
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 18 April 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1161742
This article is part of the Research TopicMultimodal Treatment of Recurrence and Distant Metastases of Colorectal CancerView all 12 articles
 Tao Li1,2
Tao Li1,2 Yahang Liang1,2
Yahang Liang1,2 Daqiang Wang1,2
Daqiang Wang1,2 Zhen Zhou1,2
Zhen Zhou1,2 Haoran Shi1,2
Haoran Shi1,2 Mingming Li1,2
Mingming Li1,2 Hualin Liao1,2
Hualin Liao1,2 Taiyuan Li1,2*
Taiyuan Li1,2* Xiong Lei1,2*
Xiong Lei1,2*Background: The morbidity and mortality of young-onset colorectal cancer (YO-CRC) patients have been increasing in recent years. Moreover, YO-CRC patients with synchronous liver-only metastases (YO-CRCSLM) have various survival outcomes. Therefore, the purpose of this study was to construct and validate a prognostic nomogram for patients with YO-CRCSLM.
Methods: The YO-CRCSLM patients were rigorously screened from the Surveillance, Epidemiology, and End Results (SEER) database in January 2010 and December 2018 and then assigned to a training and validation cohort randomly (1488 and 639 patients, respectively). Moreover, the 122 YO-CRCSLM patients who were enrolled in The First Affiliated Hospital of Nanchang University were served as a testing cohort. The variables were selected using the multivariable Cox model based on the training cohort and then developed a nomogram. The validation and testing cohort were used to validate the model’s predictive accuracy. The calibration plots were used to determine the Nomogram’s discriminative capabilities and precision, and the decision analysis (DCA) was performed to evaluate the Nomogram’s net benefit. Finally, the Kaplan-Meier survival analyses were performed for the stratified patients based on total nomogram scores classified by the X-tile software.
Results: The Nomogram was constructed including ten variables: marital status, primary site, grade, metastatic lymph nodes ratio (LNR), T stage, N stage, carcinoembryonic antigen (CEA), Surgery, and chemotherapy. The Nomogram performed admirably in the validation and testing group according to the calibration curves. The DCA analyses showed good clinical utility values. Low-risk patients (score<234) had significantly better survival outcomes than middle-risk (234–318) and high-risk (>318) patients (P < 0.001).
Conclusion: A nomogram predicting the survival outcomes for patients with YO-CRCSLM was developed. In addition to facilitating personalized survival prediction, this nomogram may assist in developing clinical treatment strategies for patients with YO-CRCSLM who are undergoing treatment.
Colorectal cancer (CRC) is a prevalent and aggressive malignancy of the gastrointestinal tract, ranking third in morbidity and second in mortality among malignant tumors globally (1). CRC patients aged 50 years and older have experienced a reduction in the incidence and mortality rates due to the general screening by colonoscopy (2), while the morbidity of young-onset CRC (YO-CRC) patients age younger than 50 years has been growing (3), with an increasing speeding at annually 3.2% from 1974-2013. The average age of diagnosis for YO-CRC is 40 years, with a comparable incidence in men and women (4). In 2010, the proportion of young-onset colon and rectal cancers was 4.8% and 9.5%, respectively, and is expected to increase to 10.9% and 22.5% by 2030 (5).
YO-CRC is characterized by the presence of microsatellite instability-high (MSI-H, 10%-30%), poorly differentiated tumor cells, and an abundance of signet-ring cell components (6). Especially, YO-CRC had a higher rate of synchronous liver metastasis than later-onset colorectal cancer (LO-CRC) patients, possibly due to diagnostic delays (7). A retrospective study by Cheng et al. demonstrated that 12.2% of YO-CRC patients who were under surgical resection developed liver metastases (8). James et al. showed that the 5-year survival rate of young colorectal patients with synchronous liver-only metastases was only 18% (9). According to another study, the median survival time for individuals with young-onset colorectal live metastases who received both primary and metastatic resection was 35 months. However, the median survival rate dropped to 18% for those patients who did not have any surgery. The 5-year survival rate of colorectal cancer with liver metastasis was only 28% (10). Radical excision of the primary lesion and metastasis is the only method for patients with liver metastases of colorectal cancer to achieve long-term survival (11). Therefore, investigating the prognosis factor affecting those patients is valuable. Previous research has investigated factors affecting the prognosis of young colorectal cancer with liver-only metastases. A retrospective study by Ding et al. showed the 5-year cancer-specific survival rate was influenced by some independent factors, such as primary tumor location, chemotherapy, and histopathological grade (12). Another indicated that the excision of the original tumor and liver metastases was substantially linked with the OS for YO-CRCSLM (9). However, a more effective model for long-term prognostic factors regarding YO-CRC with synchronous Liver-Only metastasis (YO-CRCSLM) needed to be explored.
Therefore, this study aimed to develop and evaluate a more effective model that incorporates clinicopathological factors and blood indicators to predict survival in YO-CRCSLM. Our findings may offer clinicians a more individualized and thorough outlook for YO-CRCSLM receiving treatment.
YO-CRCSLM patients between January 2010 and December 2018 were retrospectively extracted from the SEER database. The inclusion criteria include (1): CRC was the only primary tumor (2), patients aged 18 to 49 at the time of diagnosis, and (3) complete prognostic information. Patients who lacked or had insufficient clinicopathological data of interest, such as age, gender, histological differentiation, primary site, tumor size, and treatment, were excluded. According to the above inclusion and exclusion criteria, 2127 pathologically proven YO-CRCSLM patients were ultimately identified for model construction and were then randomly assigned to the training cohort (approximately 70%, n = 1488) to create the prediction model and the validation cohort (remaining 30%; n = 639). Moreover, 122 YO-CRCSLM patients recruited from the First Affiliated Hospital of Nanchang University during January 2012 to December 2020 were finally selected as a testing cohort. The First Affiliated Hospital of Nanchang University Ethics Committee approved this observational retrospective investigation, and patients who participated in the study signed informed consent forms (2022)CDYFYYLK(12-003).
We collected the following sixteen demographic and clinicopathologic variables: gender, age, marital status, tumor size, primary site, histological type, grade, metastatic lymph nodes ratio (LNR), perineural invasion, T stage, N stage, carcinoembryonic antigen (CEA), Surgery, radiotherapy, and chemotherapy. The patients were divided into three groups based on their primary site: the right-side colon (cecum, ascending colon, hepatic flexure of the colon, transverse colon), the left-side colon (splenic flexure of the colon, descending colon, sigmoid colon, rectosigmoid), and the rectum. The variable of Surgery included primary site surgery (Surg Prim Site), distant metastasis surgery (Surg Dis Site), primary and distant metastasis site combined surgery Surg Com Site and no surgery. Specifically, the information about R0 (Microscopically negative margins) or R1(Microscopically positive margins) resection performed on primary or metastasis sites is unavailable. The LNR was defined as the ratio of the number of lymph nodes with pathologically confirmed tumor infiltration to the total number of lymph nodes cleared. The primary outcome was overall survival (OS), defined as the time from diagnosis of YO-CRCSLM to death from any cause or the last follow-up.
Univariate Cox proportional hazards regression was used to identify potential risk factors, and the statistically significant variables were found as independent prognostic factors in a multivariate Cox regression analysis, and a nomogram was developed to predict the OS of patients with YO-CRCSLM.
The Nomogram’s performance was evaluated using discriminative ability and calibration (13). The calibration was used to evaluate the predictive performance of the Nomogram. The receiver operating characteristic (ROC) curve was used to evaluate the discriminatory ability of the Nomogram. Kaplan-Meier curves were drawn for additional examination based on the nomogram-predicted score categorized by the X-tile software. Finally, the decision curve analysis (DCA) was then utilized to evaluate the model’s net benefit.
The Chi-square test was utilized to compare categorical data represented as numbers and percentages. A COX proportional risk model was used to analyze the prognosis of YO-CRCSLM patients, and a nomogram map was drawn using the R package “rms”. Calibration curves were plotted using a bootstrap of 1000 samples to evaluate the nomogram fit. Calibration plots were assessed using the R package “rms”. The R package “DCA” was used for the net benefits analysis of the model, and time points of 1, 2, and 3 years were selected, respectively. R statistical software was used for statistical analysis of the data, X-tile software was used for risk stratification according to the Nomogram prediction score, and survival curves were drawn to compare the prognosis of patients at different risks. All tests were bilateral, and P < 0.05 was considered statistically significant.
Following the inclusion criteria, the SEER database was eventually used to acquire 2127 eligible YO-CRCSLM patients, who were then assigned into a training group (n=1488) and a validation cohort (n=639) by the stratified random sampling method at a 7:3 ratio. Moreover, a cohort of 122 CRCSLM patients from the First Affiliated Hospital of Nanchang University was defined as the testing cohort, among whom 70 patients died at the follow-up. Figure 1 shows a detailed flow chart of standard procedures for the patient screening process.
Table 1 shows the baseline clinicopathological features of the training and validation sets. Among all patients, the median follow-up was 38 months (3-57 months). The vast majority of the age at diagnosis was 40-49(74.89%), followed by 30-39(20.87%) and 18-29(4.23%). The male patients were more than females (55.10% vs. 44.90%). The most common tumor location was the left-side colon, which accounted for more than the right-sided colon and rectum combined (50.68% vs. 49.32%). Most patients were colorectal adenocarcinomas (89.89% vs 10.11%). Surgical resection of the primary lesion is performed in most patients (85.00% vs 15.00%). In contrast, Surgery for Liver-Only metastases is rare (19.00% vs 81.00%). Most patients received chemotherapy (88.34% vs 11.66%), but fewer received radiation therapy (13.35% vs 86.65%). There was no significant difference between the training and validation cohorts in the baseline demographic and clinicopathological characteristics, so the random of these two cohorts was comparable (Table 1). The mortality was 69.34% and 57.38% for all YO-CRCSLM patients in the SEER and testing cohorts, respectively (Supplemental Table 1).
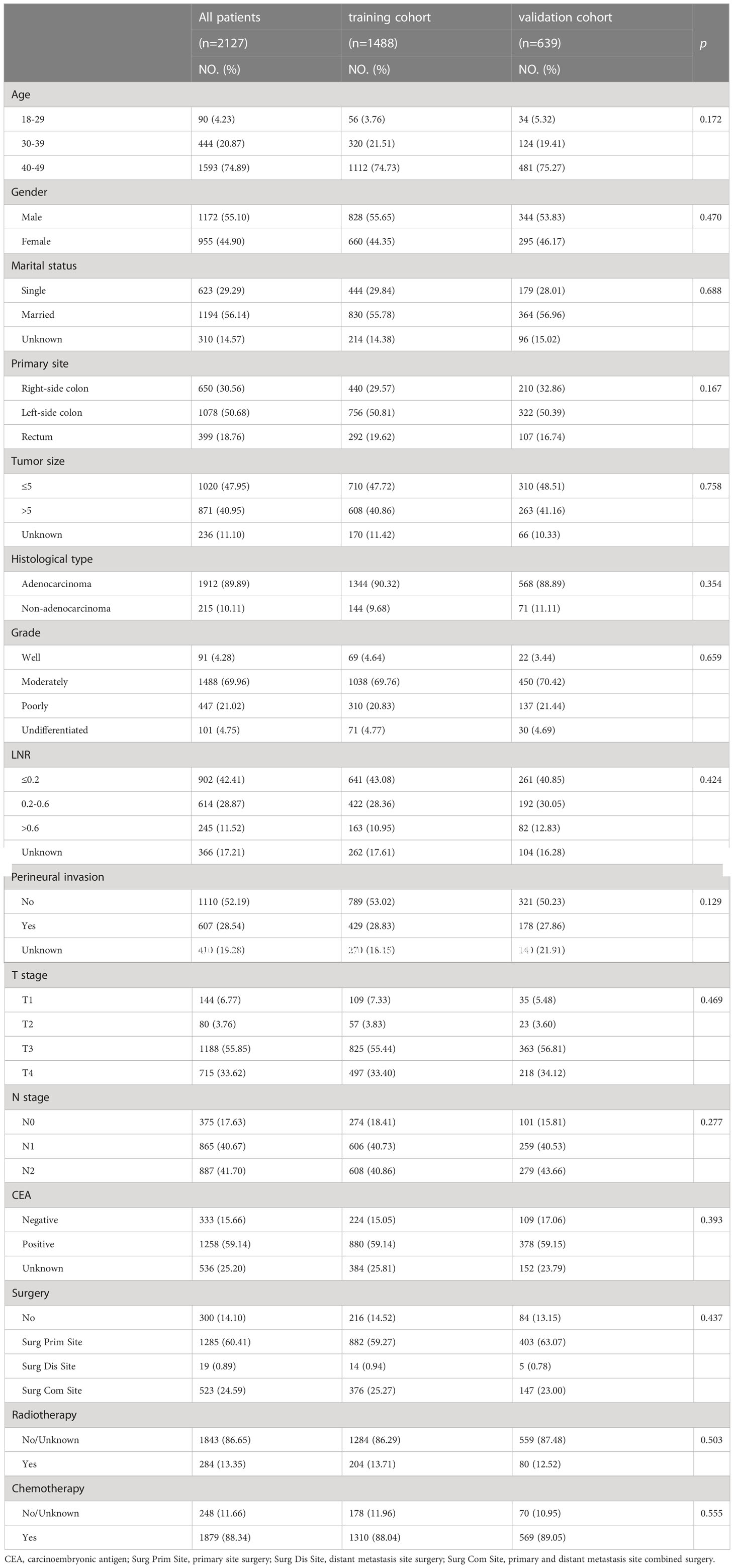
Table 1 Clinicopathological characteristics of Young-onset colorectal cancer with synchronous liver metastasis patients from 2010 to 2018.
Moreover, we included sixteen clinicopathological factors to explore their association with overall survival in the training cohort (Table 2). Finally, we obtained nine prognostic factors with significant differences by multivariate Cox regression analysis. The results showed poorly differentiated grade (HR = 2.14, 95%CI = 1.52 – 3.01, P < 0.001), higher LNR (>0.6: HR = 1.92, 95%CI = 1.50 – 2.45, P < 0.001), higher N stage (N2: HR = 1.43, 95%CI = 1.12 – 1.82, P < 0.001) and positive CEA (HR = 1.26, 95%CI = 1.04 – 1.52, P = 0.019) were related to the worse prognosis. In addition, the primary site (left-side colon: HR = 0.63, 95%CI = 0.54 – 0.72, P < 0.001; rectum: HR = 0.52, 95%CI = 0.41 – 0.65, P < 0.001), Surgery (Surg Prim Site: HR = 0.46, 95%CI = 0.31 – 0.69, P < 0.001; Surg Dis Site: HR = 0.54, 95%CI = 0.28 – 0.89, P < 0.001; Surg Com Site: HR = 0.29, 95%CI = 0.19 – 0.45, P < 0.001) and chemotherapy (HR = 0.49, 95%CI = 0.40 – 0.60, P < 0.001) were significantly associated with better survival outcome. Interestingly, married status was also a protective factor for these patients’ prognosis.
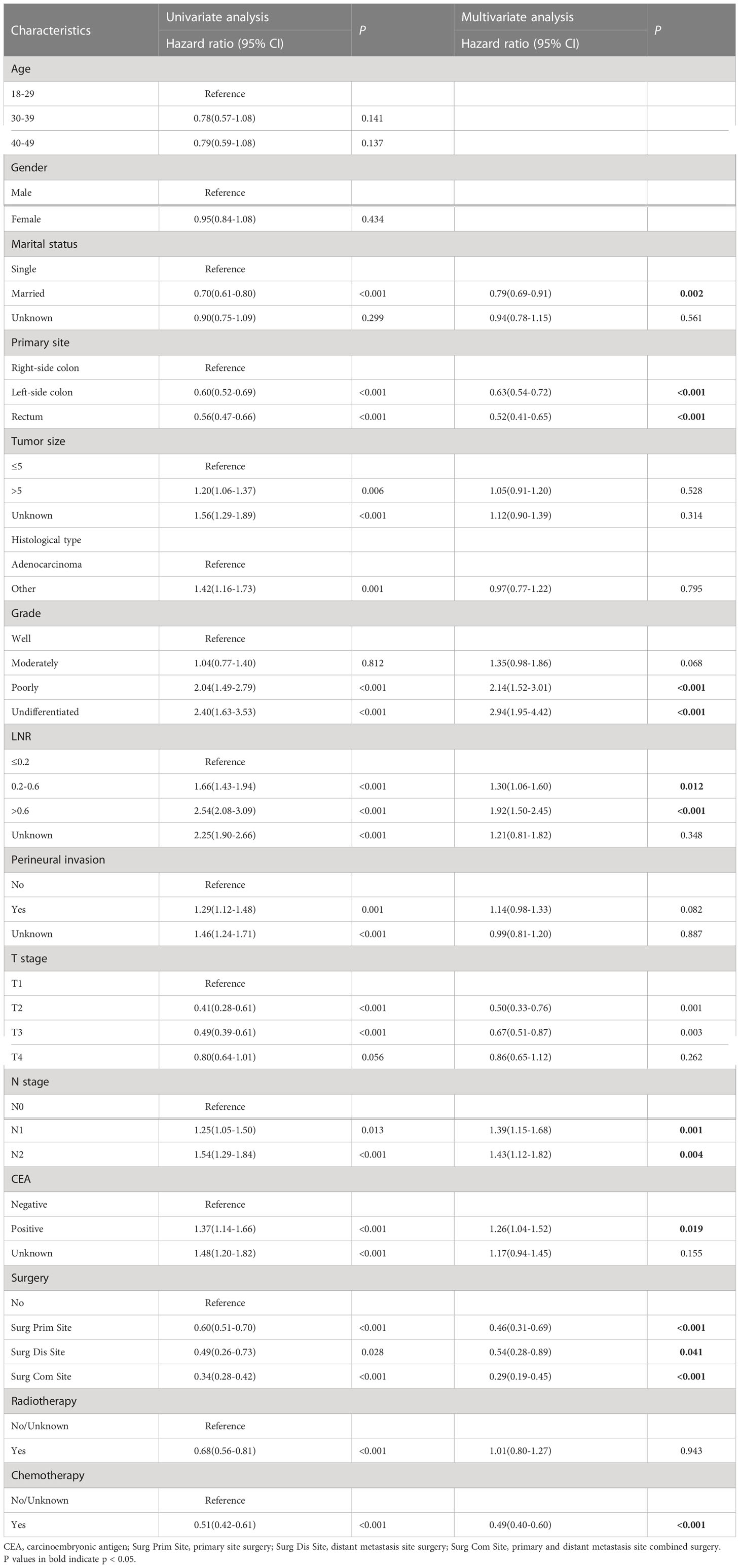
Table 2 Univariate and multivariate cox regression analysis of overall survival in the training cohort.
Then a predictive nomogram model was established based on the factors identified above (Figure 2). The risk score of each variable was obtained according to this Nomogram and then added to get the total score to predict the OS of each patient at 1, 2, and 3 years (Figure 2).
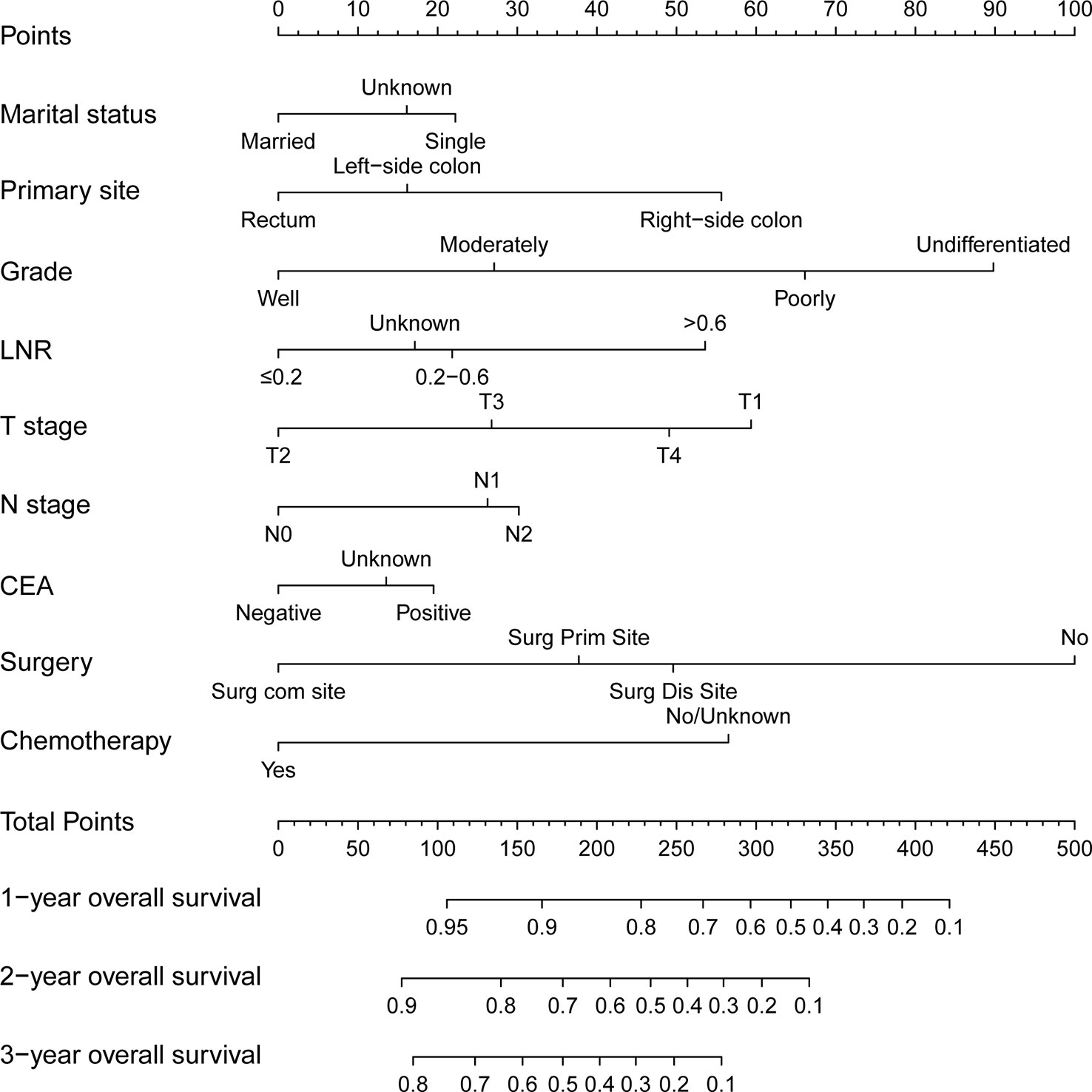
Figure 2 A nomogram for predicting 1-, 2- and 3-year overall survival of patients with young-onset colorectal cancer with synchronous liver-only metastases.
In this study, we evaluated the discriminatory ability of the Nomogram by the ROC curve. In the training cohort, the AUC values of the Nomogram for the probability of survival at 1- (Figure 3A), 2- (Figure 3B), and 3- (Figure 3C) years had excellent discriminatory power. Meanwhile, the AUC values of the monogram 0.778, 0.776, and 0.744 (Figures 3D–F) and 0.755, 0.885, and 0.908 in the validation and testing cohort, respectively (Figures 3G–I), suggesting the model’s discriminatory ability. Then we evaluated our Nomogram’s calibration using the calibration plots, and the result indicated a good agreement between the actual observation and the nomogram prediction in both the training and validation cohorts (Figures 4A–I). Moreover, the DCA analyzed the Nomogram’s clinical usefulness in the training, validation, and testing cohort, indicating excellent positive net benefits (Figures 5A–I).
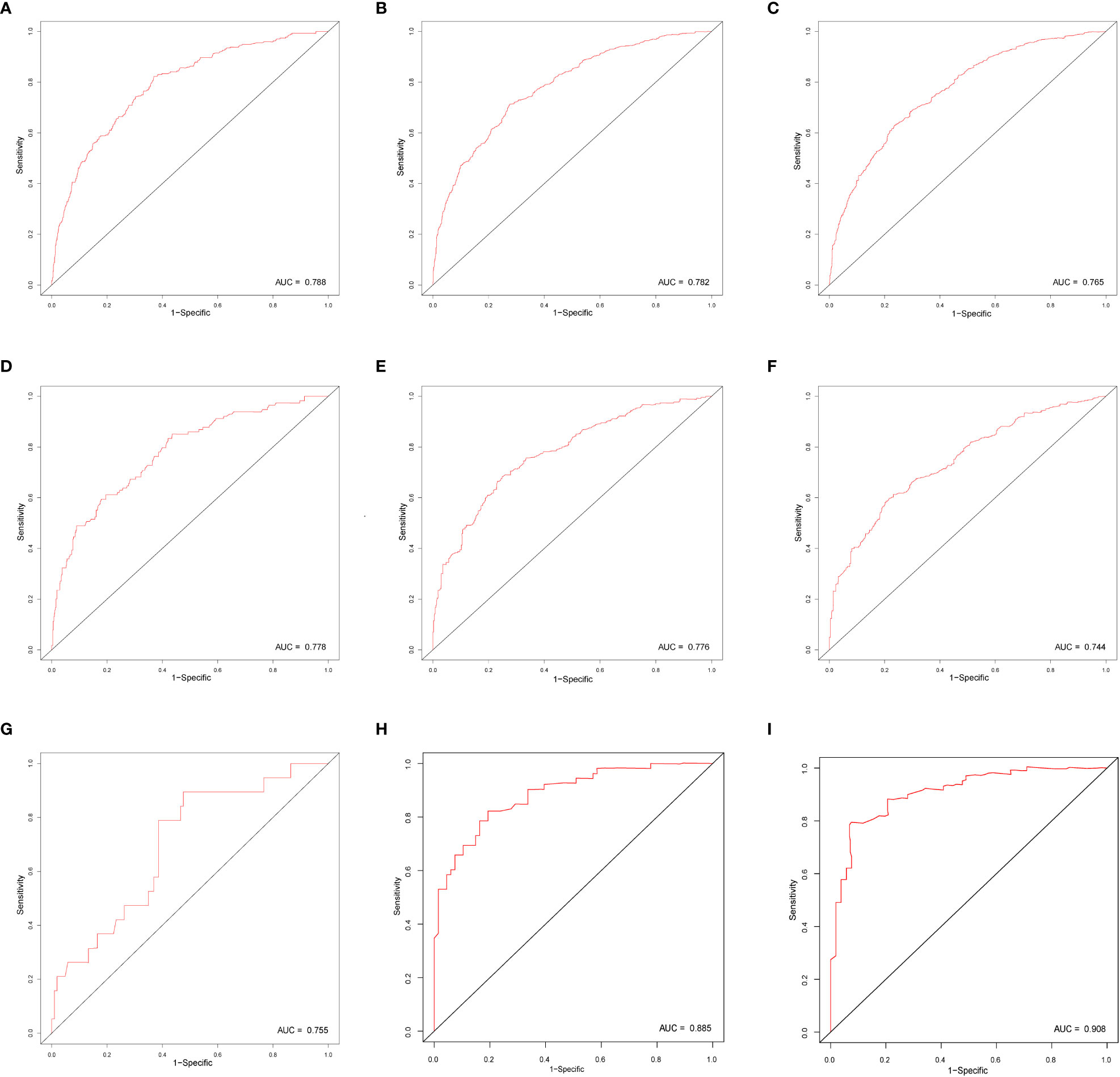
Figure 3 The ROC curve of nomograms to predict 1-, 2-, and 3-year overall survival in the training cohort (A–C), validation cohort (D–F), and testing cohort (G–I).
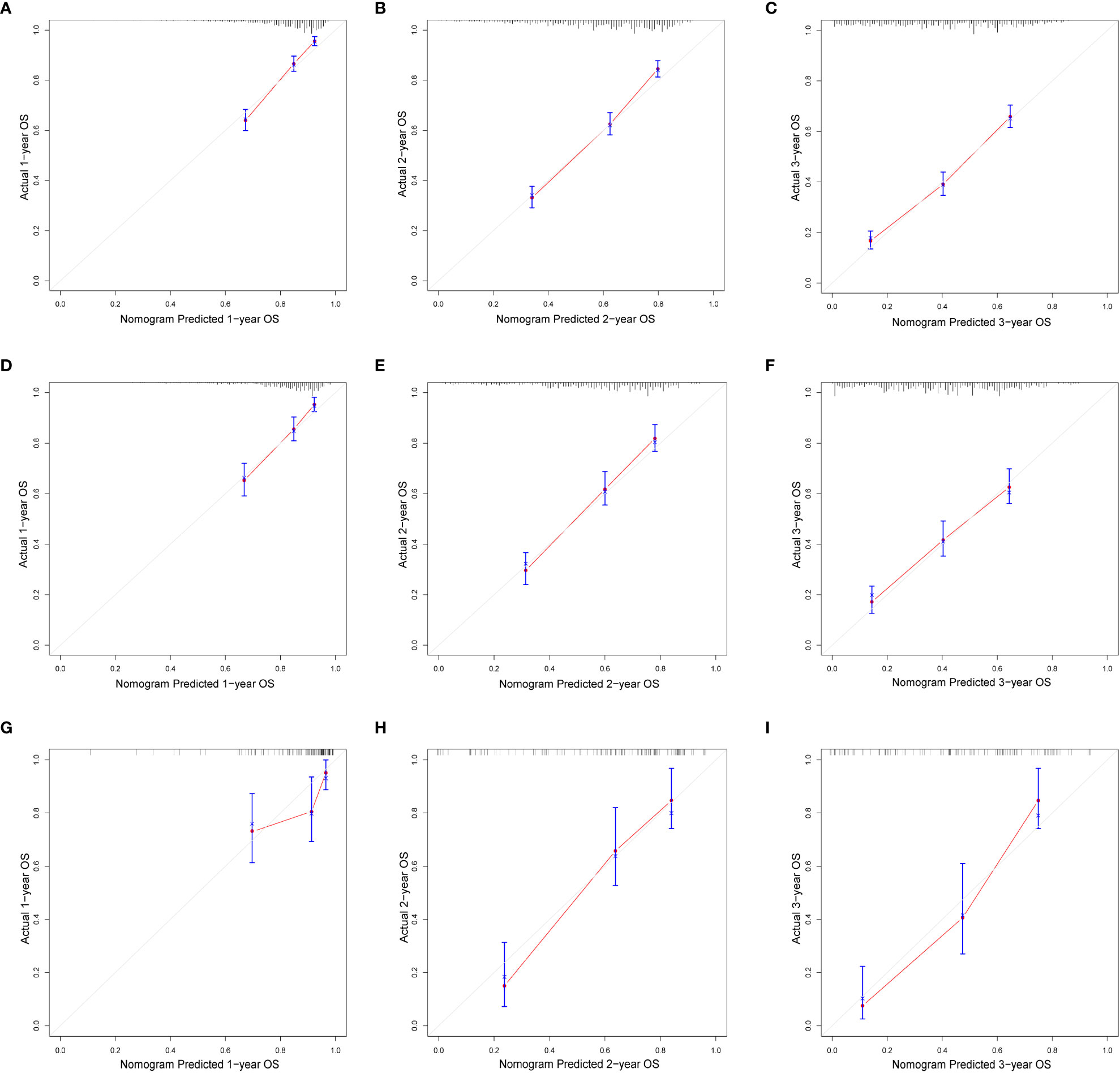
Figure 4 The calibration of nomograms to predict 1-, 2-, and 3-year overall survival in the training cohort (A–C), validation cohort (D–F), and testing cohort (G–I).
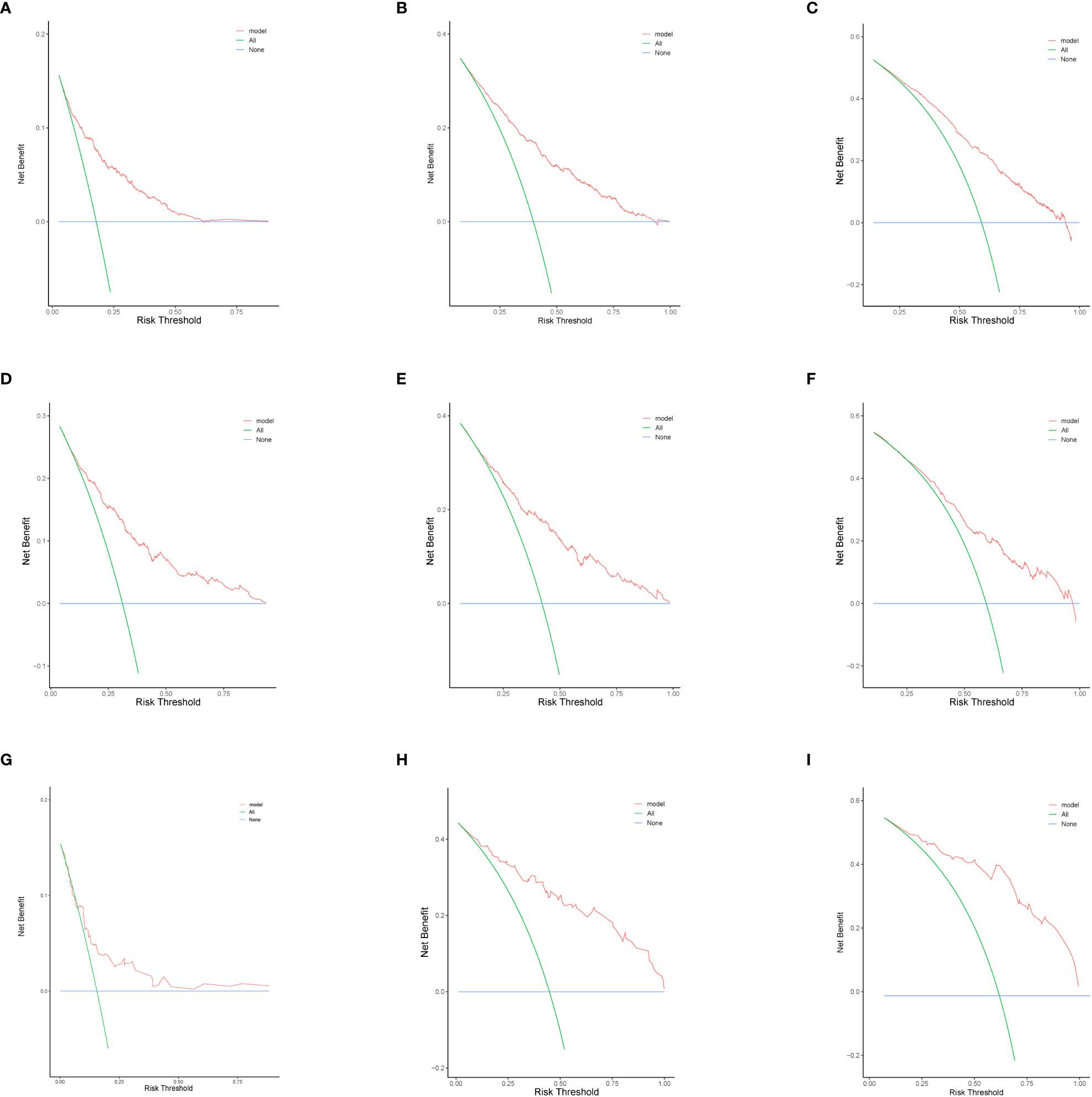
Figure 5 The decision curve analysis of nomograms to predict 1-, 2-, and 3-year overall survival in the training cohort (A-C) validation cohort (D–F), and testing cohort (G–I).
The training cohort was analyzed using the R language to calculate nomogram scores. Then, our nomogram scores for clinicopathological variables are displayed in Table 3. Based on the risk scores of patients in the Nomogram model, we divided patients into a low-risk group (defined as a total score less than 234), a medium-risk group (defined as a total score from 234 to 318), and a high-risk group (Defined as a total score more than 318) by x-tile software (Supplemental Figure 1). As expected, based on risk stratification, we performed a Kaplan-Meier survival analysis for the three groups. As expected, the result showed that the low-risk cohort had better survival outcomes compared to the middle-risk and high-risk groups in the training cohort (Figure 6A), testing cohort (Figure 6B) and validation cohort (Figure 6C).
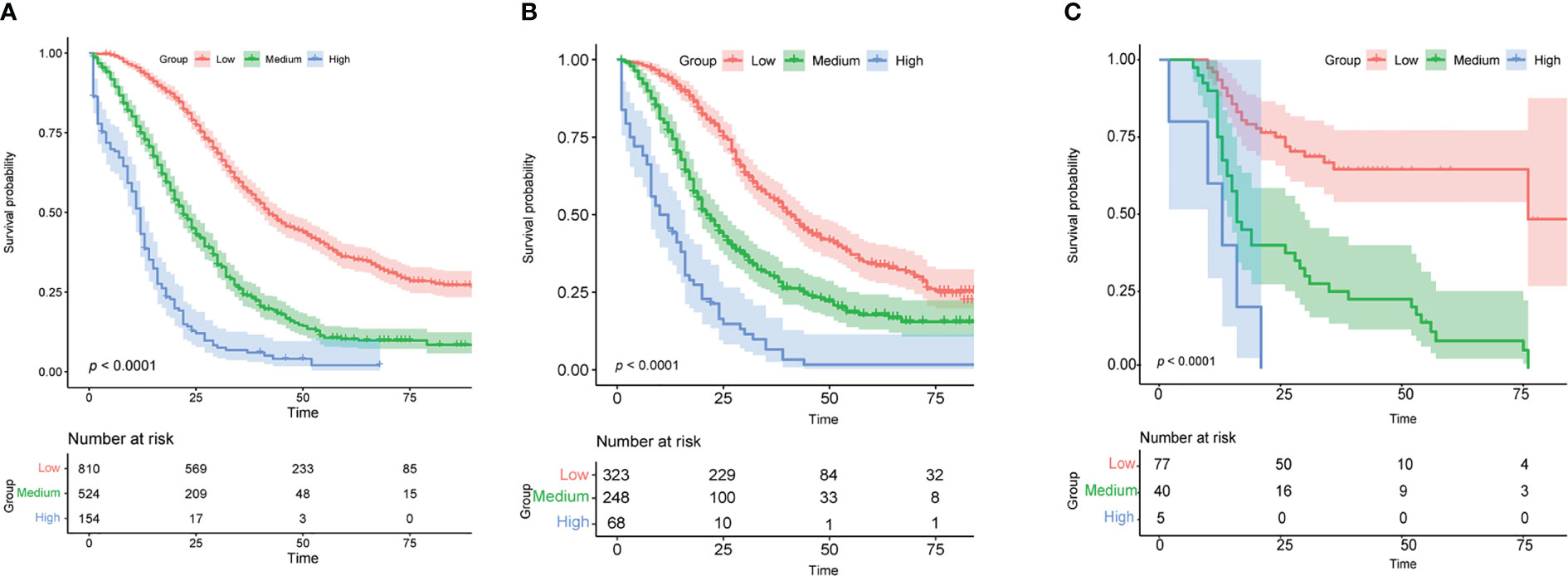
Figure 6 Analysis of survival based on risk stratification. Kaplan-Meier for patients categorized as low-risk, medium-risk, or high-risk in the training cohort (A), validation cohort (B), and testing cohort (C).
The number of individuals diagnosed with EO-CRC has been on the rise, in contrast to the declining trend seen in the elderly since the middle of the 1990s. In addition, colorectal cancer metastases occur most frequently in the liver, which is also a common cause of cancer-related death (14). Therefore, to determine patients’ prognoses and make individual treatment decisions, it is essential to arrive at an accurate survival prediction for YO-CRCSLM patients. To the best of our knowledge, this is the first study to evaluate the prognosis of YO-CRCSLM patients and develop an OS prediction model.
In this study, we evaluated the independent predictive factors influencing survival in 2,127 YO-CRCSLM patients based on the SEER database who were diagnosed between 2010 and 2018 and then constructed a nomogram, including marital status, primary site, grade, LNR, T and N stage, CEA, Surgery and chemotherapy. In addition, 122 YO-CRCSLM hospitalized patients from our hospital were collected and analyzed as external validation cohorts to validate the established nomogram. Moreover, this nomogram can be used as a practical and reliable predictive model in clinical practice to assist doctors in decision-making.
Previous studies have predicted the survival outcomes in CRC patients with liver metastasis (15). however, there is a vital problem that has not received attention, which is that patients with liver metastasis from CRC are susceptible to complicated metastasis from other sites, including the lung, brain, and bone. If only colorectal cancer liver metastasis is analyzed without eliminating the combined metastasis of other organs, this will impact the prognosis of survival for individuals with colorectal cancer liver metastasis. To further investigate the factors independently determining the prognosis of patients with liver metastasis, we analyzed 2,127 individuals with only liver metastasis to fill this gap.
Nine parameters were considered in our model and assigned to various risk scores, which might reflect their influence on the decision. The current findings confirmed our hypothesis and made several important discoveries. Our Nomogram shared some variables with earlier research on predicting the survival of CRC with Liver-Only metastases. In our model, some characteristics, such as grade, T stage, N stage, primary tumor location, and chemotherapy, were assigned a high-risk score, which was also acknowledged mainly in other research (15, 16).
In our Nomogram, marital status was revealed as a significant prognostic factor, and the prognosis of married patients is better than that of unmarried patients, which was similar to several cancers in the previous study (17–19). The reason may be that unmarried cancer patients exhibit more remarkable anguish, sadness, and anxiety than their married counterparts (20), and married patients are more likely to adhere to therapy, which may improve cancer management (21). The T1 stage had the highest risk ratings, indicating those patients had the poorest survival prognosis. It is evident that this phenomenon is contrary to our common sense. However, a study by Lupo Wu et al. also linked this occurrence to the different genetic makeup of T1-stage tumors (22). The results demonstrated the need for increased surveillance and screening of YO-CRCSLM with an early T stage. Moreover, a higher N stage and a poorer tumor grade predicted worse survival, which was similar to the previous study (23). The primary tumor locations served were a significant risk factor that might impact survival prognosis in this models, and this observation has also been confirmed by other studies (24–26). One research showed that patients with right-sided disease had worse survival outcomes than those with left -sided disease (12). Moreover, according to Shida et al.’s national multicenter retrospective study, right-sided CRC (RCRC) patients had a significantly lower OS than left-sided CRC (LCRC) patients (27). Several studies showed that this phenomenon was influenced considerably by histology and molecular traits because RCRC and LCRC have entirely different gene profiles (27–29). RCRC tends to exhibit an advanced clinical behavior than LCRC due to it has more mucinous histopathology, microsatellite instability, CpG island methylation, and BRAF mutations. In contrast, LCRC features many p53 and KRAS alterations (28, 30). Some previous studies demonstrated that preoperative serum CEA significantly affected the prognosis of CRC patients, which was consistent with our result (31, 32). Therefore, CEA might be crucial in the prognosis of CRCSLM, but more research was required to confirm the findings. This study concurs with previous findings that a high lymph node ratio (LNR) is strongly associated with poor overall and disease-free survival in metastatic colorectal cancer (33, 34). Surgery is crucial to the prognosis of cancer patients undergoing treatment. The advantages of primary tumor resection in CRCLM are still up for debate. In fact, primary tumor surgery has been performed in more than two-thirds of older individuals with stage IV CRC (35). This is because primary tumors may stimulate the development of metastasis and have severe consequences, such as obstruction, perforation, and bleeding, that can dramatically diminish patients’ survival rates (36, 37). In addition, the CRC patients’ autoimmunity may be enhanced through primary tumor resection (36). Previous studies had demonstrated the benefit of removing the primary tumor (10, 38, 39), while others had shown no clinical advantages for primary tumor resection (40, 41). In our study, initial tumor excision resulted in notable patient OS increases, which may enhance the survival and quality of life of YO-CRCSLM patients. Especially, our study suggests that performing surgery to remove both the primary tumor and synchronous liver metastasis may provide a substantial improvement in OS for YO-CRCSLM patients, and Chua et al. demonstrated that there were no significant statistical differences between simultaneous liver resection and staged liver resection in terms of overall survival in patients with synchronous liver metastasis from colorectal cancer (42), which indicates that simultaneous resection of primary colorectal cancer and liver metastases as a treatment strategy for YO-CRCSLM is safe and effective. In addition, chemotherapy is crucial in treating CRC patients and is one of the most prevalent techniques for treating colorectal cancer metastases. A retrospective study by Liu et al. indicated that CRCLM patients with chemotherapy had a better prognosis than those not (43). Similarly, in the current study, chemotherapy was demonstrated beneficial to OS.
This study has several advantages over prior research. On the one hand, our data underwent external validation in addition to internal validation, which increased the model’s reliability. Moreover, our Nomogram included distinct factors, such as LNR, which were also found to be an important prognostic factor. However, our current study still remained several limitations, including its inevitable selection bias as a retrospective study. First, some critical information, such as chemotherapy medications and surgical procedures, was missing from the predictive model, which could impact its accuracy. Second, we developed the Nomogram from the extensive SEER database in the training cohort, whereas the testing cohort remained relatively small. Thus, large populations are needed to confirm the Nomogram’s prediction capabilities. Third, it should be noted that a significant number of variables require a high level of information integrity in the constructed Nomogram, which may compromise its usefulness.
In summary, a prognostic nomogram based on nine variables was constructed to predict overall survival in YO-CRCSLM patients, which could be a valuable tool for clinicians’ decision-making. Finally, further research is needed in order to determine whether it is applicable to other patient groups.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TYL and XL conceived and designed the study, acquisition and interpretation of data. TL was involved in interpretation of data and drafting of the manuscript; YL, ZZ and HS were involved in analysis, acquisition and interpretation of data; DW, ML and HL were involved in acquisition of data; TYL and XL were involved in critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by National Natural Science Foundation of China (grant no. 81860519; 82160561), key project of Natural Science Foundation of Jiangxi, China (grant no. 20192ACBL21043).
The authors thank the National Cancer Institute for providing the Surveillance, Epidemiology, and End Results (SEER).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1161742/full#supplementary-material
Supplementary Figure 1 | The cut-off values were determined using X-tile and the total patient scores from the training set. Patients in the training set were classified as low-risk (score < 234), moderate-risk (234≤score< 318), or high-risk (score≥318) according to the Nomogram for OS.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin Gastroenterol Hepatol (2017) 15:903–9.e6. doi: 10.1016/j.cgh.2016.08.037
3. Sinicrope FA. Increasing incidence of early-onset colorectal cancer. N Engl J Med (2022) 386:1547–58. doi: 10.1056/NEJMra2200869
4. Stoffel EM, Koeppe E, Everett J, Ulintz P, Kiel M, Osborne J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology (2018) 154:897–905.e1. doi: 10.1053/j.gastro.2017.11.004
5. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the united states, 1975-2010. JAMA Surg (2015) 150:17–22. doi: 10.1001/jamasurg.2014.1756
6. Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal cancer. Gastroenterology (2021) 160:1041–9. doi: 10.1053/j.gastro.2020.12.068
7. Willauer AN, Liu Y, Pereira AAL, Lam M, Morris JS, Raghav KPS, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer (2019) 125:2002–10. doi: 10.1002/cncr.31994
8. Cheng J, Lao YJ, Wang Q, Huang K, Mou JL, Feng JH, et al. Predicting distant metastasis in young-onset colorectal cancer after surgery: a retrospective study. Front Oncol (2022) 12:804038. doi: 10.3389/fonc.2022.804038
9. Smith JD, Lowery MA, Fell D, Gallagher DJ, Nash GM, Kemeny NE. Young patients with synchronous colorectal liver metastases. J Surg Oncol (2016) 113:473–6. doi: 10.1002/jso.24181
10. Gulack BC, Nussbaum DP, Keenan JE, Ganapathi AM, Sun Z, Worni M, et al. Surgical resection of the primary tumor in stage iv colorectal cancer without metastasectomy is associated with improved overall survival compared with chemotherapy/radiation therapy alone. Dis Colon Rectum (2016) 59:299–305. doi: 10.1097/dcr.0000000000000546
11. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer (2015) 136:E359–86. doi: 10.1002/ijc.29210
12. Ding X, Yang X, Wu D, Huang Y, Dai Y, Li J, et al. Nomogram predicting the cancer-specific survival of early-onset colorectal cancer patients with synchronous liver metastasis: a population-based study. Int J Colorectal Dis (2022) 37:1309–19. doi: 10.1007/s00384-022-04175-x
13. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. Jama (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
14. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2014) 25 Suppl 3:iii1–9. doi: 10.1093/annonc/mdu260
15. Tang M, Wang H, Cao Y, Zeng Z, Shan X, Wang L. Nomogram for predicting occurrence and prognosis of liver metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis (2021) 36:271–82. doi: 10.1007/s00384-020-03722-8
16. Wu Q, Wang WJ, Huang YQ, Fang SY, Guan YJ. Nomograms for estimating survival in patients with liver-only colorectal metastases: a retrospective study. Int J Surg (2018) 60:1–8. doi: 10.1016/j.ijsu.2018.10.032
17. Zhou H, Zhang Y, Song Y, Tan W, Qiu Z, Li S, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: an analysis of the surveillance, epidemiology, and end results (seer) database. Clin Res Hepatol Gastroenterol (2017) 41:476–86. doi: 10.1016/j.clinre.2017.02.008
18. Zhang W, Wang X, Huang R, Jin K, Zhangyuan G, Yu W, et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci Rep (2017) 7:41695. doi: 10.1038/srep41695
19. Hinyard L, Wirth LS, Clancy JM, Schwartz T. The effect of marital status on breast cancer-related outcomes in women under 65: a seer database analysis. Breast (2017) 32:13–7. doi: 10.1016/j.breast.2016.12.008
20. Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol (2010) 21:877–83. doi: 10.1093/annonc/mdp398
21. Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med (2003) 57:2137–47. doi: 10.1016/s0277-9536(02)00546-4
22. Wu L, Fu J, Chen Y, Wang L, Zheng S. Early t stage is associated with poor prognosis in patients with metastatic liver colorectal cancer. Front Oncol (2020) 10:716. doi: 10.3389/fonc.2020.00716
23. Wang X, Mao M, Xu G, Lin F, Sun P, Baklaushev VP, et al. The incidence, associated factors, and predictive nomogram for early death in stage iv colorectal cancer. Int J Colorectal Dis (2019) 34:1189–201. doi: 10.1007/s00384-019-03306-1
24. Luo Z, Fu Z, Li T, Zhang Y, Zhang J, Yang Y, et al. Development and validation of the individualized prognostic nomograms in patients with right- and left-sided colon cancer. Front Oncol (2021) 11:709835. doi: 10.3389/fonc.2021.709835
25. Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F, et al. The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol (2016) 114:803–9. doi: 10.1002/jso.24425
26. Elizabeth McCracken EK, Samsa GP, Fisher DA, Farrow NE, Landa K, Shah KN, et al. Prognostic significance of primary tumor sidedness in patients undergoing liver resection for metastatic colorectal cancer. HPB (Oxford) (2019) 21:1667–75. doi: 10.1016/j.hpb.2019.03.365
27. Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, et al. Prognostic impact of primary tumor location in stage iii colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol (2020) 55:958–68. doi: 10.1007/s00535-020-01706-7
28. Imperial R, Ahmed Z, Toor OM, Erdoğan C, Khaliq A, Case P, et al. Comparative proteogenomic analysis of right-sided colon cancer, left-sided colon cancer and rectal cancer reveals distinct mutational profiles. Mol Cancer (2018) 17:177. doi: 10.1186/s12943-018-0923-9
29. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol (2017) 3:211–9. doi: 10.1001/jamaoncol.2016.4227
30. Mukund K, Syulyukina N, Ramamoorthy S, Subramaniam S. Right and left-sided colon cancers - specificity of molecular mechanisms in tumorigenesis and progression. BMC Cancer (2020) 20:317. doi: 10.1186/s12885-020-06784-7
31. Sudo M, Furuya S, Shimizu H, Nakata Y, Iino H, Shiraishi K, et al. Long-term outcomes after surgical resection in patients with stage iv colorectal cancer: a retrospective study of 129 patients at a single institution. World J Surg Oncol (2019) 17:56. doi: 10.1186/s12957-019-1599-3
32. Kawai K, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Miyata H, et al. Nomograms for predicting the prognosis of stage iv colorectal cancer after curative resection: a multicenter retrospective study. Eur J Surg Oncol (2015) 41:457–65. doi: 10.1016/j.ejso.2015.01.026
33. Ahmad A, Reha J, Saied A, Espat NJ, Somasundar P, Katz SC. Association of primary tumor lymph node ratio with burden of liver metastases and survival in stage iv colorectal cancer. Hepatobiliary Surg Nutr (2017) 6:154–61. doi: 10.21037/hbsn.2016.08.08
34. Jiang C, Wang F, Guo G, Dong J, Liu S, He W, et al. Metastatic lymph node ratio as a prognostic indicator in patients with stage iv colon cancer undergoing resection. J Cancer (2019) 10:2534–40. doi: 10.7150/jca.29216
35. Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage iv colorectal cancer. J Clin Oncol (2004) 22:3475–84. doi: 10.1200/jco.2004.10.218
36. Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res (2004) 64:2205–11. doi: 10.1158/0008-5472.can-03-2646
37. van der Wal GE, Gouw AS, Kamps JA, Moorlag HE, Bulthuis ML, Molema G, et al. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg (2012) 255:86–94. doi: 10.1097/SLA.0b013e318238346a
38. Maroney S, de Paz CC, Reeves ME, Garberoglio C, Raskin E, Senthil M, et al. Benefit of surgical resection of the primary tumor in patients undergoing chemotherapy for stage iv colorectal cancer with unresected metastasis. J Gastrointest Surg (2018) 22:460–6. doi: 10.1007/s11605-017-3617-5
39. Park JH, Kim TY, Lee KH, Han SW, Oh DY, Im SA, et al. The beneficial effect of palliative resection in metastatic colorectal cancer. Br J Cancer (2013) 108:1425–31. doi: 10.1038/bjc.2013.94
40. Massarweh NN, Li LT, Sansgiry S, Berger DH, Anaya DA. Primary tumor resection and multimodality treatment for patients with metastatic colon cancer. Ann Surg Oncol (2016) 23:1815–23. doi: 10.1245/s10434-015-5073-3
41. Matsumoto T, Hasegawa S, Matsumoto S, Horimatsu T, Okoshi K, Yamada M, et al. Overcoming the challenges of primary tumor management in patients with metastatic colorectal cancer unresectable for cure and an asymptomatic primary tumor. Dis Colon Rectum (2014) 57:679–86. doi: 10.1097/dcr.0000000000000025
42. Chua HK, Sondenaa K, Tsiotos GG, Larson DR, Wolff BG, Nagorney DM. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum (2004) 47:1310–6. doi: 10.1007/s10350-004-0586-z
Keywords: survival model, YO-CRCSLM, survival, nomogram, SEER
Citation: Li T, Liang Y, Wang D, Zhou Z, Shi H, Li M, Liao H, Li T and Lei X (2023) Development and validation of a clinical survival model for young-onset colorectal cancer with synchronous liver-only metastases: a SEER population-based study and external validation. Front. Oncol. 13:1161742. doi: 10.3389/fonc.2023.1161742
Received: 08 February 2023; Accepted: 03 April 2023;
Published: 18 April 2023.
Edited by:
Andrea Balla, San Raffaele Scientific Institute (IRCCS), ItalyReviewed by:
Diego Coletta, Sapienza University of Rome, ItalyCopyright © 2023 Li, Liang, Wang, Zhou, Shi, Li, Liao, Li and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taiyuan Li, anlsaXRhaXl1YW5AMTI2LmNvbQ==; Xiong Lei, bGVpeGlvbmdsaW50eUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.