- 1Digestive Diseases Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
- 2Guangdong Provincial Key Laboratory of Digestive Cancer Research, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong, China
- 3School of Medicine, Sun Yat-sen University, Shenzhen, China
- 4School of Public Health, Guangzhou Medical University, Guangzhou, China
- 5Clinical Research Center, Big Data Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 6Evidence Based Social Science Research Center, School of Public Health, Lanzhou University, Lanzhou, China
- 7The First School of Clinical Medicine, Lanzhou University, Lanzhou, Gansu, China
- 8Department of General Surgery, The First Hospital of Lanzhou University, Lanzhou, Gansu, China
- 9School of Public Health, Sun Yat-sen University, Shenzhen, China
Background: Metabolic syndrome has been linked to an increased risk of colorectal cancer (CRC) incidence and mortality, but whether adopting a healthy lifestyle could attenuate the risk of CRC conferred by metabolic syndrome remains unclear. The aim of the study is to investigate the individual and joint effects of modifiable healthy lifestyle and metabolic health status on CRC incidence and mortality in the UK population.
Methods: This prospective study included 328,236 individuals from the UK Biobank. An overall metabolic health status was assessed at baseline and categorized based on the presence or absence of metabolic syndrome. We estimated the association of the healthy lifestyle score (derived from 4 modifiable behaviors: smoking, alcohol consumption, diet, physical activity and categorized into “favorable,” “intermediate”, and “unfavorable”) with CRC incidence and mortality, stratified by metabolic health status.
Results: During a median follow-up of 12.5 years, 3,852 CRC incidences and 1,076 deaths from CRC were newly identified. The risk of incident CRC and its mortality increased with the number of abnormal metabolic factors and decreased with healthy lifestyle score (P trend = 0.000). MetS was associated with greater CRC incidence (HR = 1.24, 95% CI: 1.16 – 1.33) and mortality (HR = 1.24, 95% CI: 1.08 – 1.41) when compared with those without MetS. An unfavorable lifestyle was associated with an increased risk (HR = 1.25, 95% CI: 1.15 – 1.36) and mortality (HR = 1.36, 95% CI: 1.16 – 1.59) of CRC across all metabolic health status. Participants adopting an unfavorable lifestyle with MetS had a higher risk (HR = 1.56, 95% CI: 1.38 – 1.76) and mortality (HR = 1.75, 95% CI: 1.40 – 2.20) than those adopting a favorable healthy lifestyle without MetS.
Conclusion: This study indicated that adherence to a healthy lifestyle could substantially reduce the burden of CRC regardless of the metabolic status. Behavioral lifestyle changes should be encouraged for CRC prevention even in participants with MetS.
Introduction
CRC is one of the most diagnosed malignancies worldwide, leading to nearly 1 million deaths per year (1). Both hereditary and environmental risk factors contribute to the development of CRC. Although the etiology of CRC has not been fully elucidated, accumulating epidemiologic evidence have suggested that metabolic syndrome (MetS) is the major risk factor for CRC incidence and mortality (2, 3). MetS and its related factors, including central obesity, hypertension, hyperglycemia, low HDL cholesterol and hyperlipidemia that might independently contribute to processes like angiogenesis and oxidative stress, could potentiate the risk of CRC (4–8). Therefore, a global trend with highly prevalent metabolic syndrome components may lead to a heavier CRC burden (9–11).
Since primary prevention based on risk factors of CRC may have the lowest cost and best effect among all strategies, then lifestyle modification is essential. Indeed, unhealthy lifestyle, including smoking (12, 13), alcoholic consumption (14), poor diet (15, 16), and physical inactivity (17) have been linked with the increased risk and mortality of CRC. Individuals with MetS may suffer a higher risk of CRC incidence and mortality (2, 18, 19), whether the increased risk could be offset by a healthy lifestyle remains hypothetical. No studies have systematically assessed the relationship of combined lifestyle factors with the risk of CRC incidence and mortality across a population at different degrees of metabolic risk.
We performed this prospective analysis based on the UK biobank database to assess the individual and joint effect of metabolic risk and healthy lifestyle on the risk of CRC incidence and mortality. We further examined the beneficial reduction in CRC risk provided by these modifiable factors across groups stratified by metabolic risk for CRC.
Materials and methods
Study design and study population
UK biobank is a large, prospective cohort study based on over 0.5 million participants recruited from 2006 to 2010 across England, Wales, and Scotland. Details about study design and information extraction have been reported previously (20). The UKB cohort was approved by North West Multicenter Research Ethics Committee. Written informed consent was obtained from all participants before the study.
In the study, participants with any diagnosed malignancy prior to baseline (except for nonmelanoma skin cancer, n = 26,819), or with missing data on lifestyle or other covariates (n = 146,172) were excluded, leaving 328,236 participants in the final analysis (Figure S1).
Assessment of outcome
CRC cases were identified through linkage to cancer and death registries by using the International Classification of Diseases, 10th Revision (ICD-10). The endpoints of present study were incidence of and mortality from CRC (ICD-10 C18-C20). Eligible patients contributed person-years from recruitment date until date of CRC diagnosis, date of death, date of withdrawal from the study, or last date of follow-up (31 Dec 2021), whichever came first.
Assessment of healthy lifestyle factors
In order to assess adherence to a healthy lifestyle, based on previous knowledge (21–23), 4 factors (smoking, alcohol consumption, diet, physical activity) were used to construct healthy lifestyle score, excluding body mass index (BMI), as it is strongly related to MetS. Based on the World Cancer Research Fund/American Institute of Cancer Research [WCRF/AICR] recommendations, smoking was defined as ideal if individuals were non-smokers, poor intermediate if previous smokers, or poor if current smokers. As for alcohol, the ethanol content was calculated, and then converted into standard units (g/d) (24). Thereafter, alcohol was considered as ideal if individuals were non-drinker (0 g/d), intermediate if moderate alcohol consumers (0 < n ≤ 28 g/d for males and n ≤ 14 g/d for females, or poor if excess alcoholic drinker (n > 28 g/d for males and n > 14 g/d for females) (25). Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) (26, 27), and then grouped into three groups: low (0 < n ≤ 600 MET minutes/week), moderate (600 < n ≤ 3000 MET minutes/week), high (n ≥ 3000 MET minutes/week) (28, 29). A food frequency questionnaire was used to obtain dietary information. According to previous studies (15, 30, 31), 4 main food components (whole grains, vegetables, fruits, red and processed meats) that have associations with CRC are used to compose a diet score which is then categorized into 3 groups (favorable, intermediate, unfavorable).
A healthy lifestyle score (HLS) was created based on recommendations that may confer some benefit (25). For smoking, alcohol consumption and physical activity, 1 point was assigned for high, 0.5 point for mediate, 0 point for low. Total score for diet was 2 points, as it included several factors. Details about point assignment could be seen in SUPPLEMENT 1. A healthy lifestyle score was subsequently categorized into 3 groups: favorable (n ≥ 3.5 points), intermediate (3.5 points > n ≥ 2.75 points) and unfavorable (n < 2.75 points).
Assessment of metabolic status
The metabolic status was categorized two groups according to the presence or absence of metabolic syndrome. Metabolic syndrome (MetS) includes at least 3 component as mentioned below (4): (1) waist circumference (WC) of ≥ 85 cm in women or ≥ 90 cm in men; (2) fasting plasma glucose of ≥ 100 mg/dL or ongoing drug treatment for diabetes mellitus (DM); (3) blood pressure of ≥130/85 mmHg or ongoing drug treatment for hypertension; (4) serum HDL-C of < 50 mg/dL in women or < 40 mg/dL in men; and (5) serum triglyceride of ≥ 150 mg/dL.
Assessment of covariates
Information on sociodemographic characteristics, health and medical history and lifestyle factors was collected through touchscreen questionnaire at baseline, including age, ethnicity, gender, index of multiple deprivation (IMD), family history of cancer, sleep time, medication use [non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs), vitamin supplement, mineral supplement, aspirin]. Anthropometric data, including height, body weight, and waist circumference was measured in the assessment center. The components of abnormal metabolism were measured during physical examination. Further details on the derivation of these variables could be seen via the website (http://www.ukbiobank.ac.uk).
Statistical analysis
Cox proportional hazards models were performed to evaluate the effect of metabolic status, healthy lifestyle and their combination on the risk of CRC incidence and mortality. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for lifestyle categories within each metabolic status stratum. Cox models were adjusted for age, gender, assessment center, ethnicity, family history of cancer, sleep time, use of vitamin supplement, use of mineral supplement, aspirin, use of non-aspirin NSAIDs, and use of statin. The interaction between metabolic status and healthy lifestyle score was tested by adding an interaction term in the Cox regression models.
To investigate possible effect modification, we conducted additional stratified analyses according to gender, age, history of bowel screening and family history of cancer. In addition, we performed several sensitivity analyses to validate the robustness of the main findings. Firstly, we excluded participants who developed CRC or died during the first two years of follow-up to minimize reverse causality. Secondly, we used competing risk regression to account for the competing risk of death, and diagnosis of any other cancer (except for non-melanoma skin cancer). All statistical analyses were performed using R software (version 3.5.3, R Foundation for Statistical Computing, Vienna, Austria) and a two-sided P-value of < 0.05 was defined as a significant difference.
Results
Baseline characteristics of study population
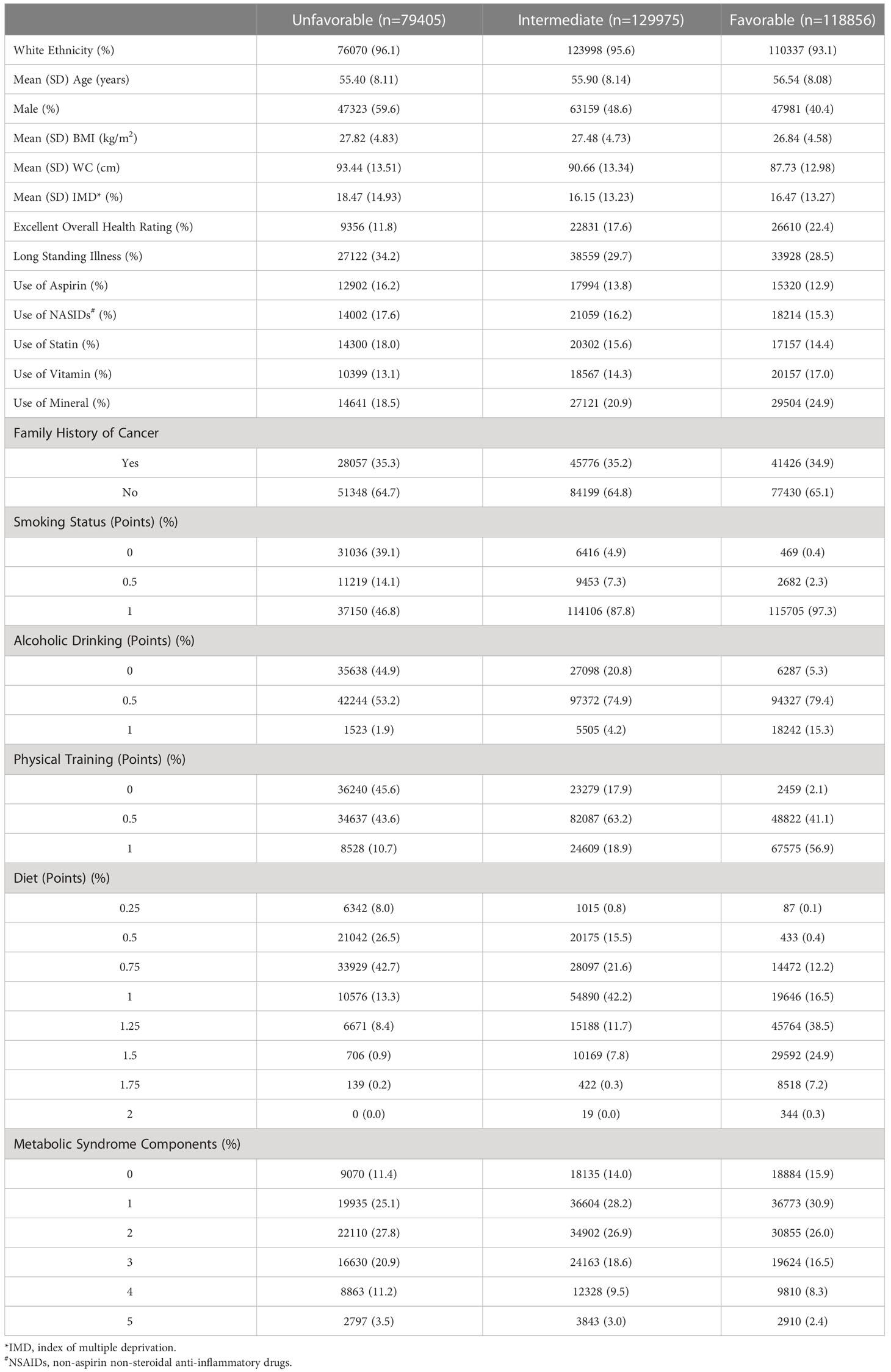
In total, 328,236 participants were finally included in the final analysis. Table 1 shows better the baseline characteristics of the participants by the healthy lifestyle categories. The study population had a mean age of 56 years with 48% males. Compared with individuals adopting an unfavorable lifestyle (24%, n = 79,405), individuals with an intermediate or favorable healthy lifestyle (76%, n = 248,831) tended to be female, with a lower WC, a better general health condition, a higher prevalence of MetS, long standing illness, and medication use (aspirin, NASIDs, and statin). During a median follow-up of 12.5 years, 3,852 incidences of CRC and 1,076 deaths were registered.
Association of metabolic status with CRC incidence and mortality
The risk of CRC incidence and mortality were monotonically associated with the number of abnormal metabolic components (Ptrend < 0.01; Figures S2A, B). Compared with those without MetS, participants with MetS had a higher risk of CRC incidence (HR = 1.24, 95% CI: 1.16 – 1.33, P < 0.01) (Figure 1A), and mortality (HR = 1.24, 95% CI: 1.08 – 1.41, P < 0.01) (Figure 1B). These results were unchanged after adjusting for lifestyle factors (Table S1A), revealing that metabolic status was independently associated with the risk of CRC incidence and mortality.
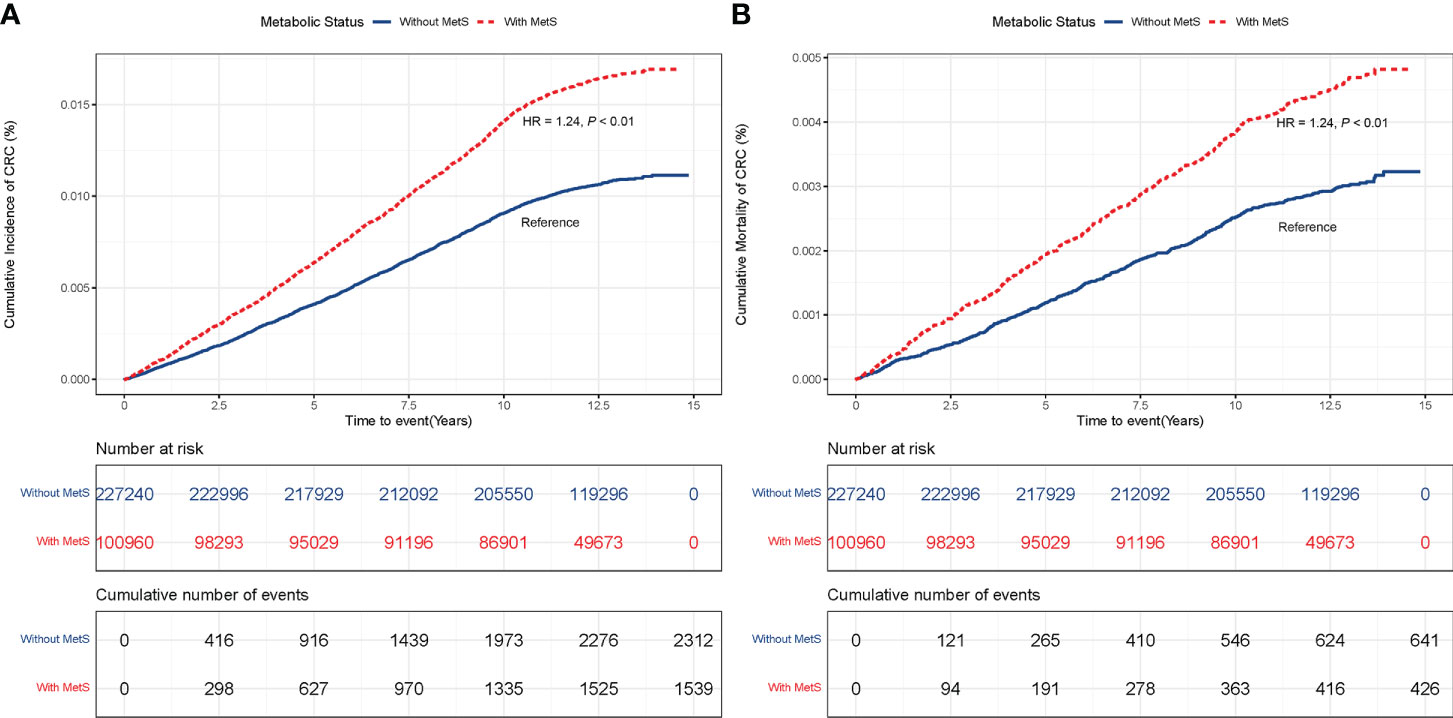
Figure 1 (A) CRC incidence in participants with and without MetS. (B) CRC mortality in participants with and without MetS.
Association of healthy lifestyle and CRC incidence and mortality
As shown in Figures 2A, B adopting a healthy lifestyle was associated with a lower risk of CRC incidence and mortality. The relative risk of CRC incidence and mortality were lower in participants with an intermediate (incidence: HR = 0.88, 95% CI: 0.81 – 0.95; mortality: HR = 0.85, 95% CI: 0.73 – 0.99, respectively), and favorable lifestyle (HR 0.80, 95% CI: 0.74 – 0.87; HR = 0.74, 95% CI: 0.63 – 0.86, respectively), as compared with participants with unfavorable lifestyle. These estimates were unchanged after adjusting for metabolic status, indicating that lifestyle was associated with CRC risk independently (Table S1B). The same pattern of results was noted when the number of healthy lifestyle factors was used instead of lifestyle categories (Figures S3A, B).
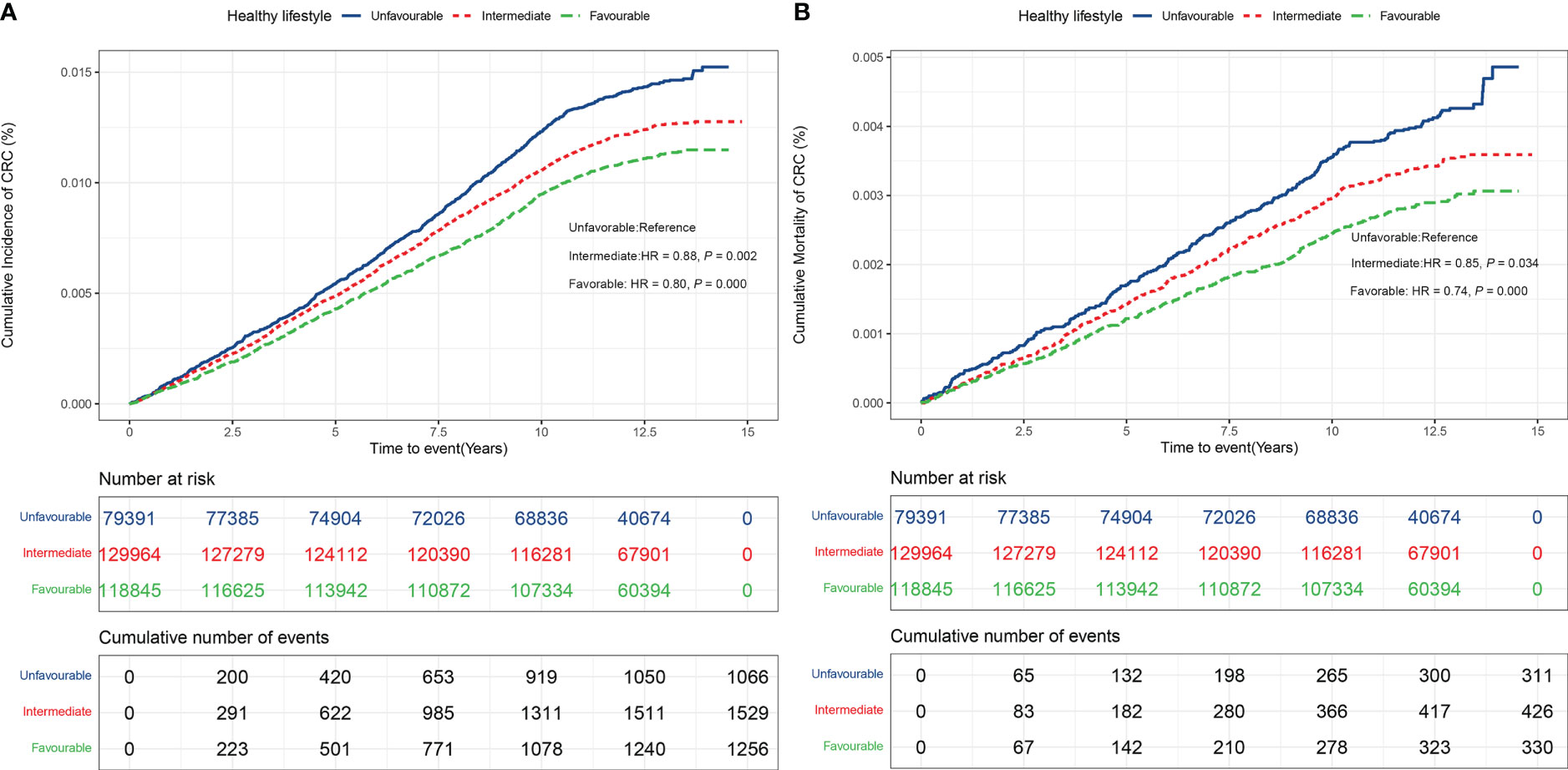
Figure 2 (A) CRC incidence in participants stratified by healthy lifestyle (Unfavorable, Intermediate, Favorable). (B) CRC mortality in participants stratified by healthy lifestyle (Unfavorable, Intermediate, Favorable).
Joint effect of healthy lifestyle and metabolic status on the risk and mortality of CRC
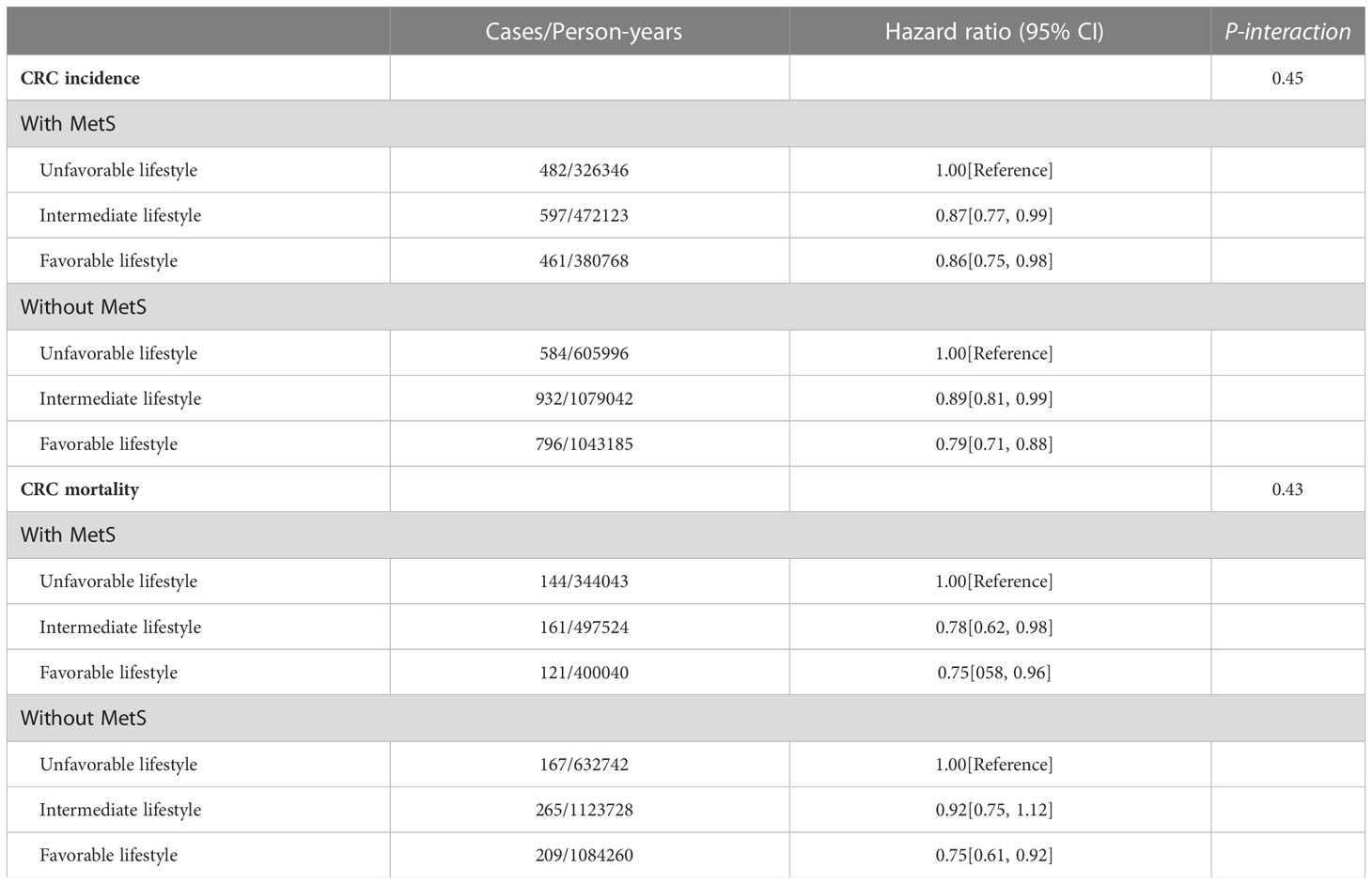
Table 2 shows the associations of adopting a healthy lifestyle with the risk of CRC incidence and mortality stratified by metabolic status. Adopting an intermediate or favorable lifestyle was associated with the lower risk of incident CRC across subgroups of population defined by baseline metabolic status (Table 2).
Similarly, we found that participants adopting a favorable lifestyle had a 25% lower risk of CRC and mortality as compared to participants with an unfavorable lifestyle across all metabolic status subgroups (HR = 0.75, 95% CI: 0.58 – 0.96; HR = 0.75, 95% CI: 0.61 – 0.92, respectively). Compared with an unfavorable lifestyle, intermediate lifestyle was associated with a 22% reduction in risk of CRC mortality in participants with MetS (HR = 0.78, 95% CI: 0.62 – 0.98), but was not associated with CRC mortality in participants without MetS (HR = 0.92, 95% CI: 0.75 – 1.12).
We also noted a joint effect of metabolic and lifestyle factors on the risk of CRC incidence (Figure 3A) and mortality (Figure 3B). Participants with MetS and an unfavorable lifestyle had the highest risk of CRC incidence and mortality as compared with those without MetS and a favorable lifestyle (HR = 1.56, 95% CI: 1.38 – 1.76; HR = 1.75, 95% CI: 1.40 – 2.20, respectively).
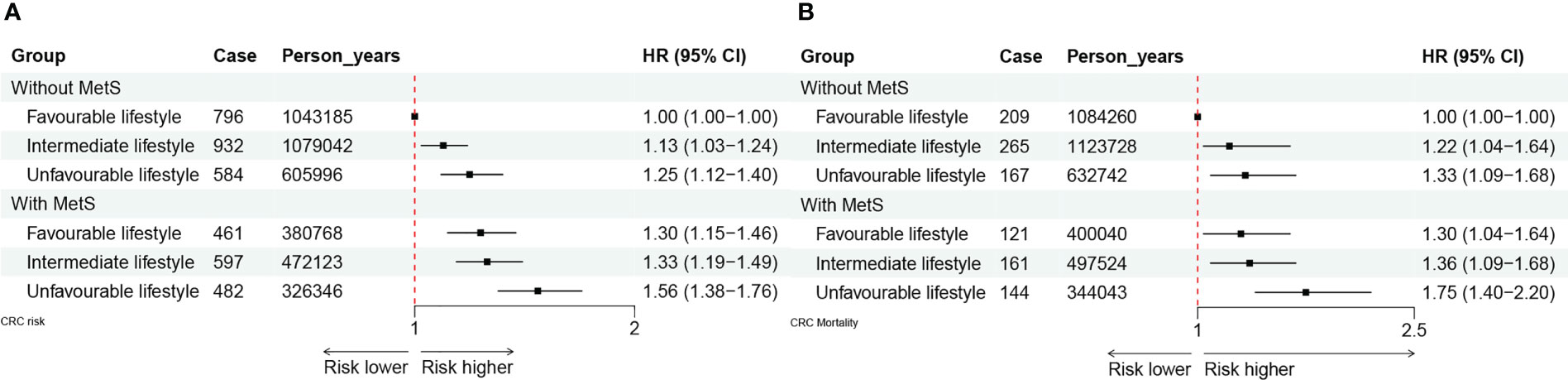
Figure 3 (A) The joint effect of healthy lifestyle and metabolic status on CRC incidence. (B) The joint effect of healthy lifestyle and metabolic status on CRC mortality.
These observed pattern of associations of metabolic status and healthy lifestyle factors with the risk of CRC incidence and mortality did not differ by gender (P = 0.11; P = 0.26), history of bowel screening (P = 0.11; P = 0.37), age (P = 0.73; P = 0.10), and family history of cancer (P = 0.88; P = 0.55) (Table S2). These estimated effect size remain essentially unchanged in several sensitivity analyses that excluded the events occurred during the first 2 years of follow-up, and using the competing risk proportional subdistribution hazards modes (Table S3).
Discussion
In this prospective cohort study, we investigated the individual and joint effect of metabolic risk and healthy lifestyle on the risk of CRC incidence and mortality using over 340,000 participants in the UK Biobank. MetS could increase 24% overall risk of CRC incidence and mortality compared with those without MetS regardless of healthy lifestyle. Adopting an unfavorable lifestyle could further increase CRC incidence and mortality by 20% and 26% respectively when compared with a favorable lifestyle regardless of metabolic status. Participants with MetS and an unfavorable lifestyle profile had a nearly 1.5- and 2- fold risk of CRC incidence and mortality compared to those without MetS and a favorable lifestyle.
Consistent with our findings, previous studies have demonstrated that MetS is associated with a higher risk of CRC incidence and mortality (32–35). For example, a recent nested case–control study in the U.S. showed that MetS was associated with a 25% higher risk of early-onset CRC (HR = 1.25; 95% CI 1.09 – 1.43) (33). A meta-analysis involving 18 studies with CRC incidence and 12 studies with CRC mortality found that patients with MetS had a 25% higher CRC incidence, and a 15% higher CRC mortality (34). However, many established risk factors for CRC, like family history of CRC, history of colonoscopy, smoking, alcohol consumption and diet were not always fully controlled for in most previous studies (33–35), which might lead to residual confounding. In present study, we adjusted for known risk factors for CRC and found that MetS was independently associated with the risk of CRC incidence and mortality. Consistent findings from diverse populations and different studies implied the importance of MetS on the aetiology and preventative strategies of CRC.
We also found that adopting a favorable lifestyle could decrease the risk of CRC incidence and mortality among patients with or without MetS after adjusting for known risk factors, which were in agreement with previous epidemiological studies (36–39). For example, a large cohort study based on the Nurses’ Health Study and the Health Professionals Follow-up Study suggested that adherence to a healthy lifestyle was associated with a reduced CRC incidence and mortality regardless of endoscopic screening (36). Another study based on the DACHS (Darmkrebs Chancen der Verhu¨tung durch Screening) study found that a healthy lifestyle score was increasingly associated with a lower risk of CRC independent of patient’s genetic predisposition (37). However, although importance of screening in preventing CRC has been recognized (40), whether adhering to a healthy lifestyle could attenuate the increased risk caused by MetS remains unclear. To our knowledge, this is the first to examine the joint beneficial association of lifestyle and metabolic health factors with CRC incidence and mortality. The present study has bridged this gap so it would be nice to really emphasize this important new knowledge.
Several previous studies suggested that the associations of lifestyle factors with the risk of CRC incidence and mortality may vary by an individual’s metabolic risk status (41). A prospective cohort study in the United States indicated that moderate to vigorous metabolic status physical activity was associated with a reduced risk of colon cancer in non-diabetics, but not in diabetic patients (41). The present study did not find sufficient evidence that the association of lifestyle factors with CRC risk could be modified by metabolic status. We did observe a synergistic effect of lifestyle and metabolic factors with CRC incidence and mortality, which was in line with previous findings (42). For example, a case-control study in China found that patients with an unfavorable lifestyle and a high level of comorbidity risk had a 10.33-fold increased CRC risk (OR = 10.33, 95% CI: 6.59 – 16.18) (42). These findings, including ours, suggested that people belonging to this group had a higher risk of CRC and require targeted support and services.
Mechanisms linking MetS and CRC incidence had been partly elucidated before. Insulin resistance induced by MetS might promote carcinogenesis through insulin, insulin-like growth factor 1 signaling, and systemic inflammation (6, 43, 44). Recent researches also suggested that the gut microbiota might promote cancer development by modulating the bile acid-microbiota crosstalk and microbe-derived proinflammatory molecules, like lipopolysaccharide (45, 46). Factors included in the healthy lifestyle, like physical training and diet, is helpful to prevent central obesity, and to restore microbiota dysfunction in rats (47, 48). Based on current knowledge we have before concluding this study, we hypothesized that adopting a healthy lifestyle could decrease the risk and mortality by partly intervening or reversing MetS. However, the reduction in HR of risk and mortality of CRC was almost the same in both population with or without MetS, and no multiplicative interactions between healthy lifestyle and metabolic status were identified, which indicate that a healthy lifestyle might act through additional mechanisms. Further investigations would be necessary to clarify these connections.
The major strengths of the present study include the study of a healthy lifestyle and metabolic status in the large sample size of the UK Biobank. Furthermore, we studied the combined effects of metabolic status and healthy lifestyle, systematically investigated the association between them and risk, mortality of CRC, and provided solid evidence for CRC prevention and prognosis in individuals with varied metabolic status. The present study has several limitations. Firstly, some data were not available, the influence of other pathogenic factors on CRC like genetic predisposition could not be calculated in the study, and the risk of CRC may not be so specific. Secondly, due to the limitations of the UK biobank, the findings may not be generalizable to other ethnic groups, and as such, large-scale studies involving other races and ethnicities may be required to confirm the conclusions. Thirdly, lifestyle factors were self-reported, hence the lifestyle risk levels could be misclassified.
Conclusion
In summary, associations of a healthy lifestyle with the risk of CRC in individuals with varied metabolic health have been analyzed quantitively in a large cohort study. The findings show that adopting a healthy lifestyle could decrease the overall risk and mortality of CRC, even in individuals with MetS.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ukbiobank.ac.uk/.
Author contributions
BX contributed to conception and design of the study. JY organized the database and performed the statistical analysis. PX wrote the first draft and QH, BX modified the manuscript. ZK checked for possible linguistic problems. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (grants 82003524, 82003408, 82103913, and 82204123), the Startup Fund for the 100 Top Talents Program, SYSU (392012), Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006) and He Yulong Expert Workstation (202104AC100001-B03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1162221/full#supplementary-material
Abbreviations
BMI, body mass index; CI, confidence interval; CRC, colorectal cancer; DACHS, Darmkrebs Chancen der Verhu¨tung durch Screening; DM, diabetes mellitus; HDL-C, high density liptein cholesterol; HLS, healthy lifestyle score; HR, hazard ratio; ICD-10, International Classification of Diseases, 10th Revision; IMD, index of multiple deprivation; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent; MetS, metabolic syndrome; NSAID, non-aspirin non-steroidal anti-inflammatory drug; WC, waist circumstance; WCRF/AICR, World Cancer Research Fund/American Institute of Cancer Research; UKB, United Kingdom Biobank; US, United States.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care (2012) 35(11):2402–11. doi: 10.2337/dc12-0336
3. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology (2010) 138(6):2029–43.e10. doi: 10.1053/j.gastro.2010.01.057
4. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. a consensus statement from the international diabetes federation. Diabetes Med (2006) 23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x
5. Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci (2011) 7(7):1003–15. doi: 10.7150/ijbs.7.1003
6. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr (2007) 86(3):s836–42. doi: 10.1093/ajcn/86.3.836S
7. Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol (2006) 169(5):1505–22. doi: 10.2353/ajpath.2006.051090
8. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. Jama (2003) 289(2):187–93. doi: 10.1001/jama.289.2.187
9. Qiao Q. Comparison of different definitions of the metabolic syndrome in relation to cardiovascular mortality in European men and women. Diabetologia (2006) 49(12):2837–46. doi: 10.1007/s00125-006-0438-6
10. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep (2018) 20(2):12. doi: 10.1007/s11906-018-0812-z
11. Borch-Johnsen K. The metabolic syndrome in a global perspective. the public health impact–secondary publication. Dan Med Bull (2007) 54(2):157–9.
12. Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer (2009) 124(10):2406–15. doi: 10.1002/ijc.24191
13. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA (2008) 300(23):2765–78. doi: 10.1001/jama.2008.839
14. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer (2015) 112(3):580–93. doi: 10.1038/bjc.2014.579
15. Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol (2021) 36(9):937–51. doi: 10.1007/s10654-021-00741-9
16. Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology (2011) 141(1):106–18. doi: 10.1053/j.gastro.2011.04.013
17. Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst (2012) 104(20):1548–61. doi: 10.1093/jnci/djs354
18. Shen X, Wang Y, Zhao R, Wan Q, Wu Y, Zhao L, et al. Metabolic syndrome and the risk of colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis (2021) 36(10):2215–25. doi: 10.1007/s00384-021-03974-y
19. Chung KC, Juang SE, Chen HH, Cheng KC, Wu KL, Song LC, et al. Association between metabolic syndrome and colorectal cancer incidence and all-cause mortality: a hospital-based observational study. BMC Gastroenterol (2022) 22(1):453. doi: 10.1186/s12876-022-02505-5
20. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. Uk biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
21. Chudasama YV, Khunti K, Gillies CL, Dhalwani NN, Davies MJ, Yates T, et al. Healthy lifestyle and life expectancy in people with multimorbidity in the uk biobank: a longitudinal cohort study. PloS Med (2020) 17(9):e1003332. doi: 10.1371/journal.pmed.1003332
22. Carr PR, Weigl K, Edelmann D, Jansen L, Chang-Claude J, Brenner H, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study. Gastroenterology (2020) 159(1):129–38 e9. doi: 10.1053/j.gastro.2020.03.016
23. Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the national health and nutrition examination survey iii mortality study. Am J Public Health (2011) 101(10):1922–9. doi: 10.2105/ajph.2011.300167
24. Goddard E. Estimating alcohol consumption from survey data: updated method of converting volumes to units. Newport:Office for National Statistics Cardiff (2007).
25. Shams-White MM, Brockton NT, Mitrou P, Romaguera D, Brown S, Bender A, et al Operationalizing the 2018 world cancer research fund/American institute for cancer research (WCRF/AICR) cancer prevention recommendations: a standardized scoring system. Nutrient. (2019) 11(7):1572. doi: 10.3390/nu11071572
26. Guo W, Bradbury KE, Reeves GK, Key TJ. Physical activity in relation to body size and composition in women in uk biobank. Ann Epidemiol (2015) 25(6):406–13.e6. doi: 10.1016/j.annepidem.2015.01.015
27. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc (2003) 35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
28. Guo W, Bradbury KE, Reeves GK, Key TJ. Physical activity in relation to body size and composition in women in uk biobank. Ann Epidemiol (2015) 25(6):406–13.e6. doi: 10.1016/j.annepidem.2015.01.015
29. Celis-Morales CA, Lyall DM, Anderson J, Iliodromiti S, Fan Y, Ntuk UE, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 uk-biobank participants. Eur Heart J (2017) 38(2):116–22. doi: 10.1093/eurheartj/ehw249
30. Zhao J, Zhu Y, Du M, Wang Y, Vallis J, Parfrey PS, et al. Association between dietary fiber intake and mortality among colorectal cancer survivors: results from the Newfoundland familial colorectal cancer cohort study and a meta-analysis of prospective studies. Cancers (Basel) (2022) 14(15). doi: 10.3390/cancers14153801
31. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Bmj (2011) 343:d6617. doi: 10.1136/bmj.d6617
32. Stürmer T, Buring JE, Lee IM, Gaziano JM, Glynn RJ. Metabolic abnormalities and risk for colorectal cancer in the physicians' health study. Cancer Epidemiol Biomarkers Prev (2006) 15(12):2391–7. doi: 10.1158/1055-9965.Epi-06-0391
33. Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut (2021) 70(6):1147–54. doi: 10.1136/gutjnl-2020-321661
34. Han F, Wu G, Zhang S, Zhang J, Zhao Y, Xu J. The association of metabolic syndrome and its components with the incidence and survival of colorectal cancer: a systematic review and meta-analysis. Int J Biol Sci (2021) 17(2):487–97. doi: 10.7150/ijbs.52452
35. Jin EH, Han K, Lee DH, Shin CM, Lim JH, Choi YJ, et al. Association between metabolic syndrome and the risk of colorectal cancer diagnosed before age 50 years according to tumor location. Gastroenterology (2022) 163(3):637–48.e2. doi: 10.1053/j.gastro.2022.05.032
36. Wang K, Ma W, Wu K, Ogino S, Chan AT, Giovannucci EL, et al. Healthy lifestyle, endoscopic screening, and colorectal cancer incidence and mortality in the united states: a nationwide cohort study. PloS Med (2021) 18(2):e1003522. doi: 10.1371/journal.pmed.1003522
37. Carr PR, Weigl K, Jansen L, Walter V, Erben V, Chang-Claude J, et al. Healthy lifestyle factors associated with lower risk of colorectal cancer irrespective of genetic risk. Gastroenterology (2018) 155(6):1805–15.e5. doi: 10.1053/j.gastro.2018.08.044
38. Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a Large European cohort study. BMC Med (2014) 12:168. doi: 10.1186/s12916-014-0168-4
39. Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the united states. JAMA Oncol (2016) 2(9):1154–61. doi: 10.1001/jamaoncol.2016.0843
40. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol (2019) 16(12):713–32. doi: 10.1038/s41575-019-0189-8
41. Schmid D, Behrens G, Matthews CE, Leitzmann MF. Physical activity and risk of colon cancer in diabetic and nondiabetic us adults. Mayo Clin Proc (2016) 91(12):1693–705. doi: 10.1016/j.mayocp.2016.08.017
42. Hang J, Cai B, Xue P, Wang L, Hu H, Zhou Y, et al. The joint effects of lifestyle factors and comorbidities on the risk of colorectal cancer: a Large Chinese retrospective case-control study. PloS One (2015) 10(12):e0143696. doi: 10.1371/journal.pone.0143696
43. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer (2008) 8(12):915–28. doi: 10.1038/nrc2536
44. Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol (2019) 17(2):275–89. doi: 10.1016/j.cgh.2018.07.012
45. Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol (2018) 15(2):111–28. doi: 10.1038/nrgastro.2017.119
46. Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol (2018) 15(11):671–82. doi: 10.1038/s41575-018-0025-6
47. Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PloS One (2014) 9(3):e92193. doi: 10.1371/journal.pone.0092193
Keywords: CRC, healthy lifestyle, metabolic syndrome, incidence, mortality
Citation: Xie P, Wu S, Kuo Z, Tian H, He Q, Li Y, Mi N, Hu L, Zhao H, Li W, Xia B, Yuan J, Yang K, Zhang C and He Y (2023) Association of modifiable lifestyle with colorectal cancer incidence and mortality according to metabolic status: prospective cohort study. Front. Oncol. 13:1162221. doi: 10.3389/fonc.2023.1162221
Received: 13 February 2023; Accepted: 16 May 2023;
Published: 30 May 2023.
Edited by:
Mark Daniel Ross, Heriot-Watt University, United KingdomReviewed by:
Marie Mclaughlin, University of the West of Scotland, United KingdomRichard Metcalfe, Swansea University, United Kingdom
Copyright © 2023 Xie, Wu, Kuo, Tian, He, Li, Mi, Hu, Zhao, Li, Xia, Yuan, Yang, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kehu Yang, a2VodXlhbmdlYm0yMDA2QDEyNi5jb20=; Changhua Zhang, emhjaGFuZ2hAbWFpbC5zeXN1LmVkdS5jbg==; Yulong He, ZG9jdG9yeXVsb25nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
 Peng Xie
Peng Xie Siqing Wu
Siqing Wu Zichong Kuo
Zichong Kuo Huidong Tian4
Huidong Tian4 Qiangsheng He
Qiangsheng He Yanfei Li
Yanfei Li Ningning Mi
Ningning Mi Haitong Zhao
Haitong Zhao Wenjing Li
Wenjing Li Bin Xia
Bin Xia Jinqiu Yuan
Jinqiu Yuan Kehu Yang
Kehu Yang Changhua Zhang
Changhua Zhang Yulong He
Yulong He