
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 June 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1152575
 Jinhao Wang1†
Jinhao Wang1† Yaxin Liu2†
Yaxin Liu2† Yuehua Liang2†
Yuehua Liang2† Yue Zhang1
Yue Zhang1 Hang Dong1
Hang Dong1 Tiantian Zheng1
Tiantian Zheng1 Jianjun Yu1
Jianjun Yu1 Pan Du3
Pan Du3 Shidong Jia3
Shidong Jia3 Bonnie L. King3
Bonnie L. King3 Jing Wang2
Jing Wang2 Xiaoran Liu2
Xiaoran Liu2 Huiping Li2*
Huiping Li2*Background: Poor outcomes have been widely reported for younger vs. older breast cancer patients, but whether this is due to age itself or the enrichment of aggressive clinical features remains controversial. We have evaluated the clinicopathologic characteristics and genomic profiles of real-world hormone receptor-positive (HR+)/HER2-negative (HER2-) metastatic breast cancer (MBC) patients to examine the determinants of outcome for younger vs. older patients in a single clinical subtype undergoing treatment in the same clinic.
Patients and methods: This study included patients presenting at the Peking University Cancer Hospital with primary stage IV or first-line metastatic HR+/HER2- breast cancer who consented to an additional blood draw for genomic profiling prior to treatment. Plasma samples were analyzed with a targeted 152-gene NGS panel to assess somatic circulating tumor DNA (ctDNA) alterations. Genomic DNA (gDNA) extracted from peripheral blood mononuclear cells was analyzed for germline variants using a targeted 600-gene NGS panel. Kaplan-Meier survival analysis was performed to analyze disease free survival (DFS), progression free survival (PFS) and overall survival (OS) in association with clinicopathologic and genomic variables.
Results: Sixty-three patients presenting with HR+/HER2- MBC were enrolled in this study. Fourteen patients were < 40 years, 19 were 40-50 years, and 30 were > 50 years at the time of primary cancer diagnosis. No significant associations were observed between age and DFS, PFS or OS. Shorter OS was associated with de novo Stage IV disease (p = 0.002), Luminal B subtype (p = 0.006), high Ki67 index (p = 0.036), resistance to adjuvant endocrine therapy (p = 0.0001) and clinical stage (p = 0.015). Reduced OS was also observed in association with somatic alterations in FGFR1 (p = 0.008), CCND2 (p = 0.012), RB1 (p = 0.029) or TP53 (p = 0.029) genes, but not in association with germline variants.
Conclusion: In this group of real-world HR+/HER2- MBC breast cancer patients younger age was not associated with poor outcomes. While current guidelines recommend treatment decisions based on tumor biology rather than age, young HR+ breast cancer patients are more likely to receive chemotherapy. Our findings support the development of biomarker-driven treatment strategies for these patients.
Breast cancer is generally a disease of advanced age, with peak incidence occurring after the age of 50 (1, 2). However, while breast cancer is relatively less common before the age of 50, it is the most frequently diagnosed malignancy in younger women worldwide, and a leading cause of cancer deaths in this age group (3). Of the 2.1 million breast cancer cases diagnosed globally in 2018, approximately 645,000 cases and more than 130,000 deaths occurred in women below the age of 50, with incidence and mortality rates varying according to geographic region and socioeconomic levels (4). As a proportion of all breast cancer cases, the highest rates of premenopausal breast cancer incidence and mortality are reported in Africa and Asia (4). Although the greatest burden of premenopausal cases and deaths is observed in countries with a low human development index (HDI), the incidence of premenopausal breast cancer has been climbing in high-income countries (4). Thus, despite being relatively less common, breast cancer in younger women constitutes a significant component of the global breast cancer burden.
Many previous studies have suggested that breast cancer in younger women is a more aggressive disease compared to breast cancer in older women (reviewed) (5–7). Young age at diagnosis has been reported to be an independent risk factor for worse outcomes in many studies, with young patients more likely to die of breast cancer relative to older patients (8–17). Compared to older breast cancer patients, younger breast cancer patients have been identified as more likely to present with de novo stage IV disease (11, 16). Young women who present with earlier stage breast cancer have also been reported as more likely to progress to metastatic disease and are more likely to develop brain metastases (18, 19). Studies have also found that young breast cancer patients are more likely to be diagnosed with larger, higher-grade tumors, a higher Ki67 index and positive axillary nodes (8, 10–12, 16, 18, 20, 21). Relative to older breast cancer patients, younger patients have also been characterized for a higher incidence of the more aggressive TNBC and HER2+ clinical subtypes (8, 9, 17, 18, 21–24), and a higher likelihood of harboring BRCA1/2 germ line mutations (24–26). In addition, comprehensive genomic profiling, which enables the identification of tumor-related alterations, has demonstrated that younger breast cancer patients have distinct somatic genomic profiles, methylation patterns and gene expression signatures associated with the dysregulation of genes including ESR1, GATA3 and FGFR1 (24, 27–29). Thus, as a group, young breast cancer patients appear to harbor a variety of clinical, biological and genetic features consistent with a more aggressive form of disease.
However, despite the enrichment of TNBC and HER2+ subtypes in younger breast cancer patients, similar to older patients, the majority of cases are HR+/HER2- (9, 17, 18, 21, 23, 24, 27). Moreover, while germline mutations in BRCA1/2 are found with higher frequency among younger vs. older patients, the majority of younger patients are not BRCA1/2 mutation carriers (25, 30). On the other hand, menopausal status across younger breast cancer patients varies considerably as there is wide deviation around the worldwide average age of menopause at 50 (31). Despite this variation, age is often used as a proxy for menopausal status in epidemiologic and clinical studies (4, 32). Thus, the overlap and variable presence of features across younger breast cancer patients complicates the issue of whether breast cancer in younger patients represents a distinct biologic entity that drives aggressive disease with poor outcomes, or alternatively, reflects a variable enrichment of unfavorable clinical features (17). Two studies focusing solely on HER2+ (33) and TNBC (34) breast cancer patients did not find that young age was associated with poor outcomes. A refined understanding of these issues is needed to optimize management paradigms for young breast cancer patients.
In the present study we have characterized a cohort of 63 real world HR+ Chinese metastatic breast cancer patients undergoing treatment in a single clinic to compare clinical outcomes in association with age and other clinicopathologic features, treatment regimens and genomic profiles to address whether younger patients experience worse outcomes relative to older breast cancer patients.
Patients presenting at the Peking University Cancer Hospital with first-line metastatic or primary stage IV, HR+/HER2- breast cancer from December 2015 –March 2019 who consented to an additional blood draw for genomic profiling were enrolled in this study. This study was approved by an Institutional Review Board (ethic No. 2016KT75 and ethic No.2017KT40), and all patients signed written informed consent for additional blood collection for genomic profiling. Blood samples (10mL) were prospectively collected from 63 patients, before any treatment was initiated in the metastatic setting (Figure 1). Criteria for enrollment included a pathologically confirmed diagnosis of metastatic relapse or de novo metastatic disease, with classification of the primary tumor as HR+/HER2-, as confirmed by immunohistochemical staining. HR+ was defined as ≥1% positive staining for tumor nuclear estrogen receptor (ER) and/or progesterone receptor (PR). HER2 staining was evaluated using a range of (0-3+), with 0 and 1+ classified as negative, 2+ as equivocal, and 3+ as positive. Fluorescence in-situ hybridization tests were used to confirm HER2 status when immunohistochemistry results were equivocal. Menopausal status at the time of primary breast cancer diagnosis was determined for a subset of patients using the following criteria to define the postmenopausal state: i) prior bilateral oophorectomy, ii) age > 60 years iii) age < 60 years and amenorrheic for 12 or more months in the absence of chemotherapy, tamoxifen, toremifene, or ovarian suppression with follicle-stimulating hormone (FSH) and estradiol levels in the postmenopausal ranges, and iv) if taking tamoxifen or toremifene, and age < 60 years, with FSH and plasma estradiol levels in the postmenopausal ranges. Endocrine resistance was defined by a relapse within 2 years of adjuvant endocrine treatment or disease progression during the first 6 months of first-line endocrine therapy for MBC per EMSO 2020 guidelines (35).
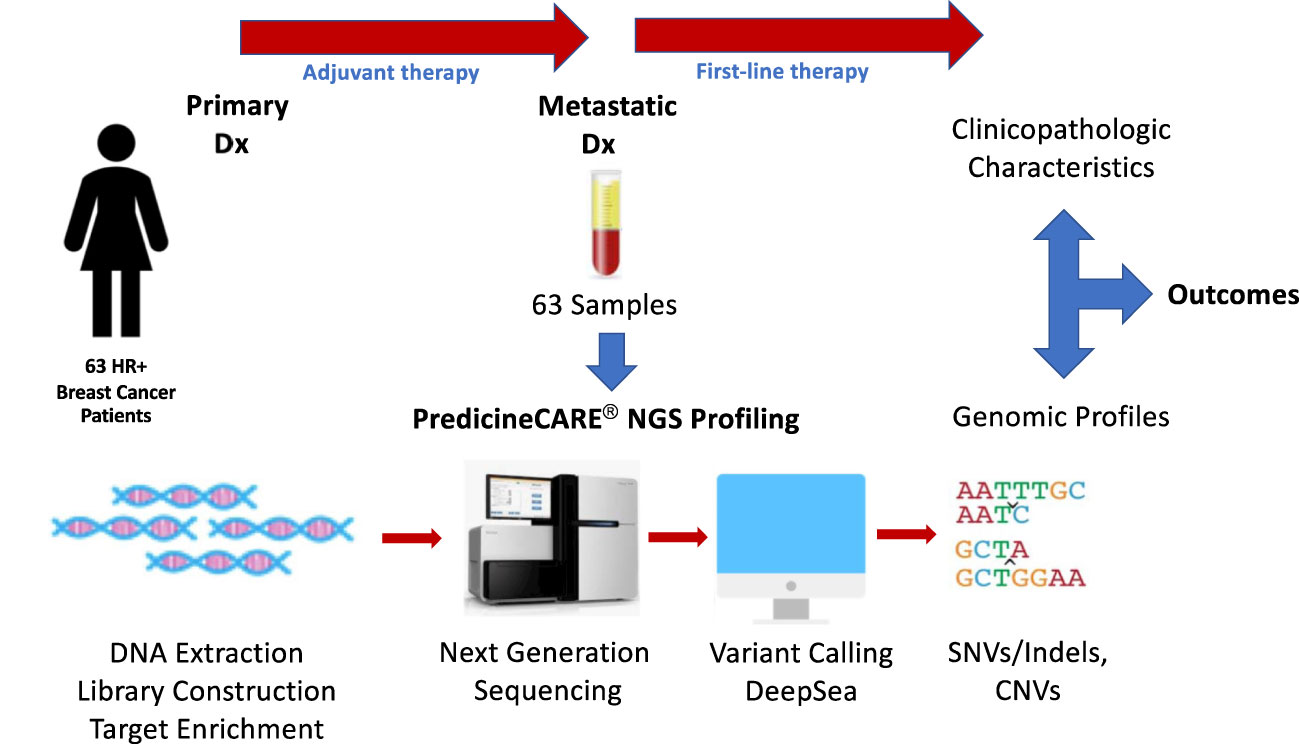
Figure 1 Study design. Plasma samples were collected from 63 HR+/Her2- MBC patients and subjected to NGS profiling using a targeted 152-gene panel to detect somatic mutations and copy number variations. Outcomes were analyzed in association with patient clinicopathological characteristics, treatment regimens and genomic profiles.
Cell-free DNA (cfDNA) testing was performed at the College of American Pathologists (CAP) accredited laboratory at Huidu Shanghai Medical Sciences, Ltd. using the harmonized 152-gene PredicineCARE™ NGS hybrid capture-based sequencing assay. The list of genes covered by this panel is shown in Supplementary Figure 1. The details of sample processing and profiling, including library preparation, amplification, hybrid capture and sequencing are described in Supplementary Methods and have been described previously (36). In brief, cfDNA was extracted from plasma samples using the QIAamp circulating nucleic acid kit, after which the quantity and quality of purified cfDNA were assessed using a Qubit fluorometer and Bioanalyzer 2100. Five to 30 ng of extracted cfDNA was prepared for library construction in a sequential process involving end-repair, dA-tailing, adapter ligation, and PCR amplification. Amplified DNA libraries with sufficient yields were advanced to hybrid capture with PredicineCARE™ panel probes. Purified libraries underwent quality control analysis with a Bioanalyzer 2100 and were then subjected to paired-end 2x150bp sequencing using the Illumina NGS platform.
Sequencing data were analyzed using Predicine’s in-house analysis pipeline, which encompasses the initial analysis of raw sequencing data and culminates in final mutation calling, as described in Supplementary Methods and a previous publication (36). Briefly, the process included adapter trimming, barcode checking, and correction. Cleaned, paired FASTQ files generated by the pipeline were further aligned to the human reference genome build hg19 using the Burrows-Wheeler Aligner (BWA) (version 0.7.15) alignment tool. Consensus binary alignment map (BAM) files were then derived by merging paired-end reads originated from the same molecules (based on mapping location and unique molecular identifiers) as single strand fragments. Single strand fragments from complementary double strand DNA molecules were further merged as double stranded. Both sequencing and PCR errors were deeply suppressed during this process. Candidate variants, consisting of single nucleotide variants (SNVs), small insertions and deletions (Indels), and copy number variations (CNVs) were identified across the targeted regions covered by the panel. The data analysis was conducted in China.
Genomic DNA (gDNA) was extracted from peripheral blood monocytes and was analyzed for the presence of cancer-associated germline variants with NGS-based sequencing using the comprehensive 600-cancer gene PredicineATLAS™ panel as described in Supplementary Methods and a previous publication (37) and to control for mutations resulting from CHIP (Clonal Hematopoiesis of Indeterminant Potential). The data analysis was conducted in China.
To examine the proportion of mutations within key oncogenic signaling pathways within the cohort and across age groups, we filtered the list of genes included in a previous publication describing oncogenic signaling pathways (38) to include only those identified as breast cancer driver genes (39, 40), as described in the Supplementary Methods. The frequency of SNVs across these genes was analyzed for the entire cohort as well as across age groups, and statistical significance was evaluated using the Fisher’s Exact Test. The data analysis was conducted in China.
Fisher’s exact and Pearson’s chi-squared tests were performed to compare the proportions of patients with defined clinicopathologic variables across age categories and to assess the frequency of genomic alterations across patient subgroups, with significance set at p ≤ 0.05. Kaplan-Meier survival analysis was performed to analyze patient outcomes including disease free survival (DFS), progression free survival (PFS) and overall survival (OS) in association with patient age at the time of primary cancer diagnosis. Outcomes were also analyzed at the univariate level in association with menopausal status, germline status, de novo Stage IV disease, type of adjuvant endocrine treatment, treatment with adjuvant chemotherapy, endocrine resistance to adjuvant and relapse therapy, molecular subtype, clinical stage and somatic gene alterations detected at the time of metastatic disease diagnosis. OS was also evaluated in association with age in separate multivariate models that included de novo Stage IV disease, molecular subtype, Ki67 index, resistance to adjuvant endocrine therapy and the presence of ctDNA alterations as co-variates using Cox proportional hazards regression. P-values were calculated for univariate and multivariate analyses using the log-rank test, with significance set at p ≤ 0.05. All statistical analyses were performed using R (version 4.1.0).
This study cohort included 63 HR+/HER2- metastatic breast cancer patients who presented at the Peking University Cancer Hospital from December 2015 – March 2019 with metastatic relapse or de novo Stage IV metastatic disease. Last follow up was in December 2022. Classification of patients based on clinicopathological characteristics across the three age groups is summarized in Table 1.
All patients were female and were between the ages of 27 and 82 years (mean = 50.3 years, median = 48.2 years) at the time of primary cancer diagnosis. Fourteen patients were < 40 years of age, 19 patients were between 40-50 years of age, and 30 patients were > 50 years of age. Menopausal status at the time of primary cancer diagnosis was confirmed for 48/63 patients according to the criteria defined in Materials and Methods and varied significantly across age groups (p = 5.44E-10), with the majority of patients under 50 years classified as premenopausal. Further breakdown of menopausal status for the three age groups is presented in Table 1.
Germline mutations were detected in 11 genes across 16/63 (25.4%) patients. BRCA2 mutations were detected in 5 patients and DNMT3A alterations were detected in 2 patients. ALOX12B, ATM, BARD1, BRCA1, CHEK2, IDH1, NOTCH3, PMS and RECQL, mutations were each detected only once. Three patients were characterized for the presence of two germline mutations: (ATM and BRIP1), (RAD50 and PMS2) and (IDH1 and TP53) (37). Although the proportion trended highest in patients < 40 years, no significant variation in frequencies of germline mutations were observed across the three age groups (Table 1).
Significant variation across age groups was observed for other clinicopathological variables, including de novo Stage IV disease (p < 0.01), type of adjuvant endocrine therapy (p = 6.87E-05), and molecular subtype classification (p = 0.04). The highest prevalence of de novo Stage IV disease was observed in patients > 50 years (40%). Aromatase inhibitors (AIs) were administered as adjuvant endocrine therapy to 76.92% of women above the age of 50 vs. 22.22% of women aged 40-50 years and 0% of women age < 40 years, respectively. The proportion of patients classified as Luminal B was highest in patients > 50 years (86.21%) and < 40 years (76.92%) and lowest in women aged 40-50 years (52.63%). In keeping with the molecular subtype finding, similar, but nonsignificant trends were observed for lower prevalence of other high-risk features among women aged 40-50 years relative to the other age groups.
To assess the contributions of clinicopathologic variables that could potentially influence clinical outcomes for patients in this cohort, univariate analyses were performed to evaluate patient outcomes in association with age and menopausal status at the time of primary disease diagnosis, germline mutation status, de novo Stage IV disease, endocrine treatment in the adjuvant and first-line settings, molecular subtype, Ki67 index, clinical stage and resistance to adjuvant and relapse endocrine therapy. Disease free survival (DFS) was measured from the time of surgery following primary diagnosis to the time of diagnosis of first-line metastatic relapse, with a median time to metastasis of 57.05 months (Table 1). DFS was measured for 48/49 patients with relapsed metastatic disease but was not determined for one patient that did not undergo surgery due to poor health. Progression free survival (PFS) was measured from the time at which treatment for metastatic disease was initiated to the time of metastatic progression, with a median time to progression of 13.27 months (Table 1). PFS was measured for 62/63 patients but was not determined for 1 patient for whom follow up could not be obtained. OS was measured from the time of surgery or treatment for de novo disease following primary diagnosis to the time of death, with a median OS of 103.97 months (Table 1). OS was determined for all patients except the patient who did not undergo surgery at the time of primary diagnosis.
At the univariate level no significant associations were observed between DFS (p = 0.83), PFS (p = 0.87) or OS (p = 0.17) with age (Figures 2A–C). Median OS was 89.9, 127.4 and 75.5 months for women <40, 40-50 and >50 years, respectively (Table 1). A similar pattern across age groups was observed for DFS (50.4, 71.4 and 52.7 months) but not PFS (12.2, 10.2 and 15.5 months) (Table 1). No significant association between age and OS was observed in separate multivariate analyses after adjustment for de novo stage IV disease, ctDNA alterations, luminal B subtype, resistance to adjuvant therapy or high Ki67 index (Table 2). However, a number of associations were observed between outcomes and several other clinicopathologic variables. Significantly shorter DFS was observed in association with the Luminal B subtype (p = 0.0016) (Figure 3A), high Ki67 index (p = 0.007) (Figure 3B), and resistance to adjuvant endocrine therapy (p = 0.0001) (Figure 3C). Significantly shorter PFS was observed in association with postmenopausal status at the time of primary diagnosis (p = 0.037) (Figure 3D), endocrine resistance to relapse endocrine therapy (p = 0.0001) (Figure 3E), and type of first-line therapy (p = 0.0002) (Figure 3F). No significant associations were observed between postmenopausal status and DFS or OS (Supplementary Figure 2). Shorter OS was observed in association with de novo Stage IV disease (p = 0.0018) (Figure 4A), luminal B molecular subtype (p = 0.0061) (Figure 4B), high Ki67 index (p = 0.036) (Figure 4C), resistance to adjuvant endocrine therapy (p = 0.0001) (Figure 4D) and clinical stage at primary diagnosis (p = 0.015) (Figure 4E). No significant associations were observed between any survival outcomes and germline mutation status (Supplementary Figure 2), type of adjuvant endocrine therapy or receipt of adjuvant chemotherapy (Supplementary Figure 3). PFS was not significantly shorter for patients presenting with de novo vs. recurrent Stage IV disease (Supplementary Figure 4). DFS, PFS and OS were not significantly shorter for patients when de novo Stage IV patients were excluded from the analysis (Supplementary Figure 5).
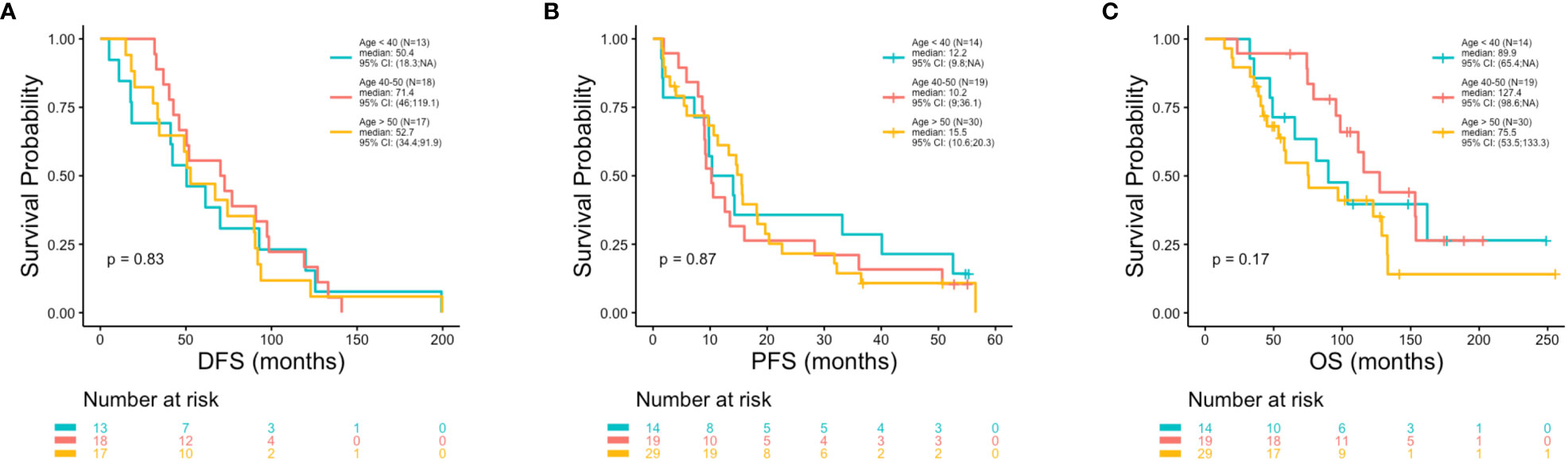
Figure 2 Poor outcomes were not observed in association with younger vs. older age. Kaplan-Meier survival analysis was performed to analyze patient outcomes including disease free survival (DFS), progression free survival (PFS) and overall survival (OS), in association with age. P-values were calculated using the log-rank test, with significance set at p ≤ 0.05. No significant associations were observed between DFS (A) or PFS (B) or OS (C) with age.
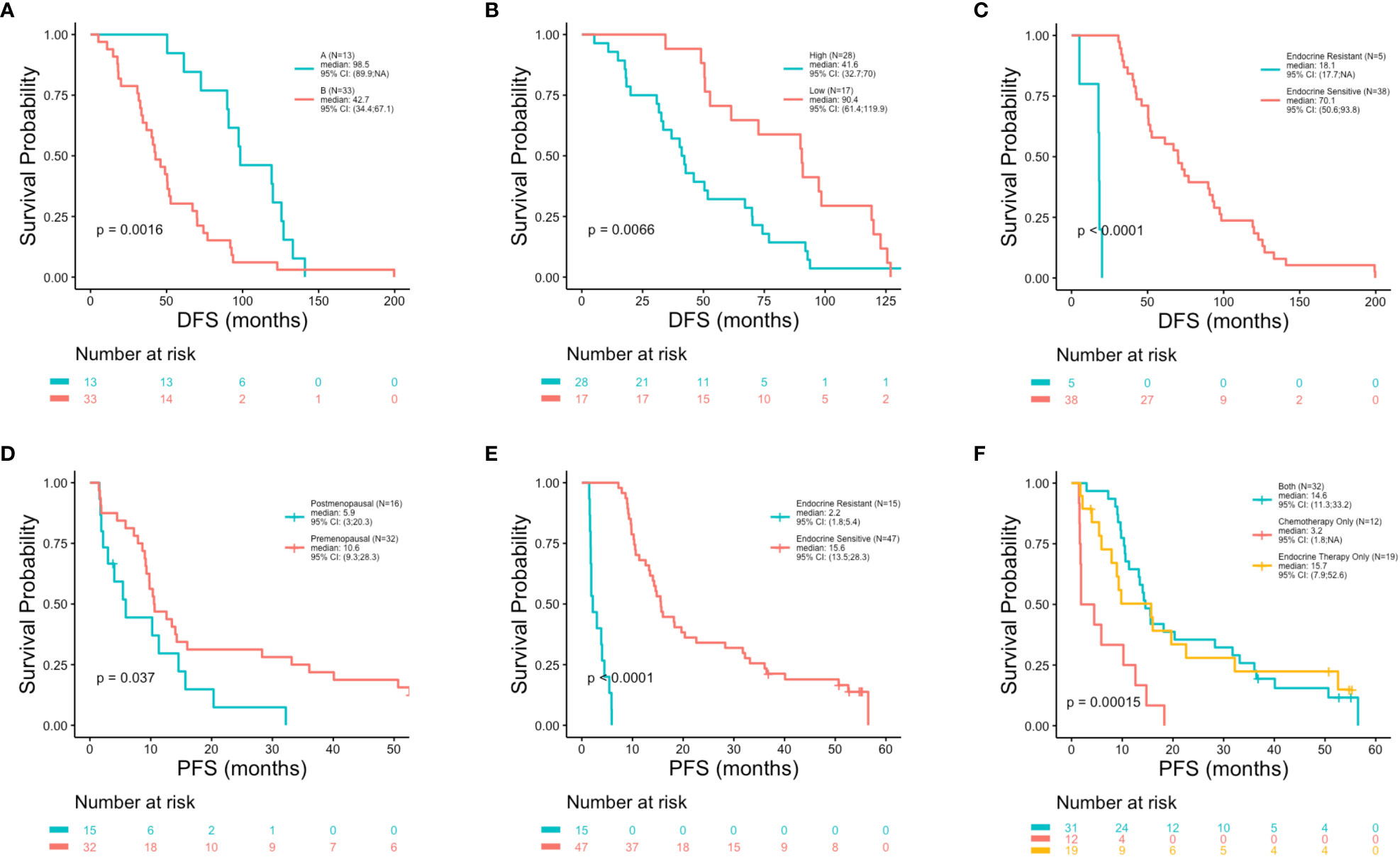
Figure 3 Shorter disease free survival and progression free survival in association with clinicopathologic characteristics. Kaplan-Meier survival analysis was performed to analyze disease free survival (DFS) and progression free survival (PFS) in association with clinicopathologic features. Shorter DFS was observed in associations with luminal subtype (p = 0.0016) (A), high Ki67 index (p = 0.0066) (B) and resistance to adjuvant endocrine therapy (p = 0.0001) (C). Shorter PFS was observed in association with postmenopausal status at primary cancer diagnosis (p = 0.037) (D), resistance to relapse endocrine therapy (p = 0.0001) (E) and type of first-line therapy regimen (p = 0.00015) (F).
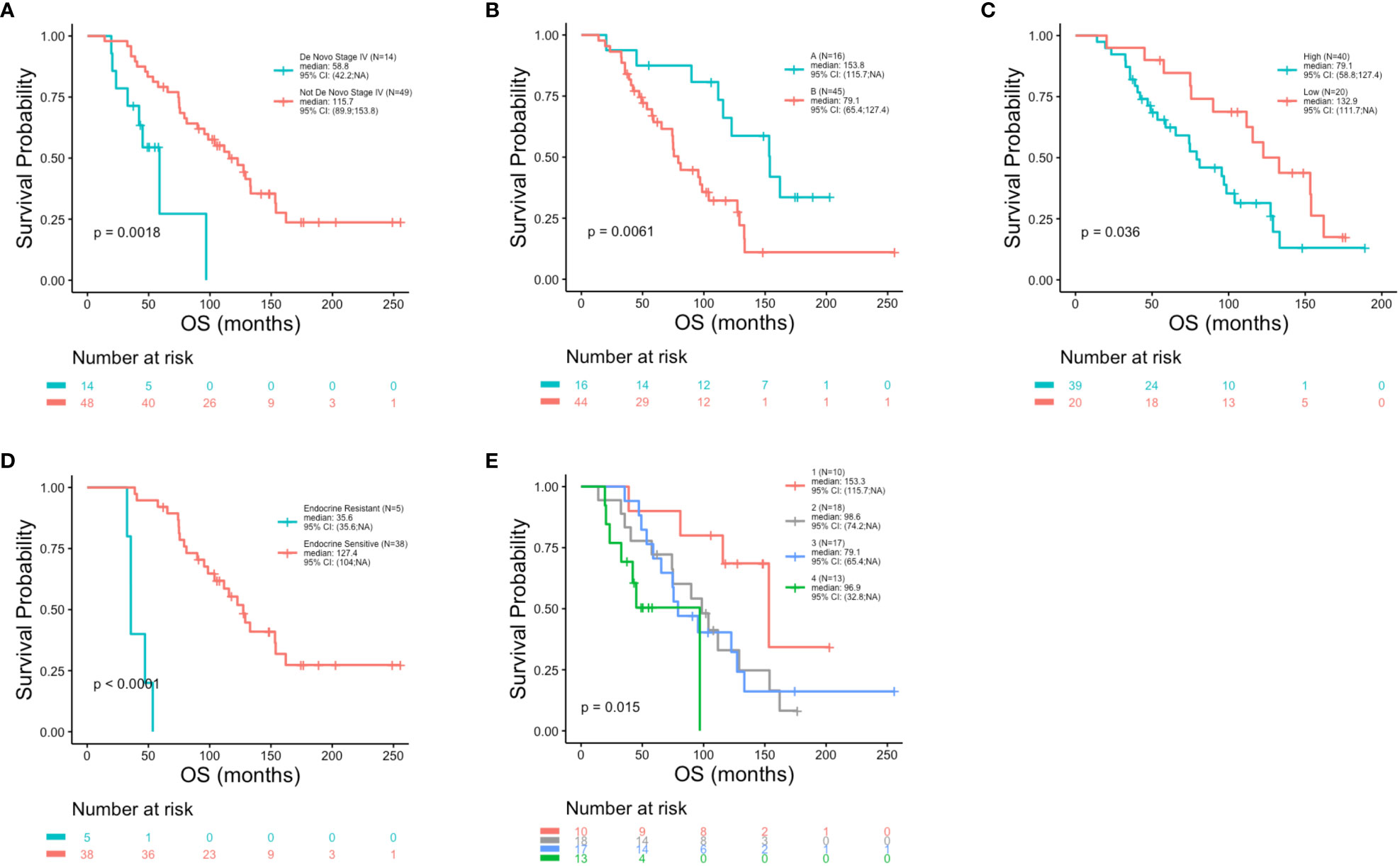
Figure 4 Reduced overall survival in association with clinicopathologic characteristics. Kaplan-Meier survival analysis was performed to analyze overall survival (OS) in association with clinicopathological features. Reduced OS was observed in association with de novo stage IV disease (p = 0.0018) (A), luminal B molecular subtype (p = 0.0061) (B), high Ki67 index (p = 0.036) (C), resistance to adjuvant endocrine therapy (p = 0.0001) (D) and clinical stage (p = 0.015) (E).
Blood samples were collected from 63 patients at the time of metastatic disease diagnosis, prior to the initiation of treatment (Figure 1). As such, the mutational profiles detected in these samples reflect the process of primary breast cancer progression and the impact of adjuvant therapies. The landscape of genomic alterations for the entire cohort shows that the top 10 most frequently altered genes across all patients at this timepoint were PIK3CA (46%), TP53 (37%), FGFR1 (25%), ATM (19%) CCND1 (17%), ARID1A (16%), AKT3 (14%), MYC (14%), ESR1 (13%) and CCND2 (13%) (Figure 5), similar to previously reported findings for an overlapping cohort (36). To examine gene alterations in relation to key patient clinicopathologic variables, we compared somatic genomic profiles across patients grouped according to age at primary cancer diagnosis, menopausal status at primary cancer diagnosis, germline mutation status, type of adjuvant endocrine therapy, molecular subtype, Ki67 index and tumor grade. No significant differences in gene alteration frequencies were observed across age groups, menopausal status, germline mutation status, molecular subtype, Ki67 index, or tumor grade. However, comparison of profiles across women who received adjuvant endocrine therapy revealed a significantly higher prevalence of FGFR1 (p = 0.04), and RB1 (p = 0.03) alterations in patients treated with AIs vs. SERMS (Figure 6).
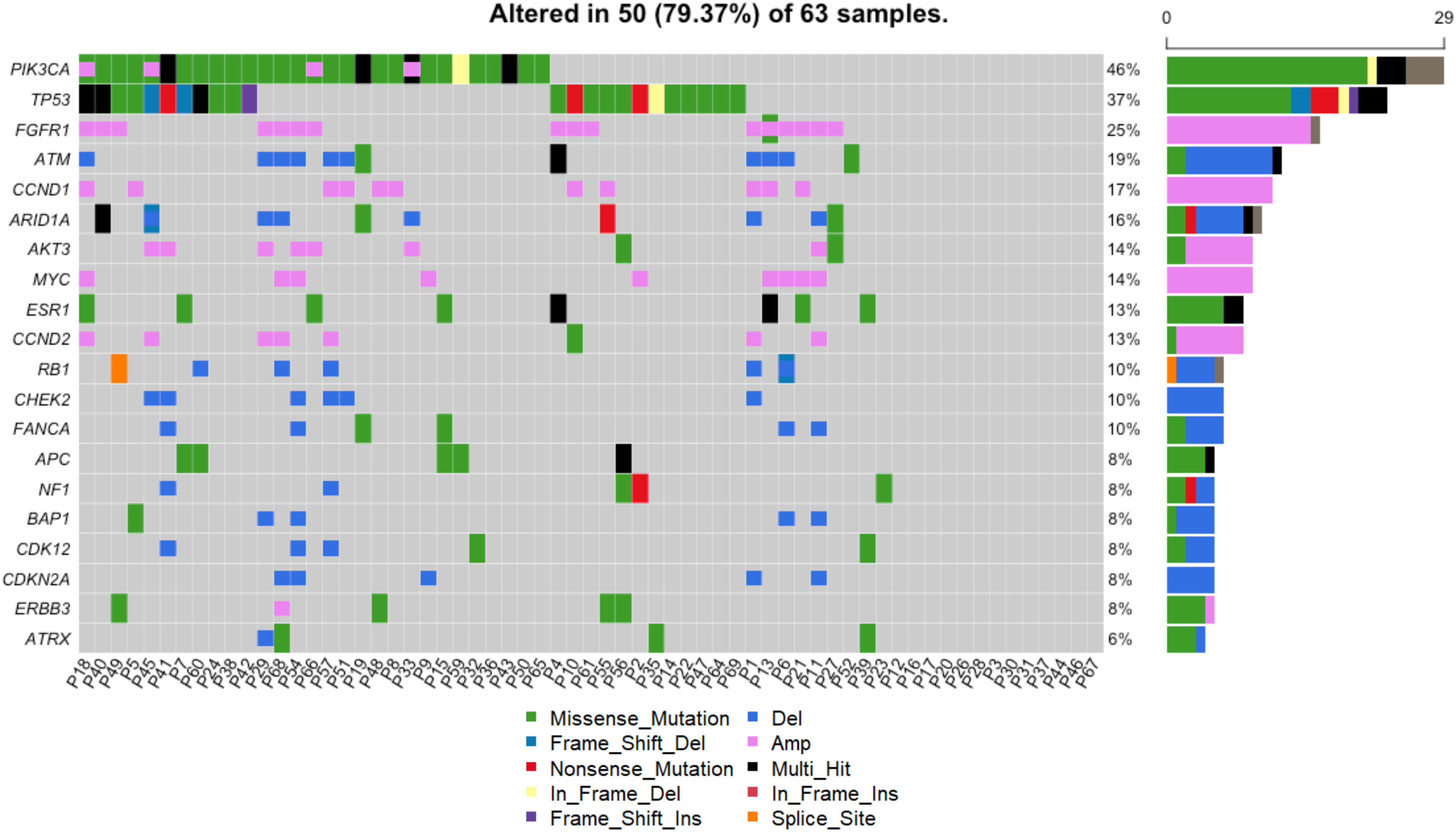
Figure 5 Genomic landscape across all patients. Alterations including SNVs and CNVs were detected in 50/63 (79%) of patients. The heatmap shows alterations of the top 20 most frequently altered genes. Each column shows the alterations for a given patient. The frequency and absolute counts of gene alterations across all patients are shown to the right of the heatmap.
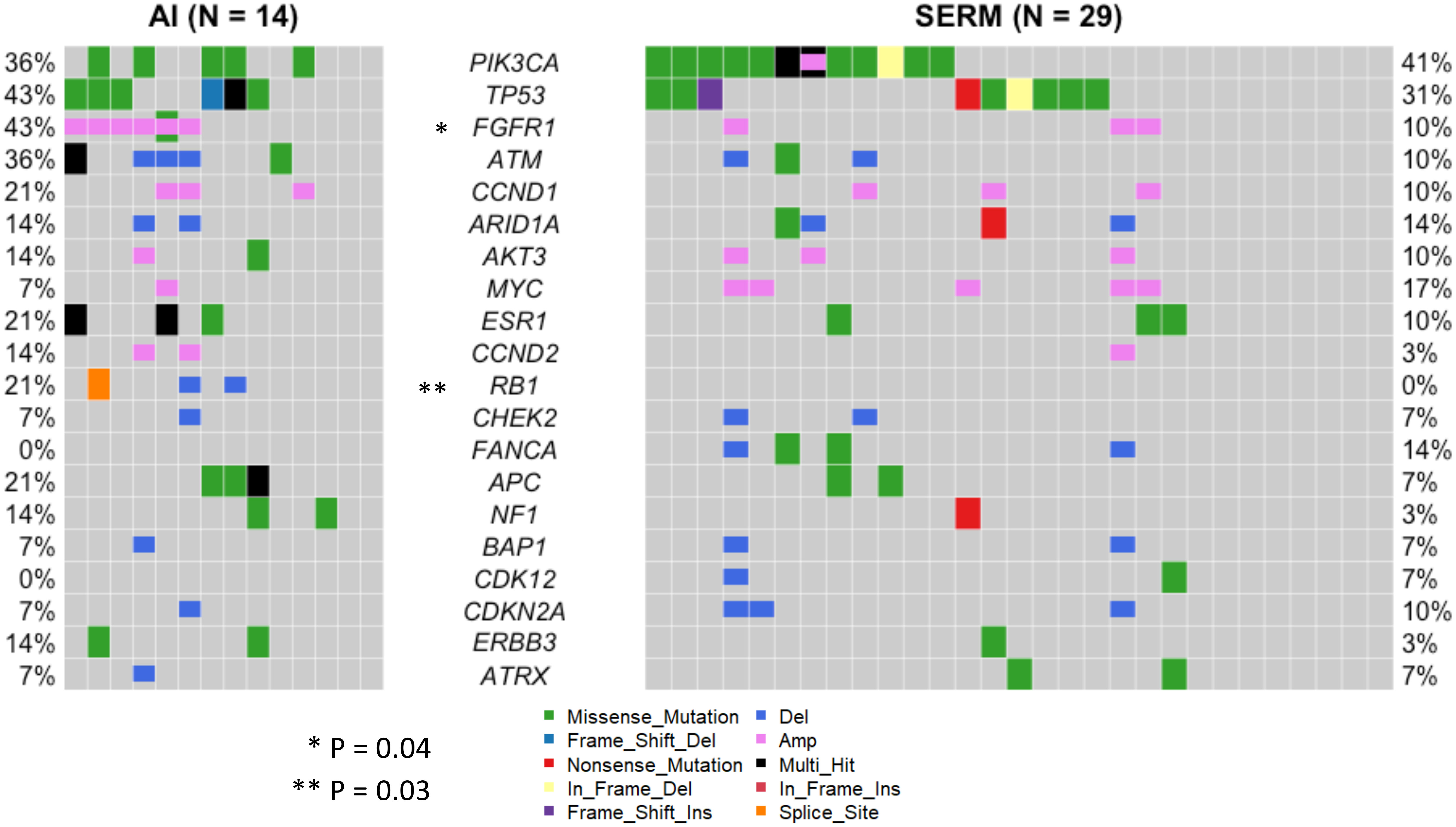
Figure 6 Landscape of genomic alterations across clinicopathological subgroups. Somatic genomic profiles were compared across patients grouped according to age at primary cancer diagnosis, menopausal status at primary cancer diagnosis, germline mutation status, type of adjuvant endocrine therapy, molecular subtype, Ki67 index and tumor grade. Patients who received AI adjuvant endocrine therapy (N=14) had a significantly higher prevalence of FGFR1 (p = 0.04), and RB1 (p = 0.03) alterations than patients treated with SERMS (N=29). No significant differences in gene alteration frequencies were observed across age groups, menopausal status, germline mutation status, molecular subtype, Ki67 index, or tumor grade.
Patient outcomes (DFS, PFS and OS) were examined in association with the top 10 most frequent ctDNA alterations (PIK3CA, TP53, FGFR1, ATM, CCND1, ARID1A, AKT3, MYC, ESR1 and CCND2) detected across all patients at the time of metastatic disease diagnosis (Figure 5). Significantly shorter DFS was observed in association with alterations in FGFR1 (p = 0.005) (Figure 7A), TP53 (p = 0.014) (Figure 7B), and MYC (p = 0.045) (Figure 7C). Significantly shorter PFS was observed in association with alterations in TP53 (p = 0.004) (Figure 7D) and APC (p = 0.011) (Figure 7E). Significantly shorter OS was observed in association with alterations in FGFR1 (p = 0.008) (Figure 8A), TP53 (p = 0.029) (Figure 8B), CCND2 (p = 0.012) (Figure 8C), and RB1 (p = 0.029) (Figure 8D).
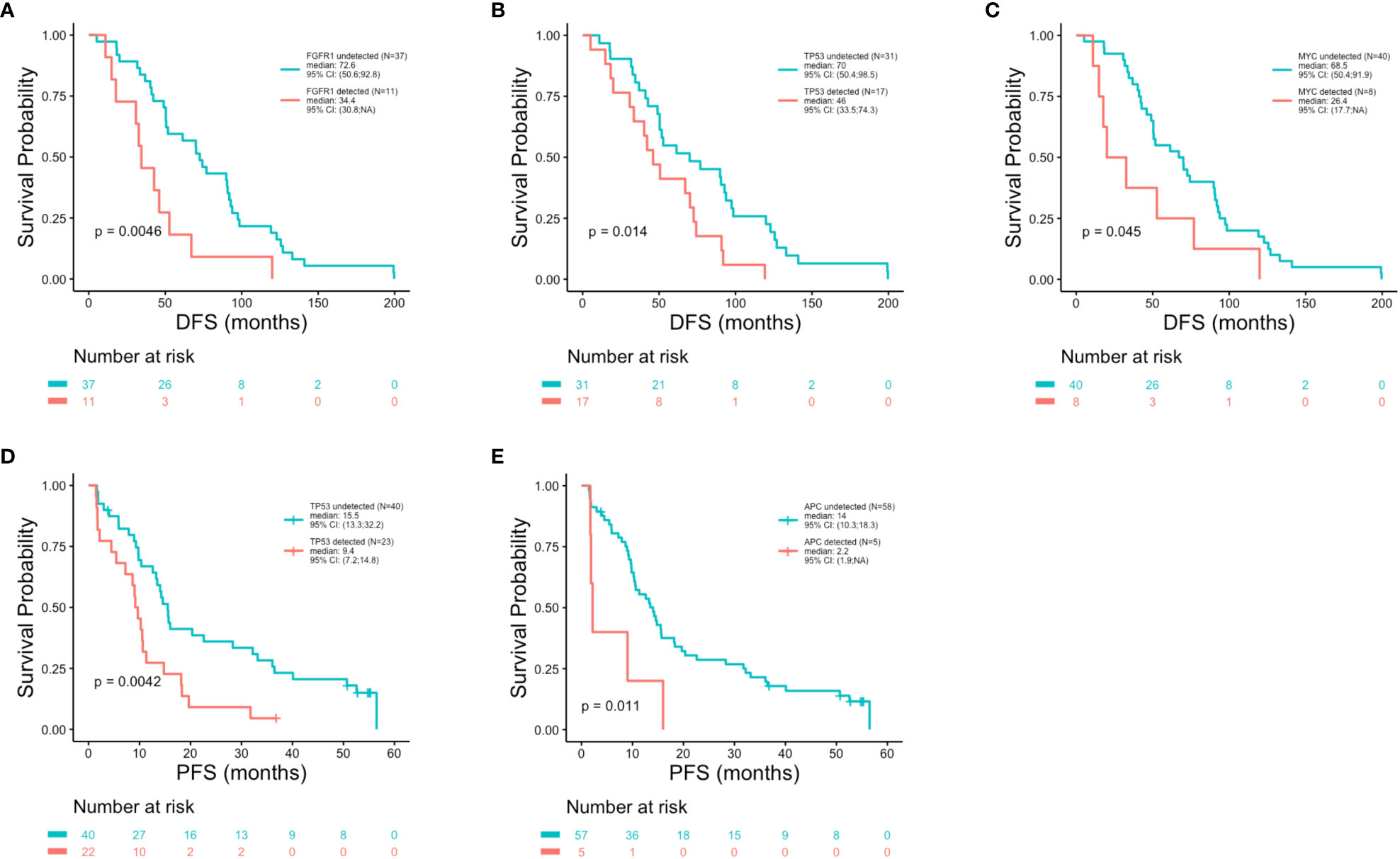
Figure 7 Shorter disease free survival and progression free survival in association with genomic alterations. Kaplan-Meier survival analysis was performed to analyze disease free survival (DFS) and progression free survival (PFS) in association with the top 20 most altered genes. P-values were calculated using the log-rank test, with significance set at p ≤ 0.05. Shorter DFS was associated with the presence of an alteration in FGFR1 (p = 0.0046) (A), TP53 (p = 0.014) (B) and MYC (p = 0.045) (C). Reduced PFS was observed in association with alterations in TP53 (p = 0.0042) (D) and APC (p = 0.011) (E).
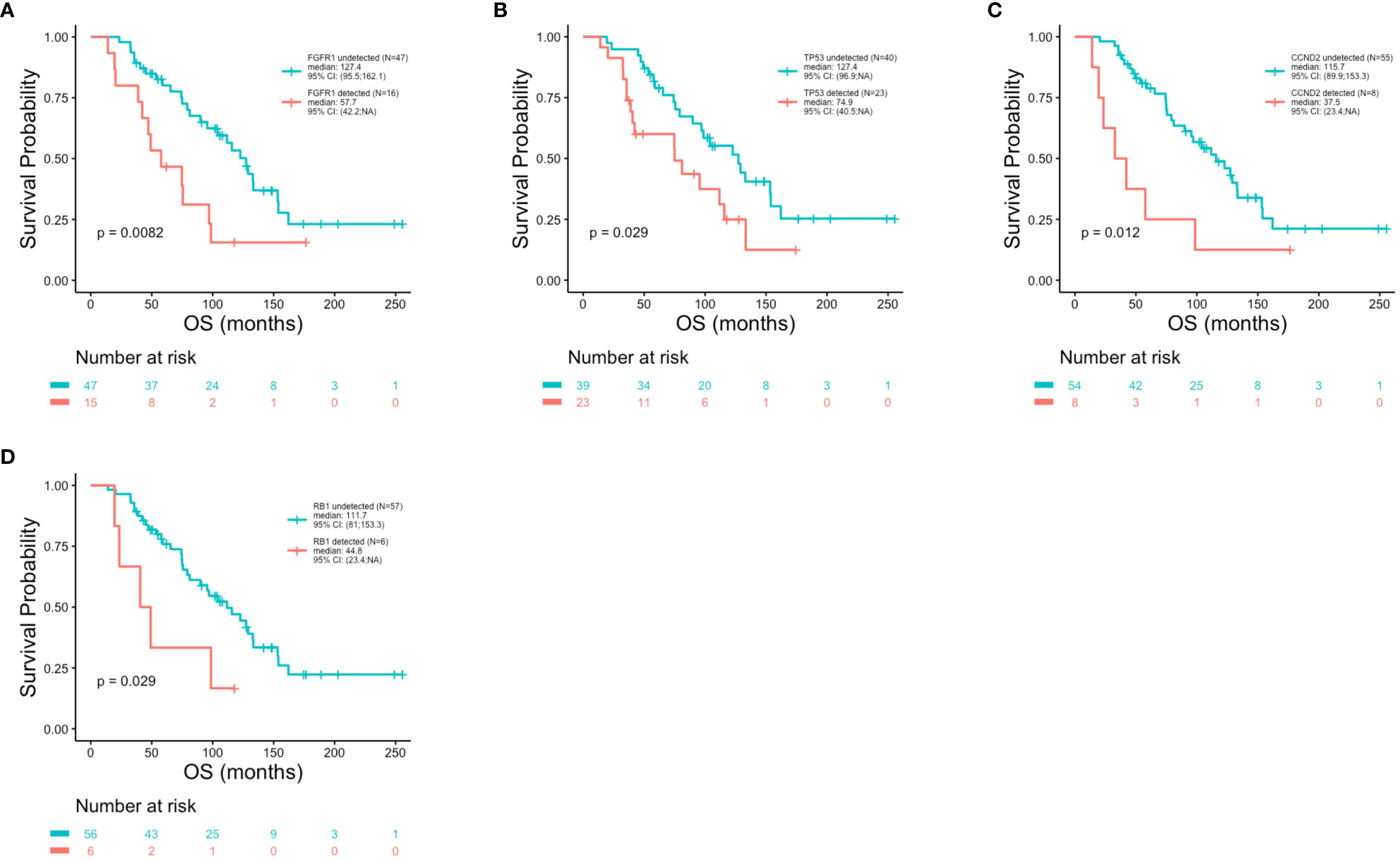
Figure 8 Reduced overall survival in association with genomic alterations. Kaplan-Meier survival analysis was performed to analyze overall survival (OS) in association with specific genomic alterations. P-values were calculated using the log-rank test, with significance set at p ≤ 0.05. Shorter OS was observed in association with FGFR1 (p = 0.0082) (A), TP53 (p = 0.029) (B), CCND2 (p = 0.012) (C) and RB1 (p = 0.029) (D) alterations.
The proportion of patients in the entire cohort with one or more mutations in a specific oncogenic signaling pathways were: DDR (DNA Damage Response) (60.32%), PI3K (52.38%), TP53 (52.38%), RTK/RAS (23.81%), Cell Cycle (14.29%), HRD (Homologous Recombination Deficiency) (7.94%), Hippo (6.35%) and NOTCH (1.59%). No statistically significant differences were observed across age groups.
Breast cancer in younger women has been widely characterized as a more aggressive form of disease relative to breast cancer in older women (5–7). This has been attributed to a variety of factors including the enrichment of aggressive TNBC and HER2+ clinical subtypes among young breast cancer patients (8, 9, 17, 18, 21–24). However, the majority of young breast cancer cases are HR+ (9, 17, 18, 21, 23, 24, 27). While HR+ breast cancer patients as a group have the most favorable prognosis relative to other clinical subtypes, comparatively poor outcomes have also been reported for younger vs. older HR+ patients (8–10, 13, 14, 17, 41). Breast cancer in younger women is particularly common in China (42), where the average age of diagnosis is 10 years younger than in the U.S (43). In the current study half of the real-world HR+/HER- MBC patients were below the age of 50 at the time of primary cancer diagnosis, providing an opportunity to compare their outcomes with the older patients presenting in the same clinic. We have compared clinical outcomes for these patients in association with clinicopathologic features, treatment regimens and genomic profiles. In this study cohort, younger patients did not experience inferior outcomes compared to older patients.
The relationship between age and breast cancer outcomes is complex and is complicated by a number of factors. First, the cutoff for delineating younger vs. older breast cancer patients varies widely across studies, ranging from <35, < 40, <45 and <50 years (9, 10, 13–17, 44, 45). In the present cohort, we compared outcomes and clinicopathologic variables across three age groups: <40 years, 40-50 years, and >50 years. While no significant differences in survival outcomes were detected across these age groups, we did observe significant enrichment of negative prognostic features and shorter median DFS and OS in women <40 vs. 40-50 years. These trends are consistent with numerous studies reporting worse outcomes for very young (<35-40 years) women vs. women aged 40-50 years (10, 11, 13, 16, 17, 44–46) and suggesting that breast cancer patients below 40 years of age may be clinically distinct from those aged 40-50 years. Relatively poor outcomes for very young women in these studies were attributed to a number of factors including incomplete chemotherapy-induced amenorrhea, nonadherence to treatment, lower ER expression levels, hormone resistance, elevated Ki67 levels and higher tumor grade. Additional variation across studies results from the comparison of data from large population-based registries with results from smaller clinical cohorts, both of which have advantages and disadvantages. While large population-based studies are better powered to detect differences across age groups, they are more likely to encompass lower economic levels that have reduced access to health care, resulting in enrichment of poor prognostic features. Although patients in smaller single center cohorts receive the same care, trends often fail to reach significance. And finally, variable control of key clinicopathologic features has generated variation in results across studies. Several reports suggest that the association of young age with poor outcomes is confined to early-stage breast cancer patients (11, 12, 15, 16), while other studies controlling for or focusing exclusively on specific clinical subtypes have failed to observe an association between young age and poor patient outcomes (22, 33, 34).
In contrast to age, poor outcomes were significantly associated with several other clinicopathologic variables in the present study. Reduced OS was observed in association with de novo Stage IV disease, Luminal B subtype, high Ki67 index, resistance to adjuvant endocrine therapy, and clinical stage at primary diagnosis. While a diagnosis of de novo Stage IV disease has been reported as more frequent in younger breast cancer patients and has been invoked to support a more aggressive classification of breast cancer diagnosed at young age (11, 16), in the present study de novo Stage IV disease was more common in women over the age of 50. As aromatase inhibitors constitute standard of care adjuvant endocrine treatment for postmenopausal women, this therapy regimen was administered to the majority of patients > 50 years, while those in the younger age groups mostly received SERMS (47). While no associations were observed between outcomes and type of adjuvant endocrine therapy, patients treated with AIs had a higher frequency of FGFR1 alterations, which were significantly associated with poor outcomes.
In this study, genomic profiling was performed at the time of metastatic breast cancer diagnosis, prior to the initiation of treatment for metastatic disease. Thus, the mutational profiles reflect the process of primary breast cancer progression and the impact of adjuvant therapies. Outcomes, including DFS, PFS and OS were examined in association with the top ten most frequent gene alterations observed in the entire study cohort. Poor outcomes were variously associated with APC, CCND2, FGFR1, MYC, RB1, and TP53 gene alterations. In previous studies FGFR1 gene alterations have been observed in ~ 7-27% of all breast cancers (48), have been associated with poor prognosis, and constitute a critical mechanism underlying the development of treatment resistance to endocrine suppression via AIs and SERMs (48–50). While FGFR1 alterations, consisting predominantly of gene amplification events, were detected in 25% of all the patients in this study, they were significantly more frequent in women treated with AIs (43%) vs. SERMS (10%). Interestingly, while FGFR1 alterations were significantly associated with reduced OS and DFS, these alterations were not associated with shorter PFS. These data suggest that the emergence of an FGFR1 alteration during adjuvant therapy may initiate resistance to adjuvant endocrine therapy that enables accelerated progression to metastatic relapse.
This study has several limitations relating to the small size of the patient cohort, which restricted comparisons and could result in biased analyses. First, due to the small number of patients, it was not possible to evaluate outcomes in association with genomic alterations within age subgroups. Second, due to the small size of the entire cohort, the limited number of events precluded simultaneous evaluation of all pertinent variables within a single multivariate analysis of OS. And third, as adjuvant endocrine therapy was determined by menopausal status, with most patients over 50 receiving AIs, and those under 50 receiving SERMS, it was not possible to incorporate this variable into multivariate analysis. Nevertheless, despite these limitations, all individual multivariate analyses supported the lack of association between OS and age identified in univariate analysis.
In summary, in this study, inferior clinical outcomes were not observed for younger HR+//HER2- metastatic breast cancer patients. Our findings are consistent with other subtype-specific studies that did not find worse outcomes for young HER2+ or TNBC patients (33, 34). While current guidelines advocate that treatment decisions should be based on individual tumor biology rather than age (51), young HR+ breast cancer patients are more likely to receive chemotherapy (52–54). The successful combination of ovarian function suppression with endocrine therapies (55) and the recent inclusion of premenopausal metastatic HR+ breast cancer patients in phase III clinical trials testing CDK4/6 inhibitors clearly demonstrated benefit for young HR+ patients (56), presenting an expanded choice of treatment options. Our findings illustrate the complexity of clinical, biological and genetic variables that underly outcomes for HR+ breast cancer patients of different ages, underscoring the need for biomarker-based treatment strategies for all patients.
All data in the main text are publicly available after publication. The supporting data of this study are available on request from the corresponding author (H.L.).
This study was approved by an Institutional Review Board (ethic No. 2016KT75 and ethic No.2017KT40), and all patients signed written informed consent for additional blood collection for genomic profiling. The study was performed following Good Clinical Practice and the Declaration of Helsinki under protocols approved by the Ethics Committee of Peking University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Conception and design of study: BK, HL. Collection of clinical data: YXL, YHL, JGW. Supervision of sample processing, generation and analysis of sequencing data: HL, YZ, HD. Statistical analysis: JHW, TZ. Interpretation of data: JHW, BK, XL, JY, PD, SJ. Drafting of the manuscript: BK. Critical revision of the manuscript for important intellectual data: BK, SJ, HL. Obtaining funding: HL. All authors contributed to the article and approved the submitted version.
The present study was supported by the Beijing Natural Science Foundation (Grant No. 7212011).
The authors gratefully acknowledge all the patients who participated in this study.
JHW, YZ, HD, TZ and JY were stockholders and/or employed by the company Huidu Shanghai Medical Sciences, Ltd. PD, SJ and BK were stockholders and employed by the company Predicine, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1152575/full#supplementary-material
AI, aromatase inhibitor; BAM, binary alignment map; BWA, Burroughs-Wheeler Aligner; cfDNA, cell free DNA; CHIP, clonal hematopoiesis of indeterminant potential; CNV, copy number variation; DDR, DNA damage response; DFS, disease free survival; EOBC, early onset breast cancer; FSH, follicle-stimulating hormone; HDI, human development index; Her2-, human epidermal growth factor receptor negative; HR+, hormone receptor positive; HRD, Homologous recombination deficiency; Indel, small insertion or deletion; LOBC, late onset breast cancer; MBC, metastatic breast cancer; NGS, next generation sequencing; OS, overall survival; PCR, polymerase chain reaction; PFS, progression free survival; RTK, Receptor tyrosine kinase; SERM, selective estrogen receptor modulator; SNV, single nucleotide variation; TNBC, triple negative breast cancer.
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin (2019) 69(6):438–51. doi: 10.3322/caac.21583
2. Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond) (2021) 41(11):1183–94. doi: 10.1002/cac2.12207
3. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol (2017) 18(12):1579–89. doi: 10.1016/S1470-2045(17)30677-0
4. Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health (2020) 8(8):e1027–e37. doi: 10.1016/S2214-109X(20)30215-1
5. Azim HA Jr., Partridge AH. Biology of breast cancer in young women. Breast Cancer Res (2014) 16(4):427. doi: 10.1186/s13058-014-0427-5
6. Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis (2013) 5 Suppl 1:S2–8. doi: 10.3978/j.issn.2072-1439.2013.05.24
7. Johnson RH, Anders CK, Litton JK, Ruddy KJ, Bleyer A. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. (2018) 65(12):e27397. doi: 10.1002/pbc.27397
8. Azim HA Jr., Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res (2012) 18(5):1341–51. doi: 10.1158/1078-0432.CCR-11-2599
9. Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol (2010) 21(10):1974–81. doi: 10.1093/annonc/mdq072
10. El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer (2006) 6:194. doi: 10.1186/1471-2407-6-194
11. Fredholm H, Magnusson K, Lindstrom LS, Garmo H, Falt SE, Lindman H, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat (2016) 160(1):131–43. doi: 10.1007/s10549-016-3983-9
12. Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg (2009) 208(3):341–7. doi: 10.1016/j.jamcollsurg.2008.12.001
13. Han W, Kang SY, Korean Breast Cancer S. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat (2010) 119(1):193–200. doi: 10.1007/s10549-009-0388-z
14. Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol (2011) 29(29):3885–91. doi: 10.1200/JCO.2011.36.1105
15. Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ (2000) 320(7233):474–8. doi: 10.1136/bmj.320.7233.474
16. Kataoka A, Iwamoto T, Tokunaga E, Tomotaki A, Kumamaru H, Miyata H, et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese breast cancer registry. Breast Cancer Res Treat (2016) 160(1):163–72. doi: 10.1007/s10549-016-3984-8
17. Sheridan W, Scott T, Caroline S, Yvonne Z, Vanessa B, David V, et al. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat (2014) 147(3):617–29. doi: 10.1007/s10549-014-3125-1
18. Akrami M, Sepahdar A, Arasteh P, Tahmasebi S, Zangouri V, Askari A, et al. Do site and type of metastasis in breast cancer show a changing pattern with increased age? a cross comparison of clinicopathological characteristics between age groups. World J Surg Oncol (2018) 16(1):147. doi: 10.1186/s12957-018-1435-1
19. Hung MH, Liu CY, Shiau CY, Hsu CY, Tsai YF, Wang YL, et al. Effect of age and biological subtype on the risk and timing of brain metastasis in breast cancer patients. PloS One (2014) 9(2):e89389. doi: 10.1371/journal.pone.0089389
20. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol (2008) 26(20):3324–30. doi: 10.1200/JCO.2007.14.2471
21. Plichta JK, Thomas SM, Vernon R, Fayanju OM, Rosenberger LH, Hyslop T, et al. Breast cancer tumor histopathology, stage at presentation, and treatment in the extremes of age. Breast Cancer Res Treat (2020) 180(1):227–35. doi: 10.1007/s10549-020-05542-4
22. Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol (2011) 29(1):e18–20. doi: 10.1200/JCO.2010.28.9199
23. Loibl S, Jackisch C, Lederer B, Untch M, Paepke S, Kummel S, et al. Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat (2015) 152(2):377–87. doi: 10.1007/s10549-015-3479-z
24. Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun (2018) 9(1):1725. doi: 10.1038/s41467-018-04129-4
25. Ponder BAJ, Day NE, Easton DF, Pharoah PDP, Lipscombe JM, Redman KPrevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. anglian breast cancer study group. Br J Cancer (2000) 83(10):1301–8. doi: 10.1054/bjoc.2000.1407
26. Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res (2006) 66(16):8297–308. doi: 10.1158/0008-5472.CAN-06-0503
27. Azim HA Jr., Nguyen B, Brohee S, Zoppoli G, Sotiriou C. Genomic aberrations in young and elderly breast cancer patients. BMC Med (2015) 13:266. doi: 10.1186/s12916-015-0504-3
28. Liao S, Hartmaier RJ, McGuire KP, Puhalla SL, Luthra S, Chandran UR, et al. The molecular landscape of premenopausal breast cancer. Breast Cancer Res (2015) 17:104. doi: 10.1186/s13058-015-0618-8
29. Siddig A, Tengku Din T, Mohd Nafi SN, Yahya MM, Sulong S, Wan Abdul Rahman WF. The unique biology behind the early onset of breast cancer. Genes (Basel). (2021) 12(3):1-21. doi: 10.3390/genes12030372
30. Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol (2018) 19(2):169–80. doi: 10.1016/S1470-2045(17)30891-4
31. Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol. (2014) 43(5):1542–62. doi: 10.1093/ije/dyu094
32. Shah AN, Carroll KJ, Gerratana L, Lin C, Davis AA, Zhang Q, et al. Circulating tumor cells, circulating tumor DNA, and disease characteristics in young women with metastatic breast cancer. Breast Cancer Res Treat (2021) 187(2):397–405. doi: 10.1007/s10549-021-06236-1
33. Partridge AH, Gelber S, Piccart-Gebhart MJ, Focant F, Scullion M, Holmes E, et al. Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol (2013) 31(21):2692–8. doi: 10.1200/JCO.2012.44.1956
34. Aine M, Boyaci C, Hartman J, Hakkinen J, Mitra S, Campos AB, et al. Molecular analyses of triple-negative breast cancer in the young and elderly. Breast Cancer Res (2021) 23(1):20. doi: 10.1054/bjoc.2000.1407
35. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol (2020) 31(12):1623–49. doi: 10.1016/j.annonc.2020.09.010
36. Liu X, Davis AA, Xie F, Gui X, Chen Y, Zhang Q, et al. Cell-free DNA comparative analysis of the genomic landscape of first-line hormone receptor-positive metastatic breast cancer from the US and China. Breast Cancer Res Treat (2021) 190(2):213–26. doi: 10.1007/s10549-021-06370-w
37. Zhang J, Wang N, Zheng T, Lu T, Zhang R, Ran R, et al. Germline mutational landscape in Chinese patients with advanced breast cancer. Front Oncol (2022) 12:745796. doi: 10.3389/fonc.2022.745796
38. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell (2018) 173(2):321–37 e10. doi: 10.1016/j.cell.2018.03.035
39. Dietlein F, Weghorn D, Taylor-Weiner A, Richters A, Reardon B, Liu D, et al. Identification of cancer driver genes based on nucleotide context. Nat Genet (2020) 52(2):208–18. doi: 10.1038/s41588-019-0572-y
40. Martinez-Jimenez F, Muinos F, Sentis I, Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, et al. A compendium of mutational cancer driver genes. Nat Rev Cancer. (2020) 20(10):555–72. doi: 10.1038/s41568-020-0290-x
41. Sun H, Huang W, Ji F, Pan Y, Yang L. Comparisons of metastatic patterns, survival outcomes and tumor immune microenvironment between young and non-young breast cancer patients. Front Cell Dev Biol (2022) 10:923371. doi: 10.3389/fcell.2022.923371
42. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol (2014) 15(7):e279–89. doi: 10.1016/S1470-2045(13)70567-9
43. Song QK, Li J, Huang R, Fan JH, Zheng RS, Zhang BN, et al. Age of diagnosis of breast cancer in china: almost 10 years earlier than in the united states and the European union. Asian Pac J Cancer Prev (2014) 15(22):10021–5. doi: 10.7314/APJCP.2014.15.22.10021
44. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea–a report from the Korean breast cancer society. J Clin Oncol (2007) 25(17):2360–8. doi: 10.1200/JCO.2006.10.3754
45. Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Orlando L, Ghisini R, et al. Role of endocrine responsiveness and adjuvant therapy in very young women (below 35 years) with operable breast cancer and node negative disease. Ann Oncol (2006) 17(10):1497–503. doi: 10.1093/annonc/mdl145
46. Chia KS, Du WB, Sankaranarayanan R, Sankila R, Wang H, Lee J, et al. Do younger female breast cancer patients have a poorer prognosis? results from a population-based survival analysis. Int J Cancer (2004) 108(5):761–5. doi: 10.1002/ijc.11632
47. Xu B, Li H, Jiang Z, Gu L, Tang J, Xie H, et al. Adjuvant tamoxifen switched to exemestane treatment in postmenopausal women with estrogen receptor-positive early breast cancer: a pragmatic, multicenter, and prospective clinical trial in China. Chin J Cancer Res (2022) 34(6):592–600. doi: 10.21147/j.issn.1000-9604.2022.06.07
48. Drago JZ, Formisano L, Juric D, Niemierko A, Servetto A, Wander SA, et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR(+)) breast cancer. Clin Cancer Res (2019) 25(21):6443–51. doi: 10.1158/1078-0432.CCR-19-0138
49. Giltnane JM, Hutchinson KE, Stricker TP, Formisano L, Young CD, Estrada MV, et al. Genomic profiling of ER(+) breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci Transl Med (2017) 9(402):1-35. doi: 10.1126/scitranslmed.aai7993
50. Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res (2010) 70(5):2085–94. doi: 10.1158/0008-5472.CAN-09-3746
51. Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA Jr., Bianchi-Micheli G, et al. ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4). Ann Oncol (2020) 31(6):674–96. doi: 10.1016/j.annonc.2020.03.284
52. Dalal AA, Gauthier G, Gagnon-Sanschagrin P, Burne R, Guerin A, Niravath P, et al. Treatment and monitoring patterns among premenopausal women with HR+/HER2- advanced breast cancer. Adv Ther (2018) 35(9):1356–67. doi: 10.1007/s12325-018-0764-3
53. Hartkopf AD, Huober J, Volz B, Nabieva N, Taran FA, Schwitulla J, et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors - data from the German PRAEGNANT breast cancer registry. Breast (2018) 37:42–51. doi: 10.1016/j.breast.2017.10.002
54. Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the southeast Netherlands breast cancer consortium. Ann Oncol (2016) 27(2):256–62. doi: 10.1093/annonc/mdv544
55. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med (2018) 379(2):122–37. doi: 10.1056/NEJMoa1803164
56. Shah AN, Metzger O, Bartlett CH, Liu Y, Huang X, Cristofanilli M. Hormone receptor-Positive/Human epidermal growth receptor 2-negative metastatic breast cancer in young women: emerging data in the era of molecularly targeted agents. Oncologist (2020) 25(6):e900–e8. doi: 10.1634/theoncologist.2019-0729
Keywords: hormone receptor-positive breast cancer, metastatic breast cancer, cfDNA, liquid biopsy, biomarkers, prognosis
Citation: Wang J, Liu Y, Liang Y, Zhang Y, Dong H, Zheng T, Yu J, Du P, Jia S, King BL, Wang J, Liu X and Li H (2023) Clinicopathologic features, genomic profiles and outcomes of younger vs. older Chinese hormone receptor-positive (HR+)/HER2-negative (HER2-) metastatic breast cancer patients. Front. Oncol. 13:1152575. doi: 10.3389/fonc.2023.1152575
Received: 27 January 2023; Accepted: 23 May 2023;
Published: 08 June 2023.
Edited by:
Mangesh A. Thorat, Guy’s and St Thomas’ NHS Foundation Trust, United KingdomReviewed by:
Kitt Dokal, Imperial College London, United KingdomCopyright © 2023 Wang, Liu, Liang, Zhang, Dong, Zheng, Yu, Du, Jia, King, Wang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiping Li, aHVpcGluZ2xpMjAxMkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.