- 1Department of Gastrointestinal Surgery, Pengzhou People's Hospital, Chengdu, Sichuan, China
- 2Department of Urology, Pengzhou People's Hospital, Chengdu, Sichuan, China
- 3Department of Respiratory Medicine, First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, China
Background: Minimally invasive total mesorectal excision (MiTME) and transanal total mesorectal excision (TaTME) are popular trends in mid and low rectal cancer. However, there is currently no systematic comparison between MiTME and TaTME of mid and low-rectal cancer. Therefore, we systematically study the perioperative and pathological outcomes of MiTME and TaTME in mid and low rectal cancer.
Methods: We have searched the Embase, Cochrane Library, PubMed, Medline, and Web of Science for articles on MiTME (robotic or laparoscopic total mesorectal excision) and TaTME (transanal total mesorectal excision). We calculated pooled standard mean difference (SMD), relative risk (RR), and 95% confidence intervals (CIs). The protocol for this review has been registered on PROSPERO (CRD42022374141).
Results: There are 11010 patients including 39 articles. Compared with TaTME, patients who underwent MiTME had no statistical difference in operation time (SMD -0.14; CI -0.31 to 0.33; I2=84.7%, P=0.116), estimated blood loss (SMD 0.05; CI -0.05 to 0.14; I2=48%, P=0.338), postoperative hospital stay (RR 0.08; CI -0.07 to 0.22; I2=0%, P=0.308), over complications (RR 0.98; CI 0.88 to 1.08; I2=25.4%, P=0.644), intraoperative complications (RR 0.94; CI 0.69 to 1.29; I2=31.1%, P=0.712), postoperative complications (RR 0.98; CI 0.87 to 1.11; I2=16.1%, P=0.789), anastomotic stenosis (RR 0.85; CI 0.73 to 0.98; I2=7.4%, P=0.564), wound infection (RR 1.08; CI 0.65 to 1.81; I2=1.9%, P=0.755), circumferential resection margin (RR 1.10; CI 0.91 to 1.34; I2=0%, P=0.322), distal resection margin (RR 1.49; CI 0.73 to 3.05; I2=0%, P=0.272), major low anterior resection syndrome (RR 0.93; CI 0.79 to 1.10; I2=0%, P=0.386), lymph node yield (SMD 0.06; CI -0.04 to 0.17; I2=39.6%, P=0.249), 2-year DFS rate (RR 0.99; CI 0.88 to 1.11; I2=0%, P = 0.816), 2-year OS rate (RR 1.00; CI 0.90 to 1.11; I2=0%, P = 0.969), distant metastasis rate (RR 0.47; CI 0.17 to 1.29; I2=0%, P = 0.143), and local recurrence rate (RR 1.49; CI 0.75 to 2.97; I2=0%, P = 0.250). However, patients who underwent MiTME had fewer anastomotic leak rates (SMD -0.38; CI -0.59 to -0.17; I2=19.0%, P<0.0001).
Conclusion: This study comprehensively and systematically evaluated the safety and efficacy of MiTME and TaTME in the treatment of mid to low-rectal cancer through meta-analysis. There is no difference between the two except for patients with MiTME who have a lower anastomotic leakage rate, which provides some evidence-based reference for clinical practice. Of course, in the future, more scientific and rigorous conclusions need to be drawn from multi-center RCT research.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42022374141.
1 Introduction
Rectal cancer ranks third among the most common malignant tumors worldwide (1), and about 65% of rectal cancer is in the middle to low position. Total mesorectal excision (TME) is currently the standard surgical procedure for rectal cancer (2, 3). Some factors related to the recurrence, prolonged operation time (OP), and increased complications of rectal cancer have been identified, including male patients, pelvic stenosis, obese patients, and tumor size (4, 5). With the advancement of medical engineering technology, minimally invasive total mesorectal excision (MiTME) has gradually replaced open total mesorectal excision (OpTME) (6). Compared to OpTME, MiTME has a clear field of vision and a more precise operation process, which can obtain high-quality TME (7). However MiTME, especially in patients with difficult pelvic conditions, may not provide a clearer view and high-quality TME, and taTME has emerged, overcoming the drawbacks of previous MiTME techniques (8). There is currently a lack of meta-analysis that integrates laparoscopic and robotic versus transanal total mesorectal excision (TaTME). Therefore, the purpose of the meta-analysis is to analyze the perioperative, postoperative, and oncology outcomes of MiTME versus TaTME for mid and low rectal cancer.
2 Methods
2.1 Protocol and guidance
The study was performed according to Preferred Reporting Items for Systematic Reviews and the meta-analysis (PRISMA) (9) and the quality evaluation of this article was scored using the Newcastle-Ottawa Scale (NOS) score. The protocol for this review has been registered on PROSPERO (CRD42022374141).
2.2 Search strategy
This study involved literature published in the Embase, PubMed, Cochrane Library, Medline, and Web of Science up to September 18, 2022. We defined the eligibility criteria according to the population(P), intervention(I), comparator(C), outcome, and study design approach(O). P: The patients with mid and low rectal cancer. I: undergoing MiTME. C: TaTME was performed as a comparator. O: one or more of the following outcomes: perioperative period, postoperative indices, and oncologic outcomes. The search terms included (laparotomy OR laparoscopy OR laparoscopic OR minimally invasive OR robot OR robotic) AND (transanal OR perineal OR natural orifice) AND (colorectal cancer OR rectal cancer OR mesorectal excision OR TME OR proctectomy OR anterior resection OR abdominoperineal excision). The search strategy was not limited by language or year. The ethics or institutional review committee did not request it due to the study being designed as a systematic review and meta-analysis.
2.3 Inclusion and exclusion criteria
We have included the literature by the following criteria. Comparative data were available on the treatment of mid and low-rectal cancer through MiTME (RaTME and LaTME) and TaTME. Outcome indexes should include at least one of the following, perioperative period, postoperative indices, and oncologic outcomes. Any study which did not confirm the above inclusion criteria was excluded.
2.4 Data extraction and outcome measures
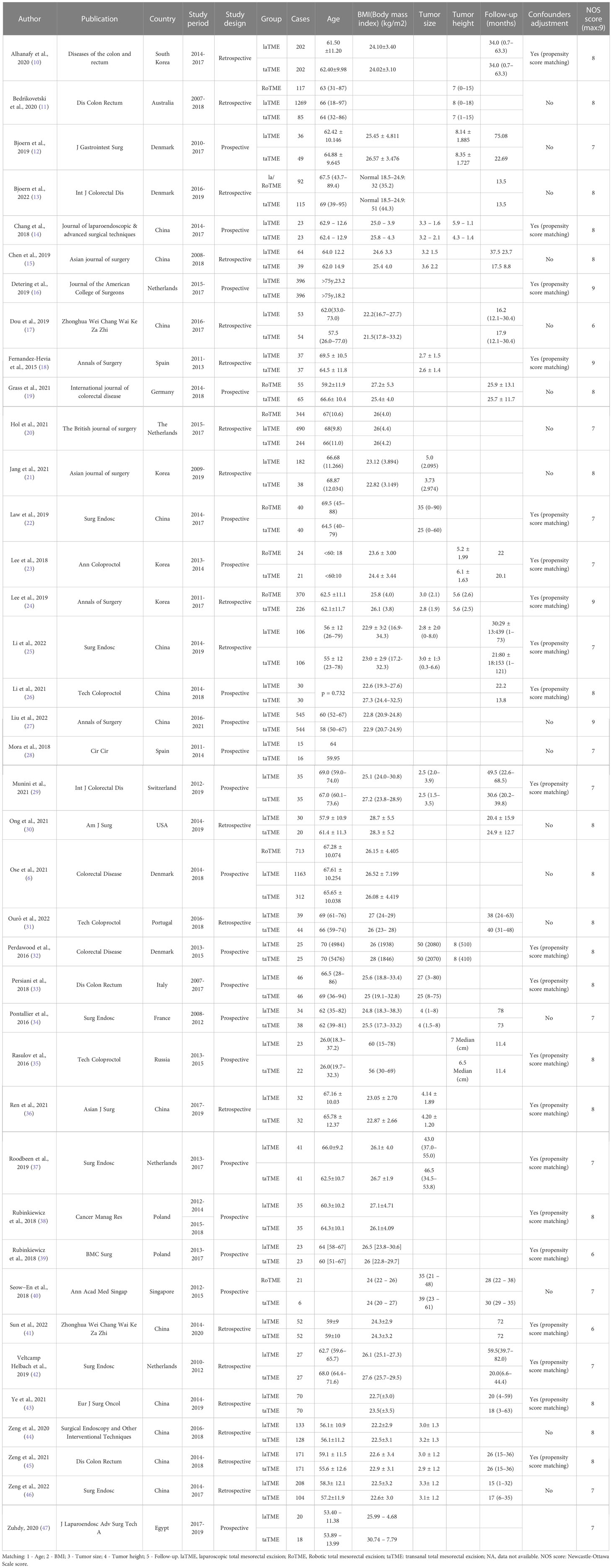
Two researchers (L.D. and Y.L.) independently reviewed the retrieved literature by the inclusion and exclusion criteria. The third researcher (Z.Y.C) was asked to participate in the discussion to decide whether to include when disagreements were encountered. The extracted data included the first author, publication, country, study type, group, age, follow-up, tumor height, and tumor size (if mentioned) (Table 1).
2.5 Statistical analysis
Statistical analysis was performed by Stata v.12.0 (Stata Corp LLC, College Station, TX, USA). For this meta-analysis, if the heterogeneity test was I2>50%, P<0.1, we used the random effect model; if the heterogeneity test was I2<50%, P>0.1, we used the fixed utility model. The combined r values and 95% confidence intervals (CIs) of each study were calculated, and the forest map displayed the characteristics of each study result. The quality of the included literature was evaluated using the Newcastle–Ottawa scale (NOS). Begg’s and Egger’s tests were used to test the publication bias. The P<0.05 was indicated as statistically significant.
3 Results
3.1 Eligible studies and study characteristics
We initially searched 6059 records. 3376 literature that was published repeatedly and cross-published were deleted. After reading the title and abstract, 2399 articles were excluded. After the remaining 284 pieces of literature were searched for full text, reading, and quality assessment, 39 pieces of literature (11010 patients: MiTME: 6268 vs TaTME: 4742) were eventually included (Figure 1). The detailed information on this literature was listed in Table 1.
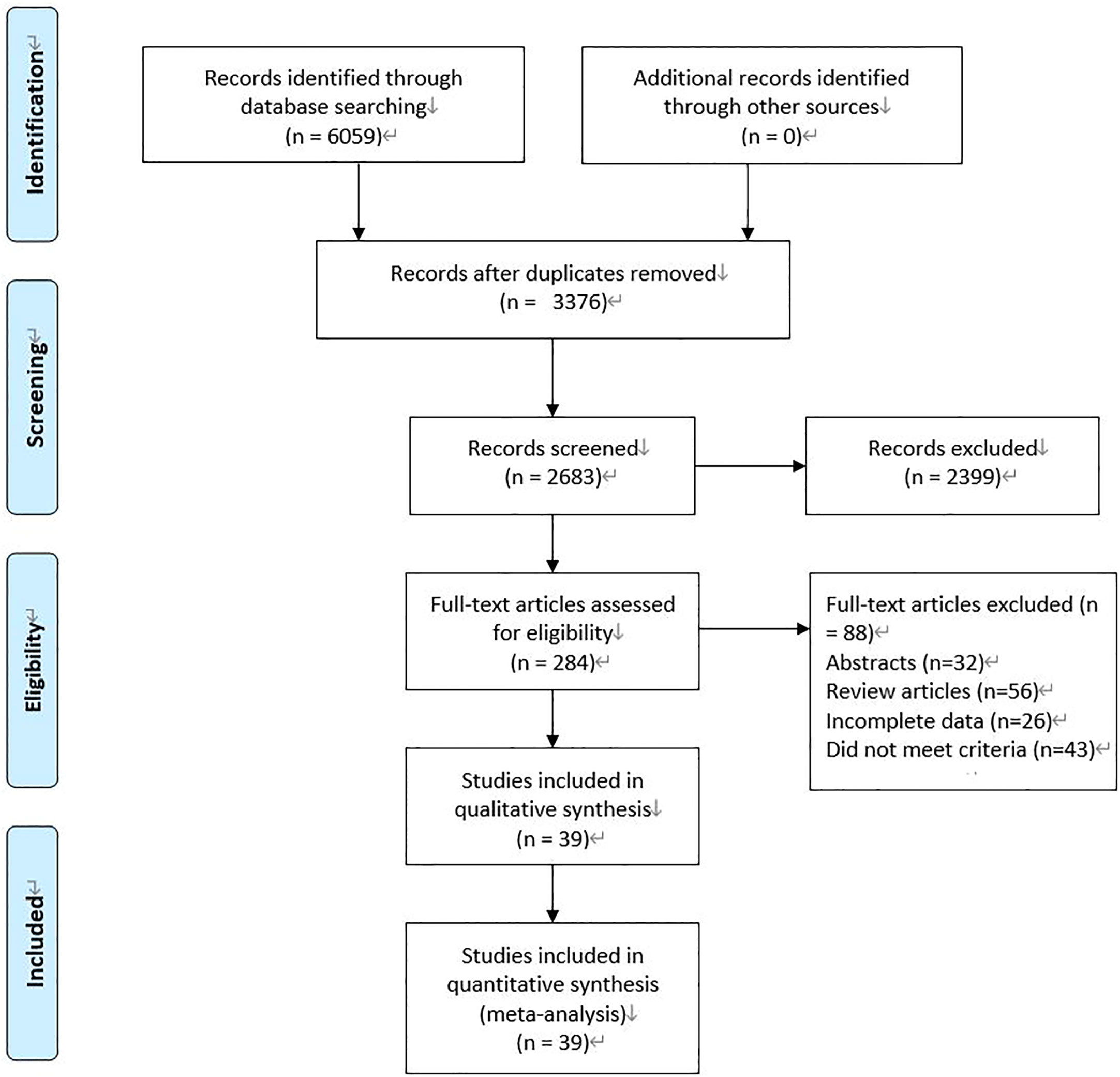
Figure 1 Flowchart for records selection process of the meta-analysis. (According to PRISMA template: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal. Pmed 1000097).
3.2 Perioperative outcomes
Data on operation time (OP) were reported in 21 studies (6, 14, 15, 17–21, 23, 25, 27, 30, 36, 38, 41, 44–49). Compared with TaTME, patients who underwent MiTME had no statistical difference (SMD -0.00; CI -0.06 to 0.06; I2 = 84.7%, P=0.885). Owing to high heterogeneity (I2 = 84.7%), we chose subgroup analysis. Compared with TaTME, patients who underwent RoTME or LaTME had no statistical difference (SMD -0.03; CI -0.37 to 0.31; I2 = 82.5%, P=0.866; SMD -0.18; CI -0.40 to 0.04; I2 = 86.0%, P=0.102). Sensitivity analysis and subgroup analysis cannot reduce heterogeneity. Therefore, we choose random effect model results (SMD -0.14; CI -0.31 to 0.33; I2 = 84.7%, P=0.116) (Figure 2A). We included 11 studies (6, 14, 15, 17, 19, 23, 25, 30, 36, 38, 44) about estimated blood loss (EBL). Compared with TaTME, patients who underwent MiTME had no statistical difference (SMD 0.00; CI -0.09 to 0.09; I2 = 61.2%, P=0.955). Owing to high heterogeneity (I2 = 61.2%), sensitivity analysis was carried out by Stata 12.0. After removing the studies by Grass et al (19) and Ong et al (30) as the sample that was “left out”, the pooled results did not change substantially but the heterogeneity was significantly reduced (SMD 0.05; CI -0.05 to 0.14; I2 = 48%, P=0.338) (Figure 2B). Data on postoperative hospital stays were reported in 7 studies (14, 15, 17, 19, 23, 30, 44). Compared with TaTME, patients who underwent MiTME had no statistical difference (SMD 0.08; CI -0.07 to 0.22; I2 = 0%, P=0.308) (Figure 2C).
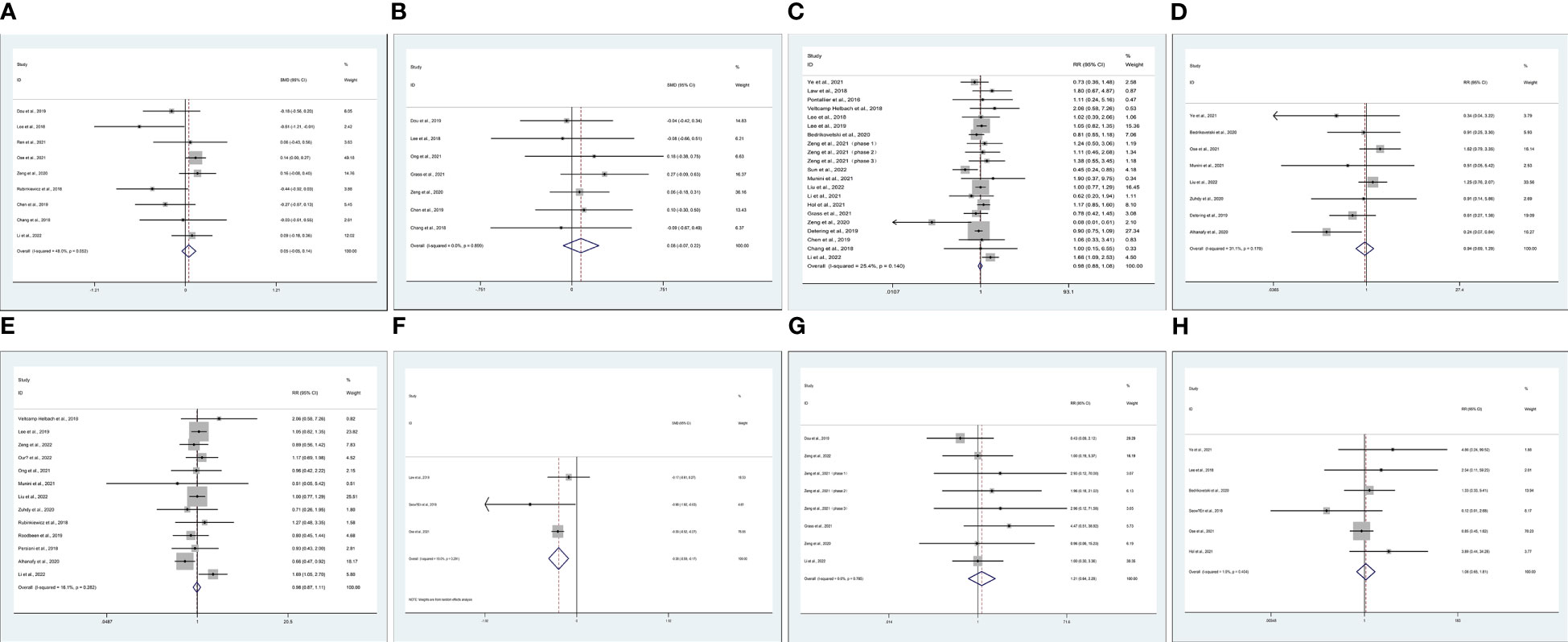
Figure 2 Meta-analysis of minimally invasive total mesorectal excision vs transanal total mesorectal excision for mid and low rectal cancer in (A) operation time, (B) estimated blood loss (C) postoperative hospital stays (D) over complications, (E) intraoperative or postoperative complications, (F) anastomotic leak rates, (G) anastomotic stenosis, (H) wound infection.
Data on over complications were reported in 20 studies (14–16, 19–21, 23, 24, 26, 27, 29, 34, 41, 43–45, 49–51). Compared with TaTME, patients who underwent MiTME had no statistical difference (RR 0.98; CI 0.88 to 1.08; I2 = 25.4%, P=0.644) (Figure 2D). Compared with TaTME, patients who underwent MiTME had no statistical difference in intraoperative (RR 0.94; CI 0.69 to 1.29; I2 = 31.1%, P=0.712) (Figure 2E-1) or postoperative complications (RR 0.98; CI 0.87 to 1.11; I2 = 16.1%, P=0.789) (Figure 2E-2). Compared with TaTME, patients who underwent MiTME had less anastomotic leak rates (SMD -0.38; CI -0.59 to -0.17; I2 = 19.0%, P<0.0001) (Figure 2F), patients who underwent MiTME had no statistical difference in anastomotic stenosis (RR 0.85; CI 0.73 to 0.98; I2 = 7.4%, P=0.564) (Figure 2G), and patients who underwent MiTME had no statistical difference for wound infection (RR 1.08; CI 0.65 to 1.81; I2 = 1.9%, P=0.755) (Figure 2H).
3.3 Postoperative outcomes
Data on circumferential resection margin (CRM) were reported in 19 studies (11–13, 16, 19, 23–27, 31, 36–38, 43, 44, 49). Compared with TaTME, patients who underwent MiTME had no statistical difference (RR 1.10; CI 0.91 to 1.34; I2 = 0%, P=0.322) (Figure 3A). Data on distal resection margin (DRM) were reported in 7 studies (24, 25, 27, 36, 38, 45, 46). Compared with TaTME, patients who underwent MiTME had no statistical difference (RR 1.49; CI 0.73 to 3.05; I2 = 0%, P=0.272) (Figure 3B). Data on major low anterior resection syndrome (LARS) were reported in 9 studies (12, 17, 19, 26, 28, 30, 34, 38, 50). Compared with TaTME, patients who underwent MiTME had no statistical difference (RR 0.93; CI 0.79 to 1.10; I2 = 0%, P=0.386) (Figure 3C). Data on lymph node yield were reported in 11 studies (14, 15, 19, 23, 24, 30, 36, 41, 43, 48, 49). Compared with TaTME, patients who underwent MiTME had no statistical difference (SMD 0.06; CI -0.04 to 0.17; I2 = 39.6%, P=0.249) (Figure 3D).

Figure 3 Meta-analysis of minimally invasive total mesorectal excision vs transanal total mesorectal excision for mid and low rectal cancer in (A) circumferential resection margin, (B) distal resection margin, (C) major low anterior resection syndrome, and (D) lymph node yield.
3.4 Oncological outcomes
5 studies recorded on 2-year disease-free survival (DFS) rate (15, 25, 29, 43, 46), 5 studies recorded on 2-year overall survival (OS) rate (15, 25, 31, 43, 46), 3 studies recorded on distant metastasis (23, 31, 43), and 8 studies recorded on local recurrence (15, 23, 25, 29, 31, 43, 46, 48). There are similarities between MiTME and TaTME for the 2-year DFS rate (RR 0.99; CI 0.88 to 1.11; I2 = 0%, P = 0.816) (Figure 4A), 2-year OS rate (RR 1.00; CI 0.90 to 1.11; I2 = 0%, P = 0.969) (Figure 4B), distant metastasis rate (RR 0.47; CI 0.17 to 1.29; I2 = 0%, P = 0.143) (Figure 4C), and local recurrence rate (RR 1.49; CI 0.75 to 2.97; I2 = 0%, P = 0.250) (Figure 4D).
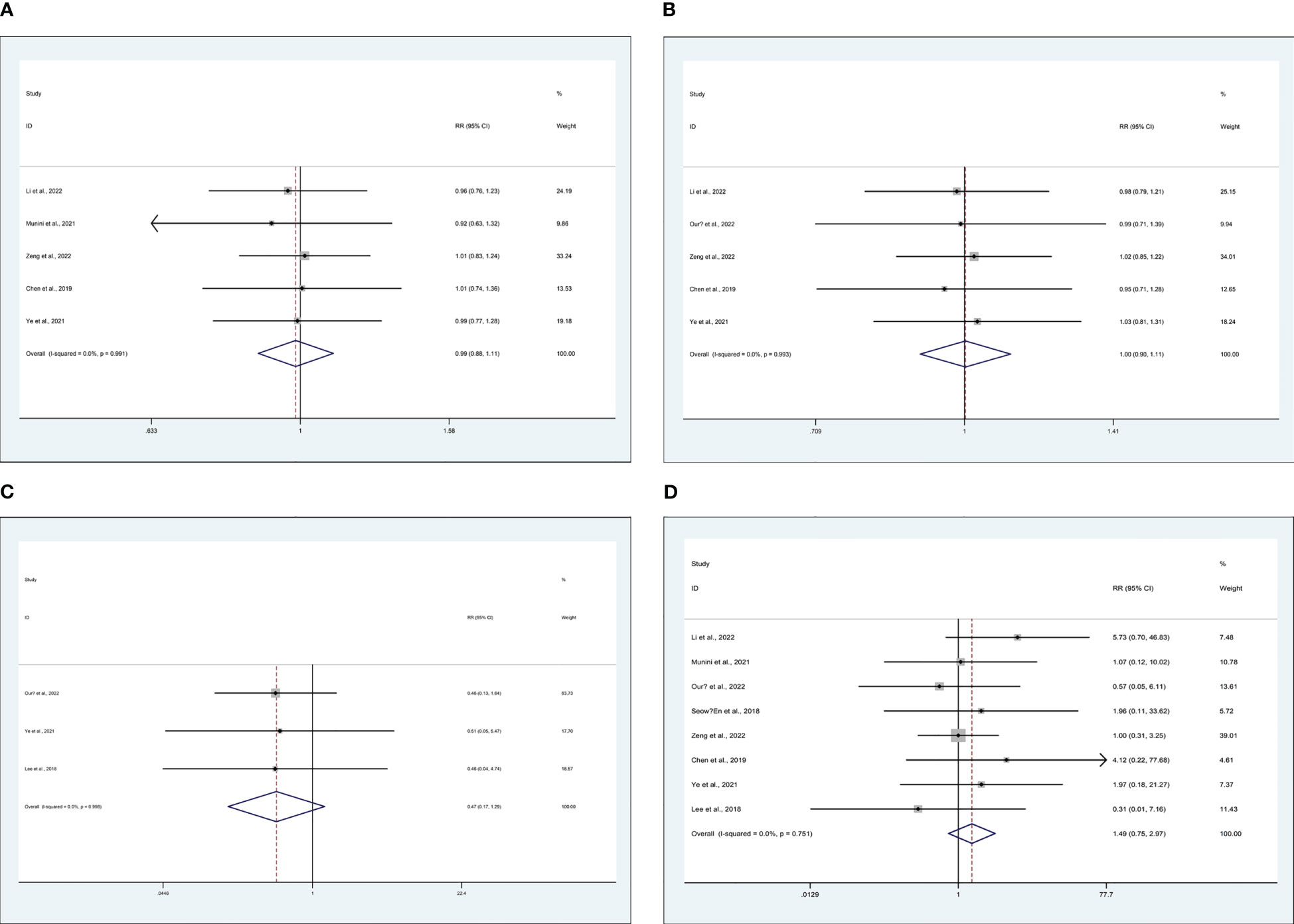
Figure 4 Meta-analysis of minimally invasive total mesorectal excision vs transanal total mesorectal excision for mid and low rectal cancer in (A) 2-year DFS rate, (B) 2-year OS rate, (C) distant metastasis rate, and (D) local recurrence rate.
4 Publication bias
We conducted publication bias on more than 15 included studies using Begg’s test. For OP, Begg’s test results revealed that t=-1.87, P=.075 in Supplementary Figure 1A. For over complications. Begg’s test results revealed that t=0.81, P=.427 in Supplementary Figure 1B. For the circumferential resection margin, Begg’s test results revealed that t=4.20, P=.001 in Supplementary Figure 1C. There is no publication bias except circumferential resection margin in the above.
5 Discussion
As TaTME has reported more and more in recent years, so has its controversy (52). The main focus is on whether TaTME can get better safety and efficacy with mid to low-rectal cancer in patients. The results of this study show that patients who underwent MiTME had fewer anastomotic leak rates. Compared with TaTME, patients who underwent MiTME had no statistical difference in OP, EBL, postoperative hospital stay, over complications, intraoperative complications, postoperative complications, anastomotic stenosis, wound infection, CRM, DRM, major LARS, lymph node yield, 2-year DFS rate, 2-year OS rate, distant metastasis rate, and local recurrence rate. The absence of heterogeneity in postoperative hospital stays, circular differential recovery margin, total recovery margin, major low adverse recovery syndrome, 2-year disease-free survival, 2-year overall survival rate, distance metastasis rate, and local recurrence rate indicates that these results are reliable. The slightly lower heterogeneity of postoperative hospital stays, over applications, intra-operational applications, postoperative applications, analytical leak rates, analytical stenosis, and weak node yield indicates that these results are relatively reliable. The heterogeneity of EBL is slightly higher, which may be related to different surgeons. The high heterogeneity of OP indicates the low reliability of these results.
CRM positive rate is a good evaluation index for tumor outcome (53). This study’s results suggest no significant difference in the positive rate of CRM, DRM, lymph node yield between TaTME and MITME. This indicates that there is no difference in the treatment effectiveness between the two. In secondary outcomes, there is no significant difference between the two in terms of OP, EBL, postoperative hospital stays, CRM, DRM, LARS, lymph node yield, and incidence of intraoperative and postoperative complications. However, it is expected to achieve better results with the technique becomes more proficient in the application of mid and low rectal cancer (54). For oncological outcomes, only a small portion of studies have reported differences in late local recurrence and survival between the two groups. The Zeng (46) et al.’s study was found that the local recurrence rate was 3.8% in both groups of patients and another study confirmed that local recurrence is only 3% after TaTME for rectal cancer (55). However, our research results showed that there was no difference in DFS, OS, distance metastasis rate, and local recurrence rate between the two groups at 2 years. Currently, larger RCT studies are underway (56), and more reliable results are expected.
Both types of rectal cancer surgery have a certain impact on a patient’s quality of life (57), mainly LARS (58). A study suggests that some patients develop severe LARS after TaTME (59). Another article found a low incidence of mild/severe LARS in patients after TaTME (60). There was no significant difference in LARS between the two groups in this study. It shows that the probability of anal sphincter injury function damage is not increased after the anal operation of TaTME. This conclusion also adds a strong backing for the application of TaTME.
Of course, our research also has some limitations: 1. The included studies are retrospective studies or prospective cohort studies, which will inevitably be affected by selection bias. 2. In terms of the baseline report of the cases included in the literature, only some of them were provided. Of course, we analyzed the baseline data that can be extracted from the included literature, but we still lacked the comprehensiveness of the data, and could not conduct subgroup analysis according to general characteristics, such as male-female ratio, BMI value, etc. 3. In the data analysis, although we conducted a sensitivity analysis on highly heterogeneous outcome indicators, some results did not identify the source of their heterogeneity. 4. In terms of analysis indicators, the long-term efficacy, such as local tumor recurrence rate, was not analyzed by subgroup according to the follow-up time, while only 5 articles were included in the 2-year DFS and 2-year OS, and the number of articles included in the analysis was insufficient. 5. At present, the follow-up time of various studies is limited, and not enough long-term efficacy data is provided for analysis. In terms of functional outcome data, only kinds of literature mention it and it is not uniformly quantified, which causes certain difficulties in analysis.
6 Conclusion
This study comprehensively and systematically evaluated the safety and efficacy of MiTME and TaTME in the treatment of mid to low rectal cancer through meta-analysis. There is no difference between the two except for patients with MiTME who have a lower anastomotic leakage rate, which provides some evidence-based reference for clinical practice. Of course, in the future, more scientific and rigorous conclusions need to be drawn from multi-center RCT research.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
Conceptualization: LD, ZD. Data curation: LD, LY, ZC. Formal analysis: LD, LY. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific Research Foundation of Health and Family Planning Commission of Chengdu (2022124) and the Scientific Research Foundation of Health and Family Planning Commission of Sichuan Province (20PJ236).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1167200/full#supplementary-material
Supplementary Figure 1 | Egger's publication bias plot to detect publication bias.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke. P, van Krieken. JH. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol (2002) 20:1729–34. doi: 10.1200/JCO.2002.07.010
3. . An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis (2018) 20(Suppl 6):33–46. doi: 10.1111/codi.14376
4. Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, et al. Can we predict immediate outcome after laparoscopic rectal surgery? multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg (2008) 247:642–9. doi: 10.1097/SLA.0b013e3181612c6a
5. Di Saverio S, Gallo G, Davies RJ, Bergamaschi R, Wheeler J, Sileri P, et al. Robotic-assisted transanal total mesorectal excision for rectal cancer: more questions than answers. Tech Coloproctol (2021) 25:987–8. doi: 10.1007/s10151-020-02402-7
6. Ose. I, Perdawood. SK. A nationwide comparison of short-term outcomes after transanal, open, laparoscopic, and robot-assisted total mesorectal excision. Colorectal Dis (2021) 23:2671–80. doi: 10.1111/codi.15809
7. Young. M, Pigazzi. A. Total mesorectal excision: open, laparoscopic or robotic. Recent Results Cancer Res (2014) 203:47–55. doi: 10.1007/978-3-319-08060-4_6
8. Creavin B, Kelly ME, Ryan É J, Ryan. OK, Winter. DC. Oncological outcomes of laparoscopic versus open rectal cancer resections: meta-analysis of randomized clinical trials. Br J Surg (2021) 108:469–76. doi: 10.1093/bjs/znaa154
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
10. Alhanafy MK, Park SS, Park SC, Park B, Kim MJ, Sohn DK, et al. Early experience with transanal total mesorectal excision compared with laparoscopic total mesorectal excision for rectal cancer: a propensity score-matched analysis[J]. Dis Colon Rectum (2020) 63:1500–10.
11. Bedrikovetski S, Dudi-Venkata NN, Kroon HM, Moore JW, Hunter. RA, Sammour. T. Outcomes of minimally invasive versus open proctectomy for rectal cancer: a propensity-matched analysis of bi-national colorectal cancer audit data. Dis Colon Rectum (2020) 63:778–87. doi: 10.1097/DCR.0000000000001654
12. Bjoern MX, Nielsen. S, Perdawood. SK. Quality of life after surgery for rectal cancer: a comparison of functional outcomes after transanal and laparoscopic approaches. J Gastrointest Surg (2019) 23:1623–30. doi: 10.1007/s11605-018-4057-6
13. Bjoern MX, Clausen FB, Seiersen M, Bulut O, Bech-Knudsen F, Jansen JE, et al. Quality of life and functional outcomes after transanal total mesorectal excision for rectal cancer-results from the implementation period in Denmark. Int J Colorectal Dis (2022) 45:2197–2202. doi: 10.1007/s00384-022-04219-2
14. Chang. TC, Kiu. KT. Transanal total mesorectal excision in lower rectal cancer: comparison of short-term outcomes with conventional laparoscopic total mesorectal excision. J Laparoendosc Adv Surg Tech A (2018) 28:365–9. doi: 10.1089/lap.2017.0520
15. Chen Y-T, Kiu K-T, Yen. M-H, Chang. T-C. Comparison of the short-term outcomes in lower rectal cancer using three different surgical techniques: transanal total mesorectal excision (TME), laparoscopic TME, and open TME. Asian J Surg (2019) 42:674–80. doi: 10.1016/j.asjsur.2018.09.008
16. Detering R, Roodbeen SX, van Oostendorp SE, Dekker J-WT, Sietses C, Bemelman WA, et al. Three-year nationwide experience with transanal total mesorectal excision for rectal cancer in the Netherlands: a propensity score-matched comparison with conventional laparoscopic total mesorectal excision. J Am Coll Surgeons (2019) 228:235–244.e1. doi: 10.1016/j.jamcollsurg.2018.12.016
17. Dou R, Sun W, Luo S, Hou Y, Zhang. C, Kang. L. Comparison of postoperative bowel function between patients undergoing transanal and laparoscopic total mesorectal excision. Zhonghua Wei Chang Wai Ke Za Zhi (2019) 22:246–54. doi: 10.3760/cma.j.issn.16710274.2019.03.011
18. Fernandez-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, del Gobbo GD, et al. Transanal total mesorectal excision in rectal cancer short-term outcomes in comparison with laparoscopic surgery. Ann Surg (2015) 261:221–7. doi: 10.1097/SLA.0000000000000865
19. Grass J-K, Persiani R, Tirelli F, Chen C-C, Caricato M, Pecorino A, et al. Robotic versus transanal total mesorectal excision in sexual, anorectal, and urinary function: a multicenter, prospective, observational study. Int J colorectal Dis (2021) 36:2749–61. doi: 10.1007/s00384-021-04030-5
20. Hol JC, Burghgraef TA, Rutgers MLW, Crolla RMPH, van Geloven NAW, Hompes R, et al. Comparison of laparoscopic versus robot-assisted versus transanal total mesorectal excision surgery for rectal cancer: a retrospective propensity score-matched cohort study of short-term outcomes. Br J Surg (2021) 108:1380–7. doi: 10.1093/bjs/znab233
21. Jang HB, Kang SB, Lee H, Choi. BJ, Lee. SC. Anastomotic leakage and chronic presacral sinus after transanal total mesorectal excision (taTME) for rectal cancer: a comparative study to laparoscopic TME. Asian J Surg (2021) 277:1–6. doi: 10.1016/j.asjsur.2021.11.009
22. Law W, Foo DCC. Comparison of early experience of robotic and transanal total mesorectal excision using propensity score matching[J]. Surg Endoscopy (2019) 33:757–63.
23. Lee KY, Shin JK, Park YA, Yun SH, Huh JW, Cho YB, et al. Transanal endoscopic and transabdominal robotic total mesorectal excision for mid-to-Low rectal cancer: comparison of short-term postoperative and oncologic outcomes by using a case-matched analysis. Ann Coloproctol (2018) 34:29–35. doi: 10.3393/ac.2018.34.1.29
24. Lee L, de Lacy B, Gomez Ruiz M, Liberman AS, Albert MR, Monson JRT, et al. A multicenter matched comparison of transanal and robotic total mesorectal excision for mid and low-rectal adenocarcinoma. Ann Surg (2019) 270:1110–6. doi: 10.1097/SLA.0000000000002862
25. Li Z, Xiao J, Hou Y, Zhang X, Jie H, Liu H, et al. Transanal versus laparoscopic total mesorectal excision in Male patients with low tumor location after neoadjuvant therapy: a propensity score-matched cohort study. Gastroenterol Res Pract (2022) 2022:2387464. doi: 10.1155/2022/2387464
26. Li Y, Bai X, Niu B, Zhou J, Qiu H, Xiao Y, et al. A prospective study of health related quality of life, bowel and sexual function after TaTME and conventional laparoscopic TME for mid and low rectal cancer. Tech Coloproctol (2021) 25:449–59. doi: 10.1007/s10151-020-02397-1
27. Liu H, Zeng Z, Zhang H, Wu M, Ma D, Wang Q, et al. Morbidity, mortality, and pathologic outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer short-term outcomes from a multicenter randomized controlled trial. Ann Surg (2022) 37:1997–2011. doi: 10.1097/SLA.0000000000005523
28. Mora L, Zarate A, Serra-Aracil X, Pallisera A, Serra.S. Navarro-Soto. [Functional impairment S. And quality of life after rectal cancer surgery]. Cir Cir (2018) 86:140–7. doi: 10.24875/CIRU.M18000022
29. Munini M, Popeskou SG, Galetti K, Roesel R, Mongelli. F, Christoforidis. D. Transanal (TaTME) vs. laparoscopic total mesorectal excision for mid and low rectal cancer: a propensity score-matched analysis of early and long-term outcomes. Int J Colorectal Dis (2021) 36:2271–9. doi: 10.1007/s00384-021-04019-0
30. Ong GK, Tsai B, Patron RL, Johansen O, Lane F, Melbert RB, et al. Transanal total mesorectal excision achieves equivalent oncologic resection compared to laparoscopic approach, but with functional consequences. Am J Surg (2021) 221:566–9. doi: 10.1016/j.amjsurg.2020.11.013
31. Ourô S, Ferreira M, Roquete. P, Maio. R. Transanal versus laparoscopic total mesorectal excision: a comparative study of long-term oncological outcomes. Tech Coloproctol (2022) 26:279–90. doi: 10.1007/s10151-022-02570-8
32. Perdawood SK, Khefagie GAA. Transanal vs laparoscopic total mesorectal excision for rectal cancer: initial experience from Denmark[J]. Colorectal Dis Off J Assoc Coloproctology Great Britain Ireland (2016) 18:51–8.
33. Persiani R, Biondi A, Pennestrì F, Fico V, De Simone V, Tirelli F, et al. Transanal total mesorectal excision vs laparoscopic total mesorectal excision in the treatment of low and middle rectal cancer: a propensity score matching analysis[J]. Dis Colon Rectum (2018) 61:809–16.
34. Pontallier A, Denost Q, Van Geluwe B, Adam JP, Celerier. B, Rullier. E. Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg Endosc (2016) 30:4924–33. doi: 10.1007/s00464-016-4833-x
35. Rasulov AO, Mamedli ZZ, Gordeyev SS, Kozlov NA, Dzhumabaev HE. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer[J]. Tech Coloproctol (2016) 20:227–34.
36. Ren J, Liu S, Luo H, Wang. B, Wu. F. Comparison of short-term efficacy of transanal total mesorectal excision and laparoscopic total mesorectal excision in low rectal cancer. Asian J Surg (2021) 44:181–5. doi: 10.1016/j.asjsur.2020.05.007
37. Roodbeen SX, Penna M, Mackenzie H, Kusters M, Slater A, Jones OM, et al. Transanal total mesorectal excision (TaTME) versus laparoscopic TME for MRI-defined low rectal cancer: a propensity score-matched analysis of oncological outcomes. Surg Endosc (2019) 33:2459–67. doi: 10.1007/s00464-018-6530-4
38. Rubinkiewicz M, Nowakowski M, Wierdak M, Mizera M, Dembiński M, Pisarska M, et al. Transanal total mesorectal excision for low rectal cancer: a case-matched study comparing TaTME versus standard laparoscopic TME. Cancer Manag Res (2018) 10:5239–45. doi: 10.2147/CMAR.S181214
39. Rubinkiewicz M, Nowakowski M, Wierdak M, Mizera M, Dembiński M, Pisarska M, et al. Transanal total mesorectal excision for low rectal cancer: a case-matched study comparing TaTME versus standard laparoscopic TME[J]. Cancer Manag Res (2018) 10:5239–45.
40. Seow-En I, Seow-Choen F. An initial experience comparing robotic total mesorectal excision (RTME) and transanal total mesorectal excision (taTME) for low rectal Tumours[J]. Ann Acad Med Singap (2018) 47:188–90.
41. Sun R, Cong L, Qiu HZ, Lin GL, Wu B, Niu BZ, et al. [Safety and prognosis analysis of transanal total mesorectal excision versus laparoscopic mesorectal excision for mid-low rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi (2022) 25:522–30. doi: 10.3760/cma.j.cn4415302021081100321
42. Veltcamp Helbach JM, Koedam TWA, Knol JJ, Velthuis S, Bonjer HJ, Tuynman JB, et al. Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision[J]. Surg Endosc (2019) 33:79–87.
43. Ye J, Tian Y, Li F, van Oostendorp S, Chai Y, Tuynman J, et al. Comparison of transanal total mesorectal excision (TaTME) versus laparoscopic TME for rectal cancer: a case matched study. Eur J Surg Oncol (2021) 47:1019–25. doi: 10.1016/j.ejso.2020.11.131
44. Zeng ZW, Luo SL, Chen JJ, Cai YH, Zhang. XW, Kang. L. Comparison of pathological outcomes after transanal versus laparoscopic total mesorectal excision: a prospective study using data from randomized control trial. Surg Endoscopy Other Interventional Techniques (2020) 34:3956–62. doi: 10.1007/s00464-019-07167-1
45. Zeng Z, Liu Z, Huang L, Liu H, Jie H, Luo S, et al. Transanal total mesorectal excision in mid-low rectal cancer: evaluation of the learning curve and comparison of short-term results with standard laparoscopic total mesorectal excision. Dis Colon Rectum (2021) 64:380–8. doi: 10.1097/DCR.0000000000001816
46. Zeng Z, Liu Z, Luo S, Liang Z, Huang L, Ruan L, et al. Three-year outcomes of transanal total mesorectal excision versus standard laparoscopic total mesorectal excision for mid and low rectal cancer. Surg Endosc (2022) 36:3902–10. doi: 10.1007/s00464-021-08707-4
47. Zuhdy M, Elmore U, Shams N, Hegazy MAF, Roshdy S, Eldamshety O, et al. Transanal versus laparoscopic total mesorectal excision: a comparative prospective clinical trial from two centers. J Laparoendosc Adv Surg Tech A (2020) 30:769–76. doi: 10.1089/lap.2019.0828
48. Seow-En. I, Seow-Choen. F. An initial experience comparing robotic total mesorectal excision (RTME) and transanal total mesorectal excision (taTME) for low rectal tumours. Ann Acad Med Singap (2018) 47:188–90. doi: 10.47102/annals-acadmedsg.V47N5p188
49. Law. WL, Foo. DCC. Comparison of early experience of robotic and transanal total mesorectal excision using propensity score matching. Surg Endosc (2019) 33:757–63. doi: 10.1007/s00464-018-6340-8
50. Veltcamp Helbach M, Koedam TWA, Knol JJ, Diederik A, Spaargaren GJ, Bonjer HJ, et al. Residual mesorectum on postoperative magnetic resonance imaging following transanal total mesorectal excision (TaTME) and laparoscopic total mesorectal excision (LapTME) in rectal cancer. Surg Endosc (2019) 33:94–102. doi: 10.1007/s00464-018-6279-9
51. Bednarski BK. Minimally invasive rectal surgery: laparoscopy, robotics, and transanal approaches. J Surg Oncol (2020) 122:78–84. doi: 10.1002/jso.25925
52. Jiang TY, Ma. JJ, Zheng. MH. Controversies and consensus in transanal total mesorectal excision (taTME): is it a valid choice for rectal cancer? J Surg Oncol (2021) 123(Suppl 1):S59–s64. doi: 10.1002/jso.26340
53. Tilney HS, Rasheed S, Northover. JM, Tekkis. PP. The influence of circumferential resection margins on long-term outcomes following rectal cancer surgery. Dis Colon Rectum (2009) 52:1723–9. doi: 10.1007/DCR.0b013e3181b54fbd
54. Francis N, Penna M, Mackenzie H, Carter. F, Hompes. R. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc (2017) 31:2711–9. doi: 10.1007/s00464-017-5562-5
55. Roodbeen SX, Spinelli A, Bemelman WA, Di Candido F, Cardepont M, Denost Q, et al. Local recurrence after transanal total mesorectal excision for rectal cancer: a multicenter cohort study. Ann Surg (2021) 274:359–66. doi: 10.1097/SLA.0000000000003757
56. Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc (2016) 30:3210–5. doi: 10.1007/s00464-015-4615-x
57. Frick MA, Vachani CC, Hampshire MK, Bach C, Arnold-Korzeniowski K, Metz JM, et al. Survivorship after lower gastrointestinal cancer: patient-reported outcomes and planning for care. Cancer (2017) 123:1860–8. doi: 10.1002/cncr.30527
58. Koedam TW, van Ramshorst GH, Deijen CL, Elfrink AK, Meijerink WJ, Bonjer HJ, et al. Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient-reported quality of life and functional outcome. Tech Coloproctol (2017) 21:25–33. doi: 10.1007/s10151-016-1570-z
59. Tirelli F, Lorenzon L, Biondi A, Neri I, Santoro. G, Persiani. R. Functional outcomes after transanal total mesorectal excision (TaTME): a random forest analysis to predict patients' outcomes. Tech Coloproctol (2023), 1–10. doi: 10.1007/s10151-023-02775-5
60. De Simone V, Persiani R, Biondi A, Litta F, Parello A, Campennì P, et al. One-year evaluation of anorectal functionality and quality of life in patients affected by mid-to-low rectal cancer treated with transanal total mesorectal excision. Updates Surg (2021) 73:157–64. doi: 10.1007/s13304-020-00919-y
Keywords: minimally invasive total mesorectal excision, transanal total mesorectal excision, mid and low-rectal cancer, systematic review, meta-analysis
Citation: Gang DY, Dong L, DeChun Z, Yichi Z and Ya L (2023) A systematic review and meta-analysis of minimally invasive total mesorectal excision versus transanal total mesorectal excision for mid and low rectal cancer. Front. Oncol. 13:1167200. doi: 10.3389/fonc.2023.1167200
Received: 21 February 2023; Accepted: 10 May 2023;
Published: 12 June 2023.
Edited by:
Emanuele Damiano Urso, University of Padua, ItalyReviewed by:
Ugo Grossi, University of Padua, ItalyBeatriz Martin-Perez, University Hospital of Badajoz, Spain
Copyright © 2023 Gang, Dong, DeChun, Yichi and Ya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Dong, OTEzNDg3MjkwQHFxLmNvbQ==
 Du Yong Gang1
Du Yong Gang1 Lin Dong
Lin Dong