
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 June 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1175580
This article is part of the Research Topic Advances in the Surgical Management of Gastric and Colorectal Cancers View all 35 articles
Background: To explore the safety, efficacy, and survival benefits of laparoscopic digestive tract nutrition reconstruction (LDTNR) combined with conversion therapy in patients with unresectable gastric cancer with obstruction.
Methods: The clinical data of patients with unresectable gastric cancer with obstruction who was treated in Fujian Provincial Hospital from January 2016 to December 2019, were analyzed. LDTNR was performed according to the type and degree of obstruction. All patients received the epirubicin + oxaliplatin + capecitabine regimen as conversion therapy.
Results: Thirty-seven patients with unresectable obstructive gastric cancer underwent LDTNR, while thirty-three patients received chemotherapy only. In LDTNR group patients, the proportion of nutritional risks gradually decreased, the rate of severe malnutrition decreased, the proportion of neutrophil-lymphocyte ratio (NLR) <2.5 increased, the proportion of prognosis nutrition index (PNI) ≥45 increased, and the Spitzer QOL Index significantly increased at day 7 and 1 month postoperatively (P<0.05). One patient (6.3%) developed grade III anastomotic leakage and was discharged after the endoscopic intervention. The median chemotherapy cycle of patients in LDTNR group was 6 cycles (2-10 cycles), higher than that in Non-LDTNR group (P<0.001). Among those who received LDTNR therapy, 2 patients had a complete response, 17 had a partial response, 8 had stable disease, and 10 had progressive disease, which was significantly better than the response rate in Non-LDTNR group(P<0.001). The 1-year cumulative survival rates of the patients with or without LDTNR were 59.5% and 9.1%. The 3-year cumulative survival rate with or without LDTNR was 29.7% and 0%, respectively (P<0.001).
Conclusions: LDTNR can improve the inflammatory and immune status, increase compliance with chemotherapy, and have potential benefits in improving the safety and effectiveness of and survival after conversion treatment.
Gastric cancer is frequently diagnosed at an unresectable advanced stage and has a poor prognosis (1). Gastric obstruction (GO), a common complication in such patients, has various symptoms, such as nausea and vomiting, depriving them of further anticancer treatments (2, 3). A previous study demonstrated that fewer chemotherapy cycles and an objective response rate (ORR) could be achieved in patients with gastric outlet obstruction (GOO) (4). Therefore, ameliorating GO plays an important role in the continuation of subsequent anticancer treatments.
The National Comprehensive Cancer Network (NCCN) guidelines recommend the restoration of enteral nutrition with palliative methods, including gastrojejunostomy (GJ), gastrostomy (GT), and jejunostomy (JT) (5). Moreover, with the application of laparoscopic techniques, these palliative methods tend to afford quicker resumption of enteral nutrition, less surgical trauma, and better compliance with chemotherapy (6). Our previous study also indicated that laparoscopic gastrojejunostomy (LGJ) combined with conversion therapy could improve the overall survival (OS) of these patients (4). However, few studies have focused on multimodal therapy of laparoscopic digestive tract nutrition reconstruction (LDTNR), including GJ, GT, and JT, combined with conversion therapy. Therefore, the present study was designed to determine the safety and efficacy of LDTNR combined with conversion therapy in patients with GO. The results of this study may aid in clinical decisions to enable the management of symptoms caused by GO.
A retrospective study was conducted on all cases of unresectable gastric cancer treated at the Fujian Provincial Hospital, Fuzhou, Fujian, China, between January 2016 and December 2019. The inclusion criteria were as follows (1): pathological and radiological diagnosis of gastric cancer and presence of non-curable factors (No.16 lymph node metastasis, peritoneum metastasis and other organ invasion or metastasis) (7); (2) GO confirmed by endoscopy; (3) difficulty in oral intake caused by GO; (4) tolerance to general anesthesia and laparoscopic surgery; and (5) written informed consent. Exclusion criteria were as follows: (1) combined with other gastrointestinal obstructions; (2) combined with other malignant tumors; (3) patient received other anticancer treatments, such as chemoradiotherapy, before surgery; (4) severe dysfunction of the heart, lungs, kidneys, and other important organs; (5) patient completed less than two chemotherapy cycles; and (6) incomplete clinicopathological data. Based on the inclusion criteria, 70 patients were enrolled in the study. A multidisciplinary team determined the strategy for each patient. Gastric outlet obstruction scoring system (GOOSS) was scored as followed: 0 = no oral intake, 1 = liquids only, 2 = soft food and 3 = solid food. Patients with a GOOSS score of 2 were categorized into the Non-LDTNR group, and GOOSS score of 0 or 1 were categorized into the LDTNR group. The study was approved by the Ethics Committee of the Fujian Provincial Hospital. All procedures were performed in accordance with the Declaration of Helsinki of 1964 and its later versions.
All patients in LDTNR group received general anesthesia with endotracheal intubation and were placed in a supine position with splayed legs. We used the “five-hole method” to establish the laparoscopic hole. In the “five-hole method,” a 10 mm Trocar was inserted 2 cm below the umbilicus as the observation hole. A 12 mm Trocar was inserted 2 cm below the costal margin of the left anterior axillary line as the main operating hole. Trocars of 5 mm were inserted 2 cm above the plain umbilical of the left midclavicular line and 2 cm below the costal margin of the right anterior axillary line as an auxiliary operation hole. And a 5 mm Trocar was inserted 2 cm above the right midclavicular plain umbilicus.
(1) LGJ surgery was performed in patients with gastric pyloric cancer: a) The omentum of the greater curvature of the stomach was dissociated. We made holes in the greater curvature of the stomach (> 5 cm from the tumor edge) and jejunum (approximately 15 cm from the ligament of Treitz). b) The linear stapler was placed in the greater curvature of the stomach and closed with the jejunum to form a gastrojejunal anastomosis (Figure 1).
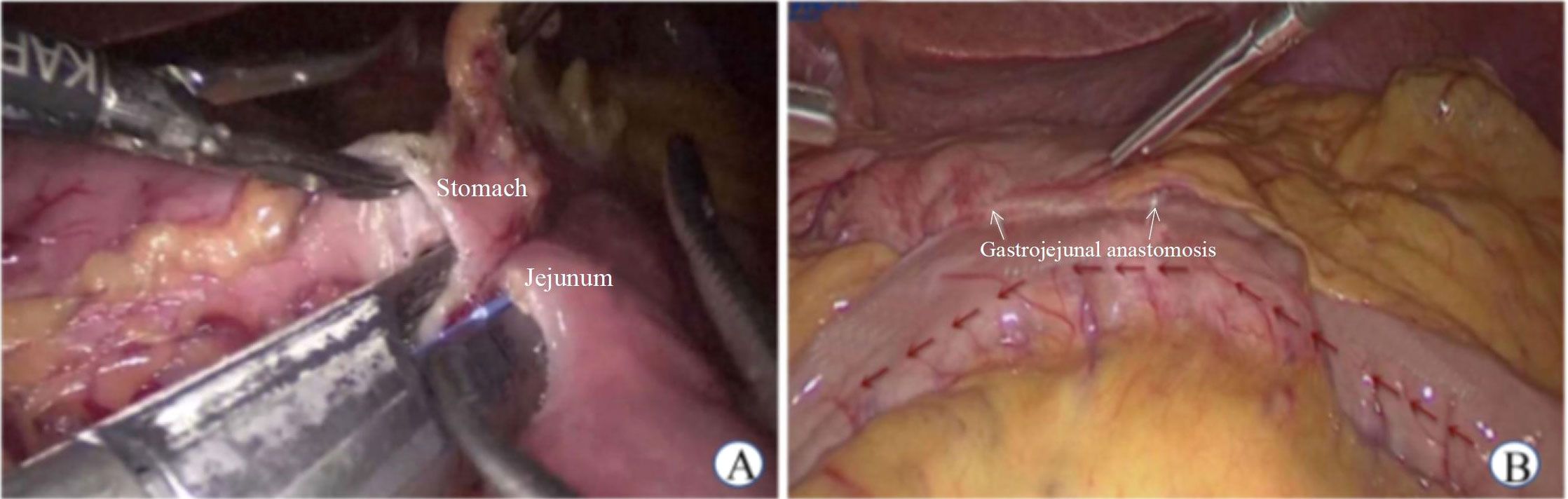
Figure 1 Laparoscopic gastrojejunostomy. (A) Side to side gastrojejunostomy (B) Postoperative status.
(2) Laparoscopic gastrostomy (LGT) was performed in patients with gastric cardia cancer: a) The omentum of the greater curvature of the stomach was dissociated, and the gastric wall was cut to 2 cm. b) The purse-string needle was inserted into the anterior wall of the stomach (2 cm from the incision of the gastric wall) and the abdominal wall from the inside, and the gastrostomy tube was placed by traction. c) The gastric wall incision was closed using a linear stapler, and the inner and outer latches of the fistula tube were fixed to the abdominal wall (Figure 2).
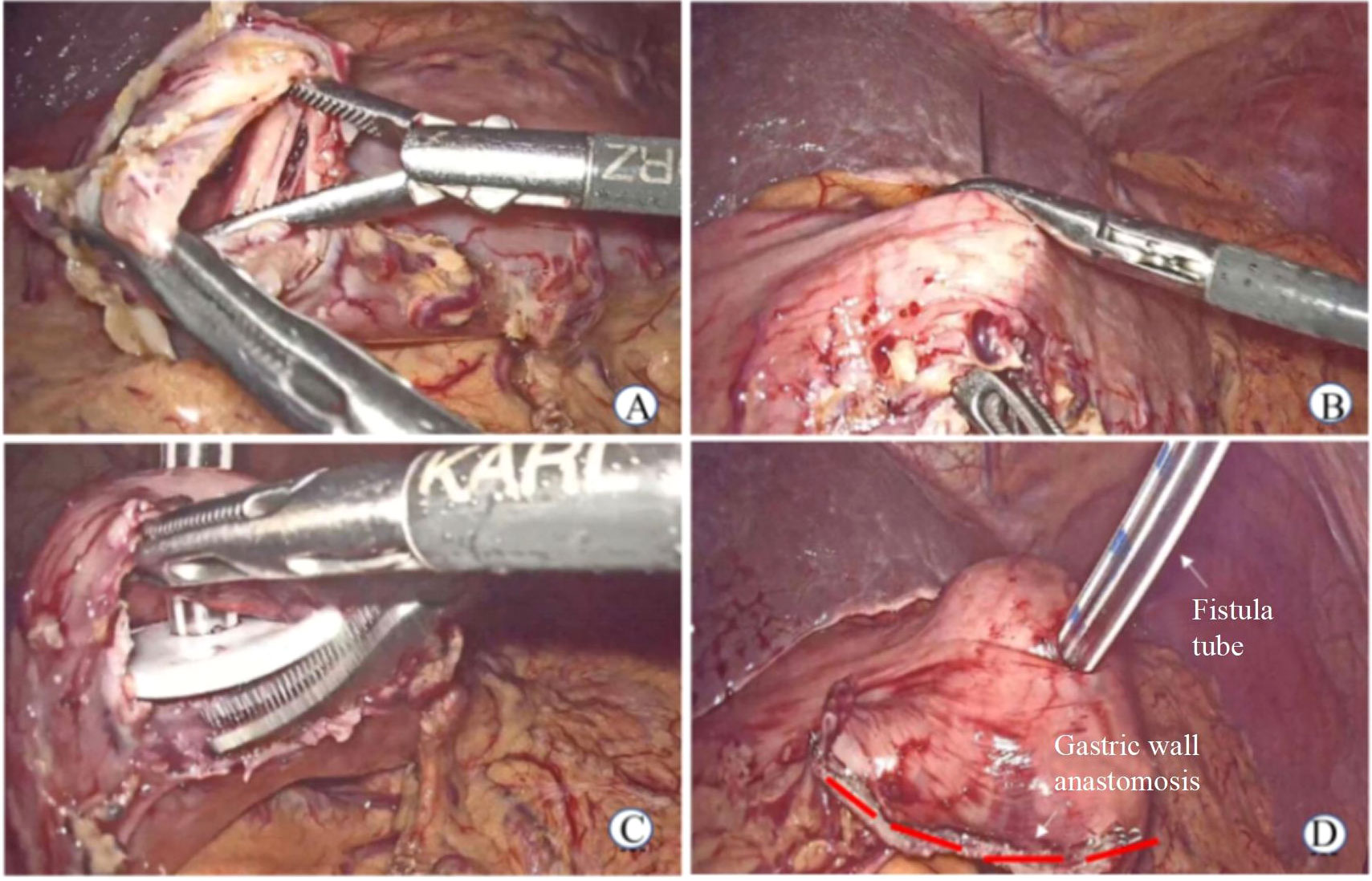
Figure 2 Laparoscopic gastrostomy. (A) Cut open gastric wall (B) The purse-string needle was inserted into the anterior wall of the stomach (C) The gastrostomy tube was placed (D) Postoperative status.
(3) Laparoscopic jejunostomy (LJT) was performed in patients with diffuse-type cancer. a) The jejunum was cut, using a linear stapler, 10–15 cm from the ligament of Treitz. b) The mesentery of the proximal jejunum was fully dissociated, and holes were made on the side of the proximal jejunum and the side of the jejunum 10 cm from the distal jejunum. A linear stapler was placed to perform side-to-side anastomosis of the proximal and distal jejunums. c) The distal jejunal stump was cut 2 cm, a purse-string needle was inserted into the intestinal wall (2 cm from the jejunal stump incision) and the abdominal wall from the inside, and the jejunostomy tube was placed. d)The opening of the distal jejunal stump was closed using a linear stapler, and the inner and outer latches of the fistula tube were fixed to the abdominal wall (Figure 3).
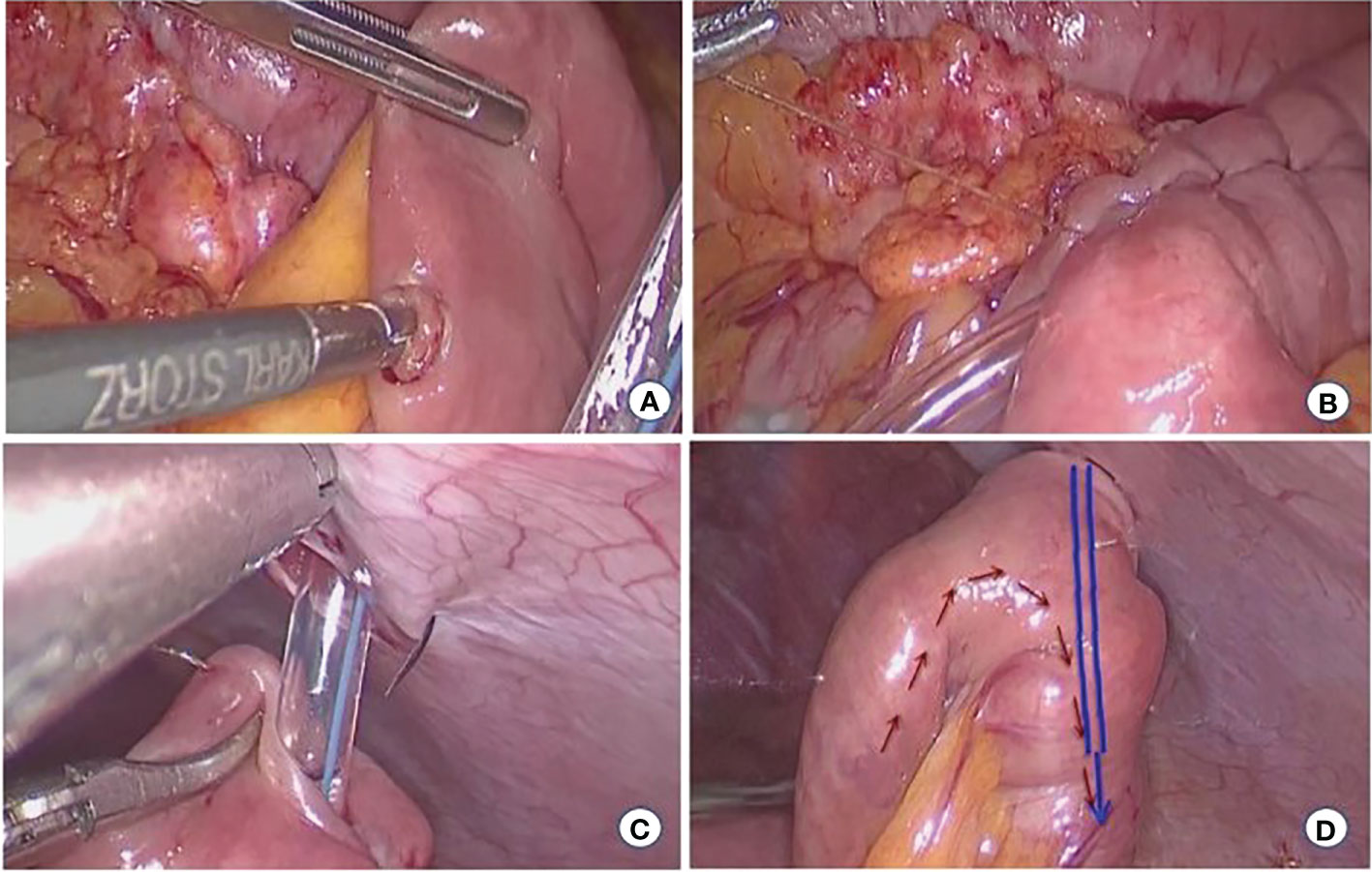
Figure 3 Laparoscopic jejunostomy. (A) Jejunum puncture (B) Embedding suture (C) Attach the fistula to the abdominal wall (D) Postoperative status.
All patients received the EOX (epirubicin + oxaliplatin + capecitabine) regimen, and the EOX regimen was administered to patients who underwent LDTNR, 7–14 days post surgery. The EOX regimen was epirubicin 100 mg/m2 on day 1, oxaliplatin 130 mg/m2 on day 1, and capecitabine 825 mg/m2 on days 1-14, and the chemotherapy cycles repeated every 3 weeks. Imaging evaluations were performed after every two cycles of chemotherapy. After a multidisciplinary discussion, the tumor was evaluated for radical surgery. In the case of tumor progression or National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) (8) grade 3-4 chemotherapy adverse events, subsequent treatments were formulated according to the multidisciplinary discussion.
(1) Nutritional Risk Assessment: The total score of the Nutrition Risk Screening 2002 (NRS2002) was calculated (9). For nutritional status assessment, BMI and the Patient-Generated Subjective Global Assessment (PG-SGA) overall assessment grading were used (10). The Prognosis Nutrition Index (PNI) and Neutrophil-Lymphocyte ratio (NLR) were used to assess the inflammatory and immune status. We divided the patients into two groups: PNI (<45 vs. ≥45) and NLR (<2.5 vs. ≥2.5) (11, 12). The Quality of life Assessment: Spitzer QOL Index was used to evaluate patients’ mobility, daily life, health status, support, and knowledge of disease and life (13, 14). Nutritional risk, nutritional status, inflammatory and immune status, and quality of life were evaluated before surgery and 7 days and 1 month after surgery of patients in LDTNR group.
(2) Response to chemotherapy: The efficacy of chemotherapy was evaluated according to RECIST 1.1 criteria. The overall response rate (ORR) was calculated as follows: cases of complete response + cases of partial response)/total number ×100%, and the disease control rate (DCR) as follows: cases of complete response + cases of partial response + number of stable disease)/total number ×100%.
(3) Postoperative follow-up: Postoperative complications were graded according to the Clavien-Dindo classification, and patients were followed up by telephone, outpatient examination, and inpatient review until the death of the patient or the last follow-up date (November 1, 2021). OS was defined as the time from the start of treatment to the date of the last follow-up or death.
The SPSS Statistics 23.0 (IBM, USA) statistical software was used for statistical analysis. Continuous variables were presented as x ± s. Kruskal-Wallis H test, t-test, or Wilcoxon rank sum test was used for continuous variables. Categorical variables were described as absolute numbers or percentages and tested using the chi-square or Fisher’s exact test. The Kaplan-Meier method was used to analyze survival, and the log-rank test was used for survival analysis. Prognostic factors of OS were analyzed using univariate and multivariate logistic regression. A P-value of less than 0.05 was considered to indicate a statistically significant difference.
During the study period, we obtain data of 37 patients who received LDTNR therapy, and 33 patients who received chemotherapy only. The baseline characteristics of all eligible patients are outlined in Table 1. No significant differences were detected in sex, age, cancer types, ECOG, clinical stage, non-curable factors and baseline nutritional and inflammatory status. Baseline GOOSS was better in Non-LDTNR group in comparison to the LDTNR group (P<0.001).
After LDTNR, the proportion of patients with nutritional risk (94.6% vs. 70.3% vs. 18.9%) and severe malnutrition (89.2% vs. 51.4% vs. 0) decreased gradually. The proportion of patients with NLR <2.5 (21.6% vs. 48.6% vs. 70.3%), PNI ≥45 (24.3% vs. 56.8% vs. 73.0%), and Spitzer QOL Index (P<0.05) increased gradually (Tables 2, 3). There was no significant difference in the BMI (P>0.05) at these time points.
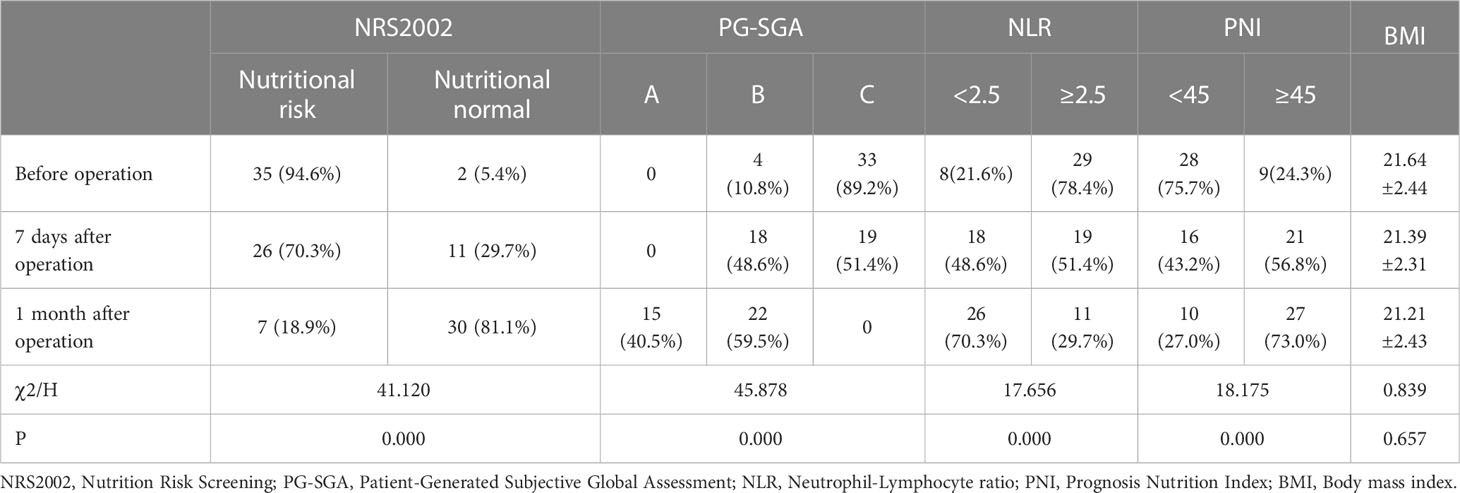
Table 2 The nutritional risk, inflammatory and immune status before operation, 7 days and 1 month after operation.
The median chemotherapy cycle of patients in LDTNR group was 6 cycles (2-10 cycles), higher than that in Non-LDTNR group (P<0.001). Among those who received LDTNR therapy, 2 patients had a complete response, 17 had a partial response, 8 had stable disease, and 10 had progressive disease, which was significantly better than the response rate in Non-LDTNR group(P<0.001) (Table 4). In the LDTNR group patients, peritoneal metastasis (receiving hyperthermic intraperitoneal chemotherapy) turned negative in eight patients, the No.16 lymph nodes disappeared or decreased in four patients, the depth of tumor invasion reduced to T4a stage in one patient with pancreatic invasion, and the liver metastasis significantly reduced or disappeared in three patients. While none of the Non-LDTNR group patients had resolution of non-curable factors.
Conversion surgery was performed in 16 (43.2%) LDTNR group patients. Four patients underwent gastrectomy and D3 lymph node dissection, and 12 underwent gastrectomy and D2 lymph node dissection (including 3 patients with liver metastasis resection). R0 resection was achieved in 13 patients (81.2%) and R1 resection in 3 patients (18.8%) (Table 5). One patient (6.3%) developed grade III anastomotic leakage and was discharged after the endoscopic intervention. All patients were followed for 1.2-50.3 months, with a median time of 12.5 months. The 1-year cumulative survival rates of the patients with or without LDTNR were 59.5% and 9.1%. The 3-year cumulative survival rate with or without LDTNR was 29.7% and 0%, respectively. The difference in survival between the two groups was statistically significant (P<0.001) (Figure 4). Further analysis showed that the survival time of the 13 patients with R0 resection was 41.9 ± 9.4 months and that of the 3 patients with R1 resection 18.4 ± 1.5 months, and the difference was statistically significant (P<0.001) (Figure 5). Univariate and multivariate analysis identified LDTNR therapy and subsequent gastrectomy were associated with better long-term prognosis (Table 6).
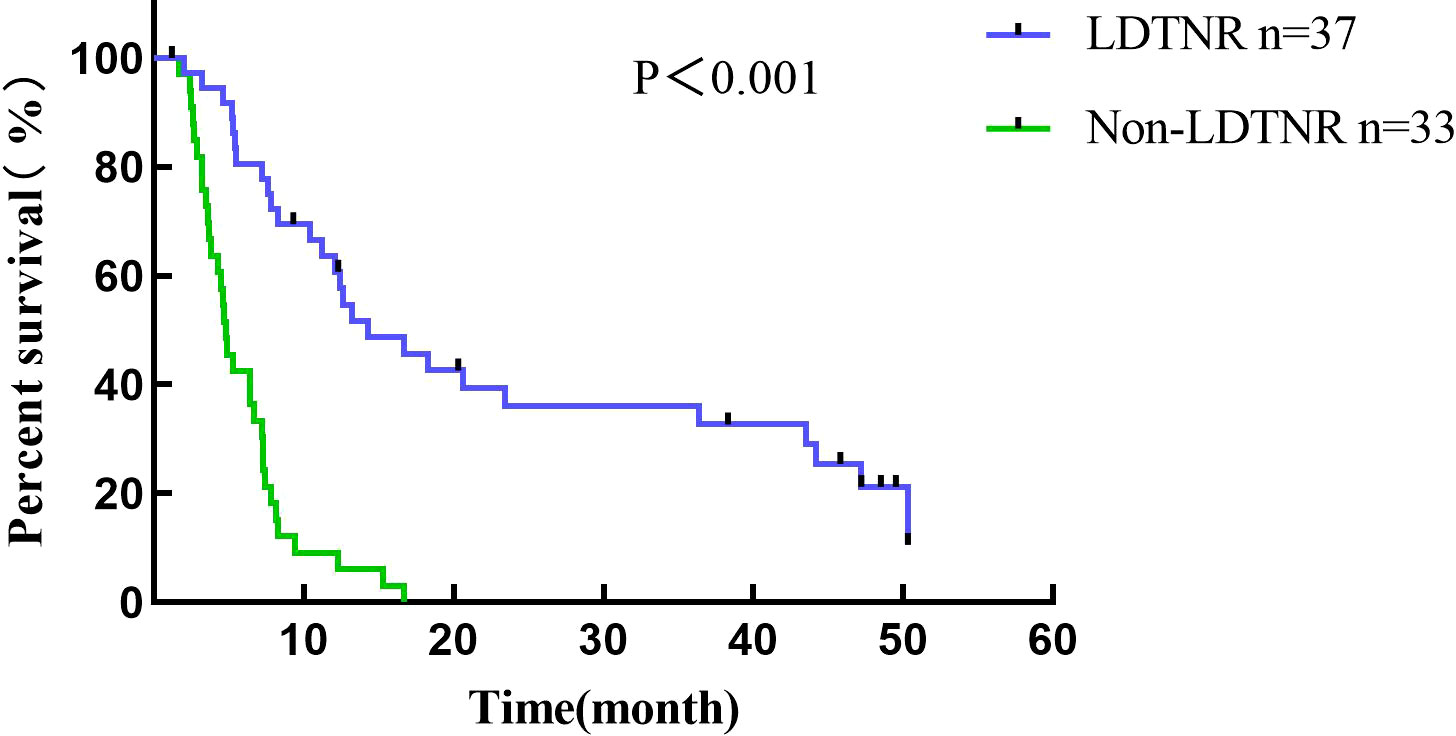
Figure 4 Survival curve of all patients enrolled in the present study. The 1-year cumulative survival rates of the patients with or without LDTNR were 59.5% and 9.1%. The 3-year cumulative survival rate with or without LDTNR was 29.7% and 0%, respectively.
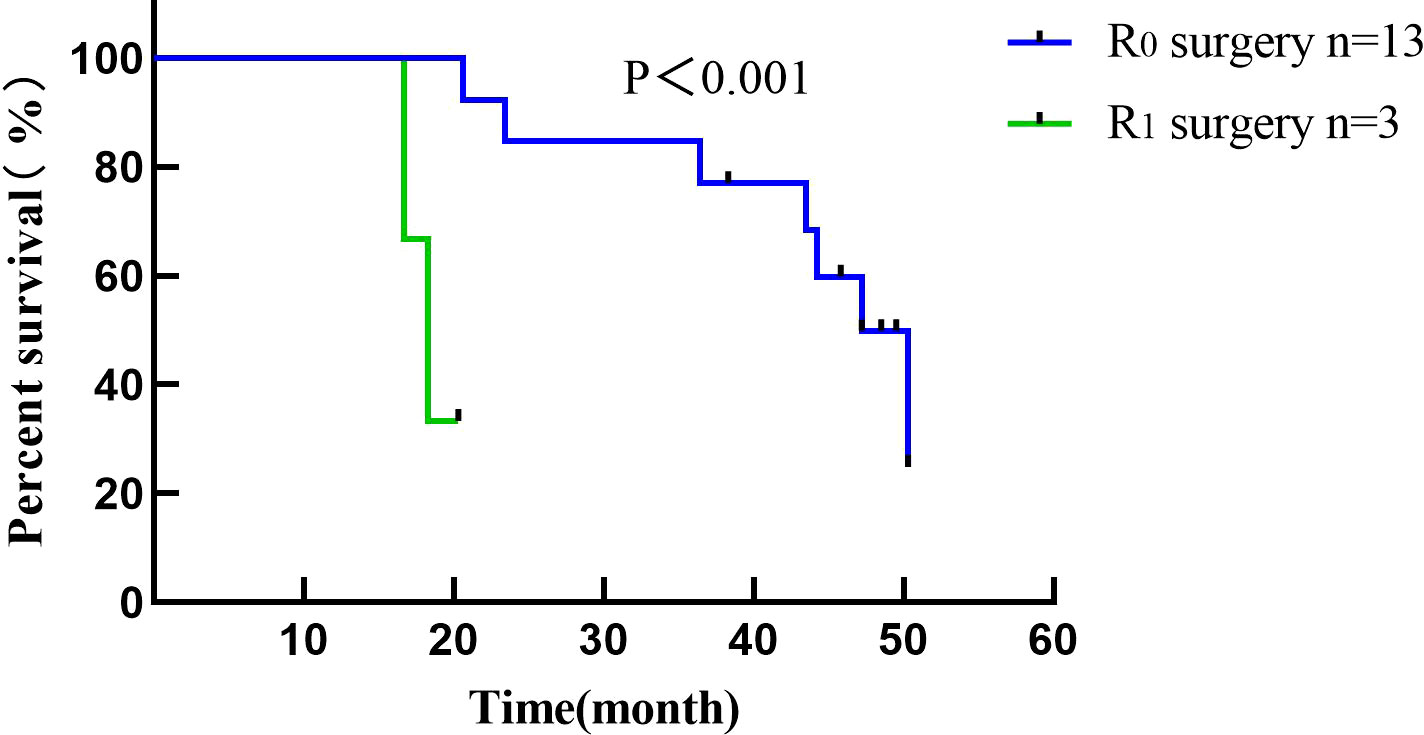
Figure 5 Survival curve of patients receiving radical gastrectomy in the present study. The survival time of patients with R0 resection was 41.9 ± 9.4 months and that of patients with R1 resection was 18.4 ± 1.5 months.
In previous studies, palliative resection was often used for patients with unresectable advanced gastric cancer with obstructive symptoms, focusing less on the long-term prognosis of such patients (14). A previous study (15) showed that tumor cells are released into the blood after palliative resection, and the activity of residual tumor cells is enhanced under surgical stress and an inflammatory response, which greatly enhances proliferation, invasion, and metastasis. In addition, the influence of gastrectomy on the quality of life, chemotherapy compliance, and tolerance will also affect the subsequent treatment effects in patients with gastric cancer. The REGATTA study (14) demonstrated that palliative resection could not prolong patient survival. LDTNR can bypass obstruction without stimulating the primary tumor, relieve the symptoms caused by obstruction, and restore enteral nutrition. It is expected to overcome the problem of undertaking subsequent treatments in patients with obstruction due to long-term insufficient nutrient intake and a metabolic state of high decomposition and low synthesis of nutrients (14, 16, 17). To the best of our knowledge, this study is the first to analyze the role of LDTNR in improving the inflammatory nutritional immune status and survival time of patients with unresectable advanced gastric cancer with obstructive symptoms.
In patients with gastric cancer obstruction, improvement in nutritional status enhances long-term survival. Therefore, relieving obstruction plays an important role. LDTNR is associated with less trauma load, shorter recovery time for enteral nutrition, and high compliance of patients with subsequent treatment (6). While reducing the stress response, it also avoids failure of endoscopic catheterization caused by obstruction and gastric retention, stent displacement, and secondary interventions due to tumor progression (18). After LDTNR, the first time to liquid food was after 1.43 ± 0.50 days, which was shorter than that after an open procedure (19), indicating that LDTNR results in less trauma and faster recovery of intestinal function. In addition, the latest general principles of nutritional therapy indicate that it supplements insufficient nutrients, enhances the body’s immune function, and reduces the inflammatory response (20). After LDTNR, the nutritional status and quality of life gradually increased, the inflammatory indicators gradually decreased, and the differences were statistically significant (P<0.001).
In survival analysis, LDTNR therapy offers a survival benefit in patients with unresectable and obstructive gastric cancer. The 3-year cumulative survival rate of patients with LDTNR was 29.7%, which is higher than that in Non-LDTNR group (P<0.001). In addition, multivariate analysis identified that LDTNR were associated with better long-term survival, compared with Non-LDTNR (HR, 5.198; 95% CI 1.019-3.368,P<0.001). For patients with GO caused by unresectable advanced gastric cancer, subsequent gastric resection, specifically R0 resection, is key to determining long-term prognosis. In this study, the survival of patients treated with radical resection was significantly better than those without this treatment. The median survival time of patients with R0 resection was also significantly longer than that of patients with R1 resection, which was similar to the previous report (21). Such patients have a low completion rate of conversion therapy owing to their poor nutritional status. In this study, 37 patients with unresectable advanced gastric cancer received LDTNR; the ORR was higher than that in Non-LDTNR group, with a resection rate of 43.2% and an R0 resection rate of 81.2%, which are also higher than those previously reported (22). The basic nutritional status of patients with gastric cancer and obstruction is poor. Preoperative chemotherapy often leads to a decreased nutritional status and suppression of cellular immunity, which increases the risk of postoperative complications. In this study, only one patient who underwent LDTNR had grade III postoperative complications, which was lower than that reported in the literature (20), and no deaths occurred. Enteral nutrition can effectively reduce gastric wall edema and malnutrition caused by obstruction and reduce the occurrence of anastomotic leakage, bleeding, and other complications. However, there was no significant difference in BMI among the patients in this study, and 10 patients could not tolerate the toxic effects of chemotherapy. This suggests that after the removal of the obstruction, individualized enteral nutrition, appropriate loading exercise, and efficacy evaluation are still needed for the safety and effectiveness of conversion therapy.
LDTNR results in better nutritional and inflammatory immune status of patients with obstruction caused by gastric cancer and improves the long-term prognosis of these patients. However, the effect of combination therapy with conversion therapy on the efficacy of chemotherapy, postoperative complications, and OS of these patients still needs to be verified using larger sample size and long-term follow-up data.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Fujian Provincial Hospital. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, BH and GG. Methodology, RY and CW. Formal analysis, RY and CW. Resources, RY and CW. Writing—original draft preparation, RY and CW. Writing—review and editing, RY, CW, BH and GG. Funding acquisition, GG. All authors contributed to the article and approved the submitted version.
This work was supported by the Talent programs granted from The First Affiliated Hospital of Fujian Medical University (YJRC3600) for data collection and analysis.
We wish to thank all colleagues and nurses who provided care to the patients in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
2. Del Piano M, Ballarè M, Montino F, Todesco A, Orsello M, Magnani C, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc (2005) 61(3):421–6. doi: 10.1016/S0016-5107(04)02757-9
3. Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study) : a multicenter randomized trial. Gastrointest Endosc (2010) 71(3):490–9. doi: 10.1016/j.gie.2009.09.042
4. Wang C, Lin S, Zhang X, Yang C, Li W. Laparoscopic gastrojejunostomy with conversion therapy in gastric outlet obstruction caused by incurable advanced gastric cancer. Cancer Manage Res (2021) 13:6847–57. doi: 10.2147/CMAR.S322569
5. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN (2022) 20(2):167–92. doi: 10.6004/jnccn.2022.0008
6. Tanaka T, Suda K, Satoh S, Kawamura Y, Inaba K, Ishida Y, et al. Effectiveness of laparoscopic stomach-partitioning gastrojejunostomy for patients with gastric outlet obstruction caused by advanced gastric cancer. Surg Endosc (2017) 31(1):359–67. doi: 10.1007/s00464-016-4980-0
7. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer (2016) 19(2):329–38. doi: 10.1007/s10120-015-0575-z
8. Zhou C, Ma T, Shi M, Xi W, Wu J, Yang C, et al. Dose-finding study of modified FLOT (mFLOT) regimen as first-line treatment in Chinese patients with metastatic adenocarcinoma of stomach. Cancer Chemother Pharmacol (2020) 85(1):113–9. doi: 10.1007/s00280-019-03982-4
9. Li YF, Nie RC, Wu T, Li SM, Chen S, Wang W, et al. Prognostic value of the nutritional risk screening 2002 scale in metastatic gastric cancer: a Large-scale cohort study. J Cancer (2019) 10(1):112–9. doi: 10.7150/jca.27729
10. Feijó PM, Rodrigues VD, Viana MS, Dos Santos MP, Abdelhay E, Viola JP, et al. Effects of ω-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: a randomized controlled trial. Nutr (Burbank Los Angeles County Calif) (2019) 61:125–31. doi: 10.1016/j.nut.2018.11.014
11. Mimatsu K, Fukino N, Ogasawara Y, Saino Y, Oida T. Utility of inflammatory marker- and nutritional status-based prognostic factors for predicting the prognosis of stage IV gastric cancer patients undergoing non-curative surgery. Anticancer Res (2017) 37(8):4215–22. doi: 10.21873/anticanres.11812
12. Yoshida N, Baba Y, Shigaki H, Harada K, Iwatsuki M, Kurashige J, et al. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg (2016) 40(8):1910–7. doi: 10.1007/s00268-016-3549-3
13. Khan L, Cramarossa G, Lemke M, Nguyen J, Zhang L, Chen E, et al. Symptom clusters using the spitzer quality of life index in patients with brain metastases–a reanalysis comparing different statistical methods. Supportive Care Cancer (2013) 21(2):467–73. doi: 10.1007/s00520-012-1540-6
14. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA) : a phase 3, randomised controlled trial. Lancet Oncol (2016) 17(3):309–18. doi: 10.1016/S1470-2045(15)00553-7
15. Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Trans Med (2018) 10(436):3464. doi: 10.1126/scitranslmed.aan3464
16. Aoyama T, Kawabe T, Fujikawa H, Hayashi T, Yamada T, Tsuchida K, et al. Loss of lean body mass as an independent risk factor for continuation of s-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol (2015) 22(8):2560–6. doi: 10.1245/s10434-014-4296-z
17. Oida T, Mimatsu K, Kawasaki A, Kano H, Kuboi Y, Amano S. Modified devine exclusion with vertical stomach reconstruction for gastric outlet obstruction: a novel technique. J Gastrointest Surg (2009) 13(7):1226–32. doi: 10.1007/s11605-009-0874-y
18. Krishnamoorthi R, Bomman S, Benias P, Kozarek RA, Peetermans JA, McMullen E, et al. Efficacy and safety of endoscopic duodenal stent versus endoscopic or surgical gastrojejunostomy to treat malignant gastric outlet obstruction: systematic review and meta-analysis. Endosc Int Open (2022) 10(6):E874–e97. doi: 10.1055/a-1794-0635
19. Ojima T, Nakamori M, Nakamura M, Katsuda M, Hayata K, Yamaue H. Laparoscopic gastrojejunostomy for patients with unresectable gastric cancer with gastric outlet obstruction. J Gastrointest Surg (2017) 21(8):1220–5. doi: 10.1007/s11605-017-3387-0
20. Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and s-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol (2015) 41(10):1354–60. doi: 10.1016/j.ejso.2015.04.021
21. Tanizawa Y, Terashima M, Tokunaga M, Bando E, Kawamura T, Sugisawa N, et al. [Conversion therapy of stage IV gastric cancer]. Gan to kagaku ryoho Cancer Chemotherapy (2012) 39(13):2469–73.
Keywords: advanced gastric cancer, digestive tract reconstruction, conversion therapy, immune and nutrition status, inflammatory status
Citation: Ye R, Wang C, Hu B and Guan G (2023) Safety and efficacy of laparoscopic digestive tract nutrition reconstruction combined with conversion therapy for patients with unresectable and obstructive gastric cancer. Front. Oncol. 13:1175580. doi: 10.3389/fonc.2023.1175580
Received: 08 March 2023; Accepted: 22 May 2023;
Published: 08 June 2023.
Edited by:
Bo Zhang, Sichuan University, ChinaReviewed by:
Zsolt Kaposztas, Somogy County Kaposi Mór Teaching Hospital, HungaryCopyright © 2023 Ye, Wang, Hu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoxian Guan, Zmp4aGdneEAxNjMuY29t; Bo Hu, aHVibzMzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.