- 1Department of Medical Oncology, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan
- 2Department of Medical Oncology/Hematology, Kakogawa Central City Hospital, Hyogo, Japan
Response evaluation criteria in solid tumors version 1.1 (RECIST ver1.1) has been widely adopted to evaluate treatment efficacy in solid tumors, including breast cancer (BC), in clinical trials and clinical practice. RECIST is based mainly on computed tomography (CT) images, and the role of fluorodeoxyglucose-positron emission tomography (FDG-PET) is limited. However, because the rate of tumor shrinkage on CT does not necessarily reflect the potential remaining tumor cells, there may be a discrepancy between the treatment response and prognosis in some cases. Here we report a case of metastatic human epidermal growth factor receptor 2 (HER2)-positive BC where FDG-PET was preferable to CT for evaluation of the treatment response. A 40-year-old woman became aware of a lump in her right breast in September 201X. She was pregnant and underwent further examinations, including a biopsy, in November. The diagnosis was HER2-positive BC (cT2N2bM1, stage IV). Trastuzumab plus pertuzumab plus docetaxel (TPD) therapy was initiated in December 201X. CT performed in February 201X+1 showed cystic changes in the metastatic lesions in the liver, and the treatment response was stable disease (SD) according to RECIST. However, FDG-PET in March 201X+1 did not detect abnormal uptake of FDG in the hepatic lesions. The disease remained stable thereafter. Thus, tumor shrinkage may not be apparent in situations where the response to treatment results in rapid changes in blood flow within the tumor, which is associated with cystic changes. When patients with hypervascular liver metastases receive treatment with highly effective regimens, the target lesion may show cystic changes rather than shrinkage, as observed in the present case. Therefore, FDG-PET is sometimes superior to CT in judging a tumor response.
1 Introduction
Response evaluation criteria in solid tumors version 1.1 (RECIST ver1.1) has been widely adopted to evaluate treatment efficacy in solid tumors, including breast cancer (BC), in clinical trials and clinical practice (1). RECIST ver1.1 is mainly based on computed tomography (CT) images and is useful for the evaluation of cytotoxic anticancer therapy as well as molecular-targeted drug therapy (2). The role of 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) in the determination of the treatment efficacy is limited. However, because tumor shrinkage based on CT images does not always correspond to tumor cell residuals, scattered cases have been reported in which the treatment efficacy determination and prognosis are divergent (3–7). Conversely, FDG-PET can evaluate tumor activity by glucose uptake. Hence, in Europe and the United States, quantitative treatment response determination by FDG-PET has been attempted, with the recommendation of FDG-PET by the European organization for research and treatment of cancer (8) and the PET Response Criteria in Solid Tumors (9). Although several studies have used FDG-PET to determine the efficacy of neoadjuvant chemotherapy against human epidermal growth factor receptor 2 (HER2)-positive BC (10–13), few studies have examined the utility of FDG-PET in determining the efficacy of treatment for metastatic HER2-positive BC (14). Here we report a case of metastatic HER2-positive BC where FDG-PET was preferable to CT for evaluation of the treatment response.
2 Case report
A 40-year-old woman became aware of a lump in her right breast in September 201X. Because she was pregnant, she underwent a cesarean section in mid-November and underwent further examinations, including a core needle biopsy, in late November. Physical examination at the initial visit to our department revealed a body temperature of 36.5°C; a heart rate of 78 beats/min; blood pressure of 122/74 mmHg; a respiratory rate of 12 breaths/minute; no eyelid conjunctiva pallor; no heart murmur; flat, soft, non-tender abdomen; no edema; a palpable, 2-cm, elastic, firm mass in the upper outer quadrant of the right breast; and palpable and swollen right axillary lymph nodes. Breast ultrasound revealed a hypoechoic mass measuring 32.6 × 16.2 mm and showing well-defined borders and a heterogeneous interior in the upper outer quadrant of the right breast. Blood tests showed mildly elevated liver enzymes, high serum alkaline phosphatase and serum lactate dehydrogenase (LDH) levels, and markedly elevated carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3) levels (Table 1). FDG-PET/CT revealed high FDG accumulation in the upper outer quadrant of the right breast (standardized uptake value (SUV) max, 7.519), enlarged lymph nodes, and high FDG accumulation in the level I–II region of the right axilla and internal mammary lymph node region (SUV max, 3.525), numerous low-density areas with high FDG accumulation in the liver (SUV max, 7.816), and high FDG accumulation in the left iliac bone (SUV max, 7.356) (Figure 1). The histopathological diagnosis based on core needle biopsy from the breast mass was invasive ductal carcinoma of the breast (estrogen receptor (ER)-positive, progesterone receptor-negative, HER2 3+, Ki-67 40%). The clinical stage by imaging was cT2N2bM1[OSS, HEP], stage IV. Trastuzumab plus pertuzumab plus docetaxel (TPD) therapy for metastatic HER2-positive BC was initiated in December 201X. Blood tests on the day after treatment showed the following: aspartate aminotransferase (AST), 341 IU/L; alanine aminotransferase (ALT), 155 IU/L; LDH, 4021 IU/L; and liver dysfunction. However, there were no findings indicating suspected tumor lysis syndrome, with a serum creatinine level of 0.48 mg/dL, uric acid level of 5.2 mg/dL, potassium level of 3.9 mmol/L, and phosphorus level of 3.6 mg/dL. Blood tests performed 2 days after the start of chemotherapy showed the following: AST, 187 IU/L; ALT, 143 IU/L; and LDH, 2151 IU/L, with liver dysfunction and LDH levels also showing an improvement trend. At the start of the second course of treatment, the patient’s liver enzymes were within normal limits, and she continued treatment. In February 201X+1, the CEA and CA15-3 levels were 90.2 ng/mL and 33.0 IU/mL, respectively. CT performed in the same period showed cystic changes in the metastatic lesions in the liver, and the treatment response was stable disease according to RECIST (Figure 2). However, FDG-PET performed in March 201X+1 did not detect abnormal uptake of FDG in the hepatic lesions (Figure 2; Supplementary Figure 1). CT performed in June 201X+1 showed shrinkage of the liver metastases, and the disease remained stable for more than three years (Figure 2).
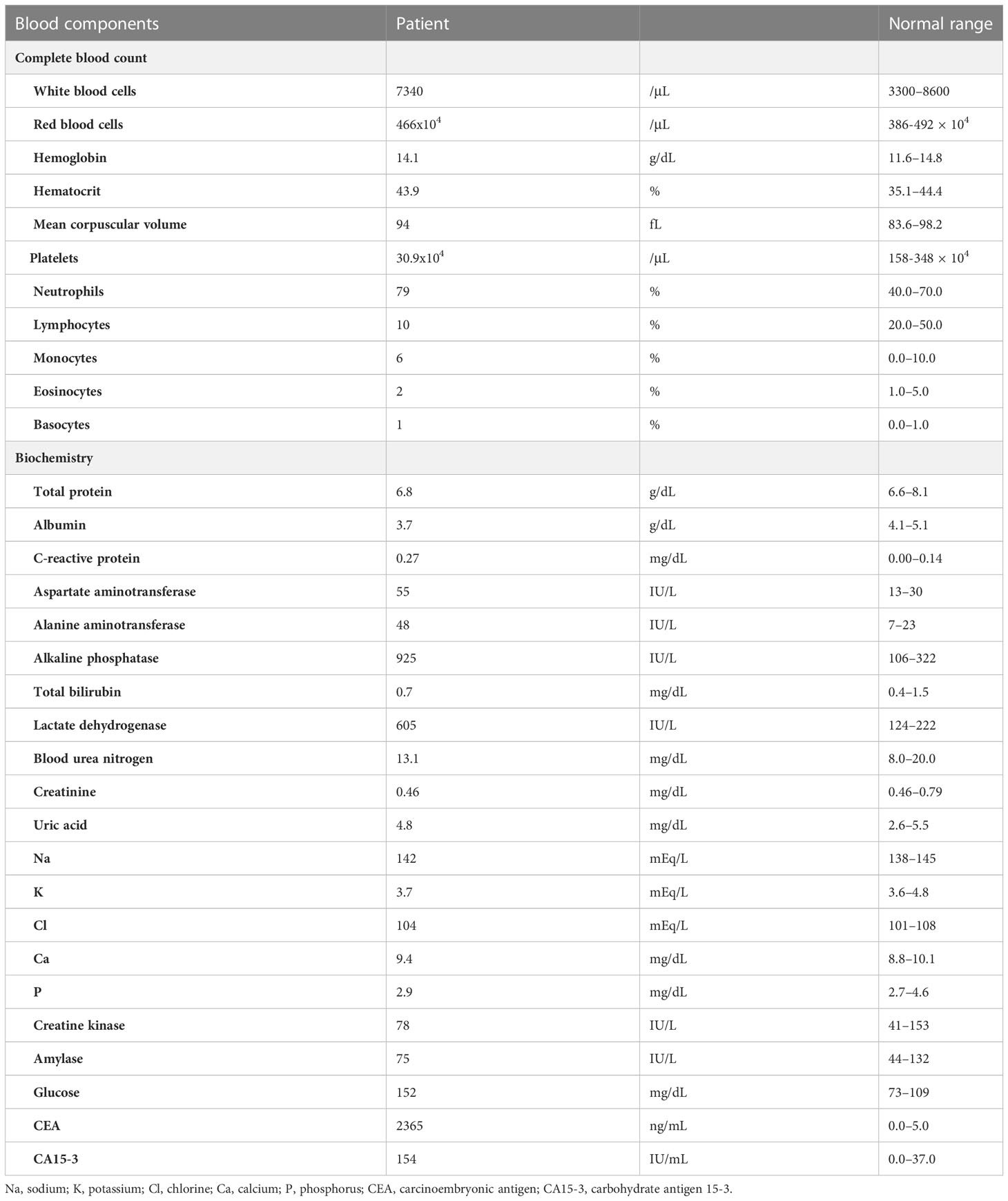
Table 1 Laboratory data obtained at the initial visit to our department for a patient with human epidermal growth factor receptor 2-positive breast cancer.
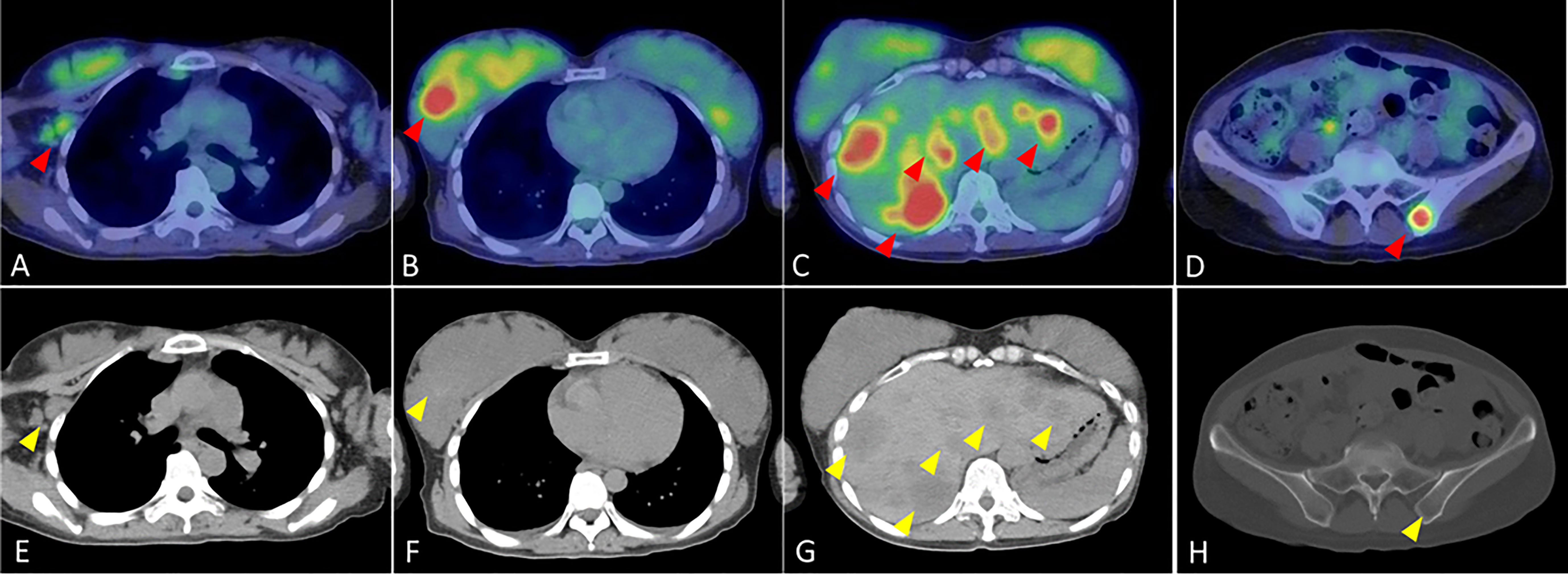
Figure 1 Fluorodeoxyglucose-positron emission tomography/computed tomography findings at the initial visit to our department for the patient with human epidermal growth factor receptor 2-positive breast cancer. (A) High FDG accumulation in the level I–II region of the right axilla (red arrow) (B) High FDG accumulation in the upper outer quadrant of the right breast (red arrow) (C) Numerous foci of high FDG accumulation in the liver (red arrows). (D) High FDG accumulation in the left iliac bone (red arrow). (E) Enlarged lymph node in the level I-II region of the right axilla (yellow arrow). (F) Mass in the upper outer right breast (yellow arrow). (G) Multiple low density areas in the liver (yellow arrows). (H) Low density area in pelvic region (yellow arrow).
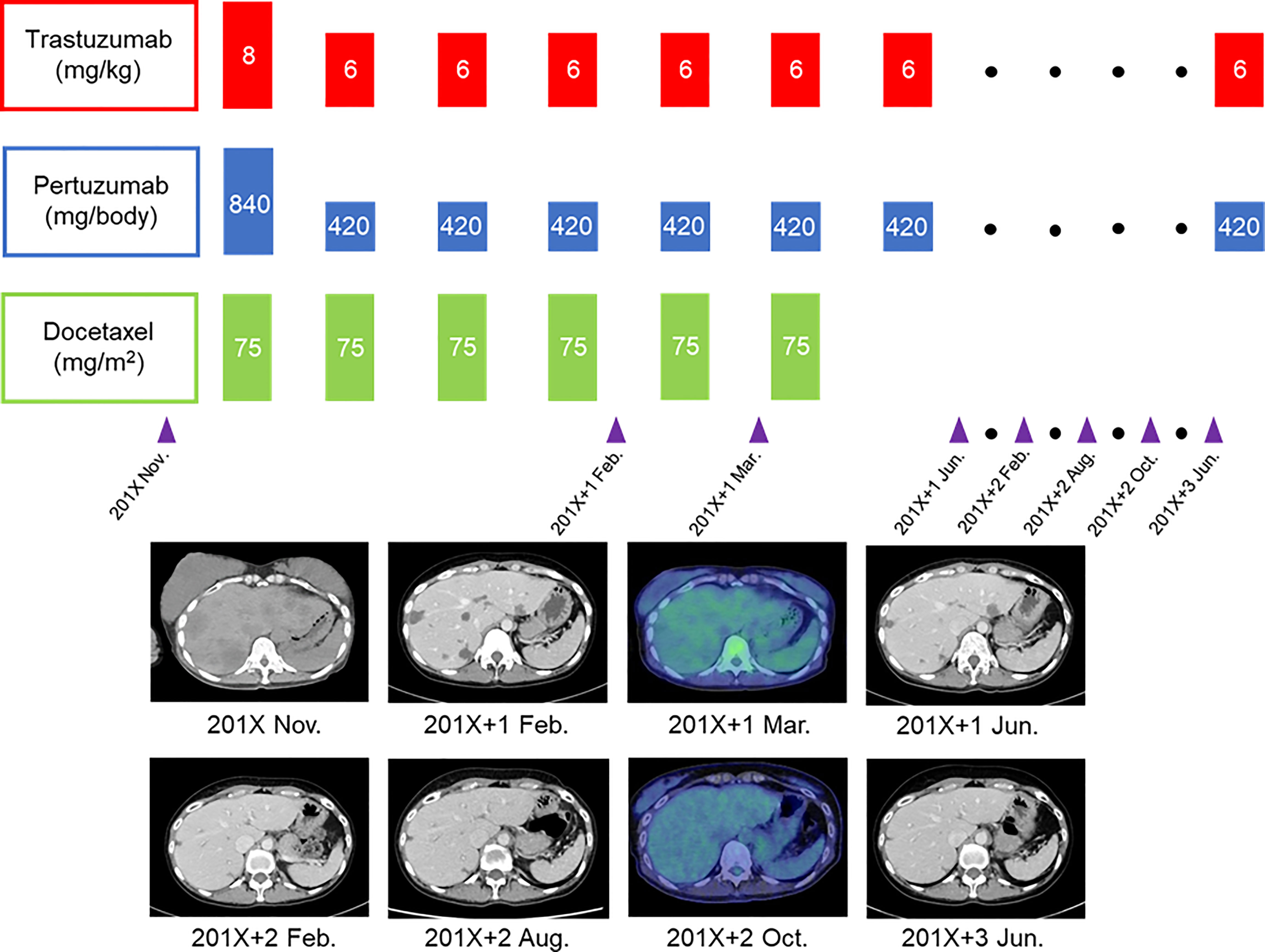
Figure 2 Course of treatment and imaging changes in multiple liver metastases for the patient with human epidermal growth factor receptor 2-positive breast cancer.
3 Discussion
We presented a case of HER2-positive BC with liver metastasis where FDG-PET was valuable for the assessment of the therapeutic response. The patient, who showed an early response according to FDG-PET, continued to respond to treatment three years after the start of treatment.
In some reports, the pathological complete response rate after neoadjuvant chemotherapy for HER2-positive BC has correlated with the treatment response evaluated by FDG-PET (10–13, 15–21), whereas no correlation has been observed in other studies (22–26). Furthermore, for BC, the utility of FDG-PET may differ between primary sites and metastatic lymph nodes (27). Furthermore, the ability of PET to detect breast cancer is highly dependent on tumor size: the sensitivity for tumors less than 1 cm in diameter was 25%, whereas the sensitivity for tumors between 1 cm and 2 cm in diameter was 84.4% (28). On the other hand, RECIST ver1.1, based on CT imaging, reportedly shows efficacy in determining the therapeutic effect of molecular-targeted drug therapy (2). Therefore, the routine use of FDG-PET for determining the treatment response in BC is not recommended.
However, HER2/ER-positive breast cancer may be the most suitable breast cancer subtype for FDG-PET. The rationale for their suitability is that glucose transporters (GLUT) on cell membranes and cell proliferative capacity influence FDG accumulation (29). The Phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is also involved in the expression and function of GLUTs, which are involved in glucose uptake (30). HER2/ER-positive breast cancer often has high Ki67 levels, a marker of cell proliferative potential, and the PI3K/Akt/mTOR pathway is also activated (31). If treatment for this breast cancer subtype is successful, a decrease in FDG accumulation may be detected earlier than morphological shrinkage by CT because of the expected reduced expression of GLUT and Ki67 values. Furthermore, there are reports that FDG-PET affects the prognosis of breast cancer patients (32, 33). That is because FDG-PET has a high diagnostic ability for distant metastasis, especially in breast cancer patients with bone metastasis (34, 35). Therefore, FDG-PET may be useful not only for detecting distant metastases that are difficult to detect with CT in staging but also for follow-up.
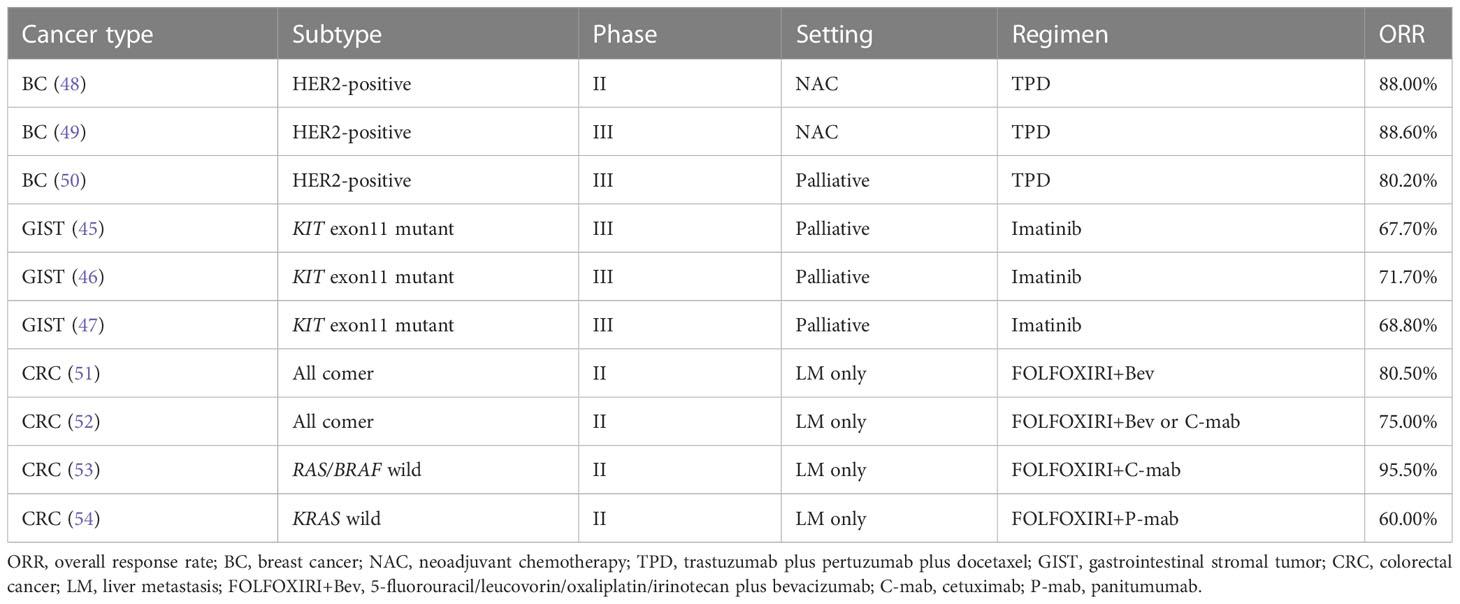
In addition, FDG-PET is useful for determining the response to drug treatment in patients with gastrointestinal stromal tumors (GISTs) (3, 4, 36–38). Therefore, FDG-PET is preferred over RECIST for evaluation of the response to treatment (39). The characteristics of GISTs and their treatment include the presence of hypervascular liver metastases (40–42) and a high response rate to imatinib therapy (43). Approximately two-thirds of GISTs have KIT exon11 mutations (40, 44). The response rate for imatinib in patients with untreated metastatic GISTs with KIT exon11 mutations reportedly ranges from 68% to 72% (45–47) (Table 2). High-response chemotherapy for hypervascular tumors leads to rapid blood flow changes. This can result in internal necrosis and cystic transformation without tumor shrinkage, which may occur during the treatment of GISTs (55). In such cases, FDG-PET is more suitable for determining the treatment response than RECIST.
The response rate for the TPD regimen used for untreated HER2-positive BC reportedly ranges from 80.2% to 88.6% (48–50) (Table 2), and some cases of hepatic metastases from BC show hypervascular patterns (56, 57). In addition, the response rate for triplet plus bevacizumab or anti-epidermal growth factor receptor antibody treatment in patients with untreated colorectal cancer (CRC) with liver metastases ranges from 60.0% to 95.5% (51–54) (Table 2). However, liver metastases from CRC are generally hypovascular tumors (55). Therefore, they are less frequently cystic, similar to GISTs. Meanwhile, when angiogenesis inhibitors are administered, the tumor blood flow is rapidly altered and the liver metastases from CRC may become cystic; this suggests that RECIST is inappropriate for determining the treatment efficacy (58).
The present case involved untreated HER2-positive BC with liver metastases, and the LDH levels after initiation of the TPD regimen suggested a high response within a few days. Patients with such a significant reaction to hypervascular liver metastases within a few days are prone to cystic transformation of the liver metastases.
In summary, when liver metastases do not shrink and become cystic despite a high response to chemotherapy, FDG-PET may be more suitable than CT-based RECIST for determination of the treatment response.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patient provided written informed consent to participate in this study. Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
Conceptualization, HS and AO. Methodology, HS and AO. Investigation, HS, YI, and AO. Data curation, HS, YI, and AO. Writing—original draft preparation, HS. Writing—review and editing, HS, YI, and AO. Supervision, AO. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1158797/full#supplementary-material
References
1. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours. revised recist guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
2. Litière S, Isaac G, De Vries EGE, Bogaerts J, Chen A, Dancey J, et al. Recist 1.1 for response evaluation apply not only to chemotherapy-treated patients but also to targeted cancer agents: a pooled database analysis. J Clin Oncol (2019) 37(13):1102–10. doi: 10.1200/JCO.18.01100
3. Goldstein D, Tan BS, Rossleigh M, Haindl W, Walker B, Dixon J. Gastrointestinal stromal tumours: correlation of f-fdg gamma camera-based coincidence positron emission tomography with ct for the assessment of treatment response–an agitg study. Oncology (2005) 69(4):326–32. doi: 10.1159/000089765
4. Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, et al. 18fdg-positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (glivec). Eur J Cancer (2003) 39(14):2012–20. doi: 10.1016/s0959-8049(03)00073-x
5. Pietrantonio F, Orlandi A, Inno A, Da Prat V, Spada D, Iaculli A, et al. Bevacizumab-based neoadjuvant chemotherapy for colorectal cancer liver metastases: pitfalls and helpful tricks in a review for clinicians. Crit Rev Oncol Hematol (2015) 95(3):272–81. doi: 10.1016/j.critrevonc.2015.04.008
6. Koh Y, Lee HE, Oh DY, Kim JH, Lee SH, Kim SH, et al. The lack of cd34 expression in gastrointestinal stromal tumors is related to cystic degeneration following imatinib use. Jpn J Clin Oncol (2012) 42(11):1020–7. doi: 10.1093/jjco/hys138
7. Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA (2009) 302(21):2338–44. doi: 10.1001/jama.2009.1755
8. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18f]-fluorodeoxyglucose and positron emission tomography: review and 1999 eortc recommendations. European organization for research and treatment of cancer (eortc) pet study group. Eur J Cancer (1999) 35(13):1773–82. doi: 10.1016/s0959-8049(99)00229-4
9. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From recist to percist: evolving considerations for pet response criteria in solid tumors. J Nucl Med (2009) 50 Suppl 1(Suppl 1):122s–50s. doi: 10.2967/jnumed.108.057307
10. Humbert O, Cochet A, Riedinger JM, Berriolo-Riedinger A, Arnould L, Coudert B, et al. Her2-positive breast cancer: 18f-fdg pet for early prediction of response to trastuzumab plus taxane-based neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging (2014) 41(8):1525–33. doi: 10.1007/s00259-014-2739-1
11. Gebhart G, Gámez C, Holmes E, Robles J, Garcia C, Cortés M, et al. 18f-fdg pet/ct for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in her2-positive breast cancer: results from neo-altto. J Nucl Med (2013) 54(11):1862–8. doi: 10.2967/jnumed.112.119271
12. Groheux D, Giacchetti S, Hatt M, Marty M, Vercellino L, de Roquancourt A, et al. Her2-overexpressing breast cancer: fdg uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer (2013) 109(5):1157–64. doi: 10.1038/bjc.2013.469
13. Hatschek T, Foukakis T, Bjöhle J, Lekberg T, Fredholm H, Elinder E, et al. Neoadjuvant trastuzumab, pertuzumab, and docetaxel vs trastuzumab emtansine in patients with erbb2-positive breast cancer: a phase 2 randomized clinical trial. JAMA Oncol (2021) 7(9):1360–7. doi: 10.1001/jamaoncol.2021.1932
14. Hildebrandt MG, Naghavi-Behzad M, Vogsen M. A role of fdg-pet/ct for response evaluation in metastatic breast cancer? Semin Nucl Med (2022) 52(5):520–30. doi: 10.1053/j.semnuclmed.2022.03.004
15. Groheux D, Majdoub M, Sanna A, de Cremoux P, Hindié E, Giacchetti S, et al. Early metabolic response to neoadjuvant treatment: fdg pet/ct criteria according to breast cancer subtype. Radiology (2015) 277(2):358–71. doi: 10.1148/radiol.2015141638
16. Humbert O, Lasserre M, Bertaut A, Fumoleau P, Coutant C, Brunotte F and Cochet A. Breast cancer blood flow and metabolism on dual-acquisition (18)f-fdg pet: correlation with tumor phenotype and neoadjuvant chemotherapy response. J Nucl Med (2018) 59(7):1035–41. doi: 10.2967/jnumed.117.203075
17. Sheikhbahaei S, Trahan TJ, Xiao J, Taghipour M, Mena E, Connolly RM, et al. Fdg-pet/ct and mri for evaluation of pathologic response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis of diagnostic accuracy studies. Oncologist (2016) 21(8):931–9. doi: 10.1634/theoncologist.2015-0353
18. Antunovic L, De Sanctis R, Cozzi L, Kirienko M, Sagona A, Torrisi R, et al. Pet/ct radiomics in breast cancer: promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging (2019) 46(7):1468–77. doi: 10.1007/s00259-019-04313-8
19. Tőkés T, Szentmártoni G, Torgyík L, Kajáry K, Lengyel Z, Györke T, et al. Response evaluation after primary systemic therapy of her2 positive breast cancer – an observational cross-sectional study. Croat Med J (2015) 56(2):128–38. doi: 10.3325/cmj.2015.56.128
20. Cheng L, Zhang J, Wang Y, Xu X, Zhang Y, Zhang Y, et al. Textural features of (18)f-fdg pet after two cycles of neoadjuvant chemotherapy can predict pcr in patients with locally advanced breast cancer. Ann Nucl Med (2017) 31(7):544–52. doi: 10.1007/s12149-017-1184-1
21. Akimoto E, Kadoya T, Kajitani K, Emi A, Shigematsu H, Ohara M, et al. Role of (18)f-pet/ct in predicting prognosis of patients with breast cancer after neoadjuvant chemotherapy. Clin Breast Cancer (2018) 18(1):45–52. doi: 10.1016/j.clbc.2017.09.006
22. Cheng J, Wang Y, Mo M, Bao X, Zhang Y, Liu G, et al. 18f-fluorodeoxyglucose (fdg) pet/ct after two cycles of neoadjuvant therapy may predict response in her2-negative, but not in her2-positive breast cancer. Oncotarget (2015) 6(30):29388–95. doi: 10.18632/oncotarget.5001
23. Koolen BB, Pengel KE, Wesseling J, Vogel WV, Vrancken Peeters MJ, Vincent AD, et al. Fdg pet/ct during neoadjuvant chemotherapy may predict response in er-positive/her2-negative and triple negative, but not in her2-positive breast cancer. Breast (2013) 22(5):691–7. doi: 10.1016/j.breast.2012.12.020
24. Koolen BB, Pengel KE, Wesseling J, Vogel WV, Vrancken Peeters MJ, Vincent AD, et al. Sequential (18)f-fdg pet/ct for early prediction of complete pathological response in breast and axilla during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging (2014) 41(1):32–40. doi: 10.1007/s00259-013-2515-7
25. Wu S, Wang Y, Li J, Zhang N, Mo M, Klimberg S, et al. Subtype-guided (18) f-fdg pet/ct in tailoring axillary surgery among patients with node-positive breast cancer treated with neoadjuvant chemotherapy: a feasibility study. Oncologist (2020) 25(4):e626–33. doi: 10.1634/theoncologist.2019-0583
26. Connolly RM, Leal JP, Solnes L, Huang CY, Carpenter A, Gaffney K, et al. Updated results of tbcrc026: phase ii trial correlating standardized uptake value with pathological complete response to pertuzumab and trastuzumab in breast cancer. J Clin Oncol (2021) 39(20):2247–56. doi: 10.1200/JCO.21.00280
27. van Ramshorst MS, Teixeira SC, Koolen BB, Pengel KE, Gilhuijs KG, Wesseling J, et al. Additional value of (18)f-fdg pet/ct response evaluation in axillary nodes during neoadjuvant therapy for triple-negative and her2-positive breast cancer. Cancer Imaging (2017) 17(1):15. doi: 10.1186/s40644-017-0117-5
28. Rosé C, Dose J, Avril N. Positron emission tomography for the diagnosis of breast cancer. Nucl Med Commun (2002) 23(7):613–8. doi: 10.1097/00006231-200207000-00004
29. Nguyen XC, Lee WW, Chung JH, Park SY, Sung SW, Kim YK, et al. Fdg uptake, glucose transporter type 1, and ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol (2007) 62(2):214–9. doi: 10.1016/j.ejrad.2006.12.008
30. Sharma S, Guthrie PH, Chan SS, Haq S, Taegtmeyer H. Glucose phosphorylation is required for insulin-dependent mtor signalling in the heart. Cardiovasc Res (2007) 76(1):71–80. doi: 10.1016/j.cardiores.2007.05.004
31. Miricescu D, Totan A, Stanescu S, Badoiu SC, Stefani C, Greabu M. Pi3k/akt/mtor signaling pathway in breast cancer: from molecular landscape to clinical aspects. Int J Mol Sci (2020) 22(1):173. doi: 10.3390/ijms22010173
32. Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Prognostic value of fdg-pet and dwi in breast cancer. Ann Nucl Med (2018) 32(1):44–53. doi: 10.1007/s12149-017-1217-9
33. Urso L, Quartuccio N, Caracciolo M, Evangelista L, Schirone A, Frassoldati A, et al. Impact on the long-term prognosis of fdg pet/ct in luminal-a and luminal-b breast cancer. Nucl Med Commun (2022) 43(2):212–9. doi: 10.1097/MNM.0000000000001500
34. Kitajima K, Miyoshi Y. Present and future role of fdg-pet/ct imaging in the management of breast cancer. Jpn J Radiol (2016) 34(3):167–80. doi: 10.1007/s11604-015-0516-0
35. Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison of 18 fdg pet-ct and bone scintigraphy for detection of bone metastases in breast cancer patients. a meta-analysis. Surg Oncol (2013) 22(2):86–91. doi: 10.1016/j.suronc.2013.01.002
36. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol (2007) 25(13):1753–9. doi: 10.1200/JCO.2006.07.3049
37. Chacón M, Eleta M, Espindola AR, Roca E, Méndez G, Rojo S. And pupareli c: assessment of early response to imatinib 800 mg after 400 mg progression by 18f-fluorodeoxyglucose pet in patients with metastatic gastrointestinal stromal tumors. Future Oncol (2015) 11(6):953–64. doi: 10.2217/fon.14.292
38. Yokoyama K, Tsuchiya J, Nakamoto Y, Tateishi U. Additional value of [(18)f]fdg pet or pet/ct for response assessment of patients with gastrointestinal stromal tumor undergoing molecular targeted therapy: a meta-analysis. Diagnostics (Basel) (2021) 11(3):475. doi: 10.3390/diagnostics11030475
39. Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using recist, at least in gist. J Clin Oncol (2007) 25(13):1760–4. doi: 10.1200/JCO.2006.07.3411
40. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer (2011) 11(12):865–78. doi: 10.1038/nrc3143
41. von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol (2018) 36(2):136–43. doi: 10.1200/JCO.2017.74.9705
42. Betz M, Kopp HG, Spira D, Claussen CD, Horger M. The benefit of using ct-perfusion imaging for reliable response monitoring in patients with gastrointestinal stromal tumor (gist) undergoing treatment with novel targeted agents. Acta Radiol (2013) 54(7):711–21. doi: 10.1177/0284185113484642
43. Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers (2021) 7(1):22. doi: 10.1038/s41572-021-00254-5
44. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol (2003) 21(23):4342–9. doi: 10.1200/JCO.2003.04.190
45. Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, et al. Kit mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer (2006) 42(8):1093–103. doi: 10.1016/j.ejca.2006.01.030
46. Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, et al. Correlation of kinase genotype and clinical outcome in the north american intergroup phase iii trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: calgb 150105 study by cancer and leukemia group b and southwest oncology group. J Clin Oncol (2008) 26(33):5360–7. doi: 10.1200/JCO.2008.17.4284
47. Blay JY, Shen L, Kang YK, Rutkowski P, Qin S, Nosov D, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (enestg1): a randomised phase 3 trial. Lancet Oncol (2015) 16(5):550–60. doi: 10.1016/S1470-2045(15)70105-1
48. Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early her2-positive breast cancer (neosphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol (2012) 13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9
49. Shao Z, Pang D, Yang H, Li W, Wang S, Cui S, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced erbb2-positive breast cancer in asia: the peony phase 3 randomized clinical trial. JAMA Oncol (2020) 6(3):e193692. doi: 10.1001/jamaoncol.2019.3692
50. Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med (2012) 366(2):109–19. doi: 10.1056/NEJMoa1113216
51. Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mfolfox-6 or folfoxiri in patients with initially unresectable liver metastases from colorectal cancer: the olivia multinational randomised phase ii trial. Ann Oncol (2015) 26(4):702–8. doi: 10.1093/annonc/mdu580
52. Ychou M, Rivoire M, Thezenas S, Guimbaud R, Ghiringhelli F, Mercier-Blas A, et al. Chemotherapy (doublet or triplet) plus targeted therapy by ras status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. the unicancer prodige-14 randomised clinical trial. Br J Cancer (2022) 126(9):1264–70. doi: 10.1038/s41416-021-01644-y
53. Hu H, Wang K, Huang M, Kang L, Wang W, Wang H, et al. Modified folfoxiri with or without cetuximab as conversion therapy in patients with ras/braf wild-type unresectable liver metastases colorectal cancer: the foculm multicenter phase ii trial. Oncologist (2021) 26(1):e90–8. doi: 10.1634/theoncologist.2020-0563
54. Bendell JC, Zakari A, Peyton JD, Boccia R, Moskowitz M, Gian V, et al. A phase ii study of folfoxiri plus panitumumab followed by evaluation for resection in patients with metastatic kras wild-type colorectal cancer with liver metastases only. Oncologist (2016) 21(3):279–80. doi: 10.1634/theoncologist.2015-0439
55. Ozaki K, Higuchi S, Kimura H, Gabata T. Liver metastases: correlation between imaging features and pathomolecular environments. Radiographics (2022) 42(7):1994–2013. doi: 10.1148/rg.220056
56. Silva AC, Evans JM, McCullough AE, Jatoi MA, Vargas HE, Hara AK. Mr Imaging of hypervascular liver masses: a review of current techniques. Radiographics (2009) 29(2):385–402. doi: 10.1148/rg.292085123
57. Sadigh G, Applegate KE, Baumgarten DA. Comparative accuracy of intravenous contrast-enhanced ct versus noncontrast ct plus intravenous contrast-enhanced ct in the detection and characterization of patients with hypervascular liver metastases: a critically appraised topic. Acad Radiol (2014) 21(1):113–25. doi: 10.1016/j.acra.2013.08.023
Keywords: breast cancer, HER2, liver metastasis, CT, FDG-PET, RECIST
Citation: Suto H, Inui Y and Okamura A (2023) Is CT or FDG-PET more useful for evaluation of the treatment response in metastatic HER2-positive breast cancer? a case report and literature review. Front. Oncol. 13:1158797. doi: 10.3389/fonc.2023.1158797
Received: 04 February 2023; Accepted: 05 April 2023;
Published: 20 April 2023.
Edited by:
Nan Wang, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Sung Hoon Sim, National Cancer Center, Republic of KoreaKazunori Kubota, Dokkyo Medical University Saitama Medical Center, Japan
Jingyi Cheng, Shanghai Proton and Heavy Ion Center (SPHIC), China
Christelle Levy, Centre François Baclesse, France
Copyright © 2023 Suto, Inui and Okamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hirotaka Suto, aGlyb3Rha2Euc3V0b0BqZmNyLm9yLmpw
 Hirotaka Suto
Hirotaka Suto Yumiko Inui2
Yumiko Inui2 Atsuo Okamura
Atsuo Okamura