
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 April 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1158104
This article is part of the Research Topic Innovative Strategies and New Insights for the Treatment of Stage III Non-Small Cell Lung Cancer View all 10 articles
Introduction: The efficacy of postoperative radiotherapy (PORT) is still unclear in non-small cell lung cancer (NSCLC) patients with pIIIA-N2 disease. Estrogen receptor (ER) was proven significantly associated with poor clinical outcome of male lung squamous cell cancer (LUSC) after R0 resection in our previous study.
Methods: A total of 124 male pIIIA-N2 LUSC patients who completed four cycles of adjuvant chemotherapy and PORT after complete resection were eligible for enrollment in this study from October 2016 to December 2021. ER expression was evaluated using immunohistochemistry assay.
Results: The median follow-up was 29.7 months. Among 124 patients, 46 (37.1%) were ER positive (stained tumor cells≥1%), and the rest 78 (62.9%) were ER negative. Eleven clinical factors considered in this study were well balanced between ER+ and ER- groups. ER expression significantly predicted a poor prognosis in disease-free survival (DFS, HR=2.507; 95% CI: 1.629-3.857; log-rank p=1.60×10-5). The 3-year DFS rates were 37.8% with ER- vs. 5.7% with ER+, with median DFS 25.9 vs. 12.6 months, respectively. The significant prognostic advantage in ER- patients was also observed in overall survival (OS), local recurrence free survival (LRFS), and distant metastasis free survival (DMFS). The 3-year OS rates were 59.7% with ER- vs. 48.2% with ER+ (HR, 1.859; 95% CI: 1.132-3.053; log-rank p=0.013), the 3-year LRFS rates were 44.1% vs. 15.3% (HR=2.616; 95% CI: 1.685-4.061; log-rank p=8.80×10-6), and the 3-year DMFS rates were 45.3% vs. 31.8% (HR=1.628; 95% CI: 1.019-2.601; log-rank p=0.039). Cox regression analyses indicated that ER status was the only significant factor for DFS (p=2.940×10-5), OS (p=0.014), LRFS (p=1.825×10-5) and DMFS (p=0.041) among other 11 clinical factors.
Conclusions: PORT might be more beneficial for ER negative LUSCs in male, and the examination of ER status might be helpful in identifying patients suitable for PORT.
Radical surgery and dissection of mediastinal lymph node is the standard therapy for non-small cell lung cancer (NSCLC) patients with resectable lymph node(s) if the operation is endurable. Multiple randomized clinical trials (RCTs) confirmed a definitive survival benefit brought by adjuvant chemotherapy in selected patients (1–3). Nevertheless, disease-free survival (DFS) is still suboptimal, with considerable local failures leading to high risk in disease recurrence and worse overall survival (OS), especially in stage III N2 patients, even after adjuvant chemotherapy (4).
However, the evidences for postoperative radiation therapy (PORT) of R0 resected NSCLC are quite controversial. PORT has been found to be detrimental for pathologic N0/1 disease based on OS in meta-analyses (majorly population-based analysis of data from SEER database of small RCTs) (5, 6). Some meta-analyses showed a prognostic advantage of PORT in patients with pathologic N2 disease (6–8). However, the evidences from these meta-analyses were highly flawed. Most of these enrolled researches were from 1960s, when no definite staging system had ever been established. Moreover, the majority of patients received outdated radiotherapy technologies, for instance, 2-dimension conventional radiotherapy and Cobalt-60 equipment, leading to enormous unevenness in dose distribution and great heterogeneity in dose prescriptions, target volumes, and fractionations. Additionally, clinical information, including margin status, performance status, use of adjuvant chemotherapy and subsequent clinical implementations, was not available in these public databases, which was certainly not discussed in these meta-analyses. Besides, these analyses only took OS into consideration to evaluate the survival benefit brought by PORT, giving us no information about the DFS, local recurrence free survival (LRFS), and distant metastasis free survival (DMFS), which are also important to evaluate the therapeutic advantage of PORT after R0 resection.
Despite of some approval from meta-analyses, the therapeutic benefit of PORT in pIIIA-N2 patients was still unclear based on RCTs, especially in patients after R0 radical surgery and adjuvant chemotherapy. The ANITA retrospective RCT found that PORT increased OS in patients with pathologic N2 disease after adjuvant chemotherapy (9), whereas both LungART (10) and PORT-C (11) studies, the so-far only two completed prospective RCTs, failed in validating this survival advantage of PORT in stage IIIA-N2 patients. Therefore, the grim prospect of PORT in this subgroup of patients implies that a molecular predictor is urgently needed to identify the particular section of patients who can actually benefit from PORT.
Estrogen has been extensively reported to have an important function in NSCLC (12, 13). Some studies attempted to establish the correlation between estrogen receptor (ER) expression and NSCLC using immunohistochemistry (IHC) stain. Nevertheless, the reported results are contradictory and hard to interpret (14–17). Notably, the majority of these studies were only focusing upon female patients (18–20), probably caused by the stereotypical thinking that only women are subjected to the biofunction of estrogen. Moreover, the majority of ER-related studies in NSCLC were focusing on adenocarcinoma, while lung squamous cell cancer (LUSC) was seldom paid attention to let alone male LUSC patients. The treatment modality for lung adenocarcinoma has been ushered into a new era during the past decades. The tyrosine kinase inhibitors (TKIs) of EGFR and ALK have been proven remarkably beneficial in bringing a better clinical outcome in patients with lung adenocarcinoma in both adjuvant or salvage settings (21, 22), whose impact upon the observation of PORT efficacy was not considered by these RCTs. Thus, it is greatly necessary to analyze the efficacy of PORT in lung adenocarcinoma and LUSC separately, in order to eliminate the bias caused by targeted therapy.
LUSC patients are mainly male, and ER expression was reported as a significant unfavorable predictor of the clinical outcome in male LUSCs after radical resection in our previous study (23). In this study, we specifically focused on male stage IIIA-N2 LUSC who received sequential adjuvant chemotherapy and PORT, in attempt to establish the correlation between ER status and the prognosis of these patients. Despite the fact that the therapeutic effects of these inhibitors have been seldom discussed in LUSC patients (24), the EGFR mutation rate was reported around 5% in LUSCs, indicating these LUSCs might benefit from EGFR TKIs (25, 26). Therefore, in order to avoid the masking effect upon PORT by targeted therapy, molecular testing of EGFR mutation and ALK fusions was conducted in all the enrolled patients to exclude those with sensitive mutations of EGFR or ALK.
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. Ethical standards of national and institutional research committee were strictly followed in all the procedures involving human participants. Written informed consent was provided by all the enrolled participants.
Enrollment criteria in this study were as follows: male LUSC patients with the age 18 to 70 years old, weight loss < 10% before surgery, and Eastern Cooperative Oncology Group performance (ECOG) score < 2. Patients were excluded if they had any kind of neo-adjuvant treatments, a history of other cancer(s), EGFR sensitive mutation (including 19 exon deletion and 21 exon L858R mutation), ALK fusions, pneumonectomy, moderate/severe interstitial pulmonary disease, or uncontrolled infections. All the patients underwent thorough staging evaluations at most 60 days before surgery, including enhanced CT scan of the chest and abdomen; enhanced MRI of the brain; ultrasound test of supraclavicular lymph nodes and bone scan. Enrolled patients must be confirmed as pathologic stage IIIA-N2 (pT1-3N2) LUSC based on the seventh edition of American Joint Committee on Cancer staging system after R0 radical resection. Only those who completed the whole process of platinum-based adjuvant chemotherapy and PORT were enrolled.
After the diagnosis of LUSC through biopsy, patients were evaluated by a multiple disciplinary team (MDT), including at least a radiologist, a thoracic surgeon, a pathologist, a radiation oncologist, and a medical oncologist, to achieve consensus as follows: (a) technically resectable tumor. (b) N2 disease to the extent that adjuvant sequential chemoradiotherapy should be applied according to the knowledge at that time. All of the enrolled patients received lobectomy/bilobectomy of R0 resection, and complete dissection and exploration of the mediastinal lymph nodes, at least including the levels 4 (if accessible), 5, 6, 7, and 10 for left LUSC, and levels 4, 7, and 10 for right LUSC. All the resected lymph nodes were separately labeled with their corresponding locations for pathological examination. R0 resection was all confirmed by thoracic surgeons and two independent experienced pathologists.
Four cycles of platinum-based doublet regimen were administrated in adjuvant chemotherapy, i.e., GP [gemcitabine (1,000 mg/m2 intravenously on days 1 and 8) and cisplatin (40 mg/m2 intravenously on days 1-2) for every 21 days] or TP [paclitaxel (135 mg/m2 intravenously on days 1) and cisplatin (40 mg/m2 intravenously on days 1-2) for every 21 days]. Only intensity-modulated radiotherapy (IMRT) was adopted as the technique for PORT, with the clinical target volume (CTV) including the stump of the central lesions, the ipsilateral hilum, subcarinal region, and the region of bilateral mediastinum. The planning target volume (PTV) was formed by extending 0.5-0.8 cm margins from CTV (adjusted based on the irradiation and the condition of the residual lung). The total dose of radiation was up to 50 Gy at 2 Gy per fraction, 5 days per week, with 6 MV X-rays. Dose constraints for normal tissues were required as follows: the maximum dose should be ≤45 Gy for spinal cord; the mean dose should be ≤12Gy for lung, and ≤ 5% of the residual normal lung received 20Gy (V20 <25%); and the mean dose should be ≤30Gy for heart, with V30 < 40% and V40 <30%. PORT should proceed within six weeks from the fourth cycle of chemotherapy. The total interruption of PORT for any reason should be no more than 10 days.
The primary tumors embedded with formalin-fixed paraffin were collected. Slides of the tumors were stained with anti-ERα antibody (Zhongshan Bio-chemistry, China), and then incubated with anti-mouse secondary antibody (Zhongshan Bio-chemistry, China). The positivity of staining was evaluated based on PV-6000 detecting system, and each slide was then counterstained with hematoxylin. The microscope system of Olympus BX37 was adopted to obtain digital images. Each slide was examined by two blinded, experienced, and independent pathologists. The tumor was regarded as ER positive if IHC showed more than 1% of the cells were stained.
DFS was defined as the duration from the date of operation to the date of any disease recurrence, death due to any cause or the last follow-up. OS was defined as the time span from the date of surgery to the date of death due to any cause or the last follow-up. LRFS was defined as the duration from the date of surgery to the date of loco-regional disease recurrence, death due to any cause or the last follow-up. DMFS was defined as the duration from the date of surgery to the date of distant metastasis of this disease, death due to any cause or the last follow-up. Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) was adopted to grade the radiation toxicity related to PORT. R programming system (Version 4.0.3) was used in all the data analyses of this study. Log-rank test and Kaplan–Meier analysis were conducted to demonstrate the survival difference (significant if p<0.05). As for Cox analysis, variables of interest were first tested in univariate analysis, and those indicated as significant (if p<0.05) were further included in multivariate analysis to test their independence (significant if p<0.05). If only one variable was significant in univariate cox analysis, no further multivariate analysis was needed.
In this study, 124 male LUSC patients who received complete resection in Department of Thoracic Surgery in the Affiliated Hospital of Qingdao University from October 2016 to December 2021 were enrolled based on aforementioned criteria. Clinical target volume (CTV) and planning target volume (PTV) were shown in Figure 1. The median follow-up time was 29.7 months, with the range from 3.4 to 65.3 months. Among these patients, 46 (37.1%) were ER positive, and the other 78 (62.9%) were ER negative according to IHC assay (Table 1 and Figure 2). Eleven clinical factors were considered in baseline characteristic analysis, including age (<60 vs. ≥60 years old), ECOG score (0 vs. 1), grade (G1-2 vs. G3), pathological tumor size (pT, T1-2 vs. T3), visceral pleura invasion (positive vs. negative), vascular invasion (positive vs. negative), location (left vs. right), chemotherapy regimen (GP vs. TP), detected lymph nodes (DLNs, <20 vs. ≥20), positive N2 lymph nodes (PLNs, <3 vs. ≥3), and stations of N2 lymph nodes (<2 vs. ≥2). Table 1 showed that all the clinico-pathological factors were well balanced between ER+ and ER- groups.
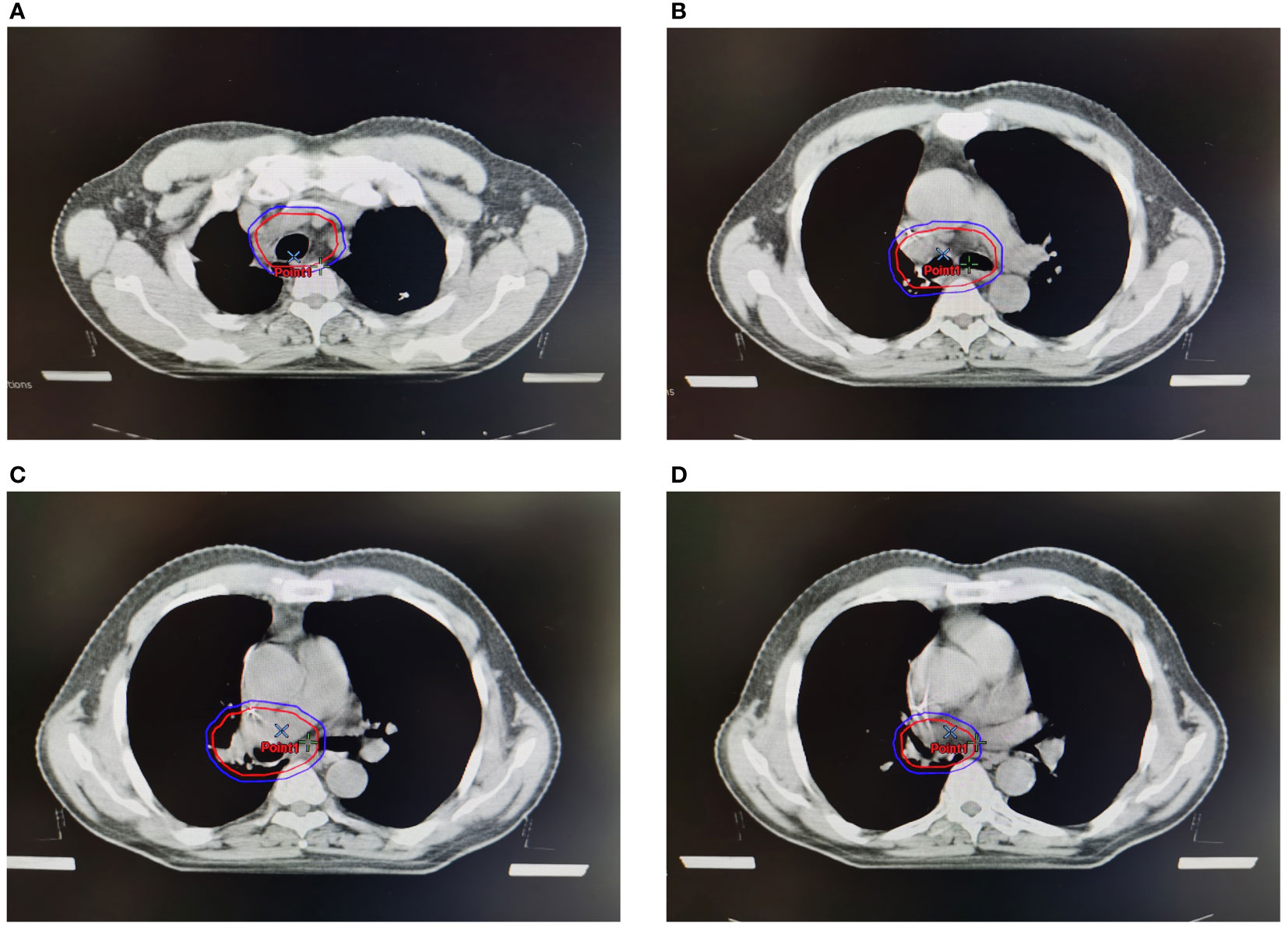
Figure 1 Clinical target volume (CTV) and planning target volume (PTV) of PORT. Red lines represented CTV, and blue lines represented PTV. (A). CTV and PTV at the level of sternoclavicular joint. (B) CTV and PTV at the level of trachea carina. (C) CTV and PTV at the stump of the bronchia. (D) CTV and PTV at the level of ipsilateral hilum.
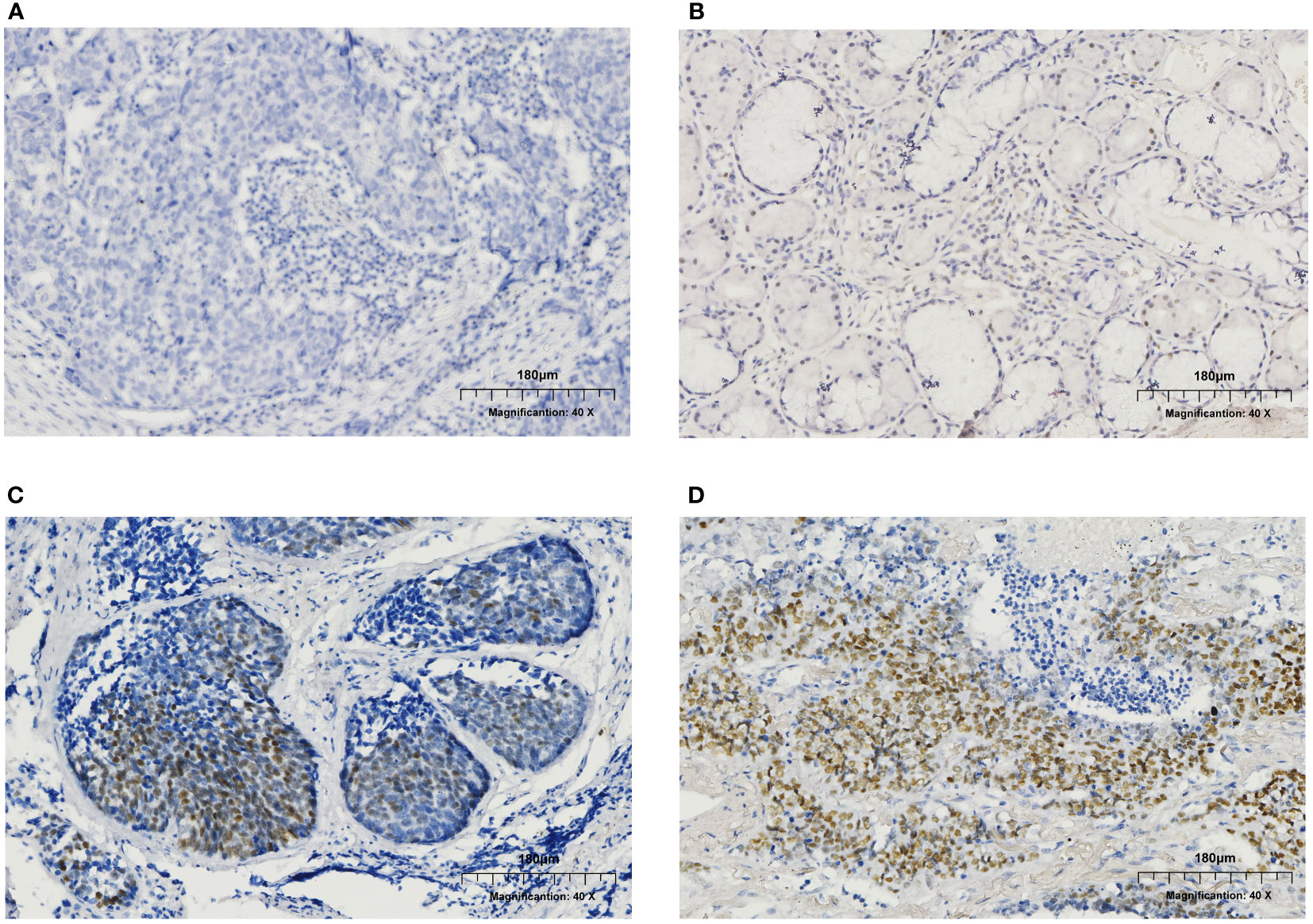
Figure 2 Immunohistochemistry (IHC) results of ER expression in male patients. (A) ER negative. (B) ER expression at the level of 5-10%. (C) ER expression at the level of 30-40%. (D) ER expression at the level of 70-80%.
Acute toxicities related to PORT was defined as the adverse events happening within the duration between the beginning of PORT and the 3 months after PORT (Table 2). Twenty-two patients (17.7%) suffered from grade 1 (n=16) or grade 2 (n=6) acute pneumonitis. Additionally, 58 patients (46.8%) experienced grade 1 (n=48) or grade 2 acute esophagitis (n=10). No patients with grade 3 or higher acute pneumonitis or/and esophagitis were observed. There were 3 patients with grade 3 neutropenia and 5 patients with grade 3 thrombocytopenia. No patient with grade 4 or higher acute toxicities was observed (Table 2). There was no difference between two arms in respect to acute toxicities. As for late toxicity, only 4 patients (3.2%, 1 patient with ER+ and 3 with ER-) experienced pulmonary fibrosis. No treatment-related deaths have been observed for all the enrolled patients.
In this study, 86 DFS events (44 in ER- arm and 42 in ER+ arm) were observed at the time of the last follow-up of this study. The median DFS for ER- patients was 23.8 [95% confidence interval (CI), 14.6-NA] months, while the median DFS for ER+ arm was only 11.2 (95% CI, 10.2-13.9) months. The 3-year DFS for ER- and ER+ patients was 37.8% and 5.7%, respectively, showing a significant difference [hazard ratio (HR) = 2.507; 95% confidence interval (CI): 1.629-3.857; log-rank p=1.60×10-5; Figure 3A and Table 3].
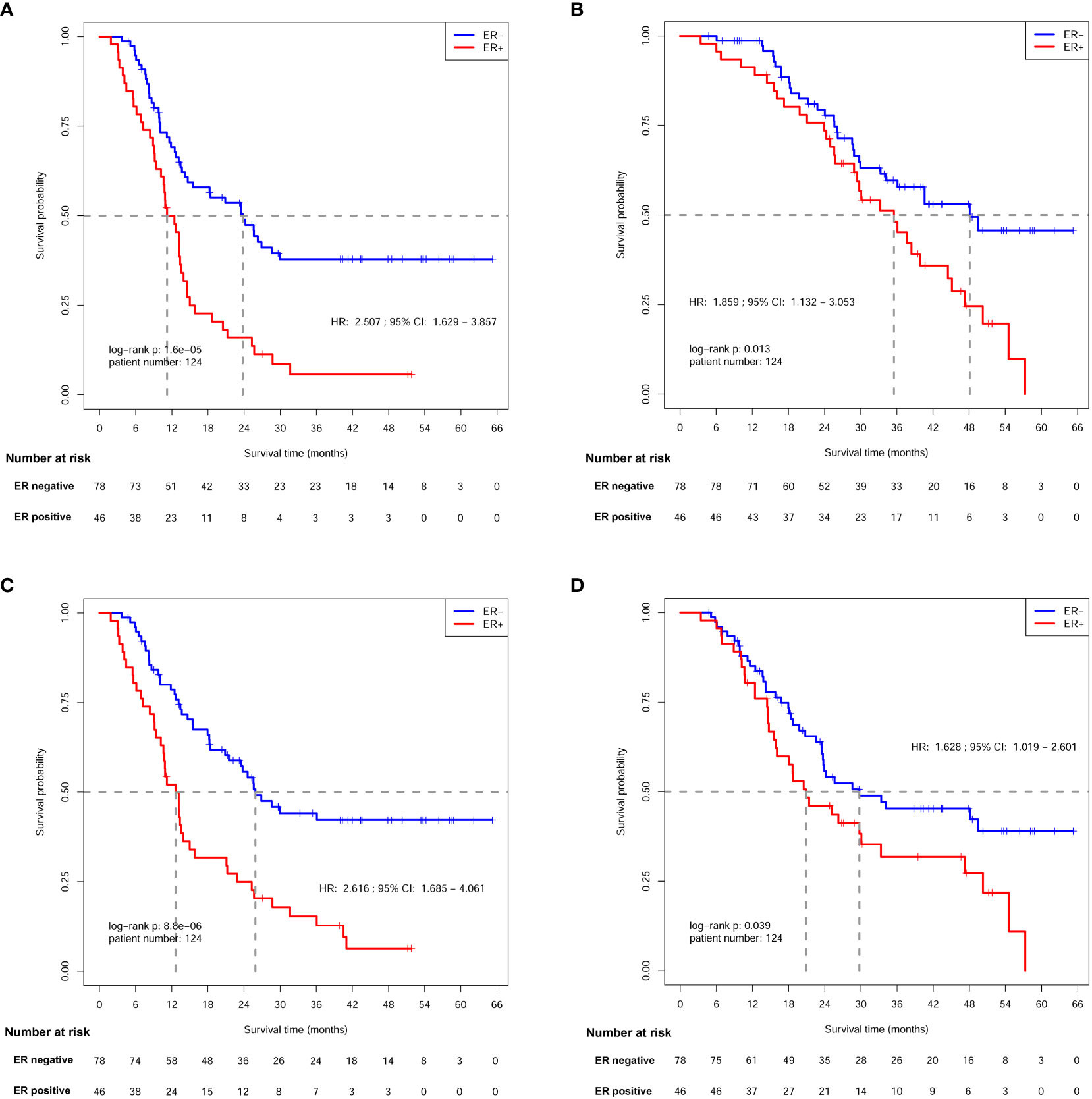
Figure 3 Survival analysis of patients between ER- and ER+ arms. (A) Disease free survival (DFS) analysis. (B) Overall survival (OS) analysis. (C) local recurrence free survival (LRFS) analysis. (D) Distant metastasis free survival (DMFS) analysis.
Sixty-three deaths (31 in ER- arm and 32 in ER+ arm) were observed at the time of last follow-up. The median OS was 48.1 (95% CI: 34.1-NA) months for ER- patients, and 35.5 (95% CI, 28.9-45.1) months for ER+ patients. The 3-year OS rates were 59.7% and 48.2% for each arm, respectively, indicating a significant OS difference between patients with different ER status (HR, 1.859; 95% CI: 1.132-3.053; log-rank p=0.013; Figure 3B and Table 3).
Eighty-one patients (40 in ER- arm and 41 in ER+ arm) suffered from loco-regional recurrence. The median LRFS was 25.9 (95% CI: 21.5-NA) months in ER- patients, and the median LRFS was 12.6 (95% CI: 10.6-15.8) months for ER+ patients. The 3-year LRFS rates were 44.1% and 15.3% for ER- and ER+ arms, respectively, indicating a significant difference (HR=2.616; 95% CI: 1.685-4.061; log-rank p=8.80×10-6; Figure 3C and Table 4).
Seventy-one patients (38 in ER- arm and 33 in ER+ arm) suffered from distant metastasis. The median DMFS was 29.7 (95% CI: 23.5-NA) months in ER- patients, while the median DMFS was 20.9 (95% CI: 15.9-47.3) months in ER+ patients. The 3-year DMFS rates were 45.3% and 31.8% for ER- and ER+ arms, respectively, and a significant difference was observed between the two arms (HR=1.628; 95% CI: 1.019-2.601 log-rank p=0.039; Figure 3D and Table 4).
Cox regression analyses of DFS, OS, LRFS, and DMFS (Tables 3, 4) were conducted among ER status and the other 11 clinico-pathological factors in these 124 patients, respectively. The result indicated that ER status was the only significant prognostic factor for DFS (p=2.940×10-5), OS (p=0.014), LRFS (p=1.825×10-5), and DMFS (p=0.041).
No concrete evidence has ever been established to support the prognostic advantage of PORT in pIIIA-N2 NSCLCs using modern radiotherapy techniques after R0 radical surgery and adjuvant chemotherapy, let alone for the subgroup of male LUSC patients. The landmark meta-analyses and RCTs were only concentrating on the clinical factors associated with the outcomes of PORT. However, the conflicting results of these studies demonstrated that only clinical factors were not sufficient to fulfill the mission, and molecular biomarkers should certainly be taken into consideration.
LUSC and lung adenocarcinoma, the two major components of NSCLC, were proven with great distinction on the basis of both pathology and treatment modality. Two milestone prospective RCTs, including ADAURA and EVIDENCE studies, demonstrated that EGFR TKIs could significantly improve clinical outcome and have a better tolerability profile in patients with EGFR-mutant NSCLCs after radical surgery (27, 28). Since almost all the EGFR-mutant NSCLCs were lung adenocarcinoma (more than 95% in ADAURA trial), EGFR TKI, instead of sequential chemoradiotherapy, was currently the standard of treatment for stage IIIA-N2 EGFR-mutant lung adenocarcinoma after complete resection. Therefore, LUSC and lung adenocarcinoma should be discussed separately in terms of adjuvant clinical implementations, and patients with driver gene mutations, including EGFR sensitive mutations or ALK fusions, were excluded from the present study in order to eliminate the systematic bias.
Although the optimal sequence of sequential adjuvant chemoradiotherapy is not established, PORT is generally administered after postoperative chemotherapy (29–31). Sequential adjuvant chemoradiotherapy in this study was strictly conducted according to PORT-C trial. Only those who completed all the four cycles of GP or TP chemotherapy and subsequent PORT of 2Gy×25 fractions were enrolled in attempt to decrease the potential bias causing by different clinical managements. Notably, only IMRT was adopted as the radiation technique to reduce the potential bias brought by other techniques, for instance, 3-dimensional conformal radiotherapy (3D-CRT) used by LungART and PORT-C studies. Modern radiation technology brings a very low toxicity, which hopefully might be translated into prognostic advantage of PORT. For instance, no grade 4 or higher adverse event related to PORT using IMRT has been observed in our study. The CTV in our radiation center includes the contralateral mediastinum but not supraclavicular region (Figure 1), of which the target volume is between PORT-C (ipsilateral mediastinum and subcarinal region) and LungART study (bilateral mediastinum and supraclavicular region). Superior 5-year OS advantage has been reported in N2 NSCLC patients who received PORT with the total dose between 45 to 54 Gy (32), while the prognostic advantage was not observed if the total dose > 54Gy because of an increased cardiac toxicity (33). Thus, all the enrolled patients received PORT with the dosage of 50Gy, in an attempt to balance between efficacy and toxicity. Both LungART and PORT-C studies failed in observing prognostic advantage of PORT in respect to DFS and OS, while both studies demonstrated the prognostic advantage of PORT in reducing local failure. It is possible since pIII-N2 NSCLC is highly heterogeneous, and thus only a part of patients could benefit from PORT.
The relationship between ER and NSCLC’s clinical outcome varies tremendously, and the most of these studies only focused on female adenocarcinoma. The remarkable controversy is probably due to many reasons, for instance, the patient population selected for research, the heterogeneous definitions of positivity, the differences in detecting methodology, and so on (14, 15, 34). Our previous finding indicated that the expression of ER predicted a poor clinical outcome in male LUSCs after receiving radical operation, which was also demonstrated by IHC assay (23). In present study, ER was significantly associated with DFS, OS, LRFS, and DMFS in male LUSCs after adjuvant sequential chemoradiotherapy. Currently, no effective biomarker has been confirmed to predict the therapeutic efficacy of PORT, and ER might be a promising biomarker to fulfill the mission. The result indicated that PORT might be more beneficial for ER negative LUSCs in male, and the examination of ER status might be helpful to identify male LUSCs suitable for PORT. As for ER positive male LUSCs with much worse prognosis, it is very intriguing that ER antagonist might be beneficial for treating these patients in adjuvant clinical setting.
The primary limitation of this study is the limited patient number (n=124), since we set a very strict enrollment criterion to reduce the potential bias. We only focused on male stage IIIA-N2 LUSCs with definitive molecular information of their EGFR and ALK status, and only patients strictly completed the sequential adjuvant chemoradiotherapy were enrolled, trying to validate the hypothesis inspired by our previous study (23). Additionally, this study is a single-center retrospective study. As we know, single-center studies have certain limitations in providing robustness and generalizability (35), but they might also reduce the bias brought by the inconsistency among different centers. However, external validations with more patients are certainly needed to further demonstrate the association between ER expression and PORT. PORT might be more beneficial for ER negative LUSCs in male, and the examination of ER status might be helpful in identifying patients with stage-IIIA N2 LUSC who are suitable for PORT.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of the Affiliated Hospital of Qingdao University. The patients/participants provided their written informed consent to participate in this study.
Data curation: LW, XJ, RX, HML, HJL, and ZY; formal analysis: NA and LW; funding acquisition: NA and XY; investigation: NA and XY; methodology: NA, XY, and LW; project administration: NA, XY, LW, XJ, and RX; resources: LW, XJ, RX, NA, and HJL; software: NA and LW; supervision: ZY; validation: XY; visualization: LW, XJ, and RX; writing-original draft: NA and XY; writing review and editing: all authors. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81801734 to XY, 81802271 to NA), the Natural Science Foundation of Shandong Province, China (ZR2019QH003 to XY) and by Qilu Health Outstanding Young Talents Training Project (to NA No.3843).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med (2005) 352:2589–97. doi: 10.1056/NEJMoa043623
2. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol (2008) 26:3552–9. doi: 10.1200/JCO.2007.13.9030
3. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med (2004) 350:351–60. doi: 10.1056/NEJMoa031644
4. Higgins KA, Chino JP, Berry M, Ready N, Boyd J, Yoo DS, et al. Local failure in resected N1 lung cancer: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys (2012) 83:727–33. doi: 10.1016/j.ijrobp.2011.07.018
5. PORT meta-analysis trialists group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT meta-analysis trialists group. Lancet (1998) 352:257–63. doi: 10.1016/S0140-6736(98)06341-7
6. Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol (2006) 24:2998–3006. doi: 10.1200/JCO.2005.04.6110
7. Robinson CG, Patel AP, Bradley JD, DeWees T, Waqar SN, Morgensztern D, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the national cancer data base. J Clin Oncol (2015) 33:870–6. doi: 10.1200/JCO.2014.58.5380
8. Patel SH, Ma Y, Wernicke AG, Nori D, Chao KS, Parashar B. Evidence supporting contemporary post-operative radiation therapy (PORT) using linear accelerators in N2 lung cancer. Lung Cancer (2014) 84:156–60. doi: 10.1016/j.lungcan.2014.02.016
9. Douillard JY, Rosell R, De Lena M, Riggi M, Hurteloup P, Mahe MA, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant navelbine international trialist association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys (2008) 72:695–701. doi: 10.1016/j.ijrobp.2008.01.044
10. Le Pechoux C, Pourel N, Barlesi F, Lerouge D, Antoni D, Lamezec B, et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23:104–14. doi: 10.1016/S1470-2045(21)00606-9
11. Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, et al. Effect of postoperative radiotherapy for patients with pIIIA-N2 non-small cell lung cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-c randomized clinical trial. JAMA Oncol (2021) 7:1178–85. doi: 10.1001/jamaoncol.2021.1910
12. Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst (1996) 88:183–92. doi: 10.1093/jnci/88.3-4.183
13. Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol (2001) 2:506–13. doi: 10.1016/S1470-2045(01)00457-0
14. Baik CS, Eaton KD. Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel) (2012) 4:969–88. doi: 10.3390/cancers4040969
15. Kawai H. Estrogen receptors as the novel therapeutic biomarker in non-small cell lung cancer. World J Clin Oncol (2014) 5:1020–7. doi: 10.5306/wjco.v5.i5.1020
16. Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res (2005) 11:5084–9. doi: 10.1158/1078-0432.CCR-05-0200
17. Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res (2005) 11:7280–7. doi: 10.1158/1078-0432.CCR-05-0498
18. Honma N, Hosoi T, Arai T, Takubo K. Estrogen and cancers of the colorectum, breast, and lung in postmenopausal women. Pathol Int (2015) 65:451–9. doi: 10.1111/pin.12326
19. Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol (2006) 24:59–63. doi: 10.1200/JCO.2005.02.9827
20. Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, Manson JE, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s health initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet (2009) 374:1243–51. doi: 10.1016/S0140-6736(09)61526-9
21. Pasche B, Grant SC. Non-small cell lung cancer and precision medicine: a model for the incorporation of genomic features into clinical trial design. JAMA (2014) 311:1975–6. doi: 10.1001/jama.2014.3742
22. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol (2018) 19:139–48. doi: 10.1016/S1470-2045(17)30729-5
23. Yang X, Jin X, Xu R, Yu Z, An N. ER expression associates with poor prognosis in male lung squamous carcinoma after radical resection. BMC Cancer (2021) 21:1043. doi: 10.1186/s12885-021-08777-6
24. Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res (2012) 18:2443–51. doi: 10.1158/1078-0432.CCR-11-2370
25. Cheung AH, Tong JH, Chung LY, Chau SL, Ng CS, Wan IYP, et al. EGFR mutation exists in squamous cell lung carcinoma. Pathology (2020) 52:323–8. doi: 10.1016/j.pathol.2019.12.003
26. Taniguchi Y, Matsumoto Y, Furukawa R, Ohara S, Usui K. The clinical features of squamous cell lung carcinoma with sensitive EGFR mutations. Int J Clin Oncol (2018) 23:452–7. doi: 10.1007/s10147-017-1233-8
27. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-Small-Cell lung cancer. N Engl J Med (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
28. He J, Su C, Liang W, Xu S, Wu L, Fu X, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir Med (2021) 9:1021–9. doi: 10.1016/S2213-2600(21)00134-X
29. Burdett S, Stewart L, PORT Meta-analysis Group. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer (2005) 47:81–3. doi: 10.1016/j.lungcan.2004.09.010
30. Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brooks BJ, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern cooperative oncology group. N Engl J Med (2000) 343:1217–22. doi: 10.1056/NEJM200010263431703
31. Bradley JD, Paulus R, Graham MV, Ettinger DS, Johnstone DW, Pilepich MV, et al. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: promising long-term results of the radiation therapy oncology group–RTOG 9705. J Clin Oncol (2005) 23:3480–7. doi: 10.1200/JCO.2005.12.120
32. Corso CD, Rutter CE, Wilson LD, Kim AW, Decker RH, Husain ZA. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the national cancer database. J Thorac Oncol (2015) 10:148–55. doi: 10.1097/JTO.0000000000000406
33. Karakoyun-Celik O, Yalman D, Bolukbasi Y, Cakan A, Cok G, Ozkok S. Postoperative radiotherapy in the management of resected non-small-cell lung carcinoma: 10 years’ experience in a single institute. Int J Radiat Oncol Biol Phys (2010) 76:433–9. doi: 10.1016/j.ijrobp.2009.02.010
34. Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol (2014) 41:5–16. doi: 10.1053/j.seminoncol.2013.12.009
Keywords: postoperative radiotherapy, lung squamous cell cancer, stage IIIA-N2, survival, estrogen
Citation: Yang X, Wang L, Jin X, Xu R, Yu Z, Li H, Lu H and An N (2023) ER predicts poor prognosis in male lung squamous cell cancer of stage IIIA-N2 disease after sequential adjuvant chemoradiotherapy. Front. Oncol. 13:1158104. doi: 10.3389/fonc.2023.1158104
Received: 03 February 2023; Accepted: 04 April 2023;
Published: 27 April 2023.
Edited by:
Aakash Desai, Mayo Clinic, United StatesReviewed by:
Wencheng Zhang, Tianjin Medical University Cancer Institute and Hospital, ChinaCopyright © 2023 Yang, Wang, Jin, Xu, Yu, Li, Lu and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning An, YW5ubmluZzA5MjFAcWR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.