
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 June 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1153131
This article is part of the Research Topic Real-World Data and Real-World Evidence in Lung Cancer View all 23 articles
Objective: The histological conversion of lung adenocarcinoma (LUAD) into small-cell lung cancer (SCLC) is an important resistance mechanism for epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI)-resistant LUAD. Anlotinib has been recommended as the third-line treatment for SCLC patients. The efficacy of etoposide/platinum (EP) as the main treatment is very limited for patients with transformed SCLC. However, little is known about EP plus anlotinib for transformed SCLC. The present study retrospectively explored the clinical response to EP combined with anlotinib in patients with transformed SCLC from LUAD after EGFR-TKI failure.
Methods: A total of 10 patients who underwent SCLC transformation from EGFR-TKI-resistant LUAD were retrospectively reviewed from September 1, 2019, to December 31, 2022, in three regional hospitals. All of the patients were treated with the combination regimen of EP and anlotinib for four to six cycles, followed by anlotinib maintenance therapy. The clinical efficacy indices including objective response rate (ORR), disease control rate (DCR), median progression-free survival (mPFS), median overall survival (mOS), and toxicities were evaluated.
Results: The median time from EGFR-TKI treatment to SCLC conversion was 20.1 ± 2.76 months (17–24 months). Genetic examination after transformation showed that 90% of the patients retained their original EGFR gene mutations. Additional driver genes were found, including BRAF mutation (10%), PIK3CA mutation (20%), RB1 loss (50%), and TP53 mutation (60%). The ORR and DCR were 80% and 100%, respectively. The mPFS was 9.0 months (95% CI, 7.9–10.1 months), and the mOS was 14.0 months (95% CI, 12.0–15.9 months). Less than 10% of grade 3 toxicities were observed, and no grade 4 toxicity and death events were reported.
Conclusion: The EP plus anlotinib regimen appears to be a promising and safe strategy in transformed SCLC patients after EGFR-TKI resistance, which warrants further investigation.
Epidermal growth factor receptor (EGFR) is the most prominent driving gene in non-small-cell lung cancer (NSCLC), mainly including EGFR exon 19 deletion and L858R mutation. EGFR-tyrosine kinase inhibitors (EGFR-TKIs) have been listed as the preferable standard of care in EGFR-mutant NSCLC patients, in particular for lung adenocarcinoma (LUAD). However, nearly all patients inevitably experience acquired resistance to EGFR-TKI. Among these patients, 5–15% display histological transformation from NSCLC to small-cell lung cancer (SCLC) (1). The underlying mechanisms are very complicated, and most of them remain unclear. For transformed SCLC, chemotherapy with etoposide/platinum (EP) is the most common regimen, but the clinical prognosis is dismal, with a median overall survival (mOS) of merely 6–10.9 months (2, 3).
As the principal angiogenic growth factor, vascular endothelial growth factor (VEGF) modulates the process of angiogenesis during the growth, invasion, and metastasis of tumors (4). In SCLC, antiangiogenic agents targeting VEGF have not become an important therapeutic strategy until the advent of anlotinib. As an oral antiangiogenic tyrosine kinase inhibitor (TKI), anlotinib has been recommended by Chinese Society of Clinical Oncology (CSCO) as a third-line treatment for SCLC and NSCLC (5, 6). Majority of patients with transformed SCLC after EGFR-TKI resistance of LUAD have received more than one systemic treatment. Furthermore, the combination of anlotinib with EP regimen has been administered as the first-line treatment of extensive-stage SCLC with an objective response rate (ORR) of 87.2% and a median progression-free survival (mPFS) of 9.0 months (7). However, little is known about the efficacy of the combination regimens in transformed SCLC from EGFR-TKI-resistant LUAD. Therefore, this study retrospectively analyzed the clinical efficacy and safety of the EP regimen plus anlotinib for patients with the histological conversion from EGFR-TKI-resistant LUAD to SCLC.
This was a multicenter retrospective observational study. All transformed SCLC patients who received anlotinib combined with EP chemotherapy were from three regional hospitals, namely the Affiliated Hospital of Jiujiang University, Ruichang People Hospital, and Lushan People Hospital, between September 1, 2019, and December 31, 2022. The clinical data of the patients were collected, including age, sex, ECOG PS, histopathology, molecular examination, TNM stage, anlotinib dose, and adverse reaction.
The inclusion criteria for patients were as follows: (1) 18–75 years of age; (2) pathologically confirmed histological transformation from EGFR-mutant LUAD to SCLC; (3) more than one systemic treatment of EGFR-TKI before transformation; (4) ECOG PS ≤ 2; and (5) TNM stage: IIIB–IV; (6) no obvious abnormality in liver and kidney function; (7) no obvious hematological abnormality; (7) no active bleeding and coagulation abnormalities; (8) no clinically significant electrocardiograph abnormality.
The exclusion criteria for patients were as follows: (1) age >75 years; (2) initial histopathological diagnosis of SCLC; (3) ECOG PS >2; and (4) presentation of active bleeding; (5) presence of contraindications to chemotherapy.
DNA was extracted from tumor tissue and matched pleural fluid samples. Next-generation sequencing (NGS) was performed via a panel of at least 73 genes in Daan Gene Co., Ltd. (Guangzhou, China), covering all exons of EGFR gene with a mean coverage depth of >800×.
All of the patients were treated with a combination regimen of EP and anlotinib, i.e., 80 mg/m2 etoposide (Qilu Phar., Jinan, China) for days 1–3, carboplatin (Qilu Phar., Jinan, China) (AUC = 5) on day 1, and anlotinib (10 mg/day) (Chia-Tai Tianqing Phar., Nanjing, China) orally for days 1–14. The cycle was repeated every 3 weeks for four to six cycles, and then anlotinib was maintained every 21 days. Dose adjustment was made according to the patients’ actual situation. The treatment was terminated if disease progression, death, or unacceptable toxicity occurred.
The clinical efficacy was evaluated according to the RECIST standard (ver. 1.1). The objective responses were classified as complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). The primary end points were ORR (CR + PR) and mPFS, and the secondary end points were DCR (CR + PR + SD) and median overall survival (mOS).
The adverse reaction grades were classified following the Common Terminology Criteria for Adverse Events (CTCAE) (ver. 5.0).
PFS was defined as the period from the initial date of chemotherapy plus anlotinib to disease progression or death. OS was determined from the start of chemotherapy with anlotinib to death or the date of last follow-up evaluation. Time to SCLC transformation was calculated from the initial date of EGFR-TKI treatment to confirmation of transformed SCLC.
The cutoff date for follow-up was December 31, 2022. The Kaplan–Meier method was used to analyze the median PFS, OS, and 95% confidence interval (CI). All of the statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, ver. 20.0, Chicago, Illinois, U.S.A) and GraphPad Prism (ver. 7.0, San Diego, California, U.S.A).
Out of 152 patients with EGFR mutations, a total of 10 patients (6.57%) with transformed SCLC were enrolled in the present study. Their baseline clinical features are given in Tables 1, 2. All of the included patients were in IIIB–IVB stage. The initial mutation status included EGFR exon 19 Del (60%, 6/10) and EGFR exon 21 L858R mutation (40%, 4/10). One patient (no. 10) had a concurrent T790M mutation. 80% (8/10) of the patients received osimertinib (AstraZeneca Phar., London, UK) as the first-line treatment, while only 20% (2/10) of the patients received aumolertinib (HanSoh Phar., Lianyungang, China) treatment. The median interval from initial treatment to transformation was 20.1 ± 2.76 months (17–24 months).
All of the patients underwent the second genetic testing, and the specimens included tissue, pleural fluid, and lymph node. Compared with the initial gene mutation, the second mutation status of transformed SCLC was very complicated. Except for patient no. 4, who lost the initial EGFR exon 19 deletion, the nine remaining patients retained their original EGFR gene mutations. These mutations were accompanied by additional driver gene mutations, including TP53 mutation (60%), RB1 loss (50%), PIK3CA mutation (20%), BRAF mutation (10%), PTEN (10%), CDK6 (10%), CCNE(10%), NF1 (10%) and MYC (10%). Of note, patient no. 3 carried TP53 mutation but did not experience RB1 loss, while patient no. 10 harbored RB1 loss and TP53 mutation but lost T790M mutation.
All the patients discontinued osimertinib or aumolertinib treatment after disease progression, and then receive the combination treatment of EC plus anlotinib. In the present study, four patients were in stage IIIB, but they exhibited poor performance status (PS=2) due to the comorbidities including as chronic bronchitis (no. 1 and 4), obstructive emphysema (no.5), and chronic bronchial asthma (no.7), respectively. So, they only received EGFR-TKI therapy alone without thoracic radiotherapy.
Except for one patient (no. 8), who only received four cycles of the combination treatment, the remaining nine patients received six cycles of EP and anlotinib treatment. All of the patients received anlotinib as maintenance therapy after the completion of the combination treatment. One patient achieved CR, seven patients achieved PR, and two patients had SD (Figure 1). The ORR was 80%, and the DCR was 100% (Table 3). The median PFS was 9.0 months (95% CI, 7.9–10.1 months), and the median OS was 14.0 months (95% CI, 12.0–15.9 months) (Figure 2). The median follow-up time was 15.2 months (95% CI, 13.4-16.8 months).
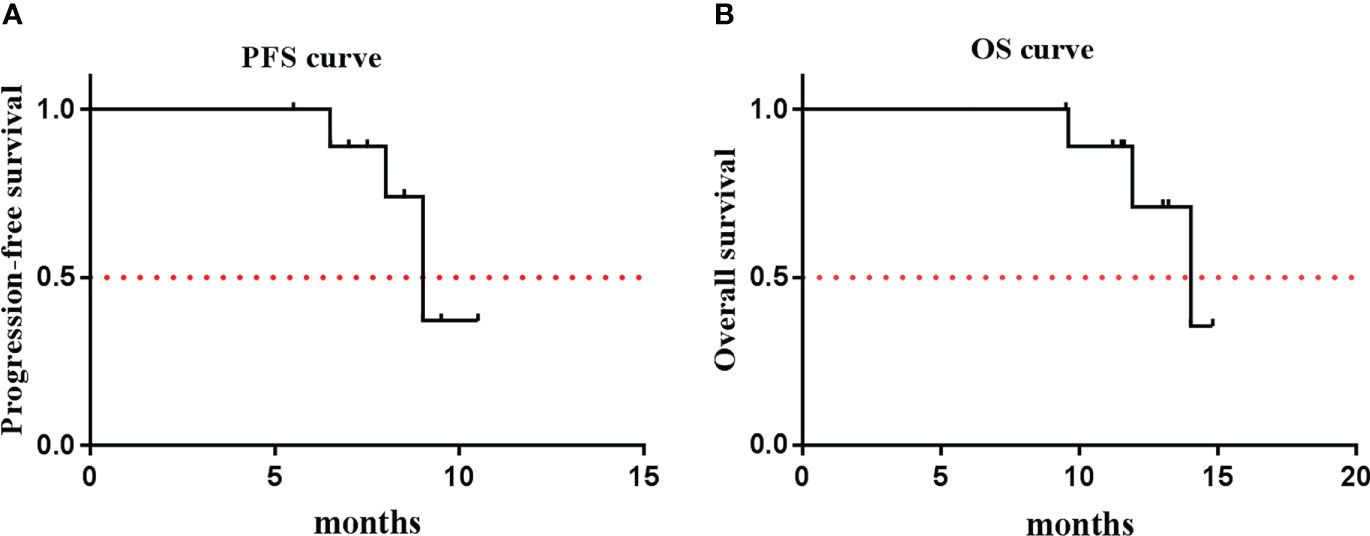
Figure 2 Kaplan-Meier Estiamtes of survival. (A) PFS: 9.0 months (95% CI: 7.9~10.1); (B) OS: 14.0 months (95% CI: 12.0~15.9).
All of the patients were included in the safety assessment. Adverse reactions were assessed from the start of the combination treatment until disease progression or the last follow-up date. The treatment-related adverse effects included vomiting and nausea, granulocytopenia, leukopenia, thrombocytopenia, hypertension, proteinuria, fatigue, hand–foot syndrome, and leukopenia (Table 4). The grade 3 toxicities were granulocytopenia (10%), leukopenia (5%), and hypertension (10%). No grade 4 toxicities were recorded, and no deaths were observed.
In 2006, a female NSCLC patient carrying EGFR exon 19 deletion was first reported to transform to SCLC (8). Since then, cases of LUAD conversion to SCLC have been continually presented (9–11). Statistically, 4–14% of EGFR-mutant NSCLC patients experience histological transformation to SCLC after EGFR-TKI failure. The histological conversion to SCLC represents one of the important mechanisms governing EGFR-TKI resistance. Three prevailing mechanisms may be proposed to explain the histological conversion from LUAD to SCLC. First, twin clones (i.e., both LUAD and SCLC clones) coexist in the tumor sites in the initial stages of tumorigenesis. LUAD cells are the dominant clones during the early stage, which become constrained under the pressure of EGFR-TKI treatment. Accordingly, the new clones of SCLC emerge and replace the previously predominant clones. Second, both SCLC and LUAD originate from the common precursor, i.e., alveolar type II cells. With long exposure to EGFR-TKI, the resistant clones survive and then convert to SCLC type. Finally, secondary gene alterations appear in the process of transformation, including RB1 loss, TP53 mutation, and PIK3CA and BRAF mutation. Recent studies have revealed some novel gene alterations that are associated with the course of transformation, which include WNK1 mutation (12), SPP1 upregulation (13), REST inactivation (14), and ETV1 mutation (15). In addition, Xie et al. reported that the conversion of LUAD to SCLC may result from somatic copy number variation (CNV) events rather than from mutational events. The burden of CNV is closely associated with the interval time to transformed SCLC and OS after SCLC conversion (16). The definitive mechanisms behind the histological transformation are very complicated and remain to be fully clarified.
Currently, an increasing number of researchers prefer the shared-origin theory. Logistically, if the theory of twin clones is true, it is difficult to explain PR or even CR response to first-line EGFR-TKI treatment. In the present study, 80% of the patients achieved more than PR response (including CR in one patient). Importantly, 90% of the patients retained their prior EGFR gene mutations. These results strongly support the common-precursor doctrine of LUAD and SCLC. Furthermore, RB1 loss was found in 50% of the patients, and TP53 mutation occurred in 60% of the patients. One patient (no. 3) harbored only gene alterations of TP53 but without RB1 loss. These findings indicate that RB1 loss and TP53 mutation are not universally present in transformed SCLC patients, which has also been confirmed by others (12, 17). Additionally, PIK3CA mutation was found in 20% of the patients, and BRAF mutation occurred in 10% of the patients, suggesting the molecular heterogeneity of transformation from LUAD to SCLC. Finally, the interval time from EGFR-TKI treatment to histological transformation was 20.1 months, which is consistent with previous reports of 17.8–22.7 months (2, 12, 18).
Currently, antiangiogenesis therapy targeting VEGF has become an indispensable strategy for cancer treatment. VEGF overexpression has been found in almost 80% of SCLC patients, indicating highly vascularized tumor of SCLC. The anti-VEGF agents, such as thalidomide, sorafenib, and sunitinib, showed disappointing clinical efficacy but increased treatment-related toxicity (19–21). In extensive-stage SCLC patients, bevacizumab plus EP regimen prolonged the PFS (6.7 vs. 5.7 months, P=0.03) but didn’t translate into the benefit of OS (8.9 vs. 9.8 months, P=0.113) compared with EP regimen (22). Obviously, the role of antiangiogenic drugs remains controversial in the treatment of SCLC until the advent of anlotinib.
In ALTER 1202 study, the novel antiangiogenic agent anlotinib as a third- or further-line treatment achieved better mPFS (4.1 vs. 0.7 months, P < 0.0001) and mOS (7.3 vs. 4.9 months, P = 0.0029) than the placebo group for patients with extensive-stage SCLC (ES-SCLC) (23). Consequently, the Chinese Society of Clinical Oncology (CSCO) recommended anlotinib as the only antiangiogenic agent for refractory ES-SCLC in China on August 30, 2019. Furthermore, a prospective study of ACTION-2 reported that EP plus anlotinib regimen as the first-line treatment for ES-SCLC achieved an ORR of 87.2%, a DCR of 97.7%, an mPFS of 9.0 months, and an mOS of 19.0 months (7). A single-arm trial showed that anlotinib plus EP as the first-line treatment for ES-SCLC achieved an ORR of 85.71%, a DCR of 94.29%, an mPFS of 8.02 months, and an mOS of 15.87 months (24). Inspired by this, we attempted to explore the combination of EP with an anlotinib regimen in transformed SCLC patients.
In de novo extensive SCLC, immune-combination therapy has been recommended as the first-line treatment with mOS reaching 13–15.4 months (25–27). Conversely, no objective responses were observed in 17 transformed SCLC cases who received immunotherapy (2). The EP regimen is the most common therapy for the transformed SCLC but with limited efficacy (only 3.2–4.0 months of mPFS and 8.0–10.9 months of mOS) (2, 12, 18, 28, 29). Wang et al. reported that the ORR and mPFS of EP chemotherapy for transformed SCLC were 44.4% and 3.5 months, respectively, but the ORR and mPFS of anlotinib alone were 66.7% and 6.2 months, respectively, indicating anlotinib as an optional choice in this population (29). In the present study, the combination of EP with an anlotinib regimen was used to treat transformed SCLC patients. The mPFS and mOS were 9.0 months (95% CI, 7.9–10.1 months) and 14.0 months (95% CI, 12.0–15.9 months), respectively. Additionally, the combination regimen was associated with a favorable safety profile, with less than 10% of grade 3 toxicities and no grade 4 toxicities and deaths. Therefore, EP plus anlotinib regimen in our study seems to achieve higher clinical efficacy than EP chemotherapy or anlotinib alone in previous studies.
Heretofore, no treatment guidelines have been established for the transformed SCLC. Our study revealed that EP combined with anlotinib may be a better choice for transformed SCLC originating from EGFR-TKI-resistant LUAD compared with EP or anlotinib alone treatment. However, this study also has some shortcomings. On the one hand, the sample size was small. On the other hand, the present study was retrospective in nature, and the results were observational. Due to the study limitations, further well-designed prospective studies with large sample sizes should be performed to confirm these findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Jiujiang University Affiliated Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization and writing: JD. Patients’ data collection: ZL, HG and XJ. Data processing: YS. All of the authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
The research received funding from the Key project of Jiangxi Natural Science Foundation (No. 20224ACB206038), China.
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yin X, Li Y, Wang H, Jia T, Wang E, Luo Y, et al. Small cell lung cancer transformation: from pathogenesis to treatment. Semin Cancer Biol (2022) 86:595–606. doi: 10.1016/j.semcancer.2022.03.006
2. Marcoux N, Gettinger SN, O'Kane G, Arbour KC, Neal JW, Husain H, et al. Egfr-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol (2019) 37:278–85. doi: 10.1200/JCO.18.01585
3. Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treat Rev (2017) 59:117–22. doi: 10.1016/j.ctrv.2017.07.007
4. Montanino A, Manzo A, Carillio G, Palumbo G, Esposito G, Sforza V, et al. Angiogenesis inhibitors in small cell lung cancer. Front Oncol (2021) 11:655316. doi: 10.3389/fonc.2021.655316
5. Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled phase 2 study. Br J Cancer. (2021) 125:366–71. doi: 10.1038/s41416-021-01356-3
6. Gong J, Wan Q, Shang J, Qian X, Su D, Sun Z, et al. Cost-effectiveness analysis of anlotinib as third- or further-line treatment for relapsed small cell lung cancer (sclc) in china. Adv Ther (2021) 38:5116–26. doi: 10.1007/s12325-021-01889-2
7. Zhang W, Deng P, Kong T, Zhang B, Qian F, Dong Y, et al. Safety and efficacy of anlotinib in combination with standard chemotherapy as first-line treatment for extensive-stage small cell lung cancer: a multi-center, prospective study (action-2). Lung Cancer. (2022) 173:43–8. doi: 10.1016/j.lungcan.2022.09.003
8. Zakowski MF, Ladanyi M, Kris MG. Egfr mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med (2006) 355:213–15. doi: 10.1056/NEJMc053610
9. Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J, Zhong W, et al. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma: a case report and review of literature. Medicine (2016) 95:e2752. doi: 10.1097/MD.0000000000002752
10. Lee PH, Huang YH, Lin H, Hsu KH, Chen KC, Tseng JS, et al. Histological transformation after acquired resistance to the third-generation egfr-tki in patients with advanced egfr-mutant lung adenocarcinoma. Medicina (2022) 58:908. doi: 10.3390/medicina58070908
11. Shastri M, Gupta P, Gupta N, Singh N, Bal A, Srinivasan R, et al. Sequential small cell transformation and t790m mutation in an epidermal growth factor-mutant lung adenocarcinoma: a rare occurrence with significant management implications. Cytopathology. (2022) 33:732–37. doi: 10.1111/cyt.13168
12. Yu L, Bazhenova L, Gold K, Tran L, Hilburn V, Vu P, et al. Clinicopathologic and molecular characteristics of egfr-mutant lung adenocarcinomas that transform to small cell lung cancer after tki therapy. Transl Lung Cancer Res (2022) 11:452–61. doi: 10.21037/tlcr-21-665
13. Wang Z, Zhang L, Xu W, Li J, Liu Y, Zeng X, et al. The multi-omics analysis of key genes regulating egfr-tki resistance, immune infiltration, sclc transformation in egfr-mutant nsclc. J Inflammation Res (2022) 15:649–67. doi: 10.2147/JIR.S341001
14. Masawa M, Sato-Yazawa H, Kashiwagi K, Ishii J, Miyata-Hiramatsu C, Iwamoto M, et al. Rest inactivation and coexpression of ascl1 and pou3f4 are necessary for the complete transformation of rb1/tp53-inactivated lung adenocarcinoma into neuroendocrine carcinoma. Am J Pathol (2022) 192:847–61. doi: 10.1016/j.ajpath.2022.03.007
15. Zhou Y, Bai H, Xia J, Xu WY, Cheng L, Xiong L. Novel etv1 mutation in small cell lung cancer transformation resistant to egfr tyrosine kinase inhibitors. Ann Transl Med (2021) 9:1150. doi: 10.21037/atm-21-2625
16. Xie T, Li Y, Ying J, Cai W, Li J, Lee KY, et al. Whole exome sequencing (wes) analysis of transformed small cell lung cancer (sclc) from lung adenocarcinoma (luad). Transl Lung Cancer Res (2020) 9:2428–39. doi: 10.21037/tlcr-20-1278
17. Mambetsariev I, Arvanitis L, Fricke J, Pharaon R, Baroz AR, Afkhami M, et al. Small cell lung cancer transformation following treatment in egfr-mutated non-small cell lung cancer. J Clin Med (2022) 11:1429. doi: 10.3390/jcm11051429
18. Xu J, Xu L, Wang B, Kong W, Chen Y, Yu Z. Outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after egfr tyrosine kinase inhibitors resistance: a systematic review and pooled analysis. Front Oncol (2021) 11:766148. doi: 10.3389/fonc.2021.766148
19. Lee SM, Woll PJ, Rudd R, Ferry D, O'Brien M, Middleton G, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst (2009) 101:1049–57. doi: 10.1093/jnci/djp200
20. Sharma N, Pennell N, Nickolich M, Halmos B, Ma P, Mekhail T, et al. Phase ii trial of sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs (2014) 32:362–68. doi: 10.1007/s10637-013-0061-6
21. Ready NE, Pang HH, Gu L, Otterson GA, Thomas SP, Miller AA, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase ii study-calgb 30504 (alliance). J Clin Oncol (2015) 33:1660–65. doi: 10.1200/JCO.2014.57.3105
22. Tiseo M, Boni L, Ambrosio F, Camerini A, Baldini E, Cinieri S, et al. Italian, Multicenter, phase iii, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the goirc-aifa farm6pmfjm trial. J Clin Oncol (2017) 35:1281–87. doi: 10.1200/JCO.2016.69.4844
23. Liu Y, Cheng Y, Li K, Shi J, Liu Y, Wu L, et al. Effect of prior thoracic radiotherapy on prognosis in relapsed small cell lung cancer patients treated with anlotinib: a subgroup analysis of the alter 1202 trial. Transl Lung Cancer Res (2021) 10:3793–806. doi: 10.21037/tlcr-21-632
24. Deng P, Hu C, Chen C, Cao L, Gu Q, An J, et al. Anlotinib plus platinum-etoposide as a first-line treatment for extensive-stage small cell lung cancer: a single-arm trial. Cancer Med (2022) 11:3563–71. doi: 10.1002/cam4.4736
25. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (caspian): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22:51–65. doi: 10.1016/S1470-2045(20)30539-8
26. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (capstone-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23:739–47. doi: 10.1016/S1470-2045(22)00224-8
27. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the astrum-005 randomized clinical trial. Jama. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
28. Ferrer L, Giaj LM, Brevet M, Antoine M, Mazieres J, Rossi G, et al. A brief report of transformation from nsclc to sclc: molecular and therapeutic characteristics. J Thorac Oncol (2019) 14:130–34. doi: 10.1016/j.jtho.2018.08.2028
29. Wang W, Xu C, Chen H, Jia J, Wang L, Feng H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with egfr tyrosine kinase inhibitors: a multicenter retrospective study. Lung Cancer. (2021) 155:20–7. doi: 10.1016/j.lungcan.2021.03.006
Keywords: etoposide/platinum (EP), anlotinib, transformation, small-cell lung cancer (SCLC), lung adenocarcinoma (LUAD), epidermal growth factor receptor (EGFR)
Citation: Ding J, Leng Z, Gu H, Jing X and Song Y (2023) Etoposide/platinum plus anlotinib for patients with transformed small-cell lung cancer from EGFR-mutant lung adenocarcinoma after EGFR-TKI resistance: a retrospective and observational study. Front. Oncol. 13:1153131. doi: 10.3389/fonc.2023.1153131
Received: 28 January 2023; Accepted: 30 May 2023;
Published: 09 June 2023.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Lorenzo Belluomini, University of Verona, ItalyCopyright © 2023 Ding, Leng, Gu, Jing and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianghua Ding, ZG9jdG9yMDkyMkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.