- Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Objective: Many clinical trials of immune checkpoint inhibitors (ICIs) in combination with chemotherapy in the first-line treatment of extensive-stage small cell lung cancer (ES-SCLC) have been initiated, but the conclusions of these trials are not identical. This meta-analysis aimed to comprehensively collect these randomized clinical controlled trials (RCTs) to evaluate the efficacy and safety of ICIs combined with chemotherapy in the first-line treatment of ES-SCLC.
Methods: We systematically searched PubMed, Embase, and ClinicalTrials databases, to find relevant studies published until October 2022.RevMan 5.4 software was used for statistical analysis. The Cochrane Risk of Bias Tool was adopted to evaluate the risk of bias in the included studies. The primary outcome of this study was overall survival (OS), while the secondary outcomes were progression-free survival (PFS), objective response rate (ORR), all grand AEs (AEs), and ≥ 3 grand adverse events (≥ 3 AEs).
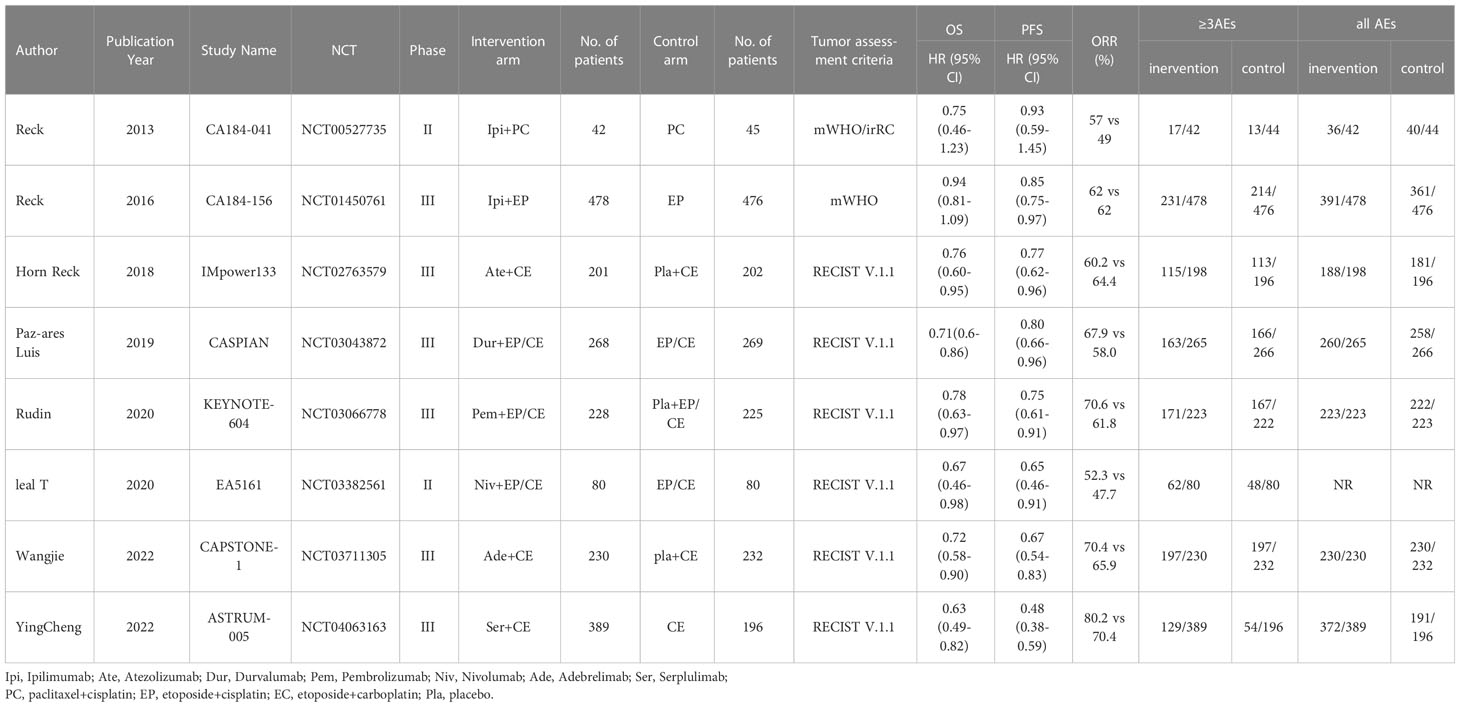
Results: A total of 780 articles were obtained in the initial examination, which was screened by layer and finally included 8 studies including 3367 patients. Six studies evaluated the efficacy of PD-1/PD-L1 inhibitors (Pembrolizumab, Nivolumab, Atezolizumab, Durvalumab, Adebrelimab, Serpulimab) combined with chemotherapy, and two studies evaluated the efficacy of CTLA-4 inhibitors (Ipilimumab) in combination with chemotherapy. The results showed that compared to chemotherapy alone, ICIs combined with chemotherapy significantly improved patients’ OS (HR=0.8, 95% CI (0.72-0.85), P<0.05), PFS (HR = 0.72, 95% CI (0.63-0.83), P < 0.05), and ORR(RR=1.08, 95% CI: 1.03-1.13, P<0.05), but patients would experience more any grand AEs and ≥3 grand AEs. Subgroup analysis showed that the PD-1/PD-L1 group performed better than the CTLA-4 group in both efficacy and safety. And ICIs plus chemotherapy significantly improved OS and PFS in patients regardless of age, gender, and performance status.
Conclusion: The addition of ICIs to chemotherapy resulted in significant improvements in both PFS and OS for patients with ES-SCLC, but patients would experience more AEs.
1 Introduction
Globally, lung cancer is the second most common cancer and one of the main causes of death due to cancer. In 2020, 1.8 million people died from lung cancer (1). Small cell lung cancer (SCLC) accounts for about 15% of lung cancer, characterized by high aggressiveness and lethality. Due to its rapid progression and early onset of metastasis, about 60-70% of SCLC patients are diagnosed with extensive small cell lung cancer (ES-SCLC) when found diseased (2). SCLC is highly sensitive to chemotherapy, so chemotherapy usually shows obvious treatment effects in the early stage, and the remission rate can reach 60% ~ 80%. However, most patients will progress or relapse in a short time after early chemotherapy, and the relapsed tumor is resistant to further treatment (3, 4). In the past 40 years, etoposide or irinotecan and platinum remain the standard first-line treatments for ES-SCLC. But even with standard first-line chemotherapy, the median overall survival (OS) of patients is only about 10 months (5, 6), the average OS is only 2 ~ 4 months (7, 8), and the 5-year survival rate is only about 7% (9). Therefore, finding better treatment options for the majority of patients has become a new goal for researchers.
In recent years, the advent of immunotherapy, especially immune checkpoint inhibitors (ICIS), has changed the traditional treatment paradigm for multiple tumors. Tumor cells can escape from attacks by the immune system through multiple mechanisms, and their cell surface-expressed immunosuppressive molecules can secrete immunosuppressive factors and can also recruit other suppressive immune cell groups (10). Specific inhibitors against checkpoint receptors can block this immunosuppression, thereby increasing the specific immune response of T lymphocytes and eliciting an antitumor response (11, 12). Currently, the most common ICIS used in clinical practice is monoclonal antibodies (mAbs) against programmed death receptor 1 (PD-1) and its ligand 1 (PD-L1), cytotoxic T-lymphocyte antigen 4 (CTLA-4).ICIs have achieved phased success in the treatment of non-small cell lung cancer, melanoma, gastric cancer, liver cancer, bladder cancer, kidney cancer, and other malignant tumors in the past decade (13).
Based on the results of the checkmate-032 (14) study, nivolumab was approved by the US Food and Drug Administration (FDA) in 2018 for the third-line treatment of ES-SCLC. The phase III randomized trial IMpower-133, CASPIAN demonstrated the addition of Atezolizumab or Durvalumab to first-line chemotherapy can improve progression-free survival (PFS) and OS, respectively, with results still favoring the combination arm after three years of follow-up. Therefore, the FDA subsequently approved Atezolizumab or Durvalumab combined with chemotherapy for the first-line treatment of ES-SCLC (15). But not all researches show the benefit of ICIs for ES-SCLC patients. In KEYNOTE-604 (16), the improved OS of patients treated with Pembrolizumab plus chemotherapy compared with chemotherapy alone was not evident. The same was true for the combination of Nivolumab and chemotherapy in another phase II study, EA5161, in which the median PFS was 5.5 months and 4.7 months for Nivolumab plus chemotherapy and chemotherapy alone, respectively.
Therefore, this study aims to collect data from these trials more comprehensively around the world, evaluate the efficacy and safety of ICIs for ES-SCLC, and provide some reference for the clinical selection of treatment for small cell lung cancer.
2 Methods
2.1 Search strategy
According to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (17, 18), we systematically searched PubMed, Embase, and ClinicalTrials databases, to find relevant studies published until October 2022. The main search terms and combinations include extensive staging, SCLC, ICIs, and randomized controlled trials. The manual search of the list of references for all available reviews was also conducted to confirm the final selection. Three reviewers independently conducted literature searches. Three researchers conducted the literature search independently.
2.2 Inclusion and exclusion criteria
Establish inclusion and exclusion criteria based on the principles of PICOS (Population, Intervention, Comparison, Output, Study design).
Inclusion criteria: (1) randomized controlled trial (RCT); (2) Patients with pathologically confirmed ES-SCLC who have not previously received treatment, regardless of race, age, or gender; (3) Intervention measures: ICIs plus chemotherapy versus placebo combined with chemotherapy or chemotherapy alone; (4) Outcome measures: overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and ≥ 3 grand adverse events (AEs).
Exclusion criteria: (1) Non-randomized controlled trials or retrospective studies; (2) Unable to obtain outcome measures indicators or full text; (3) Repeated publications; (4) Non-Chinese and English literature.
2.3 Data extraction
Two researchers independently screen the literature and extract data. If there are differences, they will negotiate with a third researcher to resolve them. Data extraction included: the author, year of publication, study design, number of patients, specific intervention plans, and related outcome measures. The Cochrane Risk of Bias Tool was adopted to evaluate the risk of bias in the included studies (19).
2.4 Statistical analysis
RevMan 5.4 software was used for statistical analysis. Hazard ratio (HR) for survival outcomes (OS and PFS), odds ratio (OR) for dichotomous outcomes (ORR and AEs), and their 95% confidence interval (CI) were used to measure outcomes and safety. Heterogeneity between included studies was quantified by combining I2 to determine the magnitude of heterogeneity. If the heterogeneity across the studies is small, a fixed effect model was used for meta-analysis; If there is significant heterogeneity across studies, the source of heterogeneity was further analyzed, and a random effects model was used for meta-analysis after excluding the effect of obvious clinical heterogeneity. P < 0.05 was taken as statistically significant.
3 Results
3.1 Literature search
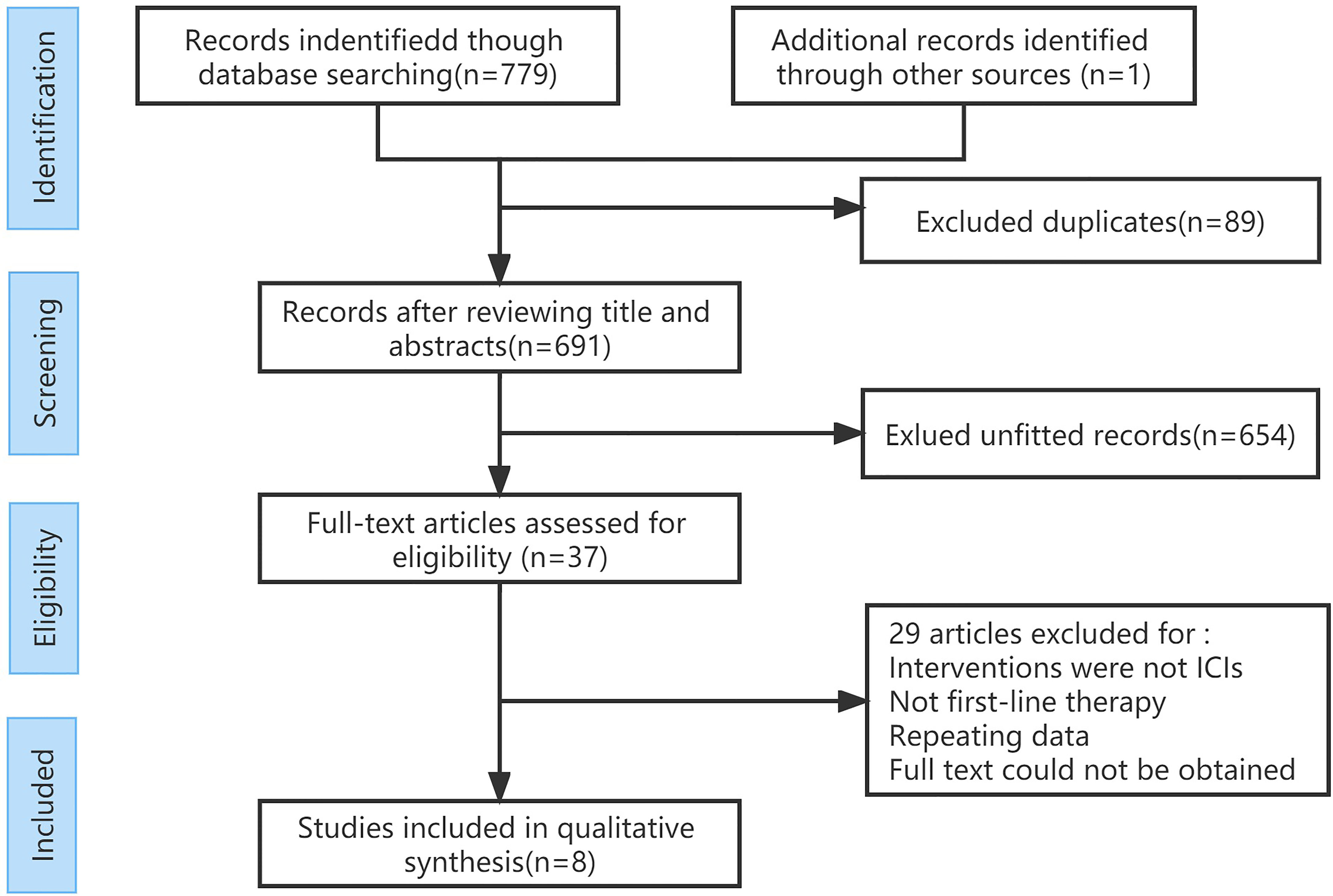
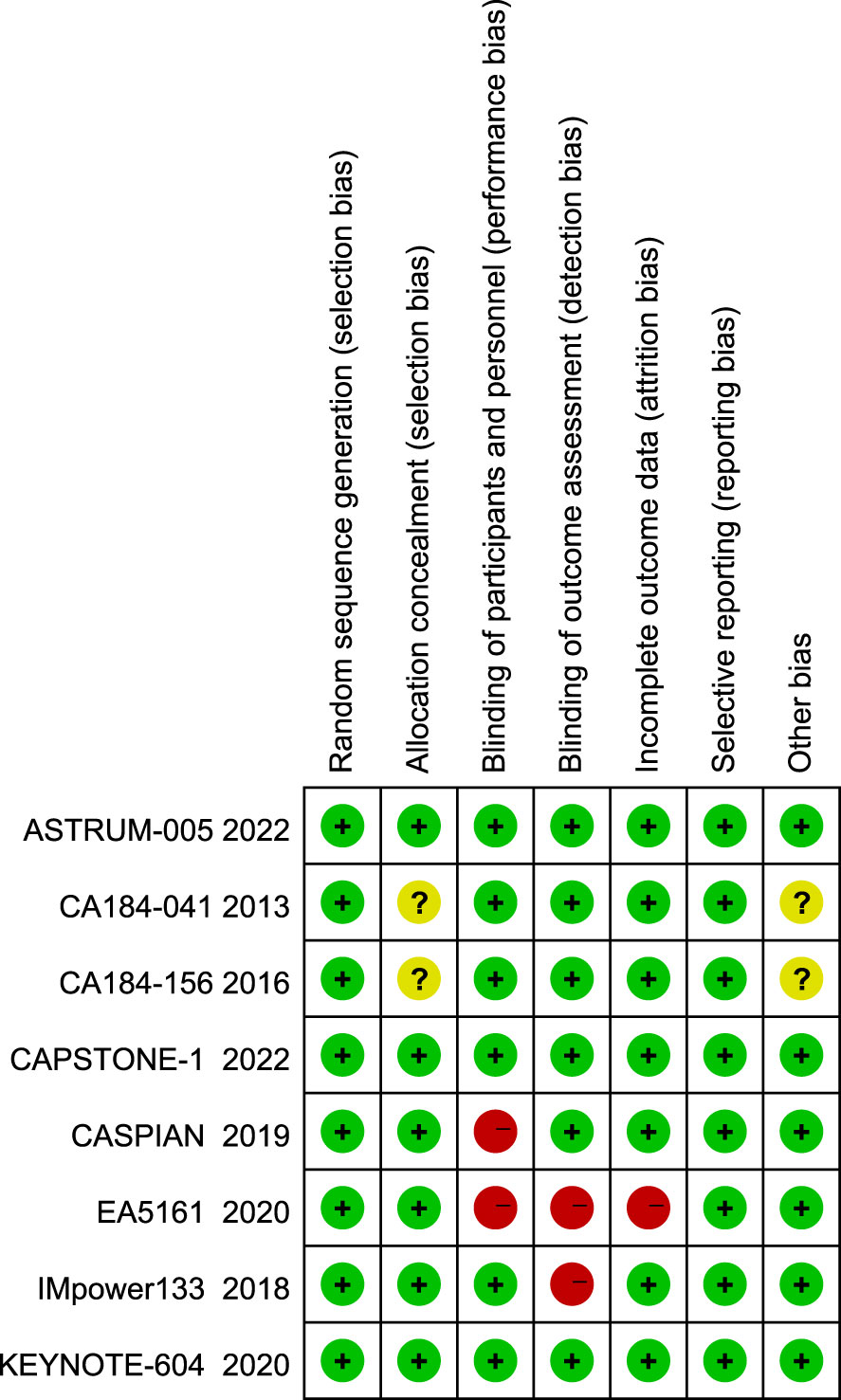
A total of 780 articles were obtained in the initial examination, which was screened by layer and finally included 8 studies including 3367 patients. The literature screening flow is shown in Figure 1. Table 1 summarizes the basic characteristics of all included studies. We adopted the Cochrane risk of bias tool to evaluate the quality of the included studies, and the results of the studies are shown in Figure 2.
Six studies evaluated the efficacy of PD-1/PD-L1 inhibitors (Pembrolizumab, Nivolumab, Atezolizumab, Durvalumab, Adebrelimab, Serpulimab) combined with chemotherapy, and two studies evaluated the efficacy of CTLA-4 inhibitors (Ipilimumab) in combination with chemotherapy. All studies reported on OS, PFS, ORR, and AEs, and we also performed subgroup analyses by gender, age, and performance status subgroups based on information published by the studies.
3.2 Results of meta-analysis
The primary outcome of this study was OS, while the secondary outcomes were PFS, ORR, and AEs.
3.2.1 OS
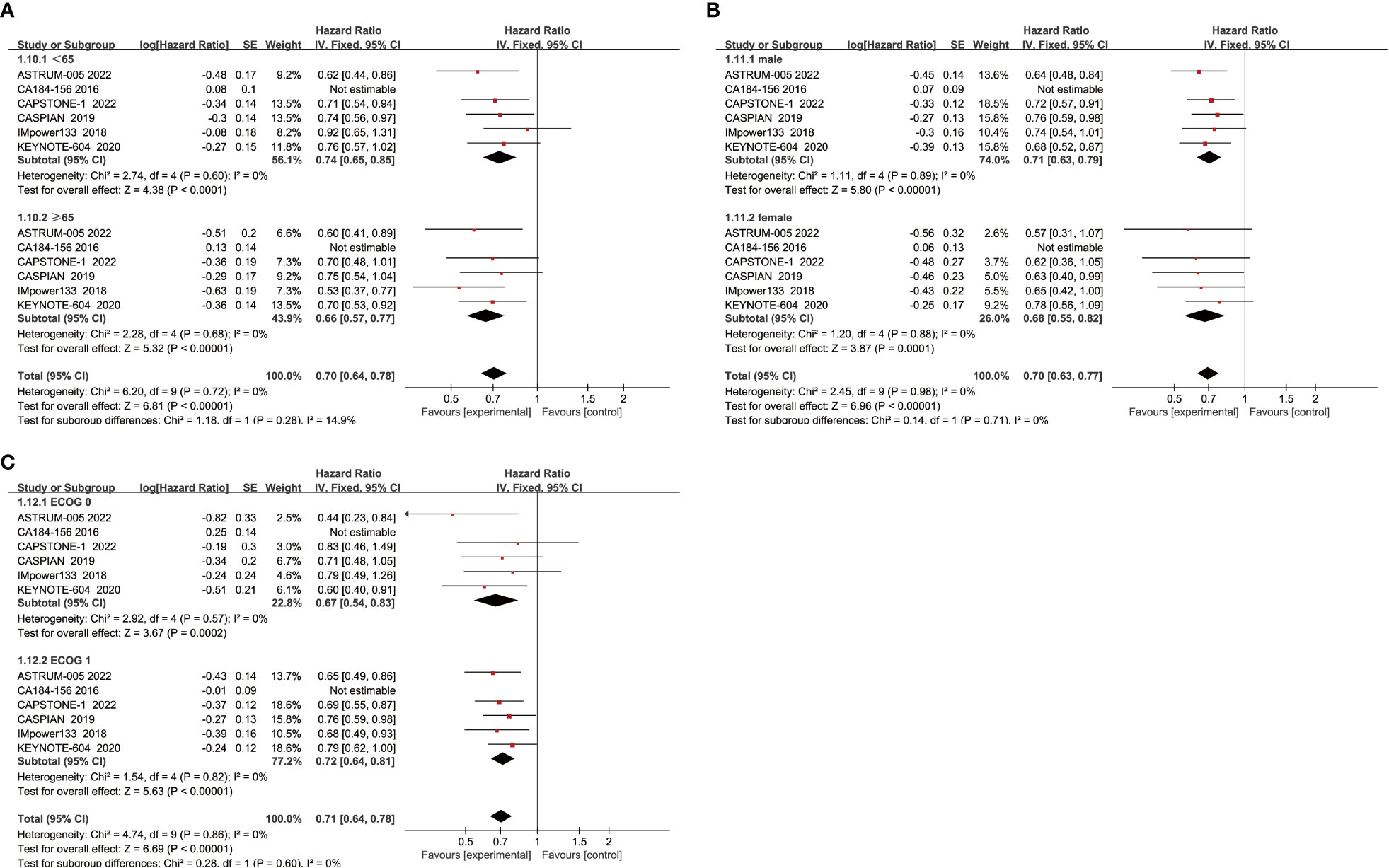
Regarding OS, eight studies have reported OS, with no significant heterogeneity among all studies (I2 = 39%; Figure 3). The results showed that compared to chemotherapy alone, ICIs combined with chemotherapy significantly improved patients’ OS (HR=0.78, 95% CI:0.72-0.85, P<0.05).
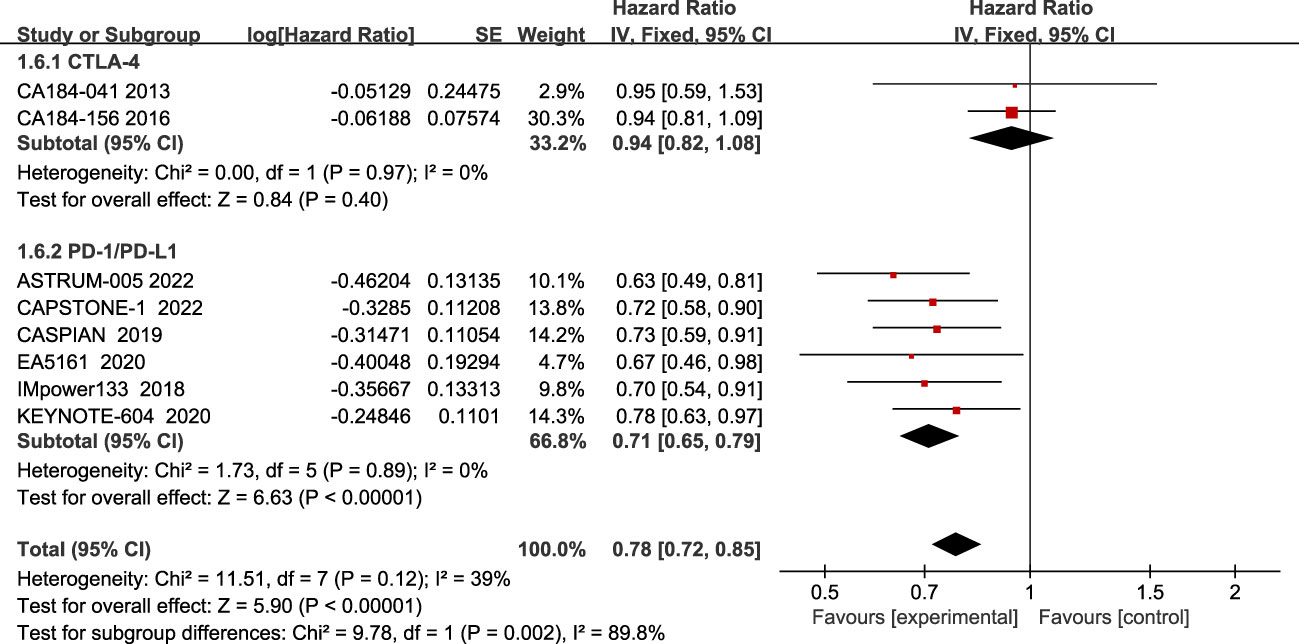
Figure 3 Forest plot of OS comparison between ICIs combined with chemotherapy and chemotherapy alone and subgroup analysis.
The results of the subgroup analysis showed that PD-1/PD-L1 inhibitors combined with chemotherapy significantly improved OS in patients(HR=0.71, 95% CI:0.65-0.79, P<0.05), while there was no statistically significant improvement in the CTLA-4 inhibitor group(HR=0.94, 95% CI:0.82-1.08, P=0.40).
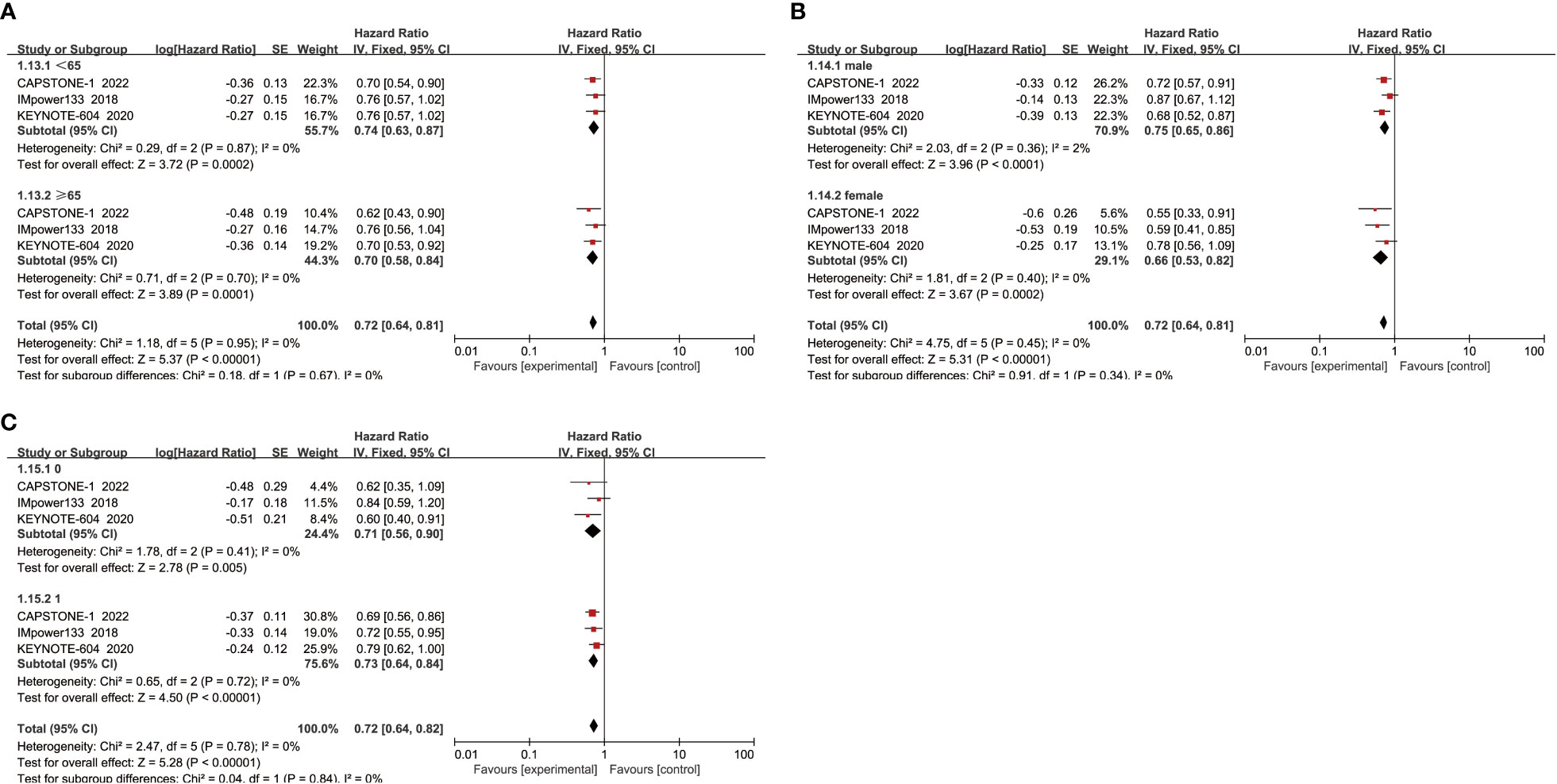
When subgroup analysis was based on age, gender, and performance status, the results showed that ICIs combined with chemotherapy significantly improved OS in patients compared with chemotherapy alone, regardless of whether they were male(HR=0.71, 95% CI:0.63-0.79, P<0.05) or female(HR=0.68, 95% CI:0.55-0.82, P<0.05), aged ≥65 years(HR=0.66, 95% CI:0.57-0.77, P<0.05) or <65 years(HR=0.74, 95% CI:0.65-0.85, P<0.05), and performance status score of 0(HR=0.67, 95% CI:0.54-0.83, P<0.05) or 1(HR=0.72, 95% CI:0.64-0.81, P<0.05) (Figure 4).
3.2.2 PFS
Regarding PFS, all eight included studies reported PFS outcome measures, and a random effects model was used because of heterogeneity among studies (I2 = 69%; Figure 5). The results showed that the treatment regimen of ICIS combined with chemotherapy significantly improved PFS in patients, and the difference was statistically significant (HR = 0.72, 95% CI:0.63-0.83, P < 0.05).

Figure 5 Forest plot of PFS comparison between ICIs combined with chemotherapy and chemotherapy alone and subgroup analysis.
Subgroup analysis found a large heterogeneity among PD-1/PD-L1 inhibitor groups, and after excluding the study ASTRUM-005 using a case-by-case exclusion method, there was no heterogeneity (I2 = 0%), and the results showed that PD-1/PD-L1 inhibitors significantly improved PFS in patients(HR=0.74, 95% CI:0.67-0.82, P<0.05).In contrast, there was no heterogeneity between studies in the CTLA-4 group, indicating that its combination with chemotherapy improves patients’ PFS compared to chemotherapy alone(HR=0.86, 95% CI:0.76-0.97, P<0.05).
When subgroup analysis was conducted for age, gender, and performance status, it was found that regardless of age<65 years(HR=0.74, 95% CI:0.63-0.87, P<0.05) or ≥ 65 years(HR=0.70, 95% CI:0.58-0.84, P<0.05), male(HR=0.75, 95% CI:0.65-0.86, P<0.05) or female(HR=0.66, 95% CI:0.53-0.82, P<0.05), and ECOG score was 1(HR=0.73, 95% CI:0.64-0.84, P<0.05) or 0(HR=0.71, 95% CI:0.56-0.90, P<0.05), ICIs combined with chemotherapy could effectively improve the PFS of patients, with a statistically significant difference (Figure 6).
3.2.3 ORR
Regarding ORR, eight studies have reported ORR, and the heterogeneity among the studies is not significant (I2 = 34%; Figure 7). Compared with the chemotherapy group, the ORR of the combined treatment group was significantly improved (68.2% vs 62.2%), (RR=1.08, 95% CI: 1.03-1.13, P<0.05). Subgroup analysis showed that patients in the PD-1/PD-L1 inhibitor group had significantly improved ORR (RR=1.10, 95% CI:1.04-1.16, P<0.05), while the CTLA-4 inhibitor group did not show a significant difference in improving ORR compared to the chemotherapy alone (RR=1.02, 95% CI: 0.93-1.13, P=0.63).
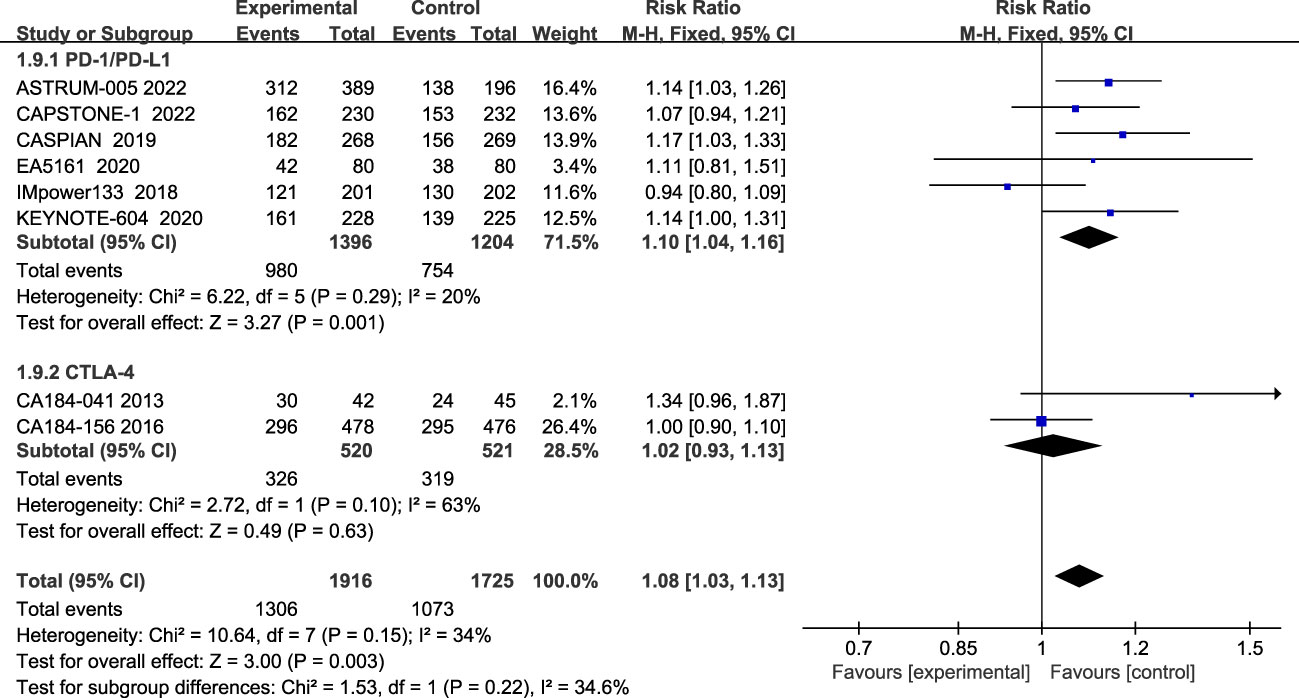
Figure 7 Forest plot of ORR comparison between ICIs combined with chemotherapy and chemotherapy alone and subgroup analysis.
3.2.4 Safety analysis
Seven studies reported all treatment-related adverse events, and heterogeneity among studies was not evident(I2 = 0%; Figure 8). The most common of all grade AEs were neutropenia, anemia, leukopenia, thrombocytopenia, nausea, diarrhea, alopecia, etc. The results showed that patients treated with ICIs plus chemotherapy had more all-grade treatment-related adverse events than those treated with chemotherapy alone (HR=1.33, 95% CI:1.03-1.73, P<0.05). Subgroup analysis showed that patients in the CTLA-4 inhibitor group (HR=1.37, 95% CI:1.01-1.85, P<0.05) and patients treated with PD-1/PD-L1 inhibitors (HR=1.24, 95% CI:0.74-2.07, P=0.41) all had more all-grade treatment-related adverse events than chemotherapy alone, although the difference was not statistically significant.
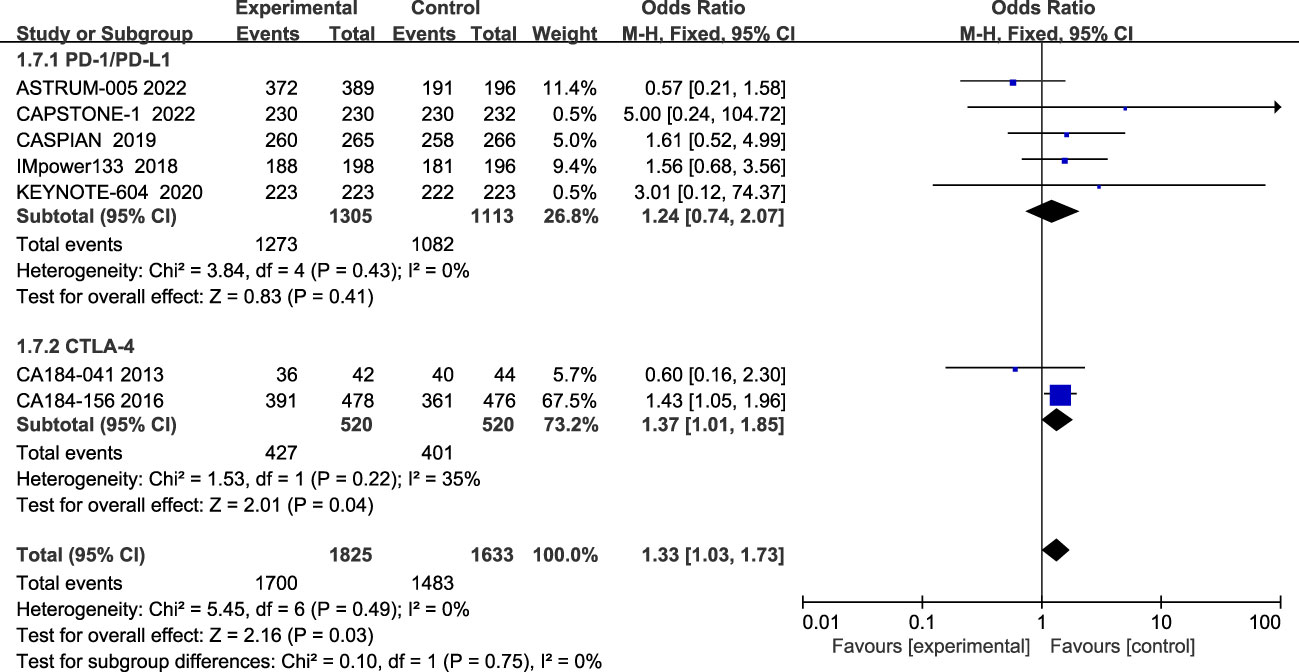
Figure 8 Forest plot of all grade AEs comparison between ICIs combined with chemotherapy and chemotherapy alone and subgroup analysis.
The incidence of grade 3 and above AES during treatment was reported in eight studies, with insignificant heterogeneity among studies (I2 = 29%; Figure 9). The most common grade 3 and above AEs were mainly in the hematologic, such as neutropenia, anemia, thrombocytopenia, leucopenia, etc., while the main manifestations outside the hematological system are nausea, alopecia, and impaired liver function, etc. According to the results, patients treated with ICIs plus chemotherapy had more grade 3 and higher chemotherapy-related adverse events than patients treated with chemotherapy alone (RR=1.06; 95% CI:1.00-1.12; P=0.04). Subgroup analysis showed that patients in the CTLA-4 inhibitor group (RR=1.11; 95% CI (0.97-1.27); P=0.12) and patients in the PD-1/PD-L1 inhibitor group (RR=1.04; 95% CI, 0.98-1.10; P=0.16) both had more grade 3 and higher adverse events than chemotherapy alone, although the differences were not statistically significant.
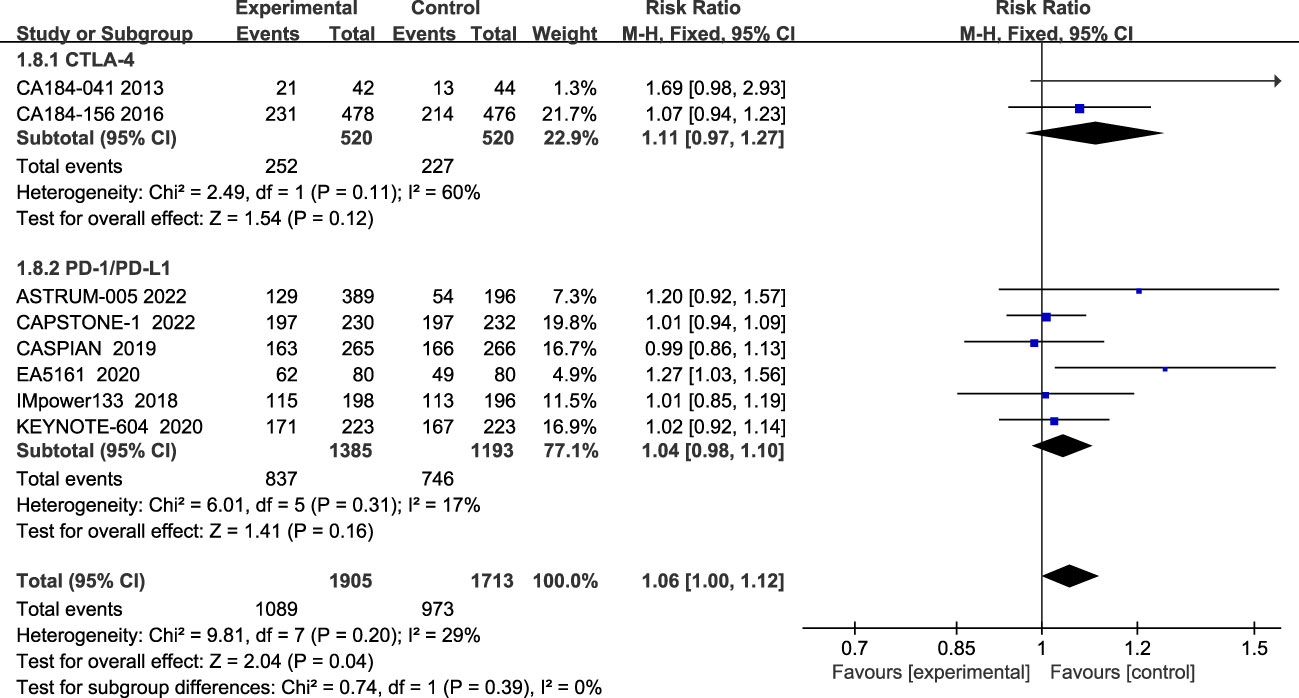
Figure 9 Forest plot of grade≥3AEs comparison between ICIs combined with chemotherapy and chemotherapy alone and subgroup analysis.
3.3 Heterogeneity and publication bias
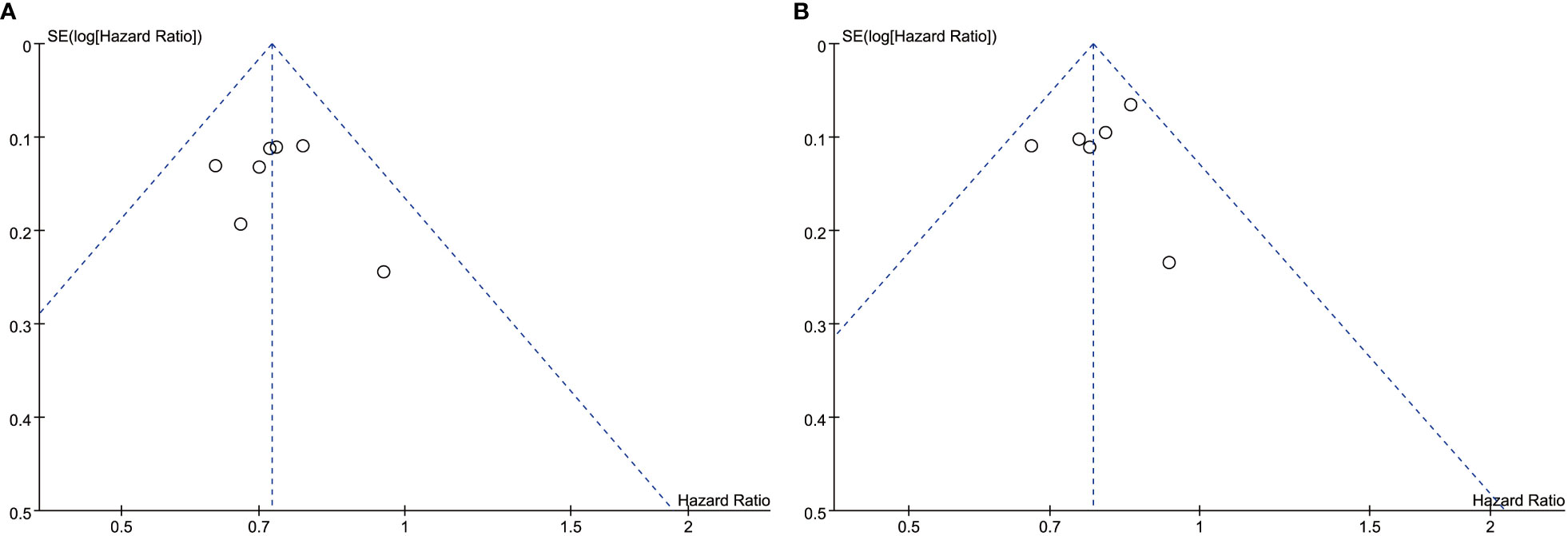
In the present study, the only significantly heterogeneous outcome was PFS (I2 = 69%). After excluding ASTRUM-005 from this study there was no significant heterogeneity (I2 = 0%), which may be because ASTRUM-005 was the most recently conducted trial, and the duration of follow-up was relatively short by the date of data cutoff.
The overall quality of the included studies was high. The ones that included RCT were open-label with some risk of bias. Because all trials have been properly randomized, the risk of confounding in RCTs is minimal. Funnel plot asymmetry was not obvious for any outcome (Figure 10).
4 Discussion
Through genomic analyses of SCLC, investigators have discovered mutations in two tumor suppressor genes in SCLC, p53, and Rb1, which can induce genomic instability and lead to the production of tumor-related antigens (20). Moreover, long-term exposure to smoking carcinogens causes SCLC to become one of the tumors with the highest mutational burden (TMB), which is also closely associated with a large number of potentially immunogenic neoantigens present in the tumors (21, 22). Some researches show an association between high TMB and sensitivity to ICIS, which is associated with the promotion of tumor-specific CD8 + T cell responses (23–25). However, according to the current clinical research results, the correlation between elevated TMB in SCLC and the benefit of ICIs is much lower than that observed in non-small cell lung cancer. This may be due to the limited infiltration of immune cells into the tumor microenvironment, reduced PD-L1 expression, and lack of antigen presentation, which to some extent diminishes the efficacy of immunotherapy in SCLC (26–28). But even so, compared to conventional standard chemotherapy in the past, the application of antibodies that block the pathway of immune checkpoints in SCLC is still promising. Moreover, SCLC is a malignant neuroendocrine tumor with a high frequency of tumor suppressor factors and genetic aberrations of oncogenes, making it feasible to treat SCLC with ICIs.
This study collected 8 RCTs, including CTLA-4 and PD-1/PD-L1 inhibitors, and conducted a meta-analysis of their updated follow-up data (29–31). The results showed that adding ICIs to ES-SCLC first-line chemotherapy significantly improved patients’ OS, PFS, and ORR compared to chemotherapy alone, but patients would experience more AEs. Such results are consistent with the observed application of ICIs in the real world (32). Patients treated with ICIs combined with chemotherapy exhibit higher ORR, indicating that immunotherapy can effectively mobilize the body’s anti-tumor system in ES-SCLC patients, and that can benefit patients more, making ICIs combined with chemotherapy a promising therapeutic strategy for SCLC. Although more grade 3 and above adverse events were observed in patients treated with ICIs plus chemotherapy, after early intervention treatment, most adverse events can also be well resolved. This also reminds us that in clinical applications, more attention should be paid to adverse events during the treatment of ICIs, and patients should be treated as soon as possible and properly managed.
Subgroup analysis showed that ICIs plus chemotherapy significantly improved OS and PFS in patients regardless of age, gender, and performance status. And the PD-1/PD-L1 group performed better than the CTLA-4 group in both efficacy and safety. Results from two studies evaluating CTLA-4 inhibitors demonstrated its ability to prolong PFS, but no clear improvement in OS was observed. This is similar to the results of patients with advanced squamous non-small cell lung cancer receiving Ipilimumab combined chemotherapy. One possible explanation is that Ipilimumab may stimulate early T-cell activation, which may not produce effective antitumor responses in the local tumor environment without corresponding effector T-cell activation. Currently, there are few studies related to CTLA-4 inhibitors, and more studies are needed in the future to determine the role of CTLA-4 inhibitors in the treatment of ES-SCLC.
Of note, our study included the most recent RCTs, such as CAPSTONE-1, which evaluates Adebrelimab, and ASTRUM-005, which evaluates Serplulimab, as well as some recent follow-up results from previous studies that were not contained in previous Meta-analysis studies.
Of course, the included studies and this study also have limitations: there are not many RCTs included; In the included studies, the combined chemotherapy regimen was not completely consistent; The characteristics of eligible patients in each RCT are not the same; Some studies have corporate support, which may lead to unpublished negative results; The impact of potential factors such as smoking history, region, and PD-L1 expression level in patients.
5 Conclusion
In conclusion, this study is the latest and most comprehensive study comparing ICIs plus chemotherapy with chemotherapy alone for first-line treatment of ES-SCLC, which verified the efficacy and safety through meta-analysis and provided some drug references for the clinical treatment. However, this study is still flawed and needs to be validated by further large samples and high-quality randomized controlled trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CC and XF contributed to the conception and design of the study. CC, PT, and JZ collected and assessed the literature. CC and PT performed the statistical analysis. CC wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer (2008) 113(1):5–21. doi: 10.1002/cncr.23542
3. Marchetti C, De Felice F, Romito A, Iacobelli V, Sassu CM, Corrado G, et al. Chemotherapy resistance in epithelial ovarian cancer: Mechanisms and emerging treatments. Semin Cancer Biol (2021) 77:144–66. doi: 10.1016/j.semcancer.2021.08.011
4. Costa M. Twenty-third heidelberger symposium on cancer research. Semin Cancer Biol (2021) 76:1–2. doi: 10.1016/j.semcancer.2021.08.010
5. Lara PN, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, et al. Phase III trial of Irinotecan/Cisplatin compared with Etoposide/Cisplatin in extensive-stage small-cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol (2009) 27(15):2530–5. doi: 10.1200/JCO.2008.20.1061
6. Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J Clin Oncol (2012) 30(14):1692–8. doi: 10.1200/JCO.2011.40.4905
7. Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol OncolJ Hematol Oncol (2019) 12(1):47. doi: 10.1186/s13045-019-0736-3
8. Blackhall F, Frese KK, Simpson K, Kilgour E, Brady G, Dive C. Will liquid biopsies improve outcomes for patients with small-cell lung cancer? Lancet Oncol (2018) 19(9):e470–81. doi: 10.1016/S1470-2045(18)30455-8
9. Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol (2017) 14(9):549–61. doi: 10.1038/nrclinonc.2017.71
10. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
11. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol (2002) 3(11):991–8. doi: 10.1038/ni1102-991
12. Pardoll D. Cancer and the immune system: Basic concepts and targets for intervention. Semin Oncol (2015) 42:523–38. doi: 10.1053/j.seminoncol.2015.05.003
13. Proto C, Ferrara R, Signorelli D, Lo Russo G, Galli G, Imbimbo M, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev (2019) 75:39–51. doi: 10.1016/j.ctrv.2019.03.004
14. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/S1470-2045(16)30098-5
15. Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC). Pharmacol Ther (2017) 180:16–23. doi: 10.1016/j.pharmthera.2017.06.002
16. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
17. Moher D, Liberati A, Tetzlaff J, Altman DG. For the PRISMA group. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339(jul21 1):b2535–5. doi: 10.1136/bmj.b2535
18. Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg (2015) 4(2):112–22. doi: 10.3978/j.issn.2225-319X.2015.02.04
19. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev (2019). doi: 10.1002/14651858.ED000142
20. George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature (2015) 524(7563):47–53. doi: 10.1038/nature14664
21. Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, et al. Mutational signatures associated with tobacco smoking in human cancer. Science (2016) 354(6312):618–22. doi: 10.1126/science.aag0299
22. Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature (2010) 463(7278):184–90. doi: 10.1038/nature08629
23. McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (2016) 351(6280):1463–9. doi: 10.1126/science.aaf1490
24. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell (2018) 33(5):853–861.e4. doi: 10.1016/j.ccell.2018.04.001
25. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther (2017) 16(11):2598–608. doi: 10.1158/1535-7163.MCT-17-0386
26. Calles A, Aguado G, Sandoval C, Álvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol (2019) 21(8):961–76. doi: 10.1007/s12094-018-02011-9
27. Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: Beyond traditional treatment approaches. J Thorac Oncol (2013) 8(5):587–98. doi: 10.1097/JTO.0b013e318286cf88
28. Tian Y, Zhai X, Han A, Zhu H, Yu J. Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J Hematol OncolJ Hematol Oncol (2019) 12(1):67. doi: 10.1186/s13045-019-0753-2
29. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol Off J Am Soc Clin Oncol (2021) 39(6):619–30. doi: 10.1200/JCO.20.01055
30. Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open (2022) 7(2):100408. doi: 10.1016/j.esmoop.2022.100408
31. Cheng Y, Wang J, Zhou C, Yao W, Wang Q, Min X, et al. Abstract CT038: Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage SCLC: A phase 3 trial. Cancer Res (2022) 82(12_Supplement):CT038–8. doi: 10.1158/1538-7445.AM2022-CT038
Keywords: small cell lung cancer, chemotherapy, immune checkpoint inhibitors, randomized controlled trial, meta-analysis
Citation: Chen C, Tian P, Zhong J and Fan X (2023) Efficacy and safety of immune checkpoint inhibitors combined with chemotherapy in patients with extensive-stage small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Front. Oncol. 13:1151769. doi: 10.3389/fonc.2023.1151769
Received: 26 January 2023; Accepted: 28 March 2023;
Published: 19 April 2023.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Maria De Miguel, Centro Integral Oncológico Clara Campal, SpainQibiao Wu, Macau University of Science and Technology, Macao SAR, China
Copyright © 2023 Chen, Tian, Zhong and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianming Fan, ZnhtMTI5QDE2My5jb20=
 Chunlan Chen
Chunlan Chen Peng Tian
Peng Tian