332202
17. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol (2015) 16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8
18. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol (2010) 31:363–72. doi: 10.1007/s13277-010-0042-8
19. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol (2016) 13:516–24. doi: 10.1038/nrclinonc.2016.30
20. Frey B, Rubner Y, Wunderlich R, Weiss EM, Pockley AG, Fietkau R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr Med Chem (2012) 19:1751–64. doi: 10.2174/092986712800099811
21. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med (2012) 366:925–31. doi: 10.1056/NEJMoa1112824
22. Wersall PJ, Blomgren H, Pisa P, Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol (2006) 45:493–7. doi: 10.1080/02841860600604611
23. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol (2017) 14:365–79. doi: 10.1038/nrclinonc.2016.211
24. Kovacs A, Stenmark Tullberg A, Werner Rönnerman E, Holmberg E, Hartman L, Sjöström M, et al. Effect of radiotherapy after breast-conserving surgery depending on the presence of tumor- infiltrating lymphocytes: A long-term follow-up of the sweBCG91RT randomized trial. J Clin Oncol (2019) 37:1179–87. doi: 10.1200/JCO.18.02157
25. Tramm T, Vinter H, Vahl P, Özcan D, Alsner J, Overgaard J. Tumor-infiltrating lymphocytes predict improved overall survival after post-mastectomy radiotherapy: a study of the randomized DBCG82bc cohort. Acta Oncol (2022) 61:153–62. doi: 10.1080/0284186X.2021.1989629
26. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights: breast cancer, version 1.2017. J Natl Compr Canc Netw (2017) 15:433–51. doi: 10.6004/jnccn.2017.0044
27. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol (2015) 26:259–71. doi: 10.1093/annonc/mdu450
28. Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, et al. Development of tumor- infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res (2001) 7:3025–30.
29. Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys (2012) 84:879–80. doi: 10.1016/j.ijrobp.2012.06.020
30. Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol (2010) 22:113–24. doi: 10.1016/j.smim.2010.03.001
31. Sjöström M, Lundstedt D, Hartman L, Holmberg E, Killander F, Kovács A, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol (2017) 35:3222–9. doi: 10.1200/JCO.2017.72.7263
32. Okoh V, Deoraj A, Roy D. Estrogen-induced reactive oxygen species-mediated signalings contribute to breast cancer. Biochim Biophys Acta (2011) 1815:115–33. doi: 10.1016/j.bbcan.2010.10.005
33. Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol (2008) 26:1419–26. doi: 10.1200/JCO.2007.14.5565
34. Foster JS, Henley DC, Bukovsky A, Seth P, Wimalasena J. Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol Cell Biol (2001) 21:794–810. doi: 10.1128/MCB.21.3.794-810.2001
35. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res (2011) 71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820
36. Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol (2008) 180:3132–9. doi: 10.4049/jimmunol.180.5.3132
Keywords: tumor-infiltrating lymphocytes, breast cancer, postmastectomy radiotherapy, prognosis, local recurrence
Citation: Zhang L, Tang T, Liu L, Li C, Li Y and Geng C (2023) Effect of tumor-infiltrating lymphocytes depending on the presence of postmastectomy radiotherapy on the prognosis in pT1-2N1M0 breast cancer. Front. Oncol. 13:1175965. doi: 10.3389/fonc.2023.1175965
Received: 28 February 2023; Accepted: 21 July 2023;
Published: 04 August 2023.
Edited by:
Yutian Zou, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Lixue Xuan, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaXinzhao Wang, Shandong University, China
Copyright © 2023 Zhang, Tang, Liu, Li, Li and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuizhi Geng, Z2VuZ2N1aXpoaUBob3RtYWlsLmNvbQ==
 Lina Zhang
Lina Zhang Tiantian Tang
Tiantian Tang Cuizhi Geng
Cuizhi Geng