Abstract
Background:
Overexpression of heat shock proteins (HSPs) has been observed in a wide range of human tumors, and there is an increasing evidence demonstrated that HSPs play a key role in tumor progression. Several studies were conducted to explore the clinicopathological characteristics and prognostic value of HSPs in hepatocellular carcinoma (HCC), but the results remain controversial. To address this gap, we conducted a systematic review and meta-analysis.
Methods:
The eligible literature was obtained from PubMed, Cochrane library, Web of science, Embase, Chinese National Knowledge Infrastructure and Wan Fang databases. We used the odds ratio (OR) and hazard ratio (HR) as the suitable parameters to assess the clinicopathological features and prognostic value of HSPs in HCC patients.
Results:
The meta-analysis results showed that HSPs expression was associated with overall survival (OS) of HCC patients (HR = 1.61, 95%CI = 1.22-2.13, P=0.001, I2 = 62.7%). In addition, the pooled results suggested that HSPs expression was significantly correlated with tumor differentiation (OR = 1.33, 95%CI = 1.08-1.65, P = 0.907), vascular invasion (OR = 1.31, 95%CI = 1.02-1.69, P = 0.921) and lymphatic metastasis (OR=1.98, 95%CI= 1.70-2.31, P = 0.740). Meanwhile, the subgroup analysis showed a significant correlation between the expression of HSP27 (HR=1.69, 95%CI = 1.24-2.31, P = 0.674) and HSP90α (HR=2.03, 95%CI = 1.73-2.40, P = 0.743) with OS of HCC patients.
Conclusions:
Our meta-analysis confirms that HSPs expression is closely associated with a worse prognosis in HCC patients, and may be directly involved in tumor differentiation and distant metastasis. In addition, the subgroup analysis results demonstrate that the expression of HSP27 and HSP90α can be served as potential prognostic predictors of HCC.
Introduction
Primary liver cancer is one of the most aggressive malignant tumors worldwide, with the sixth highest incidence rate and the third highest mortality rate among cancers (1, 2). About 906,000 new cases and more than 830,000 cancer-related deaths were estimated to have occurred in 2020 globally, and hepatocellular carcinoma (HCC) accounts for about 75–85% of all liver cancers (2). Approximately half of all HCC cases and HCC-related deaths occur in China (3). Majority of patients are diagnosed at an advanced stage because of lacking typical clinical symptoms and signs, and less than 20% of HCC patients can undergo surgery for complete resection (4–6). Despite the advances in diagnostic and therapeutic approaches over the past decades, the recurrence rates of HCC patients were high and long-term survival remained disappointed (7–9). The 5-year overall survival of HCC was reported 18% in western countries, and decreased to 12.1% from 20% in China (10, 11). A series of studies have been conducted to explore the molecular and biological mechanism that lead to carcinoma, and researches in identifying novel biomarkers for accurately predicting the clinical features and prognosis of HCC have become a hot topic.
Heat shock proteins (HSPs) are highly conversed molecular chaperones that produced by cells in response to stressful conditions. They are generally classified into five groups based on their molecular weight or systematic gene symbols, including HSP60, HSP70, HSP90, HSP110 and small HSPs (12). This class of proteins were found to be involved in various cellular processes, both physiological and pathological, and profoundly impact tumor progression by manipulating cancer hallmarks such as anti-apoptosis, proliferation, invasion, migration, metastasis, and angiogenesis (13–19). Overexpression of HSPs was observed in a wide range of human malignant tumors, including liver, colorectal, cervical, breast, prostate and lung cancers (20–22). Of particular interest, increasing evidence has demonstrated that HSPs are potential biomarkers for tumor diagnosis, prognosis and therapeutic targets (13). Its prognostic value has been confirmed in various types of solid tumors such as oral, breast and prostate cancers (23–25). Recently, numerous studies attempted to clarify the clinical and prognostic significance of HSPs expression in HCC patients, but their results remained controversial and even paradoxical (26–47). Ji et al. demonstrated that the expression of HSPgp96 was associated with lower tumor, node and metastasis (TNM) stage and favorable prognosis, but they found did not find HSPgp96’s expression association with other clinicopathological features (26). King et al. reported the opposite result, their findings indicated that the expression of HSP27 was significantly related to tumor histology grade (27). In addition, HSP27 expression was found to be significantly related to poor overall survival (OS) (27). Moreover, Kang et al. found that the expression of HSP70 was not correlated with the main clinicopathological features and OS in HCC patients (28). Therefore, we performed this system review and meta-analysis to more precisely investigate the relationships between HSPs expression and the main clinicopathological features of HCC patients. We also evaluated the prognostic value of HSPs expression in patients with HCC.
Methods
Literature search
Without any restrictions in terms of publication status, language, and year of publication, all relevant primary studies published on or before May 30, 2023, which assessed the relationship between HSPs expression and the clinicopathological features or prognosis were searched in the following databases: PubMed, Cochrane library, Web of science, Embase, Chinese National Knowledge Infrastructure (CNKI) and Wan Fang databases. The literature search was performed by two researchers independently. The search terms are presented in Supplementary Table 1 and included the following keywords: heat shock protein and hepatocellular carcinoma. Additional eligible articles in the references list were obtained by manual searching.
Inclusion and exclusion criteria
Two independent authors screened all relevant articles on the basis of titles and abstracts, and skimmed the full-text according to the inclusion and exclusion criteria. Any disagreement was resolved by discussion with the third author to reach a final consensus. The inclusion criteria of the current system review and meta-analysis were as follows: 1) patients in this study should be clearly diagnosed with HCC; 2) the study was published in Chinese or English, and the full-text is available; 3) the expression of HSPs is measured by immunohistochemistry (IHC) analysis or enzyme-linked immunosorbent assay (ELISA); 4) the article should assess the relationships between HSPs expression and the HCC clinicopathological features or prognosis, and the study include at least one primary outcome of interest; 5) the follow-up periods in assessing prognosis should be more than two years. In addition, the exclusion criteria were applied: letters, reviews, conference abstracts, nonhuman subject studies, case reports, duplicated reports, and data unavailable.
Data extraction and quality assessment
Two authors independently extracted the following data: first author, publication year, country, HSPs types, sample size, cut-off values, follow-up time, detecting methods, recruitment time, hazard ratios (HRs) or adds ratios (ORs) with 95% confidence intervals (95%CIs) for overall survival (OS) and clinicopathological parameters. The clinicopathological parameters included age(≥60 versus <60), HSPs expression (high/positive versus low/negative), gender (male versus female), tumor differentiation (low versus moderate or high), hepatitis B virus surface antigen (HBsAg) (positive versus negative), alpha-fetoprotein (AFP) (≥ 400ng/ml versus < 400ng/ml), tumor size (≥ 5cm versus < 5cm), vascular invasion (yes versus low), TNM stage (I +II versus III +IV), tumor number (single versus multiple), portal vein tumor thrombus (PVTT) (yes versus no), and lymphatic metastasis (yes versus no).
Newcastle-Ottawa Scale (NOS) was applied to evaluate the quality of original non-randomized studies (48). Three perspectives including selection, comparability and exposure were considered for estimations of the quality and potential bias risks. Studies with a NOS score of ≥6 were considered as a good quality, and ≤ 5 were regarded as a poor quality.
Statistical analysis
Statistical analysis was performed using the Stata 16.0 software. ORs with 95%CIs were used to evaluate the relationship between HSPs and clinicopathological characteristics in HCC patients. To determine the association between HSPs expression and the prognosis of patients with HCC, HRs with 95% CI were used as the summarized estimates. In case of articles does not provide data directly, the Engauge Digitizer 4.1 software (http://sourceforge.net) was used to extract the survival data from the Kaplan-Meier curves to measure the accuracy of estimated HR according to the guidelines established by Tierney et al. (49). Heterogeneity was estimated using I2 value and Cochran’s Q test. With I2>50% or P<0.05 suggesting significant heterogeneity (50), the meta-analysis was conducted using a random-effect model. Otherwise, a fixed-effect model was used. To further investigate the source of heterogeneity, we conducted a meta-regression. Sensitivity analysis was performed to determine the stability of aggregated results. In addition, we also assessed potential small-trail effects and publication bias by using Begg’s and Egger’s tests for all available groups with sufficient studies (≥10 studies), and P<0.05 indicated the existence of publication bias.
Results
Characteristics of included studies
A total of 1312 records were screened in a primary search for possible related between HSPs expression and the clinicopathological features or prognosis in HCC. Figure 1 illustrates the process of literature research and selection. Ultimately, 22 eligible studies were included in this meta-analysis (26–47). As shown in Table 1, these studies were published from 2000 to 2022, and included a total of 3175 HCC patients. The sample size ranged from 40 to 2150 patients, with 15 of the studies including > 60 patients and 7 of the studies including ≤60 patients. Fourteen studies evaluated patients from China, three from Korea, two from Japan. The NOS score of all these included studies were ≥6, which implied that they were of high quality.
Figure 1
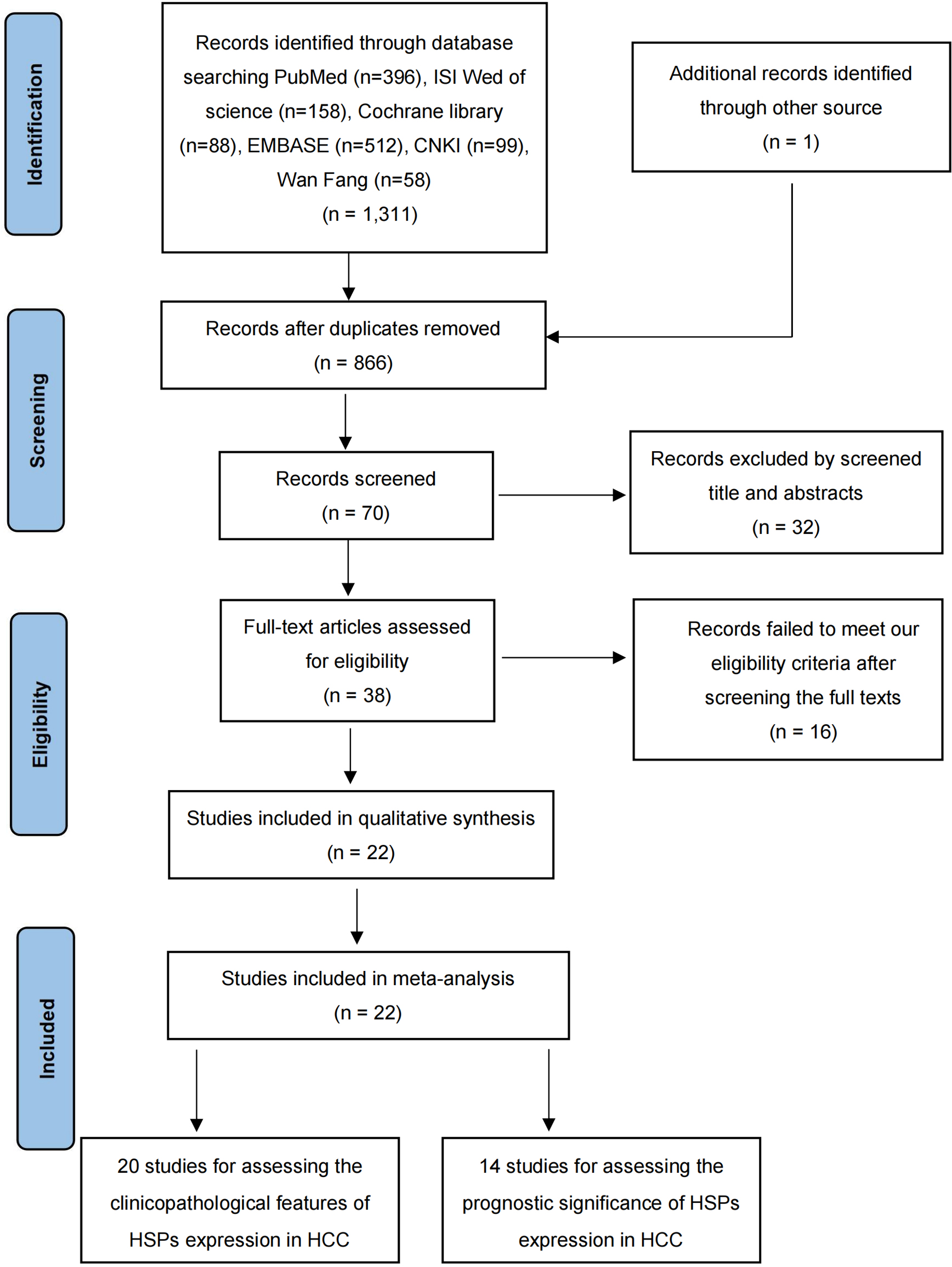
Flow chart outlining the selection of studies.
Table 1
| Author | year | Country | HSPs subtype | No.of patients | Cancer type | Detecting methods | Cut-off Value | Investigating fields | Estimates | Recruitment time | NOS Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | Prognosis | |||||||||||
| Ming et al | 2016 | China | HSP70 | 110 | HCC | IMH | 25% | √ | √ | OR, HR | NR | 8 |
| Kang et al | 2017 | China | HSPA9 | 49 | HCC | IMH | 50% | √ | √ | OR, HR | 2006-2010 | 6 |
| Kang et al | 2014 | korea | HSP70 | 83 | HCC | IMH | 5% | √ | √ | OR, HR | 1999-2011 | 8 |
| Hung at al | 2017 | China | HSP27 | 80 | HCC | IMH | NR | √ | √ | OR, HR | NR | 7 |
| Ji et al | 2018 | China | HSPgp96 | 84 | HCC | IMH | 5% | √ | √ | OR, HR | 2007-2009 | 8 |
| Harimoto et al | 2004 | Japan | HSP27 | 60 | HCC | IMH | 25% | √ | √ | OR, HR | 1991-1998 | 8 |
| King et al | 2000 | China | HSP27 | 58 | HCC | IMH | 1% | √ | √ | OR, HR | NR | 7 |
| Xu et al | 2017 | China | HSP90 | 100 | HCC | IMH | 10% | √ | √ | OR, HR | NR | 8 |
| Zhang et al | 2016 | China | HSP27 | 167 | HCC | IMH | 25% | √ | HR | NR | 8 | |
| Liu et al | 2016b | China | HSP90 | 60 | HCC | IMH | 10% | √ | OR | 2007-2010 | 7 | |
| DAIMEI et al | 2016 | Japan | HSP27 | 194 | HCC | IMH | NR | √ | OR | 1993-1997 | 8 | |
| John M et al | 2006 | China | HSP27 | 67 | HCC | IMH | NR | √ | OR | 1998-2004 | 8 | |
| HSP70 | 67 | HCC | IMH | NR | √ | OR | 1998-2004 | 8 | ||||
| GRP78 | 67 | HCC | IMH | NR | √ | OR | 1998-2004 | 8 | ||||
| Hua et al | 2008 | China | HSPgp96 | 32 | HCC | IMH | 10% | √ | OR | 2004-2007 | 6 | |
| Guo et al | 2011 | China | HSP27 | 68 | HCC | IMH | 5% | √ | OR | NR | 6 | |
| Liang et al | 2011 | China | HSP27 | 34 | HCC | IMH | 25% | √ | OR | 2008-2009 | 6 | |
| Mee et al | 2005 | korea | HSP27 | 71 | HCC | IMH | 10% | √ | OR | 1996-2003 | 8 | |
| HSP70 | 71 | HCC | IMH | 10% | √ | OR | 1996-2003 | 8 | ||||
| Eun et al | 2011 | korea | HSP70 | 412 | HCC | IMH | NR | √ | OR | 1995-2004 | 8 | |
| Sun et al | 2003 | China | HSP90α | 40 | HCC | IMH | 10% | √ | OR | 1994-2000 | 6 | |
| Dai et al | 2009 | China | HSP27 | 45 | HCC | IMH | 5% | √ | OR | 2003-2005 | 6 | |
| Chen et al | 2022 | China | HSP90α | 97 | HCC | ELISA | 135ng/mL | √ | √ | OR, HR | 2019-2020 | 7 |
| Su et al | 2022 | China | HSP90α | 2150 | HCC | ELISA | 143.5ng/ml | √ | √ | OR, HR | 2016-2021 | 8 |
| Zhang et al | 2021 | China | HSP90α | 112 | HCC | ELISA | 69.3ng/ml | √ | OR, HR | 2014-2016 | 8 | |
Characteristics of included studies.
HCC, hepatocellular carcinoma; CF, clinicopathological features; HSP, heat shock protein; OR, odds ratio; HR, hazard ratio; NR, not reported. "√" represents that the article provides such research data.
Association between HSPs expression and clinicopathological characteristics in HCC patients
In this study, we evaluated the correlation between HSPs expression and clinicopathological features of patients with HCC, and the results were shown in Table 2 and Supplementary Figure 1 , 2. Fourteen studies, including a total of 1653 HCC patients, were used to evaluate the differential expression of HSPs in HCC and non-HCC tissues. The results revealed that the expression of HSPs in HCC was significantly higher than that in the non-HCC tissues (OR =2.21, 95%CI = 1.50-3.25, P = 0.005). The ORs for tumor differentiation were reported in 16 studies that included 1131 cases. The results evaluation of these data indicated that HSPs expression was associated with tumor differentiation (OR = 1.33, 95%CI = 1.08-1.65, P = 0.907). Ten studies containing 756 patients showed a significant relationship between HSPs expression and vascular invasion in HCC (OR = 1.31, 95%CI = 1.02-1.69, P = 0.921). A greater proportion of patients with high HSPs expression had vascular invasion than those who had low HSPs expression. The ORs for lymphatic metastasis were reported in 6 studies on 2432 patients, and the pooled OR with 95%CI was 1.98 (95%CI = 1.70-2.31, P = 0.740), indicating the high HSPs expression was associated with lymphatic metastasis. The pooled results of 16 studies including 3528 patients showed a significant relationship between HSPs expression and gender (OR = 1.19, 95%CI = 1.02-1.39, P = 1.000). However, the HSPs expression were not significantly associated with any of the following parameters: HBsAg (OR = 1.03, 95%CI = 0.90-1.18, P = 0.907), TNM stage (OR = 1.10, 95%CI = 0.89-1.34, P = 0.463), tumor size (OR = 1.18, 95%CI = 0.72-1.96, P = 0.000), tumor number (OR = 1.18, 95%CI = 0.77-1.81, P = 0.035), AFP (OR = 1.23, 95%CI = 0.57-2.65, P = 0.020), PVTT (OR = 1.41, 95%CI = 0.81-2.47, P = 0.004).
Table 2
| Clinicopathological characteristics | N | No. of included patients | Heterogeneity | Model | Pooled OR with 95%CI | |||
|---|---|---|---|---|---|---|---|---|
| Total | HE | LE | I-squared | P-value | ||||
| Gender (male vs female) | 16 | 3528 | 1581 | 1947 | 0.00% | 1.000 | Fixed | 1.19(1.02-1.39) |
| Age (≥60 vs <60) | 3 | 259 | 122 | 137 | 0.00% | 0.779 | Fixed | 1.12(0.71-1.78) |
| Expression (high vs low) | 14 | 1653 | 935 | 718 | 56.40% | 0.005 | Random | 2.21(1.50-3.25) |
| Differentiation (low vs moderate or high) | 16 | 1131 | 701 | 430 | 0.00% | 0.907 | Fixed | 1.33(1.08-1.65) |
| HBsAg (posotive vs negative) | 14 | 3403 | 1510 | 1893 | 0.00% | 0.907 | Fixed | 1.03(0.90-1.18) |
| TNM stage (III+IV vsI+II) | 14 | 1063 | 666 | 397 | 0.00% | 0.463 | Fixed | 1.10(0.89-1.34) |
| Tumor size (≥5 cm vs < 5cm) | 9 | 2875 | 1172 | 1703 | 82.70% | 0.000 | Random | 1.18(0.72-1.96) |
| Vascular invasion (yes vs no) | 10 | 756 | 442 | 314 | 0.00% | 0.921 | Fixed | 1.31(1.02-1.69) |
| Tumor number (multiple vs single) | 5 | 2618 | 1054 | 1564 | 61.40% | 0.035 | Random | 1.18(0.77-1.81) |
| AFP (≥400ng/ml vs <400ng/ml) | 3 | 2310 | 849 | 1461 | 71.70% | 0.020 | Random | 1.23(0.57-2.65) |
| Lymphatic metastasis (yes vs no) | 6 | 2432 | 969 | 1463 | 0.00% | 0.740 | Fixed | 1.98 (1.70-2.31) |
| PVTT (yes vs no) | 7 | 2505 | 975 | 1530 | 68.90% | 0.004 | Random | 1.41(0.81-2.47) |
Meta analysis of clinicopathological features of heat shock proteins (HSPs) expression in patients with hepatocellular carcinoma (HCC).
N, number of included studies; HE, positive or high expression; NE, negative or low expression; OR, odds ratio, AFP, alpha-fetoprotein, PVTT, Portal vein tumor thrombus.
Meta-analysis of OS associated with the expression of HSPs in patients with HCC
Twelve articles (Harimoto et al. and Su et al. had two set of data) included fourteen studies have reported the relationship between HSPs expression and OS in HCC patients. A total of 2519 HCC patients were included, including 1022 patients with high/positive HSPs expression and 1497 patients with low/negative HSPs expression. As shown in Figure 2, the pooled HR of the fourteen studies was 1.61 (95%CI = 1.22-2.13, P = 0.001, I2 = 62.7%), indicating significant association betwen HSPs and OS of patients with HCC.
Figure 2
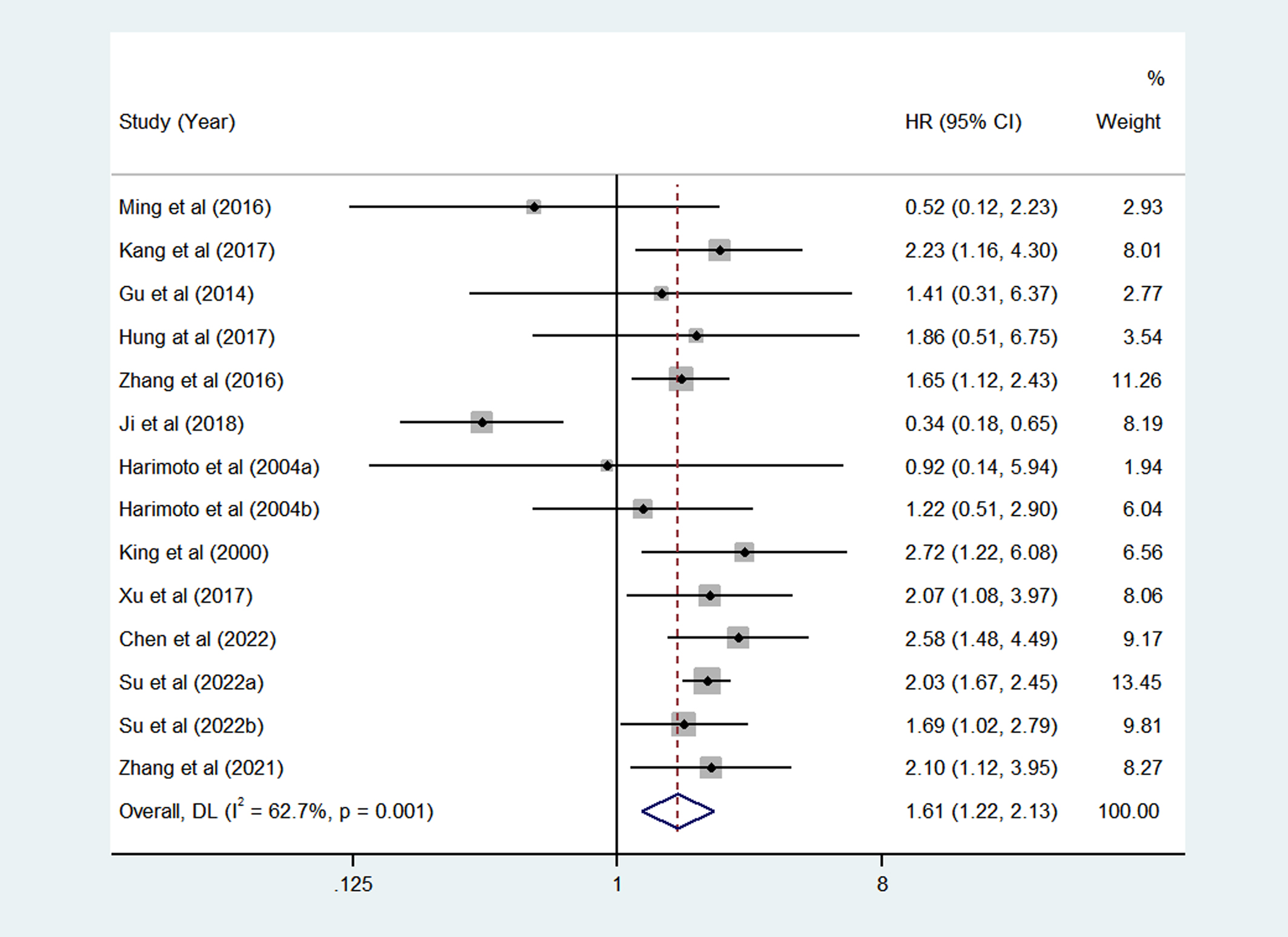
Pooled analyses for assessing the prognostic value of heat shock proteins (HSPs) expression for overall survival (OS) in hepatocellular carcinoma (HCC) patients.
Subgroup analysis
Considering the impact of different HSPs type, we did further analysis. According to the Figure 3, the expression of HSP27 and HSP90α were significantly associated with poor OS of HCC, with corresponding pooled HR was 1.69 (95%CI = 1.24-2.31, P=0.674) and 2.03 (95%CI = 1.73-2.40, P=0.743), respectively. However, the pooled results of HSP70 (HR=1.43, 95%CI = 0.62-3.31, P=0.196) and HSP90 (HR=0.84, 95%CI = 0.15-4.89, P=0.000) showed that the expression of these HSPs were not significantly associated with OS of HCC patients.
Figure 3
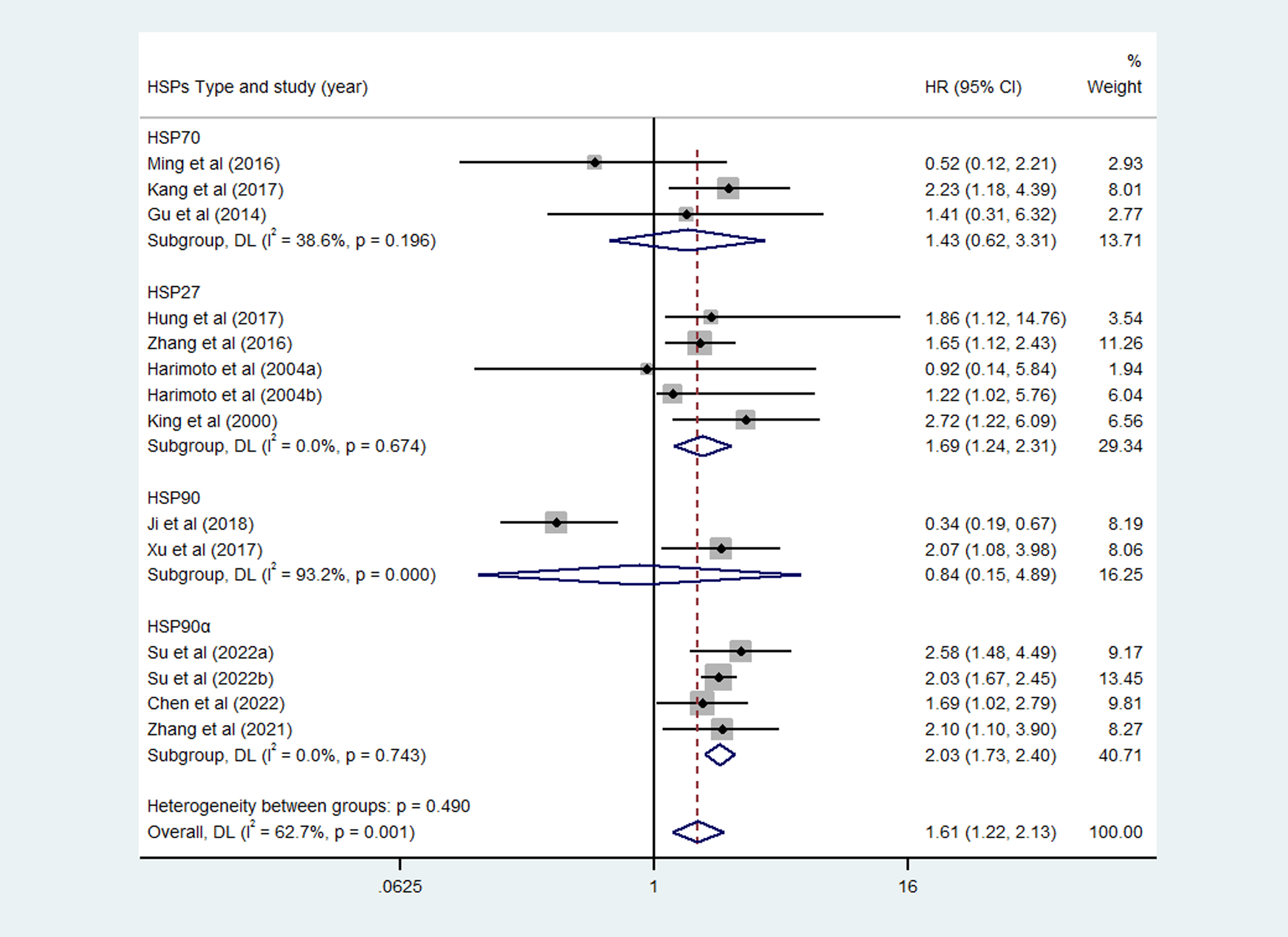
Subgroup analysis of overall survival (OS) of different heat shock proteins (HSPs).
Meta-regression
To further explore the possible sources of heterogeneity, we conducted a meta-regression based on sample size (≥60, <60), NOS score, country, detecting methods and HSPs type. The results of meta-regression were summarized in Supplementary Table 2, and none of the above covariates were found to be a significant source of heterogeneity.
Publish bias and sensitivity analysis
No publish bias was found for expression, tumor differentiation, HBsAg, TNM stage, and OS, according to the Begg’s and Egger’s test. However, publish bias existed for gender and vascular invasion. The funnel plots obtained from the Begg’s test are shown in Supplementary Figure 3, and the corresponding P values from Egger’s test are presented in Table 3. A sensitivity analysis was also calculated to determine whether individual studies influence the pooled ORs or HRs, and there were no significant impacts on ORs or HRs after excluding any one study, indicating the results were reliability.
Table 3
| Groups of outcomes | N | Estimates | Egger’s test (P value) | Publication bias |
|---|---|---|---|---|
| Clinicopathological characteristics | ||||
| Gender (male vs female) | 16 | OR with 95%CI | 0.020 | Significant |
| Expression (high vs low) | 14 | OR with 95%CI | 0.340 | Not significant |
| Tumor differentiation (low vs moderate or high) | 16 | OR with 95%CI | 0.760 | Not significant |
| HBsAg (posotive vs negative) | 14 | OR with 95%CI | 0.276 | Not significant |
| TNM stage (III+IV vsI+II) | 14 | OR with 95%CI | 0.332 | Not significant |
| Vascular invasion (yes vs no) | 10 | OR with 95%CI | 0.028 | Significant |
| Prognostic significance | ||||
| Overall survival (OS) | 14 | HR with 95%CI | 0.241 | Not significant |
Evaluations for the potential bias within the meta-analysis.
PVTT, Portal vein tumor thrombus; N, number of included studies.
Discussion
The role of HSPs in the prognosis and clinicopathological features of HCC has been investigated in several studies, but the results are inconsistent. Therefore, we performed this meta-analysis. The pooled results showed that HSPs expression was associated with poor prognosis in HCC patients (pooled HR = 1.61, 95%CI = 1.22-2.13, P=0.001), and the HSPs expression was higher in HCC patients than in normal tissues (pooled HR = 2.21, 95%CI = 1.50-3.25, P=0.005). Furthermore, the subgroup analysis confirmed that the expression of HSP27 (pooled HR = 1.69, 95%CI = 1.24-2.31, P=0.674) and HSP90α (pooled HR = 2.03, 95%CI = 1.73-2.40, P=0.743) can be used as potential prognostic predictors of HCC patients. To further investigate the role of HSPs in HCC, we also analyzed the relationship between HSPs status and clinicopathological features of HCC, the results showed that patients with high expression of HSPs have lower tumor differentiation (pooled OR = 1.33, 95%CI = 1.08-1.65, P = 0.907), a higher likelihood of lymphatic metastasis (pooled OR = 1.98, 95%CI = 1.70-2.31, P = 0.740), and more prone to vascular invasion (pooled OR = 1.31, 95%CI = 1.02-1.69, P = 0.921). Importantly, all of these parameters generally indicate a poor tumor prognosis.
HCC remains a serious challenge to public health due to its high incidence and mortality, and the long-term prognosis of HCC patients is relatively poor. Prognostic markers are widely used in clinical practice and have high clinical value as determinants factors for effective treatment (51). AFP is by far the most widely and extensively studied prognostic marker in HCC. However, the controversy has been raised regarding which specific cut-off for recurrence or survival is selected (52). Moreover, AFP negative tumors account for 30-40% pathologically diagnosed HCC patients, which significantly hampers the application of AFP in HCC prognosis (53–55). Therefore, new prognostic markers are urgently needed.
HSPs were first discovered in 1962 and have since become a hotspot of study due to their ubiquitous presence in all celled organisms (56, 57). Many oncoproteins require high levels of HSPs to maintain their function, and increased levels of HSPs have been found to be significantly higher in tumor cells than that in normal cells, including liver, colorectal, cervical, breast, prostate and lung cancers (20–22). Various molecular mechanisms of HSPs involved in tumor progression and invasion have been investigated over the past few decades, and most of these studies have focused on the expression of HSP27, HSP70 and HSP90 in tumors (17–19, 58–60). HSP27 is an important regulator of the Salvador-Warts-Hippo pathway, which controls tumor inhibition, progression, metastasis, and cancer stem cell programming (13). Eric et al. reported that elevated levels of HSP27 increased the metastatic spread of prostate cancer cells, and confirmed HSP27 as an independent prediction of poor clinical outcome predictor in prostate cancer patients (17). In HCC and lung cancer, HSP70 was reported to promote cancer progression by binding to TLR2 receptor, and inducing MyD88 dependent and independent NF-κB activation and pro-inflammatory gene transcription (18, 58). Furthermore, Kaido et al. reported that HSP70 positively regulates transforming growth factor-α (TGF-α) induced HCC cell migration via the AKT signaling pathway (19). In addition, previous studies have demonstrated that the HSP90 expression was associated with tumor proliferation and metastasis (59, 60). HSP90α, a subtype of HSP90, has been proved to participate in induction of tumor cell migration and invasion by binding to LRP1 and activating the ERK and AKT pathways (61). Taken together, HSPs have a significant impact on tumor prognosis and may serve as a predictive or prognostic factor for malignancy.
The most recent meta-analysis in 2018 reported a significant relationship between HSP27 expression and poor prognosis of HCC (62). Hua et al. conducted another meta-analysis on the prognostic role of HSPs in HCC, and the results showed that there was no significant correlation existed between HSPs expression and the prognosis of HCC, but only three eligible articles were included (63). In this study, we included fourteen studies to investigate the prognostic value of HSPs in HCC patients, and finally reached a completely opposite conclusion to the previous meta-analysis (63). Our results confirmed that increased HSPs levels were significantly associated with OS in HCC patients, indicating that HSPs may serve as a potential prognostic predictor of HCC. In addition, we further analyzed the prognostic value of HCC patients among different subtypes of HSPs, including HSP27, HSP70, HSP90 and HSP90α. The pooled results showed that the expression of HSP27 was correlated with poor OS in HCC patients, which was consistent with the previous studies (62). More importantly, our study also confirmed that HSP90α was correlated with poor prognosis of HCC patients, suggesting that HSP90α, like HSP27, was an effective prognostic biomarker for HCC patients. Therefore, HCC patients with increased expression of HSP27 or HSP90α should be advised to shorten the follow-up interval and adjust the treatment plan. A large multicenter clinical trial found that plasma HSP90α decreased after treatment and increased with tumor recurrence (64). Monitoring the expression level of HSPs, especially HSP27 and HSP90α, is a potential approach to predict disease progression and guide in deciding the next treatment strategy. Our findings may also further provide a new insight into the prognosis of HCC patients.
Several limitations should be taken into consideration in this meta-analysis. First, all of the included studies were conducted in Asia population, including China, Korea and Japan, which somewhat limits the universality of our conclusions and may bring bias to draw conclusions about other ethnic groups. Second, there may be a languages bias in this meta-analysis because we only included studies published in English or Chinese. Third, all the included studies were retrospective, which may induce the potential of bias. More high-quality articles are needed to confirm our conclusions. Fourth, the included studies used different detection methods, including IMH and ELISA to test HSPs expression, which may cause some heterogeneity and may also affect the results. Fifth, we used the Kaplan-Meier survival curves to obtain HRs and 95%CIs if the study did not report these data, which may cause bias. Finally, although we conducted meta-regression and sensitivity analysis, the source of heterogeneity could not be fully explained. Overall, the results should be interpreted with caution. More high-quality studies are needed to confirm the results of this meta-analysis.
Conclusions
In conclusion, our meta-analysis confirms that the expression of HSPs is correlated with poor OS in HCC patients, and may be directly involved in tumor differentiation and distant metastasis. Meanwhile, the subgroup analysis results demonstrate that the expression of HSP27 and HSP90α can severed as effective prognostic predictors of HCC. Additionally, owing to the limitations, more higher quality studies, especially randomized controlled trials, are needed to more precisely validate the clinical role of HSPs in HCC.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to this work. Study design and writing: DX. Constitute figures, tables and data analysis: DX and MJ. Screening of abstracts and articles: MJ, YC and CL. Critically reviewing manuscript: LL. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1169979/full#supplementary-material
Supplementary Figure 1Pooled analyses for assessing the associations between heat shock proteins (HSPs) expression and (A) Gender, (B) Age, (C) HSPs expression, (D) Differentiation, (E) HBsAg, and (F) TNM stage.
Supplementary Figure 2Pooled analyses for assessing the associations between heat shock proteins (HSPs) expression and (A) Tumor size, (B) Vascular invasion, (C) Tumor number, (D) alpha-fetoprotein (AFP), (E) Lymphatic metastasis, and (F) Portal vein tumor thrombus (PVTT).
Supplementary Figure 3Publication bias for included studies. (A) Gender, (B) HSPs expression, (C) Tumor differentiation, (D) HBsAg, (E) TNM stage, (F) Vascular invasion, and (G) Overall survival (OS).
Abbreviations
HSPs, heat shock proteins; HSP27, heat shock protein 27; HSP70, heat shock protein 70; HSP90, heat shock protein 90; HCC, hepatocellular carcinoma; OR, odds ratio; HR, hazard ratio; HBsAg, hepatitis B virus surface antigen; AFP, alpha-fetoprotein; TNM stage, tumor, node and metastasis stage; NOS, Newcastle-Ottawa Scale.
References
1
Siegel RL Miller KD Fuchs HE Jemal A . Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3
Chen W Zheng R Baade PD Zhang S Zeng H Bray F et al . Cancer statistics in china, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
4
Cauchy F Soubrane O Belghiti J . Liver resection for HCC: patient's selection and controversial scenarios. Best Pract Res Clin Gastroenterol (2014) 28:881–96. doi: 10.1016/j.bpg.2014.08.013
5
Wang H Lu Z Zhao X . Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol (2019) 12:133. doi: 10.1186/s13045-019-0806-6
6
Sun VC Sarna L . Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs (2008) 12:759–66. doi: 10.1188/08.CJON.759-766
7
El-Serag HB Rudolph KL . Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology (2007) 132:2557–76. doi: 10.1053/j.gastro.2007.04.061
8
El-Serag HB . Hepatocellular carcinoma. N Engl J Med (2011) 365:1118–27. doi: 10.1056/NEJMra1001683
9
Mazzanti R Gramantieri L Bolondi L . Hepatocellular carcinoma: epidemiology and clinical aspects. Mol Aspects Med (2008) 29:130–43. doi: 10.1016/j.mam.2007.09.008
10
Siegel RL Miller KD Jemal A . Cancer statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
11
Zhu XD Tang ZY Sun HC . Targeting angiogenesis for liver cancer: Past, present, and future. Genes Dis (2020) 7:328–35. doi: 10.1016/j.gendis.2020.03.010
12
Kampinga HH Hageman J Vos MJ Kubota H Tanguay RM Bruford EA et al . Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones (2009) 14:105–11. doi: 10.1007/s12192-008-0068-7
13
Yun CW Kim HJ Lim JH Lee SH . Heat shock proteins: agents of cancer development and therapeutic targets in anti-cancer therapy. Cells (2019) 9:60. doi: 10.3390/cells9010060
14
Shevtsov M Balogi Z Khachatryan W Gao H Vígh L Multhoff G . Membrane-associated heat shock proteins in oncology: from basic research to new theranostic targets. Cells (2020) 9:1263. doi: 10.3390/cells9051263
15
Boliukh I Rombel-Bryzek A Żuk O Radecka B . The role of heat shock proteins in neoplastic processes and the research on their importance in the diagnosis and treatment of cancer. Contemp Oncol (Pozn) (2021) 25:73–9. doi: 10.5114/wo.2021.106006
16
Seclì L Fusella F Avalle L Brancaccio M . The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell Mol Life Sci (2021) 78:4069–83. doi: 10.1007/s00018-021-03764-3
17
Voll EA Ogden IM Pavese JM Huang X Xu L Jovanovic BD et al . Heat shock protein 27 regulates human prostate cancer cell motility and metastatic progression. Oncotarget (2014) 15:5:2648–63. doi: 10.18632/oncotarget.1917
18
Somensi N Brum PO de Miranda Ramos V Gasparotto J Zanotto-Filho A Rostirolla DC et al . Extracellular HSP70 activates ERK1/2, NF-kB and pro-inflammatory gene transcription through binding with RAGE in A549 human lung cancer cells. Cell Physiol Biochem (2017) 42:2507–22. doi: 10.1159/000480213
19
Kobayashi K Matsushima-Nishiwaki R Yamada N Migita S Hioki T Mizutani D et al . Heat shock protein 70 positively regulates transforming growth factor-α-induced hepatocellular carcinoma cell migration via the AKT signaling pathway. Heliyon (2020) 23:6:e05002. doi: 10.1016/j.heliyon.2020.e05002
20
Nahleh Z Tfayli A Najm A El Sayed A Nahle Z . Heat shock proteins in cancer: targeting the 'chaperones'. Future Med Chem (2012) 4:927–35. doi: 10.4155/fmc.12.50
21
Ciocca DR Calderwood SK . Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones (2005) 10:86–103. doi: 10.1379/CSC-99r.1
22
Calderwood SK Khaleque MA Sawyer DB Ciocca DR . Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci (2006) 31:164–72. doi: 10.1016/j.tibs.2006.01.006
23
Buttacavoli M Di Cara G D'Amico C Geraci F Pucci-Minafra I Feo S et al . Prognostic and functional significant of heat shock proteins (HSPs) in breast cancer unveiled by multi-omics approaches. Biol (Basel) (2021) 10:247. doi: 10.3390/biology10030247
24
Lu W Wang Y Gan M Duan Q . Prognosis and predictive value of heat-shock proteins expression in oral cancer: A PRISMA-compliant meta-analysis. Med (Baltimore) (2021) 100:e24274. doi: 10.1097/MD.0000000000024274
25
Saini J Sharma PK . Clinical, prognostic and therapeutic significance of heat shock proteins in cancer. Curr Drug Targets (2018) 19:1478–90. doi: 10.2174/1389450118666170823121248
26
Ji F Zhang Y Zhu ZB Guo Y Shen SL Cao QH et al . Low levels of glycoprotein 96 indicate a worse prognosis in early-stage hepatocellular carcinoma patients after hepatectomy. Hum Pathol (2019) 86:193–202. doi: 10.1016/j.humpath.2018.11.025
27
King KL Li AF Chau GY Chi CW Wu CW Huang CL et al . Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer (2000) 88:2464–70. doi: 10.1002/1097-0142(20000601)88:11<2464::AID-CNCR6>3.0.CO;2-W
28
Kang GH Lee BS Lee ES Kim SH Lee HY Kang DY . Prognostic significance of p53, mTOR, c-Met, IGF-1R, and HSP70 overexpression after the resection of hepatocellular carcinoma. Gut Liver (2014) 8:79–87. doi: 10.5009/gnl.2014.8.1.79
29
Kang Q Zou H Liu LX Zhao SL Zhang WH Zhang XW . Expression and clinical significance of HSPA9 in hepatocellular carcinoma. Chongqing Med (2017) 46:2343–46. doi: 10.3969/j.issn.1671-8348.2017.17.012
30
Hung CS Huang CY Lee CH Chen WY Huang MT Wei PL et al . IGFBP2 plays an important role in heat shock protein 27-mediated cancer progression and metastasis. Oncotarget (2017) 8:54978–92. doi: 10.18632/oncotarget.18989
31
Harimoto N Shimada M Aishima S Kitagawa D Itoh S Tsujita E et al . The role of heat shock protein 27 expression in hepatocellular carcinoma in Japan: special reference to the difference between hepatitis B and C. Liver Int (2004) 24:316–21. doi: 10.1111/j.1478-3231.2004.0927.x
32
Xu Q Tu J Dou C Zhang J Yang L Liu X et al . HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol Cancer (2017) 16:178. doi: 10.1186/s12943-017-0748-y
33
Zhang Y Tao X Jin G Jin H Wang N Hu F et al . A targetable molecular chaperone hsp27 confers aggressiveness in hepatocellular carcinoma. Theranostics (2016) 6:558–70. doi: 10.7150/thno.14693
34
Liu X Chen S Tu J Cai W Xu Q . HSP90 inhibits apoptosis and promotes growth by regulating HIF-1α abundance in hepatocellular carcinoma. Int J Mol Med (2016) 37:825–35. doi: 10.3892/ijmm.2016.2482
35
Eto D Hisaka T Horiuchi H Uchida S Ishikawa H Kawashima Y et al . Expression of HSP27 in hepatocellular carcinoma. Anticancer Res (2016) 36:3775–9.
36
Luk JM Lam CT Siu AF Lam BY Ng IO Hu MY et al . Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics (2006) 6:1049–57. doi: 10.1002/pmic.200500306
37
Hua TY Huang JF Zhang H Huang DF Wei Q . Expression of heat shock protein gp96, myeloid cell leukemia sequence-1 and prohibitin in liver cirrhosis and hepatocellular carcinoma. Word Chin J Dig (2008) 16:2661–5. doi: 10.11569/wcjd.v16.i23.2661
38
Guo W Chen Q Li SQ Yu YQ . Expression of HSP27 and VEGF in HCC and its clinical implication. Shandong Med J (2011) 51:13–4. doi: 10.3969/j.issn.1002-266X.2011.30.007
39
Liang DQ Lu XP Zhan LL Tang XH . Expression and their clinical significance of Cyr61, VEGF and HSP27 in hepatocellular carcinoma. Shandong Med J (2011) 51:19–21. doi: 10.3969/j.issn.1002-266X.2011.09.010
40
Joo M Chi JG Lee H . Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci (2005) 20:829–34. doi: 10.3346/jkms.2005.20.5.829
41
Shin E Ryu HS Kim SH Jung H Jang JJ Lee K . The clinicopathological significance of heat shock protein 70 and glutamine synthetase expression in hepatocellular carcinoma. J Hepatobiliary Pancreat Sci (2011) 18:544–50. doi: 10.1007/s00534-010-0367-0
42
Sun SP Wu SL Geng ZM Niu XJ Yang W . Expression of heat shock protein 90α in hepatocellular carcinoma and its significance. Chin J Bases Clinics Gen Surgery (2003) 10:243–5. doi: 10.3969/j.issn.1007-9424.2003.03.025
43
Dai FJ Feng MH Xie W Yu JL Duan L Wu HY et al . Expression of HSP27 in hepatocellular carcinoma (HCC) and its clinical implication. Chin J Clin Oncol (2009) 36:578–81. doi: 10.3969/j.issn.1000-8179.2009.10.011
44
Liu M Miao N Li JZ Wang ZQ Wang Z Li MY et al . Correlation of HSP70, P53 and Bmi-1 in hepatitis, cirrhosis and hepatocellular carcinoma. Int J Clin Exp Pathol (2016) 9:473–80.
45
Su K Liu Y Wang P He K Wang F Chi H et al . Heat-shock protein 90α is a potential prognostic and predictive biomarker in hepatocellular carcinoma: a large-scale and multicenter study. Hepatol Int (2022) 16:1208–19. doi: 10.1007/s12072-022-10391-y
46
Zhang B Chen RH Yang W Xu G . Application of serum HSP90α and PIVKA-II levels in prognosis of patients with hepatocellular carcinoma. J Prac Hepatol (2021) 24:548–51. doi: 10.3969/j.issn.1672-5069.2021.04.024
47
Chen Q Yao HH Li B . Influence of serum heat shock protein 90α on the prognosis of patients with hepatocellular carcinoma after transarterial chemoembolization. J Clin Hepatol (2022) 38:577–81. doi: 10.14218/JCTH.2021.00289
48
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
49
Tierney JF Stewart LA Ghersi D Burdett S Sydes MR . Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
50
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
51
Rich N Murphy C Yopp A Tiro J Marrero J Singal AG et al . Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther (2020) 52(4):701–9. doi: 10.1111/apt.15917
52
Piñero F Dirchwolf M Pessôa MG . Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells (2020) 9(6):1370. doi: 10.3390/cells9061370
53
Farinati F Marino D De Giorgio M Baldan A Cantarini M Cursaro C et al . Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol (2006) 101(3):524–32. doi: 10.1111/j.1572-0241.2006.00443.x
54
Giannini E Marenco S Borgonovo G Savarino V Farinati F Del Poggio P et al . Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identifed during surveillance in compensated cirrhosis. Hepatology (2012) 56(4):1371–9. doi: 10.1002/hep.25814
55
Agopian V Harlander-Locke M Markovic D Zarrinpar A Kaldas F Cheng E et al . Evaluation of patients with hepatocellular carcinomas that do not produce α-fetoprotein. JAMA Surg (2017) 152(1):55–64. doi: 10.1001/jamasurg.2016.3310
56
Ritossa F . A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia (1962) 18:571–3. doi: 10.1007/BF02172188
57
Milner CM Campbell RD . Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics (1990) 32:242–51. doi: 10.1007/BF00187095
58
Wu FH Yuan Y Li D Liao SJ Yan B Wei JJ et al . Extracellular HSPA1A promotes the growth of hepatocarcinoma by augmenting tumor cell proliferation and apoptosis-resistance. Cancer Lett (2012) 317:157–64. doi: 10.1016/j.canlet.2011.11.020
59
Zhang T Yang X Xu W Wang J Wu D Hong Z et al . Heat shock protein 90 promotes RNA helicase DDX5 accumulation and exacerbates hepatocellular carcinoma by inhibiting autophagy. Cancer Biol Med (2021) 18(3):693–704. doi: 10.20892/j.issn.2095-3941.2020.0262
60
Nouri-Vaskeh M Alizadeh L Hajiasgharzadeh K Mokhtarzadeh A Halimi M Baradaran B . The role of HSP90 molecular chaperones in hepatocellular carcinoma. J Cell Physiol (2020) 235(12):9110–20. doi: 10.1002/jcp.29776
61
Sahu D Zhao Z Tsen F Cheng CF Park R Situ AJ et al . A potentially common peptide target in secreted heat shock protein-90α for hypoxia-inducible factor-1α-positive tumors. Mol Biol Cell (2012) 23:602–13. doi: 10.1091/mbc.e11-06-0575
62
Liang C Xu Y Ge H Li G Wu J . The clinicopathological and prognostic value of HSP27 in hepatocellular carcinoma: a systematic review and meta-analysis. Onco Targets Ther (2018) 11:1293–303. doi: 10.2147/OTT.S154227
63
Ge H Yan Y Guo L Tian F Wu D . Prognostic role of HSPs in human gastrointestinal cancer: a systematic review and meta-analysis. Onco Targets Ther (2018) 11:351–9. doi: 10.2147/OTT.S155816
64
Fu Y Xu X Huang D Cui D Liu L Liu J et al . Plasma heat shock protein 90alpha as a biomarker for the diagnosis of liver cancer: an official, large-scale, and multicenter clinical trial. EBioMedicine (2017) 24:56–63. doi: 10.1016/j.ebiom.2017.09.007
Summary
Keywords
heat shock proteins, hepatocellular carcinoma, prognosis, clinicopathological significance, meta-analysis
Citation
Xiang D, Jiang M, Chen Y, Liu C and Li L (2023) Clinicopathological and prognostic significance of heat shock proteins in hepatocellular carcinoma: a systematic review and meta-analysis. Front. Oncol. 13:1169979. doi: 10.3389/fonc.2023.1169979
Received
11 April 2023
Accepted
17 July 2023
Published
04 August 2023
Volume
13 - 2023
Edited by
Fabio Melandro, Pisana University Hospital, Italy
Reviewed by
Duilio Pagano, Mediterranean Institute for Transplantation and Highly Specialized Therapies (ISMETT), Italy; Irene Scalera, University of Bari Medical School, Italy
Updates
Copyright
© 2023 Xiang, Jiang, Chen, Liu and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leilei Li, lileilei@wchscu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.