
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 15 May 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1169616
This article is part of the Research Topic Advances in the Surgical Management of Gastric and Colorectal Cancers View all 35 articles
Background: Rectal cancer has a high risk of recurrence and metastasis, with median survival ranging from 24 months to 36 months. K-RAS mutation is a predictor of poor prognosis in rectal cancer. Advanced rectal cancer can be stopped in its tracks by pelvic exenteration.
Case summary: A 51-year-old woman was diagnosed with advanced rectal cancer (pT4bN2aM1b, stage IV) with the KRAS G12D mutation due to a change in bowel habits. The patient had experienced repeated recurrences of rectal cancer after initial radical resection, and the tumor had invaded the ovaries, sacrum, bladder, vagina and anus. Since the onset of the disease, the patient had undergone a total of seven surgeries and long-term FOLFIRI- or XELOX-based chemotherapy regimens, with the targeted agents bevacizumab and regorafenib. Fortunately, the patient was able to achieve intraoperative R0 resection in almost all surgical procedures and achieve tumor-free survival after pelvic exenteration. The patient has been alive for 86 months since her diagnosis.
Conclusions: Patients with advanced rectal cancer can achieve long-term survival through active multidisciplinary management and R0 surgery.
According to the latest global cancer statistics, colorectal cancer (CRC) is the third most common type of cancer worldwide and the second leading cause of cancer-related deaths (1, 2). The number of CRC cases has been rising steadily in recent years, and there is a trend towards an earlier age of onset (3, 4). Patients with early CRC lack typical symptoms. Most CRCs are at an advanced stage when patients present with changes in bowel habits, blood in the stool, anemia or abdominal pain (5). In addition, 20% of CRC patients already have metastases at the time of diagnosis, with the liver, lung, peritoneum, and local lymph nodes being the most common metastatic sites (6, 7). The prognosis of metastatic CRC is poor, with a 5-year survival rate of only 12% (8). As newly developed chemotherapeutic agents and targeted drugs continue to be put into clinical use, coupled with the continued maturation of multidisciplinary oncology treatment, new hope has been brought to extend the overall survival(OS) of patients with advanced metastatic CRC (2). Rectal cancer accounts for approximately 30% of all CRCs and is one of the most common cancers of the gastrointestinal tract (9). Herein, we introduce a female patient with advanced rectal cancer who experienced multiple tumor recurrences, metastases, and underwent seven surgeries, and has survived for up to 86 months as of today.
In October 2015, a 51-year-old lady was admitted to Zhujiang Hospital because of a change in her bowel habits that had been going on for more than six months. Six months ago, she noticed an increase in the frequency of her bowel movements, which were typically thin and unformed stools with two to three drops of blood after the stool, and she also had a feeling of anal drop before defecation, tenesmus and endless defecation. Thirteen years ago, she underwent a total hysterectomy for uterine fibroids. The patient had a history of diabetes mellitus and was advised to control her diet without taking medications. There were no other comorbidities. There was no history of alcohol or tobacco abuse and no family history of hereditary disorders. On examination, the patient was found to have tenderness pain in the lower abdomen, and rectal palpation revealed a mass of approximately 3×3 cm on the right posterior wall of the rectum 7 cm from the anus. There were no abnormal findings on physical examination of other systems.
A recent colonoscopy performed on the patient at the local hospital revealed a cauliflower-like mass located 7 cm from the anus, blocking the bowel lumen and restricting access to the colonoscope. Pathological analysis of the specimen indicated rectosigmoid junction cancer. The patient had elevated tumor markers, with blood levels of 85.2 μg/L for CEA and 82.1 kU/L for CA199. Body CT showed significant local bowel luminal narrowing, a soft tissue mass shadow with uneven density and enhancement, and significant thickening of the bowel wall at the junction of the rectum and sigmoid colon. Many enlarged lymph nodes were seen around this section of the bowel. The results of the abdominal CT and chest x-ray ruled out liver and lung metastases. According to the patient’s condition and her wishes, we performed a laparoscopic radical surgery for rectal cancer. Intraoperative exploration revealed a 5cm × 4 cm mass in the right wall of the rectum, which penetrated the plasma membrane layer and was strongly adherent to the right peritoneum and ovary. Two white metastatic cancer nodules could be seen at the top of the vagina. The right ovary and metastatic cancer nodules were also removed simultaneously with the consent of the family and with the assistance of the gynecology department. The postoperative pathology revealed a moderately differentiated adenocarcinoma of the rectum with a mucinous component, infiltrating the entire intestinal wall, invading the vasculature and nerves, with metastatic cancerous tissue visible in the mesenteric lymph nodes (4/14) and metastatic tissue from the right ovary. The patient’s final diagnosis was a moderately differentiated adenocarcinoma of the rectum (pT4bN2aM1b stage IV). (Figure 1) Immunohistochemistry showed CK (+), CDX-2 (+) and approximately 30% P53 in cancer cells. The patient had a microsatellite stable tumor microenvironment (TME) (MSS: MLH1 (+), MSH2 (+), PMS2 (+)) and Ki-67 approximately 40% (+). Genetic testing suggested mutations at the G12D locus of the KRAS gene.

Figure 1 Preoperative CT and postoperative pathology for first radical rectal cancer: CT scan showed obvious thickening of the intestinal wall at the junction of the rectum and sigmoid colon, and soft tissue mass shadow with uneven density (A). CT enhanced scan showed uneven enhancement of soft tissue masses at the rectosigmoid junction and local intestinal cavity was obviously narrow (B). Part of intestinal duct (rectal mass), 18 cm long, 6-8 cm in circumference, 6cm away from the cut edge at one end, and a cauliflower-like mass 8 cm from the cut edge at the other end, 7cm x 5cm x 2 cm in size (C). The cancer tissue of rectal mass was arranged in irregular glandular tubular and sieve shape, infiltrating the whole intestinal wall and breaking through the serous layer. The cancer cells were obviously heteromorphic, with large nuclei, deep staining and frequent mitotic images (D).
The first and second surgeries: In October 2015, the patient underwent laparoscopic radical surgery for rectal cancer and right ovariectomy. This was followed by six rounds of the XELOX (oxaliplatin and capecitabine) chemotherapy. However, 22 months after the surgery, the patient underwent a pelvic CT and plain and enhanced MRI at follow-up, which showed that the metastases had progressed and that there was a significantly larger mass anterior to the left external iliac artery than previously seen. In August 2017, laparoscopic exploration revealed a 3cm × 4cm irregular mass in front of the left iliac vessels, completely encapsulating the left ovary below, so the left ovary and metastases were removed. And six cycles of FOLFIRI (irinotecan, 5-FU, and calcium folinic acid) chemotherapy were started after the procedure.
The third surgery: At review in November 2019, imaging showed an occupying lesion on the right side of the anastomosis, poorly demarcated from the right wall of the vaginal stump, invading the surrounding tethered fascia and the right levator ani muscle, and tumor recurrence was considered. Once the diagnosis was clear, the patient was localized for radiotherapy and treated with local radiotherapy and a second-line chemotherapy regimen: FOLFIRI and bevacizumab. Due to 2 or 3 loose stools per day with painful bowel movements, the patient returned to hospital in March 2021. Since non-surgical treatment could not remove the lesion and prolong life, we performed a laparoscopic partial rectal resection and sigmoid colostomy for her.
The fourth surgery: In June 2021, a review revealed that the occupying lesion on the rectal stump continued to progress and increase in size, and tumor recurrence was considered. After multidisciplinary discussion, the final decision was made to perform abdominal radiofrequency ablation and particle implantation in collaboration with the interventional and radiology departments. The multidisciplinary consultation agreed that the patient’s lesion had progressed after previous first- and second-line treatment, and that she should be switched to third-line therapy with regorafenib, while TAC102 could be considered.
The fifth to seventh surgeries: At the follow-up in July 2022, a rectal stump mass was again observed, together with an extended invasive lesion on the right side of rectal stump. Considering that almost all medical drugs had failed to control the disease, and radiotherapy and particle implantation had failed to control the tumor progression, we proposed pelvic exenteration to the patient after discussion with the multidisciplinary team. We actively sought the patient’s opinion and her main concern was to remove the tumor and reduce the threat to her life. She was willing to undergo aggressive surgical treatment and accepted our proposal. Therefore, we performed pelvic exenteration (transsacral resection of rectum, anus, bladder, vagina and sacrum) for the patient. Due to the large size of the surgical wound which was difficult to heal on its own, we performed a free myocutaneous flap graft and skin and subcutaneous necrotic tissue debridement on the lower back in September 2022. (Figures 2, 3).
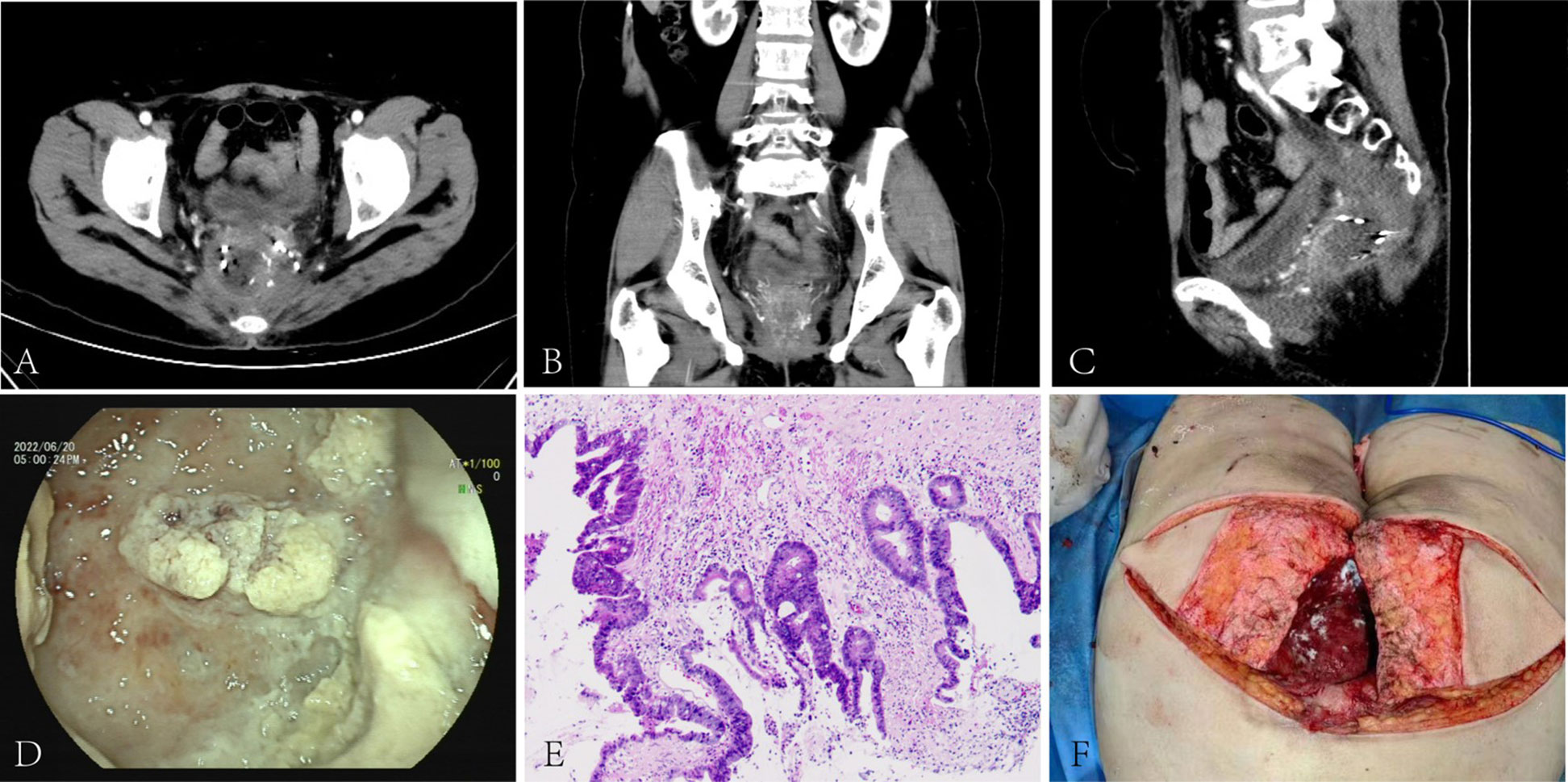
Figure 2 Pre-pelvic exenteration colonoscopy, CT, post-operative pathology and reconstruction: CT scan showed a mass-like slightly hypodense shadow next to the right side of the rectal stump. The lesion was poorly demarcated from the right vaginal wall and presacral soft tissue and invaded the surrounding tethered fascia and right levator ani muscle. The rectal cavity was compressed and deformed(A-C). The blind end was observed at 8cm away from the anus through the natural anus, and the intestinal mucosa was covered with white contents(D). The cancer tissue invaded the entire bowel wall, the entire vagina, involving the surrounding striated muscle tissue and adipose tissue, the local prolusion of the bladder mucosa, and localized necrosis of the serous layer and the muscular layer(E). After pelvic exenteration, the patient underwent free myocutaneous flap graft (F).
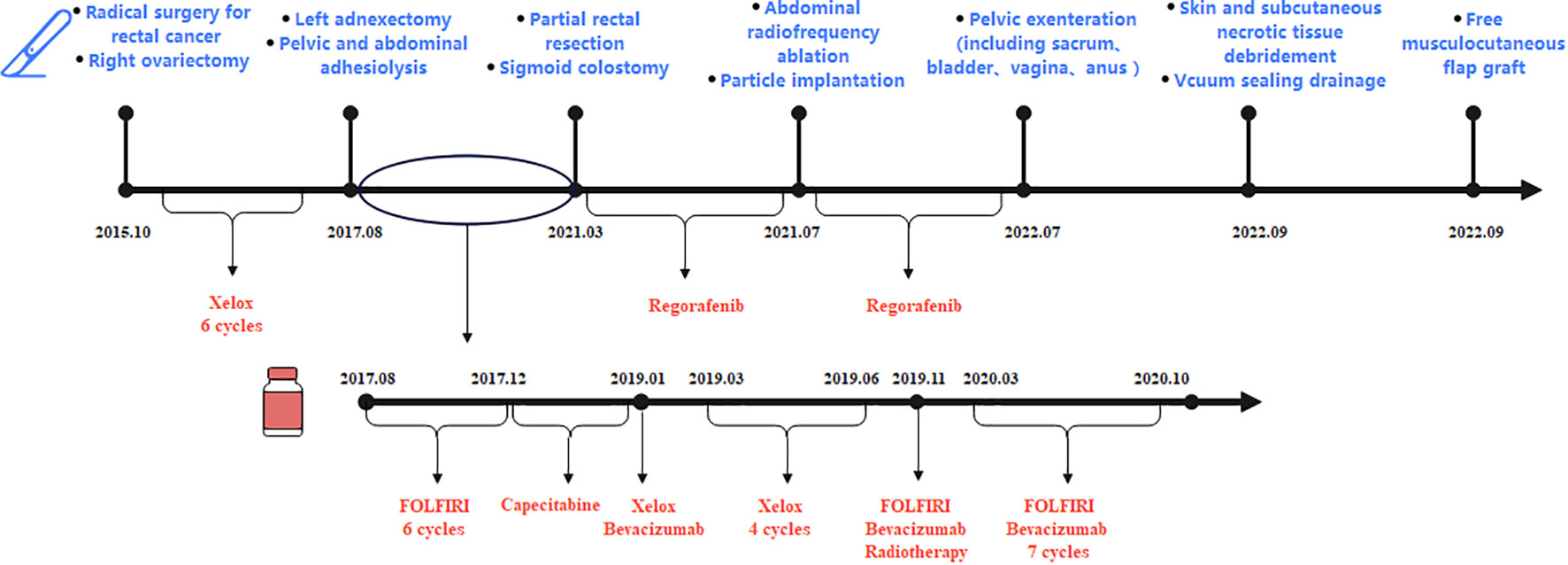
Figure 3 The patient had undergone seven surgeries (radical rectal cancer surgery and right ovariectomy in October 2015, left ovariectomy in August 2017, partial rectal resection in March 2021, radiofrequency ablation and particle implantation in July 2021, pelvic exenteration in July 2022, and two reconstructive surgeries in September 2022). During treatment, the patient had been taking chemotherapy drugs, including first-line chemotherapy drugs: XELOX (oxaliplatin and capecitabine), second-line chemotherapy drugs: FOLFIRI (irinotecan, leucovorin, and fluorouracil) and bevacizumab, and third-line therapy drugs: regorafenib.
By 2023-1, the patient had been diagnosed with advanced rectal cancer for more than 7 years, had undergone 7 surgeries, and had survived up to 86 months. The patient’s tumor markers were not elevated during regular post-operative follow-ups after pelvic exenteration. The patient’s psychological burden was greatly reduced when we informed her that the tumor was most likely to be completely removed. Although she lived with a fistula bag after surgery, she was able to live independently with little restriction in general physical activity at our follow-up. We carried out the FACT-C (Functional Assessment of Cancer Therapy-Colorectal) test for the patient and her quality of life was quite satisfactory.
The patient was still quite young when she was diagnosed with rectal cancer at the age of 51. In recent years, early-onset colorectal cancer (EOCRC) has gained widespread attention due to an alarming increase in its morbidity. Recent data from European registry studies show that the incidence of CRC in patients aged 20-49 has risen sharply over the past 25 years (10, 11). It is estimated that by 2030, one in ten colon cancers and one in four rectal cancers will be diagnosed in people under the age of 50 (12). Although the high incidence of diseases in young people is often due to genetic factors, this does not explain the rapid increase in recent years, so potential factors may also include westernized diets, obesity, antibiotics, infections and changes in the gut microbiome (13). Several studies have shown that the clinical characteristics and biological behavior of EOCRC differ significantly from those of conventional CRC. More invasive pathological factors are found in EOCRC, including higher tumor grade, lymphovascular infiltration, perineural infiltration, and elevated serum CEA, and are associated with worse OS (14). Current guidelines in the United States recommend starting screening at the age of 50 for average-risk individuals, which may lead to delayed diagnosis in EOCRC (15). In a study of 1,514 patients with rectal cancer, the median time from symptom onset to treatment is 217 days for those younger than 50, compared with 29.5 days for those older than 50 (16). Delayed diagnosis may lead to the development of cancer to a more advanced stage with a poorer prognosis (17, 18). Therefore, it is still worth investigating whether the age of screening for CRC needs to be brought forward and when to start screening to get the most benefit.
Colorectal cancer is known to have a poor prognosis as the second leading cause of cancer-related death. Fewer than 15% of patients with stage IV CRC survive within 5 years of diagnosis (19), and the majority of patients with metastatic colorectal cancer currently have a survival of 24 to 36 months (2). This patient was diagnosed with stage IV rectal cancer at the time of detection, and several factors suggested that she would have a much shorter survival. Firstly, the patient had a K-RAS mutation. As the most common and first discovered mutation in colorectal cancer, KRAS mutations are seen in between 30% and 40% of cases and are often considered a poor prognostic indicator (20, 21). On one hand, KRAS mutant cancers are highly invasive (22). On the other hand, KRAS mutations have also been found to be an important predictor of lack of response to treatment with epidermal growth factor receptor monoclonal antibodies (23–25). In addition, studies have shown that KRAS G12D mutant tumors have a poorer response to chemotherapy and radiotherapy, and significantly shorter progression-free survival (PFS) and OS (26). The patient in our case report had the G12D mutation, which largely predicted her poor prognosis. Secondly, ovarian metastases were found during the treatment. Metastases from the gastrointestinal tract to the ovary are known as Krukenburg tumors, which are very rare metastatic malignancies of the ovary with an incidence of 0.16/100,000 (27). The prognosis for Krukenburg tumors is poor, with a median survival of 23 months for limited metastases and an average of 14 months for widespread metastases, regardless of the treatment method (28). Remarkably, the patient has lived for 86 months so far. Our analysis of her prolonged survival could be as follows:
First, despite numerous recurrences and involvement of nearby organs, the patient showed no evidence of liver or lung metastases. Metastases to secondary organs, such as the liver and lung, are thought to be a major contributor to colorectal cancer-related mortality (29). According to a review of 14 randomized clinical trials, median OS was 19.1 months for CRC patients with liver metastases and 24.6 months for those with lung metastases (30). The exemption of liver and lung is beneficial for patient survival to some extent.
Second, the only salvage procedure is pelvic exenteration (PE) when advanced rectal cancer has spread to many nearby organs and recurred frequently (31, 32). To achieve a tumor-free margin, PE is a procedure that completely eradicates all pelvic malignancies (33). Recent improvements in perioperative care, surgical technique, and multidisciplinary team approaches have reduced surgical mortality and increased access to therapy for rectal cancer whose recurrences and metastases are confined to the pelvis (34, 35). In this case, we removed the patient’s vagina, bladder, anus, rectum, and sacrum. The patient underwent two further reconstructive procedures, as research suggests that myocutaneous flap reconstruction and long-term pelvic/perineal drainage after PE may reduce infection rates and improve wound healing (36). Despite the risk associated with PE, most of these patients face a median OS of approximately 7 months if surgical resection is not attempted (37). Therefore, pelvic exenteration may be the only hope for long-term survival of rectal cancer patients without distant metastases (38).
Finally, the patient had many pelvic metastases and tumor recurrences that were surgically significant, and R0 resection was achieved in almost all surgical procedures. Margin status is the most important predictive factor for the outcome of pelvic exenteration for recurrent rectal cancer, according to a number of studies. The greatest survival benefit after PE is seen in patients with negative margins or R0 resection (39–42). The American Joint Committee on Cancer was the first organization to recognize the importance of the ‘R’ status of tumor resection in 1977 (43). Histopathological evaluation of R0 resection is considered to be a circumferential margin (CRM) of >1 mm.R1 resection is the presence of microscopic residual lesions defined as CRM ≤1 mm, while R2 resection is the presence of residual sarcoid lesions (39). R0 resection status remains the most important determinant of disease-free survival and OS in resectable rectal cancer. One study showed that the median survival was 43 months for patients with R0 resection, 21 months for patients with R1 resection, and 10 months for patients with R2 resection, and three-year survival rates were 56.4%, 29.6%, and 8.1% for R0, R1, and R2, respectively (39). In addition, multiple studies have suggested that R0 resection is associated with a good prognosis (39–41, 44). The patient we report achieved R0 resection at almost all surgeries of tumor recurrence or adjacent organ involvement and is still tumor free today.
There is currently no clinical consensus on the value of resection of distant metastases in the management of patients with advanced CRC. A number of studies have shown that surgical resection is the main treatment for patients with liver and lung metastases from CRC to achieve ‘no evidence of disease’ status and long-term survival (45–50). The Japanese guidelines for the treatment of CRC recommend surgical treatment of the metastatic lesion if the patient can tolerate it and both the primary colorectal and metastatic lesions are completely resectable (7). According to the results of the Bologna Multidisciplinary Rectal Cancer Group, people who underwent metastatic resection had a longer OS (51).. However, the main problem with resection of liver metastases is the high recurrence rate, which is as high as 75% within 2 years and is the main cause of postoperative mortality in patients (52). Other studies have shown that lung metastasectomy leads to a reduced quality of life and reduced health benefits for patients after surgery (53, 54). In addition, Riccardo Lemini et al. showed no difference in survival outcomes between patients who underwent metastasectomy and those who did not (55). Therefore, more studies are needed in the future to fully understand the role of metastasectomy in order to provide more effective treatment strategies for patients with advanced CRC who develop distant metastases.
The incidence of ovarian metastases from CRC ranges from 1.6% to 6.4% (56),with approximately 40-60% of patients with ovarian metastases presenting with bilateral involvement (57, 58). It has been shown that the ovary appears to be a ‘sanctuary’ for tumor cells, providing a favorable microenvironment for tumor growth, and that metastatic ovarian tumors do not respond well to chemotherapy. Therefore, surgery appears to be the only viable alternative (57, 59). In this case, we found involvement of the right ovary during the initial radical surgery for rectal cancer, and performed resection of the metastases at the same time, and then found metastases in the left ovary 2 years later and promptly resected them. This raises the controversial question of whether prophylactic removal of the other ovary is necessary in the case of metastases from one ovary. Bilateral ovaries are interconnected by branches of the fundic artery and CRC can develop bilateral ovarian metastases by a haematogenous route (60, 61). In addition, CRC may also increase the risk of ovarian metastases by spreading through the peritoneum (62). Utku Akgor et al. argue that: Microscopic metastases can occur even in normal-appearing ovaries, and bilateral salpingo-oophorectomy should be the standard of care for all patients with ovarian metastases from CRC (59, 63).
The standard treatment for colorectal cancer is surgical resection combined with adjuvant chemoradiotherapy (64). However, for the past 30 years, patients with KRAS mutations have been in a drug-free situation, because targeting KRAS-driven cancer therapy faces several major challenges: first, KRAS has a similar GTP/GDP-binding region to other RAS family members, making it difficult to achieve targeted therapy (65). Second, the high affinity of KRAS for GTP/GDP makes it difficult to find effective inhibitors (65). There are now new developments in targeting KRAS for the treatment of CRC. Several groups have reported the development of direct KRAS small molecule inhibitors that bind directly to KRAS and inhibit nucleotide exchange or block the interaction between RAS-GTP and SOS (66–69). In addition, RT11, a cytoplasmic penetrating antibody targeting RAS mutant tumors, has been shown in preclinical studies to overcome drug resistance when combined with cetuximab (70). In addition, immunotherapy has shown clinical promise in the treatment of gastrointestinal tumors and autologous T cell transplantation for the treatment of KRAS G12D CRC is expected to enter the clinical practice (71), which may lead us to bid farewell to the era of KRAS ‘incurable’.
Although colorectal cancer patients with KRAS mutations and multiple recurrences often have a poor prognosis, the patient in our reported case, who had advanced metastatic colorectal cancer with KRAS mutations, had an exceptionally long survival. This is due to a combination of factors, including vigorous medical and surgical treatment with intraoperative R0 resection, and the fact that the patient’s liver and lungs were free of tumor metastases. The patient achieves tumor-free survival with good quality of life after pelvic exenteration. Our diagnosis and treatment provide experience and hope for patients with multiple recurrences and local metastases of colorectal cancer.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhujiang Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
YO: literature research, manuscript preparation. YZ: literature research, manuscript preparation. HC: literature research, manuscript preparation. GL: literature research. XH: literature research. HL: manuscript final version approval. ZL: manuscript final version approval. SH: manuscript final version approval. All authors contributed to the article and approved the submitted version.
This work was supported by Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (Grant No. pdjh2021a0094).
The authors are very grateful to the Southern Medical University and the related colleagues for their encouragement and support for this study, as well as to the patient and her family for their support and help for the study, and to the reviewers for their pertinent comments and careful review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin (2022) 72(4):372–401. doi: 10.3322/caac.21728
3. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin (2022) 72(5):409–36. doi: 10.3322/caac.21731
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (2019) 394(10207):1467–80. doi: 10.1016/S0140-6736(19)32319-0
6. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (2014) 383(9927):1490–502. doi: 10.1016/S0140-6736(13)61649-9
7. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for cancer of the colon and rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol (2012) 17(1):1–29. doi: 10.1007/s10147-011-0315-2
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
9. Loftas P, Sturludottir M, Hallbook O, Almlov K, Arbman G, Blomqvist L. Assessment of remaining tumour involved lymph nodes with MRI in patients with complete luminal response after neoadjuvant treatment of rectal cancer. Br J Radiol (2018) 91(1087):20170938. doi: 10.1259/bjr.20170938
10. Chambers AC, Dixon SW, White P, Williams AC, Thomas MG, Messenger DE. Demographic trends in the incidence of young-onset colorectal cancer: a population-based study. Br J Surg (2020) 107(5):595–605. doi: 10.1002/bjs.11486
11. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut (2019) 68(10):1820–6. doi: 10.1136/gutjnl-2018-317592
12. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the united states, 1975-2010. JAMA Surg (2015) 150(1):17–22. doi: 10.1001/jamasurg.2014.1756
13. Collaborative R, Zaborowski AM, Abdile A, Adamina M, Aigner F, d'Allens L, et al. Characteristics of early-onset vs late-onset colorectal cancer: a review. JAMA Surg (2021) 156(9):865–74. doi: 10.1001/jamasurg.2021.2380
14. Gabriel E, Attwood K, Al-Sukhni E, Erwin D, Boland P, Nurkin S. Age-related rates of colorectal cancer and the factors associated with overall survival. J Gastrointest Oncol (2018) 9(1):96–110. doi: 10.21037/jgo.2017.11.13
15. Kneuertz PJ, Chang GJ, Hu CY, Rodriguez-Bigas MA, Eng C, Vilar E, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg (2015) 150(5):402–9. doi: 10.1001/jamasurg.2014.3572
16. Scott RB, Rangel LE, Osler TM, Hyman NH. Rectal cancer in patients under the age of 50 years: the delayed diagnosis. Am J Surg (2016) 211(6):1014–8. doi: 10.1016/j.amjsurg.2015.08.031
17. Kim TJ, Kim ER, Hong SN, Chang DK, Kim YH. Long-term outcome and prognostic factors of sporadic colorectal cancer in young patients: a Large institutional-based retrospective study. Med (Baltimore) (2016) 95(19):e3641. doi: 10.1097/MD.0000000000003641
18. O'Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg (2004) 187(3):343–8. doi: 10.1016/j.amjsurg.2003.12.020
19. Stebbing J, Singh Wasan H. Decoding metastatic colorectal cancer to improve clinical decision making. J Clin Oncol (2019) 37(22):1847–50. doi: 10.1200/JCO.19.01185
20. George B, Kopetz S. Predictive and prognostic markers in colorectal cancer. Curr Oncol Rep (2011) 13(3):206–15. doi: 10.1007/s11912-011-0162-3
21. Bahnassy AA, Abdel-Azim YA, Ezzat S, Abdellateif MS, Zekri AN, Mohanad M, et al. The role of circulating tumor cells and K-ras mutations in patients with locally advanced rectal cancer: a prospective study. Mol Biol Rep (2020) 47(12):9645–57. doi: 10.1007/s11033-020-05973-8
22. Rasmy A, Fayed A, Omar A, Fahmy N. Effect of KRAS mutational status on disease behavior and treatment outcome in patients with metastatic colorectal cancer: intratumor heterogeneity and mutational status. J Gastrointest Oncol (2019) 10(5):886–95. doi: 10.21037/jgo.2019.05.04
23. Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res (2006) 66(8):3992–5. doi: 10.1158/0008-5472.CAN-06-0191
24. De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol (2010) 11(8):753–62. doi: 10.1016/S1470-2045(10)70130-3
25. Knickelbein K, Zhang L. Mutant KRAS as a critical determinant of the therapeutic response of colorectal cancer. Genes Dis (2015) 2(1):4–12. doi: 10.1016/j.gendis.2014.10.002
26. Bruera G, Cannita K, Tessitore A, Russo A, Alesse E, Ficorella C, et al. The prevalent KRAS exon 2 c.35 G>A mutation in metastatic colorectal cancer patients: a biomarker of worse prognosis and potential benefit of bevacizumab-containing intensive regimens? Crit Rev Oncol Hematol (2015) 93(3):190–202. doi: 10.1016/j.critrevonc.2014.10.004
27. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol (2006) 30(3):277–99. doi: 10.1097/01.pas.0000190787.85024.cb
28. Wu F, Zhao X, Mi B, Feng LU, Yuan NA, Lei F, et al. Clinical characteristics and prognostic analysis of krukenberg tumor. Mol Clin Oncol (2015) 3(6):1323–8. doi: 10.3892/mco.2015.634
29. Chandra R, Karalis JD, Liu C, Murimwa GZ, Voth Park J, Heid CA, et al. The colorectal cancer tumor microenvironment and its impact on liver and lung metastasis. Cancers (Basel) (2021) 13(24):6206. doi: 10.3390/cancers13246206
30. Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol (2016) 17(12):1709–19. doi: 10.1016/S1470-2045(16)30500-9
31. Zoucas E, Frederiksen S, Lydrup ML, Mansson W, Gustafson P, Alberius P. Pelvic exenteration for advanced and recurrent malignancy. World J Surg (2010) 34(9):2177–84. doi: 10.1007/s00268-010-0637-7
32. Wanebo HJ, Gaker DL, Whitehill R, Morgan RF, Constable WC. Pelvic recurrence of rectal cancer. options for curative resection. Ann Surg (1987) 205(5):482–95. doi: 10.1097/00000658-198705000-00006
33. Pawlik TM, Skibber JM, Rodriguez-Bigas MA. Pelvic exenteration for advanced pelvic malignancies. Ann Surg Oncol (2006) 13(5):612–23. doi: 10.1245/ASO.2006.03.082
34. Zaborowski A, Stakelum A, Winter DC. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. Br J Surg (2019) 106(8):979–87. doi: 10.1002/bjs.11171
35. Bogner A, Fritzmann J, Mussle B, Huber J, Dobroschke J, Bork U, et al. Pelvic exenteration for colorectal and non-colorectal cancer: a comparison of perioperative and oncological outcome. Int J Colorectal Dis (2021) 36(8):1701–10. doi: 10.1007/s00384-021-03893-y
36. Davidge KM, Raghuram K, Hofer SO, Ferguson PC, Wunder JS, Swallow CJ, et al. Impact of flap reconstruction on perineal wound complications following ablative surgery for advanced and recurrent rectal cancers. Ann Surg Oncol (2014) 21(6):2068–73. doi: 10.1245/s10434-014-3529-5
37. Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis (2011) 13(7):732–42. doi: 10.1111/j.1463-1318.2009.02167.x
38. Sasikumar A, Bhan C, Jenkins JT, Antoniou A, Murphy J. Systematic review of pelvic exenteration with en bloc sacrectomy for recurrent rectal adenocarcinoma: R0 resection predicts disease-free survival. Dis Colon Rectum (2017) 60(3):346–52. doi: 10.1097/DCR.0000000000000737
39. PelvEx C. Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg (2019) 269(2):315–21. doi: 10.1097/SLA.0000000000002528
40. Nishimuta M, Hamada K, Sumida Y, Araki M, Wakata K, Kugiyama T, et al. Long-term prognosis after surgery for locally recurrent rectal cancer: a retrospective study. Asian Pac J Cancer Prev (2021) 22(5):1531–5. doi: 10.31557/APJCP.2021.22.5.1531
41. Mariathasan AB, Boye K, Giercksky KE, Brennhovd B, Gullestad HP, Emblemsvag HL, et al. Beyond total mesorectal excision in locally advanced rectal cancer with organ or pelvic side-wall involvement. Eur J Surg Oncol (2018) 44(8):1226–32. doi: 10.1016/j.ejso.2018.03.029
42. Radwan RW, Jones HG, Rawat N, Davies M, Evans MD, Harris DA, et al. Determinants of survival following pelvic exenteration for primary rectal cancer. Br J Surg (2015) 102(10):1278–84. doi: 10.1002/bjs.9841
43. Carr DT. The manual for the staging of cancer. Ann Intern Med (1977) 87(4):491–2. doi: 10.7326/0003-4819-87-4-491
44. PelvEx C. Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. Br J Surg (2018) 105(6):650–7. doi: 10.1002/bjs.10734
45. Cao G, Cheng D, Ye L, Pan Y, Yang F, Lyu S. Surgical resection of pulmonary metastases from colorectal cancer: 11 years of experiences. PloS One (2017) 12(4):e0175284. doi: 10.1371/journal.pone.0175284
46. de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg (2009) 250(3):440–8. doi: 10.1097/SLA.0b013e3181b4539b
47. Noren A, Sandstrom P, Gunnarsdottir K, Ardnor B, Isaksson B, Lindell G, et al. Identification of inequalities in the selection of liver surgery for colorectal liver metastases in Sweden. Scand J Surg (2018) 107(4):294–301. doi: 10.1177/1457496918766706
48. Giuliante F, Ardito F, Vellone M, Ranucci G, Federico B, Giovannini I, et al. Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J Surg Oncol (2009) 100(7):538–45. doi: 10.1002/jso.21393
49. Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, et al. Impact of surgical margin width on recurrence and overall survival following R0 hepatic resection of colorectal metastases: a systematic review and meta-analysis. Ann Surg (2018) 267(6):1047–55. doi: 10.1097/SLA.0000000000002552
50. Yang AD, Brouquet A, Vauthey JN. Extending limits of resection for metastatic colorectal cancer: risk benefit ratio. J Surg Oncol (2010) 102(8):996–1001. doi: 10.1002/jso.21701
51. Pinto C, Pini S, Di Fabio F, Cuicchi D, Iacopino B, Lecce F, et al. Treatment strategy for rectal cancer with synchronous metastasis: 65 consecutive Italian cases from the Bologna multidisciplinary rectal cancer group. Oncology (2014) 86(3):135–42. doi: 10.1159/000357782
52. Wong GYM, Mol B, Bhimani N, de Reuver P, Diakos C, Molloy MP, et al. Recurrence patterns predict survival after resection of colorectal liver metastases. ANZ J Surg (2022) 92(9):2149–56. doi: 10.1111/ans.17835
53. Brew-Graves C, Farewell V, Monson K, Milosevic M, Williams NR, Morris E, et al. Pulmonary metastasectomy in colorectal cancer: health utility scores by EQ-5D-3L in a randomized controlled trial show no benefit from lung metastasectomy. Colorectal Dis (2021) 23(1):200–5. doi: 10.1111/codi.15386
54. Treasure T, Farewell V, Macbeth F, Monson K, Williams NR, Brew-Graves C, et al. Pulmonary metastasectomy versus continued active monitoring in colorectal cancer (PulMiCC): a multicentre randomised clinical trial. Trials (2019) 20(1):718. doi: 10.1186/s13063-019-3837-y
55. Lemini R, Attwood K, Almerey T, Gunn J, Yeager TE, Elias AW, et al. Is metastasectomy a worthy option?-the role of surgery in metastatic colon cancer to liver and lungs. J Gastrointest Oncol (2019) 10(6):1032–48. doi: 10.21037/jgo.2019.09.06
56. Shimazaki J, Tabuchi T, Nishida K, Takemura A, Motohashi G, Kajiyama H, et al. Synchronous ovarian metastasis from colorectal cancer: a report of two cases. Oncol Lett (2016) 12(1):257–61. doi: 10.3892/ol.2016.4553
57. Kim DD, Park IJ, Kim HC, Yu CS, Kim JC. Ovarian metastases from colorectal cancer: a clinicopathological analysis of 103 patients. Colorectal Dis (2009) 11(1):32–8. doi: 10.1111/j.1463-1318.2008.01543.x
58. Fujiwara A, Noura S, Ohue M, Shingai T, Yamada T, Miyashiro I, et al. Significance of the resection of ovarian metastasis from colorectal cancers. J Surg Oncol (2010) 102(6):582–7. doi: 10.1002/jso.21675
59. Akgor U, Kuru O, Soyak B, Gunes AC, Uyanik E, Gultekin M, et al. Adnexal masses in patients with colorectal cancer. J Gynecol Obstet Hum Reprod (2021) 50(5):101898. doi: 10.1016/j.jogoh.2020.101898
60. Paramythiotis D, Goulas P, Moysidis M, Karakatsanis A, Tzioufa-Asimakopoulou V, Sotiriou S, et al. Metachronous ovarian metastases in a patient with primary colorectal cancer. a case report and review of the literature. Am J Case Rep (2019) 20:1515–20. doi: 10.12659/AJCR.917957
61. Herrera LO, Ledesma EJ, Natarajan N, Lopez GE, Tsukada Y, Mittelman A. Metachronous ovarian metastases from adenocarcinoma of the colon and rectum. Surg Gynecol Obstet (1982) 154(4):531–3.
62. Segelman J, Floter-Radestad A, Hellborg H, Sjovall A, Martling A. Epidemiology and prognosis of ovarian metastases in colorectal cancer. Br J Surg (2010) 97(11):1704–9. doi: 10.1002/bjs.7196
63. Chang GJ, Kaiser AM, Mills S, Rafferty JF, Buie WD. Standards practice task force of the American society of c, rectal s. practice parameters for the management of colon cancer. Dis Colon Rectum (2012) 55(8):831–43. doi: 10.1097/DCR.0b013e3182567e13
64. Gahagan JV, Whealon MD, Phelan MJ, Mills S, Jafari MD, Carmichael JC, et al. Improved survival with adjuvant chemotherapy in locally advanced rectal cancer patients treated with preoperative chemoradiation regardless of pathologic response. Surg Oncol (2020) 32:35–40. doi: 10.1016/j.suronc.2019.10.021
65. McCormick F. K-Ras protein as a drug target. J Mol Med (Berl) (2016) 94(3):253–8. doi: 10.1007/s00109-016-1382-7
66. Ostrem JM, Shokat KM. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat Rev Drug Discovery (2016) 15(11):771–85. doi: 10.1038/nrd.2016.139
67. Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, et al. Small-molecule ligands bind to a distinct pocket in ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci U.S.A. (2012) 109(14):5299–304. doi: 10.1073/pnas.1116510109
68. Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, et al. Discovery of small molecules that bind to K-ras and inhibit sos-mediated activation. Angew Chem Int Ed Engl (2012) 51(25):6140–3. doi: 10.1002/anie.201201358
69. Shima F, Yoshikawa Y, Ye M, Araki M, Matsumoto S, Liao J, et al. In silico discovery of small-molecule ras inhibitors that display antitumor activity by blocking the ras-effector interaction. Proc Natl Acad Sci U.S.A. (2013) 110(20):8182–7. doi: 10.1073/pnas.1217730110
70. Shin SM, Choi DK, Jung K, Bae J, Kim JS, Park SW, et al. Antibody targeting intracellular oncogenic ras mutants exerts anti-tumour effects after systemic administration. Nat Commun (2017) 8:15090. doi: 10.1038/ncomms15090
Keywords: rectal cancer, recurrence, pelvic exenteration, long-term survival, KRAS mutation
Citation: Ouyang Y, Zhu Y, Chen H, Li G, Hu X, Luo H, Li Z and Han S (2023) Case Report: Long-term survival of a patient with advanced rectal cancer and multiple pelvic recurrences after seven surgeries. Front. Oncol. 13:1169616. doi: 10.3389/fonc.2023.1169616
Received: 30 March 2023; Accepted: 04 May 2023;
Published: 15 May 2023.
Edited by:
Bo Zhang, Sichuan University, ChinaReviewed by:
Emmanuel Gabriel, Mayo Clinic, United StatesCopyright © 2023 Ouyang, Zhu, Chen, Li, Hu, Luo, Li and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Luo, aG9uZ3l1X2x1b0AxNjMuY29t; Zhou Li, Z3psaXpob3VAc211LmVkdS5jbg==; Shuai Han, Z3poYW5ibzA2MjRAc211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.