- 1Department of Oncology, the Affiliated Aoyang Hospital of Jiangsu University, Zhangjiagang, China
- 2Department of Oncology, Changhai Hospital, Naval Medical University, Shanghai, China
- 3Department of Radiation Oncology, the Affiliated Aoyang Hospital of Jiangsu University, Zhangjiagang, China
- 4Department of Health Statistics, Naval Medical University, Shanghai, China
Objective: Existing evidence suggests that palliative care (PC) is highly underutilized in metastatic gynecologic cancer (mGCa). This study aims to explore temporal trends and predictors for inpatient PC referral in mGCa patients who received specific critical care therapies (CCT).
Methods: The National Inpatient Sample from 2003 to 2015 was used to identify mGCa patients receiving CCT. Basic characteristics were compared between patients with and without PC. Annual percentage change (APC) was estimated to reflect the temporal trend in the entire cohort and subgroups. Multivariable logistic regression was employed to explore potential predictors of inpatient PC referral.
Results: In total, 122,981 mGCa patients were identified, of whom 10,380 received CCT. Among these, 1,208 (11.64%) received inpatient PC. Overall, the rate of PC referral increased from 1.81% in 2003 to 26.30% in 2015 (APC: 29.08%). A higher increase in PC usage was found in white patients (APC: 30.81%), medium-sized hospitals (APC: 31.43%), the Midwest region (APC: 33.84%), and among patients with ovarian cancer (APC: 31.35%). Multivariable analysis suggested that medium bedsize, large bedsize, Midwest region, West region, uterine cancer and cervical cancer were related to increased PC use, while metastatic sites from lymph nodes and genital organs were related to lower PC referral.
Conclusion: Further studies are warranted to better illustrate the barriers for PC and finally improve the delivery of optimal end-of-life care for mGCa patients who receive inpatient CCT, especially for those diagnosed with ovarian cancer or admitted to small scale and Northeast hospitals.
1 Introduction
Gynecologic cancer is the most common malignancy in women, encompassing ovarian cancer, uterine cancer, and cervical cancer. According to the Cancer Statistics for 2022, it is estimated that there will be approximately 19,880 new cases of ovarian cancer, 65,950 new cases of uterine cancer, and 14,100 new cases of cervical cancer in the United States (US). Meanwhile, the estimated deaths for gynecologic cancer are also less than encouraging (1). Early diagnosis and treatment could improve cancer survival, while a significant number of cases progress rapidly and are diagnosed with metastasis (1). For those admitted to intensive care units, patients are frequently administrated with critical care therapies (CCT) to provide respiratory and nutritional support for life-saving measures (2–4). These patients are usually experience severe physical, psychological and social suffering (5, 6).
Palliative care (PC) is a structured system that provides care to patients with end-stage diseases. It has been reported to improve symptom management, alleviate psychological suffering, and reduce cancer-related mortality (7). The American Society of Clinical Oncology (ASCO) and the Society of Gynecologic Oncology (SGO) have formally endorsed early palliative care for gynecologic cancer patients (8–10). Multiple studies have demonstrated the beneficial role of early PC in addressing symptoms and managing psychological concerns in patients with gynecologic oncology (11, 12). However, studies have reported that PC is highly underutilized in metastatic gynecologic cancer (mGCa) patients, with utilization rates ranging from 5% to 24% (13–17). mGCa patients receiving CCT have increased cancer-related complications and long-term morbidity, and thus are strong indications for PC referral (18). Increasing awareness and accessibility of PC in this population is clinically significant. Although several publications have examined the utilization pattern of inpatient PC across different cancers in patients receiving life-sustaining treatments (19–21), there is a dearth of data focusing specifically on PC referral in mGCa patients receiving CCT while hospitalized.
The present study aims to investigate the temporal trends, predictors and barriers for inpatient PC referral in mGCa patients who specific CCT from a national perspective using the National Inpatient Sample (NIS) database.
2 Patients and methods
2.1 Data source
Data in the study is de-identified and thus exempt from approval by an institutional review board. The NIS database is the largest publically available all-payer healthcare database in the US (22), developed by the Agency for Healthcare Research and Quality (AHRQ), as part of the Healthcare Cost and Utilization project (HCUP), which collected a stratified sample from nearly 1000 hospitals. Each hospitalization contains up to 30 inpatient diagnoses and 15 procedures that could be identified through the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
2.2 Study design and patient selection
NIS database from 2003 to 2015 was used in this cross-sectional study. Gynecologic cancers were obtained by retrieving the following diagnostic codes: 1830, 1832, 1838, 1839 (ovarian cancer), 179, 1820, 1821, 1828 (uterine cancer), 1800, 1801, 1808, 1809 (cervical cancer) (16). Cases were considered metastatic gynecologic cancer (mGCa) with the presence of bone & bone marrow, brain & spinal cord, lymph nodes, liver, respiratory organs, urinary organs, adrenal glands, gastrointestinal organs, genital organs or other organs in the field of the secondary codes (Supplementary Table 1) (23). Among the selected mGCa cases, specific CCT including invasive mechanic ventilation (IMV), total parenteral nutrition (TPN), percutaneous endoscopic gastrostomy (PEG) tube, tracheostomy and dialysis for acute kidney failure (AKF) were considered (19–21). These procedures are aggressive and commonly used during the end-of-life period to provide necessary respiratory and nutritional support.
2.3 Patient and hospital characteristics
Patient-related, cancer-related and hospital-related characteristics were collected. Patient-related characteristics included age, year of admission, race, insurance type, income category, discharge destination, primary diagnosis and Elixhauser comorbidity score. The last consisted of 29 common comorbidities that could represent the disease burdens (excluded cancer in this study) (24). Cancer-related characteristics encompassed cancer type, metastatic sites, number of metastatic sites and chemotherapy. Lastly, hospital-related characteristics were hospital type, hospital bedsize and hospital region.
2.4 Definition of principal diagnosis and inpatient PC use
The principal diagnosis was categorized using the Clinical Classifications Software codes, which collapsed diagnoses and procedures into clinically meaningful categories (22).The primary outcome was temporal trend of inpatient PC referral in mGCa patients who received specific CCT. The secondary outcome included predictors of PC referral in the overall patients and in the subgroup undergoing IMV treatment. PC referral was defined using ICD-9-CM diagnostic code V66.7, which has been validated in metastatic disease with moderate sensitivity and high specificity (25, 26). Cases involving patients under 18 years old or admitted to hospitals that did not provide PC service during the study period were excluded from the analysis.
2.5 Statistical analysis and covariates
Continuous characteristics between patients with and without PC referral were expressed as mean and compared using t-test, while categorical variables were reported as proportions and compared using chi-square tests. We calculated annual percentage change (APC) in the entire cohort and subgroups by race, hospital region, hospital bedsize, teaching status, cancer type and discharge destination. Sampling stratas, clusters and weights were considered to derive estimates from the national perspective using complex survey methods. Additionally, we preformed multivariable logistic regression analysis to explore the predictors of PC referral in mGCa patients receiving CCT, taking into account patient-related, cancer-related and hospital-related characteristics. Confidence intervals for the ORs were calculated using the Taylor series method.
A P value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 and R version 3.6.2.
3 Results
3.1 Study population
In total, 122,981 hospitalizations diagnosed with mGCa were identified from 2003 to 2015, among which 10,737 have received inpatient CCT. We further excluded 357 patents who were under 18 years old or admitted to hospitals where PC was not available. Consequently, 10,380 (weighted 51,008) mGCa patients receiving CCT were identified in the further analysis. Among these patients, 7,254 (69.88%) were diagnosed with metastatic ovarian cancer (mOCa), 1,931 (18.60%) were diagnosed with metastatic uterine cancer (mUCa) and 1,195 (11.51%) were diagnosed with metastatic cervical cancer (mCCa). Regarding specific CCT, 3,641 (35.08%) patients received IMV, 1,207 (11.63%) received PEG, 5,918 (57.01%) received TPN, 265 (2.55%) received tracheostomy and 695 (6.70%) received dialysis for AKF. Characteristics between patients with and without PC in the IMV subgroup were summarized in Supplementary Table 2.
3.2 Trends of IPC use
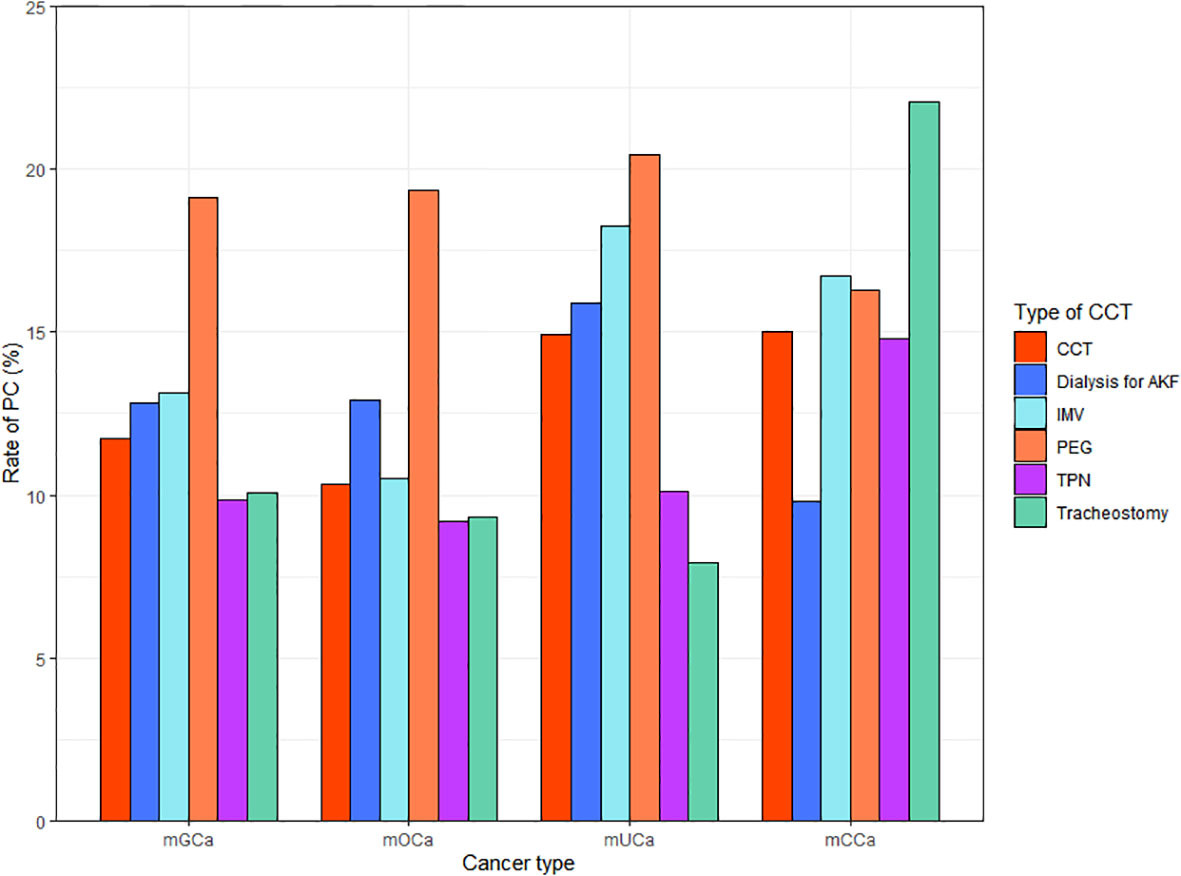
Among the included patients, 1208 (11.64%) received inpatient PC. There were 743(10.24%), 288 (14.91%) and 177(14.81%) patients who received PC in mOCa, mUCa and mCCa patients, respectively. As showed in Figure 1, the rates of PC referral varied across different types of CCT and cancer. Patients who received PC were younger (61.74 vs. 62.92), less likely to be diagnosed with mOCa (61.51% vs. 70.99%), more likely to be admitted for infections (14.32% vs. 8.18%) and admitted in Midwest (22.60% vs. 19.41%) or urban teaching hospitals (69.62% vs. 62.91%) (Table 1).
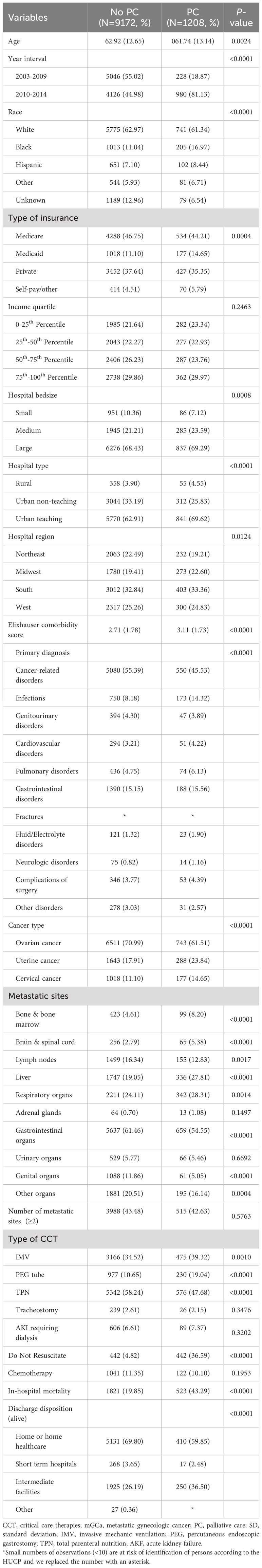
Table 1 Basic characteristics of mGCa patients receiving CCT stratified according to use of inpatient PC.
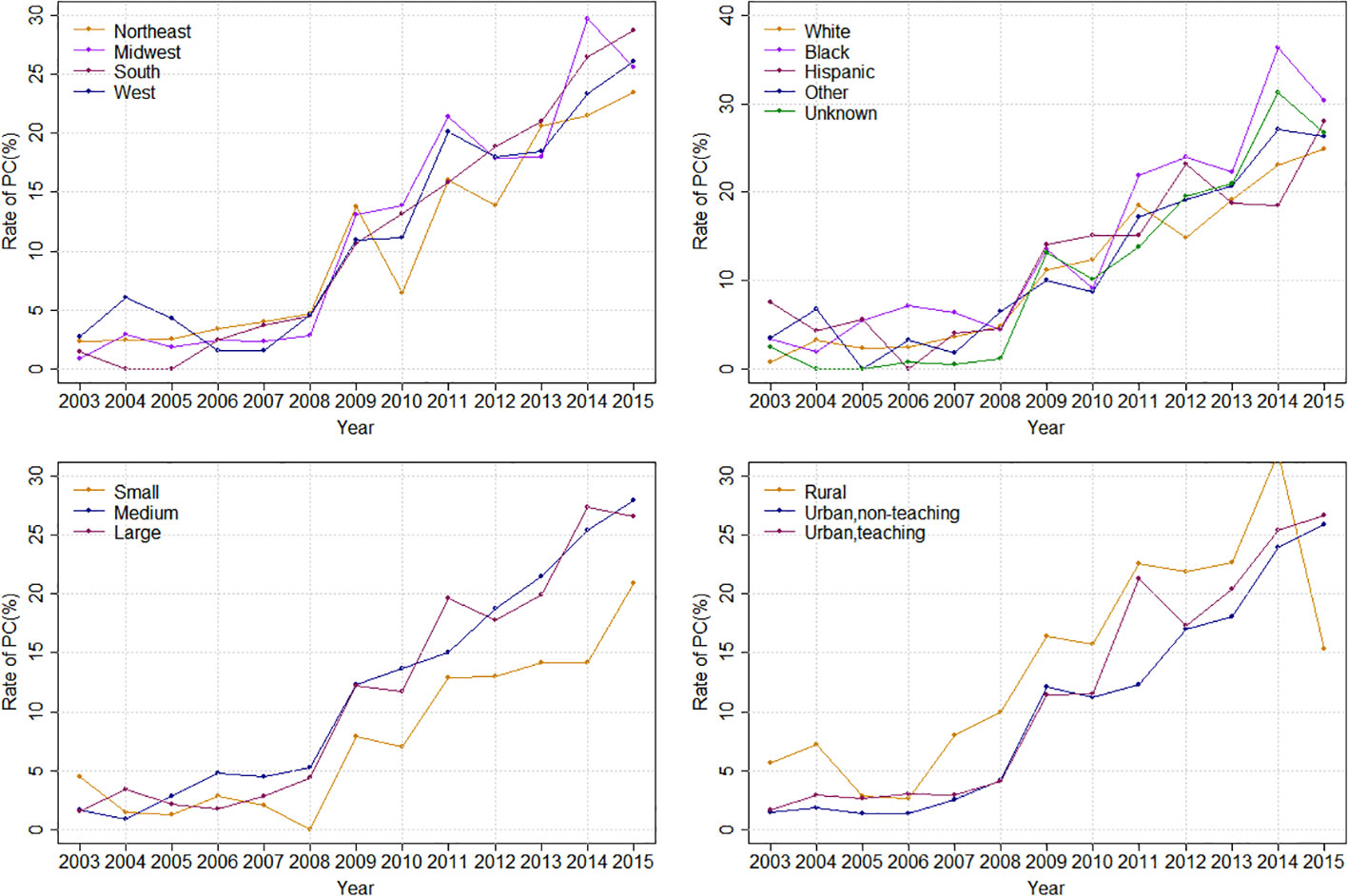
Overall, the rate of PC referral increased from 1.81% in 2003 to 26.30% in 2015 (APC: 29.08%%; p < 0.0001). Stratified by race, the PC rate increased from 0.78% to 24.93% in White (APC: 30.81%%; p < 0.0001), from 3.33% to 30.43% in Black (APC: 24.92%%; p < 0.0001) and from 7.47% to 28.07% (APC: 16.48%%; p=0.0005) in the Hispanic population (Figure 2). Stratified by bedsize, the PC rate increased from 4.46% to 20.90% in small bedsize hospitals (APC: 23.99%; p=0.0001), from 1.60% to 27.97% in medium bedsize hospitals (APC: 31.43%%; p < 0.0001) and from 1.53% to 26.63% in large bedsize hospitals (APC: 30.55%%; p < 0.0001). Stratified by hospital region, the PC rate increased from 2.29% to 23.48% in the Northeast (APC: 24.92%; p < 0.0001), from 0.87% to 25.62% in the Midwest (APC: 33.84%; p < 0.0001), from 1.40% to 28.72% in the South (APC: 31.88%; p < 0.0001) and from 2.71% to 26.15% in the West (APC: 24.35%; p=0.0004). In addition, stratified by cancer type, the rate of PC referral increased from 1.06% to 23.32% in mOCa (APC: 31.35%; p < 0.0001), from 3.06% to 33.58% in mUCa (APC: 27.68%; p < 0.0001), and from 4.81% to 28.17% in mCCa (APC: 25.80%; p < 0.0001; Figure 3). Stratified by discharge destination, the PC rate increased from 1.79% to 21.63% in patients who died during hospitalization (APC: 32.00%; p < 0.0001) and from 1.88% to 43.20% among the survivors (APC: 27.78%; p < 0.0001; Supplementary Figure 1).
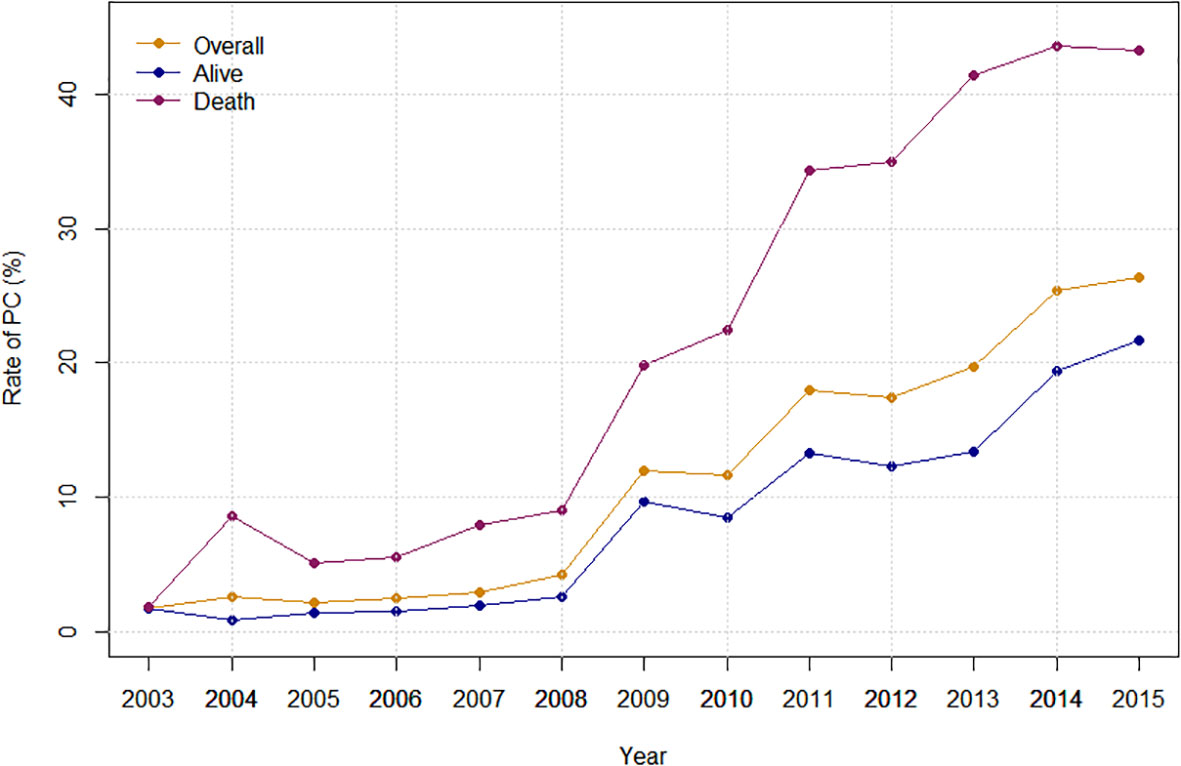
Figure 2 Inpatient palliative care referral over time, stratified by hospital region, race, hospital bedsize and hospital teaching status.
3.3 Predictors of PC use
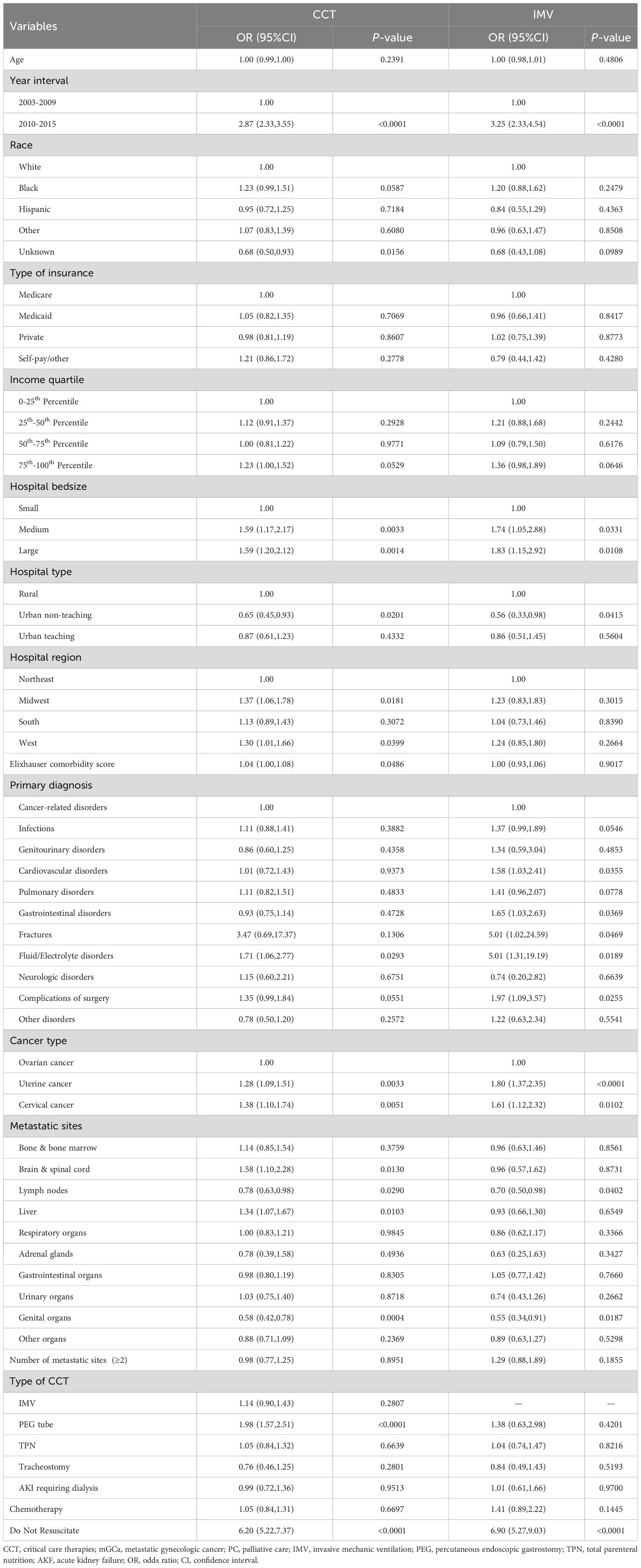
According to the multivariable analysis, year interval (odds ratio[OR]: 2.87, 95% confidence interval [CI]: 2.33-3.55), medium bedsize (OR: 1.59, 95% CI: 1.17-2.17), large bedsize (OR: 1.59, 95% CI: 1.20-2.12), Midwest region (OR: 1.37, 95% CI: 1.06-1.78), West region (OR: 1.30, 95% CI: 1.01-1.66), higher Elixhauser comorbidity score (OR: 1.04, 95% CI: 1.00-1.08), uterine cancer (OR: 1.28, 95% CI: 1.09-1.51), cervical cancer (OR: 1.38, 95% CI: 1.10-1.74), Do Not Resuscitate (OR: 6.20, 95% CI: 5.22-7.37), patients receiving PEG tube (OR: 1.98, 95% CI: 1.57-2.51), metastatic sites from brain & spinal cord (OR: 1.58, 95% CI: 1.10-2.28) and liver (OR: 1.34, 95% CI: 1.07-1.67) were associated with increased PC referral, while urban non-teaching hospitals, metastatic sites from lymph nodes and genital organs were related to lower PC referral. Additionally, predictors of PC referral in patients receiving IMV could be found in Table 2, which were similar to results in the main analysis.
4 Discussion
Although ASCO and SGO have long recommended early integration of PC to improve end-of-life care, practical evidence shows high underutilization of PC referral in mGCa patients (8, 10, 13, 16). Intensive care therapies are often provided to mGCa patients when severe treatment-related complications occurred or cancer progressed, highlighting the clinical importance and necessity of PC referral in this vulnerable population (2, 18). Our analysis suggested that approximately 11.64% of patients received inpatient PC, and the rate of PC referral increased from 1.81% in 2003 to 26.30% in 2015, with an average annual increase of 29.08%. Multivariable analysis suggested that medium bedsize, large bedsize, Midwest region, West region, higher Elixhauser comorbidity score, uterine cancer and cervical cancer were related to increased PC use, while urban non-teaching hospitals, metastatic sites from lymph nodes and genital organs were related to lower PC referral.
Overall, approximately 11.64% of mGCa patients with CCT received inpatient PC, which is more than two times higher than the reported PC rate of 5% in the entire population regardless of CCT, as reported by Rosenfeld et al. (13). However, this proportion is still far from satisfactory considering that all mGCa patients with CCT are candidates for PC referral. It is worth noting that PC referral consistently increased by 29.08% from 2003 to 2015. This phenomenon might reflect improved adherence of oncological guideline by both physicians and patients. Subgroup analysis indicated that increasing trend of PC referral was more pronounced in White and patients admitted to medium bedsize, urban non-teaching and Midwest hospitals, suggesting a wider acceptance of PC use in these patients. From the trend charts, it is evident that PC rate experienced a sharp increase since 2009, which aligns with the findings of previous publications (13, 16). As aggressive measures such as CCT can reduce quality of life in mGCa patients, this unexpected increase may be partly attributed to the landmark ENABLE II trial in 2009 that revealed the effectiveness of PC interventions in improving the quality of life for patients with advanced cancer (27).
When considering hospital region, patients hospitalized in Midwest hospitals had the highest PC rate (13.30%), followed by South (11.80%), West (11.46%) and Northeast (10.11%), accompanied by the highest APC (33.84%). Multivariable analysis accounting for potential confounders suggested that Midwest region (OR: 1.37) and West region (OR: 1.30) were associated with increased probability of PC referral compared to the Northeast region. This regional disparities in PC use has been previously reported. Milki et al. enrolled mGCa patients who subsequently died during hospitalization and found that patients in Midwest region (OR: 1.37) and West region (OR: 1.30) had increased PC use (16). Another study focusing on metastatic bladder cancer receiving CCT also described a higher PC rate in the West region (21). Further studies are warranted to understand the undelaying mechanisms for this geographic disparities and to relieve barriers for lower PC utilization in the Northeast region.
When considering hospital size, we observed that both medium bedsize (OR: 1.59) and large bedsize (OR: 1.59) were associated with increased PC use compared to small bedsize. One possible explanation for this finding might be that larger hospitals have more dedicated end-of-life specialists to provide PC services. However, research on this topic has produced conflicting results. For instance, Rosenfeld et al. conducted a study using data from the 2005 to 2011 NIS database, including all mGCa cases, and concluded that bedsize was not a predictor for PC referral (13). Another study by Milki et al. found that large bedsize was a positive predictor of PC referral (OR: 1.36) in mGCa cases who died in hospital (16). We hypothesized that the severity of dying status might result this disparity, as mGCa patients receiving CCT or died in hospital represented more severe conditions with significant symptom burden. Large bedsize hospitals are likely to form well-organized PC team and well-established relationship between physicians and mGCa patients with more severe conditions.
There has been controversy surrounding the emerging evidence on racial disparities in PC use among mGCa patients (2, 13–16). Understanding the racial and cultural differences among various racial groups can help personalize palliative care for mGCa patients receiving CCT and improve the delivery of comprehensive cancer care. Studies have reported Studies have reported that racial minority groups, such as Black or Hispanic gynecologic cancer patients, have expressed a desire for more intensive and invasive end-of-life care (2, 28), making them the potential candidates for PC delivery from the perspective of end-of-life decision-making. Consistent with previous publications (13, 29), our findings showed that Hispanic patients had the highest rate of PC use (16.83%), followed by Black patients (13.55%) and White patients (11.37%). However, this significant finding disappeared after adjustment for patient-related, cancer-related and hospital-related characteristics. Notably, Islam et al. analyzed data from the 2016 National Cancer Database and found that Hispanic and Black patients were less likely to utilize PC in metastatic ovarian cancer patients (14). In our subgroup analysis focusing exclusively on metastatic ovarian cancer patients, we did not observe such racial disparities. These discrepancies may be attributed to different population groups and data sources, especially considering that our study specifically involved patients receiving CCT during hospitalization. Therefore, further studies are needed to provide sufficient evidence to better understand the underlying racial differences and to improve equitable provision of PC among mGCa patients, irrespective of race.
For cancer types, uterine cancer ranked first in the rate of PC use (14.91%), followed by cervical cancer (14.81%) and ovarian cancer (10.24%). Although ovarian cancer patients has the lowest rate of PC use, the use of PC has dramatically increased over the study period, with the highest APC (31.35%). Previous studies have also reported lower PC use in ovarian cancer (13, 14). As we know, ovarian cancer has a higher degree of malignancy and worse survival compared to uterine cancer and cervical cancer (30). Therefore, future efforts are needed to improve and optimize PC referral in metastatic ovarian patients receiving CCT.
The present study utilized a national-level hospitalized database covering long time spans to investigate the temporal trends and predictors for inpatient PC referral in mGCa patients who frequently received CCT, including IMV, TPN, PEG tube, tracheostomy and dialysis for AKF. However, several limitations should also be considered for an accurate interpretation of our results. Firstly, PC use in the NIS database was defined based on the ICD-9-CM diagnostic code V66.7. Being an administrative database, the NIS may not capture all instances of PC discussions, and only those that are documented by physicians are recorded. Therefore, there may be a bias towards underestimating the actual number of PC use cases. However, the code was initially introduced in 1996 and has since been used in several publications, demonstrating moderate sensitivity (66.3% to 83%) and high specificity (95% to 99.1%) (25, 26). Secondly, this study focused only on specific CCTs that were frequently used in routine clinical practice. Any external extrapolation (eg, to all critically ill mGCa patients) should be interpreted with adequate caution. Thirdly, race information was unknown for nearly 12.22% of the included patients. Despite these limitations, the present study provides new evidence and insights into the understanding of PC referral in mGCa patients receiving CCT.
This analysis suggests that approximately 11.64% of patients received inpatient PC, which is still considerably below an ideal level. Further studies are necessary to elucidate the barriers to PC and ultimately enhance the provision of optimal end-of-life care for mGCa patients who receive inpatient CCT. This is particularly important for patients with ovarian cancer or those admitted to small-scale and northeast hospitals.
5 Conclusions
Despite the increase in PC referral over time, the absolute rate has remained low. The rates of PC referral in mGCa patients receiving CCT differ based on various sociodemographic and clinical factors. Thus, further studies are necessary to better understand the barriers to PC in mGCa patients undergoing inpatient CCT.
Data availability statement
The datasets presented in this article are not readily available because data are available in the NIS website: www.hcup-us.ahrq.go. Requests to access the datasets should be directed to www.hcup-us.ahrq.gov.
Ethics statement
The studies involving humans were approved by The NIS database is publically available and de-identified and thus is exempt from approval by an institutional review board in Aoyang Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The NIS database is publically available and de-identified and thus is exempt from approval by an institutional review board in Aoyang Hospital of Jiangsu University.
Author contributions
LS, LC, YZ, HH, ZL designed the study and drafted the manuscript. TC, QX edited the manuscript. In addition, each author has read and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1173438/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE and Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Taylor J, Rajan S, Zhang N, Meyer L, Ramondetta L, Bodurka D, et al. End-of-life racial and ethnic disparities among patients with ovarian cancer. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35:1829–35. doi: 10.1200/jco.2016.70.2894
3. Nevadunsky NS, Spoozak L, Gordon S, Rivera E, Harris K and Goldberg GL. End-of-life care of women with gynecologic Malignancies: a pilot study. Int J Gynecol Cancer (2013) 23:546–52. doi: 10.1097/IGC.0b013e3182842efa
4. Fauci J, Schneider K, Walters C, Boone J, Whitworth J, Killian E and Straughn JM Jr. The utilization of palliative care in gynecologic oncology patients near the end of life. Gynecol Oncol (2012) 127:175–9. doi: 10.1016/j.ygyno.2012.06.025
5. Krakauer EL, Kwete X, Kane K, Afshan G, Bazzett-Matabele L, Bien-Aime DDR, et al. Cervical cancer-associated suffering: estimating the palliative care needs of a highly vulnerable population. JCO Glob Oncol (2021) 7:862–72. doi: 10.1200/GO.21.00025
6. Taylor JS, Brown AJ, Prescott LS, Sun CC, Ramondetta LM, Bodurka DC. Dying well: How equal is end of life care among gynecologic oncology patients? Gynecol Oncol (2016) 140:295–300. doi: 10.1016/j.ygyno.2015.12.012
7. Swetz KM, Kamal AH. Palliative care. Ann Intern Med (2018) 168:ITC33–48. doi: 10.7326/AITC201803060
8. Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of clinical oncology clinical practice guideline update. J Clin Oncol (2017) 35:96–112. doi: 10.1200/JCO.2016.70.1474
9. Bauman JR, Temel JS. The integration of early palliative care with oncology care: the time has come for a new tradition. J Natl Compr Canc Netw (2014) 12:1763–71. doi: 10.6004/jnccn.2014.0177
10. Choosing wisely campaign: Society of Gynecologic Oncology (2013). Available at: http://www.choosingwisely.org/societies/society-of-gynecologic-oncology/.
11. Lefkowits C, Teuteberg W, Courtney-Brooks M, Sukumvanich P, Ruskin R and Kelley JL. Improvement in symptom burden within one day after palliative care consultation in a cohort of gynecologic oncology inpatients. Gynecol Oncol (2015) 136:424–8. doi: 10.1016/j.ygyno.2014.12.030
12. Lopez-Acevedo M, Lowery WJ, Lowery AW, Lee PS and Havrilesky LJ. Palliative and hospice care in gynecologic cancer: a review. Gynecol Oncol (2013) 131:215–21. doi: 10.1016/j.ygyno.2013.06.012
13. Rosenfeld EB, Chan JK, Gardner AB, Curry N, Delic L and Kapp DS. Disparities associated with inpatient palliative care utilization by patients with metastatic gynecologic cancers: A study of 3337 women. Am J Hosp Palliat Care (2018) 35:697–703. doi: 10.1177/1049909117736750
14. Islam JY, Saraiya V, Previs RA and Akinyemiju T. Health care access measures and palliative care use by race/ethnicity among metastatic gynecological cancer patients in the United States. Int J Environ Res Public Health (2021) 18:6040. doi: 10.3390/ijerph18116040
15. Islam JY, Deveaux A, Previs RA and Akinyemiju T. Racial disparities in palliative care utilization among metastatic gynecological cancer patients living at last follow-up: An analysis of the National Cancer Data Base. Data Brief (2021) 34:106705. doi: 10.1016/j.dib.2020.106705
16. Milki A, Mann AK, Gardner A, Kapp DS, English D and Chan JK. Trends in the utilization of palliative care in patients with gynecologic cancer who subsequently died during hospitalization. Am J Hosp Palliat Care (2021) 38:138–46. doi: 10.1177/1049909120935038
17. Uppal S, Rice LW, Beniwal A and Spencer RJ. Trends in hospice discharge, documented inpatient palliative care services and inpatient mortality in ovarian carcinoma. Gynecol Oncol (2016) 143:371–8. doi: 10.1016/j.ygyno.2016.08.238
18. Azoulay E, Schellongowski P, Darmon M, Bauer PR, Benoit D, Depuydt P, et al. The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med (2017) 43:1366–82. doi: 10.1007/s00134-017-4884-z
19. Loh KP, Abdallah M, Shieh MS, Stefan MS, Pekow PS, Lindenauer PK, et al. Use of inpatient palliative care services in patients with advanced cancer receiving critical care therapies. J Natl Compr Canc Netw (2018) 16:1055–64. doi: 10.6004/jnccn.2018.7039
20. Chen Y, Lin S, Zhu Y, Xu R, Lan X, Xiang F, et al. Prevalence, trend and disparities of palliative care utilization among hospitalized metastatic breast cancer patients who received critical care therapies. Breast (2020) 54:264–71. doi: 10.1016/j.breast.2020.11.001
21. Mazzone E, Knipper S, Mistretta FA, Palumbo C, Tian Z, Gallina A, et al. Trends and social barriers for inpatient palliative care in patients with metastatic bladder cancer receiving critical care therapies. J Natl Compr Canc Netw (2019) 17:1344–52. doi: 10.6004/jnccn.2019.7319
22. NIS database documentation. Available at: www.hcup-us.ahrq.gov.
23. Nordstrom BL, Whyte JL, Stolar M, Mercaldi C and Kallich JD. Identification of metastatic cancer in claims data. Pharmacoepidemiol Drug Saf (2012) 21 Suppl 2:21–8. doi: 10.1002/pds.3247
24. Southern DA, Quan H and Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care (2004) 42:355–60. doi: 10.1097/01.mlr.0000118861.56848.ee
25. Feder SL, Redeker NS, Jeon S, Schulman-Green D, Womack JA, Tate JP, et al. Validation of the ICD-9 diagnostic code for palliative care in patients hospitalized with heart failure within the veterans health administration. Am J Hosp Palliat Care (2018) 35:959–65. doi: 10.1177/1049909117747519
26. Hua M, Li G, Clancy C, Morrison RS and Wunsch H. Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med (2017) 20:372–7. doi: 10.1089/jpm.2016.0363
27. Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA (2009) 302:741–9. doi: 10.1001/jama.2009.1198
28. Barnato AE, Anthony DL, Skinner J, Gallagher PM and Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med (2009) 24:695–701. doi: 10.1007/s11606-009-0952-6
29. Lefkowits C, Binstock AB, Courtney-Brooks M, Teuteberg WG, Leahy J, Sukumvanich P and Kelley JL. Predictors of palliative care consultation on an inpatient gynecologic oncology service: are we following ASCO recommendations? Gynecol Oncol (2014) 133:319–25. doi: 10.1016/j.ygyno.2014.02.031
Keywords: trends, barriers, palliative care, metastatic gynecologic cancer, critical care therapies
Citation: Shen L, Chen L, Zhou Y, Chen T, Han H, Xia Q and Liu Z (2023) Temporal trends and barriers for inpatient palliative care referral in metastatic gynecologic cancer patients receiving specific critical care therapies. Front. Oncol. 13:1173438. doi: 10.3389/fonc.2023.1173438
Received: 24 February 2023; Accepted: 29 September 2023;
Published: 19 October 2023.
Edited by:
Mevhibe Hocaoglu, King’s College London, United KingdomReviewed by:
Akram Parandeh, Baqiyatallah University of Medical Sciences, IranSilvio Cavuto, IRCCS Local Health Authority of Reggio Emilia, Italy
Copyright © 2023 Shen, Chen, Zhou, Chen, Han, Xia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanguo Liu, MTM3MzA0MjQwQHFxLmNvbQ==; Qiuyan Xia, MzAxMjAwOEAxNjMuY29t
†These authors have contributed equally to this work
 Li Shen1†
Li Shen1† Hedong Han
Hedong Han Zhanguo Liu
Zhanguo Liu