Corrigendum: Interpreting flow cytometry with caution: a case report of follicular lymphoma in leukemic phase misdiagnosed as chronic lymphocytic leukemia
- 1Department of Hematology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 24+4 Medical Doctor Program, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Chronic lymphocytic leukemia (CLL) is a subtype of mature B-cell proliferative neoplasms characterized by abnormally increasing lymphocytes in circulation. The diagnosis of CLL is usually established on peripheral blood analysis and typical flow cytometric immunophenotype rather than biopsy. In particular, the high RMH (Royal Marsden Hospital Scoring System for CLL) score of immunophenotype has a highly sensitive weight for diagnostic value. However, immunophenotyping by flow cytometry may also be misleading in specific clinical situations. Here, we report a case on admission with lymphadenopathy and lymphocytosis, misdiagnosed as chronic lymphocytic leukemia by flow cytometry initially but finally confirmed as follicular lymphoma (FL) in the leukemic phase via lymph node biopsy. Since FL in the leukemic phase is uncommon at the time of diagnosis and indicates a poorer prognosis of FL, such misdiagnosis is worthy of attention. It is also thought-provoking that there had been conflicts between immunophenotype of bone marrow and immunohistochemistry of lymph node. Our case report aims to remind clinicians’ awareness that the immunophenotyping by flow cytometric analysis needs to be interpreted with caution especially when the results cannot account for all of clinical features, and it is significant to make the right decision about when to conduct further examination including lymph node biopsy for avoiding misdiagnosis.
Introduction
Chronic lymphocytic leukemia (CLL) is a common type of indolent B-cell lymphoma that occurs mainly in middle-aged and elderly people, especially in Western countries. According to recent guidelines (1–3), peripheral blood routine, smear, and flow cytometric analysis are adequate for diagnosing CLL, and it is not generally required to conduct lymph node biopsy. Despite a high RMH (Royal Marsden Hospital Scoring System for CLL) score (≥4) of immunophenotyping representing high sensitivity in distinguishing CLL from other B-cell proliferative disorders (4), the results of immunophenotype by flow cytometry need to be interpreted with caution, particularly in need of combination with clinical features. Herein, we report a case of a young male patient misdiagnosed with CLL by flow cytometry initially but finally confirmed as follicular lymphoma via lymph node biopsy, in hopes of reminding clinicians’ awareness.
Case presentation
A 42-year-old man without any past medical history was admitted in our hospital with 1 year of weight loss and night sweats. Physical examination revealed lymphadenopathy and massively enlarged spleen (reached the level of umbilicus). Peripheral blood analysis found a lymphocyte count of 16.80 × 109/l, and abnormal lymphocytes accounted for 59% of white blood cells, whereas the hemoglobin concentration (144 g/l) and platelet counts (131 × 109/l) were within the normal range. Serum biochemical examination showed an elevated lactate dehydrogenase (LDH) level of 267 U/l (upper limit of normal, 250 U/l) and a β2-microglobulin (β2-MG) level of 6.6 mg/l (reference range, 0.7–1.8 mg/l). Bone marrow smear showed an increase in mature heteromorphic lymphocytes (Figure 1), suggesting a chronic lymphoproliferative disease. The immunophenotype of bone marrow by flow cytometric analysis (Figure 2A) was an abnormal B-cell phenotype: CD19+CD20+CD5+CD23+CD200+CD22+CD10-FMC7-sIg-. Given that the RMH score calculated is 4, chronic lymphocytic leukemia was firstly diagnosed.
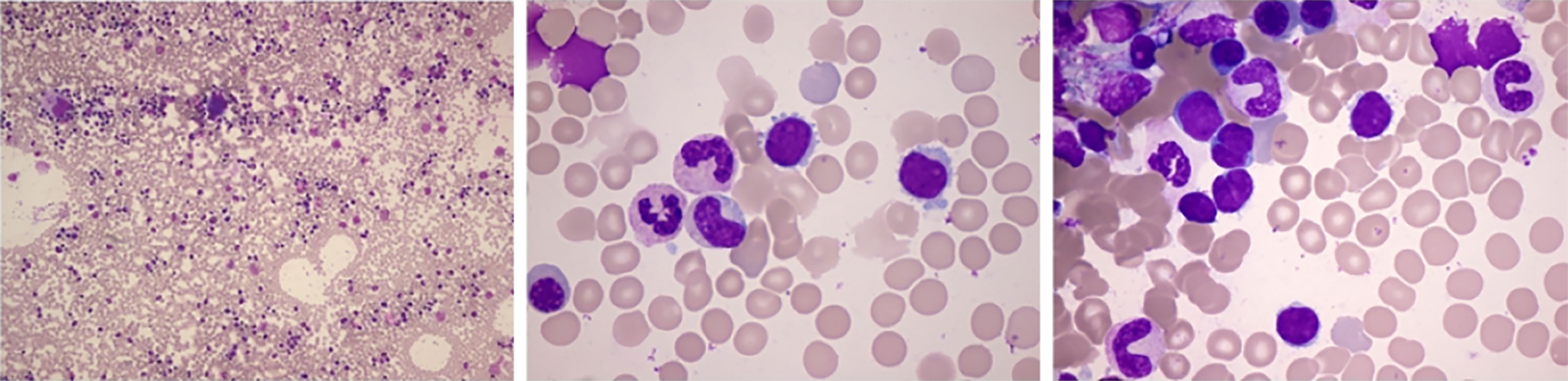
Figure 1 Bone marrow smear: active proliferation. The positive rate of periodic acid–Schiff staining was 75%. Some heteromorphic lymphocytes were small to moderate in size with mature aggregated chromatin, scant cytoplasm, and irregular nuclear contours. The smear suggested lymphoproliferative diseases.
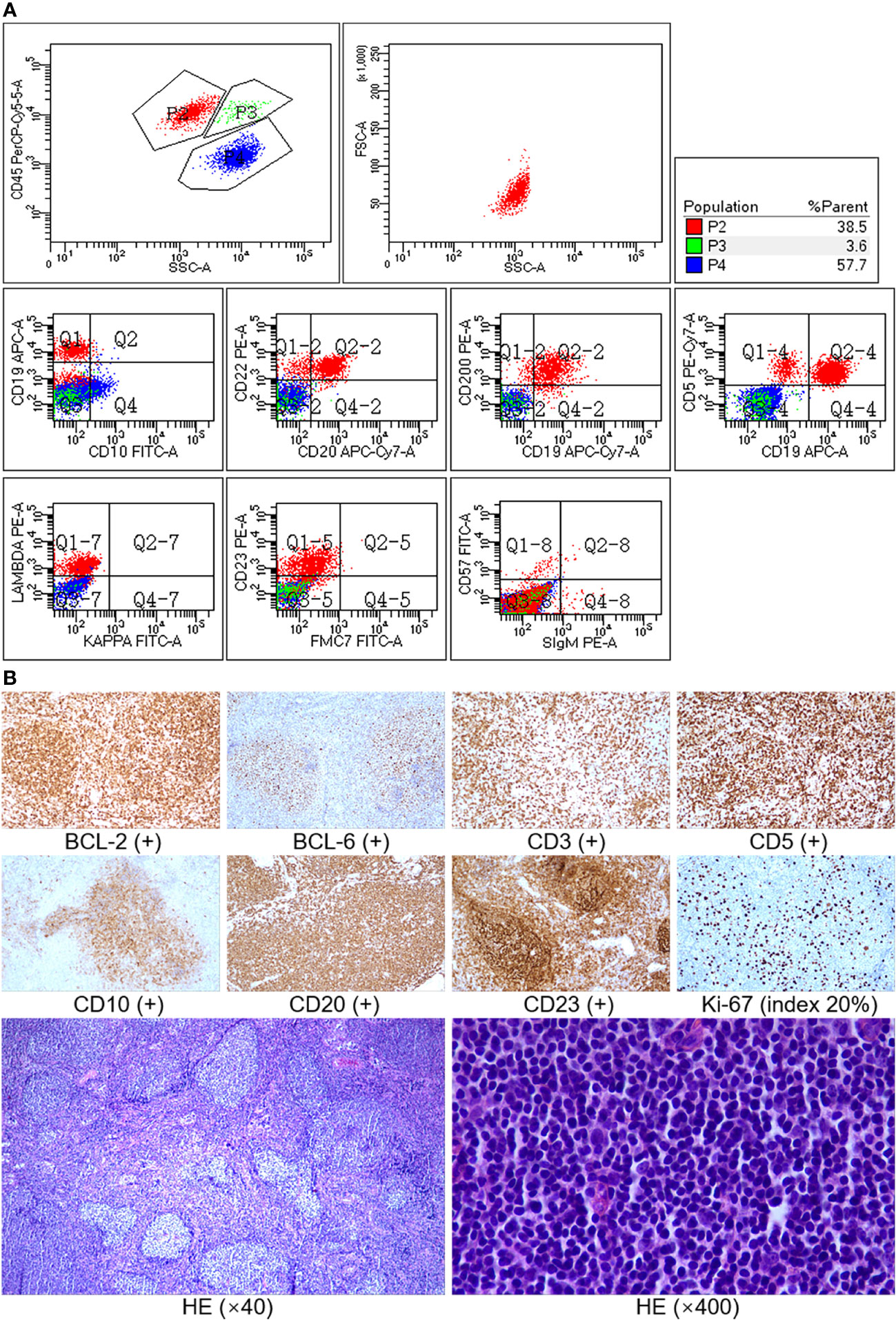
Figure 2 (A) Immunophenotype of bone marrow by flow cytometry. Method: sample staining (lyse and wash) by fluorescent-labeled monoclonal antibody was performed, a BD FACSCanto II flow cytometer was used, and the data were analyzed using BD FACSDiva software. Result: P2 (red) was lymphocytes, accounting for 38.5% of non-erythrocytes in the sample. B cells accounted for 85% of lymphocytes and mainly expressed CD19, CD20, CD22, CD5, CD23, and CD200 but did not express CD10, FMC7, and sIg (surface immunoglobulin), with lambda light-chain restriction. The immunophenotype of B cells was abnormal phenotype. (B) Immunohistochemistry and histopathology of lymph node biopsy. The neoplastic follicles were closely packed, showing an almost back-to-back pattern, and lacked mantle zones (low power, ×40). Most of the cells in the field were centrocytes (high power, ×400). Immunostaining showed that the tumor cells were positive for CD20, CD10, BCL-6, and BCL-2 and negative for CD3, CD5, and CD23, and the Ki-67 index was about 20%. CD3 and CD5 were positive for background T cells, and CD23 was positive for follicular dendritic cells, while follicular lymphoma cells did not express CD5 and CD23.
However, considering the patient’s young age and significantly enlarged spleen, we planned a further evaluation to confirm the diagnosis. PET-CT scan revealed multiple (cervical, axillary, inguinal, retroperitoneal, etc.) lymphadenopathy (maximum cross section: 10.9 cm × 10.2 cm) and obvious hepatosplenomegaly, with a maximum SUV of 5.0 (Figure 3). Biopsy of the left inguinal lymph node was scheduled (see pathological details in Figure 2B), and the patient was finally diagnosed with follicular lymphoma (FL) in the leukemic phase. Meanwhile, DNA sequencing detected CREBBP mutation, which also supports common gene features of FL (5). As per extranodal organs such as liver and bone marrow involved, the final Ann Arbor stage was IVB. FLIPI (Follicular Lymphoma International Prognostic Index)-1 and FLIPI-2 both scored 3 (high risk).
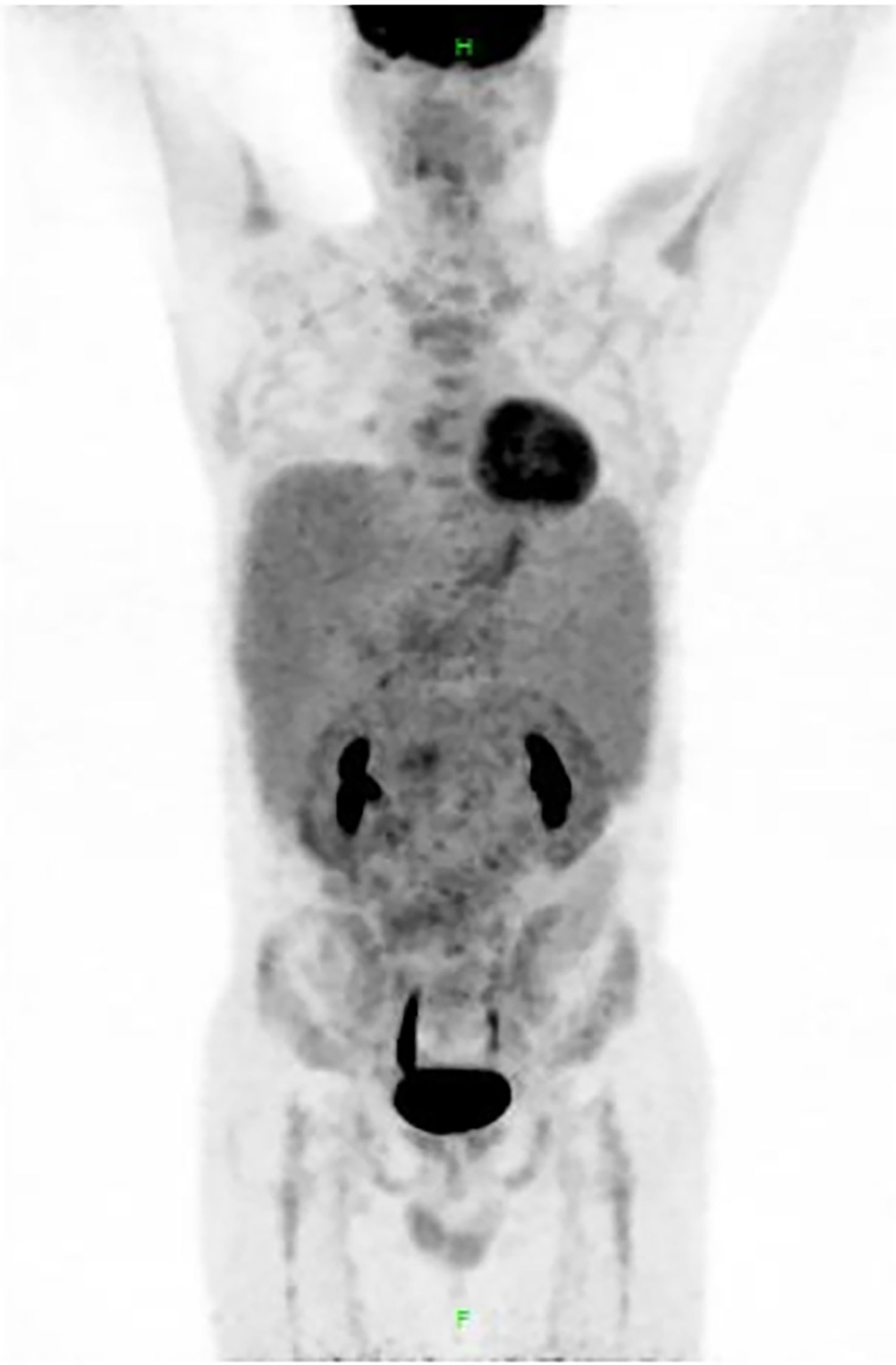
Figure 3 Results of positron emission tomography (PET)-CT. PET-CT scan revealed multiple lymphadenopathy (SUVmax 5.0) in the bilateral cervical, bilateral axillary, mediastinal, retroperitoneal, supra-mesenteric, iliac vessel, and bilateral inguinal region, and the maximum cross section of the lymph node mass was 10.9 cm × 10.2 cm. Liver (SUVmax 3.2) and spleen (SUVmax 3.0) were significantly enlarged. Metabolism of central and peripheral bone marrow was diffusely increased, with SUVmax of 3.6.
The treatment was initiated for ≥1 GELF criteria (any site >7 cm; B symptoms; splenomegaly; leukemic phase) that were met (6). Six cycles of the BR regimen (rituximab and bendamustine) were given, and the patient achieved complete remission (CR).
Discussion
Reviewing this case, the young male patient mainly presented with B symptoms, lymphadenopathy, and palpable splenomegaly, and peripheral blood examination indicated a prominent lymphocytosis accompanied by several biochemical abnormalities. Next, when interpreting the results of immunophenotype, although the RMH score of 4 is highly sensitive for diagnosing CLL, we still cannot draw a conclusion directly based on flow cytometry since there existed some clinical features of the case mismatched with CLL. Here, the reasons that drive the clinician’s critical decision to lymph node biopsy are as follows: First, the age of the patient is relatively young compared to the median age of CLL (72-year-old) at diagnosis (1). Second, the patient had marked splenomegaly with elevated serum LDH and β2-MG levels, while his lymphocyte count was only 16.80 × 109/l. Third, on PET-CT scanning CLL overall has a lower fluorine-18 fluoro-deoxyglucose avidity, namely, lower SUV value (mean: 2.5), compared with other indolent lymphomas such as FL (mean SUV: 7.7) (7). Due to the above reasons, further examination especially lymph node biopsy was essential.
In our report, of concern is that the initial immunophenotype (CD5+CD23+CD200+CD10-FMC7-sIg-) of bone marrow by flow cytometry did not coincide with immunohistochemistry (CD5-CD23-CD10+BCL-2+BCL-6+) of lymph node pathology. By reviewing a previous study of FL in the leukemic phase, we found a similar discordance. As Maeshima et al. mentioned (8), the incidence of CD10 positivity was lower in the bone marrow compared to the primary site (lymph node); even the histological grades of FL were discordant between the bone marrow and other sites. Dogan et al. illustrated (9) that because of a lack of follicle germinal center activation markers and a low proliferation fraction, diffusely infiltrating lymphoma cells in the bone marrow may be negative for CD10 expression. Regarding why the bone marrow immunophenotyping showed CLL-like characteristics in our case, we assume that the cell surface immunophenotype had been transformed when follicular lymphoma cells involved bone marrow and peripheral blood. It probably related to the unique hematopoietic microenvironment of bone marrow, resulting in changes of cell growth patterns. Such unusual presentation of the leukemic FL is not completely observed or understood. There remain several unanswered questions about the mechanism behind this phenomenon to be explored.
Additionally in previous retrospective cohort studies, the leukemic phase of FL patients is rare and associated with shorter PFS independently of the FLIPI score (10–12). Therefore, it is fairly significant that follicular lymphoma in the leukemic phase is timely and correctly diagnosed.
Conclusion
For patients with a CLL-like immunophenotype (RMH score ≥4) while clinical features are not completely consistent with CLL, the conclusion drawn from flow cytometry should be interpreted cautiously. As reported in our case, manifestations of FL in the leukemic phase in some ways are mimicking those of CLL, thus requiring careful differential diagnosis. Even if small lymphoma cells in the bone marrow resemble the CLL immunophenotype and are negative for CD10, FL cannot be excluded. In summary, the final diagnosis must not be made only on the basis of the immunophenotypic data but must be a synthesis that the clinician makes considering all the clinical and laboratory aspects. To avoid misdiagnosis, lymph node biopsy should be performed positively in the presence of atypical clinical findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YR wrote the first draft of the manuscript, CJ, MC and WZ provided the case data, and WW revised the final draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National High Level Hospital Clinical Research Funding of China (No. 2022-PUMCH-A-194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2021) 32(1):23–33. doi: 10.1016/j.annonc.2020.09.019
2. Wierda WG, Byrd JC, Abramson JS, Bilgrami SF, Bociek G, Brander D, et al. Chronic lymphocytic Leukemia/Small lymphocytic lymphoma, version 4.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18(2):185–217. doi: 10.6004/jnccn.2020.0006
3. Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet (2018) 391(10129):1524–37. doi: 10.1016/S0140-6736(18)30422-7
4. Moreau EJ, Matutes E, A'Hern RP, Morilla AM, Morilla RM, Owusu-Ankomah KA, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol (1997) 108(4):378–82. doi: 10.1093/ajcp/108.4.378
5. Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol (2020) 95(3):316–27. doi: 10.1002/ajh.25696
6. Brice P, Bastion Y, Lepage E, Brousse N, Haioun C, Moreau P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the groupe d'Etude des lymphomes folliculaires. groupe d'Etude des lymphomes de l'Adulte. J Clin Oncol (1997) 15(3):1110–7. doi: 10.1200/JCO.1997.15.3.1110
7. Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer (2006) 107(1):175–83. doi: 10.1002/cncr.21967
8. Maeshima AM, Taniguchi H, Tanioka K, Kitahara H, Miyamoto K, Fukuhara S, et al. Clinicopathological characteristics of follicular lymphoma with peripheral blood involvement. Leuk Lymphoma (2015) 56(7):2000–4. doi: 10.3109/10428194.2014.963578
9. Dogan A, Du MQ, Aiello A, Diss TC, Ye HT, Pan LX, et al. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic b-cell population in the interfollicular zone. Blood (1998) 91(12):4708–14. doi: 10.1182/blood.V91.12.4708
10. Sarkozy C, Baseggio L, Feugier P, Callet-Bauchu E, Karlin L, Seymour JF, et al. Peripheral blood involvement in patients with follicular lymphoma: a rare disease manifestation associated with poor prognosis. Br J Haematol (2014) 164(5):659–67. doi: 10.1111/bjh.12675
11. Sarkozy CM, Baseggio L, Callet-Bauchu E, Karlin L, Lebras LL, Michallet A-S, et al. Detection of leukemic phase in patients with follicular lymphoma At diagnosis: A rare event associated with poor prognosis. Blood (2012) 120(21):1594–. doi: 10.1182/blood.V120.21.1594.1594
Keywords: chronic lymphocytic leukemia, misdiagnosis, immunophenotyping, follicular lymphoma, case report
Citation: Ren Y, Jia C, Chen M, Wang W and Zhang W (2023) Interpreting flow cytometry with caution: A case report of follicular lymphoma in leukemic phase misdiagnosed as chronic lymphocytic leukemia. Front. Oncol. 13:1170598. doi: 10.3389/fonc.2023.1170598
Received: 21 February 2023; Accepted: 15 March 2023;
Published: 27 March 2023.
Edited by:
Donatella Raspadori, Siena University Hospital, ItalyReviewed by:
Emanuele Cencini, Siena University Hospital, ItalyAnna Sicuranza, University of Siena, Italy
Copyright © 2023 Ren, Jia, Chen, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2FuZ3dlaXB1bWNAMTYzLmNvbQ==
 Yuan Ren
Yuan Ren Congwei Jia3
Congwei Jia3 Miao Chen
Miao Chen Wei Wang
Wei Wang Wei Zhang
Wei Zhang