- 1Department of Breast Surgery, General Surgery Center, the First Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Breast Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
Background: The axillary lymph node positive (ypN+) rate in patients with clinically node-negative (cN0) breast cancer who have achieved breast pathologic complete response (bpCR) after neoadjuvant systemic therapy (NST) is extremely low, and this population has the potential to be exempt from sentinel lymph node biopsy (SLNB). However, an overview of the ypN+ rate in this population for different breast cancer subtypes is lacking.
Objective: To provide the pooled ypN+ rate in cN0 patients who achieved bpCR after NST in different breast cancer subtypes defined by hormone receptor (HR) status and human epidermal growth factor receptor 2 (HER2) status.
Methods: A systematic literature search was conducted in Embase and PubMed on July 20, 2022. Two authors independently selected studies that met the inclusion criteria and extracted all data. The pooled ypN+ rates for each subtype were calculated by a random-effects model using the Stata 16.0 metaprop command.
Results: The pooled analysis of 9609 cN0 patients who achieved bpCR showed that the ypN+ rate was lowest for the HR+/HER2+ (0%) subtype, followed by HR+/HER2- (5.1%), HR-/HER2+ (0.6%), and HR-/HER2- (0.3%). Additionally, 6571 cT1-T2N0 patients who achieved bpCR had a pooled ypN+ rate of 0.6%, and the ypN+ rates for different subtypes were as follows: HR+/HER2+ (1.7%), HR+/HER2- (2.7%), HR-/HER2+ (0.1%), and HR-/HER2- (0.8%).
Conclusion: Our results suggested that cN0 patients who achieve bpCR may be exempt from axillary surgery in the HR+/HER2-, HR+/HER2+, and HR-/HER2- subtypes because of the extremely low probability of residual axillary lymph node disease. However, the safety of omitting axillary surgery needs to be further confirmed by prospective studies.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier CRD42022351739.
Introduction
To provide successful disease control and to enhance the long-term quality of life of patients, the current trend in axillary management for those with early breast cancer is to focus on accuracy and safety. With the addition of targeted therapy to neoadjuvant systemic therapy (NST), the pathological complete response rate (pCR) in breast cancer patients has greatly improved, providing an opportunity to reduce or possibly eliminate surgery for certain patients (1, 2).
At present, sentinel lymph node biopsy (SLNB) is the standard of care and has replaced axillary lymph nodes dissection (ALND) as a staging procedure in clinically node-negative (cN0) patients. SLNB alone without further ALND has been found to be an appropriate, safe, and effective treatment for patients with clinically node-negative (cN0) breast cancer, as demonstrated by the fact that overall survival, disease-free survival, and regional control are not significantly different between the SLNB plus ALND group and the SLNB group (3). However, SLNB after NST remains controversial. Partial evidence has shown that SLNB is feasible in cN0 patients after NST, while the false-negative rate (FNR) can be as high as 15% in cN1 patients after NST (4). How to reduce the FNR and improve the accuracy of SLNB in these patients still needs to be further explored.
This study focused on patients with cN0 after NST. For cN0 patients, the nodal positivity (ypN+) rate following NST is low, particularly in those with breast pCR (bpCR) (5–9). It has been shown that ypN+ rates are less than 2% in patients with TNBC or HER2+ disease and bpCR (7, 10). Barron et al. (10) (n = 5377) and Tadros et al. (7) (n = 116) studied the ypN+ rate in cN0 patients with bpCR after NST and demonstrated that the ypN+ rate was 1.6% for both HER2+ and TNBC. Samiei et al. (9) (n = 986) reported that the ypN+ rate in cN0 patients with bpCR was 6.7%, 1.6%, 0%, and 1.5% in ER+/HER2-, ER+/HER2+, ER-/HER2+ and TNBC, respectively. The Moreover, the GANEA2 study showed that cN0 patients were followed up with SLNB for 3 years after neoadjuvant chemotherapy, with only one recurrence (11). Therefore, it is safe for cN0 patients to achieve bpCR for SLNB after NST. At this time, we raised a question: if the ypN+ ratio of the HER2+ and TNBC subtypes of breast cancer is less than 4%, can SLNB be exempted directly?
Therefore, the aim of this study was to pool and systematically review the ypN+ rate in cN0 patients with bpCR after NST in different subtypes, thereby providing clinicians with medical-based evidence on the safety and potential feasibility of SLNB exemption for such patients.
Methods
Literature search strategy
On July 20, 2022, studies evaluating the axillary pathological complete response (apCR) and/or nodal positivity rate (ypN+) for various breast cancer subtypes in patients with cN0 were originally searched in Embase and PubMed. Details of both search strategies are provided in the Supplementary Materials. This review protocol (No. CRD42014012901), which was registered in the International Prospective Register of Systematic Reviews (PROSPERO), adhered to the Preferred Reporting Items for Meta-Analyses (PRISMA).
Eligibility criteria for study inclusion
The following requirements had to be met for studies to be included in this review: patients who were clinically node-negative at the time of diagnosis underwent neoadjuvant chemotherapy, with or without HER2-targeted therapy, with achieved bpCR, followed by SLNB, ALND, or SLNB+ALND. The absence of suspicious or unusual lymph nodes on physical examination or ultrasound imaging was referred to as clinically node-negative. The definition of bpCR was no invasive disease (ypT0 or ypTis) by final pathologic result, and apCR was defined as ypN0/itc or ypN0 by final pathologic result. Second, among individuals with cN0, the ypN0/itc or axillary nodal positivity (ypN+) rates were reported for two or more distinct subtypes of breast cancer. Third, studies using SLNB performed prior to NST or neoadjuvant endocrine or radiation therapy were excluded. Additionally, we considered only English-language cohort studies, case−control studies, and randomized clinical trials.
Outcome measures
The rate of ypN+ following NST for various breast cancer subtypes was the study’s primary outcome. Micrometastatic or macrometastatic nodal disease was defined as ypN+. It should be emphasized that according to the attribution of isolated tumor cells (itc), the definitions of apCR in the included literature are different. Some studies define apCR as ypN0, while others define it as ypN0/itc. Therefore, if apCR was reported as an outcome event in the included literature and the ypN+ rate was not directly reported, we could also use the formula ypN+ (%) = 100%-ypN0 (or ypN0/itc) (%). To date, the definition of apCR as ypN0 or ypN0/itc is controversial among different research institutions. Conflicting results have been reported regarding the prognostic implications of ypN0 and residual isolated tumor cells.
Study selection
Two authors independently evaluated all available studies and resolved disagreements by reaching consensus (Le Ma and Heyan Chen). The Newcastle−Ottawa Scale (NOS) was utilized to appraise the validity of eligible studies (12). When two reviewers were uncertain about the quality assessment of a review, they emailed or interviewed the authors to resolve the quality differences.
Data extraction and analysis
The two reviewers separately retrieved the following study features from the included studies. Characteristics of studies, such as year, first author, research type, and country, were collected. Traits of participants, including cT category, cancer subtype, neoadjuvant systemic therapy regimens, axillary surgery, definition of bpCR and apCR, were extracted. Data extraction disagreements were settled via a consensus meeting.
The overall pooled estimate of the ypN+ rate for each subtype was computed using the Stata 16.0 metaprop command and the random-effects model for meta-analysis. Subgroup analyses were performed according to different apCR definitions. Forest plots were used to display the estimated variance in size estimates of the ypN+ effect with 95% CI and weights for each subtype. The statistical heterogeneity was measured using the I2 statistic, and values of 25%, 50%, and 75% were considered to be low, moderate, and high, respectively. The statistical heterogeneity was evaluated using the χ2 test. A two-sided P < 0.05 was regarded as statistically significant.
Results
Selected studies and methodological quality
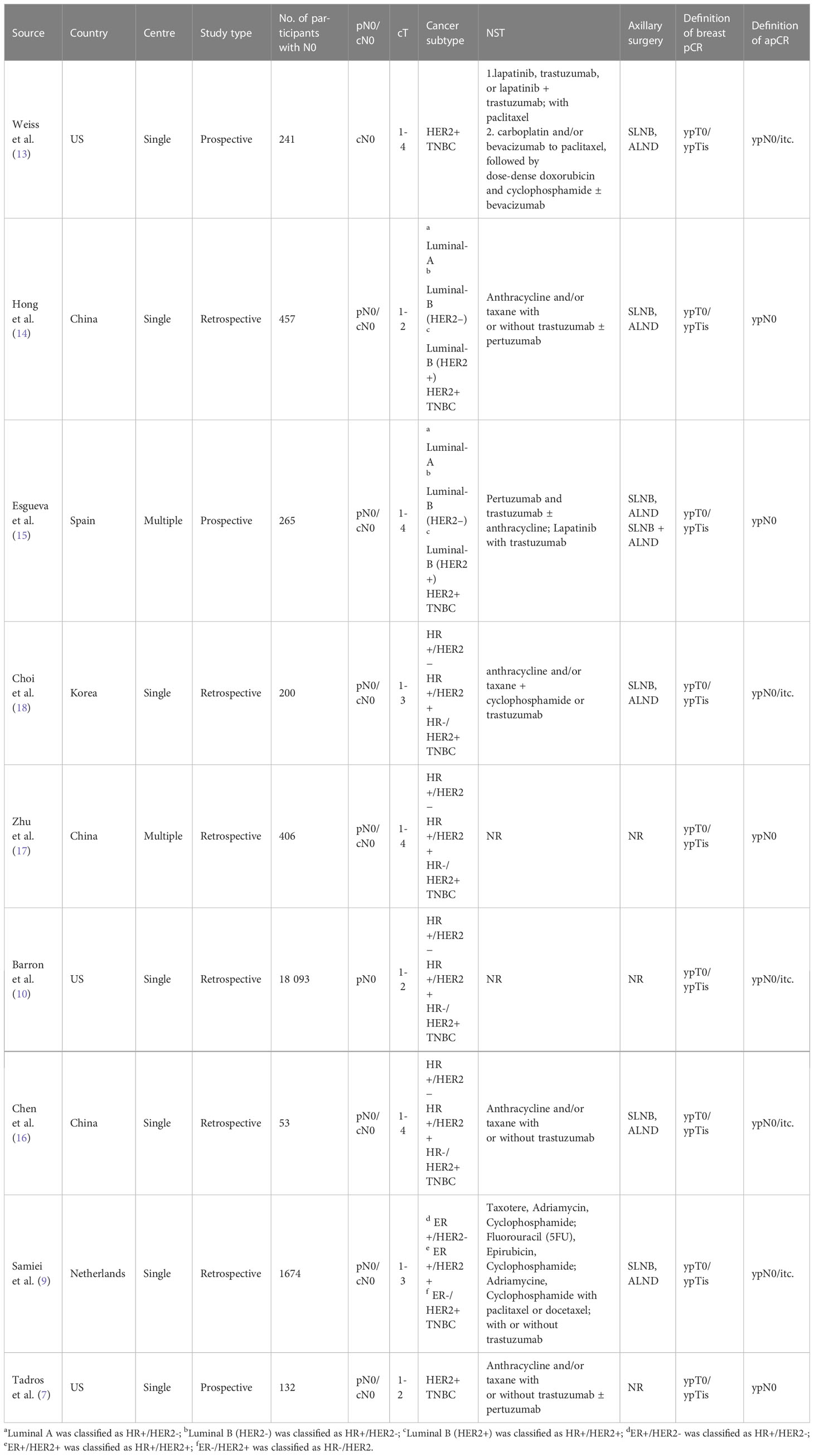
Figure 1 shows a flow diagram of the literature review procedure. The PubMed and Embase databases yielded a total of 3754 items, and 1122 duplicate papers were eliminated after being loaded into EndNote. There were 65 articles left after the initial screening of titles and abstracts using inclusion and exclusion criteria. By reading the entire articles, 56 items were ultimately eliminated, while 9 were enrolled. The quality of the included studies was evaluated using the NOS, and the outcomes are given in Supplementary Materials Table S1.
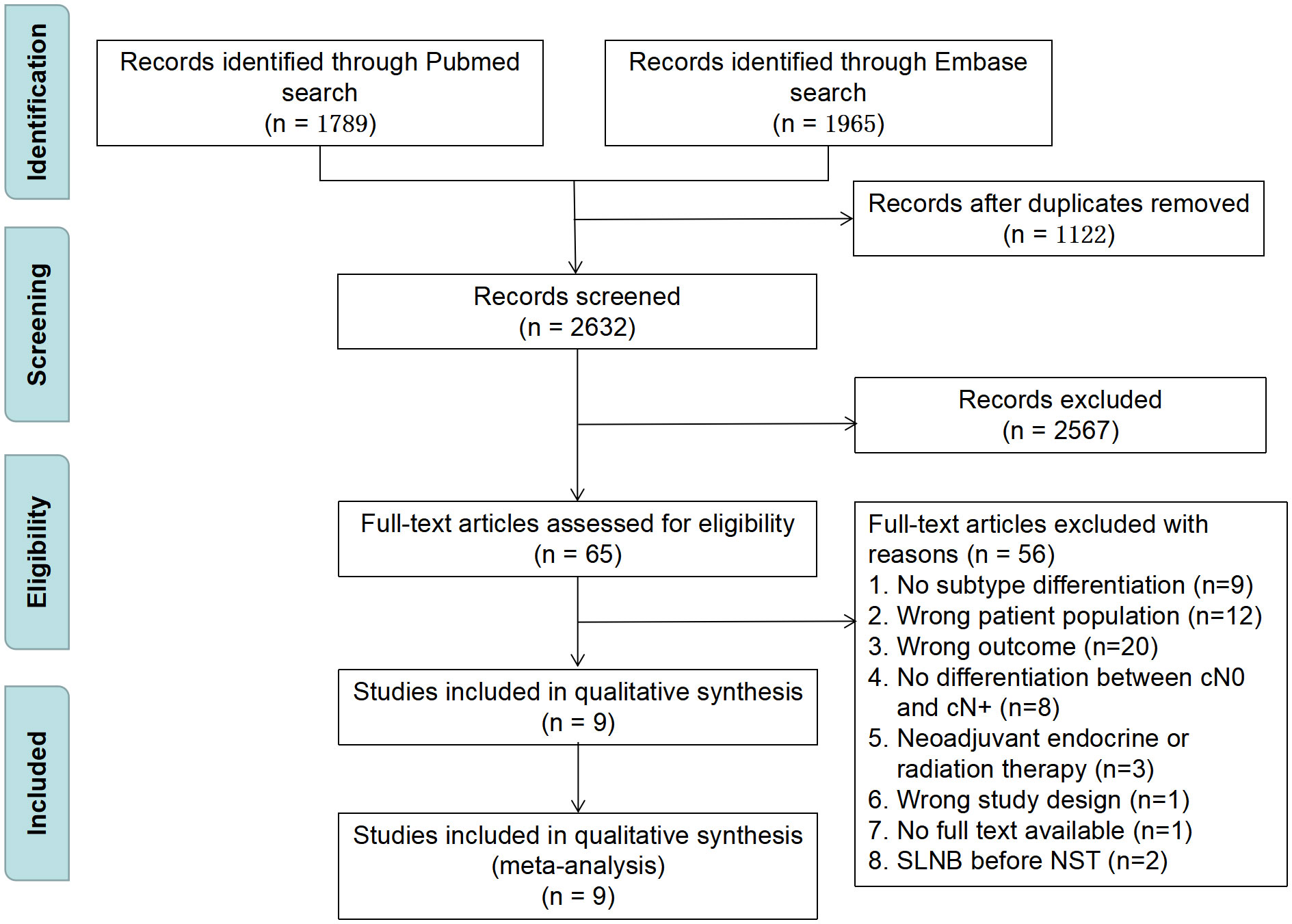
Figure 1 PRISMA flow diagram for study selection. SLNB, sentinel lymph node biopsy; NST, neoadjuvant systematic therapy.
Characteristics of studies and participants
A total of 21521 participants were enrolled in the meta-analysis across 9 studies (7, 9, 10, 13–18) (Table 1), including 9609 cN0 patients who achieved bpCR. The definition of apCR was ypN0 in 4 studies (7, 14, 15, 17) and ypN0/itc in 5 studies (9, 10, 13, 16, 18). Three of these studies were carried out in the United States (7, 10, 13), three in China (14, 16, 17), one in the Netherlands (9), one in Korea (18), and one in Spain (15). Six retrospective studies and three prospective studies were included. Four studies (7, 9, 10, 15) reported the ypN+ rate under different T stages, among which T1-T2 accounted for 99% (6571/6632). ER+/PR+, ER+/PR- or ER-/PR+ is defined as HR+, and ER-/PR- is defined as HR-.
HR+/HER2- breast cancer
Seven studies (9, 10, 14–18) involving 865 cN0 patients with bpCR who had HR+/HER2- breast cancer were published (Figure 2). The overall pooled ypN+ rate was 5.1% (95% CI, 0.7%-11.9%) (42/865 cases). With an I2 value of 71.41% between the trials, there was significant heterogeneity (P=0.002). According to three studies (9, 10, 15) (Figure 3), 762 cT1-T2N0 patients who achieved bpCR had a pooled ypN+ rate of 2.7% (95% CI, 0.1%-7.4%) (29 cases).
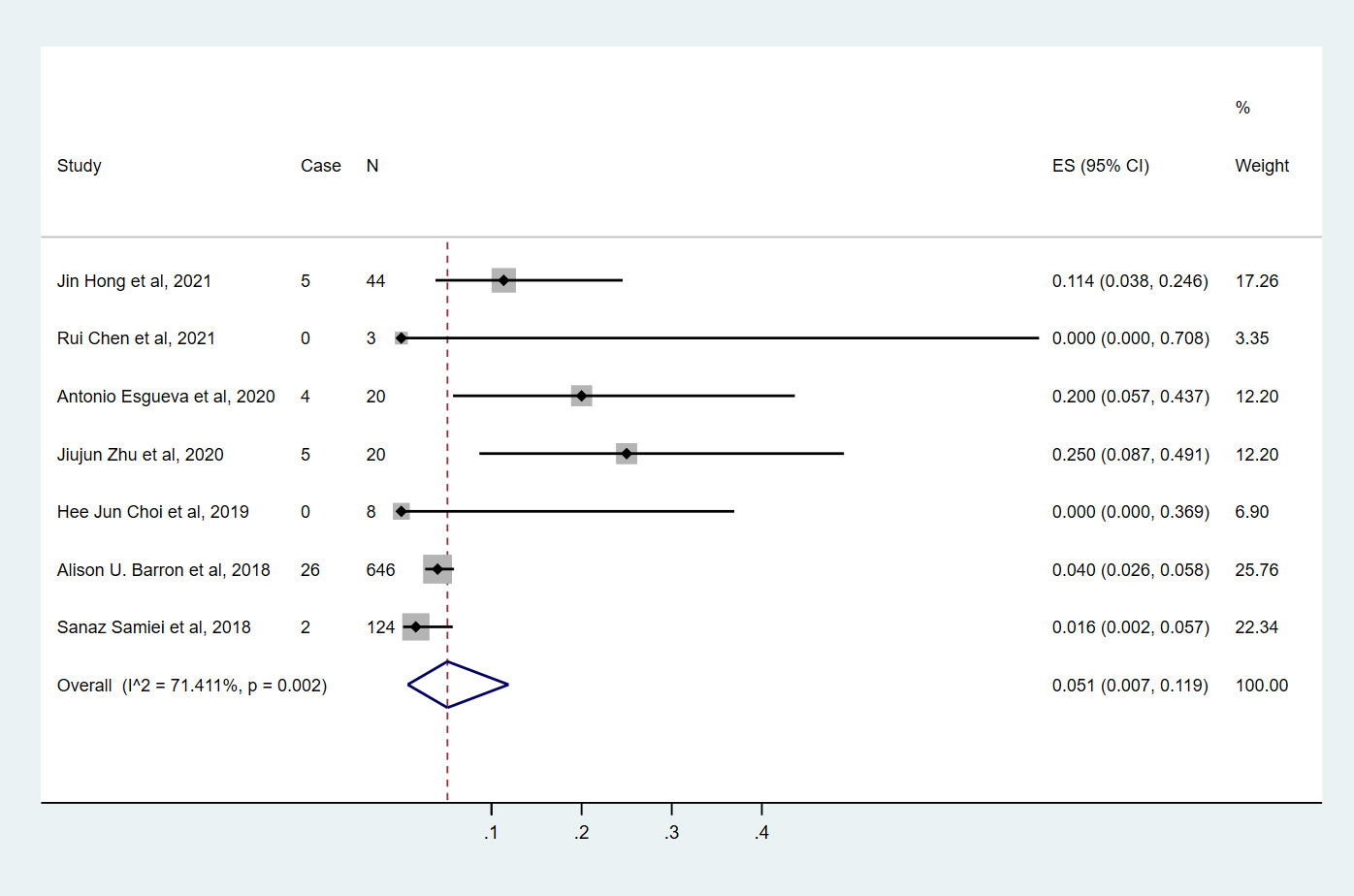
Figure 2 The overall pooled ypN+ rate of cN0 patients who achieved bpCR in HR+/HER2- breast cancer. HR, hormone receptor; HER2, human epidermal growth factor receptor 2. ES, effect size. CI, confidence interval. Effect size was used to estimate the ypN+ rate of each study. Confidence intervals determine the consistency and reliability of the mean estimated effect size. Diamonds indicate effect size.
HR+/HER2+ breast cancer
Seven studies (9, 10, 14–18) involving 1892 cN0 patients with bpCR who had HR+/HER2+ breast cancer were published (Figure 4). The overall pooled ypN+ rate was 0% (95% CI, 0%-0.1%) (39/1892 cases). With an I2 value of 42.89% between the trials, there was no significant difference in heterogeneity (P=0.105). According to two studies (9, 10) (Figure 3), 1817 cT1-T2N0 patients who achieved bpCR had a pooled ypN+ rate of 1.7% (95% CI, 1.1%-2.4%) (36/1817 cases).
Figure 3 The overall pooled ypN+ rate of cN0 patients who achieved bpCR for different breast cancer subtypes with T1-T2 tumors. HR, hormone receptor; HER2, human epidermal growth factor receptor 2. ES, effect size. CI, confidence interval. Effect size was used to estimate the ypN+ rate of each study. Confidence intervals determine the consistency and reliability of the mean estimated effect size. Diamonds indicate effect size.

Figure 4 The overall pooled ypN+ rate of HR+/HER2+ breast cancer cN0 patients who achieved bpCR. HR, hormone receptor; HER2, human epidermal growth factor receptor 2. ES, effect size. CI, confidence interval. Effect size was used to estimate the ypN+ rate of each study. Confidence intervals determine the consistency and reliability of the mean estimated effect size. Diamonds indicate effect size.
HR-/HER2+ breast cancer
Nine studies (7, 9, 10, 13–18) involving 1571 cN0 patients with bpCR who had HR-/HER2+ breast cancer were published (Figure 5). The overall pooled ypN+ rate was 0.6% (95% CI, 0%-3.2%) (22/1571 cases). With an I2 value of 50.13% between the trials, there was significant heterogeneity (P=0.042). According to four studies (7, 9, 10, 15) (Figure 3), 1428 cT1-T2N0 patients who achieved bpCR had a pooled ypN+ rate of 0.1% (95% CI, 0%-3.3%) (16/1462 cases). With an I2 index of 66.37%, significant heterogeneity was observed (P=0.030).
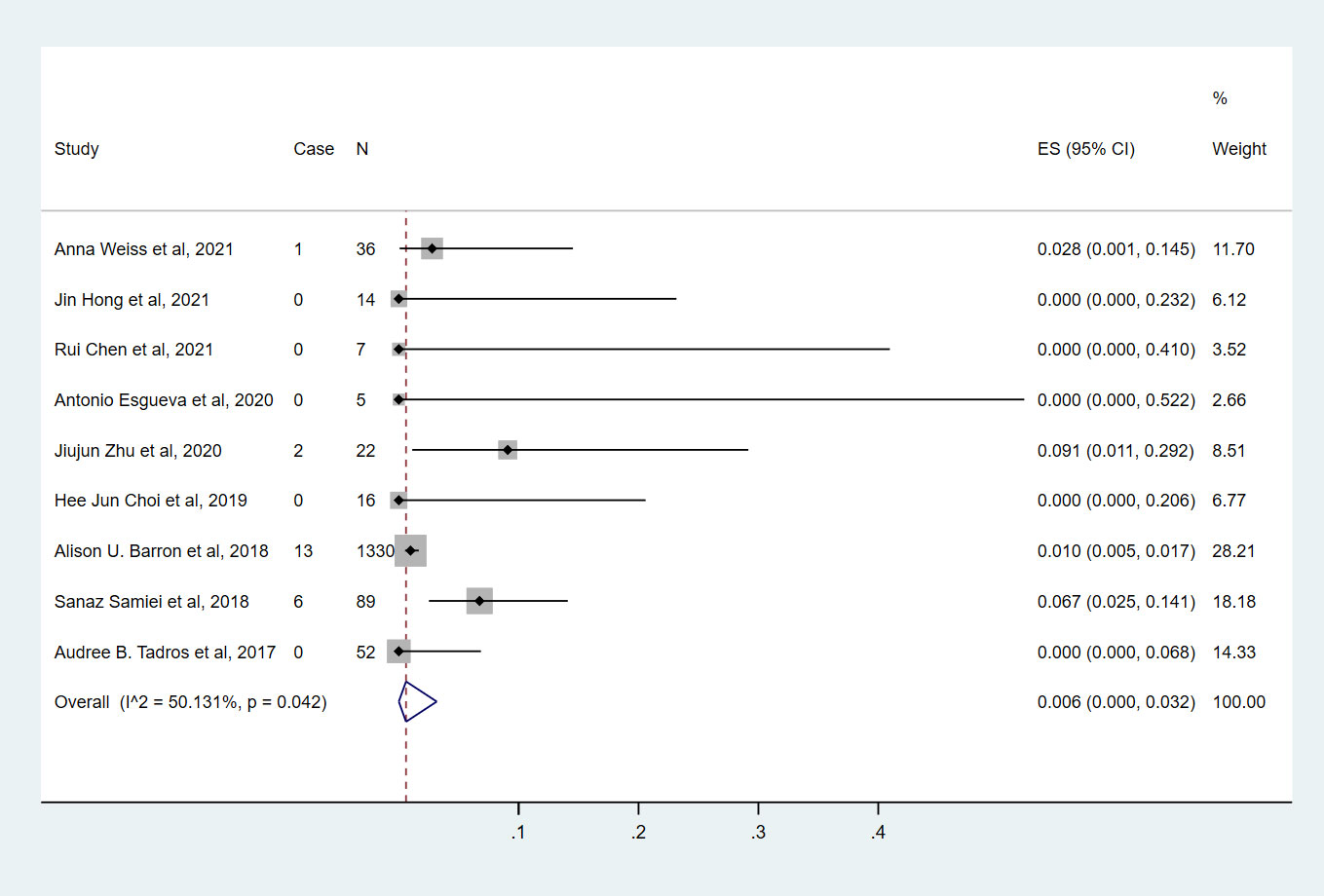
Figure 5 The overall pooled ypN+ rate of cN0 patients who achieved bpCR in HR-/HER2+ breast cancer. HR, hormone receptor; HER2, human epidermal growth factor receptor 2. ES, effect size. CI, confidence interval. Effect size was used to estimate the ypN+ rate of each study. Confidence intervals determine the consistency and reliability of the mean estimated effect size. Diamonds indicate effect size.
HR-/HER2- breast cancer
Nine studies (7, 9, 10, 13–18) involving 2682 cN0 patients with bpCR who had HR-/HER2- breast cancer were published (Figure 6). The overall pooled ypN+ rate was 0.3% (95% CI, 0%-0.7%) (43/2682 cases). With an I2 value of 0% between the trials, no statistically significant heterogeneity was observed (P=0.560). According to four studies (7, 9, 10, 15) (Figure 3), 2530 cT1-T2N0 patients who achieved bpCR had a pooled ypN+ rate of 0.8% (95% CI, 0.4%-1.3%) (37/2530 cases). With an I2 index of 0%, there was no significant heterogeneity (P=0.751).
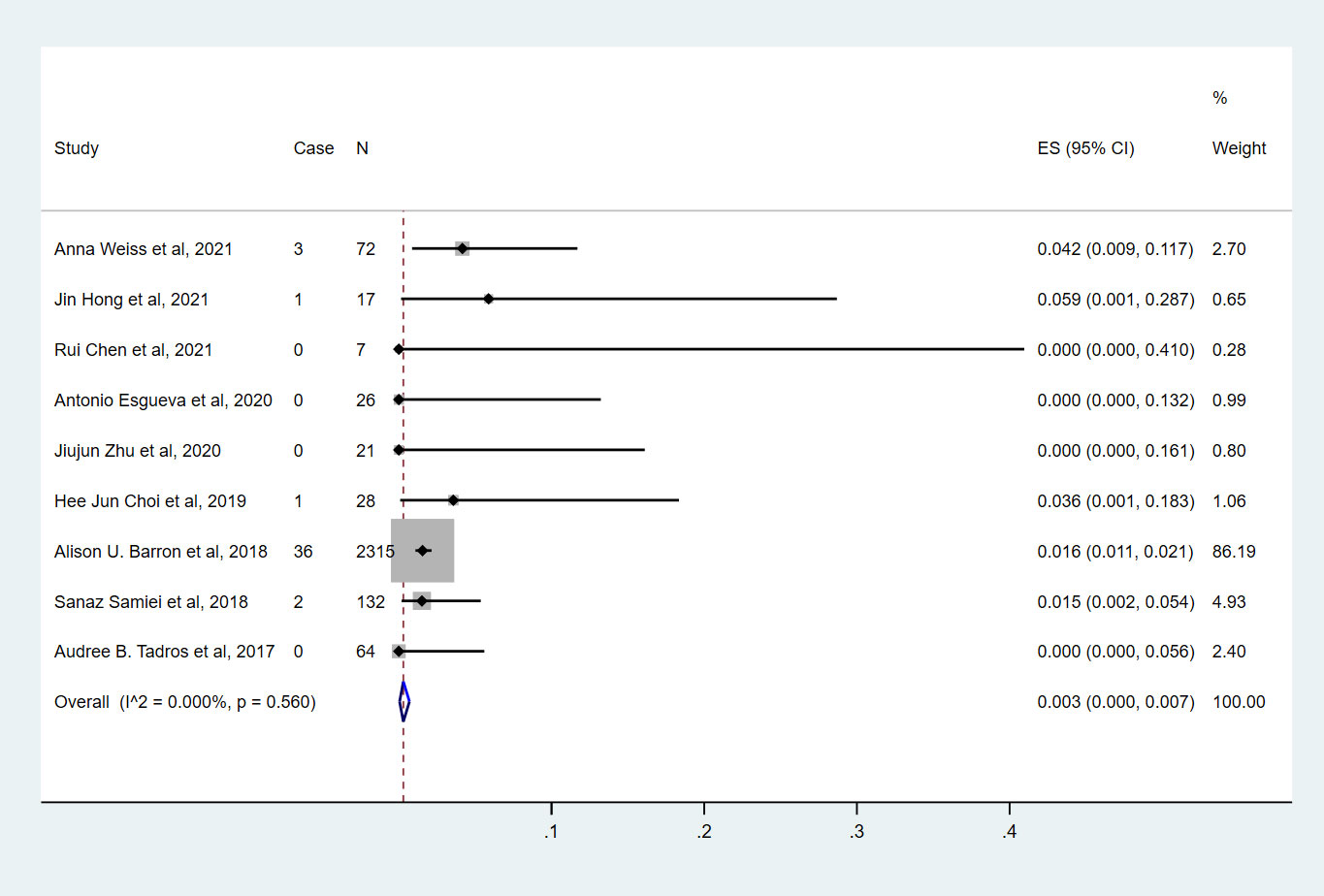
Figure 6 The overall pooled ypN+ rate of cN0 patients who achieved bpCR in HR-/HER2- breast cancer. HR, hormone receptor; HER2, human epidermal growth factor receptor 2. ES, effect size. CI, confidence interval. Effect size was used to estimate the ypN+ rate of each study. Confidence intervals determine the consistency and reliability of the mean estimated effect size. Diamonds indicate effect size.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to investigate the ypN+ rate of cN0 patients with bpCR after NST in different breast cancer subtypes. The overall pooled analysis of 9609 cN0 patients who achieved bpCR showed that the ypN+ rate was only 0.2%, and the ypN+ rate was the lowest for the HR+/HER2+ (0%) subtype, followed by the HR-/HER2- (0.3%), HR-/HER2+ (0.6%), and HR+/HER2- (5.1%) subtypes.
At present, cN0 is determined by clinical physical examination, imaging and fine needle aspiration biopsy or core needle biopsy, an assessment that is accompanied by a certain FNR before NST. Although approximately 30% of patients have axillary lymph node metastases prior to NST, only 2-6% of these patients who achieve bpCR remain SLNB positive after NST, and the rate is even lower in HER2-positive and TNBC patients (7, 9, 10, 13–19). A retrospective study (10) from the National Cancer Database (NCDB) revealed that in patients with cT1/cT2 N0 HER2-positive cancer or TNBC who attained bpCR, the nodal positivity rate was less than 2%, which supports the idea of forgoing axillary surgery in this population of patients. Another retrospective study (9) that included patients with cT1-3N0-1 breast cancer from the Netherlands Cancer Registry also revealed that the rates of ypN+ for the HR+HER2+ (1.6%), HR-/HER2- (1.5%), and HR-/HER2+ (0%) subtypes were incredibly low. Furthermore, Tadros et al. (7) demonstrated that bpCR has a significant correlation with axillary nodal status following NST. The application of SLNB omission in HR+/HER2- was constrained by the significantly lower overall rate of bpCR. Moreover, NST is also utilized less frequently in cN0 individuals with HR+/HER2- illness. Therefore, the analysis or decision of whether it is safe for such patients to be exempt from SLNB must be made with extra caution. In summary, individuals with bpCR had a nodal positivity rate of less than 10%, which is in favor of exempting axillary surgery in this population of patients. For HR-/HER2-, HER2+ breast cancer, ypN+ rates were even lower (less than 2%), and patients with these two subtypes can be relieved of axillary surgery. In light of the aforementioned findings, future clinical trials should investigate whether axillary surgery can be safely omitted in precisely chosen patients.
However, in clinical practice, there will be some problems. First, for breast cancer patients with non-bpCR, the ypN+ rate of each subtype was more than 10%, except for some studies about HR-/HER2+ and HR-/HER2- (Supplementary Table 2), and there is no evidence to suggest that it is safe to exempt axillary surgery for these patients. Residual disease can provide guidance for patients in their decisions about adjuvant systemic therapy. Studies have confirmed that HER2+ patients with non-bpCR after neoadjuvant chemotherapy can subsequently be treated with adjuvant trastuzumab emtansine (T-DM1), while HR-/HER2- patients can be treated with capecitabine, which can improve event-free survival or disease-free survival (20, 21). Therefore, for non-bpCR patients, SLNB is still required to confirm axillary lymph node metastases; otherwise, false-negative pathological complete response assessment would result in inappropriate de-escalation of axillary lymph node metastasis and inappropriate adjuvant therapy, which may bring a higher risk of recurrence. Perhaps the findings of ongoing clinical trials (22, 23) comparing complete ALND to axillary radiotherapy in patients with positive SLNB (ypN+) following NST will provide a solution for these patients.
Second, as bpCR becomes increasingly common due to improved systemic therapy in NST, identifying bpCR prior to surgery is a critical challenge when developing surgical intervention-free treatment alternatives for individuals who have attained bpCR. Radiological CR (rCR) was considered to be used to predict bpCR and thus dispense with breast surgery; however, modern imaging techniques, including ultrasound, MRI, and F-FDG PET-CT scan, are insufficiently precise to differentiate bpCR (24–26). Henry et al. demonstrated that the vacuum-assisted breast biopsy (VABB) technique is promising for eliminating breast surgery in specific breast cancer patients after neoadjuvant chemotherapy (27). Several trials (such as MICRA) also demonstrated that VABB is not accurate enough to identify bpCR in patients with a good response on MRI after NST (28–31). Therefore, for cN0 patients who have undergone NST, breast surgery can be performed first, and the next course of axillary treatment will depend on whether the bpCR is attained. If bpCR is not achieved, axillary surgery is required as a second procedure, which is in concordance with EUBREAST-01 protocol (32). EUBREAST-01 is an ongoing international, prospective, non-randomized, single-arm surgical study. Its goal is to demonstrate the cancer-related safety of not performing axillary SLNB after achieving bpCR in response to NST for TNBC and HER2-positive patients with cN0 status (32). In this trial, axillary surgery is not carried out simultaneously. This approach offers two benefits: firstly, it shortens the duration of the operation, and secondly, it reduces the risk of lymphedema and other complications for patients who don’t undergo axillary surgery.
Limitations
The greatest limitation in this meta-analysis is the presence of heterogeneity, the main possible causes of which are as follows. First, none of the included studies was a prospective randomized clinical trial; thus, the distribution of patients and the limited availability of details of axillary surgery might have biased our results regarding regional control. Second, information on systemic therapy was not included in our analysis because the specific chemotherapy regimen and number of patients for each subtype could not be extracted from the original literature. For example, this study included a large proportion of HER2+ patients. Targeted therapy in this population is known to increase bpCR and apCR rates, but it is not possible to obtain the proportion of targeted drug use in this population. Third, patients with cN0 confirmed with pathological biopsy before NST as well as others without such confirmation were included in the present study, but we did not have enough information to distinguish between them.
Conclusions
In summary, the results of this meta-analysis indicated that cN0 patients who achieved bpCR may be exempt from axillary surgery in HR+/HER2+ and HR-/HER2- subtypes because of the extremely low probability of ypN+. However, it remains unclear whether the presence of axillary lymphatic disease in this specific population affects long-term survival and recurrence. Therefore, the safety of exemption from axillary surgery needs to be further confirmed by prospective studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Concept and design: ZF, HZ and JH. The acquisition, analysis, and/or interpretation of data: LM, HC, PX and YL. Draft of the manuscript: LM, HC, PG and HZ. Supervision: ZF, HZ and JH. All other authors contributed by providing revisions to the manuscript, giving approval of the final version of the manuscript, and accepting responsibility for all aspects of the manuscript.
Funding
This work is supported by the Institutional Foundation of The First Affiliated Hospital of Xi’an Jiaotong University (2022YQPY08) and the Scientific Research Fund of the First Affiliated Hospital of Jilin University (lcpyjjl2017004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1167912/full#supplementary-material
References
1. Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? results of a prospective study. Ann Surg Oncol (2016) 23:3467–74. doi: 10.1245/s10434-016-5246-8
2. Morrow M. Leveraging the benefits of systemic therapy to tailor surgery. JAMA Surg (2017) 152:671. doi: 10.1001/jamasurg.2017.0565
3. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP b-32 randomised phase 3 trial. Lancet Oncol (2010) 11:927–33. doi: 10.1016/S1470-2045(10)70207-2
4. Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z, et al. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: An up-to-date meta-analysis of 3,578 patients. Breast (2021) 59:256–69. doi: 10.1016/j.breast.2021.07.015
5. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol (2014) 15:1303–10. doi: 10.1016/S1470-2045(14)70460-7
6. Barrio AV, Mamtani A, Eaton A, Brennan S, Stempel M, Morrow M. Is routine axillary imaging necessary in clinically node-negative patients undergoing neoadjuvant chemotherapy? Ann Surg Oncol (2017) 24:645–51. doi: 10.1245/s10434-017-5765-y
7. Tadros AB, Yang WT, Krishnamurthy S, Rauch GM, Smith BD, Valero V, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg (2017) 152:665–70. doi: 10.1001/jamasurg.2017.0562
8. Al-Hilli Z, Hoskin TL, Day CN, Habermann EB, Boughey JC. Impact of neoadjuvant chemotherapy on nodal disease and nodal surgery by tumor subtype. Ann Surg Oncol (2018) 25:482–93. doi: 10.1245/s10434-017-6263-y
9. Samiei S, van Nijnatten TJA, de Munck L, Keymeulen K, Simons JM, Kooreman LFS, et al. Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg (2020) 271:574–80. doi: 10.1097/SLA.0000000000003126
10. Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg (2018) 153:1120–6. doi: 10.1001/jamasurg.2018.2696
11. Classe JM, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat (2019) 173:343–52. doi: 10.1007/s10549-018-5004-7
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
13. Weiss A, Campbell J, Ballman KV, Sikov WM, Carey LA, Hwang ES, et al. Factors associated with nodal pathologic complete response among breast cancer patients treated with neoadjuvant chemotherapy: Results of CALGB 40601 (HER2+) and 40603 (Triple-negative) (Alliance). Ann Surg Oncol (2021) 28:5960–71. doi: 10.1245/s10434-021-09897-w
14. Hong J, Tong Y, He J, Chen X, Shen K. Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery. Ther Adv Med Oncol (2021) 13:1758835921996673. doi: 10.1177/1758835921996673
15. Esgueva A, Siso C, Espinosa-Bravo M, Sobrido C, Miranda I, Salazar JP, et al. Leveraging the increased rates of pathologic complete response after neoadjuvant treatment in breast cancer to de-escalate surgical treatments. J Surg Oncol (2021) 123:71–9. doi: 10.1002/jso.26236
16. Chen R, Li S, Li Y, Zhu Q, Shi X, Xu L, et al. Can axillary surgery be omitted in patients with breast pathologic complete response after neoadjuvant systemic therapy for breast cancer? a real-world retrospective study in China. J Cancer Res Clin Oncol (2021) 147:3495–501. doi: 10.1007/s00432-021-03763-8
17. Zhu J, Li J, Fan Z, Wang H, Zhang J, Yin Y, et al. Association of higher axillary pathologic complete response rate with breast pathologic complete response after neoadjuvant chemotherapy. Ann Transl Med (2020) 8:992. doi: 10.21037/atm-20-5172
18. Choi HJ, Ryu JM, Kim I, Nam SJ, Kim SW, Yu J, et al. Prediction of axillary pathologic response with breast pathologic complete response after neoadjuvant chemotherapy. Breast Cancer Res Treat (2019) 176:591–6. doi: 10.1007/s10549-019-05214-y
19. Cohen LF, Breslin TM, Kuerer HM, Ross MI, Hunt KK, Sahin AA. Identification and evaluation of axillary sentinel lymph nodes in patients with breast carcinoma treated with neoadjuvant chemotherapy. Am J Surg Pathol (2000) 24:1266–72. doi: 10.1097/00000478-200009000-00010
20. Mamounas EP, Untch M, Mano MS, Huang CS, Geyer CE Jr., von Minckwitz G, et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann Oncol (2021) 32:1005–14. doi: 10.1016/j.annonc.2021.04.011
21. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med (2017) 376:2147–59. doi: 10.1056/NEJMoa1612645
22. Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy . Available at: https://clinicaltrials.gov/ct2/show/NCT01901094.
23. Standard or comprehensive radiation therapy in treating patients with early-stage breast cancer previously treated with chemotherapy and surgery . Available at: https://clinicaltrials.gov/ct2/show/NCT01872975.
24. Sheikhbahaei S, Trahan TJ, Xiao J, Taghipour M, Mena E, Connolly RM, et al. FDG-PET/CT and MRI for evaluation of pathologic response to neoadjuvant chemotherapy in patients with breast cancer: A meta-analysis of diagnostic accuracy studies. Oncologist (2016) 21:931–9. doi: 10.1634/theoncologist.2015-0353
25. Schaefgen B, Mati M, Sinn HP, Golatta M, Stieber A, Rauch G, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol (2016) 23:789–95. doi: 10.1245/s10434-015-4918-0
26. Bossuyt V, Provenzano E, Symmans WF, Boughey JC, Coles C, Curigliano G, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol (2015) 26:1280–91. doi: 10.1093/annonc/mdv161
27. Kuerer HM, Smith BD, Krishnamurthy S, Yang WT, Valero V, Shen Y, et al. Eliminating breast surgery for invasive breast cancer in exceptional responders to neoadjuvant systemic therapy: a multicentre, single-arm, phase 2 trial. Lancet Oncol (2022) 23:1517–24. doi: 10.1016/S1470-2045(22)00613-1
28. Heil J, Pfob A, Sinn HP, Rauch G, Bach P, Thomas B, et al. Diagnosing pathologic complete response in the breast after neoadjuvant systemic treatment of breast cancer patients by minimal invasive biopsy: Oral presentation at the San Antonio breast cancer symposium on Friday, December 13, 2019, program number GS5-03. Ann Surg (2022) 275:576–81. doi: 10.1097/SLA.0000000000004246
29. van Loevezijn AA, van der Noordaa MEM, van Werkhoven ED, Loo CE, Winter-Warnars GAO, Wiersma T, et al. Minimally invasive complete response assessment of the breast after neoadjuvant systemic therapy for early breast cancer (MICRA trial): Interim analysis of a multicenter observational cohort study. Ann Surg Oncol (2021) 28:3243–53. doi: 10.1245/s10434-020-09273-0
30. Lee HB, Han W, Kim SY, Cho N, Kim KE, Park JH, et al. Prediction of pathologic complete response using image-guided biopsy after neoadjuvant chemotherapy in breast cancer patients selected based on MRI findings: a prospective feasibility trial. Breast Cancer Res Treat (2020) 182:97–105. doi: 10.1007/s10549-020-05678-3
31. Kuerer HM, Rauch GM, Krishnamurthy S, Adrada BE, Caudle AS, DeSnyder SM, et al. A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg (2018) 267:946–51. doi: 10.1097/SLA.0000000000002313
Keywords: breast cancer, neoadjuvant systemic therapy, axillary lymph node positive rate, sentinel lymph node biopsy, meta- analysis
Citation: Ma L, Chen H, He J, Xie P, Gao P, Li Y, Zhang H and Fan Z (2023) The nodal positivity rate in breast pCR patients with initially, clinically node-negative breast cancer after neoadjuvant systemic therapy: A systematic review and meta-analysis. Front. Oncol. 13:1167912. doi: 10.3389/fonc.2023.1167912
Received: 17 February 2023; Accepted: 17 March 2023;
Published: 29 March 2023.
Edited by:
Nicola Fusco, University of Milan, ItalyReviewed by:
Mariia Ivanova, European Institute of Oncology IRCCS, ItalyGiovanni Tazzioli, University of Modena and Reggio Emilia, Italy
Copyright © 2023 Ma, Chen, He, Xie, Gao, Li, Zhang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhimin Fan, ZmFuem1Aamx1LmVkdS5jbg==; Huimin Zhang, aHVpbWluLnpoYW5nQHhqdHUuZWR1LmNu
†These authors share first authorship
 Le Ma
Le Ma Heyan Chen2†
Heyan Chen2† Jianjun He
Jianjun He Yijun Li
Yijun Li Huimin Zhang
Huimin Zhang Zhimin Fan
Zhimin Fan