
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 31 July 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1165195
This article is part of the Research Topic Advances in the Surgical Management of Gastric and Colorectal Cancers View all 35 articles
Background: Hepatic hemangioma is among the most common benign liver lesions. However, giant pedunculated hepatic hemangiomas are exceptionally rare and associated with additional risks, such as torsion.
Case presentation: We present the case of a 63-year-old female patient who presented with abdominal distension and pain. Barium meal examination and gastroscopy revealed a large, smooth-surfaced submucosal bulge located at the fundus of the stomach. Subsequent MRI examination identified a mass measuring approximately 6.4 x 7 cm in the left upper abdomen. Surgical intervention was planned for mass removal. However, intraoperative exploration revealed the origin of the mass to be the liver, and subsequent histopathological examination confirmed it as a hemangioma.
Conclusion: We systematically summarized the characteristics of our case along with 31 previously reported cases. Giant pedunculated hepatic hemangiomas typically occur in the left lobe of the liver. Due to their atypical presentation, a combination of imaging methods such as ultrasound, CT, and/or MRI is essential for accurate diagnosis. Furthermore, surgical intervention is recommended due to the potential risks of bleeding, rupture, and torsion.
Hemangiomas are benign tumors that may occur anywhere in the abdomen, including solid organs, hollow viscera, ligaments, and abdominal wall, of which liver is the most common site (1). Hepatic hemangiomas are a prevalent type of benign liver lesion, second only to focal fat sparing and liver cysts in frequency (2, 3). The prevalence of hepatic hemangiomas detected through radiological imaging ranges from approximately 2.5% to 3.3% (3, 4). Typically, hepatic hemangiomas do not cause symptoms; however, when they reach a significant size, they may give rise to non-specific gastrointestinal manifestations (5). While imaging studies often aid in diagnosing hepatic hemangiomas, their presentation can occasionally deviate from the norm, leading to confusion with malignant lesions (6, 7). Although hepatic hemangiomas primarily occur within the liver, instances of giant pedunculated hemangiomas outside the liver capsule are exceedingly rare (8).
This report describes the case of a 63-year-old female patient who presented with a gastric submucosal tumor detected during gastroscopy. Subsequent MRI imaging revealed the presence of a large tumor located between the stomach and spleen within the abdominal cavity. Due to suspicion of a giant abdominal tumor, the patient underwent surgical intervention. However, during surgery, it was found that the tumor was a pedunculated tumor of the liver. The histopathological examination of the specimen revealed a hemangioma. This case has been documented in accordance with the CARE Guidelines (Supplementary Table S1).
A 63-year-old female patient was admitted to our hospital for examination and treatment on July 26, 2022. Six months prior to admission, she experienced upper and middle abdominal distension after eating, which was slightly relieved by anal venting. She reduced the amount of food consumed per meal and did not experience any further bloating. One month prior to admission, she developed vague right upper abdominal pain after consuming fatty foods, which was paroxysmal and tolerable and improved with a light diet. No other symptoms such as acidity, eructation, vomiting, or diarrhea were reported.
Upon initial physical examination, pain was present in the left upper abdomen upon pressure, without rebound pain. The rest of the examination was unremarkable. Further investigation through barium meal examination, endoscopy, and abdominal MRI was arranged. Barium meal examination revealed a locally depressed fundus of the stomach with a smooth mucosal surface (Supplementary Figure S1), while endoscopy showed a large submucosal bulge with a smooth surface at the fundus of the stomach (Figure 1), suspected to be gastric submucosal tumor. MRI detected a 6.4 x 7 cm mass in the left upper abdomen with low signal on T1-weighted images and high signal on T2-weighted images, with partial intensification in the arterial phase and progressive intensification in the venous phase (Figure 2).
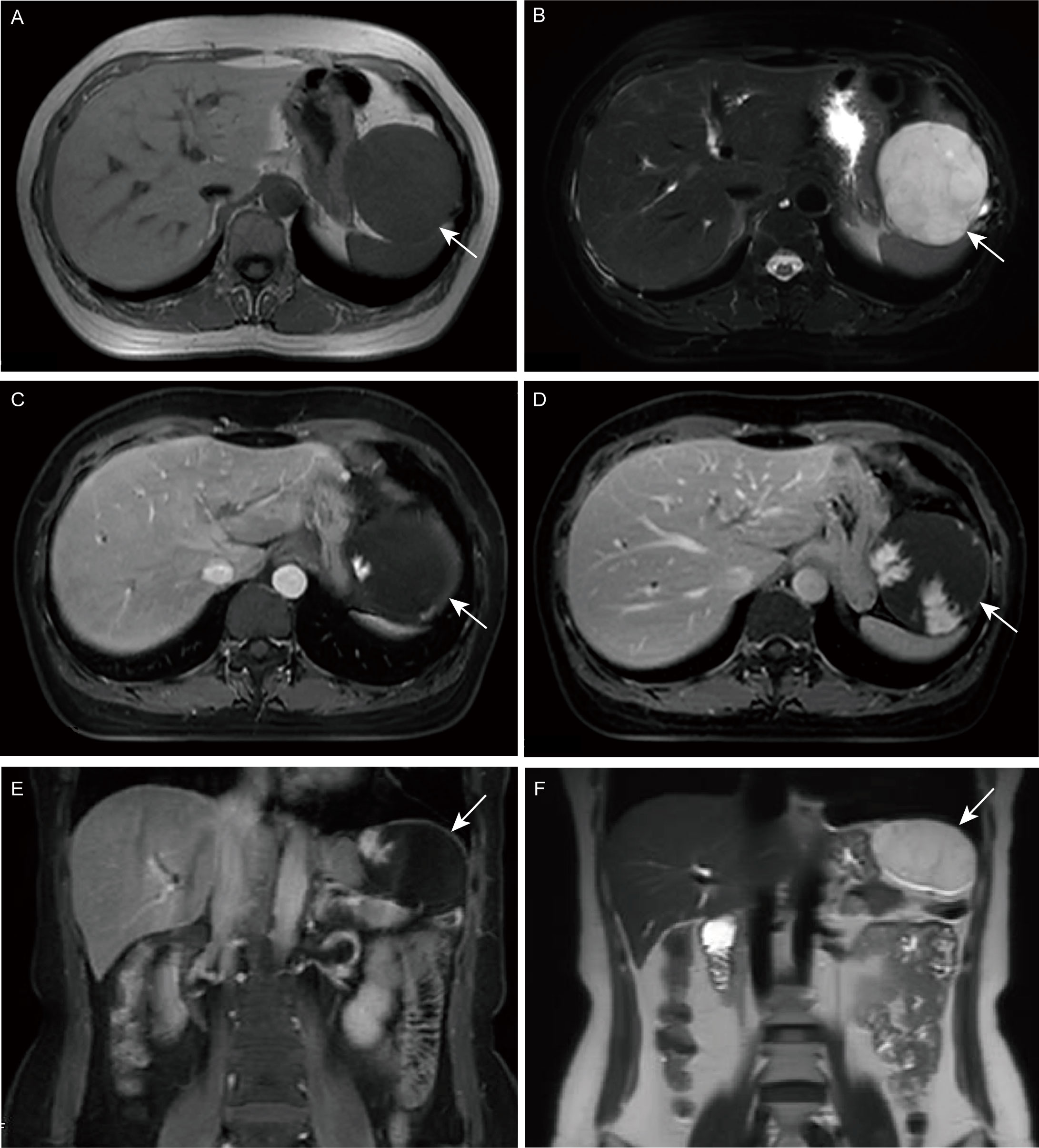
Figure 2 Magnetic resonance imaging (MRI). (A, E) T1-weighted image (T1WI); (B, F) T2-weighted image (T2WI); (C) Enhanced arterial phase; (D) Enhanced portal phase.
Laparoscopic surgery was initially planned; however, intraoperatively, it was discovered that the mass was connected to the liver by pedunculi. The mass exhibited dimensions of 9 * 8 cm and had a soft consistency with angulated surface vessels closely associated with the lesser curvature of the stomach (Figure 3). The mass was removed en bloc with 2 cm margins. The final pathology report indicated a hemangioma (Figure 4). The patient timeline of diagnostic testing and Therapeutic intervention are shown in Supplementary Figure S2. A follow-up telephone call conducted three months post-operation revealed that the patient had experienced resolution of symptoms and reported no additional discomfort.
Prior to my admission to West China Hospital of Sichuan University, I was plagued by symptoms that restricted my dietary intake, significantly diminishing my quality of life. I was apprehensive about the prospect of undergoing a partial gastrectomy, as it could potentially impact my future dietary habits. However, I was pleased to discover that my fears were unfounded, as my symptoms showed a marked improvement after being treated at West China Hospital of Sichuan University. This resulted in a significant enhancement of my overall quality of life.
Hemangioma is a benign tumor that is commonly asymptomatic when its size is less than 5 cm. Larger hemangioma may present symptoms such as abdominal pain, discomfort, and a sensation of fullness in the abdomen (9). Imaging studies, such as ultrasound, CT scans, and MRI, can aid in the diagnosis of hemangioma (10). MRI scans typically depict hemangioma as well-defined, smooth, homogenous lesions that are hypointense on T1-weighted images and hyperintense on T2-weighted images (5). Fine-needle aspiration biopsy(FNAB) is a common diagnostic tool for tumors, however, this method carries a risk of hemorrhage due to the vascular-rich nature of hemangioma (11). A study conducted by Heilo et al. found that ultrasound-guided FNAB had a false-negative rate of 15-16 out of 51 patients with suspected hepatic hemangioma (12). Several studies have suggested that the low diagnostic rate and the risk of bleeding associated with FNAB make it an unsuitable method for diagnosing hepatic hemangioma (10, 13). It is important to note that hepatic hemangioma can sometimes be mistaken for other liver diseases, such as hepatocellular adenoma, focal nodular hyperplasia, or hepatocellular carcinoma. However, cases where hepatic hemangioma mimics GIST are extremely rare.
For cases of gastric fundic masses of unknown origin, adequate investigations are required to assist in the diagnosis. Besides routine CT, MRI and endoscopy, it is necessary to perform ultrasonography (14) and ultrasound endoscopy (15) to clarify the nature of the mass and its relationship to the surrounding organs. FNAB is a valuable diagnostic tool for various types of tumors, particularly for biopsying superficial or palpable lesions in organs such as the thyroid, breast, lymph nodes, and others (16–22). While FNAB is considered a safe and effective method, it has a potential risk for bleeding and false negative results. Therefore, it may not be appropriate for all patients, particularly in cases of hepatic hemangioma (11, 23). To enhance the safety of FNAB, ultrasound guidance is recommended (24).
While hepatic hemangiomas generally exhibit a slow growth rate (25), the presence of large hepatic hemangiomas increases the risk of bleeding and rupture (4). Hemangiomas exceeding 4 cm in diameter are categorized as giant hemangiomas (26). Moreover, in the case of large, pedunculated hepatic hemangiomas, there is a potential risk of torsion, which can result in acute abdominal pain and pose a life-threatening situation (27). To date, only a limited number of cases involving giant pedunculated hepatic hemangiomas have been reported.
A systematic search was conducted in the PubMed and Embase databases to identify relevant studies. After removing duplicates, a total of 37 studies were retrieved. The search strategy employed was (((hepatic) OR (liver)) AND (hemangioma)) AND (pedunculated). Through careful evaluation of full-text articles and exclusion of studies with irrelevant topics, tumors smaller than 4 cm, and those lacking full-text availability, 19 studies remained. Furthermore, 9 additional studies were retrieved from the references of the included studies. In total, 28 studies (8, 27–52) encompassing 31 cases of giant pedunculated hepatic hemangioma were identified. A summary of the characteristics of these previous cases, as well as our own case, is provided in Table 1. The incidence of giant pedunculated hepatic hemangioma was found to be higher in females (n=22) compared to males (n=10). The median age at diagnosis was 65 years, with a range of 26 to 71 years. The left lobe of the liver was the most common location for these tumors. Out of the cases, 11 (34.4%) were asymptomatic, and 5 cases were initially suspected to be gastrointestinal interstitial tumors prior to surgery (27, 29, 41, 43, 44). Surgical resection was the predominant treatment approach in almost all cases.
In this report, we present a case of a 63-year-old female patient who was diagnosed with a large hemangioma located interposed the spleen and the stomach. The hemangioma measured 9 * 8 cm in size and was initially mistaken as a GIST based on endoscopy showed that the tumor was a spherical submucosal bulge from the fundus of the stomach (53). Although FNAB is typically a safe procedure (54, 55), it was not performed in this case due to the potential risk of hemorrhage. Given the significant size of the mass, the presence of symptoms and the risk of torsion, surgical resection was performed. During the procedure, it was revealed that the tumor originated from the liver. Pathological analysis postoperatively confirmed the diagnosis of a hemangioma.
Giant pedunculated hepatic hemangioma is a rare benign tumor primarily originated from the left lobe of the liver. The symptoms associated with this condition are typically non-specific or even asymptomatic, leading to potential confusion with various other upper abdominal masses. Accurate diagnosis of this atypical hemangioma requires a comprehensive approach utilizing multiple imaging techniques, including ultrasound, CT, and/or MRI. Considering the risks of bleeding, rupture, and torsion, surgical intervention is the recommended treatment modality for large pedunculated hepatic hemangiomas.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
TJ – manuscript write up, data collection, and patient interview. ZZ – manuscript write up, data collection, and patient interview. ZC – manuscript write up and literature review. CS – surgery perform. BZ – surgery perform. TJ and ZZ provided equal contributions and shared co-first authorship. All authors contributed to the article and approved the submitted version.
This work was supported by Beijing Bethune Charitable Foundation (Grant No. WCJZL202105).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1165195/full#supplementary-material
Supplementary Figure S1 | Barium meal examination.
Supplementary Figure S2 | Timeline.
Supplementary Table S1 | CARE-checklist.
1. Ojili V, Tirumani SH, Gunabushanam G, Nagar A, Surabhi VR, Chintapalli KN, et al. Abdominal hemangiomas: A pictorial review of unusual, atypical, and rare types. Can Assoc Radiol J (2013) 64(1):18–27. doi: 10.1016/j.carj.2011.08.004
2. Nault JC, Paradis V, Ronot M, Zucman-Rossi J. Benign liver tumours: understanding molecular physiology to adapt clinical management. Nat Rev Gastroenterol Hepatol (2022) 19(11):703–16. doi: 10.1038/s41575-022-00643-5
3. Kaltenbach TE, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (NY) (2016) 41(1):25–32. doi: 10.1007/s00261-015-0605-7
4. Mocchegiani F, Vincenzi P, Coletta M, Agostini A, Marzioni M, Baroni GS, et al. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: A large retrospective cross-sectional study. Dig Liver Dis (2016) 48(3):309–14. doi: 10.1016/j.dld.2015.09.016
5. Leon M, Chavez L, Surani S. Hepatic hemangioma: what internists need to know. World J Gastroenterol (2020) 26(1):11–20. doi: 10.3748/wjg.v26.i1.11
6. Mathew RP, Sam M, Raubenheimer M, Patel V, Low G. Hepatic hemangiomas: the various imaging avatars and its mimickers. Radiol Med (2020) 125(9):801–15. doi: 10.1007/s11547-020-01185-z
7. Noskovicova L, Kovacova M, Janik J, Marcinek J, Balogova S. Hepatic cavernous hemangioma mimicking metastasis of midgut neuroendocrine neoplasia on 18f-fluorodihydroxyphenylalanine Pet/Ct. Clin Nucl Med (2022) 47(1):76–8. doi: 10.1097/RLU.0000000000003787
8. Melfa G, Coccorullo G, Raspanti C, Falco N, Porello C, Gullo R, et al. Laparoscopic treatment of a large pedunculated hemangioma of the liver: A case report. G Chir (2016) 37(4):162–6. doi: 10.11138/gchir/2016.37.4.162
9. Sakamoto Y, Kokudo N, Watadani T, Shibahara J, Yamamoto M, Yamaue H, et al. Proposal of size-based surgical indication criteria for liver hemangioma based on a nationwide survey in Japan. J Hepatobil Pancreat Sci (2017) 24(7):417–25. doi: 10.1002/jhbp.464
10. Wesson RN, Cameron AM. Differential diagnosis of focal hepatic lesions. In: Reau N, Poordad F, editors. Primary Liver Cancer: Surveillance, Diagnosis and Treatment. Totowa, NJ: Humana Press (2012). p. 79–126.
11. Davies R. Haemorrhage after fine-needle aspiration biopsy of an hepatic haemangioma. Med J Aust (1993) 158(5):364. doi: 10.5694/j.1326-5377.1993.tb121823.x
12. Heilo A, Stenwig AE. Liver hemangioma: us-guided 18-gauge core-needle biopsy. Radiology (1997) 204(3):719–22. doi: 10.1148/radiology.204.3.9280249
13. Terriff BA, Gibney RG, Scudamore CH. Fatality from fine-needle aspiration biopsy of a hepatic hemangioma. AJR Am J Roentgenol (1990) 154(1):203–4. doi: 10.2214/ajr.154.1.2104717
14. Wu XF, Bai XM, Yang W, Sun Y, Wang H, Wu W, et al. Differentiation of atypical hepatic hemangioma from liver metastases: diagnostic performance of a novel type of color contrast enhanced ultrasound. World J Gastroenterol (2020) 26(9):960–72. doi: 10.3748/wjg.v26.i9.960
15. Deprez PH, Moons LMG, O'Toole D, Gincul R, Seicean A, Pimentel-Nunes P, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European society of gastrointestinal endoscopy (Esge) guideline. Endoscopy (2022) 54(4):412–29. doi: 10.1055/a-1751-5742
16. Eszlinger M, Lau L, Ghaznavi S, Symonds C, Chandarana SP, Khalil M, et al. Molecular profiling of thyroid nodule fine-needle aspiration cytology. Nat Rev Endocrinol (2017) 13(7):415–24. doi: 10.1038/nrendo.2017.24
17. Ha EJ, Na DG, Baek JH, Sung JY, Kim JH, Kang SY. Us fine-needle aspiration biopsy for thyroid malignancy: diagnostic performance of seven society guidelines applied to 2000 thyroid nodules. Radiology (2018) 287(3):893–900. doi: 10.1148/radiol.2018171074
18. Balasubramanian I, Fleming CA, Corrigan MA, Redmond HP, Kerin MJ, Lowery AJ. Meta-analysis of the diagnostic accuracy of ultrasound-guided fine-needle aspiration and core needle biopsy in diagnosing axillary lymph node metastasis. Br J Surg (2018) 105(10):1244–53. doi: 10.1002/bjs.10920
19. Wu SY, Li JW, Wu HL, Shao ZM, Liu GY, Hu N. Accuracy of ultrasound-guided targeted fine-needle aspiration in assessing nodal response in node-positive breast cancer after neoadjuvant chemotherapy: prospective feasibility study. Br J Surg (2022) 109(12):1194–7. doi: 10.1093/bjs/znac277
20. Wang F, Wang HL, Zhao Q. Endoscopic ultrasound-fine-needle biopsy is superior to endoscopic ultrasound-fine-needle aspiration in sampling pancreatic masses. Clin Gastroenterol Hepatol (2018) 16(5):785–7. doi: 10.1016/j.cgh.2017.12.039
21. VanderLaan PA. Percutaneous lung biopsy: point-fine-needle aspiration first. AJR Am J Roentgenol (2022) 218(5):794–5. doi: 10.2214/AJR.21.26697
22. Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol (2004) 22(15):3046–52. doi: 10.1200/JCO.2004.02.104
23. Gleeson FC, Kipp BR, Caudill JL, Clain JE, Clayton AC, Halling KC, et al. False positive endoscopic ultrasound fine needle aspiration cytology: incidence and risk factors. Gut (2010) 59(5):586–93. doi: 10.1136/gut.2009.187765
24. Al-Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD, et al. The safety of fine-needle aspiration guided by endoscopic ultrasound: A prospective study. Endoscopy (2008) 40(3):204–8. doi: 10.1055/s-2007-995336
25. Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg (2014) 149(12):1266–71. doi: 10.1001/jamasurg.2014.477
26. Hamaloglu E, Altun H, Ozdemir A, Ozenc A. Giant liver hemangioma: therapy by enucleation or liver resection. World J Surg (2005) 29(7):890–3. doi: 10.1007/s00268-005-7661-z
27. Darzi A, Taheri H, Kamali Ahangar S, Mirzapour Shafiei A, Asghari Y. Torsion of a giant pedunculated hemangioma of the liver presenting with acute abdomen: A case report. Iran Red Crescent Med J (2016) 18(8):e38198. doi: 10.5812/ircmj.38198
28. Cortes-Blanco A, Martinez-Lazaro R. Bile duct stenosis seemingly caused by a giant pedunculated hemangioma with hypogastric growth. Clin Nucl Med (2000) 25(4):299–300. doi: 10.1097/00003072-200004000-00018
29. Tsai CC, Yen TC, Tzen KY. Pedunculated giant liver hemangioma mimicking a hypervascular gastric tumor on Tc-99m Rbc spect. Clin Nucl Med (1999) 24(2):132–3. doi: 10.1097/00003072-199902000-00017
30. Ellis JV, Salazar JE, Gavant ML. Pedunculated hepatic hemangioma: an unusual cause for anteriorly displaced retroperitoneal fat. J Ultrasound Med (1985) 4(11):623–4. doi: 10.7863/jum.1985.4.11.623
31. Liang RJ, Chen CH, Chang YC, Hu RH, Sheu JC. Pedunculated hepatic hemangioma: report of two cases. J Formosan Med Assoc (2002) 101(6):437–41.
32. Nishiyama Y, Yamamoto Y, Fukunaga K, Fukuda Y, Satoh K, Ohkawa M, et al. Pedunculated hepatic hemangioma identified on Tc-99m Dtpa-I Scintigraphy. Clin Nucl Med (1999) 24(2):133–4. doi: 10.1097/00003072-199902000-00018
33. Srivastava DN, Sharma S, Yadav S, Nundy S, Berry M. Pedunculated hepatic haemangioma with arterioportal shunt: treated with angio-embolization and surgery. Australas Radiol (1998) 42(2):151–3. doi: 10.1111/j.1440-1673.1998.tb00594.x
34. Hajjam ME, Lacout A, Marzouqi MK, Lacombe P, Marcy PY. Pedunculated hepatic hemangioma masquerading as a peritoneal tumor. A case report. Polish J Radiol (2016) 81:51–3. doi: 10.12659/PJR.895327
35. Kouki S, Helal I, Ben Lassoued M. Unusual cause of a left hypochondria pain: pedunculated giant haemangioma of the liver. BMJ Case Rep (2019) 12(8)(8):e224349. doi: 10.1136/bcr-2018-224349
36. Vivarelli M, Gazzotti F, D'Alessandro L, Pinna AD. Emergency presentation of a giant pedunculated liver haemangioma. Digest Liver Dis (2010) 42(6):456. doi: 10.1016/j.dld.2008.12.096
37. Kukuk GM, Hinterthaner M, Wilhelm K. Pedunculated liver hemangioma. [German] RoFo Fortschr auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren (2004) 176(6):902–3. doi: 10.1055/s-2004-812801
38. Castañeda Puicón L, Trujillo Loli Y, Campos Medina S. Torsion of a giant pedunculated liver hemangioma: case report. Int J Surg Case Rep (2020) 75:207–10. doi: 10.1016/j.ijscr.2020.09.077
39. Guenot C, Haller C, Rosso R. [Giant pedunculated cavernous hepatic haemangioma: A case report and review of the literature]. Gastroenterol Clin Biol (2004) 28(8-9):807–10. doi: 10.1016/s0399-8320(04)95133-0
40. Bouknani N, Rami A, Kassimi M, Mahi M. Exophytic hepatic hemangioma: A case report. Radiol Case Rep (2022) 17(9):3367–9. doi: 10.1016/j.radcr.2022.06.040
41. Al Farai A, Mescam L, De Luca V, Monneur A, Perrot D, Guiramand J, et al. Giant pedunculated hepatic hemangioma: A case report and literature review. Case Rep Oncol (2018) 11(2):476–84. doi: 10.1159/000490696
42. Kudara N, Chiba T, Andoh T, Tsukahara M, Oana S, Terui T, et al. [a case of hepatic hemangioma with pedunculated extrahepatic growth simulating submucosal tumor of the stomach]. Nihon Shokakibyo Gakkai zasshi = Japanese J Gastro Enterol (2004) 101(3):293–9.
43. Moon HK, Kim HS, Heo GM, Shin WG, Kim KH, Jang MK, et al. A case of pedunculated hepatic hemangioma mimicking submucosal tumor of the stomach. Korean J Hepatol (2011) 17(1):66–70. doi: 10.3350/kjhep.2011.17.1.66
44. Krishnan V, Bajaj SK, Sethy A, Gupta N. Atypical exophytic liver mass: giant pedunculated hepatic haemangioma masquerading as a gastrointestinal stromal tumour of the gastric wall. South Afr J Radiol (2019) 23(1):a1697. doi: 10.4102/sajr.v23i1.1697
45. Tran-Minh VA, Gindre T, Pracros JP, Morin de Finfe CH, Kattan M, Peix JL. Volvulus of a pedunculated hemangioma of the liver [7]. Am J Roentgenolo (1991) 156(4):866–7. doi: 10.2214/ajr.156.4.2003458
46. Parikh VP, Iyer GN. Pedunculated hepatic hemangioma: ct findings. Am J Roentgenolo (1990) 155(5):1137–8. doi: 10.2214/ajr.155.5.2120954
47. Needleman L, Wechsler RJ, Kurtz AB. Ultrasound case of the day. Pedunculated retroperitoneal liver hemangioma. Radiographics Rev Publ Radiol Soc North America Inc (1989) 9(4):769–73. doi: 10.1148/radiographics.9.4.2667053
48. Liessi G, Sandini F, Spaliviero B, Cesari S, Avventi P, Dell'Antonio C. [Computerized tomography and ultrasonography in the study of pedunculated cavernous angioma of the liver. Description of 3 cases]. Radiol Med (1990) 80(5):758–61.
49. Ersoz F, Ozcan O, Toros AB, Culcu S, Bektas H, Sari S, et al. Torsion of a giant pedunculated liver hemangioma mimicking acute appendicitis: A case report. World J Emerg Surg (2010) 5:2. doi: 10.1186/1749-7922-5-2
50. Karatzas T, Smirnis A, Dimitroulis D, Patsouras D, Evaggelou K, Kykalos S, et al. Giant pedunculated hepatocellular carcinoma with hemangioma mimicking intestinal obstruction. BMC Gastroenterol (2011) 11:99. doi: 10.1186/1471-230X-11-99
51. Acharya M, Panagiotopoulos N, Bhaskaran P, Kyriakides C, Pai M, Habib N. Laparoscopic resection of a giant exophytic liver haemangioma with the laparoscopic habib 4x radiofrequency device. World J Gastrointest Surg (2012) 4(8):199–202. doi: 10.4240/wjgs.v4.i8.199
52. Candido Pde C, Pereira IM, Matos BA, Fontes MH, Pires TE, Costa PR. Giant pedunculated hemangioma of the liver. Radiol Bras (2016) 49(1):57–8. doi: 10.1590/0100-3984.2014.0057
53. Burkill GJ, Badran M, Al-Muderis O, Meirion Thomas J, Judson IR, Fisher C, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology (2003) 226(2):527–32. doi: 10.1148/radiol.2262011880
54. Eckardt AJ, Adler A, Gomes EM, Jenssen C, Siebert C, Gottschalk U, et al. Endosonographic large-bore biopsy of gastric subepithelial tumors: A prospective multicenter study. Eur J Gastroenterol Hepatol (2012) 24(10):1135–44. doi: 10.1097/MEG.0b013e328356eae2
55. Zoundjiekpon V, Falt P, Fojtik P, Kundratova E, Mikolajek O, Hanousek M, et al. Endosonography-guided fine-needle aspiration versus "Key-hole biopsy" in the diagnostics of upper gastrointestinal subepithelial tumors. A prospective randomized interventional study. BioMed Pap Med Fac Univ Palacky Olomouc Czech Repub (2020) 164(1):63–70. doi: 10.5507/bp.2019.013
Keywords: giant, hemangioma, pedunculated, liver, abdominal
Citation: Jiang T, Zhao Z, Cai Z, Shen C and Zhang B (2023) Case Report: Giant abdominal hemangioma originating from the liver. Front. Oncol. 13:1165195. doi: 10.3389/fonc.2023.1165195
Received: 13 February 2023; Accepted: 10 July 2023;
Published: 31 July 2023.
Edited by:
Roberto Gramignoli, Karolinska Institutet (KI), SwedenReviewed by:
Siyu Sun, China Medical University, ChinaCopyright © 2023 Jiang, Zhao, Cai, Shen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhang, emhhbmdib19zY3VAc2N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.