- 1Department of Urology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 2Department of Urology, Chengdu Xinhua Hospital Affiliated to North Sichuan Medical College, Chengdu, China
- 3Department of Anesthesia, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 4Department of Radiate, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 5Department of Urology, Lanzhou University Second Hospital, Lanzhou, China
Background: The nerve-sparing (NS) effect of robot-assisted radical prostatectomy (RARP) on patients with a high-risk prostate cancer remains unclear. The objective of this study was to compare the urinary continence, erectile function and oncology outcomes of the nerve-sparing and non-nerve-sparing (NNS) group during RARP surgeries.
Methods: We systematically searched databases including PubMed, Embase, Cochrane Library and Web of Science to identify relevant studies published in English up to December 2022. Newcastle-Ottawa Scale (NOS) was used as a quality evaluation tool to evaluate the quality of the literature parameters involved, including urinary continence, erectile function and oncologic outcomes, which were compared using the Stata 15.1 software (StataSE, USA).
Results: A total of 8 cohort studies involving 2499 patients were included. A meta-analysis of results showed that the NS group was beneficial to the recovery of urinary continence (RR 0.46, 95%CI 0.22, 0.96; p=0.045<0.05) and erectile function (RR 0.32, 95%CI 0.16, 0.63; p=0.001<0.05) 12 months after surgeries, which showed a better oncological outcome (RR 1.31, 95%CI 1.01, 1.69; p=0.01<0.05).
Conclusions: The current study results indicate that intraoperative NS during RARP is beneficial to long-term postoperative functional recovery and tumor prognosis of patients with high-risk prostate cancers. Due to interstudy interferences, the results should be interpreted with caution.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022384647.
Introduction
Prostate cancer (Pca) is the second most common solid tumor for men (1). In the United States, 191,930 new cases with Pca are expected in 2020 (2). Although the mortality of prostate has been stabilized in developed countries in recent years, it is still the leading cause of deaths for male patients with cancers (3, 4). High-risk prostate cancer was defined with a PSA≥20ng/ml, a Gleason score of 8-10 or a clinical stage ≥T3, which accounted for about 40% of the total (5). Robot-assisted radical prostatectomy (RARP) is currently a common method for treating localized Pca (6), which provides a higher field of view and more freedom compared with traditional radical prostatectomy (RP). These advantages make intraoperative NS more feasible. The perioperative and functional outcomes of the treatment of localized Pca through robot-assisted, laparoscopic or open radical prostatectomy differ (7).
Nerve sparing (NS) is a topic well worth exploring in the surgical management of patients with high-risk Pca, and many studies have confirmed that NS helps to improve the functional prognosis of patients in radical prostatectomy (8–10). Several meta-analyses have demonstrated that NS is beneficial to the postoperative recovery of urinary continence and erectile function (EF), which reduces the risk of postoperative urinary incontinence (11–13). Veneziano et al. (14) suggested that NS was beneficial for patients with urinary incontinence, which significantly improved the EF of high-risk patients. The effect of NS selection on patients in RARP is still unclear, so further exploration is very necessary.
Therefore, we conducted a meta-analysis of the published clinical studies on the outcomes of NS in RARP to provide the latest evidence for clinicians to make decisions.
Methods
We conducted a systematic review and meta-analysis following the guideline of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (15). A relevant protocol has been registered on the Prospero website (https://www.crd.york.ac.uk/PROSPERO/) with a registration number of CRD42022384647.
Literature search strategy, study selection and data collection
The databases Pubmed, Cochrane Library, Web of Science and Embase were searched, and the deadline for literature search was December 8, 2022. According to the PICOS standard definition of search terms, literature search was conducted by combining the topic with free terms: (((high-risk) AND ((prostate cancer) OR (prostate neoplasm))) AND ((NS) OR (neurovascular bundle sparing))) AND ((Robotic surgical procedures) OR (RARP)).
The literature to be searched was written in English language only, so we also manually searched and reviewed the relevant references to avoid any omission.
We used the PICOS approach to define the inclusion criteria. P (patients): all the patients were diagnosed with a high-risk prostate cancer (PSA≥20ng/ml or a Gleason score of 8-10 or a clinical stage ≥T3) and undergone RARP; I (intervention): NS, bilateral nerve-sparing (BNS), unilateral nerve-sparing (UNS) or partial nerve-sparing (PNS); C (comparator): non-nerve-sparing; O (outcome): one or more of the following outcomes: EF, urinary continence and oncologic outcomes; S (study type): cohort studies. Exclusion criteria (1): non-comparative studies; (2) editorial comments, meeting abstracts, case reports, unpublished studies, or reviews; (3) studies with unavailable data for analyses; (4) other studies that did not meet the inclusion criteria.
Data extraction was carried out independently by the two reviewers, which were as follows: (1) general information: the first author extracted the years of publication and countries; (2) population characteristics: the number of patients, age, sample size, criteria to the assess recovery of urinary continence and EF; (3) outcomes: continence recovery (defined as the use of no pad or one safety pad/day), EF recovery (defined as erections sufficient for sexual intercourse), oncologic outcomes: positive surgical margins (PSM). Any discrepancy was resolved through a consensus or consultation with a third reviewer.
Quality assessment and statistical analysis
The quality of publications was assessed based on the Newcastle-Ottawa Scale (NOS) (https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp); the quality of data extracted from each study was assessed based on case selection, comparability and outcome reporting. A meta-analysis was performed using the Stata 15.1 software (StataSE, USA). Risk ratio (RR) was used as a dichotomous variable. Statistical heterogeneity was evaluated based on I2 statistics; I2 ≥ 50% indicated a significant heterogeneity, and a random effects model was employed; when I2 < 50%, a fixed effects model was employed; p ≤ 0.05 was the threshold for statistical significance (16). Subgroup and sensitivity analyses were performed when it was necessary to explore the sources and sizes of heterogeneity through studies. However, we could not perform sensitivity analyses when comparing three or fewer studies. Publication biases were screened using a funnel plot.
Results
Baseline characteristics
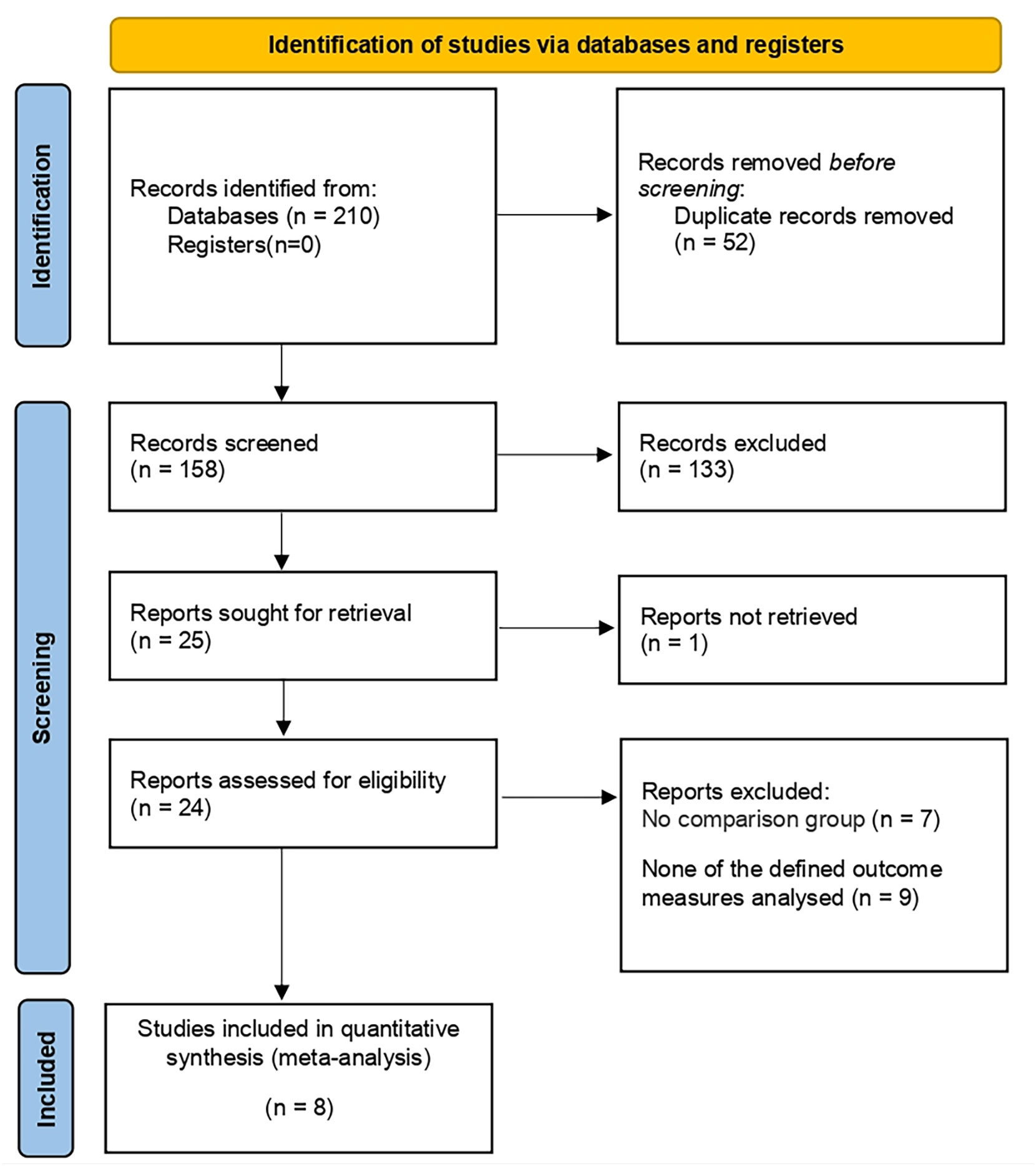
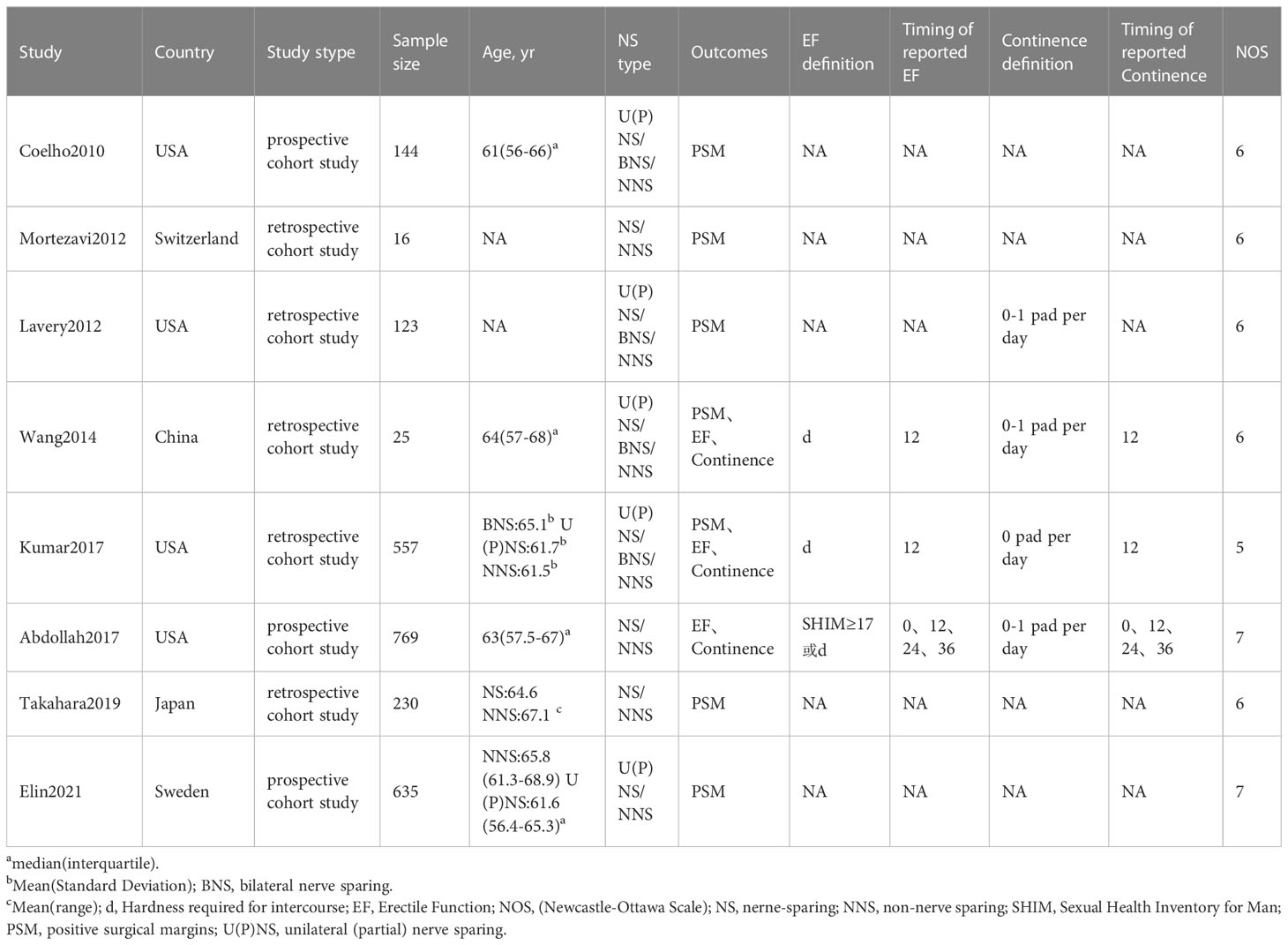
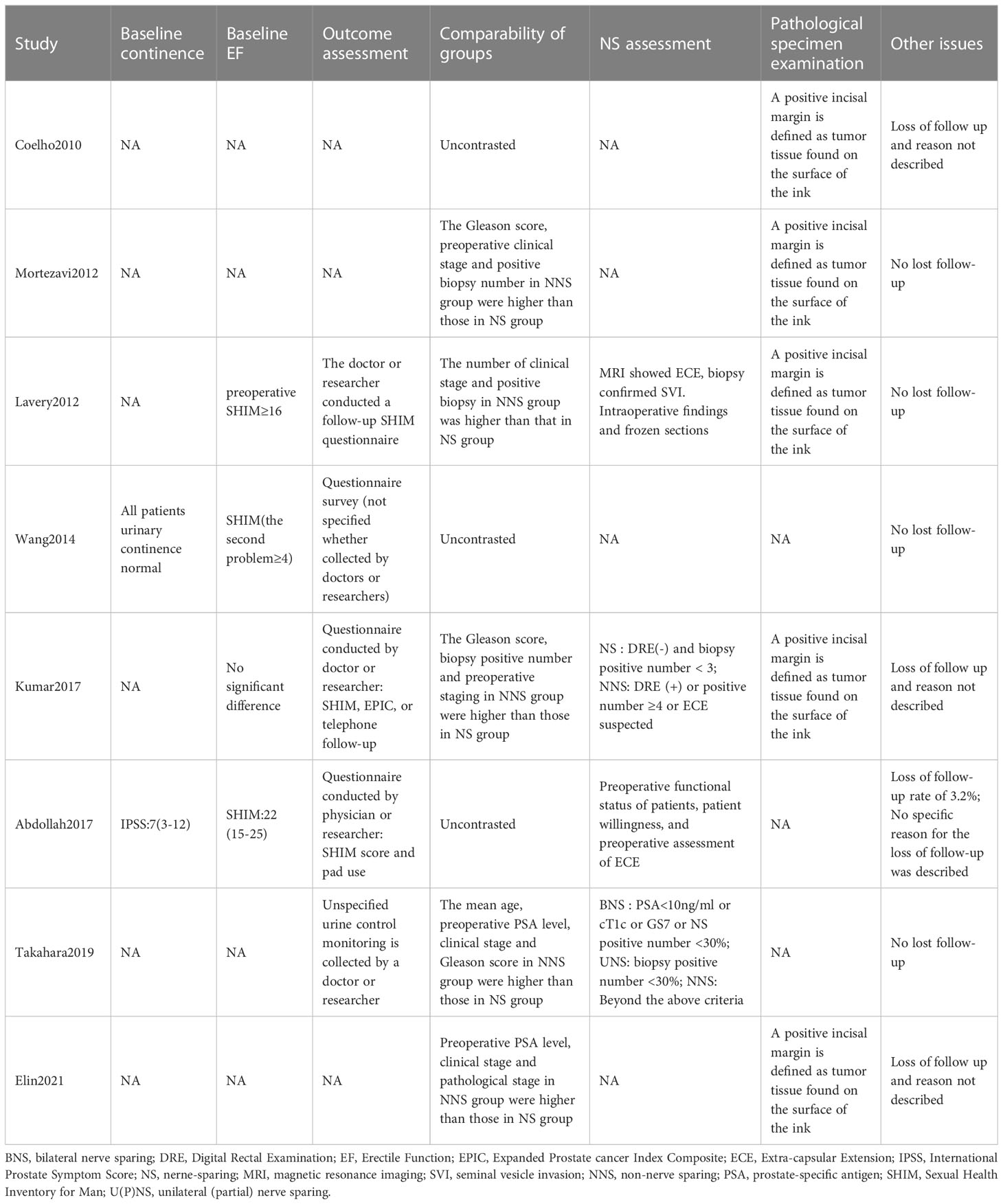
210 studies were preliminarily searched, 52 of which remained after duplicates were removed. A total of 24 relevant articles were obtained through the initial screening. We excluded 10 studies after reviewing titles and abstracts, together with 6 articles after reading and screening the full text. 8 prospective or retrospective cohort studies (17–24) were included, ranging from 16 to 769 in size, and the total number of patients included was 2499. The details of the screening processes are shown in Figure 1. 90% of the patients in these studies were from European and American countries, and 10% were from Asian countries. The baseline characteristics, the patients included and their associated preoperative variables (age, country, NS type) are summarized in Table 1. Due to the different types of NS in some studies (20, 21, 25), we divided it into three types: UNS (partial), BNS and NNS. Furthermore, the definition of urinary continence and the recovery of EF in each study are also included in this table. The preoperative urinary continence and EF of patients in each study, the comparison among studies, the NS standards adopted as well as pathological specimen examinations are summarized in Table 2.
Assessment of quality
The included studies were scored using the NOS scale. 5 studies were scored 6, 1 study was scored 5, 2 studies were scored 7 and the median NOS of all studies was 6, as is detailed in Table 3.
Outcome analysis
Urinary continence
Urinary continence recovery was defined as the use of 0/1 pad per day. The status of urinary continence after surgeries could be extracted from 3 literatures (20–22). Since only the status of urinary continence recovery 12 months after surgeries is reported in the 2 studies (20, 21), we could only conduct a meta-analysis on the data 12 months after surgeries. 1370 patients were included in the 3 studies, including 1220 in the NNS group and 150 in the NS group. The meta-analysis showed that the recovery of urinary control in the NNS group 12 months after surgeries was worse than that in the NS group (RR 0.46, 95%CI 0.22, 0.96; p=0.045 <0.05) (Figure 2), and the difference was statistically significant. Due to the small number of studies included, sensitivity analyses could not be performed.
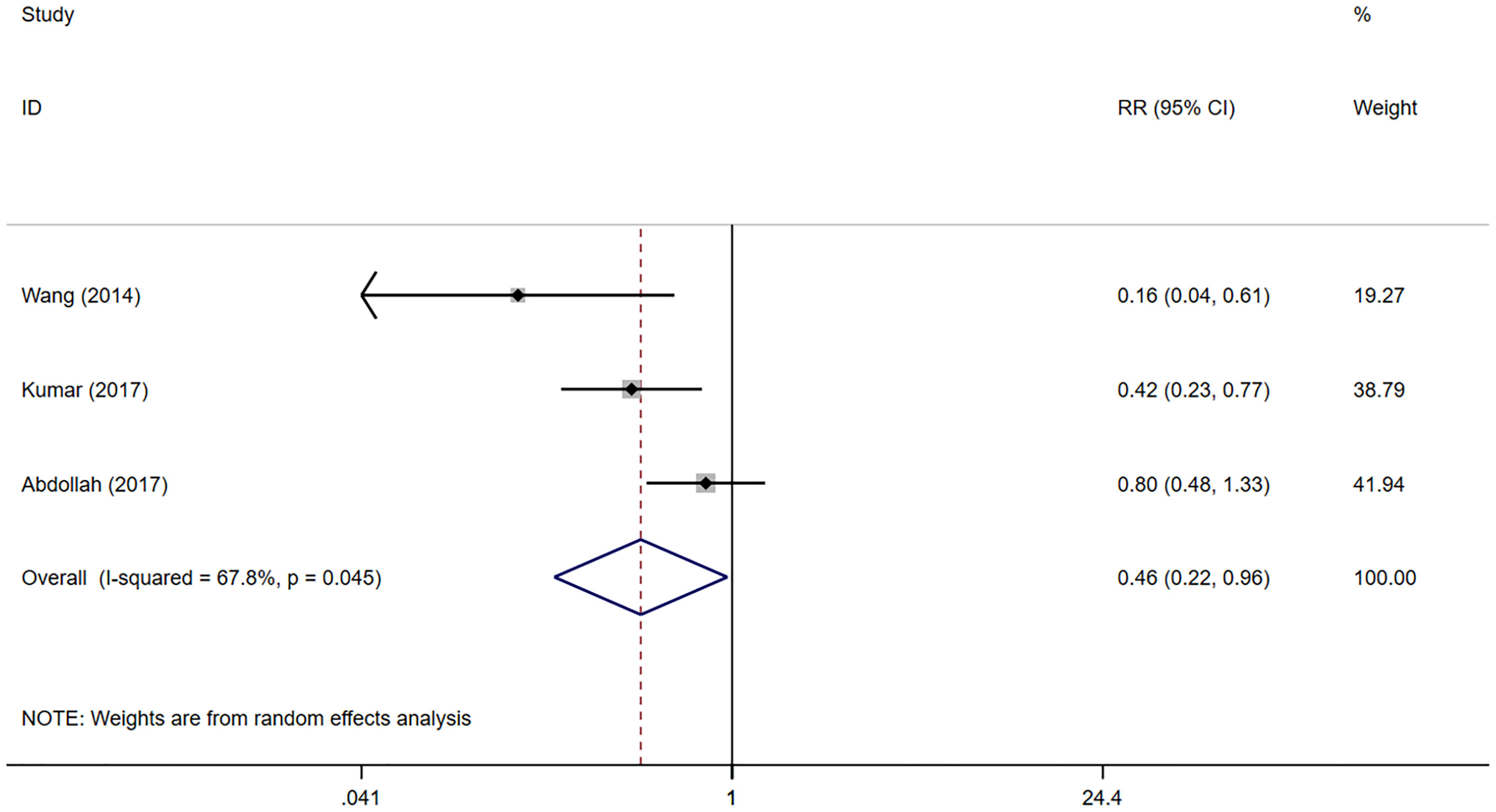
Figure 2 Forest plots of Continence recovery (12 month) for nerve sparing (NS) versus non-nerve sparing. CI, confidence interval; RR, Risk Ratio.
Erectile Function
Included in the study, EF was assessed using the SHIM (sexual health inventory for men) scale. EF recovery was defined as SHIM≥17 or an erectile hardness meeting sexual intercourse requirements. The results were evaluated via questionnaires or telephone follow-ups. EF recovery data was able to be extracted from 3 studies (20–22). However, only the recovery of EF 12 months after surgeries is reported in the 2 studies (20, 21), so we could only conduct a meta-analysis on the recovery of EF 12 months after surgeries. 1130 patients were included in the 3 studies, including 633 in the NNS group and 497 in the NS group. The results of meta-analysis showed that the EF recovery was worse in the NNS group than in the NS group 12 months after surgeries (RR 0.32, 95%CI 0.16, 0.63; p=0.001 <0.05) (Figure 3), and the difference was statistically significant. Due to the small number of studies included, sensitivity analyses could not be performed.
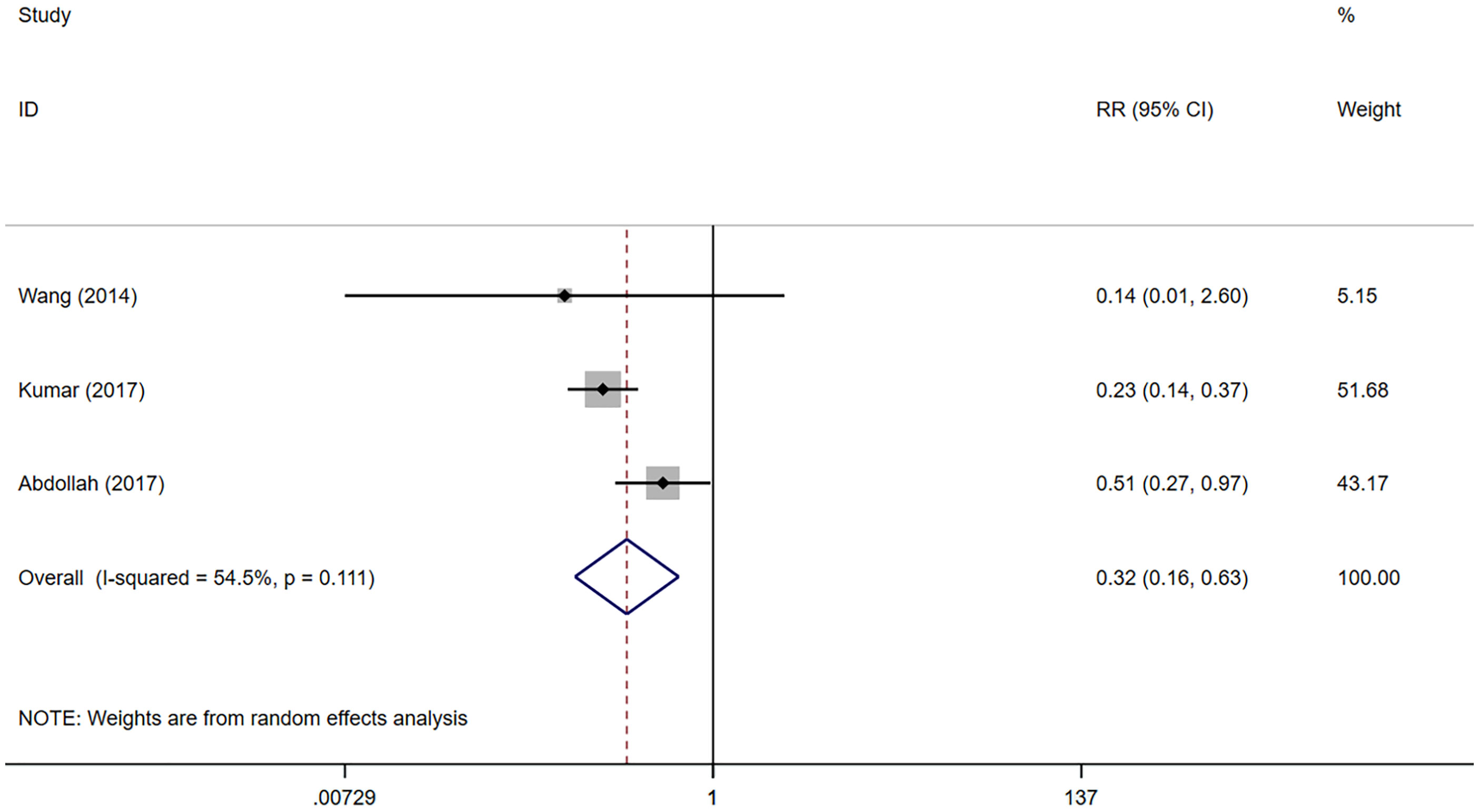
Figure 3 Forest plots of Erectile Function (12 month) for nerve sparing (NS) versus non-nerve sparing. CI, confidence interval; RR, Risk Ratio.
Oncologic outcomes
The meta-analysis showed that the NSS group presented higher positive surgical margin (PSM) rates compared with the NS group (RR 1.31, 95% CI 1.01, 1.69; p=0.042 <0.05) (Figure 4), and the difference was statistically significant.
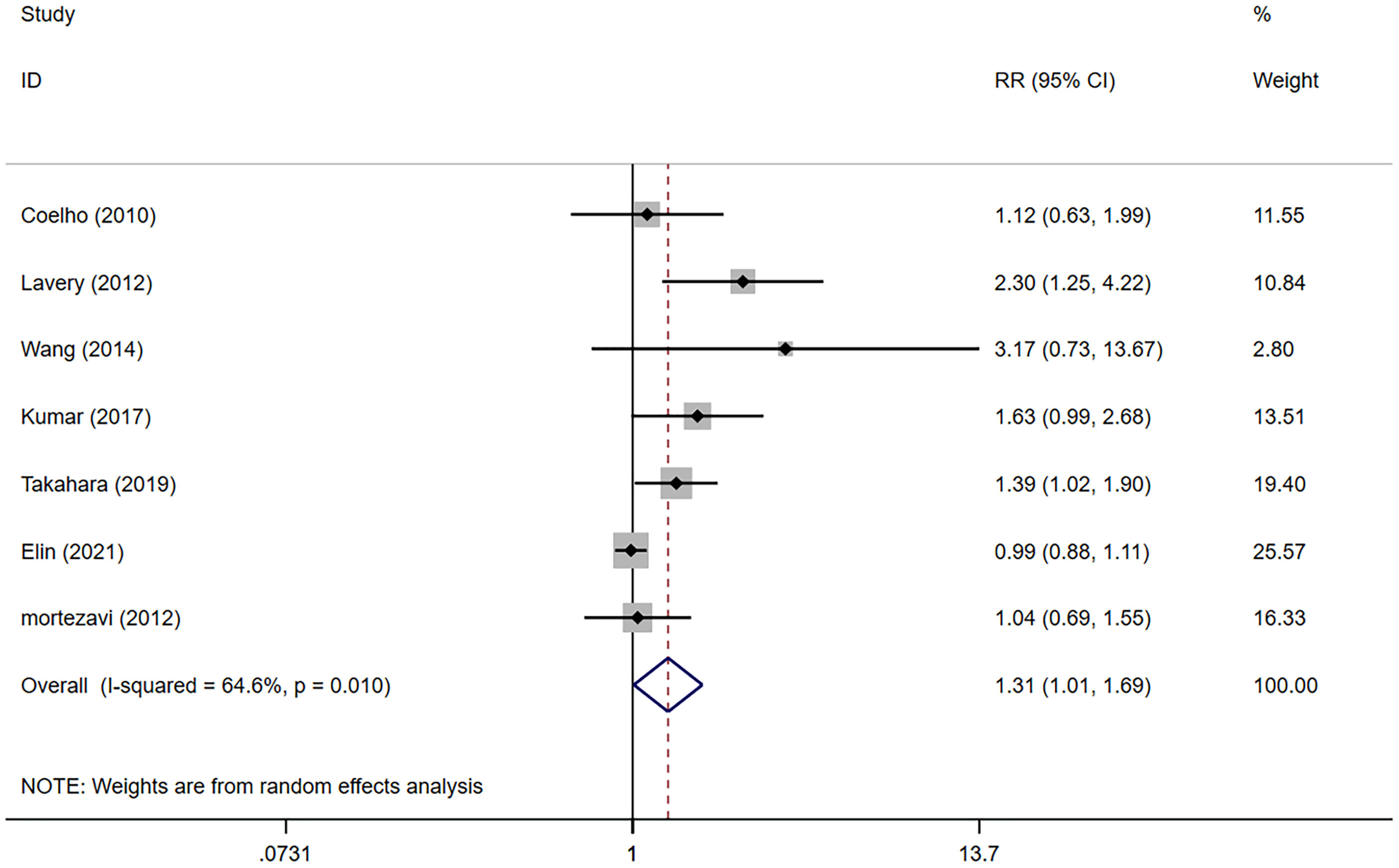
Figure 4 Forest plots of Oncologic Outcomes for non-nerve sparing versus nerve sparing (NS). CI, confidence interval; RR, Risk Ratio.
Subgroup analysis
We performed a meta-analysis on PSM according to the type of NS. The results showed that the BNS group presented lower PSM rates compared with the NNS group (RR 0.54, 95% CI 0.39, 0.73; p=0.004 <0.05). There was no significant difference in the PSM of the NNS group and the U(P)NS group (RR 0.77, 95% CI 0.57, 1.05; p>0.05). The BNS group showed lower PSM rates compared with the U(P)NS group (RR 0.55, 95% CI 0.38, 0.78; p=0.001 <0.05) (Figure 5).
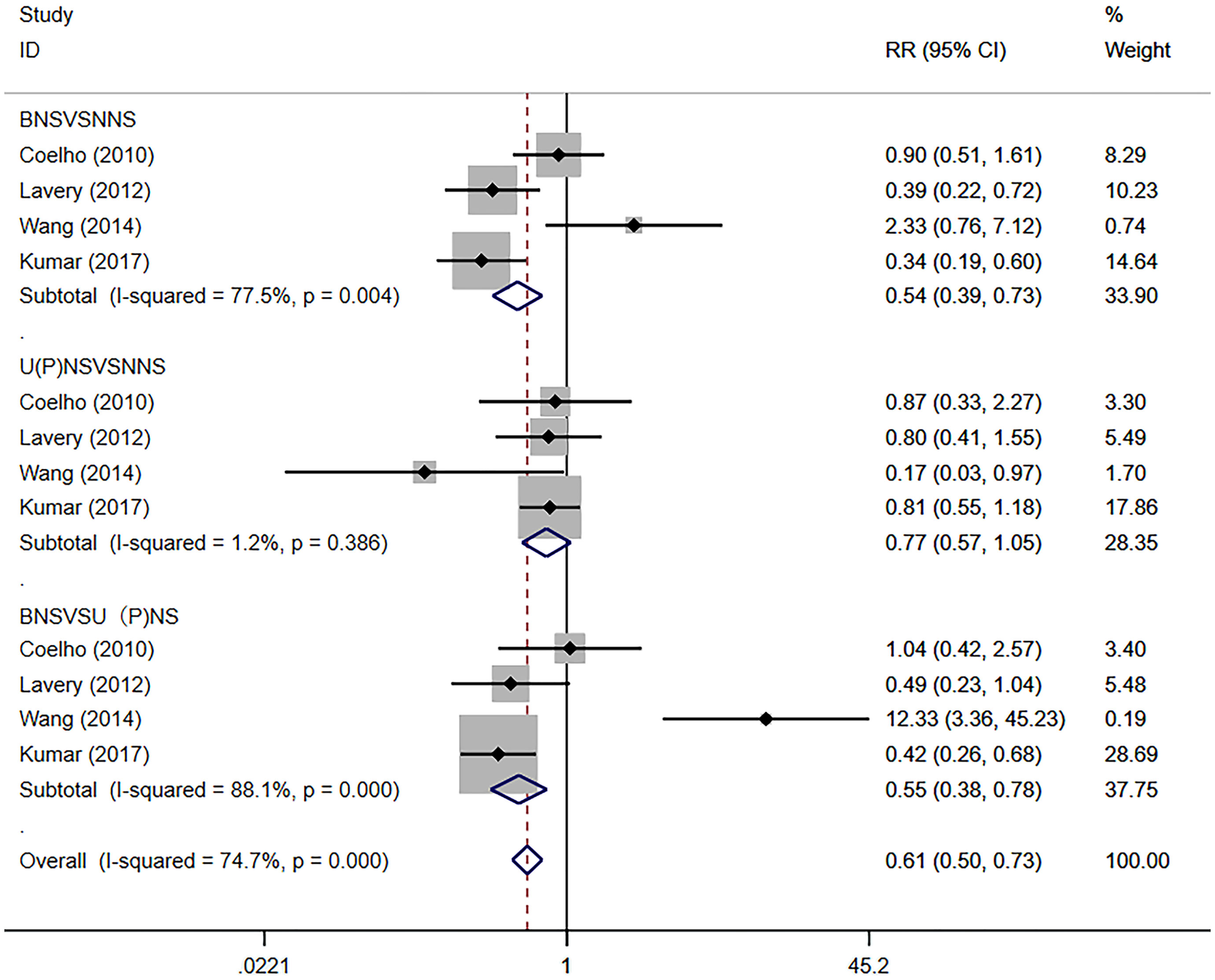
Figure 5 Forest plots of PSM Subgroup analysis for nerve sparing (NS) versus non-nerve sparing. CI, confidence interval; RR, Risk Ratio; PSM, positive surgical margins.
Publication bias and sensitivity analysis
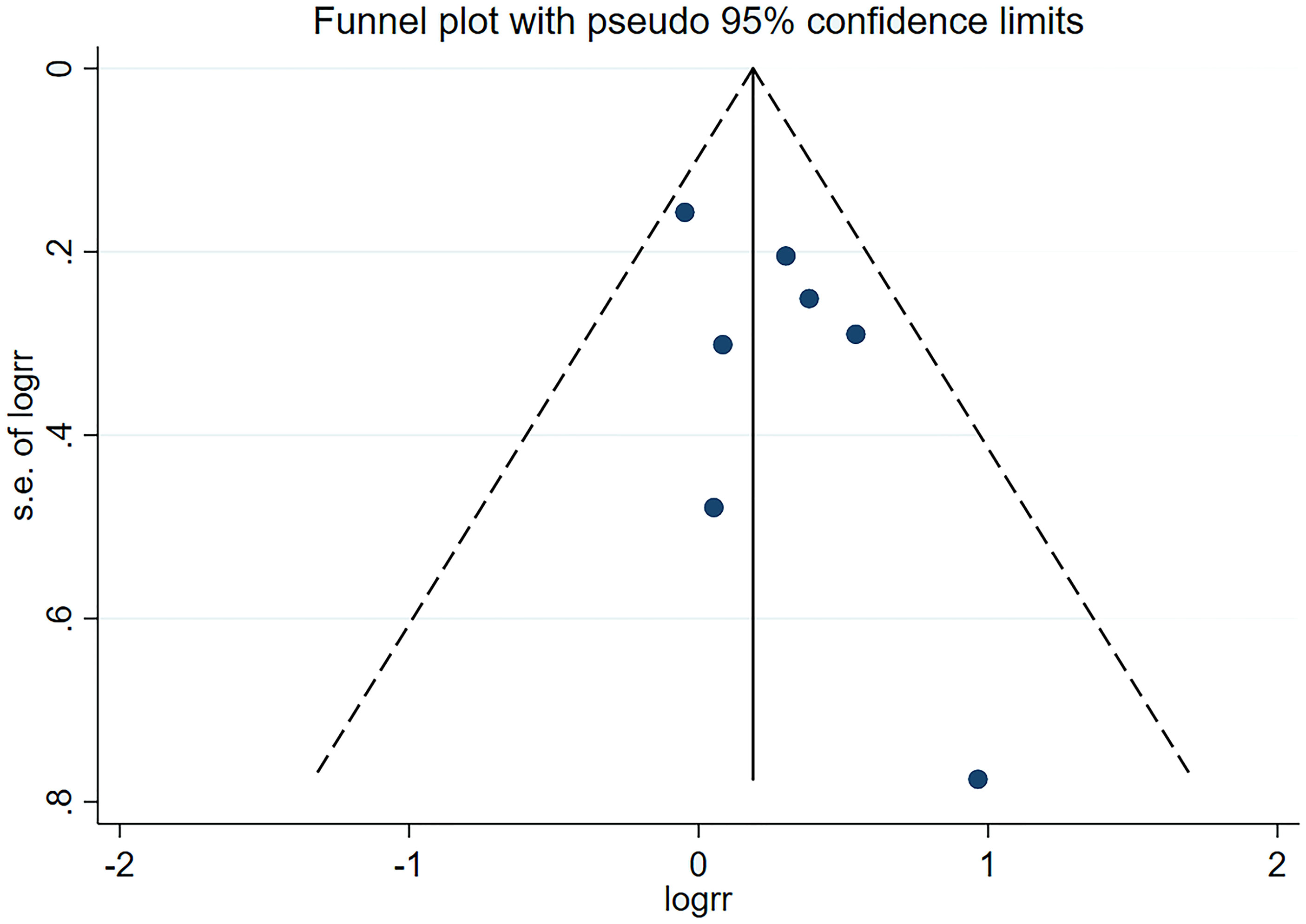
The result showed no apparent asymmetry, which indicated no obvious publication bias (Figure 6), and sensitivity analyses revealed that no significant influence was produced on the results through the removal of any article.
Discussion
Some significant findings on the urinary continence, EF and oncologic outcomes of the present study warrant in-depth discussions.
Urinary continence
Kumar and Wang et al. defined the criteria for the recovery of urinary continence as: 0 pad per day; Abdollah, Lavery et al. defined it as: 0/1 pad per day. It was found through a meta-analysis on the recovery of urinary continence 12 months after surgeries in the NNS group and the NS group that intraoperative NS was beneficial to the recovery of urinary continence 12 months after surgeries for high-risk Pca. Faieleigh et al. (13) conducted a systematic evaluation on the effect of NS during RP on postoperative urinary continence in 2015. The results showed that NS could improve the results of urinary continence recovery 6 months before and after surgeries, but there was no significant improvement in urinary continence 12 months after surgeries, which seemed different from our study. This might be due to the focus on anterior reconstruction through periurethral suspension suture as well as the posterior reconstruction via reattachment of the tendon arch (26) and pubic anterior plate to the bladder neck (26) through RARP compared to traditional laparoscopic radical prostatectomy (LRP). Although these techniques appear to have a similar efficacy to LRP 1 or 3 months after surgeries, there is a statistically-significant difference 12 months after surgeries (27). At present, the pathophysiological process of urinary incontinence after RP is not clear, and the function or structure of the striated sphincter may be the cause of urinary incontinence (28–30). The urethral sphincter is divided into two mechanisms: the striated sphincter provides active urine control under stress, and the smooth sphincter provides passive urine control (31). The two types of smooth muscles have different innervations. The smooth sphincter mainly receives autonomic innervations from the pelvic plexus, while the striated sphincter is innervated by dual nerves, namely the pelvic nerve, the pelvic branch of the pudendal nerve (32) and the abnormal innervations of the sphincter from the somatic nerve in the pelvic cavity (32–34). These studies may explain the effect of NS on postoperative urinary continence. Pelvic lymphadenectomy (LAE) is a routine procedure in the treatment of patients with high-risk Pca through RARP, which may damage the nerve fibers adjacent to the pelvic plexus. Therefore, careful attention should be paid to LAE during NS surgeries to avoid the possibility of nerve damage (35). Martin et al. summarized the current surgical approaches and anatomical techniques of RARP, which provide us with more options for surgical approaches (36). In addition, it is mentioned in the study of kumar et al. (21) that pelvic floor muscle exercise after surgeries, which can improve patients’ urine continence function recovery after surgeries (37). Unfortunately, we don’t know the statistics or follow-ups of the study, so we don’t know whether it is a contributing factor.
Erectile function
The recovery of sexual function in almost all studies has been “with or without the use of drugs, hardness is required for intercourse”. Our study shows that NS during RARP can promote EF recovery for patients with high-risk prostate 12 months after surgeries. The recovery of EF after surgeries was not entirely determined through NS. Holze et al. (38) demonstrated the potential effect of NS techniques, preoperative EF and pelvic LAE on the postoperative recovery of EF. At the same time, KIM et al. (39) also emphasized that a higher serum testosterone level before surgeries was also a factor influencing the postoperative recovery of EF. Abdollah et al. (22) also pointed out that the type of NS was largely determined by the recovery of EF. The EF recovery rate 12, 24 and 36 months after NNS was 19.6%, 19.6% and 39.7% respectively, compared with 42.1%, 67.1% and 75.3% after BNS as well as 31.2%, 47.3% and 69.5% after U (P) NS. Novara et al. (40) believed that the best effect of NS could be achieved by patients with a good EF at baseline. In other words, patients with a decreased EF before surgeries should not be treated with NS during surgeries. Combined with current studies, we believe that RARP in the treatment of high-risk Pca through NS is beneficial to the recovery of sexual dysfunction 12 months after surgeries.
Oncologic outcomes
In most studies, “tumor tissues found on the surface of ink” are taken as the definition and standard of positive surgical margins. Some studies have shown that PSM is equivalent to residual tumors as an adverse surgical outcome significantly associated with postoperative recurrence and secondary treatment (41, 42). Our meta-analysis showed that the PSM rate could increase through intraoperative NNS, a subgroup analysis showed that the PSM rate in the BNS group was lower than that in the U (P) NS group, and the difference was statistically significant. This seems to differ from the findings of Veneziano et al. (14). Lavery et al. (19) found through a comparison among study groups that patients in the NS group had lower pathological scores, showed fewer tumors, invaded extracellular membranes and seminal vesicle glands, and a higher proportion of tumor stages was T2. Kumar (21) also pointed out that the NNS group was significantly higher than the NS group in tumor invasion rate, pathological stage and tumor volume, and that there was a selection bias. The univariate and multivariate regression analysis in their study showed that NS did not affect PSM (p=0.341). These oncolologic features will be considered by the surgeons for NS treatment. In addition, PSM is also related to the age, PSA level, tumor stage (43), surgical experience (44) and tumor conformity of high-risk Pca itself. Our study confirmed that through NS in RARP, the rate of PSM can be reduced, and that the higher the degree of preservation was, the lower the risks would be. This result needs to be treated with caution.
Through a study of Kumar, Lavery and Takahara et al., we found that the mean age, biopsy Gleason score, preoperative clinical stage and preoperative PSA level of the NS group were significantly lower than those of the NNS group, suggesting that the patients included in our study had a selection bias, which might be solved through preoperative randomization, but it might violate ethics. In addition, we did not specifically group the methods of intraoperative NS. In studies on PNS (21, 24) intrafascial and interfascial PNS was also included. Again, we cannot address the effect of these classifications on the results.
Some limitations of our study should be considered before interpreting our results. First of all, all the literatures we included were cohort studies, which failed to include randomly-controlled trials with higher levels of evidence, although they were qualified by the NOS scale. The studies included involved a retrospective design and all risks of bias. Even through modern statistics methods, some issues could not be addressed (selection bias, nerve-sparing degree, postoperative protocols, etc.). Secondly, due to limited data, the situation of 1, 3, 6 and 24 months after surgeries was not further discussed. In addition, although a comparison of baseline data between the 2 treatment groups was described in the 3 studies included, fewer details were described. Therefore, it is unknown whether our interpretation of results would be affected. We expect that in follow-up studies, more attention will be paid to this point. Moreover, we have no consensus on the classification of NS, nor is there a standard classification of NS at present. Postoperative outcomes may be different due to different types of NS, and the result depends on its percentage (45, 46). There is heterogeneity in the NS techniques, which is not addressed in most studies. The authors performed different grades of NS (Kumar and Elin, etc.), which could influence its percentage. Finally, the postoperative rehabilitation protocols for potency and continence were unclear in the studies included, which could also be an issue when assessing postoperative results.
Due to the differences in postoperative follow-ups, surgical techniques and medical levels in different regions among the studies included, as well as the inevitable selective bias in the NSS group in terms of oncology characteristics. Our results need to be carefully interpreted and verified through high-quality studies.
Conclusions
NS is feasible during RARP surgeries, which is beneficial for the long-term (12 months after surgeries) urination continence and EF recovery of patients with high-risk Pca and is associated with better oncological outcomes. It is encouraging to find that NS may significantly improve the life quality of patients high-risk Pca after surgeries, which thus actively cooperates with treatments. However, due to interference factors in studies, it is not clear whether this advantage is caused by NS, and clinical decisions should be combined with various considerations on whether to perform NS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YL, X-ZD, JQ and X-SY: Protocol development, data collection and management, data analysis and manuscript writing. ZW, YJ and JH: Data management, data analysis and manuscript writing, data management, data analysis and manuscript writing. C-JW, LW, K-PL and J-HW: Data management and manuscript writing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the researchers and study participants for their contributions. Thanks to Ms. Yu Jiang for her encouragement and support to YL in his medical career.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol (2021) 4(6):877–92. doi: 10.1016/j.euo.2021.09.006
2. Crocetto F, Buonerba C, Caputo V, Ferro M, Persico F, Trama F, et al. Urologic malignancies: advances in the analysis and interpretation of clinical findings. Future Sci OA (2021) 7(4):Fso674. doi: 10.2144/fsoa-2020-0210
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
4. Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med (2018) 8(12):a030361. doi: 10.1101/cshperspect.a030361
5. Cooperberg MR, Cowan J, Broering JM, Carroll PR. High-risk prostate cancer in the united states, 1990-2007. World J Urol (2008) 26(3):211–8. doi: 10.1007/s00345-008-0250-7
6. Martin AD, Desai PJ, Nunez RN, Martin GL, Andrews PE, Ferrigni RG, et al. Does a history of previous surgery or radiation to the prostate affect outcomes of robot-assisted radical prostatectomy? BJU Int (2009) 103(12):1696–8. doi: 10.1111/j.1464-410X.2008.08276.x
7. Ilic D, Evans SM, Allan CA, Jung JH, Murphy D, Frydenberg M. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev (2017) 9(9):CD009625. doi: 10.1002/14651858.CD009625.pub2
8. Basourakos SP, Kowalczyk K, Moschovas MC, Dudley V, Hung AJ, Shoag JE, et al. Robot-assisted radical prostatectomy maneuvers to attenuate erectile dysfunction: Technical description and video compilation. J Endourol. (2021) 35(11):1601–9. doi: 10.1089/end.2021.0081
9. Bhat KRS, Covas Moschovas M, Sandri M, Dell'Oglio P, Onol FF, Rogers T, et al. A predictive preoperative and postoperative nomogram for postoperative potency recovery after robot-assisted radical prostatectomy. J Urol. (2021) 206(4):942–51. doi: 10.1097/JU.0000000000001895
10. Covas Moschovas M, Bhat S, Onol FF, Rogers T, Roof S, Mazzone E, et al. Modified apical dissection and lateral prostatic fascia preservation improves early postoperative functional recovery in robotic-assisted laparoscopic radical prostatectomy: Results from a propensity score-matched analysis. Eur Urol. (2020) 78(6):875–84. doi: 10.1016/j.eururo.2020.05.041
11. Weng H, Zeng XT, Li S, Meng XY, Shi MJ, He DL, et al. Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: a systematic review and meta-analysis. Sci Rep (2017) 7(1):11454. doi: 10.1038/s41598-017-11878-7
12. Nguyen LN, Head L, Witiuk K, Punjani N, Mallick R, Cnossen S, et al. The risks and benefits of cavernous neurovascular bundle sparing during radical prostatectomy: A systematic review and meta-analysis. J Urol. (2017) 198(4):760–9. doi: 10.1016/j.juro.2017.02.3344
13. Reeves F, Preece P, Kapoor J, Everaerts W, Murphy DG, Corcoran NM, et al. Preservation of the neurovascular bundles is associated with improved time to continence after radical prostatectomy but not long-term continence rates: Results of a systematic review and meta-analysis. Eur Urol. (2015) 68(4):692–704. doi: 10.1016/j.eururo.2014.10.020
14. Morozov A, Barret E, Veneziano D, Grigoryan V, Salomon G, Fokin I, et al. A systematic review of nerve-sparing surgery for high-risk prostate cancer. Minerva Urol Nephrol. (2021) 73(3):283–91. doi: 10.23736/S2724-6051.20.04178-8
15. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis prot ocols (PRISMA-p) 2015: elaboration and explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
16. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
17. Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, Patel VR. Predictive factors for positive surgical margins and their locations after robot-assisted laparoscopic radical prostatectomy [Article]. Eur Urology. (2010) 57(6):1022–9. doi: 10.1016/j.eururo.2010.01.040
18. Mortezavi A, Hermanns T, Seifert HH, Wild PJ, Schmid DM, Sulser T, et al. Intrafascial dissection significantly increases positive surgical margin and biochemical recurrence rates after robotic-assisted radical prostatectomy [Article]. Urologia Internationalis. (2012) 89(1):17–24. doi: 10.1159/000339254
19. Lavery HJ, Nabizada-Pace F, Carlucci JR, Brajtbord JS, Samadi DB. Nerve-sparing robotic prostatectomy in preoperatively high-risk patients is safe and efficacious. Urologic Oncology-Seminars Original Investigations. (2012) 30(1):26–32. doi: 10.1016/j.urolonc.2009.11.023
20. Wang JG, Huang J, Chin AI. RARP in high-risk prostate cancer: Use of multi-parametric MRI and nerve sparing techniques [Article]. Asian J Andrology. (2014) 16(5):715–9. doi: 10.4103/1008-682X.129942
21. Kumar A, Samavedi S, Bates AS, Mouraviev V, Coelho RF, Rocco B, et al. Safety of selective nerve sparing in high risk prostate cancer during robot-assisted radical prostatectomy [Article]. J robotic surgery. (2017) 11(2):129–38. doi: 10.1007/s11701-016-0627-3
22. Abdollah F, Dalela D, Sood A, Sammon J, Cho R, Nocera L, et al. Functional outcomes of clinically high-risk prostate cancer patients treated with robot-assisted radical prostatectomy: A multi-institutional analysis [Article]. Prostate Cancer Prostatic Diseases. (2017) 20(4):395–400. doi: 10.1038/pcan.2017.26
23. Takahara K, Sumitomo M, Fukaya K, Jyoudai T, Nishino M, Hikichi M, et al. Clinical and oncological outcomes of robot-assisted radical prostatectomy with nerve sparing vs. non-nerve sparing for high-risk prostate cancer cases [Article]. Oncol Letters. (2019) 18(4):3896–902. doi: 10.3892/ol.2019.10692
24. Axen E, Godtman RA, Bjartell A, Carlsson S, Haglind E, Hugosson J, et al. Degree of preservation of neurovascular bundles in radical prostatectomy and recurrence of prostate cancer [Journal article; clinical trial protocol]. Eur Urol Open science. (2021) 30:25–33. doi: 10.1016/j.euros.2021.06.005
25. Axén E, Godtman RA, Bjartell A, Carlsson S, Haglind E, Hugosson J, et al. Degree of preservation of neurovascular bundles in radical prostatectomy and recurrence of prostate cancer. Eur Urol Open Sci (2021) 30:25–33. doi: 10.1016/j.euros.2021.06.005
26. Patel VR, Coelho RF, Palmer KJ, Rocco B. Periurethral suspension stitch during robot-assisted laparoscopic radi cal prostatectomy: Description of the technique and continence outcome s. Eur Urol (2009) 56(3):472–8. doi: 10.1016/j.eururo.2009.06.007
27. Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary conti nence recovery after robot-assisted radical prostatectomy. Eur Urol (2012) 62(3):405–17. doi: 10.1016/j.eururo.2012.05.045
28. Chao R, Mayo ME. Incontinence after radical prostatectomy: detrusor or sphincter causes. J Urol (1995) 154(1):16–8. doi: 10.1016/s0022-5347(01)67212-4
29. Ficazzola MA, Nitti VW. The etiology of post-radical prostatectomy incontinence and correlatio n of symptoms with urodynamic findings. J Urol (1998) 160(4):1317–20. doi: 10.1016/S0022-5347(01)62525-4
30. Groutz A, Blaivas JG, Chaikin DC, Weiss JP, Verhaaren M. The pathophysiology of post-radical prostatectomy incontinence: A clin ical and video urodynamic study. J Urol (2000) 163(6):1767–70. doi: 10.1097/00005392-200006000-00030
31. Koraitim MM. The male urethral sphincter complex revisited: an anatomical concept a nd its physiological correlate. J Urol (2008) 179(5):1683–9. doi: 10.1016/j.juro.2008.01.010
32. Hollabaugh RS Jr., Dmochowski RR, Steiner MS. Neuroanatomy of the male rhabdosphincter. Urology (1997) 49(3):426–34. doi: 10.1016/S0090-4295(96)00497-9
33. Song L-J, Lu H-K, Wang J-P, Xu Y-M. Cadaveric study of nerves supplying the membranous urethra. Neurourology urodynamics (2010) 29(4):592–5. doi: 10.1002/nau.20768
34. Zvara P, Carrier S, Kour NW, Tanagho EA. The detailed neuroanatomy of the human striated urethral sphincter. Br J Urol (1994) 74(2):182–7. doi: 10.1111/j.1464-410x.1994.tb16583.x
35. Hatzichristodoulou G, Wagenpfeil S, Wagenpfeil G, Maurer T, Horn T, Herkommer K, et al. Extended versus limited pelvic lymph node dissection during bilateral nerve-sparing radical prostatectomy and its effect on continence and e rectile function recovery: Long-term results and trifecta rates of a c omparative analysis. World J Urol (2016) 34(6):811–20. doi: 10.1007/s00345-015-1699-9
36. Martini A, Falagario UG, Villers A, Dell'Oglio P, Mazzone E, Autorino R, et al. Contemporary techniques of prostate dissection for robot-assisted prostatectomy. Eur Urol. (2020) 78(4):583–91. doi: 10.1016/j.eururo.2020.07.017
37. Centemero A, Rigatti L, Giraudo D, Lazzeri M, Lughezzani G, Zugna D, et al. Preoperative pelvic floor muscle exercise for early continence after r adical prostatectomy: a randomised controlled study. Eur Urol (2010) 57(6):1039–43. doi: 10.1016/j.eururo.2010.02.028
38. Holze S, Mende M, Healy KV, Koehler N, Gansera L, Truss MC, et al. Comparison of various continence definitions in a large group of patie nts undergoing radical prostatectomy: A multicentre, prospective study. BMC Urol (2019) 19(1):70. doi: 10.1186/s12894-019-0500-6
39. Kim SC, Song C, Kim W, Kang T, Park J, Jeong IG, et al. Factors determining functional outcomes after radical prostatectomy: r obot-assisted versus retropubic. Eur Urol (2011) 60(3):413–9. doi: 10.1016/j.eururo.2011.05.011
40. Novara G, Ficarra V, D'Elia C, Secco S, De Gobbi A, Cavalleri S, et al. Preoperative criteria to select patients for bilateral nerve-sparing r obotic-assisted radical prostatectomy. J sexual Med (2010) 7(2 Pt 1):839–45. doi: 10.1111/j.1743-6109.2009.01589.x
41. Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R, et al. Positive surgical margins after radical prostatectomy: A systematic re view and contemporary update. Eur Urol (2014) 65(2):303–13. doi: 10.1016/j.eururo.2013.07.039
42. Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: A sys tematic review. Eur Urol (2007) 51(1):45–55; discussion 56. doi: 10.1016/j.eururo.2006.06.017
43. Sachdeva A, Veeratterapillay R, Voysey A, Kelly K, Johnson MI, Aning J, et al. Positive surgical margins and biochemical recurrence following minimal ly-invasive radical prostatectomy - an analysis of outcomes from a UK tertiary referral centre. BMC Urol (2017) 17(1):91. doi: 10.1186/s12894-017-0262-y
44. Nyberg M, Sjoberg DD, Carlsson SV, Wilderäng U, Carlsson S, Stranne J, et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the Swedish LAPPRO trial. BJU Int (2021) 127(3):361–8. doi: 10.1111/bju.15238
45. Moschovas MC, Patel V. Neurovascular bundle preservation in robotic-assisted radical prostatectomy: How I do it after 15.000 cases. Int Braz J urol Off J Braz Soc Urology. (2022) 48(2):212–9. doi: 10.1590/s1677-5538.Ibju.2022.99.04
Keywords: high-risk prostate cancer, robot-assisted radical prostatectomy, neurovascular bundle, outcomes, continence, meta-analysis
Citation: Liu Y, Deng X-z, Qin J, Wen Z, Jiang Y, Huang J, Wang C-j, Chen C-x, Wang L, Li K-p, Wang J-h and Yang X-s (2023) Erectile function, urinary continence and oncologic outcomes of neurovascular bundle sparing robot-assisted radical prostatectomy for high-risk prostate cancer: A systematic review and meta-analysis. Front. Oncol. 13:1161544. doi: 10.3389/fonc.2023.1161544
Received: 08 February 2023; Accepted: 23 March 2023;
Published: 05 April 2023.
Edited by:
Angelo Naselli, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Biagio Barone, University of Naples Federico II, ItalyMarcio Covas Moschovas, AdventHealth, United States
Copyright © 2023 Liu, Deng, Qin, Wen, Jiang, Huang, Wang, Chen, Wang, Li, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-song Yang, eHVlc29uZ3lhbmcyMDIyQDE2My5jb20=
†These authors have contributed equally to this work
 Yang Liu1†
Yang Liu1† Li Wang
Li Wang Kun-peng Li
Kun-peng Li Xue-song Yang
Xue-song Yang