
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 13 July 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1170220
Introduction: The prognostic role of soluble programmed death ligand 1 (sPD-L1) in digestive system cancers (DSCs) remains inconclusive. This study aimed to explore the predictive value of sPD-L1 expression in DSCs.
Methods: Comprehensive searches were run on the electronic databases (PubMed, Web of Science, EMBASE, and the Cochrane Library) to identify studies that assessed the prognostic role of sPD-L1 in DSCs. Review Manager software (version 5.3) was used for all analyses. Pooled data for survival outcomes were measured as hazard ratios (HRs), 95% confidence intervals (CIs), and odds ratios and their 95% CIs.
Results: The search identified 18 studies involving 2,070 patients with DSCs. The meta-outcome revealed that a high level of sPD-L1 was related to poorer overall survival (HR, 3.06; 95% CI: 2.22–4.22, p<0.001) and disease-free survival (HR, 2.53; 95% CI: 1.67–3.83, p<0.001) in DSCs. Individually, the prognostic significance of high level of sPD-L1 expression was the highest in hepatic cell carcinoma (HR, 4.76; p<0.001) followed by gastric cancer (HR=3.55, p<0.001).
Conclusion: sPD-L1 may be a prognostic factor in DSCs for overall survival and disease-free survival. Inflammatory cytokines, treatment approaches, and other factors may affect the expression of sPD-L1. Therefore, the prognostic value of sPD-L1 for recurrence and metastasis should be further investigated. sPD-L1 may also predict response to treatment. Well-designed prospective studies with standard assessment methods should be conducted to determine the prognostic value of sPD-L1 in DSCs.
Digestive system cancer (DSC) is a common malignant neoplasm (1). DSCs include cancers of the intestines, pancreas, esophagus, stomach, and liver (2). Colorectal cancer (CRC) is the third most common cancer, and 1.9 million new CRC cases and 935,000 deaths (3) were reported in 2020 (4). The treatment of CRC includes surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy (5). Approximately half of CRC patients with a resectable primary tumor will subsequently develop metastatic disease (6). Patients of stage IV CRC is with only 11% survival rate (7). Pancreatic cancer is with poor survival (4). Moreover, pancreatic cancer is projected to be the second leading cause of cancer-related deaths by 2030 (8). Operative way is the standard approach for resectable pancreatic cancer; however, only 20% pancreatic cancer patients are eligible for radical surgery (9). The 5-year survival rate of pancreatic cancer patients after surgery is 12%–27% (10); in advanced pancreatic cancer, the 5-year survival rate is <7% (11). Esophageal cancer ranks seventh and sixth in terms of incidence and overall mortality, respectively (4, 12, 13). The treatment approach includes surgery, chemotherapy, and radiotherapy as the mainstay of treatment for advanced esophageal cancer (14). Nevertheless, the prognosis of esophageal cancer is poor, and overall survival (OS) at 5 years is <20% (15). Survival of stomach cancer patients also remain poor (4). The incidence rate is highest in Eastern Asia. Complete resection (R0) is selected for resectable gastric cancer. However, the survival rate of stomach cancer is lower, and the 5-year survival rate is approximately 30%–35% (16). The median survival time is approximately 1 year in advanced gastric cancer patients (17). Primary liver cancer is the sixth most common cancer and the third most lethal tumor (4). The survival of hepatocellular carcinoma (HCC) at 5 years is only 18% (18). The dismay survival is due to the fact that 70%–80% of patients are diagnosed at an advanced stage (19).
Despite recent advances, the prognosis of DSCs remains unsatisfactory (20). Generally, the pathological tumor nodal metastasis (TNM) stage reflects the prognosis in different cancer (21). However, patients with the same stage may have different prognoses. Other markers such as circulating tumor DNA number of mutations have also been used to predict DSC prognosis (22). Hence, identification of valuable markers to guide clinical treatment is urgently needed.
In the tumor microenvironment, cancer immunity plays a vital role in promoting cancer cell proliferation, survival, and angiogenesis (23). In the last decade, immunotherapy has become an important treatment for cancer, and programmed cell death protein 1 (PD-1)/programmed death ligand-1 (PD-L1) are vital pathways (24). The level of PD-L1 in tumor tissues is the most effective biomarker for evaluating patients receiving immunotherapy (25). However, there are limitations that cannot be monitored during treatment, such as dynamic changes of PD-L1, which changes dynamically. PD-L1 is also called CD274 and B7-H1 (26). The soluble forms of PD-1 and PD-L1 were called soluble PD-1 (sPD-1) and soluble PD-L1 (sPD-L1), respectively (25, 27). sPD-L1 is expressed in both tumors and dendritic cells (27). sPD-L1 may be formed via the proteolytic cleavage of the extracellular portion of the membrane that binds to PD-L1 (28). sPD-L1 retains the ability to inhibit T-cell activation and proliferation (29). Moreover, activation of the PD-1/PD-L1 pathway is associated with tumor evasion, cancer development, and progression (25, 30). Normal human serum can secret sPD-L1, and the levels of sPD-L1 in human serum increases with age (27). Membrane-bound PD-L1 is a prognostic factor in several types of cancer (31). Moreover, some studies have reported that sPD-L1 can be detected in the blood of patients with cancer and is regarded as a prognostic marker (32–35). It was reported that in patients with pancreatic cancer receiving chemotherapy who achieved an objective response, sPD-L1 levels were significantly higher with disease progression. In addition, dynamic changes in sPD-L1 levels during treatment are associated with disease progression (36). Nonetheless, the prognostic value of sPD-L1 expression in cancer remains controversial (25). Several meta-analyses have been carried out to investigated the predictive role of sPD-L1 in non-small cell lung cancer (35) and solid tumors (24). There was no previous meta-analysis focusing on this topic in DSCs. Therefore, a meta-analysis was carried out to determine the prognostic value of sPD-L1 in DSCs.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in this study (37). PubMed, Web of Science, EMBASE, and the Cochrane Library electronic databases were searched. The search used the following MeSH terms and keywords: cancer, carcinoma, tumor, or neoplasm; soluble programmed cell death-ligand 1 (sPD-L1) or programmed cell death-1 or (PD-1) or PD-l1; and survival, predictive, prognosis, or prognostic. The deadline for the search was 1 February 2021. Additional searches were conducted to screen the references of the included studies for potentially missing studies that met the inclusion criteria. Two independent researchers conducted this study.
The inclusion criteria were as follows: a) patients diagnosed with malignant DSCs (such as pancreatic cancer, colorectal cancer, liver cancer, gastric cancer, esophageal cancer, and biliary tract cancer) confirmed by pathological analysis; b) the studies were conducted in English; c) human survival ([OS] or disease-free survival [DFS]) with regard to sPD-L1 levels is provided by hazard ratios (HRs) or survival curves or can be calculated from the text; and d) each study had a sample size of more than 20 cases. The exclusion criteria were as follows: a) letters to the editor, comments, reviews, and animal studies; b) the sample sizes were <20 for each cancer type; and c) survival data were not provided.
Data were extracted by two independent reviewers. Information from the included studies was reviewed and extracted. This included the following:
1) Authors, publication years, countries, histological types (differentiation), gender, tumor stage, metastases stage, initial treatment methods (surgery, chemotherapy, or radiotherapy), study types (retrospective or prospective), sample sizes, ages, the methods for sPD-L1 detection, the cutoff value of sPD-L1, and follow-up time.
2) OS and DFS and the predictive value of sPD-L1 for treatment response and metastasis.
The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to evaluate study quality, as previously described (38). The scores ranged from 0 to 9 according to the quality of the studies. A score equal to or higher than 6 was regarded as high quality. The quality assessment was performed by two independent reviewers. Any disagreements regarding the study selection, data extraction, and quality assessment were resolved by a third reviewer.
Review Manage (5.3 version) software (Nordic Cochrane Centre) and STATA software (version 12.0) were used for data evaluation (39). The correlation between sPD-L1 expression and survival outcomes were recorded using HRs and 95% confidence intervals (CI) (39). We used the χ2 and I2 tests to quantify the heterogeneity (39). Heterogeneity was evaluated using I2, and the values of 25%, 50%, and 75% were considered low, moderate, and high, respectively (40). If I2 <2.5%, data analysis was performed using a fixed-effects model. Otherwise, a random effects model was used. Statistical significance was set at p <0.05. Subgroup and sensitivity analyses were performed. Sensitivity analysis is an important method to evaluate the robustness and reliability of combined results in meta-analysis. Publication bias was evaluated using the Begg’s test (41).
The selection flowchart is shown in Figure 1. A total of 223, 464, 608, and 11 studies were identified from PubMed, Web of Science, EMBASE, and the Cochrane Library, respectively. Duplicate references (n=508) were removed using Note-express software. After screening the titles and abstracts, 22 papers were required for full-text screening. One study was excluded due to inclusion of fewer than 20 patients (42). Other studies were excluded owing to a lack of relevant survival outcome data (43–45). In all, 18 studies involving 2,070 patients met the inclusion criteria, with six studies focusing on gastric cancer (46–51), six on HCC (52–57), three on pancreatic cancer (36, 58, 59), one on biliary tract cancer (60), one on rectal cancer (61), and one on esophageal carcinoma (62). Sample sizes ranged from 25 to 313. The years of publication ranged from 2016 to 2021. The basic information of including studies and NOS scale are listed in Table 1. The sPD-L1 was detected using enzyme-linked immunosorbent assay (ELISA) in all the studies. OS was described in 17 studies, and DFS was mentioned in 10 studies. The median OS in the high sPD-L1 group and the prognostic role of sPD-L1 in terms of OS, treatment response, and metastases are summarized in Table 2.
As shown in Figure 2, an HR of 3.06 (95% CI, 2.22–4.22, p<0.001) indicated that a higher sPD-L1 level predicted worse OS in the pooled data of 18 studies. A random-effects model was applied owing to the high heterogeneity among the studies (I2 = 71, p<0.001). Furthermore, a high sPD-L1 level was correlated with unfavorable DFS by pooling the data from 10 studies using a random-effects model (HR, 2.53; 95% CI: 1.67–3.83); p-value was <0.01 with significant heterogeneity (I2 = 79, p<0.001) (Figure 3).
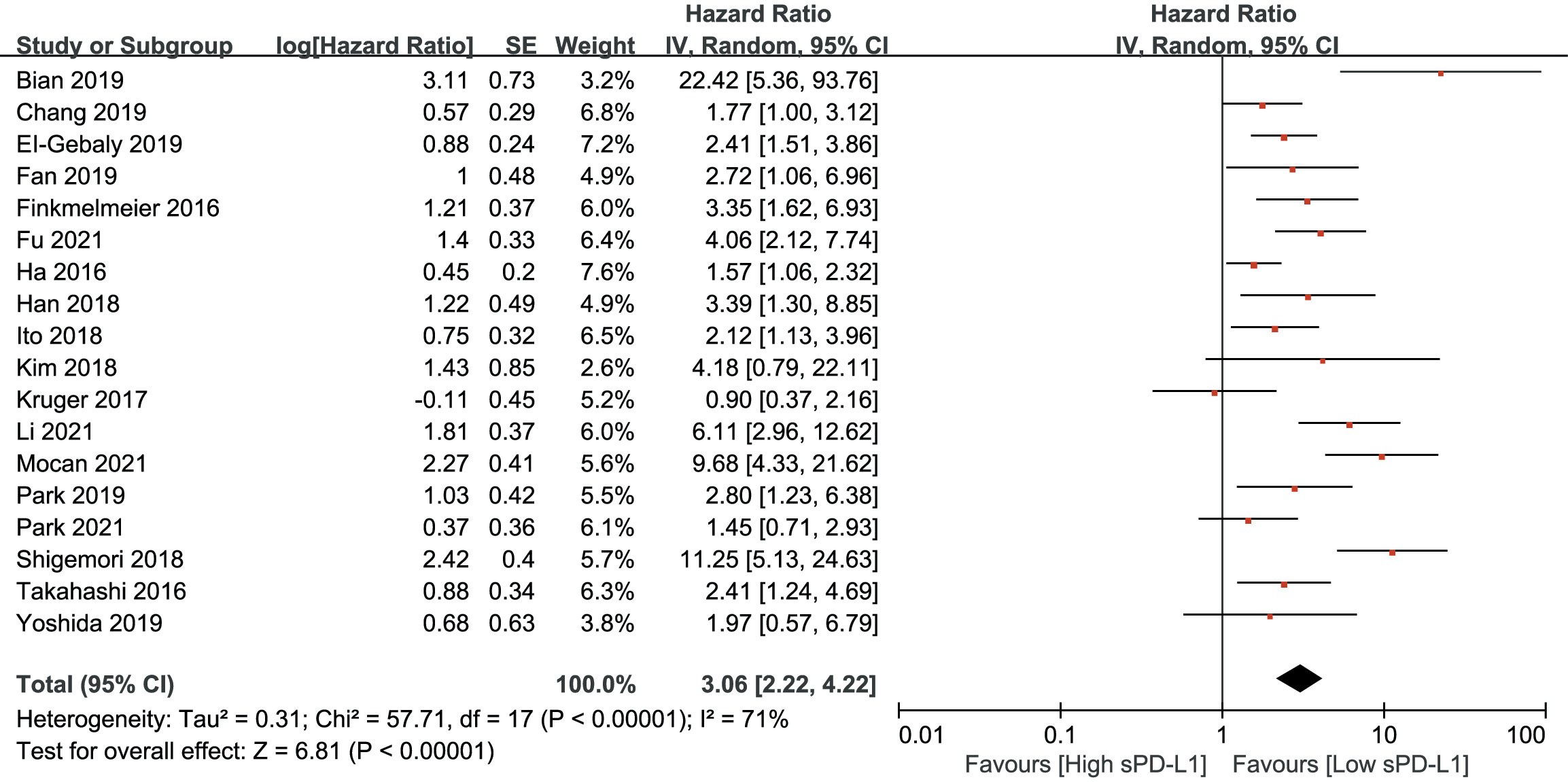
Figure 2 Forest plot of hazard ratio (HR) for the relationship between sPD-L1 level and overall survival (OS).
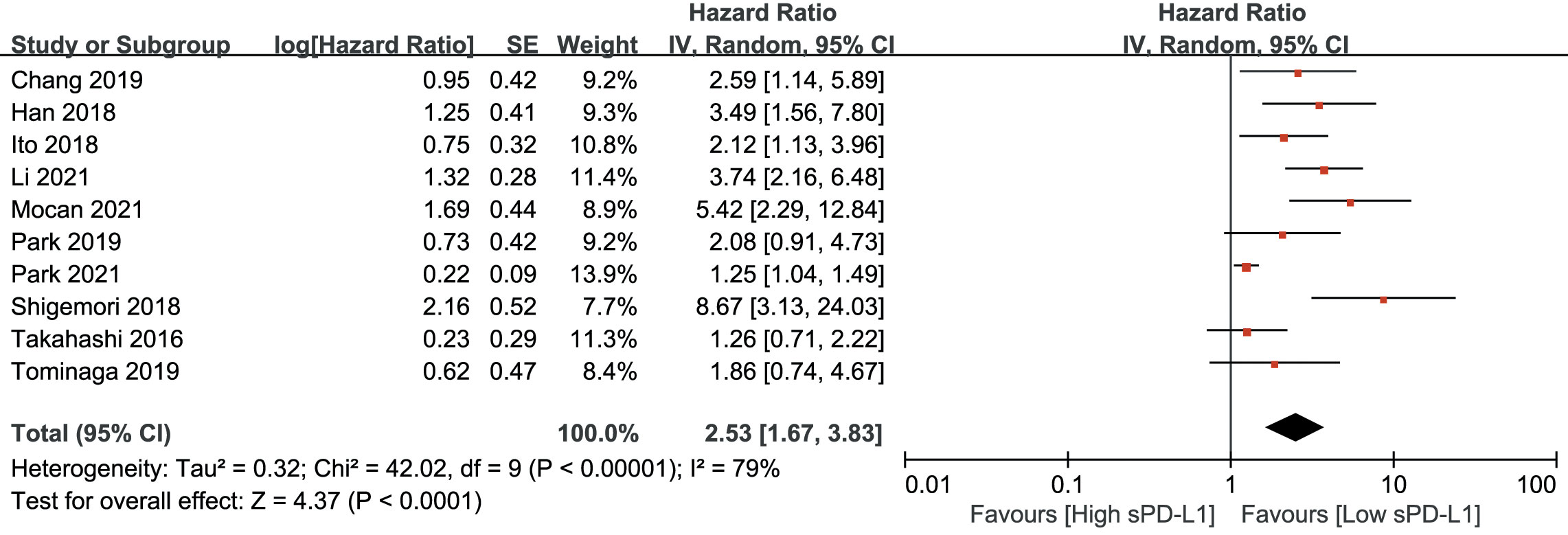
Figure 3 Forest plot of hazard ratio (HR) for the relationship between sPD-L1 level and disease-free survival (DFS).
Six studies focused on sPD-L1 levels and survival outcomes in HCC (52–57). A study by Mocan et al. (57), which included 121 patients with HCC, identified that the best cutoff value of sPD-L1 for both DFS and OS was 96 pg/ml. Patients with high sPD-L1 levels had a shorter DFS (HR, 5.42; p<0.001) and OS (HR, 9.67; p<0.001). The study of Kim et al. (56), which included 53 HCC patients, showed that high sPD-L1 level was associated with poor OS and early lung metastasis but failed to predict local failure-free or progression-free survival (PFS). A study by Han et al. (55), comprising 81 patients with hepatitis B virus-related HCC, suggested that higher sPD-L1 levels were associated with poorer OS (HR, 3.399; p=0.012) and DFS (HR, 3.503; p=0.002). Chang et al. (52) found that sPD-L1 expression was a negative predictive factor for DFS (HR, 2.58; p=0.023) and OS (HR, 1.77; p=0.048) in 120 patients with HCC. El-Gebaly et al. (53) reported that sPD-L1 was an independent prognostic factor for OS in HCC (HR, 2.397; p<0.001) on multivariable analysis. Finkelmeier et al. (54) designed a study to assess the sPD-L1 level and OS in 215 HCC patients. They found that sPD-L1 levels correlated with the Barcelona Clinic Liver Cancer staging system. They also found that high sPD-L1 levels were associated with mortality risk (HR, 3.340; p<0.001). The pooled data of these six studies indicated that a higher level of sPD-L1 was correlated with a poorer OS (HR, 3.28; 95% CI, 2.01–5.35, p<0.001).
Six studies reported sPD-L1 levels and survival outcomes in gastric cancer patients (46–51). A prospective study (49) from Korea, which included 68 patients with gastric cancer, demonstrated that a high level of sPD-L1 level at diagnosis was correlated with a poorer OS (OS, 9.5 vs. 18.3 months, p=0.057) and PFS (8.9 vs. 6.0 months, p=0.040). Li et al. (48) designed a study to assess the prognostic value of sPD-L1 in 313 patients with gastric cancer. They indicated that postoperative sPD-L1 changes correlated with poor OS (HR, 1.029; p=0.018) and recurrence-free survival (RFS) (HR, 1.029; p=0.011). Ito et al. (47) reported that in 152 patients with gastric cancer, a median sPD-L1 level of 50 pg/ml was the cutoff value and showed that a high sPD-L1 level was associated with poor OS (HR, 2.12; p=0.02). Shigemori et al. (50) designed a study that evaluated the prognostic value of sPD-L1 and tissue PD-L1 in 180 patients with gastric cancer who underwent radical surgery. They found that both tissue PD-L1 and sPD-L1 levels were associated with poorer OS (tissue PD-L1: HR, 4.28; p=0.0094; sPD-L1: HR, 11.2; p=0.0001) and poor DFS (tissue PD-L1: HR, 6.96; p=0.0002; sPD-L1: HR, 8.7; p<0.001). Takahashi et al. (51) included 75 patients with metastatic gastric cancer and found that sPD-L1 level was an independent prognostic factor for gastric cancer (optimal cutoff value: HR, 3.307; p=0.0046; median cut-off value: HR, 2.218; p=0.019). Pooled data of the six studies indicated that high level of sPD-L1 was associated with worse survival (HR, 3.55; 95% CI: 2.01–6.28, p<0.01).
A study (58) from France included 32 patients with pancreatic adenocarcinoma and showed that a high level of sPD-L1 (>0.36 ng/ml) was related with worse OS (median OS, 9.41 months in high level of sPD-L1 vs. 19.87 months in low level of sPD-L1). Kruger et al. (59) showed that sPD-L1 levels are not associated with OS in either univariate or multivariate analyses. Park et al. (36) prospectively included 60 patients with pancreatic cancer and indicated that, by multivariate analysis, patients with high levels of sPD-L1 had worse OS compared to those patients with low levels of sPD-L1 (HR, 3.249; p=0.012; median OS, 8.4 vs. 10.2 months).
A study from China (62) including 190 patients with esophageal carcinoma indicated that sPD-L1 was highly expressed in female patients with esophageal carcinoma. High sPD-L1 concentrations (≥0.63 ng/ml) were related with a shorter OS (HR, 3.71; p<0.001).
Ha et al. (60) reported on 158 patients with biliary tract cancer and measured their sPD-L1 levels. The median value of sPD-L1 was 1.20 ng/ml and patients with high concentrations of sPD-L1 (≥0.94 ng/ml) were correlated with a poorer OS than patients with low sPD-L1 (HR, 1.89; 95% CI: 1.35–2.65, p<0.01).
sPD-L1 was measured using ELISA before and after neoadjuvant chemoradiotherapy in 117 patients with rectal cancer in a study from the UK (61), which indicated that after neoadjuvant chemoradiotherapy, sPD-L1 levels significantly increased and high sPD-L1 levels before neoadjuvant chemoradiotherapy were related to younger age. High sPD-L1 levels after neoadjuvant chemoradiotherapy were associated with lymphovascular invasion and poor DFS.
To confirm the stability of the findings, a sensitivity analysis was performed by omitting any single study on OS. The results are reliable, as shown in Figure 4. To determine the reliability of the results, subgroup analyses were conducted based on country location, ages, sex, study types, initial treatment, metastases stage, year of publication, sample size, cancer type, and NOS score.
The results are summarized in Table 3. High sPD-L1 levels were associated with worse OS in all subgroup analyses except in gender data not provided, indicating reliability of the results.
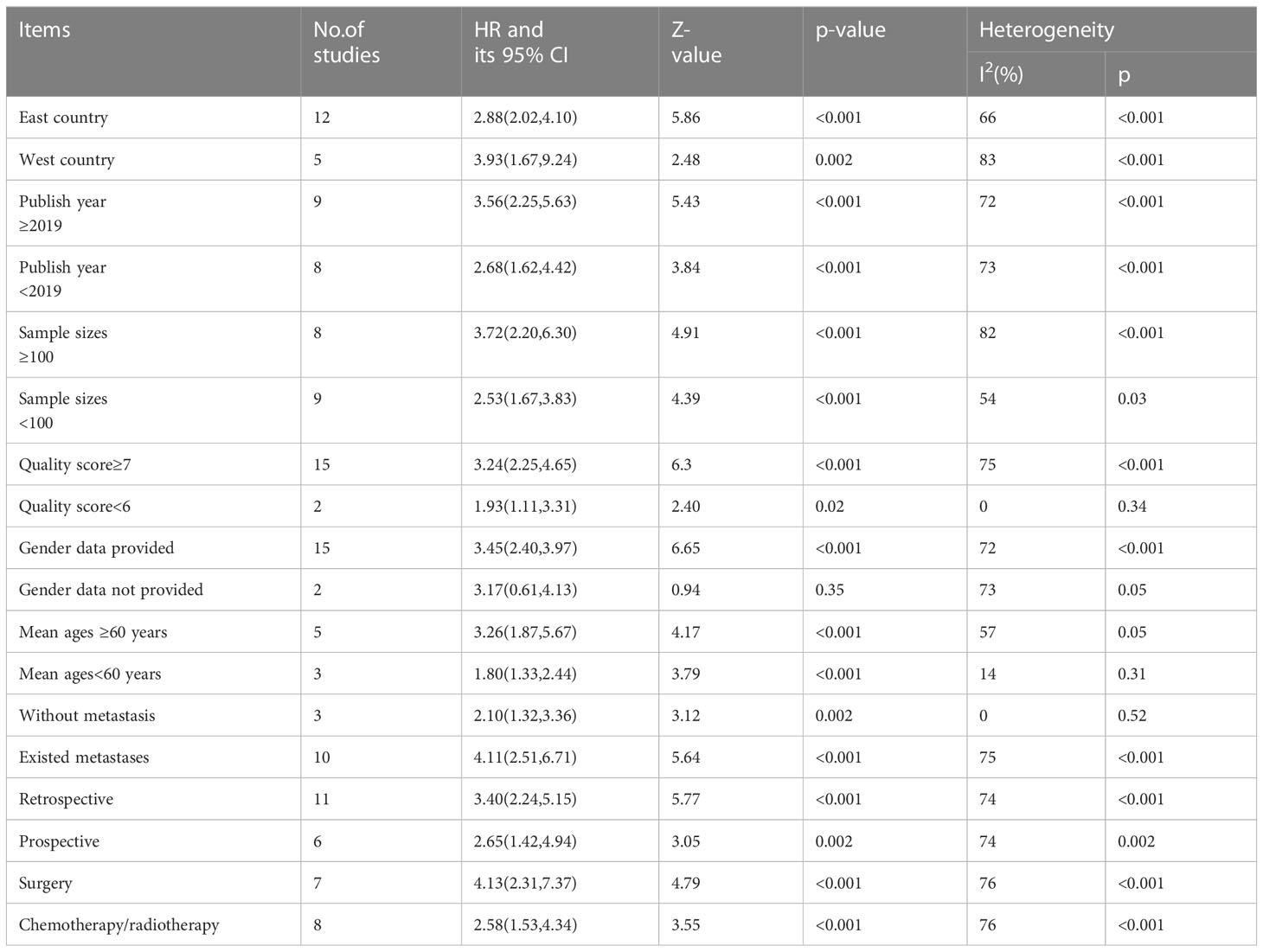
Table 3 Subgroup analyses assessing high sPD-L1 level and overall survival in patients with digestive system cancers.
Publication bias was evaluated using Begg’s test for OS. The results are shown in Figure 5 (p=0.07, Begg’s test). No significant publication bias was observed.
PD-L1 can be divided into membrane-bound PD-L1 and sPD-L1 (25). The detection of sPD-L1 in the plasma of patients with cancer has attracted great interest from researchers. Moreover, some reports indicated that sPD-L1 may be a prognostic factor in cancers (34, 35, 63–66). However, the predictive role of sPD-L1 in DSCs remains controversial.
The results of this meta-analysis revealed that high levels of sPD-L1 were associated with unfavorable OS. Several studies have reported that high sPD-L1 expression is associated with poor survival in breast cancer (25), renal cell carcinoma (63), and other solid cancers (25). However, its predictive role in digestive system cancers has not yet been fully established. Recently, the prognostic role of sPD-L1 in DSCs has been reported. Yoshida et al. reported that sPD-L1 levels are not related to OS (45). In contrast, a study reported by Fu et al. indicated that a high level of sPD-L1 predicted a worse survival outcome (62). This inconsistency requires further investigation. In pancreatic cancer, two studies indicated that higher sPD-L1 levels correlated with worse OS (58, 59). However, another study (36) revealed no significant between sPD-L1 level and survival outcomes in patients with pancreatic cancer using multivariable analysis.
Monitoring sPD-L1 levels might be helpful for predicting survival in patients with cancer and subsequently improving treatment efficacy (62). Tominaga et al. reported that the remission rate was higher in the low sPD-L1 group compared with that in the high sPD-L1 group (49). Park et al. showed that in gastric cancer, with disease progression, the sPD-L1 level increased (36). Some studies have also reported the predictive role of sPD-L1 for detecting metastasis. Kim et al. showed that patients with higher levels of sPD-L1 at 1 month (12.9 pg/ml) had poorer lung-metastasis-free survival (43). Mocan et al. indicated that high sPD-L1 predicted recurrence (57). Shigemori et al. discovered that the sPD-L1 level was a predictor of recurrence but was not related to metastases (37). Therefore, the prognostic value of sPD-L1 requires further investigation.
The potential correlation between sPD-L1 and tissue PD-L1 levels was also investigated. In rectal cancer, PD-L1 expression in biopsy specimens is not significantly different from that in serum PD-L1 (58). In gastric cancer, tissue PD-L1 expression does not correlate with sPD-L1 expression (45). Mocan et al. and Han et al. indicated that tissue PD-L1 is related to sPD-L1 in HCC (50, 52). In pancreatic cancer, no relationship has been observed between tissue PD-L1 and sPD-L1 (54). In esophageal cancer, tissue PD-L1 expression does not correlate with sPD-L1 expression (55). There was a significant correlation between sPD-L1 and tumor PD-L1 expression (51). Overall, the relationship between sPD-L1 and the expression of PD-L1 in tissue requires further investigation.
Several studies have reported the association of inflammatory cytokines with sPD-L1 level (47). In esophageal cancer, the researchers indicated that there was no correlation between sPD-L1 and C-reactive protein (CRP) (45). In another study, the investigator indicated that sPD-L1 was related with white cell count, but not correlated with CRP and other inflammatory markers. However, some studies have indicated that sPD-L1 was related with white blood cell and platelet count (49). Masaaki et al. suggested that sPD-L1 was associated with C-reactive protein levels (47). In HCC, Finkelmeier et al. indicated that sPD-L1 positively correlated with CRP (54). The relationship between sPD-L1 and inflammatory factor should be further investigated in different kinds of cancers.
Several studies indicated that sPD-L1 expression was not correlated with age and sex (36, 45, 46, 48, 50, 52, 61). One study indicated that sPD-L1 expression was related with age, but not correlated with gender (60). By contrast, another study revealed that sPD-L1 was associated with gender, but not related with age (47). However, some studies indicated that older age was associated with higher sPD-L1 (28). Furthermore, in gastric cancer, the expression of sPD-L1 was not significant difference in the intestinal type compared to that in the diffuse type (50).
Inhibition of sPD-L1 can result in a function similar to that of anti-PD-1 or anti-PD-L1 monoclonal antibodies, thereby achieving a checkpoint inhibitory effect (25). Some studies have reported that the inhibition of sPD-L1 restricting tumor growth showed a mechanism similar to that in anti-PD-L1 mAb-injected mice (67, 68). Further evaluation is required to establish the predictive ability of sPD-L1 in cancer treatment.
This study has some limitations. First, some of the studies included in this meta-analysis were retrospective studies, and there might have been selection or publication bias because the positive results were more easily published in the journal, whereas the negative results were not. Second, the cutoff values were not uniform, and heterogeneity might exist. Third, a high heterogeneity was observed in some analyses. The source of heterogeneity may be individual patients with different TNM stages and tumor types, sex, ages, study types, treatment methods, country locations, cutoff values, and follow-up times. To identify the sources of the heterogeneity, subgroup analyses were adopted but failed to determine this.
In conclusion, sPD-L1 can be a prognostic factor for DSCs. High sPD-L1 expression predicted poor OS and DFS. Inflammatory cytokines, treatment approaches, and other factors may affect the expression of sPD-L1. Therefore, the prognostic value of sPD-L1 for recurrence and metastasis should be further investigated. sPD-L1 may be a prognostic factor for treatment response. Well-designed prospective studies with standard assessment methods should be conducted to determine the prognostic value of sPD-L1 in DSCs.
Design and manuscript drafting: GL, JR, YQ, and ZZ. Editing and proving: GL, F-MK, and RX. All authors read and approved the final manuscript.
National Science of Shenzhen (No. JCYJ20220530152001002) funded this study.
We are very grateful to Thomas A. Agbaedeng for his exquisite polishing of the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin (2014) 64:9–29. doi: 10.3322/caac.21208
2. Roshani M, Baniebrahimi G, Mousavi M, Zare N, Sadeghi R, Salarinia R, et al. Exosomal long non-coding RNAs: novel molecules in gastrointestinal cancers' progression and diagnosis. Front Oncol (2022) 12:1014949. doi: 10.3389/fonc.2022.1014949
3. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer Lett (2021) 522:255–68. doi: 10.1016/j.canlet.2021.09.034
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
5. George TJ, Franke AJ, Chakravarthy AB, Das P, Dasari A, El-Rayes BF, et al. National cancer institute (NCI) state of the science: targeted radiosensitizers in colorectal cancer. Cancer (2019) 125:2732–46. doi: 10.1002/cncr.32150
6. Schimanski CC, Linnemann U, Galle PR, Arbogast R, Berger MR. Hepatic disseminated tumor cells in colorectal cancer UICC stage 4 patients: prognostic implications. Int J Oncol (2003) 23:791–96. doi: 10.3892/ijo.23.3.791
7. Yang Y, Wang Y, Wang Z. Construction of a new clinical staging system for colorectal cancer based on the lymph node ratio: a validation study. Front Surg (2022) 9:929576. doi: 10.3389/fsurg.2022.929576
8. Chiaravalli M, Reni M, O'Reilly EM. Pancreatic ductal adenocarcinoma: state-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev (2017) 60:32–43. doi: 10.1016/j.ctrv.2017.08.007
9. Javed AA, Wright MJ, Siddique A, Blair AB, Ding D, Burkhart RA, et al. Outcome of patients with borderline resectable pancreatic cancer in the contemporary era of neoadjuvant chemotherapy. J Gastrointest Surg (2019) 23:112–21. doi: 10.1007/s11605-018-3966-8
10. Yang Y, Zhang ZJ, Wen Y, Xiong L, Huang YP, Wang YX, et al. Novel perspective in pancreatic cancer therapy: targeting ferroptosis pathway. World J Gastrointest Oncol (2021) 13:1668–79. doi: 10.4251/wjgo.v13.i11.1668
11. Wang LM, Silva MA, D'Costa Z, Bockelmann R, Soonawalla Z, Liu S, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget (2016) 7:4183–94. doi: 10.18632/oncotarget.6770
12. Zhang E, Gu J, Xue J, Lin C, Liu C, Li M, et al. Accurate control of dual-receptor-engineered T cell activity through a bifunctional anti-angiogenic peptide. J Hematol Oncol (2018) 11:44. doi: 10.1186/s13045-018-0591-7
13. Setrerrahmane S, Li M, Zoghbi A, Lv X, Zhang S, Zhao W, et al. Cancer-related micropeptides encoded by ncRNAs: promising drug targets and prognostic biomarkers. Cancer Lett (2022) 547:215723. doi: 10.1016/j.canlet.2022.215723
14. Shah MA, Altorki N, Patel P, Harrison S, Bass A, Abrams JA. Improving outcomes in patients with oesophageal cancer. Nat Rev Clin Oncol (2023) 20:390–407. doi: 10.1038/s41571-023-00757-y
15. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med (2014) 371:2499–509. doi: 10.1056/NEJMra1314530
16. Chon SH, Berlth F, Plum PS, Herbold T, Alakus H, Kleinert R, et al. Gastric cancer treatment in the world: Germany. Transl Gastroenterol Hepatol (2017) 2:53. doi: 10.21037/tgh.2017.05.07
17. Basile D, Simionato F, Cappetta A, Garattini SK, Roviello G, Aprile G. State-of-the-Art of monoclonal antibodies for the treatment of gastric cancer. Biologics (2021) 15:451–62. doi: 10.2147/BTT.S290323
18. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst (2017) 109. doi: 10.1093/jnci/djx030
19. Demir T, Lee SS, Kaseb AO. Systemic therapy of liver cancer. Adv Cancer Res (2021) 149:257–94. doi: 10.1016/bs.acr.2020.12.001
20. Liu BX, Tang CT, Dai XJ, Zeng L, Cheng F, Chen Y, et al. Prognostic value of S100P expression in patients with digestive system cancers: a meta-analysis. Front Oncol (2021) 11:593728. doi: 10.3389/fonc.2021.593728
21. Bouquot M, Creavin B, Goasguen N, Chafai N, Tiret E, Andre T, et al. Prognostic value and characteristics of N1c colorectal cancer. Colorectal Dis (2018) 20:O248–55. doi: 10.1111/codi.14289
22. Zeng Z, Yang B, Liao Z. Biomarkers in immunotherapy-based precision treatments of digestive system tumors. Front Oncol (2021) 11:650481. doi: 10.3389/fonc.2021.650481
23. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med (2016) 8:324r–8r. doi: 10.1126/scitranslmed.aad7118
24. Scirocchi F, Strigari L, Di Filippo A, Napoletano C, Pace A, Rahimi H, et al. Soluble PD-L1 as a prognostic factor for immunotherapy treatment in solid tumors: systematic review and meta-analysis. Int J Mol Sci (2022) 23. doi: 10.3390/ijms232214496
25. Khan M, Zhao Z, Arooj S, Fu Y, Liao G. Soluble PD-1: predictive, prognostic, and therapeutic value for cancer immunotherapy. Front Immunol (2020) 11:587460. doi: 10.3389/fimmu.2020.587460
26. Li X, Zheng Y, Yue F. Prognostic value of soluble programmed cell death ligand-1 (sPD-L1) in various cancers: a meta-analysis. Target Oncol (2021) 16:13–26. doi: 10.1007/s11523-020-00763-5
27. Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget (2017) 8:97671–82. doi: 10.18632/oncotarget.18311
28. Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine (2011) 56:231–38. doi: 10.1016/j.cyto.2011.06.004
29. Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, et al. Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol Lett (2012) 142:78–82. doi: 10.1016/j.imlet.2011.11.001
30. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol (2001) 2:261–68. doi: 10.1038/85330
31. Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Med (Baltimore) (2017) 96:e6369. doi: 10.1097/MD.0000000000006369
32. Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large b-cell lymphoma: results from a French multicenter clinical trial. Leukemia (2014) 28:2367–75. doi: 10.1038/leu.2014.137
33. Murakami S, Shibaki R, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac Cancer (2020) 11:3585–95. doi: 10.1111/1759-7714.13721
34. Okuma Y, Wakui H, Utsumi H, Sagawa Y, Hosomi Y, Kuwano K, et al. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin Lung Cancer (2018) 19:410–17. doi: 10.1016/j.cllc.2018.04.014
35. Liao G, Zhao Z, Qian Y, Ling X, Chen S, Li X, et al. Prognostic role of soluble programmed death ligand 1 in non-small cell lung cancer: a systematic review and meta-analysis. Front Oncol (2021) 11:774131. doi: 10.3389/fonc.2021.774131
36. Park H, Bang JH, Nam AR, Eun PJ, Hua JM, Bang YJ, et al. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep (2019) 9:11131. doi: 10.1038/s41598-019-47330-1
37. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj (2009) 339:b2535. doi: 10.1136/bmj.b2535
38. Zhao Z, Liao G, Li Y, Zhou S, Zou H, Fernando S. Prognostic value of carbonic anhydrase IX immunohistochemical expression in renal cell carcinoma: a meta-analysis of the literature. PloS One (2014) 9:e114096. doi: 10.1371/journal.pone.0114096
39. Liao G, Zhao Z, Yang H, Chen M, Li X. Can prognostic nutritional index be a prediction factor in esophageal cancer?: a meta-analysis. Nutr Cancer (2020) 72:187–93. doi: 10.1080/01635581.2019.1631859
40. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
41. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
42. Ando K, Hamada K, Watanabe M, Ohkuma R, Shida M, Onoue R, et al. Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res (2019) 39:5195–201. doi: 10.21873/anticanres.13716
43. Kim JW, Lee KH, Kim JW, Suh KJ, Nam AR, Bang JH, et al. The prognostic role of soluble transforming growth factor-beta and its correlation with soluble programmed death-ligand 1 in biliary tract cancer. Liver Int (2021) 41:388–95. doi: 10.1111/liv.14636
44. Sawada R, Arai Y, Sagawa Y, Nagata Y, Nishimura T, Noguchi M, et al. High blood levels of soluble OX40 (CD134), an immune costimulatory molecule, indicate reduced survival in patients with advanced colorectal cancer. Oncol Rep (2019) 42:2057–64. doi: 10.3892/or.2019.7304
45. Yoshida J, Ishikawa T, Doi T, Ota T, Yasuda T, Okayama T, et al. Clinical significance of soluble forms of immune checkpoint molecules in advanced esophageal cancer. Med Oncol (2019) 36:60. doi: 10.1007/s12032-019-1285-x
46. Fan Y, Che X, Qu J, Hou K, Wen T, Li Z, et al. Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol (2019) 26:3745–55. doi: 10.1245/s10434-019-07431-7
47. Ito M, Oshima Y, Yajima S, Suzuki T, Nanami T, Shiratori F, et al. Is high serum programmed death ligand 1 level a risk factor for poor survival in patients with gastric cancer? Ann Gastroenterol Surg (2018) 2:313–18. doi: 10.1002/ags3.12175
48. Li G, Wang G, Chi F, Jia Y, Wang X, Mu Q, et al. Higher postoperative plasma EV PD-L1 predicts poor survival in patients with gastric cancer. J Immunother Cancer (2021) 9. doi: 10.1136/jitc-2020-002218
49. Park W, Bang JH, Nam AR, Jin MH, Seo H, Kim JM, et al. Prognostic value of serum soluble programmed death-ligand 1 and dynamics during chemotherapy in advanced gastric cancer patients. Cancer Res Treat (2021) 53:199–206. doi: 10.4143/crt.2020.497
50. Shigemori T, Toiyama Y, Okugawa Y, Yamamoto A, Yin C, Narumi A, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol (2019) 26:876–83. doi: 10.1245/s10434-018-07112-x
51. Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol (2016) 142:1727–38. doi: 10.1007/s00432-016-2184-6
52. Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother (2019) 68:353–63. doi: 10.1007/s00262-018-2271-4
53. El-Gebaly F, Abou-Saif S, Elkadeem M, Helmy A, Abd-Elsalam S, Yousef M, et al. Study of serum soluble programmed death ligand 1 as a prognostic factor in hepatocellular carcinoma in Egyptian patients. Curr Cancer Drug Targets (2019) 19:896–905. doi: 10.2174/1568009619666190718141647
54. Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer (2016) 59:152–59. doi: 10.1016/j.ejca.2016.03.002
55. Han X, Gu YK, Li SL, Chen H, Chen MS, Cai QQ, et al. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis b-related hepatocellular carcinoma. J Cancer Res Clin Oncol (2019) 145:303–12. doi: 10.1007/s00432-018-2758-6
56. Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol (2018) 129:130–35. doi: 10.1016/j.radonc.2017.11.027
57. Mocan T, Ilies M, Nenu I, Craciun R, Horhat A, Susa R, et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1): a possible biomarker in predicting post-treatment outcomes in patients with early hepatocellular carcinoma. Int Immunopharmacol (2021) 94:107467. doi: 10.1016/j.intimp.2021.107467
58. Bian B, Fanale D, Dusetti N, Roque J, Pastor S, Chretien AS, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology (2019) 8:e1561120. doi: 10.1080/2162402X.2018.1561120
59. Kruger S, Legenstein ML, Rosgen V, Haas M, Modest DP, Westphalen CB, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology (2017) 6:e1310358. doi: 10.1080/2162402X.2017.1310358
60. Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget (2016) 7:76604–12. doi: 10.18632/oncotarget.12810
61. Tominaga T, Akiyoshi T, Yamamoto N, Taguchi S, Mori S, Nagasaki T, et al. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PloS One (2019) 14:e212978. doi: 10.1371/journal.pone.0212978
62. Fu R, Jing CQ, Li XR, Tan ZF, Li HJ. Prognostic significance of serum PD-L1 level in patients with locally advanced or metastatic esophageal squamous cell carcinoma treated with combination cytotoxic chemotherapy. Cancer Manag Res (2021) 13:4935–46. doi: 10.2147/CMAR.S312690
63. Larrinaga G, Solano-Iturri JD, Errarte P, Unda M, Loizaga-Iriarte A, Perez-Fernandez A, et al. Soluble PD-L1 is an independent prognostic factor in clear cell renal cell carcinoma. Cancers (Basel) (2021) 13. doi: 10.3390/cancers13040667
64. Nukui A, Masuda A, Abe H, Arai K, Yoshida KI, Kamai T. Increased serum level of soluble interleukin-2 receptor is associated with a worse response of metastatic clear cell renal cell carcinoma to interferon alpha and sequential VEGF-targeting therapy. BMC Cancer (2017) 17:372. doi: 10.1186/s12885-017-3369-3
65. Wang Q, Zhang J, Tu H, Liang D, Chang DW, Ye Y, et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J Immunother Cancer (2019) 7:334. doi: 10.1186/s40425-019-0810-y
66. Han B, Dong L, Zhou J, Yang Y, Guo J, Xuan Q, et al. The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte. Cancer Immunol Immunother (2021) 70:2893–909. doi: 10.1007/s00262-021-02898-4
67. He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, et al. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol (2004) 173:4919–28. doi: 10.4049/jimmunol.173.8.4919
Keywords: digestive system cancers, overall survival, prognosis, soluble programmed death ligand 1, gastric cancer
Citation: Ruan J, Zhao Z, Qian Y, Xu R, Liao G and Kong F-M(S) (2023) The predictive role of soluble programmed death ligand 1 in digestive system cancers. Front. Oncol. 13:1170220. doi: 10.3389/fonc.2023.1170220
Received: 20 February 2023; Accepted: 22 June 2023;
Published: 13 July 2023.
Edited by:
Maurizio Chiriva-Internati, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Fabio Grizzi, Humanitas Research Hospital, ItalyCopyright © 2023 Ruan, Zhao, Qian, Xu, Liao and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruilian Xu, eHVydWlsaWFAMTI2LmNvbQ==; Feng-Ming (Spring) Kong, a29uZzAwMDFAaGt1Lmhr; Guixiang Liao, bGlhb2d1aXhpYW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.