
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 05 July 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1174173
This article is part of the Research Topic Breast Cancer Surgery: Silicone Implant Controversies and Current Alternatives View all 5 articles
 Grace C. Keane1
Grace C. Keane1 Alexandra M. Keane1
Alexandra M. Keane1 Ryan Diederich2
Ryan Diederich2 Kaitlyn Kennard3
Kaitlyn Kennard3 Eric J. Duncavage4
Eric J. Duncavage4 Terence M. Myckatyn1*
Terence M. Myckatyn1*Breast implants, whether placed for reconstructive or cosmetic purposes, are rarely lifetime devices. Rupture, resulting from compromised implant shell integrity, and capsular contracture caused by constriction of the specialized scar tissue that normally forms around breast implants, have long been recognized, and remain the leading causes of implant failure. It is apparent, however, that women with breast implants may also experience delayed breast swelling due to a range of etiologic factors. While a majority of delayed seromas associated with breast implants have a benign etiology, this presentation cannot be ignored without an adequate workup as malignancies such as breast implant associated anaplastic large cell lymphoma (BIA-ALCL), breast implant associated diffuse large B-cell lymphoma (BIA-DLBCL), and breast implant associated squamous cell carcinoma (BIA-SCC) can have a similar clinical presentation. Since these malignancies occur with sufficient frequency, and with sometimes lethal consequences, their existence must be recognized, and an appropriate diagnostic approach implemented. A multidisciplinary team that involves a plastic surgeon, radiologist, pathologist, and, as required, surgical and medical oncologists can expedite judicious care. Herein we review and further characterize conditions that can lead to delayed swelling around breast implants.
Breast implants remain the most common implanted medical devices in plastic surgery operating rooms. Over 350,000 women underwent cosmetic breast augmentation in the Unites States in 2021, making it the second most popular aesthetic procedure next to liposuction (1). Breast implants also represent the most common form of post-mastectomy reconstruction for the 1 in 8 women in the United States who will be diagnosed with breast cancer during their lifetimes. Though breast implants are approved by the Federal Drug Administration (FDA) for the purposes of breast augmentation or reconstruction, they are not without risk. This has come to light more recently with the discovery of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) and the considerable attention it has garnered over the last decade.
BIA-ALCL is a rare T-cell lymphoma that most often presents as a delayed seroma surrounding a textured breast implant. A mass originating in the implant capsule may develop concurrently, or as the sole finding, with most cases presenting 8-10 years post-implantation, with earlier and later cases also reported. The first case of anaplastic T-cell lymphoma in proximity to a saline-filled breast implant was described as early as 1997, although recent literature identifies a possible earlier case description in 1996 (2–4). The first FDA safety communication regarding breast implants in 2011 and the recognition of BIA-ALCL as a separate category of malignancy by the World Health Organization (WHO) in 2016 sparked heightened awareness (5). Shortly thereafter, the National Comprehensive Cancer Network (NCCN) published guidelines for the diagnosis and treatment of BIA-ALCL, emphasizing early intervention and surgical treatment (6). As of April 1, 2022, the FDA has received a total of 1,130 United States and global medical device reports (MDRs) of BIA-ALCL (7).
Despite its recent notoriety, BIA-ALCL represents only a small fraction of delayed complications associated with breast implants. Specifically, making an accurate diagnosis when a patient presents with delayed breast swelling can be challenging. Development of a swollen breast one or more years after implantation carries with it a lengthy differential diagnosis representing a wide range of potential morbidity and mortality. In addition to BIA-ALCL, other malignancies associated with breast implants have been recognized, including breast implant-associated squamous cell carcinoma (BIA-SCC) and breast implant associated diffuse large B-cell lymphoma (BIA-DLBCL). In this review, we focus on the etiology of a swollen breast that develops in a delayed manner following placement of breast implants. We expand upon each of the breast implant-associated malignancies, including a discussion on the varied presentation, etiology, diagnostic algorithm, findings, and treatment modalities for each disease. We also review common findings and treatment modalities for benign causes of delayed breast swelling, including infection, benign seroma, trauma, hematoma, double capsule, and capsular contracture.
The NCCN has recently standardized the evaluation of the delayed swollen breast in patients with a history of implants (Figure 1) (6). Patients should first be assessed with ultrasound to assess for fluid collection, breast masses, and lymphadenopathy. Complete ultrasound evaluation should include the implant; chest wall; axillary, internal mammary, and supraclavicular lymph nodes; and contralateral breast implant. If ultrasound is equivocal, breast magnetic resonance imaging (MRI) may aid in diagnosis for fluid collections or soft tissue masses. Fine needle aspiration is the standard for sampling periprosthetic fluid collections. Ultrasound guidance is recommended to obtain an appropriate sample and avoid implant injury. Any suspicious masses found during initial imaging should be biopsied and sent for histopathologic analysis. Specimens should be sent for cytology to evaluate cell morphology, immunohistochemistry (IHC) for immune cell markers, and flow cytometry to evaluate cells within the specimen. Cytologic and cell block preparations are utilized to identify neoplastic cells in aspirated effusion fluid.

Figure 1 NCCN Guidelines for BIA-ALCL Diagnosis (6).
If an implant-associated malignancy is established, a multidisciplinary team including pathologists, medical and radiation oncologists, surgical oncologists, radiologists, plastic surgeons, and the patient should be leveraged to help stage and treat disease. Pre-operative laboratory studies should include a comprehensive metabolic panel, complete blood count with differential, coagulation studies, and lactate dehydrogenase (6). Hepatitis B testing should be performed if the patient may need chemotherapy. A preoperative positron emission tomography computed tomography (PET/CT) scan is recommended for evaluating associated capsular masses, chest wall involvement, lymph node spread, and organ metastases (8). One must keep in mind that a PET/CT performed for the first three months after surgical intervention is unreliable due to post-operative inflammatory changes within the tissue (6). Treatment modality is determined by the malignancy and extent of disease (Table 1).
BIA-ALCL is a CD30-positive T-cell lymphoma that arises around textured breast implants. This disease is distinct from a primary breast lymphoma, which is typically a B-cell lymphoma that arises within the breast parenchyma. The etiology of BIA-ALCL is unknown but likely triggered by chronic inflammation. Implant texturization is indisputably a driver, while host genetic factors, and time likely play a role in tumorigenesis. Bacterial infection and biofilm formation, specifically from Raltosonia spp, or perhaps the lipopolysaccharide coat of Gram-negative bacteria was thought to a play a primary role in the pathologic inflammation leading to BIA-ALCL (9). However, more recent research suggests other potential inciting events that may obscure the exact etiologic pathway, including mechanical stress, implant toxins, and surface tribology (10–14). Curiously, a relative attenuation of circulating T-helper cells may occur in the first couple days following placement of a textured, but not smooth breast implant (15). While each of these studies proposes a different “trigger,” chronic inflammation is the common thread, and is the most likely facilitator of malignant transformation to BIA-ALCL (16).
Next generation sequencing performed on patients with BIA-ALCL has often shown activating mutations in the JAK-STAT signaling pathway, most commonly STAT3 and JAK1 (17, 18). BIA-ALCL has also recently been associated with upregulation of hypoxia signaling proteins, specifically carbonic anyhydrase-9 (19). Recently, whole exome sequencing (WES) and whole genome sequencing (WGS) have reported several genomic aberrations associated with BIA-ALCL, but no true driver mutation. These include deletions in chromosome 20q13.13, 20q11.22-q13.2, as well as a critically deleted region on chromosome 11 (11q22.3) corresponding to the ataxia-telangiectasia mutation (ATM) gene (13, 17, 20). Cytokine expression levels also help distinguish BIA-ALCL, which has been characterized by T-helper 2-associated cytokine levels and an IL10 to IL-6 ratio >0.104 (21–23). These findings not only further characterize BIA-ALCL, but open the door for novel treatments and targeted immunotherapies based on expression profile (24, 25).
All verified cases of BIA-ALCL with complete implant history have been exclusively discovered in patients with a history of textured implants, many of which have been recalled, or in some countries banned, due to this association (26–28). Of the 1,130 medical device reports of ALCL, 37 cases were found in patients with smooth implants. However, these patients either previously had textured implants or insufficient implant history (29). By contrast, implant fill (saline or silicone) and reason for implantation (augmentation or reconstruction) has no defined relationship to BIA-ALCL. Therefore, a thorough surgical history including all previous implanted devices should be obtained in all patients presenting with delayed seroma.
The incidence of BIA-ALCL in patients with a history of textured implants varies widely, reported as high as 1:355 patients to as low as 1:40,000 patients (26, 30–32). Age of diagnosis is also varied, with an median of 53 years old (ranging 24-90 years old), while time from implantation to diagnosis is consistently prolonged, with an average timeframe of 7-10 years (6, 16, 32, 33). BIA-ALCL manifests as a mass or, more commonly, a rapidly enlarging periprosthetic fluid collection surrounding an implant years after implantation (34). Other local and systemic symptoms reported in patients diagnosed with BIA-ALCL include pain, lymphadenopathy, skin rash, fevers, and capsular contracture (6, 35). In the case of advanced disease, BIA-ALCL typically spreads to the ipsilateral axillary nodes, with supraclavicular, internal mammary, or mediastinal nodal involvement occurring less commonly (36). Intraoperative findings may demonstrate intracapsular periprosthetic fluid collection containing fibrinous material with or without extracapsular spread. Herein, we describe a patient with disseminated BIA-ALCL that presented with a swollen left breast (Figure 2A). PET scan reveals T4 lesion with extracapsular disease on the left side (Figure 2B), mandating excision of adjacent axillary soft tissues in conjunction with en bloc capsulectomy (Figure 2C). Importantly, this patient has a prophylactic total capsulectomy on the contralateral right side (ie. entire capsule and breast implant as a single unit, but not adjacent margin of soft tissue) and was noted to have an occult T1 luminal capsular BIA-ALCL. We recommend contralateral prophylactic total capsulectomy in patients undergoing therapeutic en bloc capsulectomy because of a 1-3% risk of bilateral disease (6, 37–39).
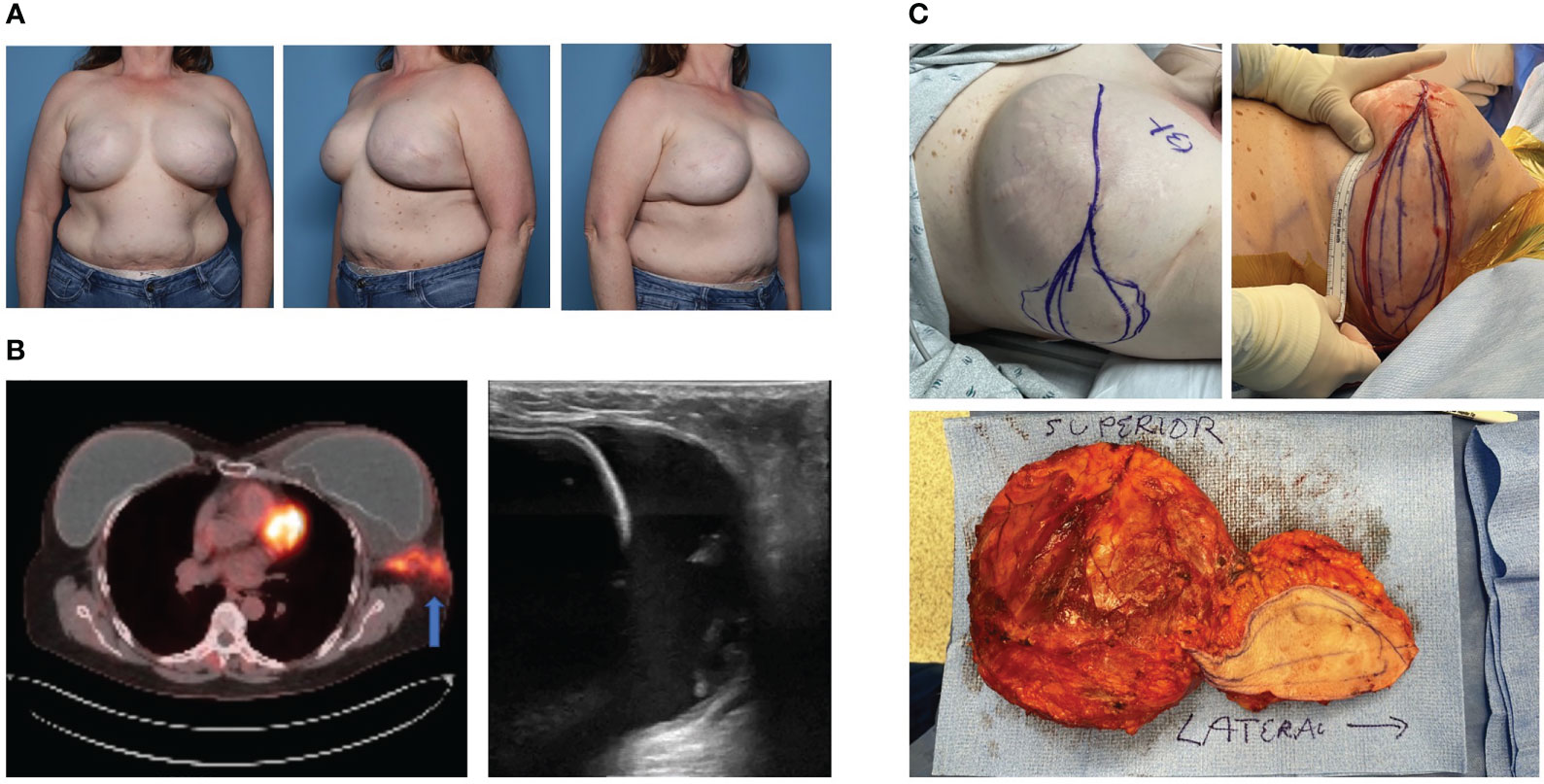
Figure 2 Case of Bilateral Disseminated BIA-ALCL. (A) A 52-year-old female with history of left stage 1 breast cancer and right ductal carcinoma in situ who underwent neoadjuvant chemotherapy, bilateral mastectomy and implant based breast reconstruction (subpectoral macrotextured breast implants) in 2008. She developed left breast swelling in 2021 and seroma aspirate was consistent with BIA-ALCL. (B) PET-CT revealed increased metabolic activity within the left capsule with associated extracapsular involvement into the ipsilateral axilla. Ultrasound revealed intracapsular fluid collection surrounding the implant. (C) Bilateral en bloc capsulectomies were performed with mass resection extending to the left axilla. Pathology revealed left Stage 4 BIA-ALCL and incidentally found right Stage 1 BIA-ALCL. The patient underwent adjuvant left chest wall radiation and brentuximab vedotin immunotherapy. The patient has had a complete metabolic response to therapy and no evidence of recurrence at 20 months post-operatively.
The diagnosis of BIA-ALCL is made through cytologic and immunohistochemical analysis of periprosthetic fluid. The neoplastic cells of BIA-ALCL are large, pleomorphic cells with anaplastic morphology. The cell nuclei are large, oval or multilobulated, with dense chromatin, and have prominent nucleoli and frequent mitoses (40). Commonly described in association with ALCL are the “hallmark” cells, which have horseshoe- or kidney-shaped nuclei and are found in a majority of cases (41). BIA-ALCL is universally CD30 positive and ALK negative. Expression of CD30 is a fundamental finding in BIA-ALCL; however, it is important to note CD30 expression alone is not diagnostic for BIA-ALCL, as it is non-specifically expressed by benign inflammatory cells (6, 42–44). Likewise, because other forms of ALCL also lack ALK expression, ALK negative IHC alone does not establish a diagnosis of BIA-ALCL. Additional biomarkers may be required to establish the diagnosis and exclude other malignancies, including CD2, CD3, CD4, CD5, CD7, CD8, and CD45 expression.
The Lugano modification of the Ann Arbor staging system is traditionally used to stage all forms of Non-Hodgkin lymphoma (45). However, nearly all BIA-ALCL cases are staged as Stage IE – lymphoma with single extranodal involvement, or Stage IIE – extranodal disease with local nodal involvement. Further, this staging system does not account for capsular involvement (46). Thus, MD Anderson Cancer Center developed a tumor, lymph node, metastasis (TNM) solid tumor staging system that was adapted by the NCCN to further characterize BIA-ALCL (Table 2). While many reported cases are categorized as stage I, over 25% of BIA-ALCL cases have extracapsular involvement at the time of diagnosis (6, 33, 46). This staging system has demonstrated improved efficacy in predicting survival and recurrence compared to the Ann Arbor staging system (46).
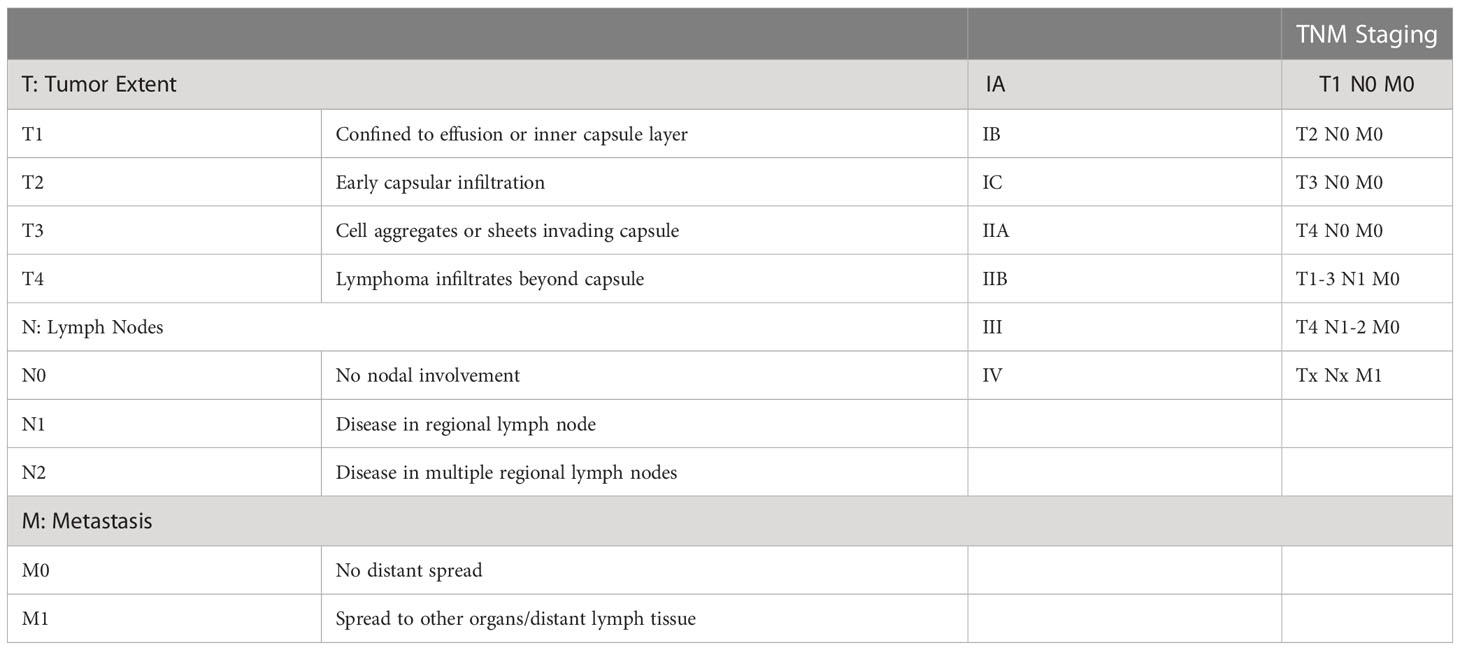
Table 2 – BIA-ALCL Tumor, Lymph Node, Metastasis (TNM) Classification and Staging (6) TNM Staging.
Following diagnosis and pre-operative imaging, the mainstay of treatment for BIA-ALCL is total capsulectomy (Figure 2C) (6, 46). There is no proven role for mastectomy, sentinel lymph node biopsy, or axillary node dissection (6). Surgery should always be conducted with an en bloc capsulectomy to remove the implant in continuity with the capsule, any associated extracapsular masses, and a margin of contiguous healthy tissue. Complete surgical excision with negative margins is associated with long-term, disease free survival, and may be adequate for disease localized to the capsule (Stage IA-IIA) (46, 47). However, disease recurrence is nearly 3-fold higher in Stage II and Stage III disease, and is more likely with incomplete resection, partial capsulectomies, or positive margins.
The use of adjuvant therapy for BIA-ALCL is limited to patients with residual or disseminated disease. The NCCN recommends radiation therapy of 24 to 36 Gray (Gy) for any local residual disease or unresectable masses due to chest wall involvement (6). In patients with disseminated disease, first-line systemic therapy should include the combination regimen CHOP (Cyclophosphamide, Adriamycin, Vincristine, and Prednisone) and brentuximab vedotin, a CD30 monoclonal antibody that has recognized survivability benefits when treating peripheral T-cell lymphomas (48–52).
Worldwide, there have been a total of 59 reported deaths related to BIA-ALCL up until April 1, 2022, with more under review (33). Overall survivability of early stage BIA-ALCL is reported as 94% at 3 years and 91% at 5 years, respectively (46). Later stage of presentation is associated with decreased survivability and higher risk of recurrence (46). Patients who reach remission should be monitored for recurrence every 3 to 6 months for 2 years, and radiologic imaging with CT or PET scan should be considered every 6 months for 2 years due to the high 3-year recurrence risk at all stages (6).
Breast implant associated squamous cell carcinoma (BIA-SCC) is a rare but aggressive malignancy that originates from the breast implant capsule. This entity was first proposed by Paletta and colleagues in 1992, who reported a case of BIA-SCC in a patient who underwent breast augmentation with silicone implants 16 years prior (53). Since this initial case report nearly 30 years ago, there have been 16 verified cases of BIA-SCC according to the American Society for Plastic Surgeons (ASPS), with more cases under review including one we present herein (34, 54–59). Though rare, this malignancy has garnered attention recently due to its aggressive nature and high mortality.
The origin of the squamous cell epithelium in this malignancy is unclear. Similar carcinogenic processes have been described with foreign bodies in other tissues, including bullet wounds and dental or orthopedic implants (60–63). It is proposed that ductal epithelium can be displaced at the time of pocket implantation, resulting in squamous epithelialization of the breast implant capsule (54, 55, 59). Additionally, macrophages and lymphocytes infiltrate the breast pocket and release cytokines to wall-off the foreign body. This may become exaggerated over a protracted course, such as in the case of a permanent breast implant. It is well-known that chronic inflammation can lead to an imbalance in inflammatory and apoptotic cell signaling pathways, resulting in tissue metaplasia (64). Such is the case for intestinal metaplasia of the esophagus and stomach in response to chronic acid exposure. Similar concepts may be applied to BIA-SCC: chronic inflammation and fibrosis surrounding the breast implant can inadvertently stimulate metaplastic squamous epithelium production within the capsule, a precursor to squamous cell carcinoma.
Age of diagnosis is highly variable, ranging from 40-81 years old (34, 54–59). The time interval from initial breast implant surgery to BIA-SCC diagnosis ranges from 11-41 years, and while consistently protracted, does overlap to an extent with the timeframe during which BIA-ALCL may develop. This dysplastic process, which may be indolent in nature, should therefore remain on the differential diagnosis along with BIA-ALCL when assessing patients who present with new breast swelling in the context of a breast implant multiple years after implantation.
Presentation of BIA-SCC includes unilateral breast pain, erythema, and fluid collection. Patients may also present with some degree of capsular contracture and implant malposition, though this is not a uniform finding at time of diagnosis. Of the 16 reported cases, eleven occurred following breast augmentation and five occurred following breast reconstruction (34, 54–59, 65). Cases have been reported in all implant types, including saline and silicone implants with either textured or smooth surfaces (54–59, 65, 66). However, in a majority of these reports, implant surface and device history are not reported and so there is insufficient information to determine whether it has etiologic relevance. Future cases including completely documented device history will help determine if an association with implant texturing exists.
The ASPS recommends FNA and cytology of any delayed seroma prior to surgical intervention (67). Most cases reported in the literature have not involved FNA of seroma fluid prior to operative intervention, as many of these cases were reported prior to new diagnostic recommendations (54–59, 65, 66). Seroma aspirates confirming BIA-SCC express epithelial carcinoma marker CK 5/6 and squamous cell transcription factor p63, and contain squamous cells and keratin. We report a case of a patient presenting with unilateral breast swelling 16 years following cosmetic breast augmentation with a macrotextured implant (Figures 3A, B). To properly evaluate for BIA-SCC, seroma fluid aspirate (Figure 3C) should be sent for IHC looking for CD30 and ALK to evaluate for BIA-ALCL, along with CK 5/6 and p63. Flow cytometry should be employed to look for T-cells, squamous cells, and keratin. One should note that seroma aspirate is not completely comprehensive for detecting malignancy, and tissue biopsy is commonly required to achieve diagnosis. For the case we present herein, initial seroma aspirate failed to reveal malignancy; squamous cell carcinoma was only found on pathology after complete capsulectomy was performed.
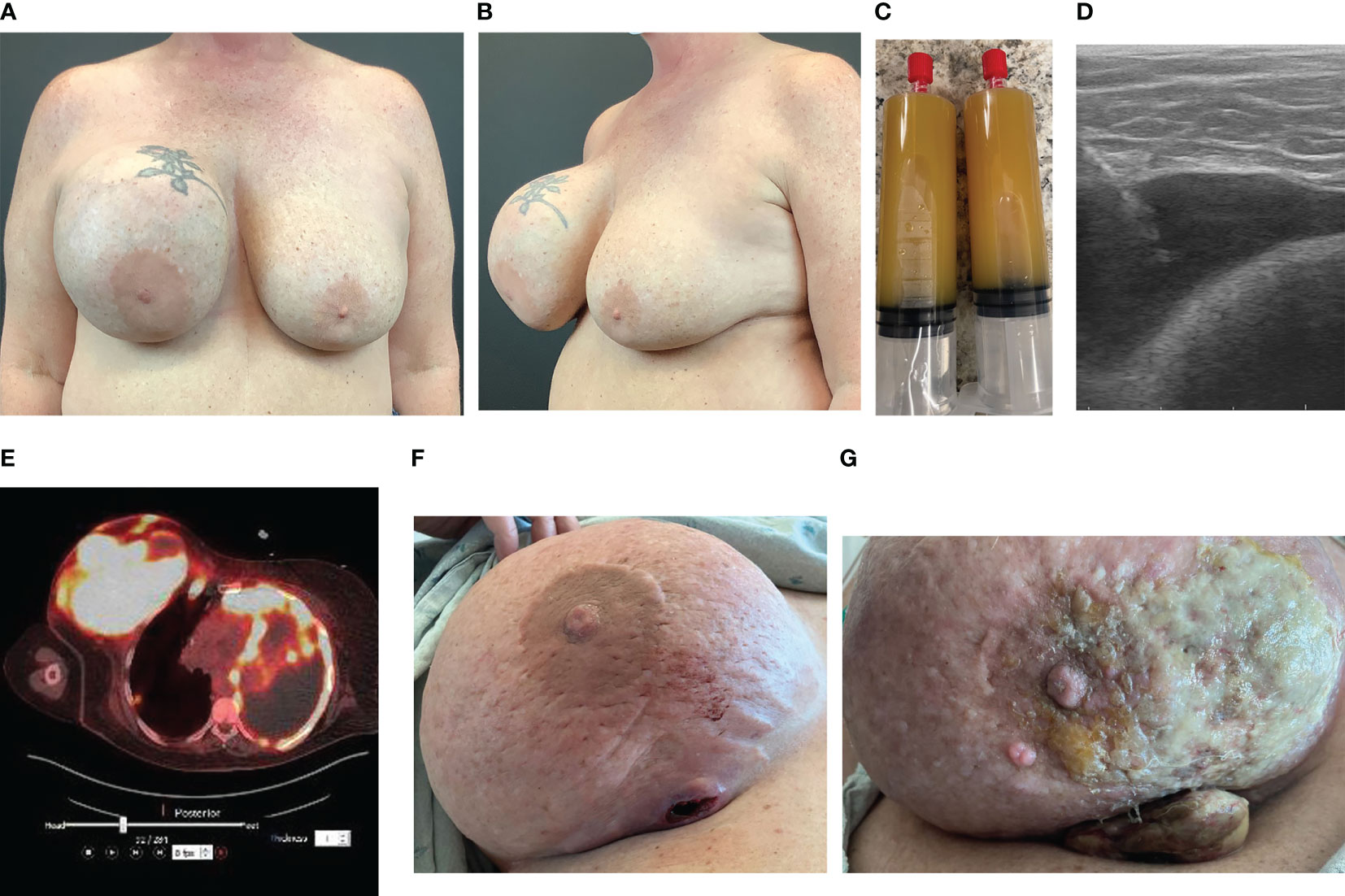
Figure 3 Case of BIA-SCC. (A) Anterior view of a 56-year-old female with history of macrotextured breast implant in 2006 presented with unilateral right breast swelling, pain, and capsular contracture 16 years after implantation. (B) Oblique view at presentation. (C) In-office seroma aspirate in May 2022 revealed copious amount of yellow, turbid fluid. Cytology and IHC analysis demonstrated acute inflammation and abundant squamous cells, but no malignant cells. BIA-ALCL workup negative. (D) Ultrasound shows fluid collection between implant and capsule. This patient then underwent bilateral implant removal with total capsulectomy. Intraoperative findings revealed thickened capsule with an associated tan-pink, indurated, nodular mass. Pathology revealed poorly differentiated SCC. The patient was placed on adjuvant pembrolizumab, abraxane, and carboplatin chemotherapy. (E) PET/CT 3 months post-implant removal revealed increased metabolic uptake throughout the right breast and axilla, and left chest wall and pleura, consistent with metastatic SCC. (F) By September 2022, the primary malignancy began to erode through the patient’s breast. (G) One month later the primary malignancy progressed despite chemotherapy. She expired weeks later from complications related to pleural metastases.
Pre-operative imaging facilitates surgical planning. Ultrasound is commonly used to guide aspiration of seroma fluid for analysis (Figure 3D). Breast MRI with and without contrast can be employed to identify any masses. Findings consistent with BIA-SCC will demonstrate an ill-defined mass arising from the breast capsule, with possible extent into the chest wall. PET-CT should be employed prior to intervention to appropriately determine extent of disease (Figure 3E).
Intraoperative findings of BIA-SCC include fungating breast capsule masses with granulomatous and keratinized debris contained within a viscous, turbid seroma fluid. In a majority of reported cases, this malignancy arises from the posterior aspect of the implant capsule, with spread of keratinaceous material into the pectoralis muscle and axillary tissue (Figures 3E–G) (53–57, 59). Capsules are commonly found intact with a thickened appearance and yellow hue. Histology from these capsules and associated granulomatous material demonstrates invasive keratinized squamous cell carcinoma and metaplasia with evidence of acute on chronic inflammation. These findings further support the theory that chronic inflammation stimulates malignant transformation.
The overall prognosis for BIA-SCC is grim, with a 6-month mortality rate of 43.8% (67). Early diagnosis and treatment can have life-lengthening benefits. Current treatment recommendations are likely to evolve as more diagnoses are described. The current treatment recommendation is surgical with en bloc capsulectomy and radical mastectomy (67). Surgeons should be aggressive, and should not hesitate to resect chest wall or axillary contents if there is suspicion for invasive malignancy. This is of utmost importance when treating BIA-SCC, as incomplete resection is associated with aggressive recurrence and increased mortality. There does not appear to be a role for adjuvant chemotherapy or radiotherapy, as the malignancy has thus far demonstrated little to no therapeutic response to either treatment modality.
Lymphomas associated with breast implants are rare and commonly have a T-cell origin. In a small minority of cases, though, delayed unilateral breast swelling years after breast implant placement (Figure 4A) have been attributed to other lymphomas. Breast implant associated-B-cell lymphomas are characterized by a more heterogenous cellular origin that includes diffuse large B-cell lymphoma, follicular lymphoma, primary cutaneous lymphoma, intravascular large-cell lymphoma, splenic marginal zone lymphoma, and plasmablastic lymphoma (68–82). Of these diagnoses, breast implant associated diffuse large B-cell lymphoma (BIA-DLBCL) has been most commonly described in the literature, is associated with a delayed seroma (Figure 4B), and has been associated, in several instances, with Epstein Barr Virus (EBV).
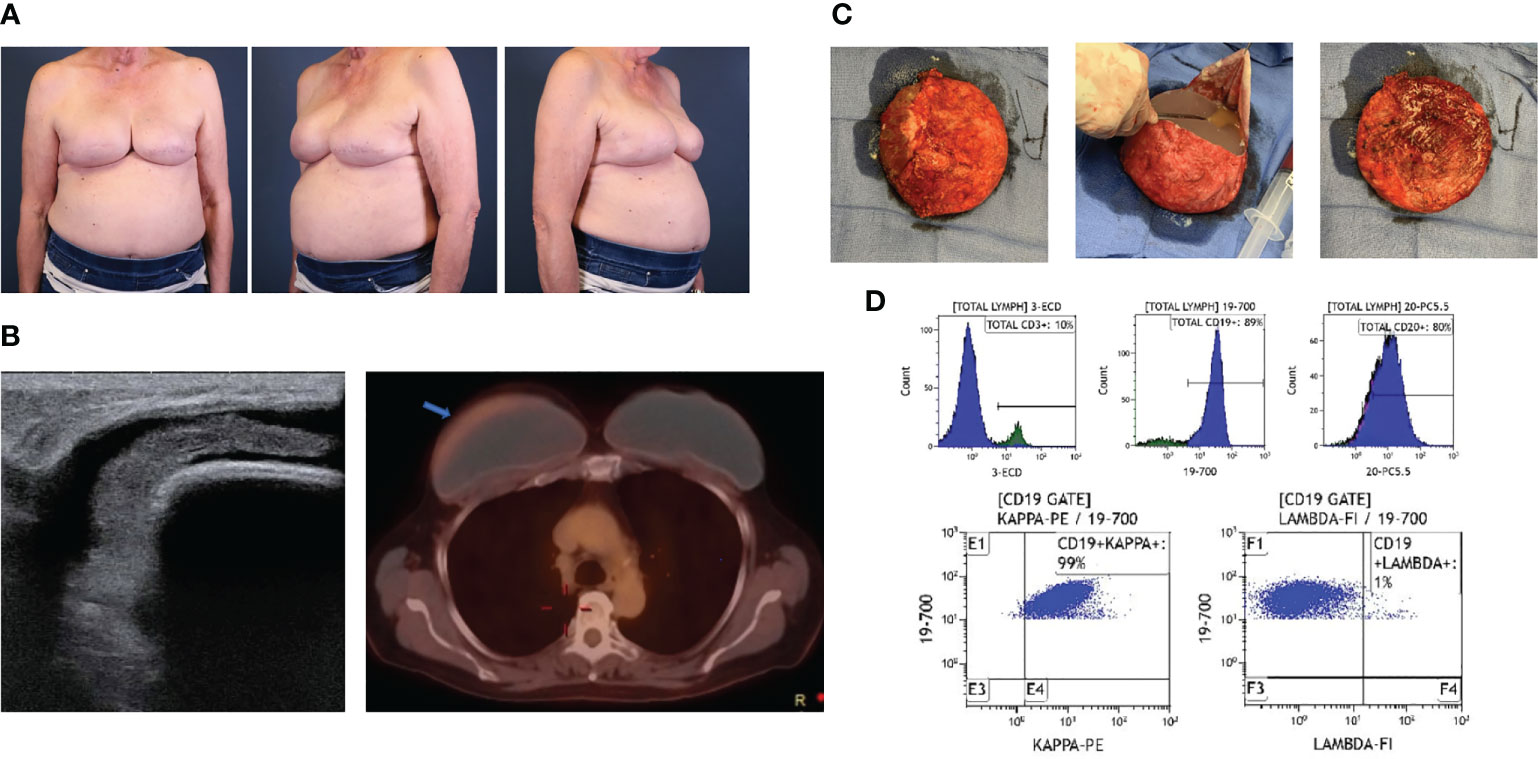
Figure 4 Case of BIA-DLBCL. (A) A 71-year-old female with history of left ductal carcinoma in situ status post bilateral mastectomies and immediate microtextured breast implant reconstruction in 2015 presented with unilateral right breast swelling three years after implantation with seroma aspirate consistent with BIA-DLBCL. (B) Ultrasound demonstrated peri-implant fluid collection and PET/CT revealed minimally increased metabolic uptake around the right breast capsule. (C) Bilateral en bloc capsulectomies were performed, demonstrating thick implant capsules and yellow-tinged intracapsular fluid collection. (D) Flow cytometry from the right breast capsule revealed a majority of cells expressing CD19 and CD20 with kappa light-chain restriction. No additional adjunct therapies were required. She has had a complete response and has no evidence of recurrence at 3 years post-operatively.
Due to the broad array of reported B-cell lymphomas, patient presentation is wide-ranging. Of the 28 reported cases, a majority of patients reported breast pain and swelling or palpable mass, while fewer presented with capsular contracture, B symptoms, hepatosplenomegaly, and lymphadenopathy (68–83). Herein, we present two cases, one of BIA-DLBCL (Figure 4) and one of breast implant associated follicular lymphoma (Figure 5). Age and time from initial implantation to diagnosis ranges from 34 – 83 years old and 6 – 44 years, respectively. Despite these differences, there are similarities, most notably the association with textured, silicone breast implants (70, 81–83).

Figure 5 Follicular cell lymphoma associated with breast implant capsule. (A) Lymphoid aggerate seen in breast capsule (2x magnification, H&E stain). (B) High power view of the aggerate shows atypical lymphocytes with irregular contours (20x magnification, H&E stain). (C) A CD3 stain shows few T-cells (20x magnification). (D) The atypical lymphocytes are CD20+ B-cells and comprise nearly all of the lymphocytes (20x magnification). (E) An adjacent lymph node shows closely packed and expanded follicles without mantle zone consistent with grade 1-2 follicular lymphoma (4x magnification). (F) A CD20 stain shows that follicles are composed of B-cells (4x magnification). (G) The neoplastic follicles are BCL2 positive (4x magnification). (H) BCL6 is positive in follicles (4x magnification).
There have been fourteen reported patients with diffuse B-cell lymphoma, twelve of which were found to be positive for Epstein-Barr Virus. EBV-positive DLBCL has been implicated in states of immunosuppression and chronic inflammation, categorized by the WHO as diffuse large B-cell lymphoma associated with chronic inflammation (DLBCL-CI), of which pyothorax-associated lymphoma is the prototype (84). The presence of longstanding chronic inflammation associated with an indwelling implant may result in proliferation of EBV-transformed B-cells, such as in the case of DLBCL-CI (85). However, the fibrinous material associated with BIA-DLBCL lends itself to a diagnosis similar to fibrin-associated DLBCL (FA-DLBCL), an indolent form of EBV-positive large B-cell lymphoma that has been categorized by the WHO as a clinically distinct subtype of DLBCL-CI (86). This form of lymphoma has been reported in association with pathologic debris surrounding atrial myxomas, endovascular graft thrombi, metallic prosthesis, and pseudocysts. These cases have a much more favorable prognosis in comparison to DLBCL-CI, and may even represent a form of EBV-positive lymphoproliferative disease rather than a lymphoma (87). However, not all cases of implant-associated DLBCL are EBV-positive, including the case we present in Figure 4. Further case collection and pathologic investigation is required to characterize this novel lymphoma.
Complete physical evaluation and diagnostic workup for the delayed swollen breast should be obtained to characterize this malignancy. Most cases are localized to the implant capsule, though few have been found to be invasive, mass-forming lymphomas. Gross pathologic findings of DLBLCL exhibit tan, thickened implant capsules with granular, gritty inner lining following en bloc capsulectomy (Figure 4C). Reported microscopic examination demonstrates focal foreign body giant cell reactions and lymphoplasmacytic aggregates of pleomorphic lymphoid cells, which may have atypical nuclei with numerous mitotic figures, heterogeneous chromatin pattern, and/or prominent nuceloli (21, 70). Immunohistochemical analysis exhibits a wide array of B-cell expression profiles, with inconsistent staining for B-cell markers CD20, CD19, CD79a, PAX-5, and BCL-6 (Figure 4D) (70, 72, 83).
En-bloc capsulectomy (Figure 4C) and implant removal has proven to be adequate in treating localized breast implant-associated B-cell lymphomas (81, 82). We have also used this approach to manage a case of follicular cell lymphoma associated with a breast implant capsule. Distinguishing this case as a follicular cell lymphoma were the findings of lymphoid aggregates seen in the breast implant capsule (Figure 5A), atypia with irregular contours (Figure 5B), few T-cells (Figure 5C), predominance of CD20+ B-cells (Figure 5D), closely packed and expanded follicles without mantle zones (Figure 5E), that were comprised of B-cells (Figure 5F). Follicles were BCL2 (Figure 5G) and BCL6 (Figure 5H) positive. Given the few case reports and overall indolent nature of the disease, a consensus on the need for adjuvant chemoradiation or radiologic disease monitoring has not been reached. Regular breast imaging has been advocated to evaluate disease recurrence. Long-term follow up data is needed to determine disease prognosis and survivability benefit for each of these treatment modalities.
Benign delayed seromas are usually defined as serous fluid collections that develop around an implant more than one year after implantation. In accordance with the NCCN guidelines (6), these are diagnosed primarily by ultrasound, but breast MRI may be required in equivocal cases or when a greater level of sensitivity and specificity are warranted. It has been theorized that benign delayed seromas are the result of trauma, hematoma, subclinical infection, or implant rupture, though they can also occur without an identified precipitating cause. It has been theorized that benign delayed seromas are the result of trauma, hematoma, subclinical infection, or implant rupture, though they can also occur without an identified precipitating cause. Benign delayed seromas are rare events, occurring in less than 1% of subjects in large multicenter trials (88–90).
Prior to the discovery of BIA-ALCL, a majority of early case reports describing delayed seromas occurred in patients who previously had macrotextured breast implants (12, 89, 91, 92). It is conceivable that early case reports of delayed seromas were undiagnosed BIA-ALCL, as all diagnosed patients had a history of textured implants. It is important to note that up to 10% of delayed fluid collections associated with breast implants are malignant on further diagnostic evaluation (42, 88, 89). Though similar in physical presentation, the pathogenesis of benign delayed seromas and BIA-ALCL are distinct. BIA-ALCL develops as a malignant effusion due to increased vascular permeability resulting from cellular production of interleukins and elevated oncotic pressure caused by the high cellularity and protein content of the fluid. Benign delayed seromas have a wide variety of purported etiologies, including an idiopathic one. Though associated with trauma and subclinical infection, benign delayed seromas typically lack a microbiologic or cytologic biomarker (92). The detection of CD30 positive cells on IHC aids the diagnosis of BIA-ALCL and helps differentiate a benign delayed seroma from a malignant one, drastically changing overall management (43).
Treatment of benign delayed seroma varies based on surgeon and patient preferences, ranging from serial aspirations to complete capsulectomy. In their multicenter retrospective review of delayed seromas, Spear et al. expounded upon a graduated approach to treating delayed seromas that included antibiotics, serial aspirations, drain placement, and surgical resection (90). A majority of patients required surgical intervention to reach full resolution, while 28.5% of patients were able to be successfully managed with aspiration or antibiotics alone. In their series, all aspirate cultures were negative for planktonic bacteria identifiable with standard culture techniques. They did not perform advanced biofilm detection techniques such as 16S rRNA sequencing, immunohistochemistry for bacteria-specific probes, or scanning electron microscopy. Likewise, routine CD30 immunohistochemistry testing was not performed as BIA-ALCL was not a well-known diagnosis at this time. We believe a similar algorithm can be used to treat delayed seromas once they are determined to be benign and non-infectious.
Another rare but benign etiology of delayed breast swelling is the double capsule. This occurs when the inner capsule envelope adheres to the implant surface while a distinct outer capsule adheres to surrounding tissues, divided by an intercapsular space that may contain a seroma (Figure 6A) (11, 12). Typically, the outer capsule can be dissected from the surrounding soft tissues while the inner capsule remains intimately associated to the textured device (Figure 6B) unless it is deliberately peeled off the implant (Figure 6C). Hall-Findlay was the first to report on her experience with double capsule formation (12). Initial patient presentation is highly variable, including persistent seroma, capsular contracture, bottoming out, and asymmetry (12, 93, 94). Similar to BIA-ALCL, this phenomenon primarily develops in patients with textured implants. It is theorized that macrotextured surfaces induce some adherence of the capsule to the implant, which can result in mechanical shearing of the implant capsule from the implant surface and seroma formation (93, 95). Bacterial biofilms may contribute to double capsule formation by weakening capsule strength and facilitating extracellular matrix delamination and double-capsule formation (96). Diagnostic evaluation should follow the NCCN guidelines. The only surgical modality efficacious in treating double capsule includes inner capsulectomy with or without outer capsulectomy and smooth implant exchange (12, 94).
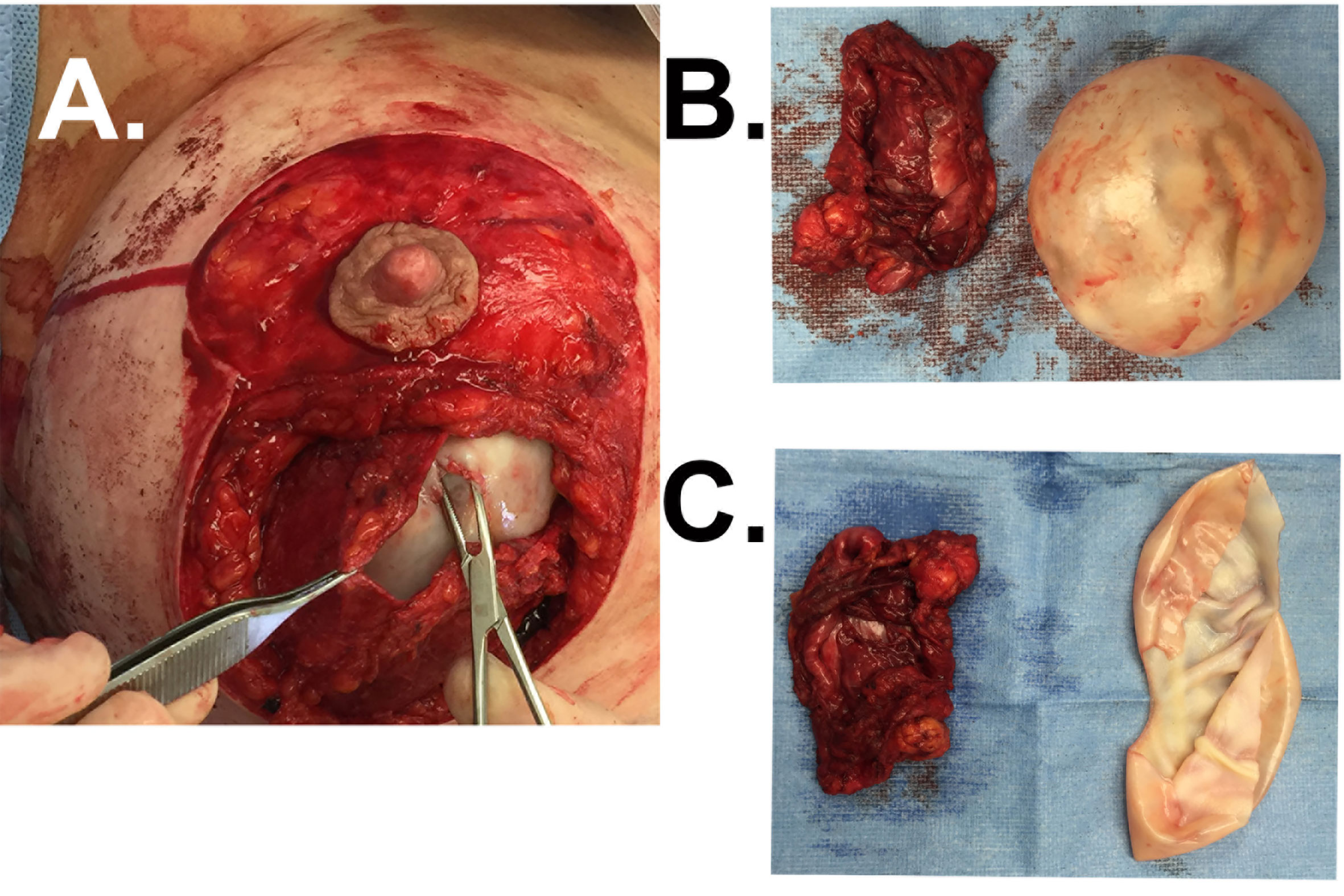
Figure 6 Case of double capsule. (A) Patient with a macrotextured saline breast implant placed for cosmetic reasons 7 years prior presents with a rapidly developing seroma of the right breast. The forcep reflects the outer capsule and the hemostat penetrates the inner capsule that is intimately associated with the implant. Clear fluid was identified between these two layers in situ. (B) The outer capsule is free from the inner capsule that is in continuity with the textured implant shown here. (C) The inner capsule now dissected free from the implant surface.
When examining the delayed swollen breast, implant infection must be taken into consideration. Reported incidence of implant infection ranges from 0-2.5% following breast augmentation, and up to 35% following breast reconstruction after mastectomy (97–100). Acute infections typically occur within weeks to months following implant placement. Patients commonly present with breast pain, drainage, and erythema, and systemic symptoms including fevers, nausea, and vomiting. Possible sources of infection include the patient’s skin or breast microbiota, contaminated implant or irrigation fluid, surgical manipulation, and hematogenous spread.
In addition to causing acute infections, many of these bacteria have evolved to adhere to implant surfaces, forming assemblages of surface-adherent bacteria encapsulated in extracellular polymers known as biofilms. The formation of these biofilms around an implant are implicated in subclinical infections, capsular contracture, and other systemic symptoms. Subacute infections, which can occur months to years after surgery, have a more indolent course, making them more difficult to distinguish from other diagnoses. Patients may present with chronic pain, persistent swelling and drainage, wound healing problems, or implant migration. Hematogenous spread of bacteria from distant sites play a crucial role for developing late onset breast implant infections.
Initial evaluation for breast implant infection should rely heavily on the patient history and physical exam findings. Full history of recent illnesses or infections and surgical interventions should be reviewed. Providers should look for subtle signs of infection including fevers, nausea and vomiting, and new breast pain, erythema, or drainage. Laboratory tests should include a comprehensive metabolic panel and complete blood count. Diagnostic evaluation for subacute infections should include complete breast ultrasound to evaluate for drainable fluid collections, cultures, and bacterioscopic smear test may be considered to confirm and characterize infection. Malignancy should also be ruled out with imaging should new breast masses or lymphadenopathy be identified, and FNA performed of seroma fluid with histologic examination. While some investigators report successfully salvaging periprosthetic implant infections using negative pressure therapy with or without irrigation (101), treatment traditionally warrants surgical washout and implant explantation.
Early hematoma directly following breast implant placement, whether it be for reconstructive or aesthetic purposes, is a well-documented post-operative complication occurring in 0.6-10.3% of all cases (102–104). Peri-prosthetic late hematomas that occur more than 6 months after surgery are considered a rare complication, many of which have unknown causes. Chest trauma is an acute inciting factor that can result in spontaneous capsular sheering and hematoma formation. Likewise, chronic inflammation or systemic therapies, such as corticosteroids, chemotherapy, or systemic anticoagulation, can damage peri-capsular arteries and lead to late capsular hematoma (105–107). In the absence of a clear inciting event, it is thought that mechanical friction between the prosthesis and the highly vascular capsule, with a consequent capsule microfractures, may play a role in delayed hematoma (104). In evaluating patients for delayed hematoma, MRI and ultrasonography may be performed, but are not helpful in distinguishing hematoma from implant rupture, and may lead to false positives. Treatment includes hematoma evacuation and implant exchange with or without capsulectomy in patients who want to maintain their breast size. Hematoma is also a risk factor for the development of capsular contracture and should be adequately addressed to avoid this latent complication.
With nearly one in eight women diagnosed with breast cancer within their lifetime, the possibility that a new breast mass or fluid collection in a patient with breast implants is related to primary or recurrent breast cancer can occur (108). Thus, physicians must have a high index of suspicion for breast cancer when evaluating the delayed swollen breast. In particular, invasion of dermal lymphatics in inflammatory breast cancer can lead to rapid swelling, erythema, and pitting edema thus presenting as delayed swelling of the breast in a women with previous implant-based breast augmentation (108). Therefore, all patients who present with delayed breast swelling should undergo diagnostic mammography, breast MRI, and/or complete breast ultrasound to evaluate for cancerous lesions (109–111). Breast MRI is an important imaging modality that can be employed in younger patients with highly dense breast tissue. Any suspicious masses or should be evaluated by a radiologist and surgical oncologist to determine need for further evaluation and treatment.
In addition to complications associated with breast implants, patient medical comorbidities must be taken into consideration when evaluating the swollen breast. Previous case reports have demonstrated that unilateral breast edema may be a manifestation of congestive heart failure, specifically in elderly patients (112–114). Patients will present with signs and symptoms of heart failure on physical exam, including jugular venous distension, pretibital pitting edema, and pulmonary congestion. Chest radiographs will demonstrate cardiomegaly and pulmonary edema, and diagnosis will be made by decreased ejection fraction on electrocardiography.
The delayed presentation of a swollen breast in patients with a history of breast implants is a diagnostic challenge to all physicians. Though many cases are benign, one must carefully follow the NCCN guidelines to properly evaluate for the malignancy, including BIA-ALCL, BIA-SCC, and BIA-DLBCL, and recurrent or new primary breast cancer. All cases of malignancies associated with breast implants should be reported to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database and the device manufacturer. To improve our understanding of these rare cancers, cases of breast implant malignancy from the United States should be reported to the PROFILE registry (https://plasticsurgery.formstack.com/forms/profile_case_submission) and equivalent registries in other countries. Moreover, genomics continue to play a critical role in the diagnosis and identification of targeted therapies to more effectively manage both breast cancers and breast-implant associated malignancies (24, 25). Further, though these malignancy are rare, all patients receiving breast implants should be counseled pre-operatively on the risk of each of these cancers, and particularly BIA-SCC due to its severity and mortality (67).
TM takes full responsibility for the integrity and accuracy of this review articles. All authors approve the final article and agree to be accountable for all aspects of the work. Concept and design: GK, AK, TM. Drafting of the manuscript: GK, AK, TM. Critical revisions of the manuscript for important intellectual content: all authors. Supervision: TM.
Authors are grateful to Jill Guess for administrative assistance with pathology slides and Michelle Bingaman for collection of patient consent forms.
TM receives grant funding from Sientra and RTI Surgical and product development royalties from RTI Surgical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. How much does breast implant removal cost? American Society of Plastic Surgeons. Available at: https://www.plasticsurgery.org/cosmetic-procedures/breast-implant-removal/cost (Accessed 13, 2021).
2. Keech JA Jr., Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg Aug (1997) 100(2):554–5. doi: 10.1097/00006534-199708000-00065
3. Lyapichev KA, Medeiros LJ, Clemens MW, Ferrufino-Schmidt MC, Marques-Piubelli ML, Chai SM, et al. Reconsideration of the first recognition of breast implant-associated anaplastic large cell lymphoma: a critical review of the literature. Ann Diagn Pathol Apr (2020) 45:151474. doi: 10.1016/j.anndiagpath.2020.151474
4. Duric V, Stockler M. Patients' preferences for adjuvant chemotherapy in early breast cancer: a review of what makes it worthwhile. Lancet Oncol Nov (2001) 2(11):691–7. doi: 10.1016/S1470-2045(01)00559-9
5. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
6. Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic Large cell lymphoma (BIA-ALCL). Aesthet Surg J (2019) 39(Suppl_1):S3–S13. doi: 10.1093/asj/sjy331
7. U.S. Food and Drug Administration. Medical device reports of breast implant-associated anaplastic large cell lymphoma. US FDA (2022). Available at: https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma.
8. Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg (2015) 135(3):695–705. doi: 10.1097/PRS.0000000000001033
9. Hu H, Johani K, Almatroudi A, Vickery K, Van Natta B, Kadin ME, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic Large-cell lymphoma. Plast Reconstr Surg (2016) 137(6):1659–69. doi: 10.1097/PRS.0000000000002010
10. Hallab NJ, Samelko L, Hammond D. The inflammatory effects of breast implant particulate shedding: comparison with orthopedic implants. Aesthet Surg J (2019) 39(Supplement_1):S36–48. doi: 10.1093/asj/sjy335
11. Giot JP, Paek LS, Nizard N, El-Diwany M, Gaboury LA, Nelea M, et al. The double capsules in macro-textured breast implants. Biomaterials (2015) 67:65–72. doi: 10.1016/j.biomaterials.2015.06.010
12. Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Comp Study Plast Reconstructive Surgery (2011) 127(1):56–66. doi: 10.1097/PRS.0b013e3181fad34d
13. Akkad N, Kodgule R, Duncavage EJ, Mehta-Shah N, Spencer DH, Watkins M, et al. Evaluation of breast implant-associated anaplastic Large cell lymphoma with whole exome and genome sequencing. Aesthet Surg J (2022). doi: 10.1093/asj/sjac282
14. Walker JN, Hanson BM, Pinkner CL, Simar SR, Pinkner JS, Parikh R, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic Large cell lymphoma. Sci Rep (2019) 9(1):10393. doi: 10.1038/s41598-019-46535-8
15. Vanni G, Materazzo M, Pellicciaro M, Amir S, Tacconi F, Ambrogi V, et al. Breast textured implants determine early T-helper impairment: BIAL2.20 study. Anticancer Res (2021) 41(4):2123–32. doi: 10.21873/anticanres.14984
16. Santanelli di Pompeo F, Clemens MW, Atlan M, Botti G, Cordeiro PG, De Jong D, et al. 2022 Practice recommendation updates from the world consensus conference on BIA-ALCL. Aesthetic Surg J (2022) 42(11):1262–78. doi: 10.1093/asj/sjac133
17. Laurent C, Nicolae A, Laurent C, Le Bras F, Haioun C, Fataccioli V, et al. Gene alterations in epigenetic modifiers and JAK-STAT signaling are frequent in breast implant-associated ALCL. Blood (2020) 135(5):360–70. doi: 10.1182/blood.2019001904
18. Blombery P, Thompson ER, Prince HM. Molecular drivers of breast implant-associated anaplastic Large cell lymphoma. Plast Reconstr Surg (2019) 143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):59S–64S. doi: 10.1097/PRS.0000000000005570
19. Oishi N, Hundal T, Phillips JL, Dasari S, Hu G, Viswanatha DS, et al. Molecular profiling reveals a hypoxia signature in breast implant-associated anaplastic large cell lymphoma. Haematologica Jun 1 (2021) 106(6):1714–24. doi: 10.3324/haematol.2019.245860
20. Los-de Vries GT, de Boer M, van Dijk E, Stathi P, Hijmering NJ, Roemer MGM, et al. Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood (2020) 136(25):2927–32. doi: 10.1182/blood.2020005372
21. Di Napoli A, Greco D, Scafetta G, Ascenzi F, Gulino A, Aurisicchio L, et al. IL-10, IL-13, eotaxin and IL-10/IL-6 ratio distinguish breast implant-associated anaplastic large-cell lymphoma from all types of benign late seromas. Cancer Immunol Immunother CII (2021) 70(5):1379–92. doi: 10.1007/s00262-020-02778-3
22. Deva AK, Turner SD, Kadin ME, Magnusson MR, Prince HM, Miranda RN, et al. Etiology of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): current directions in research. Cancers (2020) 12(12):3861. doi: 10.3390/cancers12123861
23. Hanson SE, Hassid VJ, Branch-Brooks C, Liu J, Kadin ME, Miranda R, et al. Validation of a CD30 enzyme-linked immunosorbant assay for the rapid detection of breast implant-associated anaplastic Large cell lymphoma. Aesthet Surg J (2019). doi: 10.1093/asj/sjy327
24. Dimitrov G, Atanasova M, Popova Y, Vasileva K, Milusheva Y, Trtoianova P. Molecular and genetic subtyping of breast cancer: the era of precision oncology. World Cancer Res J (2022) 2022:1–8. doi: 10.32113/wcrj_20227_2367
25. Satpathy S, Jaehnig EJ, Krug K, Kim BJ, Saltzman AB, Chan DW, et al. Microscaled proteogenomic methods for precision oncology. Nat Commun (2020) 11(1):532. doi: 10.1038/s41467-020-14381-2
26. Loch-Wilkinson A, Beath KJ, Knight RJW, Wessels WLF, Magnusson M, Papadopoulos T, et al. Breast implant-associated anaplastic Large cell lymphoma in Australia and new Zealand: high-Surface-Area textured implants are associated with increased risk. Plast Reconstr Surg (2017) 140(4):645–54. doi: 10.1097/PRS.0000000000003654
27. Swanson E. The textured breast implant crisis: a call for action. Ann Plast Surg (2019) 82(6):593–4. doi: 10.1097/sap.0000000000001963
28. Mankowski P, Carr M, Cherukupalli A, Bovill E, Lennox P, Brown MH, et al. The macro-textured implant recall: BIA-ALCL risk aversion in cosmetic and reconstructive plastic surgery practices. Aesthet Surg J (2022). doi: 10.1093/asj/sjac158
29. Akhavan AA, Wirtz EC, Ollila DW, Bhatt N. An unusual case of BIA-ALCL associated with Prolonged/Complicated biocell-textured expander, followed by smooth round breast implant exposure, and concurrent use of adalimumab. Plast Reconstr Surg (2021) 148(2):299–303. doi: 10.1097/PRS.0000000000008155
30. Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. epidemiology of breast implant-associated anaplastic Large cell lymphoma. Plast Reconstr Surg (2017) 139(5):1042–50. doi: 10.1097/PRS.0000000000003282
31. Collett DJ, Rakhorst H, Lennox P, Magnusson M, Cooter R, Deva AK. Current risk estimate of breast implant-associated anaplastic Large cell lymphoma in textured breast implants. Plast Reconstr Surg (2019) 143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):30S–40S. doi: 10.1097/PRS.0000000000005567
32. Di Pompeo FS, Panagiotakos D, Firmani G, Sorotos M. BIA-ALCL epidemiological findings from a retrospective study of 248 cases extracted from relevant case reports and series: a systematic review. Aesthet Surg J (2022). doi: 10.1093/asj/sjac312
33. Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation (1995) 59(2):256–62. doi: 10.1097/00007890-199501000-00018
34. Asaad M, Morris N, Selber JC, Liu J, Clemens MW, Adelman DM, et al. No differences in surgical and patient-reported outcomes between alloderm, surgimend, and dermacell for prepectoral implant-based breast reconstruction. Plast Reconstr Surg (2022). doi: 10.1097/PRS.0000000000010070
35. Matros E, Shamsunder MG, Rubenstein RN, Polanco TO, McCarthy CM, Nelson JA, et al. Textured and smooth implant use reported in the tracking operations and outcomes for plastic surgeons database: epidemiologic implications for BIA-ALCL. Plast reconstructive Surg Global Open (2021) 9(3):e3499. doi: 10.1097/GOX.0000000000003499
36. Ferrufino-Schmidt MC, Medeiros LJ, Liu H, Clemens MW, Hunt KK, Laurent C, et al. Clinicopathologic features and prognostic impact of lymph node involvement in patients with breast implant-associated anaplastic Large cell lymphoma. Am J Surg Pathol (2018) 42(3):293–305. doi: 10.1097/pas.0000000000000985
37. Granados R, Lumbreras EM, Delgado M, Aramburu JA, Tardio JC. Cytological diagnosis of bilateral breast implant-associated lymphoma of the ALK-negative anaplastic Large-cell type. clinical implications of peri-implant breast seroma cytological reporting. Diagn Cytopathol Jul (2016) 44(7):623–7. doi: 10.1002/dc.23485
38. Collins MS, Miranda RN, Medeiros LJ, Silva de Meneses MP, Iyer SP, Butler CE, et al. Characteristics and treatment of advanced breast implant-associated anaplastic Large cell lymphoma. Plast Reconstr Surg (2019) 143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):41s–50s. doi: 10.1097/prs.0000000000005568
39. Stuver R, Lewis NE, Ewalt MD, Dogan A, Durham BH, Plitas G, et al. First report of bilateral breast-implant associated anaplastic large cell lymphoma caused by identical T-cell clone. Leukemia Lymphoma (2022) 63(11):2747–50. doi: 10.1080/10428194.2022.2092860
40. Verde F, Vigliar E, Romeo V, Campanino MR, Accurso A, Canta L, et al. Breast implant associated anaplastic large cell lymphoma (BIA-ALCL): a challenging cytological diagnosis with hybrid PET/MRI staging and follow-up. Breast Cancer (2021) 28(2):527–32. doi: 10.1007/s12282-020-01178-w
41. Pastorello RG, D’Almeida Costa F, Osório CABT, Makdissi FBA, Bezerra SM, de Brot M, et al. Breast implant-associated anaplastic large cell lymphoma in a Li-FRAUMENI patient: a case report. Diagn Pathology (2018) 13(1):10. doi: 10.1186/s13000-018-0688-x
42. Di Napoli A, Pepe G, Giarnieri E, Cippitelli C, Bonifacino A, Mattei M, et al. Cytological diagnostic features of late breast implant seromas: from reactive to anaplastic large cell lymphoma. PloS One (2017) 12(7):e0181097. doi: 10.1371/journal.pone.0181097
43. Di Napoli A. Achieving reliable diagnosis in late breast implant seromas: from reactive to anaplastic Large cell lymphoma. Plast Reconstr Surg (2019) 143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):15s–22s. doi: 10.1097/prs.0000000000005565
44. Laporta R, Longo B, Sorotos M, Pagnoni M, Santanelli di Pompeo F. Breast reconstruction with delayed fat-Graft-Augmented DIEP flap in patients with insufficient donor-site volume. Aesthetic Plast Surg (2015). doi: 10.1007/s00266-015-0475-y
45. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/jco.2013.54.8800
46. Clemens MW, Medeiros LJ, Butler CE, Hunt KK, Fanale MA, Horwitz S, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic Large-cell lymphoma. J Clin Oncol (2016) 34(2):160–8. doi: 10.1200/JCO.2015.63.3412
47. Mehta-Shah N, Clemens MW, Horwitz SM. How I treat breast implant-associated anaplastic large cell lymphoma. Blood (2018) 132(18):1889–98. doi: 10.1182/blood-2018-03-785972
48. Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med (2010) 363(19):1812–21. doi: 10.1056/NEJMoa1002965
49. Duvic M, Tetzlaff MT, Gangar P, Clos AL, Sui D, Talpur R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol (2015) 33(32):3759–65. doi: 10.1200/jco.2014.60.3787
50. Johnson L, O'Donoghue JM, McLean N, Turton P, Khan AA, Turner SD, et al. Breast implant associated anaplastic large cell lymphoma: the UK experience. recommendations on its management and implications for informed consent. Eur J Surg Oncol (2017) 43(8):1393–401. doi: 10.1016/j.ejso.2017.05.004
51. Fitzal F, Turner SD, Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol (2019) 9(4):190006. doi: 10.1098/rsob.190006
52. Evans MG, Medeiros LJ, Marques-Piubelli ML, Wang HY, Ortiz-Hidalgo C, Pina-Oviedo S, et al. Breast implant-associated anaplastic large cell lymphoma: clinical follow-up and analysis of sequential pathologic specimens of untreated patients shows persistent or progressive disease. Mod Pathol (2021) 34(12):2148–53. doi: 10.1038/s41379-021-00842-6
53. Paletta C, Paletta FX Jr., Paletta FX Sr. Squamous cell carcinoma following breast augmentation. Ann Plast Surg (1992) 29(5):425–9; discussion 429-32. doi: 10.1097/00000637-199211000-00009
54. Kitchen SB, Paletta CE, Shehadi SI, Bauer WC. Epithelialization of the lining of a breast implant capsule. possible origins of squamous cell carcinoma associated with a breast implant capsule. Cancer. (1994) 73(5):1449–52. doi: 10.1002/1097-0142(19940301)73:5<1449::aid-cncr2820730520>3.0.co;2-j
55. Buchanan PJ, Chopra VK, Walker KL, Rudolph R, Greco RJ. Primary squamous cell carcinoma arising from a breast implant capsule: a case report and review of the literature. Aesthet Surg J (2018) 38(7):e586. doi: 10.1093/asj/sjy092
56. Olsen DL, Keeney GL, Chen B, Visscher DW, Carter JM. Breast implant capsule-associated squamous cell carcinoma: a report of 2 cases. Hum Pathol Sep (2017) 67:94–100. doi: 10.1016/j.humpath.2017.07.011
57. Zomerlei TA, Samarghandi A, Terando AM. Primary squamous cell carcinoma arising from a breast implant capsule. Plast Reconstr Surg Glob Open (2015) 3(12):e586. doi: 10.1097/gox.0000000000000567
58. Whaley RD, Aldrees R, Dougherty RE, Prieto Granada C, Badve SS, Al Diffalha S. Breast implant capsule-associated squamous cell carcinoma: report of 2 patients. Int J Surg Pathol (2022) 30(8):900–7. doi: 10.1177/10668969221086940
59. Goldberg MT, Llaneras J, Willson TD, Boyd JB, Venegas RJ, Dauphine C, et al. Squamous cell carcinoma arising in breast implant capsules. Ann Plast Surg (2021) 86(3):268–72. doi: 10.1097/SAP.0000000000002524
60. Bhatavadekar NB. Squamous cell carcinoma in association with dental implants: an assessment of previously hypothesized carcinogenic mechanisms and a case report. J Oral Implantol (2012) 38(6):792–8. doi: 10.1563/aaid-joi-d-11-00045
61. Jeelani S, Rajkumar E, Mary GG, Khan PA, Gopal H, Roy S, et al. Squamous cell carcinoma and dental implants: a systematic review of case reports. J Pharm Bioallied Sci (2015) 7(Suppl 2):S378–80. doi: 10.4103/0975-7406.163457
62. Hess SR, Rudloff NA. Incisional squamous cell carcinoma after total knee arthroplasty. Arthroplast Today (2019) 5(3):292–5. doi: 10.1016/j.artd.2019.07.005
63. Philip J. Squamous cell carcinoma arising at the site of an underlying bullet. J R Coll Surg Edinb (1982) 27(6):365–6.
64. Alikhan MB, Nassar A, Mansoor I. Squamous metaplasia on the breast implant capsule. Int J Surg Pathol (2010) 18(6):570–4. doi: 10.1177/1066896908329587
65. Zhou YM, Chaudhry HE, Shah A, Andrews J. Breast squamous cell carcinoma following breast augmentation. Cureus. (2018) 10(10):e3405. doi: 10.7759/cureus.3405
66. Liu ZL, Liu C, Zhao C, Yu Q, Zhang G, Wang X, et al. Breast prosthetic implant-associated squamous cell carcinoma: a case report and literature review (2022). Available at: https://wwwresearchsquarecom/article/rs-141167/v1 (Accessed November 13, 2022).
67. Surgeons ASoP. ASPS statement on breast implant associated-squamous cell carcinoma (BIA-SCC). Available at: https://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/asps-statement-on-breast-implant-associated-squamous-cell-carcinoma?mkt_tok=MTAxLUJTTy05OTMAAAGGxnWptWGVxMTcetEcgxYMSD28ZqQIQkvpNDuTn5ziGMsd8445wg129M42aTsnwbyAF_ZRx3FsjG6s5xIs1lVa-h0Ja74Mw45EE6j7XL_EPvI (Accessed September 10, 2022).
68. Cook PD, Osborne BM, Connor RL, Strauss JF. Follicular lymphoma adjacent to foreign body granulomatous inflammation and fibrosis surrounding silicone breast prosthesis. Am J Surg Pathology (1995) 19(6):712–7. doi: 10.1097/00000478-199506000-00012
69. Kraemer DM, Tony H-P, Gattenlohner S, Muller J. Lymphoplasmacytic lymphoma in a patient with leaking silicone implant. Haematologica (2004) 89(4):ELT01–1.
70. Medeiros LJ, Marques-Piubelli ML, Sangiorgio VF, Ruiz-Cordero R, Vega F, Feldman AL, et al. Epstein–Barr-virus-positive large b-cell lymphoma associated with breast implants: an analysis of eight patients suggesting a possible pathogenetic relationship. Modern Pathology (2021) 34(12):2154–67. doi: 10.1038/s41379-021-00863-1
71. Nichter LS, Mueller MA, Burns RG, Stallman JM. First report of nodal marginal zone b-cell lymphoma associated with breast implants. Plast Reconstr Surg (2012) 129(3):576e–8e. doi: 10.1097/PRS.0b013e3182419caa
72. Smith BK, Gray SS. Large B-cell lymphoma occurring in a breast implant capsule. Plast Reconstr Surg (2014) 134(4):670e–1e. doi: 10.1097/PRS.0000000000000535
73. Moling O, Piccin A, Tauber M, Marinello P, Canova M, Casini M, et al. Intravascular large b-cell lymphoma associated with silicone breast implant, HLA-DRB1* 11: 01, and HLA-DQB1* 03: 01 manifesting as macrophage activation syndrome and with severe neurological symptoms: a case report. J Med Case Rep (2016) 10(1):1–13. doi: 10.1186/s13256-016-0993-5
74. Geethakumari PR, Markantonis J, Shah JL, Alsuwaidan A, Shahab I, Chen W, et al. Breast implant-associated plasmablastic lymphoma: a case report and discussion of the literature. Clin Lymphoma Myeloma Leukemia (2019) 19(10):e568–72. doi: 10.1016/j.clml.2019.05.011
75. Chen VW, Hoang D, Clancy S. Breast implant-associated bilateral b-cell lymphoma. Aesthetic Surg J (2020) 40(2):NP52–8. doi: 10.1093/asj/sjy093
76. Har-Shai L, Har-Shai Y, Metanes I, Bryzgalin L, Hassan S, Sabo E, et al. Persistent extranodal marginal zone lymphoma (MZL) in the capsule of a re-explanted silicone prosthesis following breast implant-associated anaplastic Large cell lymphoma (BIA-ALCL). Israel Med Assoc Journal: IMAJ (2020) 22(2):130–1. doi: 10.1093/asj/sjz304
77. Evans MG, Miranda RN, Young PA, Pai L, Wang H-Y, Konoplev SN, et al. B-cell lymphomas associated with breast implants: report of three cases and review of the literature. Ann Diagn Pathology (2020) 46:151512. doi: 10.1016/j.anndiagpath.2020.151512
78. Bletsis PP, Janssen LE, Visser O, Offerman SR, Tellier MA, Laterveer L, et al. Bilateral breast implant associated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): a case report. Int J Surg Case Rep (2020) 71:341–5. doi: 10.1016/j.ijscr.2020.05.039
79. Rodríguez-Pinilla SM, García FJS, Balagué O, Rodríguez-Justo M, Piris MÁChecktae. Breast implant-associated Epstein-Barr virus-positive large b-cell lymphomas: a report of three cases. Haematologica (2020) 105(8):e412. doi: 10.3324/haematol.2019.232355
80. Mescam L, Camus V, Schiano J-M, Adélaïde J, Picquenot J-M, Guille A, et al. EBV+ diffuse large b-cell lymphoma associated with chronic inflammation expands the spectrum of breast implant–related lymphomas. Blood (2020) 135(22):2004–9. doi: 10.1182/blood.2019003408
81. Khoo C, McTigue C, Hunter-Smith DJ, Walker P. EBV positive fibrin/chronic inflammation associated diffuse large b-cell lymphoma: an incidental finding associated with a breast implant. Pathology (2021) 53(5):673–5. doi: 10.1016/j.pathol.2020.09.022
82. Malata CM, Madada-Nyakauru RN, Follows G, Wright P. Epstein-Barr virus-associated diffuse large b-cell lymphoma identified in a breast implant capsule: a new breast implant-associated lymphoma? Ann Plast Surgery (2021) 86(4):383–6. doi: 10.1097/SAP.0000000000002537
83. Morgan S, Tremblay-LeMay R, Lipa JE, Sur M, Delabie J, Imrie K, et al. Breast implant-associated EBV-positive diffuse large b-cell lymphoma: two case reports and literature review. Pathol Res Pract (2021) 226:153589. doi: 10.1016/j.prp.2021.153589
84. Cheuk W, Chan AC, Chan JK, Lau GT, Chan VN, Yiu HH. Metallic implant-associated lymphoma: a distinct subgroup of large b-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathology (2005) 29(6):832–6. doi: 10.1097/01.pas.0000157747.10967.f4
85. Copie-Bergman C, Niedobitek G, Mangham DC, Selves J, Baloch K, Diss TC, et al. Epstein-Barr Virus in b-cell lymphomas associated with chronic suppurative inflamation. J Pathology: A J Pathological Soc Great Britain Ireland (1997) 183(3):287–92. doi: 10.1002/(SICI)1096-9896(199711)183:3<287::AID-PATH932>3.0.CO;2-Q
86. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues Vol. 2. Lyon: International agency for research on cancer (2008).
87. Boyer DF, McKelvie PA, de Leval L, Edlefsen KL, Ko YH, Aberman ZA, et al. Fibrin-associated EBV-positive Large b-cell lymphoma: an indolent neoplasm with features distinct from diffuse Large b-cell lymphoma associated with chronic inflammation. Am J Surg Pathol Mar (2017) 41(3):299–312. doi: 10.1097/pas.0000000000000775
88. McGuire P, Reisman NR, Murphy DK. Risk factor analysis for capsular contracture, malposition, and late seroma in subjects receiving natrelle 410 form-stable silicone breast implants. Plast Reconstr Surg (2017) 139(1):1–9. doi: 10.1097/PRS.0000000000002837
89. Maxwell GP, Van Natta BW, Bengtson BP, Murphy DK. Ten-year results from the natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J (2015) 35(2):145–55. doi: 10.1093/asj/sju084
90. Spear SL, Rottman SJ, Glicksman C, Brown M, Al-Attar A. Late seromas after breast implants: theory and practice. Plast Reconstr Surg (2012) 130(2):423–35. doi: 10.1097/PRS.0b013e3182589ea9
91. Mazzocchi M, Dessy LA, Corrias F, Scuderi N. A clinical study of late seroma in breast implantation surgery. Aesthetic Plast Surg (2012) 36(1):97–104. doi: 10.1007/s00266-011-9755-3
92. Clemens MW, Nava MB, Rocco N, Miranda RN. Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules. Gland Surg (2017) 6(2):169–84. doi: 10.21037/gs.2016.11.03
93. Glicksman CA, Danino MA, Efanov JI, El Khatib A, Nelea M. A step forward toward the understanding of the long-term pathogenesis of double capsule formation in macrotextured implants: a prospective histological analysis. Aesthet Surg J (2019) 39(11):1191–9. doi: 10.1093/asj/sjy293
94. Zogheib S, Hanna C, Daou B, Mjaess G, Nasr M. Breast implant-associated double capsules: what do we know so far? a systematic review of the literature. Aesthetic Plast Surgery (2022) 46(1):35–42. doi: 10.1007/s00266-021-02443-9
95. Efanov JI, Giot JP, Fernandez J, Danino MA. Breast-implant texturing associated with delamination of capsular layers: a histological analysis of the double capsule phenomenon. Ann Chir Plast Esthet (2017) 62(3):196–201. doi: 10.1016/j.anplas.2017.02.001
96. Danino MA, Nizard N, Paek LS, Govshievich A, Giot JP. Do bacteria and biofilm play a role in double-capsule formation around macrotextured implants? Plast Reconstr Surg (2017) 140(5):878–83. doi: 10.1097/prs.0000000000003767
97. Mesa F, Cataño S, Tuberquia O. Study of infections in breast augmentation surgery with implants in 9,691 patients over 5 years. Plast Reconstr Surg Glob Open (2021) 9(8):e3752. doi: 10.1097/gox.0000000000003752
98. Jimenez RB, Packowski K, Horick N, Rosado N, Chinta S, Koh DJ, et al. The timing of acute and late complications following mastectomy and implant-based reconstruction. Ann Surg (2022). doi: 10.1097/sla.0000000000005574
99. Pyfer B, Chatterjee A, Chen L, Nigriny J, Czerniecki B, Tchou J, et al. Early postoperative outcomes in breast conservation surgery versus simple mastectomy with implant reconstruction: a NSQIP analysis of 11,645 patients. Ann Surg Oncol (2016) 23:92–8. doi: 10.1245/s10434-015-4770-2
100. Cohen JB, Carroll C, Tenenbaum MM, Myckatyn TM. Breast implant-associated infections: the role of the national surgical quality improvement program and the local microbiome. Plast Reconstr Surg (2015) 136(5):921–9. doi: 10.1097/PRS.0000000000001682
101. Knackstedt R, Scomacao I, Djohan R. Utilization of irrigating negative pressure wound therapy for breast implant salvage: long-term results and success. J Plast Reconstr Aesthet Surg (2021) 74(2):407–47. doi: 10.1016/j.bjps.2020.08.058
102. Kaoutzanis C, Winocour J, Gupta V, Ganesh Kumar N, Sarosiek K, Wormer B, et al. Incidence and risk factors for major hematomas in aesthetic surgery: analysis of 129,007 patients. Aesthetic Surg J (2017) 37(10):1175–85. doi: 10.1093/asj/sjx062
103. Seth AK, Kim JY. Acute symptomatic hematoma with defined etiology seven years after breast reconstruction: a case report and literature review. Can J Plast Surg (2010) 18(2):e27–9. doi: 10.1177/229255031001800201
104. Grippaudo FR, Renzi L, Costantino B, Longo B, Santanelli F. Late unilateral hematoma after breast reconstruction with implants: case report and literature review. Aesthetic Surg J (2013) 33(6):830–4. doi: 10.1177/1090820X13496249
105. Peters W, Fornasier V. Late unilateral breast enlargement after insertion of silicone gel implants: a histopathological study. Can J Plast Surg = J canadien chirurgie plastique Spring (2007) 15(1):19–28. doi: 10.1177/229255030701500107
106. Iorwerth A, Cochrane R, Webster D. Chronic haematoma as a late complication of cosmetic breast augmentation. Breast (2000) 9(3):158–60. doi: 10.1054/brst.1999.0117
107. Daw JL, Lewis VL, Smith JW. Chronic expanding hematoma within a periprosthetic breast capsule. Plast Reconstr Surg (1996) 97(7):1469–72. doi: 10.1097/00006534-199606000-00026
108. Kasamaki S, Tsurumaru M, Kamano T, Kobayashi S, Hino M, Kuwatsuru R. A case of inflammatory breast cancer following augmentation mammoplasty with silicone gel implants. Breast Cancer (2000) 7(1):71–4. doi: 10.1007/BF02967191
109. DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top Magn Reson Imaging (2008) 19(3):143–50. doi: 10.1097/RMR.0b013e31818a40a500002142-200806000-00001
110. Di Benedetto G, Cecchini S, Grassetti L, Baldassarre S, Valeri G, Leva L, et al. Comparative study of breast implant rupture using mammography, sonography, and magnetic resonance imaging: correlation with surgical findings. Breast J (2008) 14(6):532–7. doi: 10.1111/j.1524-4741.2008.00643.x
111. Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging (2013) 4(2):163–75. doi: 10.1007/s13244-013-0219-y
112. Jung JH, Min PK, Moon YW, Shim WH. Congestive heart failure and a swollen breast. Lancet (2004) 363(9404):210. doi: 10.1016/s0140-6736(03)15327-5
113. Oraedu CO, Pinnapureddy P, Alrawi S, Acinapura AJ, Raju R. Congestive heart failure mimicking inflammatory breast carcinoma: a case report and review of the literature. Breast J (2001) 7(2):117–9. doi: 10.1046/j.1524-4741.2001.007002117.x
Keywords: breast implant, BIA-ALCL, BIA-SCC, BIA-DLBCL, breast cancer
Citation: Keane GC, Keane AM, Diederich R, Kennard K, Duncavage EJ and Myckatyn TM (2023) The evaluation of the delayed swollen breast in patients with a history of breast implants. Front. Oncol. 13:1174173. doi: 10.3389/fonc.2023.1174173
Received: 26 February 2023; Accepted: 20 June 2023;
Published: 05 July 2023.
Edited by:
Eduardo Fleury, Instituto Brasileiro de Controle do Câncer, BrazilReviewed by:
Marco Materazzo, Policlinico Tor Vergata, ItalyCopyright © 2023 Keane, Keane, Diederich, Kennard, Duncavage and Myckatyn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Terence M. Myckatyn, bXlja2F0eW5Ad3VzdGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.