
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 July 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1166549
Objective: To explore the early onset, development and histological features of gastric signet-ring cell carcinoma (SRCC).
Methods: Three hundred and sixty-two patients with differentiated adenocarcinoma with signet-ring cells were enrolled. Histomorphological and immunohistochemical features and patterns of the specimens were observed in detail.
Results: Infection of the gastric mucosa, especially by Helicobacter pylori, can cause massive cell proliferation and transformation in the deep gastric foveola, the isthmus of the gastric gland, and the proliferative zone of the upper neck of the gland. Signet-ring-like heterocysts monoclonally proliferated after the redifferentiation and reproliferation, extending horizontally along the gastric foveola. Gastric foveolar-type SRCC grew infiltratively into the lamina propria of the mucosa and the submucosa, signet-ring cells could differentiate into undifferentiated adenocarcinoma with signet-ring cell differentiation, mucinous adenocarcinoma with signet-ring cell differentiation, gastric adenocarcinoma with signet-ring cell differentiation, and fundus gland adenocarcinoma with signet-ring cell differentiation.
Conclusion: Early SRCC developed from the proliferative zones of the fundus of the gastric foveola and the neck of the gastric gland, growing horizontally along the gastric foveola. It developed into gastric adenocarcinoma with signet-ring cell differentiation after reproliferation and retransformation in the mucosa.
Gastric signet-ring cell carcinoma (SRCC) is a special type of gastric adenocarcinoma with a high malignancy and low diagnosis rate of early SRCC (1–3). However, research in recent years has indicated a link between gastric and intestinal-related immunophenotypes and the biological behavior and prognosis of gastric SRCC (4–6). According to different marker expressions, SRCC can be divided into gastric carcinoma and intestinal carcinoma, with the gastric carcinoma being further divided into small foveolar epithelial (surface mucous epithelium) type, pyloric gland type, and fundus gland type (7, 8). Histologically, the fundus of each gastric foveola that is collected with 3–5 glands is the basic structure unit of the gastric mucosa, and is known as the gastric unit; the structure is of monoclonal origin (9). Cells proliferate actively in the isthmus and the upper neck of the gastric gland. Gastric SRCC originates in the proliferative zone (10). However, this zone contains a variety of poorly differentiated cells with distinct differentiation directions, and the origin of SRCC in the proliferative zone remains unclear (11, 12). It is widely accepted that almost all malignancies experience atypical hyperplasia before onset, with a few transforming directly from normal conditions to malignancies without experiencing atypical hyperplasia of epithelium (13–15). The World Health Organization (WHO) has classified H. pylori infection as a carcinogen of gastric carcinoma, and it has become a trend to prevent gastric carcinoma by eliminating H. pylori through H. pylori detection and treatment (16). A large number of studies have found that H. pylori causes changes in the morphology and signal transduction of gastric epithelial cells (17–20). About 10% of gastric cancer (GC) and 1% of colorectal cancer (CRC) are characterized by signet ring cell carcinoma. SRCC is associated with poor prognosis, but the underlying molecular features remain unclear (21). Regardless of the site in which SRCC occurs, it has similar molecular features. Multistage carcinogenesis of SRCC involving genetic and epigenetic aberrations is associated with stage-dependent prognosis (22). There are significant differences in clinicopathological features and prognosis between signet ring cell carcinoma (SRC) and intestinal type gastric cancer (ITGC) (23). In gastric cancer with signet ring cell (SRC) component, there is a good prognosis in stage I. However, the prognosis is poor in stage II/III (24).
The results of our previous research revealed that H. pylori selectively adhered to and destroyed the cytoplasm of mucous cells on the surface of the gastric mucosa, causing the ovoid and spherical mucus-containing particles wrapped in limitans in the cytoplasm on the nucleus to disappear, the cytoplasm showed cobweb-like vacuolar degeneration, and the epithelial cells on the surface of the gastric mucosa exfoliated, leading to the proliferation and transformation of the mucous neck cells in the proliferative zone, the formation of papillary epithelioma-like hyperplasia of mucous cells on the surface of the gastric mucosa, the extensive and segmental atrophy of the lamina propria gland of the gastric mucosa, and other changes (21, 22). Studies have shown H. pylori infection could cause foveolar-type signet-ring cell carcinoma, and the histopathological features and differential diagnosis of this type of gastric carcinoma have been proposed (23). A total of 362 patients with differentiated adenocarcinoma with signet-ring cells were enrolled in this study. Histomorphological and immunohistochemical features and patterns of the specimens were observed in detail. Our aim was to provide pathological assistance to clinicians in the precise treatment and tracking of stem cell proliferation and transformation in the proliferative zone, enabling them to intervene in the onset and development of gastric SRCC as early as possible.
From May 2020 to May 2022, 362 patients diagnosed with foveal epithelial hyperplasia of gastric mucosa, dysfunction, abnormal proliferation, and transformation by gastroscopic biopsy and who developed differentiated adenocarcinoma with signet-ring cells were enrolled from Shenzhen Longgang District Fourth People’s Hospital, the Third Affiliated Hospital of Zhengzhou University, Peking University Shenzhen Hospital, and the 990th Hospital of the PLA Joint Logistic Support Force. ESD resection or surgical local excision was conducted for 15 patients diagnosed with small signet-ring cell carcinoma-like lesions, foveolar-type signet-ring cell carcinoma, and adenocarcinoma with signet-ring cell differentiation by gastric mucosa biopsy.
Specimens were fixed with fresh 10% neutral buffered formalin solution (NBF) for 8–48 h within 30 min after detachment via biopsy and surgery, with a fixative to tissue volume ratio of 10:1. Materials were collected and sectioned in a standardized manner according to the gastric ESD specimens and early gastric carcinoma specimens (24). H&E, special staining, and immunohistochemistry (IHC) staining were performed.
Using the En Vision two-step method, the tissue sections were deparaffinized, hydrated, and rinsed with distilled water. The sections were then placed in tris-buffered saline (TBS) for 10 minutes. The endogenous peroxidase was blocked for another 5 minutes, and the sections were treated with TBS for 10 minutes. The primary antibodies (CKpan, CK7, CK20, villin, HER2, MUC5AC, ki-67, Hp) were incubated with the sections for 30 minutes at room temperature. The sections were incubated in the En Vision™ after being washed in TBS for 10 min. The sections were washed in TBS for 10 min, and were then added with the secondary antibody, and allowed to react for 10 minutes. After incubating the chromogenic substrate solution for 10 minutes, it was rinsed with distilled water. DAB was used to develop the sections and hematoxylin was used to counterstain them. The known gastric mucosa sections were used as the positive control, while the PBS buffer solution was used as the negative control instead of primary antibodies. All working solutions were purchased from MXB Biotechnologies, and the operation procedures were carried out in strict accordance with the kit instructions.
Among the 362 patients studied, there were 226 (62.4%) males and 136 (37.7%) females. Among them, there were 142 (39.2%) patients with an onset age ≤ 60 and 220 (60.8%) with an onset age > 60. The relationship between the onset age and gender is shown in Table 1.
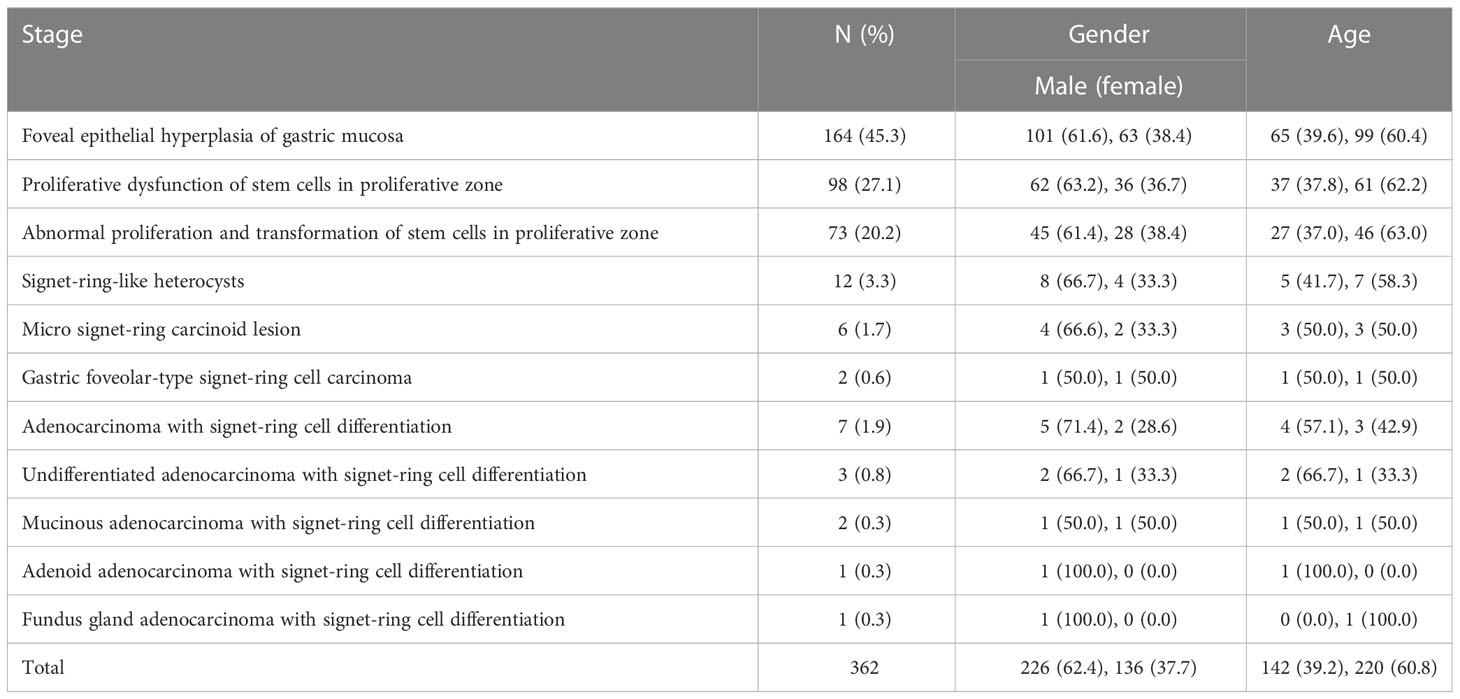
Table 1 Relationship between early onset and development of gastric signet-ring cell carcinoma and the onset age and gender.
When the gastric mucosa was affected by infection, chemical irritation, autoimmune disease, heredity, and other factors, it may result in foveal epithelial hyperplasia of gastric mucosa, dysfunction, abnormal proliferation, and transformation, which then developed into differentiated adenocarcinoma with signet-ring cells, as shown in Figure 1.
When the gastric mucosa was affected by infection, chemical irritation, autoimmune disease, heredity, and other factors, particularly H. pylori infection, it may result in foveal epithelial hyperplasia of the gastric mucosa. The cytoplasm and nucleus of the proliferative surface mucous cell showed hazy basophilic degeneration, the nucleus grew to be about twice the size of normal, and there may be a small nucleoli (Figure 2A). It then developed stem cell dysfunction in the proliferative zone (Figure 2B). Persistent dysfunction resulted in massive cell proliferation and accumulation in the proliferative zone of the gland’s upper neck, forming a lamellar heterocyst nested structure, known as abnormal proliferation and transformation of stem cells in the proliferative zone (Figure 2C). Single or multiple signet-ring cell-like cells were formed because of abnormal proliferation and transformation. This cell was 1-2 times the size of the peripheral columnar epithelium, with its nucleus being crescent or irregularly oval, forming the cytologically SRCC-like cells, which were called signet-ring cell-like heterocysts in the deep gastric foveola (Figure 2D). After redifferentiation and reproliferation, signet-ring-like heterocysts developed into classical-type signet-ring cells. The cells were round, with a diameter of 15–30 μm with reddish mucous substances in the cytoplasm; the nucleus was deviated and was signet-ring or crescent-shaped (Figure 3A). The foveolar-type signet-ring cell carcinoma was formed when signet-ring-like heterocysts and classical-type signet-ring cells extended horizontally along one third of the opening side of the fundus gland mucosa, with a length of 3–6 mm (Figure 3B). When foveolar-type signet-ring cell carcinoma infiltrated into the lamina propria of the mucosa and the submucosa, the signet-ring cell could differentiate into undifferentiated adenocarcinoma with signet-ring cell differentiation (Figure 3C), mucinous adenocarcinoma with signet-ring cell differentiation (Figure 3D), gastric adenocarcinoma with signet-ring cell differentiation (Figure 3E), and fundus gland adenocarcinoma with signet-ring cell differentiation (Figure 3F).
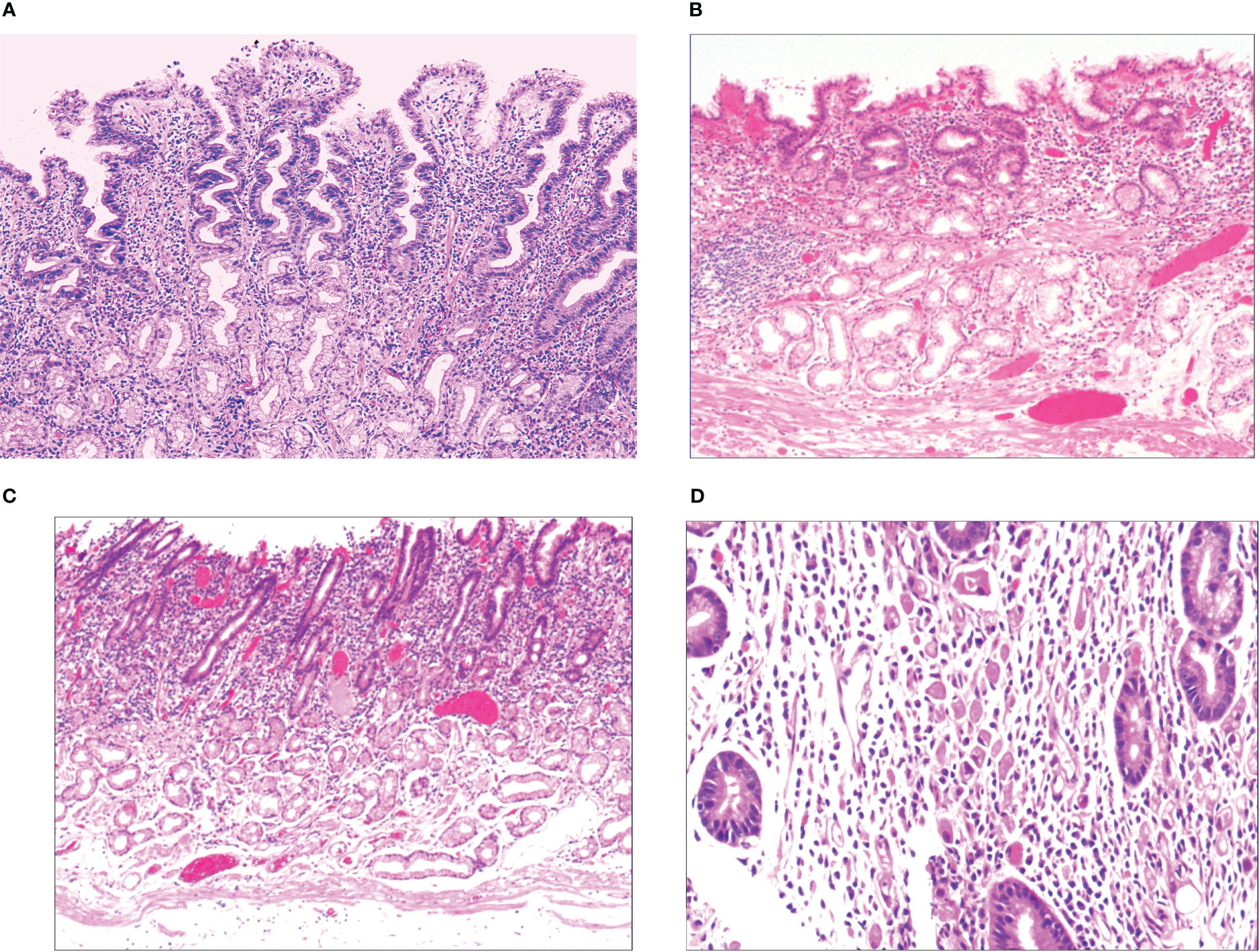
Figure 2 (A) Foveal epithelial hyperplasia of gastric mucosa, with height of gastric fovea ≥ 0.05–0.55 mm. The cytoplasm and nucleus of the proliferating surface mucous cells showed a hazy basophilic degeneration, and the nucleus volume increased, and was 1 time the volume of normal nucleus, with a small nucleolus available. HE ×100. (B) Proliferative dysfunction of stem cells in the proliferative zone. While there was an insufficient upward migration of the proliferative zone, resulting in a reduction of gastric foveal epithelium, there was an insufficient downward migration of the proliferative zone, resulting in extensive atrophy of the laminar propria glands of the gastric mucosa. HE ×100. (C) There was proliferation and transformation of stem cells in the proliferative zone, the nucleus was prolonged, with mild to moderate atypia, the nuclear chromatin increased, with a small to medium-sized nucleolus visible in approximately 20%~30% of the nucleus. HE ×100. (D) Signet-ring-like heterocysts were 1–2 times the number of cells on the peripheral columnar epithelium in quantity, and their nucleus showed a crescent shape or irregularly oval shape, forming the signet-ring cell carcinoid cells in cytology, HE ×200.
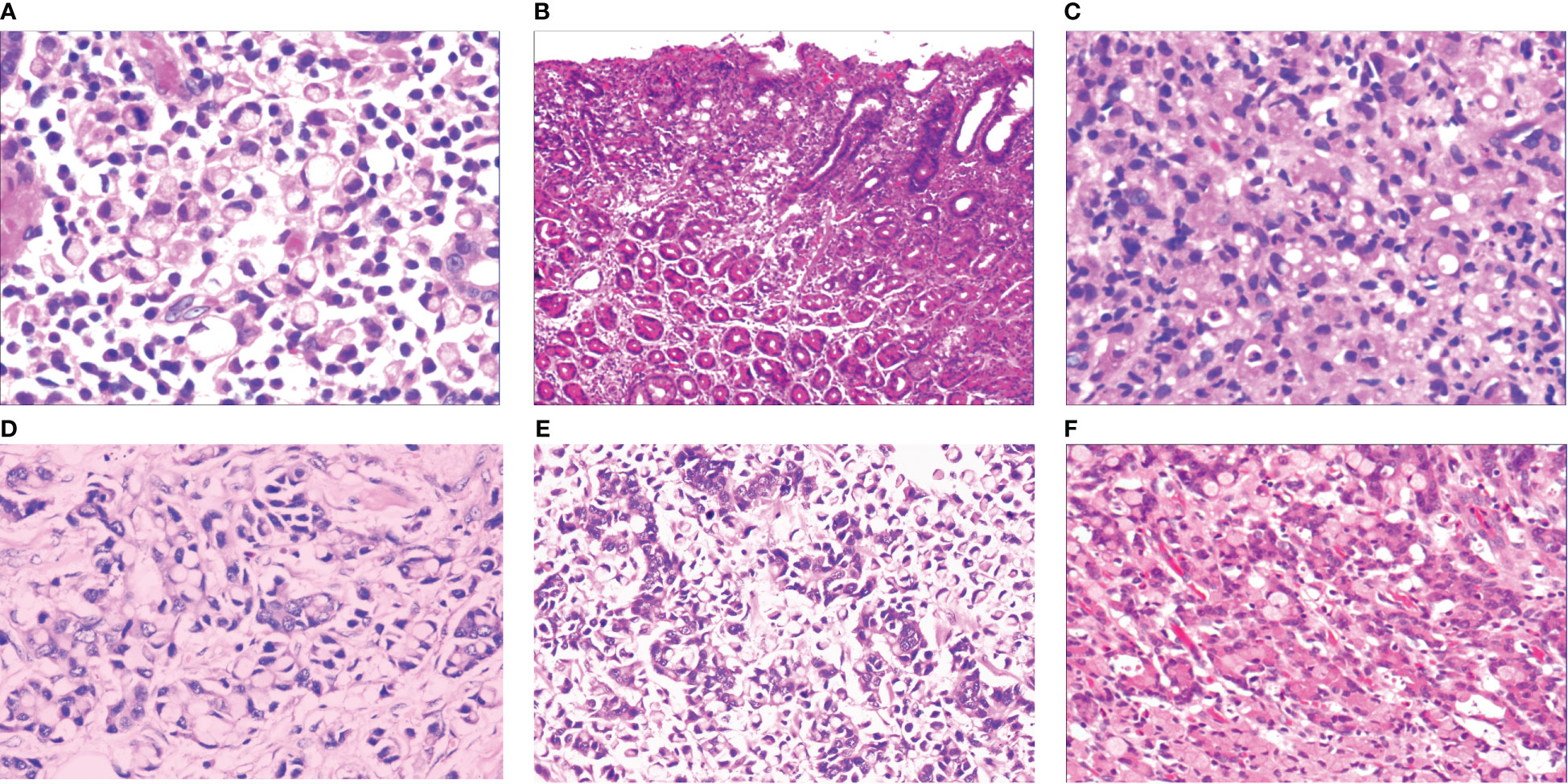
Figure 3 (A) Micro signet-ring cell carcinomatous lesion, in which multiple signet-ring cells were close to each other tightly, and were basically friendly toward surrounding normal glands, without mutual aggression. The cytoplasm on the cell was lightly eosinaceous, the envelope area was deeply stained, ringed, and about 70% of the cells were without nucleus. HE ×400. (B) Gastric foveolar-type signet-ring cell carcinoma, which also showed horizontal growth in gastric foveal area selectively. It grew slowly and by creeping into the interstitial area of the gastric fovea, and was basically friendly towards surrounding normal glands, without mutual aggression. HE ×100. (C) Undifferentiated adenocarcinoma with signet-ring cell differentiation, refers to the tumor formed by a mixture of undifferentiated cancer cells and signet-ring cells. Undifferentiated cancer cells had small volume, irregular small round shape, oval shape, less cytoplasm, a very big proportion between nucleus and cytoplasm, bare nucleus-like, and slightly basophilic staining. Signet-ring carcinoma cells accounted for 20%~80% of the tumor. HE ×200. (D) Mucinous adenocarcinoma with signet-ring cell differentiation, consists of signet-ring carcinoma and mucous tissues in terms of tumor histology. Signet-ring carcinoma cells accounted for 20%~80% of the tumor. HE ×200. (E) Adenoid adenocarcinoma with signet-ring cell differentiation, consists of signet-ring carcinoma and adenocarcinoma tissues, HE ×200. (F) Fundus gland adenocarcinoma with signet-ring cell differentiation, consists of signet-ring carcinoma and fundus gland adenocarcinoma tissues. Fundus gland adenocarcinoma tissues showed irregular tubular and branching structures, with less stroma. HE ×200.
With the HP positive cells, H. pylori adhered to and selectively destroyed the cytoplasm of the surface mucous cell (Figure 4A). MUC5AC was positively expressed on the superficial epithelium and mucous neck gland of normal gastric mucosa, and its expression decreased when stem cells were dysfunctional in the proliferative zone (Figure 4B). CEA was positively expressed in cases of abnormal proliferation and transformation of stem cells in the proliferative zone (Figure 4C). CK7 was positively expressed in signet-ring-like heterocysts (Figure 4D). CK was positively expressed in small signet-ring cell carcinoma-like lesions (Figure 5A). CK20 was positively expressed in foveolar-type signet-ring cell carcinoma (Figure 5B). In undifferentiated adenocarcinoma with signet-ring cell differentiation, CK expression showed undifferentiated cells and signet-ring cells (Figure 5C). In mucinous adenocarcinoma with signet-ring cell differentiation, signet-ring cell HER2 was positive 1+ (Figure 5D). In gastric adenocarcinoma with signet-ring cell differentiation, the villin expression showed SRCC and adenocarcinoma (Figure 5E). In fundus gland adenocarcinoma with signet-ring cell differentiation, ki67 expression showed SRCC and fundus adenocarcinoma (Figure 5F). Details are shown in Table 2. The results of immunohistochemical staining are shown in Table 3 and Figure 6.
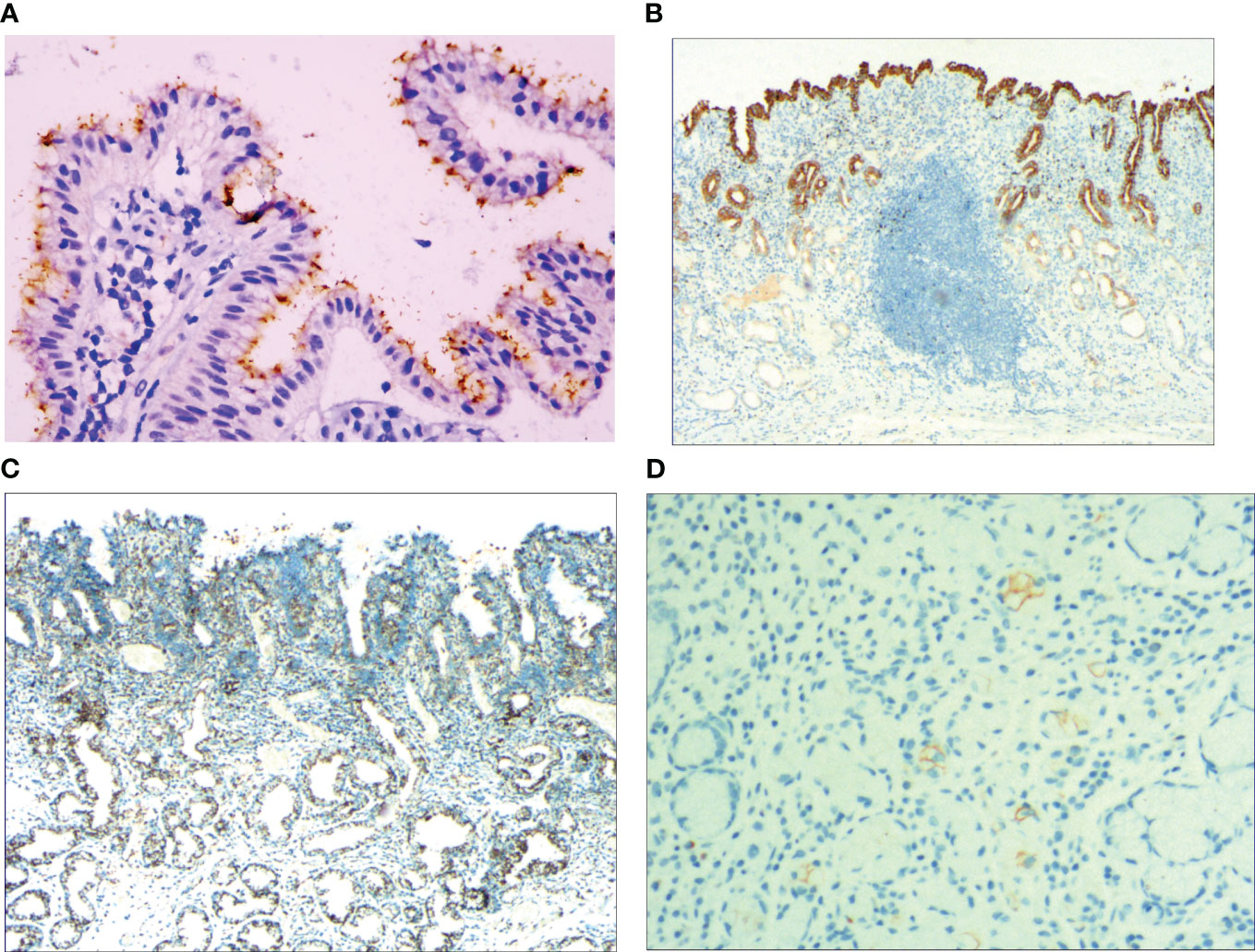
Figure 4 (A) Foveal epithelial hyperplasia of gastric mucosa, the helicobacter pylori (H. pylori) showed brown expression, by En Vision method, Hp ×400. (B) Dysfunction of stem cells in the proliferative zone, reduced MUC5AC positive cells, by En Vision method, ×200. (C) Proliferation and transformation of surface epithelial cells, CEA showed positive expression, by En Vision method, ×200. (D) Signet-ring-like heterocysts, CK7 showed positive expression, by En Vision method, ×200.
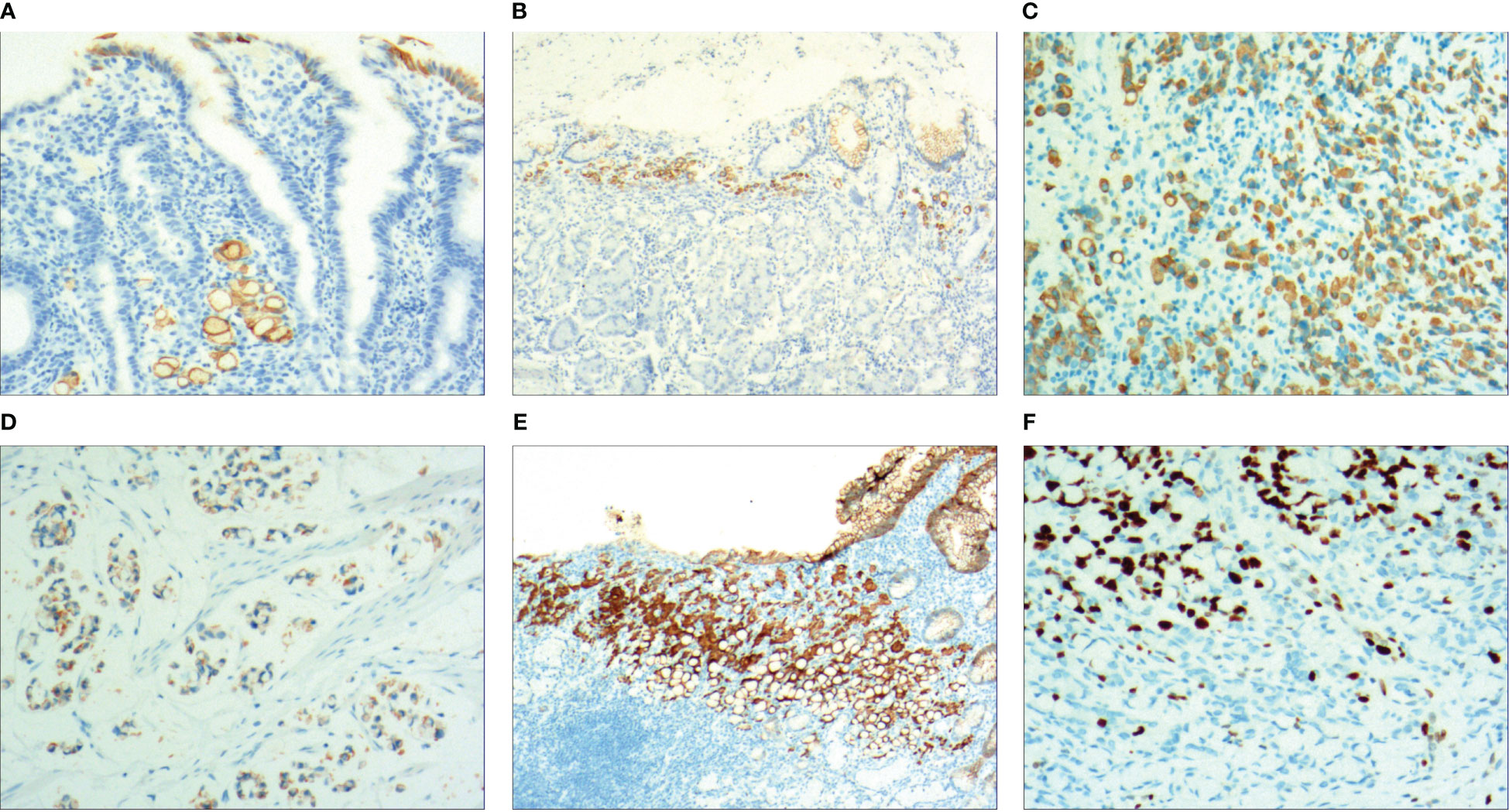
Figure 5 (A) Micro signet-ring carcinoid lesion, CK showed positive expression, by En Vision method, ×200. (B) Gastric foveolar-type signet-ring cell carcinoma, CK20 showed positive expression, by En Vision method, ×200. (C) Undifferentiated adenocarcinoma with signet-ring cell differentiation, CK showed positive expression, by En Vision method, ×200. (D) Mucinous adenocarcinoma with signet-ring cell differentiation, HER2 positive 1+, by En Vision method, ×200. (E) Adenoid adenocarcinoma with signet-ring cell differentiation, villin signet-ring carcinoma and adenocarcinoma showed positive expression, by En Vision method, ×200. (F) Fundus gland adenocarcinoma with signet-ring cell differentiation, ki67 showed positive expression, by En Vision method, Hp ×200.
Table 2 Clinical histopathological features of early onset and development of gastric signet-ring cell carcinoma.
Gastric cancer is a multifactorial disease (25). Both environmental and genetic factors, such as infection, diet, lifestyle, family history, occupational exposure, and ionizing radiation, can affect its occurrence and development (26). Atrophic gastritis, low acidity, and hypergastrinemia have also been reported in the literature as common risk factors. Especially signet ring cell carcinoma, hypergastrinemia caused by autoimmune chronic atrophic gastritis (27). H. Pylori infection leads to gastritis and gastric atrophy, progressively progresses to cancer (28). In summary, gastric cancer occurs when the gastric mucosa is affected by infection, chemical stimulation, autoimmune diseases, genetic factors, and other factors. Gastric cancer is a multi-stage disease process determined by the progressive development of mutations in the expression of various genes and epigenetic changes, which are the cause of the disease. More than half of the world’s population is affected with H. pylori, with the majority of people asymptomatic and only about 10% developing peptic ulcer, atrophic gastritis, gastric carcinoma, or MALT lymphoma (24, 29, 30). There is growing evidence that H. pylori strains, hosts, and other factors together determine the onset and development of the disease (31). Gastric flora, as an important part of the gastric microecological environment, maintains the balance of the gastric environment through various regulatory approaches. When the structure of the flora changes, the balance is bound to be disrupted, leading to the disease (32). H. pylori infection has been identified as a carcinogen of gastric carcinoma by the WHO, and it has become a trend to prevent gastric carcinoma by eliminating H. pylori through H. pylori detection and treatment (33).
The results of our study revealed that when the gastric mucosa was affected by infection, chemical irritation, autoimmune disease, heredity, and other factors, especially H. pylori infection, it resulted in foveal epithelial hyperplasia of gastric mucosa, stem cell dysfunction in the proliferative zone, and abnormal proliferation and transformation of stem cells in the proliferative zone. Subsequently, there were single or multiple signet-ring cell-like cells scattered in the proliferative zones in the isthmus of the gastric gland and the upper neck of the gland. Such cells were 1–2 times the size of the peripheral columnar epithelium, with crescent or irregularly oval nucleus, forming cytologically signet-ring cell-like heterocysts. After redifferentiation and reproliferation, signet-ring-like heterocysts, transformed into small signet-ring cell carcinoma-like lesions. In this study, we presented the characteristics of small signet-ring cell carcinoma-like lesions in the stomach: (1) it was primarily caused by H. pylori infection; (2) signet-ring cell-like heterocysts were first formed in the proliferative zones in the isthmus of the gastric gland and the upper neck of the gland; (3) signet-ring-like heterocysts, after redifferentiation and reproliferation, developed into classical-type signet-ring cells; (4) the cells were round, with a diameter of 15–30 μm with reddish mucous substances in the cytoplasm; the nucleus was deviated, and was signet-ring or crescent-shaped. The signet-ring-like heterocysts, along with classical-type signet-ring cells, formed proliferative nodules with a diameter of ≤ 0.5 mm; (5) small signet-ring cell carcinoma-like lesions in the stomach were included into the clinical pathological test report, which was extremely important in guiding clinicians in the precise treatment, tracking of malignancy transformation, and controlling the onset and development of gastric carcinoma. This can provide clinicians with very important biomarker value for early diagnosis of the disease.
One important reason for the low rate of early diagnosis of gastric SRCC is that the pathological mechanism of this gastric carcinoma as well as the histological features of precancerous lesions remain unclear (34–36). According to research, small lesions in the lamina propria of the mucosa can be difficult to diagnose by endoscopy, while the incidence of early SRCC confined to the submucosa is higher as the size is greater. Early SRCC has a much lower incidence of lymph node metastasis than moderately and poorly differentiated adenocarcinoma; when SRCC invades beyond the submucosa, tumor cells spread rapidly and widely.
The results of this study revealed that SRCC occurred when single cells, multiple cells, or lamellar signet-ring cell-like heterocysts first formed in the proliferative zone in the isthmus of the gastric gland and the upper neck of the gland, and signet-ring-like heterocysts, after redifferentiation and reproliferation, developed into small signet-ring cell carcinoma-like lesions or foveolar-type signet-ring cell carcinoma. This stage of carcinoma is the classical early gastric SRCC. It includes classical-type signet-ring cell (the cell is round, with a diameter of 15–30 μm and reddish mucous substances in the cytoplasm; the nucleus is deviated, and is signet-ring or crescent-shaped), and those that we previously reported: juvenile signet-ring cell (the cell is round or irregular, and the cytoplasm is strongly eosinophilic; the nucleus is irregularly round or oval, and deviated, and the ratio of nucleus to cytoplasm is 1:1-3; the nuclear chromatin is basophilic and eosinophilic); hyperproliferative type signet-ring cell (the cell is round or oval, the cytoplasm is uniform and slightly stained with basophilic and eosinophilic mucus; the nucleus is large with a prominent nucleolus, and is deviated); nuclear-free vacuolated signet-ring cell (the cell developed due to the relationship between sections during section preparation; the cell still maintained the cytoplastic outline and had reddish mucous substances in the cytoplasm); degenerative signet-ring cell (the size of the cell increased, and was mostly 20 mm–40 μm, or up to 50 μm; the membranes were incomplete, the nucleus was small and slightly stained, and gray, or it had no nucleus) (23). In this study, when small signet-ring cell carcinoma-like lesions or foveolar-type signet-ring cell carcinoma infiltrated into the lamina propria of the mucosa and the submucosa, signet-ring-like heterocysts and small signet-ring cell carcinoma-like lesions, after reproliferation, differentiated into various types of gastric adenocarcinoma with signet-ring cell differentiation, including undifferentiated carcinoma with signet-ring cell differentiation, mucinous adenocarcinoma with signet-ring cell differentiation, gastric gland adenocarcinoma with signet-ring cell differentiation, and fundus gland adenocarcinoma with signet-ring cell differentiation, with no pure SRCC types being found. Foveolar-type signet-ring cell carcinoma extended horizontally along the one third of the opening side of the fundus gland mucosa and crept into the stroma in the gastric foveola. It was 3–6 mm in length, but transformed into a mixed infiltrative gastric adenocarcinoma while growing infiltratively downward. However, at the time of transformation into mixed invasive gastric adenocarcinoma, quantitative differences in signet-ring cell components occur. Most scholars believe that the percentage of signet ring cells in gastric mixed carcinoma is related to the prognosis, and the number of signet ring cells is considered as an independent predictor (37, 38). Combining the quantitative changes and morphological changes of signet ring cells may offer new potential clinic biomarkers and improve the early clinical diagnosis rate. The evolution process and mechanism of gastric signet ring cell carcinoma into mixed invasive gastric adenocarcinoma need to be further studied by more cases.
The early onset, development and histological features of gastric SRCC are closely related to H. pylori infection. Early SRCC developed from the proliferative zones of the fundus of the gastric foveola and the neck of the gastric gland, growing horizontally along the gastric foveola. It developed into gastric adenocarcinoma with signet-ring cell differentiation after reproliferation and retransformation in the mucosa. However, more cases are needed to further study how H. pylori infection leads to the proliferation and transformation of cells in the proliferative zone, and the occurrence of signet-ring cell-like heterocysts, small signet-ring cell carcinoma-like lesions, and gastric foveolar epithelial SRCC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conception and design of the research: YW, SW. Acquisition of data: DR, CZ, PL, BJ. Analysis and interpretation of the data: YL. Statistical analysis: BW. Obtaining financing: YW. Writing of the manuscript: YW, SW. Critical revision of the manuscript for intellectual content: YW, SW. All authors read and approved the final draft. YW, YL, BW, DR, CZ, PL, BJ, SW. All authors contributed to the article and approved the submitted version.
Key scientific and technological research projects in Henan Province (132102310008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: impact on prognosis and specific therapeutic challenge. World J Gastroenterol (2015) 21(40):11428–38. doi: 10.3748/wjg.v21.i40.11428
2. Chon HJ, Hyung WJ, Kim C, Park S, Kim JH, Park CH, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg (2017) 265(5):946–53. doi: 10.1097/SLA.0000000000001793
3. Nichetti F, Morano F, Fabbri A, de Braud F, Di Bartolomeo M. Bilateral parotid gland metastases from gastric signet-ring cell carcinoma. Tumori (2018) 104(6):NP10–3. doi: 10.5301/tj.5000690
4. Akabah PS, Mocan S, Molnar C, Dobru D. Importance of optical diagnosis in early gastric cancer: a case report of early gastric signet ring cell carcinoma. Niger J Clin Pract (2017) 20(10):1342–5. doi: 10.4103/njcp.njcp_289_16
5. Ueyama H, Yao T, Akazawa Y, Hayashi T, Kurahara K, Oshiro Y, et al. Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type. J Gastroenterol (2021) 56(9):814–28. doi: 10.1007/s00535-021-01813-z
6. Pozos-Ochoa LI, Lino-Silva LS, León-Takahashi AM, Salcedo-Hernández RA. Prognosis of signet ring cell carcinoma of the colon and rectum and their distinction of mucinous adenocarcinoma with signet ring cells. A Comp Study Pathol Oncol Res (2018) 24(3):609–16. doi: 10.1007/s12253-017-0283-6
7. Lopes N, Bergsland C, Bruun J, Bjørnslett M, Vieira AF, Mesquita P, et al. A panel of intestinal differentiation markers (CDX2, GPA33, and LI-cadherin) identifies gastric cancer patients with favourable prognosis. Gastric Cancer (2020) 23(5):811–23. doi: 10.1007/s10120-020-01064-6
8. Odze RD, Lam AK, Ochiai A, Washington MK. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cance (2019).
9. Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int J Clin Exp Pathol (2013) 6(4):613–21.
10. Kai K, Satake M, Tokunaga O. Gastric adenocarcinoma of fundic gland type with signet-ring cell carcinoma component: a case report and review of the literature. World J Gastroenterol (2018) 24(26):2915–20. doi: 10.3748/wjg.v24.i26.2915
11. Venerito M, Link A, Rokkas T. Malfertheiner P.Review: gastric cancer-clinical aspects. Helicobacter (2019) 24(Suppl 1):e12643. doi: 10.1111/hel.12643
12. McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology (2008) 134(2):500–10. doi: 10.1053/j.gastro.2007.11.035
13. Matsuzaki J, Tsugawa H. Suzuki H.Precision medicine approaches to prevent gastric cancer. Gut Liver (2021) 15(1):3–12. doi: 10.5009/gnl19257
14. Ogutmen Koc D. Kimiloglu E.Relation of cyclooxygenase-2 expression with premalignant gastric lesions. Acta Gastroenterol Belg (2020) 83(2):249–54.
15. Piazuelo MB, Bravo LE, Mera RM, Camargo MC, Bravo JC, Delgado AG, et al. Wilson KT.The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology (2021) 160(4):1106–17.e3. doi: 10.1053/j.gastro.2020.11.017
16. Suzuki H, Mori H. World trends for h. pylori eradication therapy and gastric cancer prevention strategy by h. pylori test-and-treat. J Gastroenterol (2018) 53(3):354–61. doi: 10.1007/s00535-017-1407-1
17. Lahner E, Carabotti M, Annibale B. Treatment of helicobacter pylori infection in atrophic gastritis. World J Gastroenterol (2018) 24(22):2373–80. doi: 10.3748/wjg.v24.i22.2373
18. Lin KD, Chiu GF, Waljee AK, Owyang SY, El-Zaatari M, Bishu S, et al. Effects of anti-helicobacter pylori therapy on incidence of autoimmune diseases, including inflammatory bowel diseases. Clin Gastroenterol Hepatol (2019) 17(10):1991–9. doi: 10.1016/j.cgh.2018.12.014
19. Puccini A, Poorman K, Catalano F, Seeber A, Goldberg RM, Salem ME, et al. Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets. Oncogene (2022) 41(26):3455–60. doi: 10.1038/s41388-022-02350-6
20. Lahner E, Carabotti M, Annibale B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J Gastroenterol (2022) 24(22):2373–80. doi: 10.3748/WJGv24.I22.2373
21. Puccini A, Poorman K, Catalano F, Seeber A, Goldberg RM, Salem ME, et al. Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets. Oncogene (2022) 41(26):3455–60. doi: 10.1038/s41388-022-02350-6
22. Kumar NAN, Jose A, Usman N, Rajan K, Munisamy M, Shetty PS, et al. Signet ring cell cancer of stomach and gastro-esophageal junction: molecular alterations, stage-stratified treatment approaches, and future challenges. Langenbecks Arch Surg (2022) 407(1):87–98. doi: 10.1007/s00423-021-02314-6
23. Ma J, Meng Y, Zhou X, Guo L, Fu W. The prognostic significance and gene expression characteristics of gastric signet-ring cell carcinoma: a study based on the SEER and TCGA databases. Front Surg (2022) 9:819018. doi: 10.3389/fsurg.2022.819018
24. Dong X, Sun G, Qu H, He Q, Hao Z. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg (2021) 8:642468. doi: 10.3389/fsurg.2021.642468
25. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci (2020) 21(11):4012. doi: 10.3390/ijms21114012
26. Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev (2018) 19(3):591–603. doi: 10.22034/APJCP.2018.19.3.591
27. Fossmark R, Johannessen R, Qvigstad G, Mjønes P. Do gastric signet ring cell carcinomas and ECL-cell neuroendocrine tumours have a common origin? Medicina (Kaunas) (2022) 58(4):470. doi: 10.3390/medicina58040470
28. Sokolova O, Naumann M. Matrix metalloproteinases in helicobacter pylori-associated gastritis and gastric cancer. Int J Mol Sci (2022) 23(3):1883. doi: 10.3390/ijms23031883
29. Wang YK, Li C, Zhou YM, Zeng L, Li YY, Huang SL, et al. Histopathological features of helicobacter pylori infection in gastric mucosa. J Inflamm Res (2022) 15:6231–43. doi: 10.2147/JIR.S383075
30. Wang YK, Zhou JL, Meng NL, Zhu CY, Wang SN, Chen XD. How does helicobacter pylori infection cause gastric mucosal atrophy. Infect Drug Resist (2022) 15:3619–29. doi: 10.2147/IDR.S355981
31. Zhang ZS, Deng WY, Huang SL, Yang BF, Zhu FH, Jiang B, et al. Clinicopathological characteristics of signet-ring cell carcinoma derived from gastric fovelar epithelium. J Dig Dis (2022) 23(7):396–403. doi: 10.1111/1751-2980.13120
32. Tourani M, Habibzadeh M, Karkhah A, Shokri-Shirvani J, Barari L, Nouri HR. Association of TNF-α but not IL-1β levels with the presence of helicobacter pylori infection increased the risk of peptic ulcer development. Cytokine (2018) 110:232–6. doi: 10.1016/j.cyto.2018.01.003
33. Youn Nam S, Park BJ, Nam JH, Ryu KH, Kook MC, Kim J, et al. Association of current helicobacter pylori infection and metabolic factors with gastric cancer in 35,519 subjects: a cross-sectional study. United Eur Gastroenterol J (2019) 7(2):287–96. doi: 10.1177/2050640618819402
34. El Khadir M, Alaoui Boukhris S, Benajah DA, Ibrahimi SA, Chbani L, Bouguenouch L, et al. Helicobacter pylori CagA EPIYA-c motifs and gastric diseases in Moroccan patients. Infect Genet Evol (2018) 66:120–9. doi: 10.1016/j.meegid.2018.09.015
35. Marques MS, Melo J, Cavadas B, Mendes N, Pereira L, Carneiro F, et al. Afadin downregulation by helicobacter pylori induces epithelial to mesenchymal transition in gastric cells. Front Microbiol (2018) 9:2712. doi: 10.3389/fmicb.2018.02712
36. Sgambato D, Miranda A, Romano L, Romano M. Gut microbiota and gastric disease. Minerva Gastroenterol Dietol (2017) 63(4):345–54. doi: 10.23736/S1121-421X.17.02380-7
37. Bencivenga M, Treppiedi E, Dal Cero M, Torroni L, Verlato G, Iglesias M, et al. The amount of signet ring cells is significantly associated with tumour stage and survival in gastric poorly cohesive tumours. J Surg Oncol (2020) 121(7):1084–9. doi: 10.1002/jso.25885
Keywords: gastric foveolar epithelial type, histopathology, immunohistochemistry signet-ring cell carcinoma, signet-ring cell differentiation, signet-ring-like heterocysts
Citation: Wang Y, Li Y, Wang B, Ran D, Zhu C, Li P, Jiang B and Wang S (2023) Early onset, development and histological features of gastric signet-ring cell carcinoma. Front. Oncol. 13:1166549. doi: 10.3389/fonc.2023.1166549
Received: 15 February 2023; Accepted: 23 May 2023;
Published: 06 July 2023.
Edited by:
Luigi Marano, University of Siena, ItalyReviewed by:
Bo Cao, People’s Liberation Army General Hospital, ChinaCopyright © 2023 Wang, Li, Wang, Ran, Zhu, Li, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunan Wang, bXdhbmdzdW5hbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.