
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 20 April 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1169876
 Yun Shu1*
Yun Shu1* Zhouyu Wang2,3
Zhouyu Wang2,3 Hongjuan Shang1
Hongjuan Shang1 Wei Le1
Wei Le1 Yan Lei2,3
Yan Lei2,3 Longzhang Huang1
Longzhang Huang1 Liming Tao1
Liming Tao1 Jun Chen1
Jun Chen1 Jing Li2,3*
Jing Li2,3*C-ros oncogene 1 (ROS1) fusion is a pathogenic driver gene in non-small cell lung cancer (NSCLC). Currently, clinical guidelines from the National Comprehensive Cancer Network (NCCN) have recommended molecular pathologic tests for patients with NSCLC, including the detection of the ROS1 gene. Crizotinib is a small molecule tyrosine kinase inhibitor of anaplastic lymphoma kinase (ALK), ROS1, and mesenchymal-epithelial transition (MET). In recent years, the efficacy of crizotinib in NSCLC patients with ROS1 fusion has been reported. Here, a 77-year-old woman was diagnosed with stage IVA lung adenocarcinoma harboring a novel low-density lipoprotein receptor (LDLR)-ROS1 fusion variant. This novel LDLR-ROS1 fusion was identified by targeted DNA next-generation sequencing (NGS) panel and then verified by RNA fusion panel based on amplicon sequencing. This patient benefited from subsequent crizotinib therapy and achieved progression-free survival of 15 months without significant toxic symptoms. Our case report recommended a promising targeted therapeutic option for patients with metastatic NSCLC with LDLR-ROS1 fusion and highlighted the importance of genetic testing for accurate treatment.
Lung cancer is one of the most common malignancies, both in terms of morbidity and mortality worldwide, among which non-small cell lung cancer (NSCLC) accounts for most cases (1, 2). Most patients with NSCLC have poor prognosis. Advances in molecular pathology and targeted therapy have changed the prognosis of NSCLC (3). Until now, driver genes such as EGFR, ALK, ROS1, MET, and RET have led to a variety of changes in NSCLC.
C-ros oncogene 1 (ROS1) gene is located at 6q22, on the long arm of chromosome 6, encoding a receptor tyrosine kinase in the subclass of the insulin receptor family (4, 5). Since 2007, researchers have revealed that multiple partner genes fuse with the 3′ ROS1 fragment containing the intact tyrosine kinase domain. The expression of these genes results in autophosphorylation of the ROS1 tyrosine kinase, which activates and triggers survival signaling pathways, driving malignant cell proliferation (6). Ultimately, ROS1 fusion has been identified as a pathogenic driver gene in NSCLC, with an incidence of 1%–2% (7). Patients with ROS1 fusion have distinct clinical features such as younger age, no or slight smoking history, and histology of adenocarcinoma (7, 8). Many unknown fusions and other oncogenic mutations can be detected by next-generation sequencing (NGS), and new ROS1 fusion partner genes have been identified in lung cancer (9). Interestingly, the tyrosine kinase domain of ROS1 is structurally similar to anaplastic lymphoma kinase (ALK), another molecular driver of NSCLC (10). Considering the structural similarity, some ALK tyrosine kinase inhibitors, such as crizotinib, are also active against ROS1 (11). Crizotinib and entrectinib have been approved to treat ROS1 fusion-positive NSCLC (12). Crizotinib has received approval in 70 countries worldwide for the treatment of ROS1-positive patients with advanced NSCLC. CD74 is the most common ROS1 fusion partner (13). In addition, several novel fusion partners have been discovered in NSCLC, such as FIG, SLC34A2, and SDC4 (14). However, the duration of response may vary among patients with different clinical and genetic characteristics.
In this case, we reported a novel LDLR-ROS1 fusion variant that was identified in a patient diagnosed with advanced lung adenocarcinoma. The patient received crizotinib treatment and exhibited an evident response.
A 77-year-old woman with no smoking history presented with cough, chest tightness, and other symptoms since May 2021. The patient visited the Lushan People’s Hospital on August 15, 2021. A plain chest computed tomography (CT) scan revealed a mass, pleural nodule, and pleural effusion in the left lung. Lung adenocarcinoma was confirmed by pleural effusion cytology after closed thoracic drainage (Figure 1A). Subsequently, targeted-NGS sequencing based on pleural effusion was performed, but no gene mutations associated with targeted therapy were detected. After cis-platinum pleural perfusion, she was transferred to the Third People’s Hospital of Jiujiang on August 30, 2021.
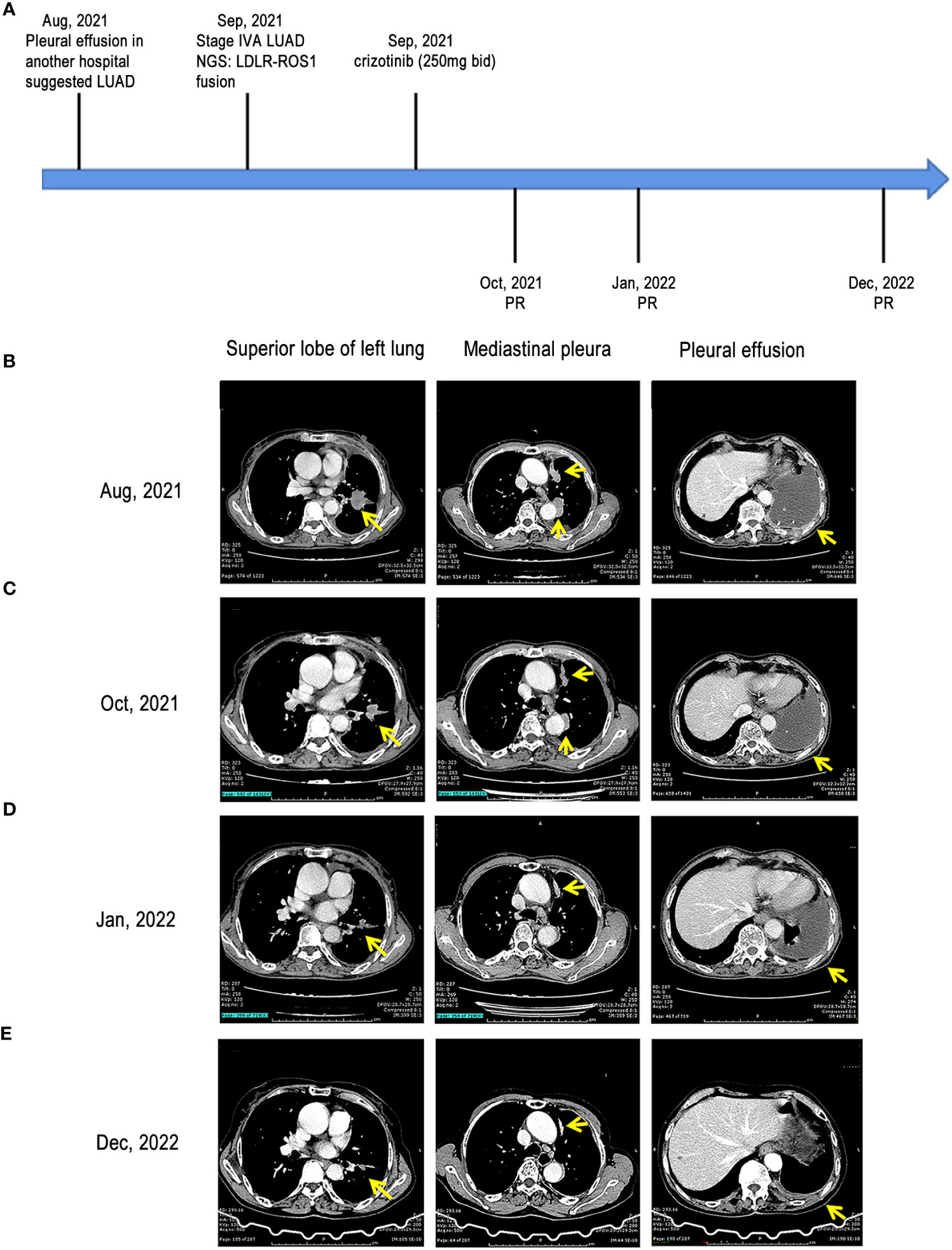
Figure 1 Treatment milestones of our case. (A) treatment timeline of the case; (B) CT scans of the left upper lobe mass, mediastinal pleural nodule, and pleural effusion at baseline; (C) CT scans of the left upper lobe mass, mediastinal pleural nodule, and pleural effusion at the first follow-up after treatment; (D) CT scans of the left upper lobe mass, mediastinal pleural nodule, and pleural effusion at the second follow-up after treatment; (E) CT scans of the left upper lobe mass, mediastinal pleural nodule, and pleural effusion at the third follow-up after treatment LUAD, lung adenocarcinoma; CT, computed tomography; LDLR, low-density lipoprotein receptor; ROS1, ROS proto-oncogene 1; PR, partial response.
After admission, the patient underwent further examinations as follows: 1) the specific tumor marker carcinoembryonic antigen (CEA) level was normal (3.52 µg/L); 2) an enhanced CT scan further revealed a mass in the left upper lobe of the lung and left pleural thickening with nodular formation (Figure 1B); 3) magnetic resonance imaging (MRI) of the head revealed no obvious abnormality; 4) single photon emission CT/CT (SPECT/CT) scan exhibited no bone metastasis. Subsequently, left pleural metastatic adenocarcinoma was diagnosed by biopsy pathology (CT2N2M1a, IVA). A few days later, the formalin-fixed paraffin-embedded (FFPE) tissue was examined using the targeted DNA NGS panel (Berry Oncology Corporation, Beijing, China) and a rare low-density lipoprotein receptor (LDLR)-ROS1 fusion variant was identified. Besides, an RNA fusion panel based on amplicon sequencing (Berry Oncology Corporation, Beijing, China) was also used to verify this fusion variant from total RNA which isolated from FFPE tissues. Both the DNA and RNA-based sequencing technology revealed LDLR exon 2-ROS1 exon 34 rearrangement in the tissue (Figure 2). This LDLR-ROS1 fusion retained the kinase domain of ROS1, which could cause constitutive kinase activity and oncogenic transformation.
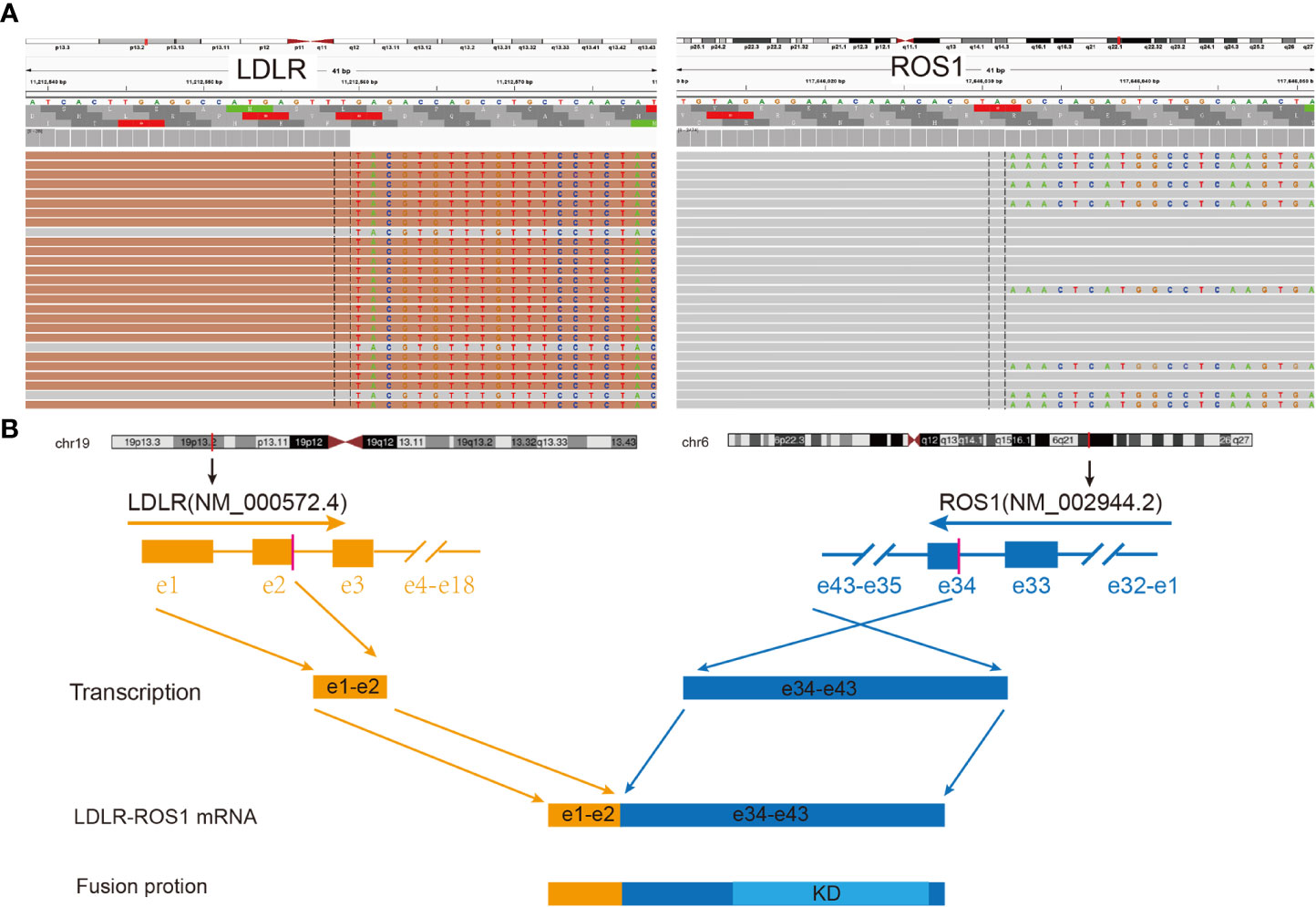
Figure 2 Identification and verification of LDLR-ROS1 fusion in this case. (A) RNA sequencing reads indicating visualization of the LDLR and ROS1 fusion regions using the Integrative Genomics Viewer (IGV) software. The fusion breakpoints are localized at chr19: p13.2: 11,212,559 and chr6: q22.1: 117,646,031, respectively; (B) Schematic of genomic rearrangement involving the fusion breakpoints at mRNA and protein levels; the transcript resulted in exons 1–2 of LDLR fused to exons 34–43 of ROS1, including kinase domain. LDLR, low-density lipoprotein receptor; ROS1, ROS proto-oncogene 1; Orange color represents LDLR; Blue color represents ROS1; KD, kinase domain; e, exon.
Based on these genetic testing results, the patient was orally administered 250 mg of crizotinib twice a day (250 mg bid) since September 10, 2021. After a few days, the patient noticed an improvement in symptoms. Subsequently, she was discharged from the hospital and continued taking medication. One month later (October 2021), the patient visited the hospital for further review. The objective response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) (15). Chest CT scan revealed that left lobe mass and mediastinal pleural nodules reduced, and pleural effusion was absorbed, indicating that this patient achieved partial response (PR) (Figure 1C). In addition, the patient presented no discomforting symptoms such as cough and chest distress. In January 2022, the chest CT scan demonstrated that the lesion shrank further, pleural effusion continued to decrease, and PR was noted (Figure 1D). Another chest CT scan conducted in December 2022 demonstrated that the lesion shrank further, pleural effusion elementally disappeared, and the treatment showed PR (Figure 1E).
In this case, we reported an infrequent LDLR-ROS1 fusion in the patient, who was an elderly woman of Asian origin without a smoking history, diagnosed with lung adenocarcinoma. The pathogenic driver gene was ROS1, and the fusion included exons 34–43 of ROS1 that retained the complete kinase domain. Once activated, ROS1 signaling promotes malignant cell growth through a series of downstream pathways, ultimately leading to cancer formation (16).
Initially, crizotinib, a MET/ALK multi-targeted receptor tyrosine kinase inhibitor, was approved for the treatment of ALK-rearranged NSCLC (17). The ATP-binding sites of kinase domains share 77% amino acid identity between the ALK and ROS1 genes (10). Furthermore, crizotinib has a high affinity for ROS1, which further effectively inhibits ROS1 signaling and cell viability in cell lines expressing ROS1 fusions (18).
The fusion partner (LDLR gene) located on chromosome 19 (19p13), consisting of 18 exons, plays a pivotal role in cholesterol homeostasis and lipid metabolism under normal physiological conditions (19, 20). Targeted-NGS sequencing based on pleural effusion at Lushan People’s Hospital did not detect an ROS-1 fusion, while targeted-DNA panel and RNA fusion panel based on needle biopsy samples at our hospital detected an ROS-1 fusion. The sensitivity of NGS based on pleural effusion for detecting actionable mutations was found to be lower than that based on tumor tissues (21, 22). In this case, the fusion included exons 1–2 of the LDLR gene and exons 34–43 of the ROS1 gene. This fusion includes kinase domains that should theoretically be sensitive to targeted therapy. It remains unclear whether patients with advanced NSCLC and LDLR-ROS1 fusion can benefit from the treatment of crizotinib. In our case, crizotinib was selected as the treatment for the patient diagnosed with advanced NSCLC with ROS1 fusion. One month later, evident changes were observed on imaging, confirming surprising therapeutic effects, and the clinical response to crizotinib continued for at least 15 months during follow-up.
In another case report, adjuvant crizotinib provided a favorable survival benefit in a patient with resectable stage IIIA NSCLC with the LDLR-ROS1 fusion (23). In this case, postoperative targeted therapy exhibited promising efficacy, and the patient’s clinical and radiological follow-ups revealed no evidence of progression or recurrence, with a relapse-free survival of more than 29 months. Our case demonstrated the use of crizotinib as the treatment in a patient with metastatic NSCLC with fusion, and the patient experienced progression-free survival of 15 months.
Our study provided clinical evidence that advanced NSCLC patients harboring LDLR-ROS1 fusion may have durable responses to crizotinib. Crizotinib may be an effective treatment option for patients with advanced NSCLC with LDLR-ROS1 fusion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethical Committee of Medical Research, Third People’s Hospital of Jiujiang City. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HS and WL contributed to patient management. YS and JL designed and reviewed the report. YS wrote the manuscript. ZW, LT, and JC reviewed and corrected the manuscript. LH, ZW and YL drew the pictures. All authors contributed to the article and approved the submitted version.
The authors thank Berry Oncology Corporation for the sequencing and analysis of tumor samples.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health (2019) 85(1). doi: 10.5334/aogh.2419
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. König D, Savic Prince S, Rothschild SI. Targeted therapy in advanced and metastatic non-small cell lung cancer. an update on treatment of the most important actionable oncogenic driver alterations. Cancers (Basel) (2021) 13(4). doi: 10.3390/cancers13040804
4. Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta (2009) 1795(1):37–52. doi: 10.1016/j.bbcan.2008.07.006
5. Satoh H, Yoshida MC, Matsushime H, Shibuya M, Sasaki M. Regional localization of the human c-ros-1 on 6q22 and flt on 13q12. Jpn J Cancer Res (1987) 78(8):772–5.
6. Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell (2007) 131(6):1190–203. doi: 10.1016/j.cell.2007.11.025
7. Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol (2012) 30(8):863–70. doi: 10.1200/JCO.2011.35.6345
8. Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med (2012) 18(3):378–81. doi: 10.1038/nm.2658
9. Shu Y, Li H, Shang H, Chen J, Su X, Le W, et al. Identification of a novel MPRIP-ROS1 fusion and clinical efficacy of crizotinib in an advanced lung adenocarcinoma patient: a case report. Onco Targets Ther (2020) 13:10387–91. doi: 10.2147/OTT.S270961
10. Huber KV, Salah E, Radic B, Gridling M, Elkins JM, Stukalov A, et al. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature (2014) 508(7495):222–7. doi: 10.1038/nature13194
11. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med (2014) 371(21):1963–71. doi: 10.1056/NEJMoa1406766
12. Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol (2019) 30(7):1121–6. doi: 10.1093/annonc/mdz131
13. Drilon A, Jenkins C, Iyer S, Schoenfeld A, Keddy C, Davare MA. ROS1-dependent cancers - biology, diagnostics and therapeutics. Nat Rev Clin Oncol (2021) 18(1):35–55. doi: 10.1038/s41571-020-0408-9
14. Bubendorf L, Büttner R, Al-Dayel F, Dietel M, Elmberger G, Kerr K, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch (2016) 469(5):489–503. doi: 10.1007/s00428-016-2000-3
15. Li Y, Duan P, Guan Y, Chen Q, Grenda A, Christopoulos P, et al. High efficacy of alectinib in a patient with advanced lung adenocarcinoma with 2 rare ALK fusion sites: a case report. Transl Lung Cancer Res (2022) 11(1):100–10. doi: 10.21037/tlcr-21-1039
16. Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol (2017) 12(11):1611–25. doi: 10.1016/j.jtho.2017.08.002
17. Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther (2011) 5:471–85. doi: 10.2147/DDDT.S19045
18. Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol (2012) 7(7):1086–90. doi: 10.1097/JTO.0b013e3182570919
19. Usifo E, Leigh SE, Whittall RA, Lench N, Taylor A, Yeats C, et al. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet (2012) 76(5):387–401. doi: 10.1111/j.1469-1809.2012.00724.x
20. Go GW, Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med (2012) 85(1):19–28.
21. Jin S, Zhou C, Hou X, Fan Z, Zhao J, Ai X, et al. A multicenter real-world study of tumor-derived DNA from pleural effusion supernatant in genomic profiling of advanced lung cancer. Transl Lung Cancer Res (2020) 9(4):1507–15. doi: 10.21037/tlcr-20-882
22. Tu HY, Li YS, Bai XY, Sun YL, Zheng MY, Ke EE, et al. Genetic profiling of cell-free DNA from pleural effusion in advanced lung cancer as a surrogate for tumor tissue and revealed additional clinical actionable targets. Clin Lung Cancer (2022) 23(2):135–42. doi: 10.1016/j.cllc.2021.09.002
Keywords: advanced non-small cell lung cancer, c-ros oncogene 1, LDLR-ROS1 fusion, crizotinib, next-generation sequencing
Citation: Shu Y, Wang Z, Shang H, Le W, Lei Y, Huang L, Tao L, Chen J and Li J (2023) Case Report: Response to crizotinib treatment in a patient with advanced non-small cell lung cancer with LDLR-ROS1 fusion. Front. Oncol. 13:1169876. doi: 10.3389/fonc.2023.1169876
Received: 20 February 2023; Accepted: 04 April 2023;
Published: 20 April 2023.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Stefania Canova, San Gerardo Hospital, ItalyCopyright © 2023 Shu, Wang, Shang, Le, Lei, Huang, Tao, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Shu, c2h1eXVuMTk3MkAxNjMuY29t; Jing Li, NDU2MDgzNjNAcXEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.